?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mung bean starches are widely used for producing transparent, good-quality noodles in the food industry. This study isolated starches from eight mung bean varieties and investigated how the amylose contents (19.1–32.9%) of the beans affected the structures, physicochemical properties, and resistant starch (RS) fractions of these starches. The starches were classified into three groups according to their amylose content: low-amylose starches with <25% amylose content (TN182, DXVN7, and DX208 varieties), intermediate-amylose starches with 25%-30% amylose contents (DX14, KPS1, and V123 varieties), and high-amylose starches with >30% amylose contents (T135 and DX044 varieties). The amylose content of mung bean starches negatively correlated with the average degree of polymerization () of the starch, whereas the mung bean variety dictated the average chain length (
, degree of order, and short-range molecular order of the starch. All mung bean starches were found to have an A-type crystalline structure with a relative crystallinity of 25.8–34.7%. The starches with higher amylose contents exhibited higher peaks, final viscosities, and setbacks than the other starches. The contents of the RS fraction were in the range of 3.6–13.0%; the starches of the DX14, T135, and DX044 varieties had higher contents of the RS fractions (9.0%, 9.9%, and 13.0%, respectively) than the starches from the other mung bean varieties.
Abbreviations: MB1, starch isolated from TN182 variety; MB2, starch isolated from DXVN7 variety; MB3, starch isolated from DX208 variety; MB4, starch isolated from DX14 variety; MB5, starch isolated from KPS1 variety; MB6, starch isolated from V123 variety; MB7, starch isolated from T135 variety; MB8, starch isolated from DX044 variety.
Introduction
Mung bean (Vigna radiata), a popular, essential edible crop of the legume family, originated from Southeast Asia thousands of years ago. The high protein, vitamin, mineral, dietary fiber, and flavonoid content has established mung bean as a functional food.[Citation1–3] Additionally, mung bean starch, which accounts for 26%-31% of the seeds, is considered an important component of transparent noodles because of its characteristic high shear resistance, restricted swelling, and good granular stability.[Citation4,Citation5]
Amylose and amylopectin, the two glucose polymers synthesized by starch synthases, form amorphous and crystalline regions within the starch granules. Their different arrangements govern the formation of texture and quality of starch-based food products. Amylose is an essentially linear molecule joined by α-(1,4)-linked D-glucopyranosyl bonds with an average degree of polymerization (n) of 324–4920 and amylopectin is a very large, highly branched chain molecule with a
n of 1278–15900 and consists of α-(1,6)-linked D-glucopyranosyl units attached to α-(1,4)-bonds.[Citation6,Citation7] Together, they give rise to alternating amorphous and semi-crystalline growth rings or shells with a radial thickness of 120–400 nm in starch granules.[Citation6] In such an arrangement, the amylose to amylopectin ratio is normally 1:3 for most starches, including legume starches. However, this ratio has been manipulated for processing purposes to create mutant genotypes of plants with increased amylose or amylopectin contents in starch. The high-amylose-containing starches have low gelatinization temperature and crystallinity, peak, breakdown, and low final viscosities of starch paste. In contrast, the low-amylose-containing starches exhibit high gelatinization temperature, transition enthalpy and crystallinity, peak viscosity, breakdown, and swelling power but low peak viscosity-temperature and final viscosity of the pastes.[Citation8] Therefore, starches with varying amylose levels have different supramolecular structures, resulting in various compositions and changes in physicochemical characteristics.
The variety and location of the mung bean starches dictate their different amylose contents. Li et al.[Citation9] found that the amylose contents of mung bean starches of 10 Chinese mung bean varieties range from 40.44–41.82%, whereas Kim et al.[Citation10] reported that 5 Korean mung bean starches contain 31.7–33.8% amylose. In general, mung bean starches have been found to contain higher amylose contents than cereal and tuber starches.[Citation9–12] The mung bean starch granules have a size of 5–40 µm in diameter with a mean value of 20.4 µm[Citation9,Citation13,Citation14] and exhibit a CA-type crystalline structure.[Citation14,Citation15] Also, the low peak viscosity, low increase in viscosity during the holding period at 95°C, and high setback viscosity of mung bean starch pastes indicate a high shear resistance and high retrogradation tendency of mung bean starches.[Citation14] Additionally, mung bean starches have a unique molecular size distribution of amylopectin that differs from other cereal and tuber starches[Citation10] and have lower slow-digesting starch than that of other legumes.[Citation16] Moreover, variations exist within starches from different mung bean cultivars with differences in their physicochemical characteristics and diverse processing properties.[Citation9] However, despite being widely used for food processing, mung bean starches have drawbacks such as high amylose content, high syneresis, and poor storage stability of the paste.[Citation10]
Recently, hybridization techniques have been used to produce mung bean varieties with different amylose contents that might improve the end-use quality of food products. However, the structural and physicochemical characteristics of these starches are not well-investigated. Therefore, this study aims to investigate the structure, physicochemical properties, and in vitro digestibility of starches isolated from different mung bean varieties with different amylose contents.
Materials and methods
Materials
Eight mung bean varieties (Vigna radiate) named TN182, VN07, DX208, DX108, KPS1, V123, T135, and DX044, coded as MB1, MB2, MB3, MB4, MB5, MB6, MB7, and MB8, respectively, were developed by the Legumes Research and Development Center, Field Crops Research Institute, Vietnam Academy of Agricultural Sciences. The fertilizer-resistant mung bean varieties are enormously grown and have high yield potential. The T135, V123, DX044, and KPS1 varieties are domestically hybridized parent genetic varieties with good agronomic characteristics. In contrast, the DX108, DX208, DXVN7, and TN182 varieties are the newly hybridized varieties with high yield, short-term growth, and wide adaptation. The enzymes and other chemicals used in this study were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Starch isolation
Mung bean starch was isolated according to the method described by Liu and Shen[Citation17] with slight modifications. Mung beans were soaked in distilled water at a solid/water ratio of 1/3 (w/v) for 12 h at 4°C. After separating the seed coat, the cotyledons were mixed with distilled water and ground for 3 min. The suspension was passed through a series of sieves (125- and 105-µm mesh sieves) and allowed to stand for 1 h. After sedimentation, the supernatant layer was decanted, and the residue was mixed with distilled water. This process was repeated thrice. Then, the starch was collected and dried in a force draft oven at 40°C for 24 h.
Yield and total carbohydrate of mung-bean starches
The yield of mung bean starches was determined as the amount of starch collected after isolation using the following equation:
Starch yield (%, w/w) =
The protein, lipid, and ash contents of the mung bean starches were determined following the standard AACC Approved Method 46–10, 30–10, and 08–01, respectively.[Citation18] The total carbohydrate content was calculated as the total dried matter subtracted from the amount of protein, lipid, and ash on a dried basis.
Morphology and structure of mung bean starches
The morphology of the starch granules was examined using a scanning electron microscope (JEOL JSM-6480LV, Jed LTD, Tokyo, Japan). The starch granules were placed on the surface of a specimen holder, coated with gold/palladium (60:40 w/w), and then observed at a magnification of 1,000 × using an accelerating potential of 15 kV.[Citation19]
The particle size distribution was determined using a laser diffraction particle size distribution analyzer (Partica LA-950, HORIBA, Japan).[Citation20] The starch granules were dispersed in ethanol (99.5%), and the size of the starch granules was captured by the LA-950 Original Optical System. The data obtained were analyzed using HORIBA LA-950 for Windows, Japan. The experiments were performed thrice for each sample.
The amylose content was determined using the method described by Hung and Morita.[Citation21] Starch (0.3 g) was well dispersed in a 1 M NaOH solution. After boiling, the starch solution was neutralized using 1 M HCl and diluted with distilled water. The starch solution (0.4 mL) was then mixed with iodine solution (0.2%) and made up to 10 mL with distilled water. After incubating for 2 h at ambient temperature, the absorbance of the starch-iodine solution was recorded at 620 nm using a spectrophotometer (Shimadzu UV-160A, Japan). The amylose content was calculated using a standard calibration curve obtained using standard amylose solutions.
The degree of polymerization () of mung bean starches was evaluated based on the method of Hizukuri et al.[Citation22] The starch suspension was prepared by mixing 30 mg of starch with 0.2 mL of ethanol and 1 mL of dimethyl sulfoxide (DMSO). After solubilization, the starch solution was used to measure the total glucose and total reducing residue concentrations.
was calculated as the total carbohydrate divided by the total reducing sugar content of starch. The average chain length (
) was calculated as the difference between total carbohydrate and total reducing residues after debranching by isoamylase (59,000 units/mL).
Branch chain length distribution (%) of mung bean starches was determined using a Dionex ICS-3000 ion chromatography system (Dionex Corporation, USA) according to the method described by Bertoft.[Citation23] The starches (4 mg) were solubilized with DMSO (1 mL) and then debranched using isoamylase (700 units/mL). The solution was incubated at ambient temperature overnight, and the enzyme was inactivated by boiling in a water bath for 5 min. After filtering through a 0.45-µm filter, the starch solution was injected into the HPIC system. A mobile phase mixed with solvent A (150 mM NaOH) and solvent B (150 mM NaOH containing 500 mM NaAc) was used. The gradient profile of the mobile phase was as follows: 15%–36% B in 0–9 min, 36%–45% B in 9–18 min, and 45%–100% B in 18–110 min.
Physicochemical characteristics of mung bean starches
X-ray diffraction analysis was performed using an X-ray diffractometer (Rigaku Co., Ltd, Rint-2000 type, Tokyo, Japan), operating from 2 = 2θ to 35° 2θ with a scanning speed of 8°/min and a scanning step of 0.02° at 40 kV and 80 mA.[Citation21] The degree of relative crystallinity (RC, %) was calculated as the ratio of the crystallinity area to the total diffraction area.
The short-range ordered structures of starches were analyzed using a high-throughput ATR PRO ONE-JASCO FT/IR-4700 spectrometer (Deutschland GmbH, Germany) following the method of Ma et al.[Citation24] The starch (2 mg, dry basis (db)) was mixed with KBr (200 mg) and then pressed into a sheet. The Fourier transform infrared (FT-IR) spectra of starch were obtained by scanning from 4,000 to 500 cm−1 at a resolution of 4 cm−1 with an accumulation of 64 scans. The degree of molecular order (DO) was calculated as the ratio of 1047/1022 cm-1, and the degree of double helix (DD) was 995/1022 cm−1.
The gelatinization temperature (GT), peak temperature (PT), maximum viscosity (MV), final viscosity (FV), breakdown (BD), and setback (SB) of mung bean starches were measured using a micro viscoamylograph (Brabender® GmbH & Co. KG, Germany). The mung bean starch was mixed with distilled water to obtain a suspension of 8% (w/v). The suspension was then heated from 30°C to 93°C at a constant rate of 7.5°C/min, kept at 93°C for 15 min, and then cooled down to 30°C at the same rate.[Citation25] The pasting profiles were obtained and interpreted.
Resistant starch (RS) content determination
RS content of the mung bean starches was evaluated using a Megazyme Resistant Starch Assay Kit (Megazyme International, Wicklow, Ireland) according to the approved AOAC 2002.02 method.[Citation26] Briefly, 100 mg of starch was weighed and mixed with 4 mL of enzyme mixture, including pancreatic α-amylase and amyloglucosidase, in a test tube. After vortexing, the tube was incubated in a shaking water bath for 16 h at 37°C to hydrolyze digestible starch. At the end of the incubation period, the suspension was mixed with 4 mL of absolute ethanol and vortexed to deactivate the enzymes. The RS was recovered as a pellet by centrifugation at 3,000 g for 10 min. The pellet was washed twice with 50% ethanol to remove digested starch. The sediment was dissolved in 2 mL of 2 M KOH by vigorous stirring for 20 min in an ice bath and then neutralized with 8 mL of sodium acetate buffer (1.2 M). After mixing with 0.1 mL of amyloglucosidase (3300 U/mL) was mixed with amyloglucosidase 50 (30 U/mL). The solution was then centrifuged at 3,000 g for 10 min, and the supernatant was recovered. An aliquot of the supernatant (0.1 mL) was mixed with 3 mL of glucose-oxidaseperoxidase-aminoantipyrine and then incubated at 50°C for 20 min. The absorbance of the samples was measured at 510 nm using a spectrophotometer. Each sample was analyzed in triplicate.
Statistical analysis
All data (means of triplicate determinations) were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test using SPSS version 16 (SPSS Inc., Chicago, IL, USA) (p < .05).
Results and discussion
Yield (%) and proximate analysis of mung bean starches
In our study, we observed that starch accounted for 36.8–41.2% of all mung bean seeds, in which the V123 mung bean variety (MB6) had the lowest starch yield (36.8%), whereas the T135 variety (MB7) had the highest yield (41.2%) (). The starch yields of mung bean varieties in this study were lower than those of ten mung bean varieties (54.73–57.99%), as described by Li et al.[Citation27] However, Hoover et al.[Citation14] reported that the starch yield of mung bean seeds was approximately 31.1 ± 2.5%, which is lower than the findings of this study. These observations led us to hypothesize that the differences in starch yields of mung beans might depend on the type of beans and isolation methods with or without the aid of cleaning agents.[Citation13,Citation28]
Table 1. Starch yields and proximate analysis of starches from different mung bean varieties1,2,3.
contains the data from the proximate analysis of the mung bean starches. Even after isolation, the mung bean starches still retained small amounts of protein (1.17–3.71%, w/w, db), lipid (0.40–0.93%, w/w, db), and ash (0.17%–0.90%, w/w, db). Therefore, the total carbohydrate content of the isolated mung bean starches ranged between 94.7–98.0%, with MB8 having the highest total carbohydrate content (98.0%, w/w, db). The presence of protein and fiber in mung bean seeds causes difficulty in purifying mung bean starches by water without affecting their quality. Our investigations also concluded that the small fiber precipitated with the starch in the sedimentation process might specifically affect the purity of the starch.[Citation29]
Morphology of mung bean starches
Here, we elaborately present the appearance of starch granules isolated from different mung bean varieties (). All varieties of mung bean starches showed a similar particle morphology, with the large granules varying from oval to elliptical and the small granules displaying a round shape. These results were consistent with those of previously published studies.[Citation23,Citation30] Moreover, the smooth surface of all the starch granules confirmed that the isolation method used in this study did not affect any of their physical properties.
Figure 1. Scanning electron microscopy of starches from different mung bean varieties. Abbreviations are the same as in .

We also found that the average diameter of mung bean starch granules ranged from 17.9 to 28.1 µm (), in which the diameters of starch granules reduced in order of the DX208 variety (MB3) > TN182 variety (MB1) = DX14 variety (MB4) > DXVN7 variety (MB2) = KPS1 variety (MB5) = DX044 (MB8) > T135 variety (MB7) > V123 variety (MB6). The granule sizes of mung bean starches used in this study were larger than those previously reported by Kim et al.[Citation31] and Phrukwiwattanakula et al.[Citation32] This led us to conclude that the varying particle sizes might significantly affect the physicochemical properties of starch, such as thermal and gelatinization characteristics, hydrolytic enzyme sensitivity, crystallization level, and solubility.[Citation33]
Table 2. Molecular fine structure of starches from different mung bean varieties1,2.
Amylose content (%) and fine structure of mung bean starches
The amylose contents of the eight mung bean starches in this study ranged from 19.1–32.9% (). Based on the amylose content, the mung bean starches were classified into three groups: low amylose starches (amylose content <25%) that included the TN182 (MB1), DXVN7 (MB2), and DX208 (MB3) varieties (19.1%, 21.0%, and 21.7% amylose, respectively), intermediate amylose starches (25% ≤ amylose content ≤ 30%), that included the DX14 (MB4), KPS1 (MB5), and V123 (MB6) varieties (26.8%, 28.1%, and 29.1% amylose, respectively), and high amylose starches (amylose content >30%), that included the T135 (MB7) and DX044 (MB8) varieties (30.6% and 32.9% amylose, respectively). Dahiya et al.[Citation1] also stated that mung bean starches had 14%–35% amylose, which is consistent with the results of this study. The amylose content was also found to significantly influence the physicochemical properties of starches, such as pasting, gelling, retrogradation, and initial gel hardness of cooked starch pastes[Citation34–36] in rice, maize, and wheat, which can be classified into four fractions: waxy starch (0%–2% amylose), low amylose starch (5%–20% amylose), intermediate starch (20%–30% amylose), and high-amylose starch (>30% amylose).[Citation34,Citation35,Citation36]
Here, we investigated the average degrees of polymerization () of mung bean starches (shown in ). The correlation analysis indicated that the
values of mung bean starches were highly negatively correlated with their amylose contents (a higher amylose content led to lower the
values) as shown in Table S1. Thus, mung bean starches with high amylopectin exhibited significantly higher
than those with high amylose. In contrast, the average chain length (
of mung bean starches was not related to the amylose content and varied depending on the mung bean variety (Table S1). The varieties of MB3, MB6, and MB8 had the longest chain lengths, the varieties of MB1, MB4, and MB5 had medium chain lengths, and the varieties of MB2 and MB7 had the shortest chain lengths (). Our results also indicated that MB3, MB6, and MB8 had the highest distribution of DP (= 24–36 and >36, whereas MB2 and MB7 had the lowest distribution of DP (6–12) and DP (13–24). The findings of Yao et al.[Citation30] that the debranched amylopectin of four Chinese mung bean starches could be grouped into four fractions: group A (DP, 6–12), group B1 (DP, 13–24), group B2 (DP, 25–36), and group B3 (DP, >36) corroborated the findings of this study. However, the molecular distribution of mung bean starches with similar amylose contents was not significantly different.[Citation30,Citation31] Thus, we conclude here that the physicochemical properties and the quality of starch-based products might be affected by the difference in amylose content and the fine structure of starch granules from different mung bean varieties.
Crystallinity of mung bean starches
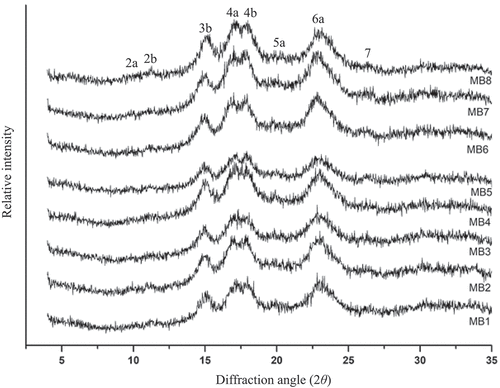
Our investigation revealed the X-ray diffraction (XRD) patterns of mung bean starches that are shown in . According to the XRD patterns, the starches of all mung bean varieties in this study were characterized as an A-type crystalline structure, similar to the findings by Ohwada et al.[Citation12] with major peaks at 10° 2θ (peak 2a), 11° 2θ (peak 2b), 15° 2θ (3b), 17° 2θ (4a), 18° 2θ (4b), and 23° 2θ (6a). However, several studies have reported that mung bean starches have a CA-type structure, which is a combination of A- and B-type polymorphic structures.[Citation14,Citation16] This difference in the polymorphic structure of mung bean starches might be due to the varying ordering of starch granules from the hilum to the periphery.[Citation37] The relative crystallinity (%) of mung bean starches are given in . We observed that the mung bean starches containing less amylose (MB1, MB2, and MB3) exhibited a higher relative crystallinity (31.9–34.7%) than the mung bean starches having intermediate (26.9–30.2%) and high (25.8–29.2%) amylose content. The correlation analysis showed that the relative crystallinity of mung bean starches was highly negatively correlated with the amylose content and positively correlated with the of the starches (Table S1). The starch granules of the MB4 and MB7 mung bean varieties, which had lower
and higher amounts of DP = 6–12 and DP = 13–24 exhibited higher relative crystallinity than the starches of the MB5, MB6, and MB8 mung bean varieties. Tester et al.[Citation38] concluded that the intertwining of the outer chains of amylopectin (A- and B1 chains) in the form of double helices created ordered regions or “crystalline lamellae” of starch granules.
Table 3. Crystalline structure of starches from different mung bean varieties1,2.
Fourier transform infrared (FT-IR) spectroscopic analysis of mung bean starches
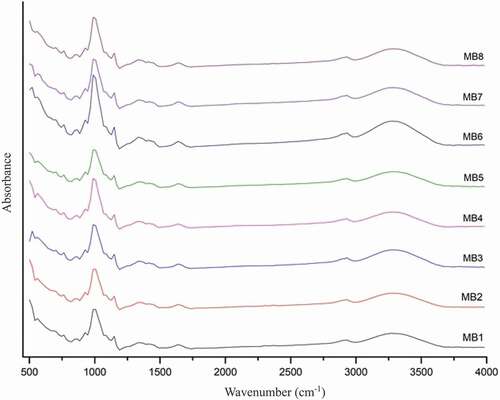
The FT-IR spectroscopic chromatograms of starches from different mung bean varieties in the range of 500–4,000 cm−1 are shown in . The absorption bands of the O-H stretching of starch ranged from 3000–3500 cm−1, the C-H stretching and bending vibrations of the methylene group were observed at 2926 cm−1 and 1400 cm−1,[Citation39] and the bending vibration of O-H of water absorbed in the amorphous regions of starch was observed at 1649 cm−1[Citation40]for the starches from all the mung bean varieties. The IR ratios of 1047/1022 cm−1 of starches from the MB1, MB2, MB3, and MB7 varieties were higher than those of the other varieties, indicating that the granules of these starches had a greater degree of order than the granules of the starches from the other mung varieties. Thus, the results of the FT-IR analysis were consistent with the results of relative crystallinity as described above. Moreover, we also found that the IR ratios of 995/1022 cm−1 for MB4, MB6, and MB8 were higher than those of the other starches, indicating that the starches of MB4, MB6, and MB8 had higher short-range molecular order than the other starches.
Pasting properties of mung bean starches
Here, we analyzed the pasting properties of mung bean starches with different amylose contents listed in . The peak viscosities of starches of the MB5, MB6, MB7, and MB8 varieties were significantly higher than those of starches of other mung bean varieties. However, the high amylose content of these mung bean starches led to an easy breakdown of their granules and rapid leaching out of amylose, resulting in lower viscosities and higher breakdown viscosities than other mung bean starches. During cooling, the high amylose-containing starches showed a higher final viscosity and setback than the low amylose starch.[Citation8] Similar to the findings by Hung et al.,[Citation8] the starches of the MB6, MB7, and MB8 varieties with higher amylose contents also exhibited higher final viscosity and setback. This correlation analysis indicated that the peak viscosities and final viscosities of mung bean starches were positively correlated with the amylose content and negatively correlated with the and relative crystallinity of the starches (Table S1). The pastes of starches of the MB1, MB2, MB3, and MB4 starches with lower peak and final viscosities and breakdown exhibited greater gel consistency, hot paste stability, and resistance against retrogradation than did other mung bean starches.
Table 4. Pasting properties of starches from different mung bean varieties1,2.
Contents of the resistant starch (RS) fraction of mung bean starches
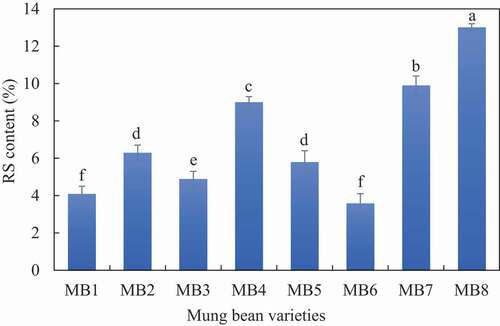
We analyzed the RS contents of mung bean starches with different amylose contents shown in . The RS contents typically ranged up to 3.0%, except for MB4, MB7, and MB8 varieties, which had higher RS contents (9.0%, 9.9%, and 13.0%, respectively) than the other mung bean starches. In contrast, the RS contents of starches of MB1 and MB6 varieties (4.1% and 3.6%, respectively) were significantly lower than those of other mung bean starches. This observation can be attributed to the fact that native starch resists digestive enzymes because of its compact structure, limiting the accessibility of the enzymes, thereby leading to higher RS content.[Citation41] In this study, the higher RS content of starches of M4, M7, and M8 varieties might be due to their higher amylose content and more compact structure of starch granules. In contrast, the starch granules of the M1 and M6 varieties might have a more porous structure that can be easily accessed by the enzymes, resulting in lower RS contents. Moreover, a positive correlation between the resistant starch content and amylose content of mung bean starch was also found in this study (Table S1).
Conclusion
In this study, we characterized the amylose content, fine structure, and physicochemical properties of starches from different mung bean varieties. Our investigation revealed that the shape of starch granules was similar for all varieties of mung bean starches (including low-, intermediate-, and high-amylose-containing starches) despite having different amylose contents. However, the of the mung bean starches decreased with increasing amylose content. While all mung bean starches exhibited an A-type crystalline structure, those with lower
and higher amounts of DP = 6–12 and DP = 13–24 exhibited higher relative crystallinity than the other starches. The starches with higher amylose contents exhibited higher peak and final viscosities and setbacks than the other starches. The resistant starch content of mung bean starches was positively correlated with the amylose content, whereas the
, degree of order, and short-range molecular order of mung bean starches were unique to each type of starch, unrelated to their amylose content.
Acknowledgments
This research is funded by Ho Chi Minh City University of Technology (HCMUT), VNU-HCM, under grant number BK-SDH-2020-1680499. The authors have declared no conflict of interest. The data available from the corresponding author upon reasonable request. Nguyen Thi Mai Huong was involved in methodology, investigation, data curation, writing (original draft). Pham Van Hung was involved in conceptualized methodology, investigation, supervision, writing (review and editing). Phan Ngoc Hoa was involved in conceptualization and supervision.
Additional information
Funding
References
- Dahiya, P. K.; Linnemann, A. R.; Van Boekel, M. A. J. S.; Khetarpaul, N.; Grewal, R. B.; Nout, M. J. R. Mung Bean: Technological and Nutritional Potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. DOI: https://doi.org/10.1080/10408398.2012.671202.
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung Bean (Vigna Radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients. 2019, 11, 1238. DOI: https://doi.org/10.3390/nu11061238.
- Mubarak, A. E.;. Nutritional Composition and Antinutritional Factors of Mung Bean Seeds (Phaseolus Aureus) as Affected by Some Home Traditional Processes. Food Chem. 2005, 89, 489–495. DOI: https://doi.org/10.1016/j.foodchem.2004.01.007.
- Oates, C. G.;. Studies on Mung Bean Starch: Granule Stability. Food Hydrocoll. 1991, 4, 365–377. DOI: https://doi.org/10.1016/S0268-005X(09)80132-X.
- Lii, C. Y.; Chang, S. M. Characterization of Red Bean (Phaseolus Radiatus Var. Aurea) Starch and Its Noodle Quality. J. Food Sci. 1981, 46, 78–81. DOI: https://doi.org/10.1111/j.1365-2621.1981.tb14535.x.
- Buleon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch Granules: Structure and Biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. DOI: https://doi.org/10.1016/S0141-8130(98)00040-3.
- Biliaderis, C. G.;. Polysaccharide Association Structures in Foods, Ed, Walter, R. H. Marcel Dekker: New York, USA, 1998; 57–168.
- Hung, P. V.; Maeda, T.; Morita, N. Study on Physicochemical Characteristics of Waxy and High‐amylose Wheat Starches in Comparison with Normal Wheat Starch. Starch/Staerke. 2007, 59, 125–131. DOI: https://doi.org/10.1002/star.200600577.
- Li, S.; Ward, R.; Gao, Q. Effect of Heat-moisture Treatment on the Formation and Physicochemical Properties of Resistant Starch from Mung Bean (Phaseolus Radiatus) Starch. Food Hydrocoll. 2011, 25, 1702–1709. DOI: https://doi.org/10.1016/j.foodhyd.2011.03.009.
- Kim, S.; Lee, B.; Baik, M.; Joo, M. H.; Yoo, S. H. Chemical Structure and Physical Properties of Mung Bean Starches Isolated from 5 Domestic Cultivars. J. Food Sci. 2007, 72, 471–477. DOI: https://doi.org/10.1111/j.1750-3841.2007.00525.x.
- Sandhu, K. S.; Lim, S.-T. Digestibility of Legume Starches as Influenced by Their Physical and Structural Properties. Carbohyd. Polym. 2008, 71, 245–252. DOI: https://doi.org/10.1016/j.carbpol.2007.05.036.
- Ohwada, N.; Ishibashi, K.; Hironaka, K.; Yamamoto, K. Physicochemical Properties of Mungbean Starch. J. Appl. Glycosci. 2003, 50, 481–485. Doi:https://doi.org/10.5458/jag.50.481.
- Abdel-Rahman, E. S. A.; EI-Fishawy, F. A.; EI-Geddawy, M. A.; Kurz, T.; EI-Rify, M. N. Isolation and Physico-chemical Characterization of Mung Bean Starches. Int. J. Food Eng. 2008, 4. Article 1. DOI: https://doi.org/10.2202/1556-3758.1184.
- Hoover, R.; Li, Y. X.; Hynes, G.; Senanayake, N. Physicochemical Characterization of Mung Bean Starch. Food Hydrocoll. 1997, 11, 401–408. DOI: https://doi.org/10.1016/S0268-005X(97)80037-9.
- Zou, J.; Xu, M.; Wang, R.; Li, W. Structural and Physicochemical Properties of Mung Bean Starch as Affected by Repeated and Continuous Annealing and Their in Vitro Digestibility. Int. J. Food Prop. 2019, 22, 898–910. DOI: https://doi.org/10.1080/10942912.2019.1611601.
- Ma, M.; Wang, Y.; Wang, M.; Jane, J.-L.; Du, S.-K. Physicochemical Properties and in Vitro Digestibility of Legume Starches. Food Hydrocoll. 2017, 63, 249–255. DOI: https://doi.org/10.1016/j.foodhyd.2016.09.004.
- Liu, W.; Shen, Q. Studies on the Physicochemical Properties of Mung Bean Starch from Sour Liquid Processing and Centrifugation. J. Food Eng. 2007, 79, 358–363. DOI: https://doi.org/10.1016/j.jfoodeng.2006.01.065.
- American Association of Cereal Chemists. Approved Methods of the AACC International, 9th ed. Methods 08-01, 30-10, 46-10. The Association St. Paul: MN, 2000.
- Jane, J.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J. F. Anthology of Starch Granule Morphology by Scanning Electron Microscopy. Starch/Stärke. 1994, 46, 121–129. DOI: https://doi.org/10.1002/star.19940460402.
- Wilson, J. D.; Bechtel, D. B.; Todd, T. C.; Seib, P. A. Measurement of Wheat Starch Granule Size Distribution Using Image Analysis and Laser Diffraction Technology. Cereal Chem. 2006, 83, 259–268. DOI: https://doi.org/10.1094/CC-83-0259.
- Hung, P. V.; Morita, N. Physicochemical Properties and Enzymatic Digestibility of Starch from Edible Canna (Canna Edulis) Grown in Vietnam. Carbohydr. Polym. 2005, 61, 314–321. DOI: https://doi.org/10.1016/j.carbpol.2005.04.021.
- Hizukuri, S.; Takeda, T.; Yasuda, M.; Suzuki, A. Multi-branched Nature of Amylose and the Action of Debranching Enzymes. Carbohydr. Res. 1981, 94, 205–213. DOI: https://doi.org/10.1016/S0008-6215(00)80718-1.
- Bertoft, E.;. On the Nature of Categories of Chains in Amylopectin and Their Connection to the Super Helix Model. Carbohydr. Polym. 2004, 57(2), 211–224. DOI: https://doi.org/10.1016/j.carbpol.2004.04.015.
- Ma, Z.; Yin, X.; Hu, X.; Li, X.; Liu, L.; Boye, J. I. Structural Characterization of Resistant Starch Isolated from Laird Lentils (Lens Culinaris) Seeds Subjected to Different Processing Treatments. Food Chem. 2018, 263, 163–170. DOI: https://doi.org/10.1016/j.foodchem.2018.04.122.
- Hung, P. V.; Huong, N. T. M.; Lan-Phi, N. T.; Tien, N. N. T. Physicochemical Characteristics and in Vitro Digestibility of Potato and Cassava Starches under Organic Acid and Heat-moisture Treatments. Int. J. Biol. Macromol. 2017, 95, 299–305. DOI: https://doi.org/10.1016/j.ijbiomac.2016.11.074.
- AOAC international. Official Methods of Analysis, 17th. Method 2002.02. Gaithersburg, MD, AOAC International, 2002.
- Li, W.; Shu, C.; Zhang, P.; Shen, Q. Properties of Starch Separated from Ten Mung Bean Varieties and Seeds Processing Characteristics. Food Bioprocess Technol. 2011, 4, 814–821. DOI: https://doi.org/10.1007/s11947-010-0421-6.
- Chung, H. J.; Liu, Q.; Hoover, R. Impact of Annealing and Heat-moisture Treatment on Rapidly Digestible, Slowly Digestible and Resistant Starch Levels in Native and Gelatinized Corn, Pea and Lentil Starches. Carbohydr. Polym. 2009, 75, 436–447.
- Wang, N.; Warkentin, T. D.; Vandenberg, B.; Bing, D. J. Physicochemical Properties of Starches from Various Pea and Lentil Varieties, and Characteristics of Their Noodles Prepared by High Temperature Extrusion. Food Res. Int. 2014, 55, 119–127.
- Yao, M.; Tian, Y.; Yang, W.; Huang, M.; Zhou, S.; Liu, X. The Multi-scale Structure, Thermal and Digestion Properties of Mung Bean Starch. Int. J. Biol. Macromol. 2019, 131, 871–878. DOI: https://doi.org/10.1016/j.ijbiomac.2019.03.102.
- Kim, Y. Y.; Woo, K. S.; Chung, H. J. Starch Characteristics of Cowpea and Mungbean Cultivars Grown in Korea. Food Chem. 2018, 263, 104–111. DOI: https://doi.org/10.1016/j.foodchem.2018.04.114.
- Phrukwiwattanakul, P.; Wichienchot, S.; Sirivongpaisal, P. Comparative Studies on Physico-Chemical Properties of Starches from Jackfruit Seed and Mung Bean. Int. J. Food Prop., 2014, 17(9), 1965–1976.
- Lindeboom, N.; Chang, P. R.; Tyler, R. T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch‐Stärke. 2004, 56(3‐4), 89–99. DOI: https://doi.org/10.1002/star.200300218.
- Zhu, L.-J.; Liu, -Q.-Q.; Wilson, J. D.; Gu, M.-H.; Shi, Y.-C. Digestibility and Physicochemical Properties of Rice (Oryza Sativa L.) Flours and Starches Differing in Amylose Content. Carbohydr Polym. 2015, 86, 1751–1759. DOI: https://doi.org/10.1016/j.carbpol.2011.07.017.
- Yu, X.; Yu, H.; Zhang, J.; Shao, S.; Xiong, F.; Wang, Z. Endosperm Structure and Physicochemical Properties of Starches from Normal, Waxy, and Super-sweet Maize. Int. J. Food Prop. 2015, 18, 2825–2839. DOI: https://doi.org/10.1080/10942912.2015.1015732.
- Hung, P. V.; Maeda, T.; Morita, N. Waxy and High-amylose Wheats - Characteristics, Functionality and Uses. Trends Food Sci. Technol. 2006, 17, 448–456. DOI: https://doi.org/10.1016/j.tifs.2005.12.006.
- Sevenou, O.; Hill, S. E.; Farhat, I. A.; Mitchell, J. R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. DOI: https://doi.org/10.1016/S0141-8130(02)00067-3.
- Tester, R. F.; Karkalas, J.; Qi, X. Starch-composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. DOI: https://doi.org/10.1016/j.jcs.2003.12.001.
- Sheng, Y.; Wang, Q.; Xu, X. C.; Jiang, W.; Gan, S. C.; Zou, H. F. Oxidation of Cornstarch Using Oxygen as Oxidant without Catalyst. LWT-Food Sci. Technol. 2011, 44, 139–144. DOI: https://doi.org/10.1016/j.lwt.2010.05.004.
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. DOI: https://doi.org/10.1021/jf011652p.
- Sajilata, M. G.; Singhal, R. S.; Kulkarni, P. R. Resistant Starch–A Review. Compr. Rev. Food Sci. 2006, 5, 1–17. DOI: https://doi.org/10.1111/j.1541-4337.2006.tb00076.x.