ABSTRACT
The current review focused on the effect of different thermal and non-thermal processing techniques on the structural and functional modifications of milk proteins. In thermal processing, denaturation in whey proteins takes place at the temperature range of 60–100°C. High temperature short time (HTST) treatment caused denaturation with the loss of secondary structure of proteins at temperature of 72°C for 15 minutes. Ultra-high temperature (UHT) treatment damaged β-lactoglobulins at higher temperature range of 135–140°C for 2 seconds. High-pressure processing (HP) (≤200-400 MPa) caused denaturation and aggregation of casein micelles with large molecular size. High-pressure homogenization with 350–400 MPa caused modification in structure of milk proteins and enhanced the solubility of whey proteins. Gamma irradiation with the dose of 3 kGy and 10 kGy crosslinked bands from α-lactalbumin and β-lactoglobulins respectively. At the dose of 32–64 kGy, crosslinking among caseinates appeared and molecular size of whey proteins increased. Dose of ultraviolet (UV) irradiation (254 nm) caused stability and improvement in the structure of whey proteins. Sonication treatment (20 W for 60 minutes) caused reduction in the size of casein micelles up to 1–4 nm.
Introduction
Among all perishable commodities, milk is highly perishable because it loses its quality and safety attributes without proper handling and processing conditions. The main role of milk processing is to reduce the risks associated with the presence of biological contaminants.[Citation1] Milk is a combination of different nutritive factors which formulate its structure. It comprises proteins, fats, carbohydrates and minerals. Milk contains various types of proteins that play a major role in milk structure.[Citation2] Milk comprises soluble protein casein which may be α-casein, β-casein, κ-casein and insoluble proteins which are β-lactoglobulin, α-lactalbumin, immunoglobulin and bovine serum albumin.[Citation3] All these milk proteins have nutritional and physicochemical impacts. Milk protein products with particular applications are manufactured by dairy industries in wide range. These milk proteins are affected by several factors and processing techniques during production, storage and consumption. Some of these techniques that affect milk proteins in different conditions will be discussed in this review. Milk proteins-based products are used as important ingredients in dairy industry and have a major contribution to food industry. Traditional products with milk proteins produced by precipitation of rennet or acid, in which adjustment of pH and high heat is involved ultimately denature the whey protein.[Citation4,Citation5]
Thermal processing techniques are integral in dairy industry for the production and manufacturing of milk-based products. Utilization of these techniques with various intensities is still underutilization without any viable results. Thermal processing treatments with different time and temperature ranges are being applied to milk products to extend the shelf life of products by eliminating pathogenic bacteria, spores and other harmful microorganisms. These techniques are suitable for favorable physicochemical changes; however, these cause considerable nutritional losses. Milk proteins like whey proteins and casein micelles undergo structural and functional variations on applications of thermal treatments.[Citation6]
Pasteurization is an important technique among different thermal techniques. High-temperature ranges are being applied to milk samples with basic purpose of deactivation and killing of pathogenic microorganisms. HTST and UHT techniques possess a significant role in milk pasteurization. In thermal pasteurization methods, HTST is the most widely used and commercial method in which sample is subjected to temperature range of 72°C for 15 minutes. This treatment causes configurational changes in milk proteins.[Citation7] Thermal treatment above pasteurization temperature is referred to as ultra-high temperature (UHT) treatment. In this treatment, sample is subjected to the temperature range of 130°C for about 1 second. UHT treatment causes disintegration of casein and its interaction with whey proteins.[Citation8,Citation9]
In preservation and processing of food products, high-pressure treatment applications are of great concern. In food industries specifically in dairy industry, HP processing with range of 100–1000 MPa is one of the most commonly used techniques. In case of high hydrostatic pressure treatment, 6000 times high pressure is applied on food products. Uniform processing is one of the best attributes of HHP processing that makes it globally acceptable.[Citation10,Citation11]
Ultra high-pressure homogenization is a technique with certain ranges of pressure. Its basic use in dairy industry is to reduce the globular size to make the fine structures. Its composition about temperature is somehow responsible for its antiseptic properties.[Citation12] Irradiation treatment plays a significant role in structural and functional modification of milk proteins such as whey proteins, casein micelles and globular proteins. Gamma irradiation treatments have shown strong effect in the improvement of mechanical properties of edible films consisting of proteins.[Citation13]
Among all irradiation treatments, UV irradiation treatment is used because of its effectiveness against lethal activities. UV irradiations cause disintegration of milk proteins at specific wavelengths and specifically it is used to kill pathogenic microorganisms such as bacteria, molds and viruses.[Citation14] Among all irradiation treatment techniques, like γ-irradiation and UV-irradiation, the sonication technique also has great importance due to its eco-friendly attributes. Ultrasound irradiation is used in all food sectors and it is specifically used in milk industries to modify the functional configuration and to eliminate undesirable substances. Its effect on milk proteins is of great concern at various times, temperatures and radiation doses.[Citation15,Citation16]
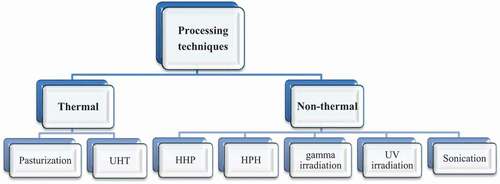
Thermal and non-thermal processing techniques have a key role in dairy sectors for product development. (). These techniques are applied for structural modification to improve functional properties, eliminating unnecessary factors and obtaining products of desirable characteristics. This review focuses on some thermal and non-thermal processing techniques and their influence on milk proteins.
Thermal processing
Heat treatments play an important role in the processing of food products and synthesis of concentrates of whey proteins. Different heat treatments like preheating, pasteurization and sterilization are being applied that alter the structure and composition of milk by changing the properties of whey proteins. Whey proteins are globular proteins with a compact structure that vary from each other in their structure and function. This is due to variability in amino acid composition and their alignment. β-lactoglobulin is abundantly present in whey protein. At room temperature, it occurs in the form of dimer which consists of two identical subunits. When temperature rises to 40°C, these dimers start to dissociate and convert into monomers. This is the most important form of β-lactoglobulin which appears as a result of heat denaturation. During heat treatments, exchange reactions with β-lactoglobulin occur which affect the functionality and solubility of proteins. Among whey proteins, α-lactalbumin is heat resistant and is the second most significant whey protein.[Citation17]
β-lactoglobulin and β-casein are soluble at the temperature of 40°C. When these globular proteins are heated up to 4–60°C they cause intermolecular association as shown in .[Citation23] This treatment also affects the functional properties of proteins. Denaturation of protein occurs at temperature range of 60–100°C. At 68°C denaturation of α-lactalbumin occurs and denaturation of lactoglobulin starts at temperature of 89°C. Heat treatment of 100°C is utilized in processing and preservation of food as shown in .[Citation24] At 80°C, partial denaturation of disulfide occurs and destabilization of residual proteins occurs at 140°C. Destabilization occurs due to the breakage of disulfide bond.[Citation25] Heat treatments of up to 140°C decrease covalent crosslinks which affect unfolding and solubility of whey proteins.
Table 1. Effect of high pressure on milk proteins
Milk heated at 70°C causes thermal denaturation of the globular whey proteins and converts them to low state order. Denatured proteins are more vulnerable to deposition by salt bridge and hydrophobic interaction. During the heating of skim milk, whey proteins and β-lactoglobulin change the structure of casein micelles. It occurs due to the association of whey proteins with casein micelles upon heating. At lower temperature (75–85°C) relatively slow increase in size occurs during heating. But at temperature limit of up to 40–100°C, a rapid increase in size occurs as shown in . At high-temperature treatment, casein micelles size increases up to 35 nm. At high temperature and time duration, the size of casein micelles increases at slower rate than denaturation of whey proteins which rapidly increases with an increase in temperature.[Citation26] Methods of heating have a great influence on whey protein denaturation, size of casein micelles and the composition of proteins.[Citation27]
Experimental studies have demonstrated that high temperature increased the size of casein micelles gradually. The relationship between casein micelles and the level of denatured whey proteins results in an increased size of micelles. The relation of whey protein with casein micelles is gradually lower than whey protein denaturation. Studies are required to investigate whether the change in size is due to denatured whey protein or aggregation of casein micelles.[Citation28,Citation29] In the preservation and shelf stability of food products especially from dairy sector, thermal treatments have a significant impact. At 100°C, milk can be preserved for longer periods but temperature fluctuations with the variation of time cause the structural changes.
Pasteurization
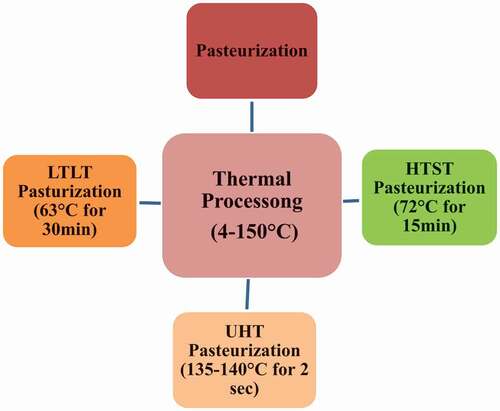
Heating milk to sufficient temperature and time to destroy and inactivate pathogenic microorganisms is called pasteurization. This technique is designed specifically to extend the shelf life of milk and to make it suitable for human consumption. Common pasteurization methods are high-temperature short time (HTST), long temperature long time (LTLT) and ultra-high temperature (UHT) as shown in . In case of HTST process, heating of milk up to 72°C for 15 minutes is performed.[Citation7] On the other hand, in UHT process treatment, temperature range of 135–140°C for 2 seconds is employed. Different treatments like standard high temperature, short time pasteurization at 72°C for 15 min (HTST), UHT 135–140°C for 2 seconds, etc., individual or with combinations are employed on milk samples.
Studies demonstrated that homogenization coupled with HTST and homogenized-HTST causes no change in apparent structure of whey proteins as compared to raw whey proteins. However, skimmed-HTST treated milk diminished its secondary structure to about 6%. On the other hand, UHT treatment causes a reduction in secondary structure of both h-UHT and s-UHT of whey proteins sample. Whey proteins with h-UHT treatment have more effect than s-UHT. The high content of α-lactalbumin is present in s-UHT as compared to h-UHT.[Citation30]
In whey analysis of skim milk, nitrogen is used that produces three casein and whey protein samples. These samples illustrate 56% denaturation in the UHT-pasteurized milk and 0.4% denaturation in raw milk.[Citation8] Analysis from far-UV CD shows that (HTST) high-temperature short time homogenization and h-HTST imposed no significant impact on secondary structure of protein as compared to raw whey proteins. UHT treatment was applied to cause reduction in secondary structure of h-UHT and s-UHT whey samples. These changes lead to the formation of irregular structures to about 7%. In case of s-UHT and h-UHT rate of α-lactalbumin is high. Overall, different studies indicated that UHT treatment damage β-lactoglobulins more severely as compared to α-lactalbumins. Far-UV CD experiments were performed to investigate the secondary structure of whey proteins. Thermal stability of whey samples was examined that depicts the stability of results as raw whole milk stability > high temperature short time > homogenized and HTST> skimmed and HTST and UHT >homogenized and UHT.[Citation30]
In long temperature long time (LTLT) pasteurization, the sample is subjected to the temperature range of 63°C for 30 minutes. LTLT has numerous uses in microbial inactivation and immunogenicity. But in comparison with other techniques like high-pressure processing, LTLT reduces the immunogenicity of immunoglobulin in milk to about 41%.[Citation31] The utilization of LTLT provides considerable results as a separate technique but in comparison with other advanced techniques did not impart any significant outcomes. LTLT pasteurization is still needed to be investigated for its effect on milk proteins. In case of HTST and UHT, stability concerns in samples of milk are different for casein micelles and whey proteins. HTST causes denaturation and loss of secondary structure of protein. HTST is a commonly accepted method as compared to UHT that severely damages the β-lactoglobulins.
High-pressure processing
Non-thermal treatments gain remarkable significance as modern technologies in processing and preservation of food products. The most commonly used method for this purpose is high-pressure processing HP (100–1000 MPa).[Citation32] This technique has a great impact because of its valuable effects on food products.[Citation33] HPP causes disintegration of casein micelles into smaller particles that ultimately increases the viscosity of milk and reduces the lightening and turbidity. The effect of high pressure on proteins is of high importance. During this treatment primary structure of proteins is never distorted.[Citation34] Although, at very high pressure variations in secondary structure of proteins occur, also at this point disulfide bonds rupture and denaturation occurs irreversibly. At >200 MPa, changes in tertiary structure of proteins occur and these changes are maintained by ionic and hydrophobic interaction.[Citation35] At relatively low pressure multimeric proteins tend to disrupt their quaternary structure which was maintained by non-covalent bonds.[Citation35,Citation36] High pressure affects the casein micelles and whey proteins in milk samples. Denaturation of whey proteins α-lactalbumin and β-lactoglobulins occur at different rates of HP treatment. α-lactalbumin is more resistant to denaturation on HP as compared to β-lactoglobulins.[Citation18,Citation21,Citation37] β-lactoglobulins tend to associate with casein micelles when milk is treated at 300–600MPa.[Citation18,Citation21] When milk is treated at HP treatment of ≤ 200 MPa then it has little effect on the size of casein micelles as shown in ().[Citation33] But in raw skim milk, disruption in casein micelles occurred when treatment of > 250 MPa was applied as showed in ().[Citation21] Casein aggregates of larger size have been observed when HP of 250–400 MPa was applied at 40–60°C.[Citation20,Citation27,Citation41]
Table 2. Influence of irradiation treatment with different dose on milk protein fractions
Raw whole milk sample of 2000 g was skimmed and centrifuged for 20 minutes at 20°C and then filtered. The ultracentrifuge process is being used to obtain the casein-micelles free milk samples. Samples were vacuum packed and HP treatment was applied.[Citation42] A gradual increase in pressure at the rate of 300 MPa per minute was established to the range of 100–800 MPa. Desired pressure was maintained at range of 1 second to 60 minutes. During treatments, temperature of HP unit vessel was maintained to 5°C, 20°C and 40°C thermostatically. The maximum increase of 15°C at 800 MPa was observed due to compressive heating. There is little influence of high-pressure treatment on casein micelles at 100 MPa for 30 minutes and temperature of 20°C. Casein micelles size enhanced by 20% when treated at 250 MPa. But in untreated milk reduction in the size of micelles up to 50% was observed at 300–800 MPa.[Citation22] HP at 300 MPa caused denaturation of β-lactoglobulins up to 80%.[Citation37] α-lactalbumins are more stable to denaturation than the β-lactoglobulins at 100–800 MPa at 20°C. Denaturation of β-lactoglobulins starts at >100 MPa and reaches up to 100% at pressure of 600 MPa. α-lactalbumin denature at pressure of 400 MPa and its denaturation reaches up to 72% at 800MPa when treated for 30 minutes as described in ().[Citation22]
High pressure treatment is applied with different pressure and time values. Casein micelles denature and start to rupture at different levels of pressure. As the pressure level increases, casein micelles along with disintegration starts interacting with whey proteins. Among α-lactalbumin and β-lactoglobulins fractions of whey proteins, the former show more stability to denaturation than the latter one. α-lactalbumin is denatured almost completely at 800 MPa and β-lactoglobulins complete their disintegration at 600 MPa. After partial denaturation at certain pressures, it starts interacting with casein micelles to form large globules of higher molecular weight. Instead of all this, the structure and functionality of the product have been improved by using this technique.
Effect of treatment time on casein micelles and whey proteins during HP
When sample was subjected to HP treatment at range of 250 MPa for 1 second to 60 minutes then it showed a substantial impact on size of casein micelles and the extent of α-lactalbumin and β-lactoglobulins in skim milk. Casein micelles when treated at 250 MPa for 1 minute not showed any significant change however, with the increase of time at this pressure, no denaturation was observed in α-lactalbumin. Reduction in the size of casein micelles up to 40% was observed when treated at 40 MPa for 1 second. Similarly, at pressure of 250 MPa and 400 MPa, no change was observed in α-lactalbumin but denatured when treated for 30 minutes.[Citation22] The combined effect of time with temperature in high-pressure treatment causes denaturation in α-lactalbumin and β-lactoglobulins.[Citation43]
High Pressure has a substantial influence on whey proteins and casein micelles. It denatures casein micelles, α-lactalbumin and β-lactoglobulins. Micelles disrupted below critical pressure (≥200).[Citation43] However, the influence of high pressure on casein micelles and whey proteins ultimately affects properties and structure of milk whey.[Citation22] HP shows variation in functionality with the combination of time by changing its structure. At lower time zone, HP did not show significant results but with the increase in time with pressure, desired structural changes can be obtained.
High hydrostatic pressure (HHP)
Among non-thermal treatments, high hydrostatic pressure (HHP) is considered the best alternative to thermal treatments used for processing. It maintains the vitamin content, protein content and ensures better sensory quality with safe and stable production as compared to other treatments.[Citation10] However, by changing the technological properties and final features, HHP may cause considerable alteration in milk proteins.[Citation44] Pressure implication causes denaturation of whey proteins which ultimately affect the structure of micelles and causes disruption of micelles, re-aggregation and removal of casein micelles.[Citation22] The structure of casein micelles and whey proteins can be modified by HHP. Casein micelles can be disaggregated and re-aggregated, and whey proteins can be denatured.
The common use of high hydrostatic pressure is due to its ability to provide food products with fresh-like taste without addition of preservatives. High hydrostatic pressure (HHP) treatment on food products enhances their shelf life, eliminates microorganisms, improves texture and increases digestibility.[Citation45] HHP has a great impact on technological and physicochemical properties of milk. When milk samples were exposed to HHP, breakage of casein micelles into smaller particles occurred. This disintegration enhances the level of calcium phosphate and casein of milk serum. In this phase, a reduction in serum nitrogen fraction and non-casein nitrogen fraction was observed.[Citation27]
When pressure treatment was applied at the range of 1000–3000 atm then it causes irreversible denaturation and reversible denaturation was observed when treated at the range of 3000 atm.[Citation46] HHP pressure in the range of 250–310 MPa causes substantial disruption of casein micelles. Nevertheless, disruption into micelles due to high pressure can be prevented by the addition of whey proteins to casein isolates.[Citation47] In milk proteins, the application of different pressure treatments imposes configurational changes. When HHP is applied, dissociation of casein micelles into smaller particles takes place. This occurs due to the reason that electrostatic and hydrophobic interactions among micelles diminished that causes the formation of large size micelles. Ultimately, enhancement in the number and size of casein micelles takes place and cluster formation of sub-micelles from spherical particles occurs.[Citation48] Rennet coagulation time reduces by this application. The same results were reported by Sivanandan et al.[Citation49]
When HHP with pressure range of 400 MPa was applied, it increased the β-lactoglobulin’s pepsin hydrolysis. It causes a decrease in the binding of immunoglobulins E of β-lactoglobulin and its antigenicity. This creates the probability for β-lactoglobulin to obtain its hyper allergenic hydrolyzates.[Citation50] Lactoglobulins denature when HHP treatment is applied in the range of 500 MPa at temperature of 25°C. Lactalbumins and immunoglobulins denaturation occurs only when high degrees of pressure and temperature in the range of 50°C have been applied.[Citation51,Citation52] Increase in hydrophobicity of whey proteinsoccurs when high hydrostatic pressure have applied. It also improves the functional properties of foods.[Citation53] HHP has a more significant impact than other techniques as it did not alter the flavor, texture and nutritional value of product. One drawback is that HHP only affects the products which contain water.[Citation10]
High hydrostatic pressure is considered a beneficial non-thermal technique as it ensures the shelf-life stability of products, lowers the microbial load, maintains nutritional status and improves the functional properties of treated samples. But in contrast, its limitation is that it shows significant results in a better way only in case of products that contain water content to some extent.
High-pressure homogenization (HPH)
In food industry, homogenization is considered the best technique to decrease the globular size especially in case of milk for creaming. In food emulsions, basic property is its stability and this can be achieved by variation in the structure of fat globules by reducing their size which ultimately increases the surface area for the absorption of proteins in milk. This stability can be accomplished by the process of homogenization. In industries, homogenization pressure is used in the range of 20 MPa.[Citation54] As several inventions take place in the design of homogenizers that permits the operation on a higher range of pressure up to 350 MPa. But it depends on the design of homogenizer.[Citation55,Citation56] Different inventions have been used to design the structure of homogenizers and high pressure technology. This makes the industry use these instruments and techniques with the pressure of up to 350–400 MPa. Due to these developments, techniques of pressure with homogenization are being used in food industries, dairy processing, pharmaceutical and cosmetics industries. Flow characteristics with variation are accessible due to the creation of a new design of homogenizers with the implication of interaction chambers of different designs and pressure valves. Ultra-high-pressure homogenization also known as dynamic high pressure is a novel technology and sustained process to provide numerous advantages in number of fields.[Citation57] UHPH give better results in the production of emulsions with finer particle size before aggregation as compared to the typical homogenization process.[Citation56,Citation58,Citation59]
Protein structure and its characteristics can be modified by UHPH.[Citation56,Citation57,Citation59] When whey protein with heat denaturation is homogenized at pressure of 150 MPa ultimately causes enhanced solubility.[Citation60] At the pressure of 41 MPa, there is no significant effect on the size of casein micelles. But size decreases to a particular level when UHPH is applied at the range of 114 and 186 MPa. At the pressure range of 150 MPa, no apparent decrease in micelles size was observed but reduction appeared at pressure of 200 Mpa.[Citation61] UHPH treatment at 186 MPa and temperature of 75–80°C, denaturation and interaction of whey proteins with casein micelles start.[Citation62] At pressure of 186 MPa, the formation of complex of β-lactoglobulins and casein take place with an increase in temperature and this complex lead toward the α-lactalbumin and β-lactoglobulins interaction.[Citation62,Citation63] Disruption of protein casein micelles takes place at 300 MPa isostatic high pressure causes its accumulation in solvent and improves the solubility.[Citation22] In the colloidal matrix, the interaction between reduced size globules of fat, accumulates of whey proteins and casein micelles is promoted by UHPH and it upgraded the sensory and rheological characteristics.[Citation61]
Reduction in the size of casein micelles observed by applying UHPH. Complexes of proteins formed which variate from those formed by heat treatment. UHPH resulted to improve the structure of casein micelles which on the other hand modify the soluble protein complexes.[Citation64] Manipulation in the structure and designs of homogenizer and improvements in technological process incoming era may cause subsequent modification in the system regarding the effect of milk proteins. Homogenization proves its place and importance regarding the stability and to decrease the size of particles to form better emulsions and to homogenize the milk samples specifically in the dairy industry.
Gamma irradiation
In irradiated techniques, γ-irradiations have a great impact on structure and composition of milk proteins like casein micelles and whey proteins. For the improvement of coatings and films which are formed by sodium and calcium caseinate or with the combination of globular proteins, γ-irradiations have significant effects.[Citation65] The firm structure of protein with an orderly arrangement of particles has formed due to crosslinking induced by radiation.[Citation66] Irradiation gave better results regarding soy proteins and caseinates. Globular proteins which are whey proteins showed effective results by heat treatment.[Citation39] When samples are subjected to irradiation or heat treatment, form protein structure with denser network specifically contains high level of β-sheets and β-strands as compared to reference proteins.[Citation13]
Edible films which contain calcium caseinates produce better crosslinked network and improved functional properties when subjected to irradiation treatment in the range of 32 kGy as shown in ().[Citation39] When irradiation treatment has applied then a significant increase in β-phase with crystalline crosslinked network takes place but on the other hand amount of α-helix phase is reduced. Irradiation treatment of milk solution indicates that better crosslinked structure of caseinates has obtained by this method than by heating. A dose of 64 kGy demonstrated the better crosslinked network among calcium caseinates on irradiation treatment as shown in .[Citation40] On whey protein concentrates, γ-irradiations generate little changes. The fact behind this condition is little amount of tyrosine in whey proteins as compared to casein micelles.[Citation67] Irradiation dose of 0.25 kGy causes an increase in the concentration of bityrosine in α-casein. But molecular weight of whey proteins increased at the dose of 32 kGy. The crosslinked network exists prominently in globular proteins, due to which molecular weight show variations to a little extent.
At irradiated treatment dose of 3 kGy, main crosslinked bands disappear from α-casein and β-lactoglobulin to the range of 10 kGy as described in . Disintegration in the protein molecules into smaller particles takes place by irradiated treatment and these particles coagulate to form larger molecules by interaction with each other. In the existence of oxygen with irradiation, coagulation takes place in α-casein and β-lactoglobulin. When irradiation dose increased then turbidity of α-casein and β-lactoglobulin enhanced due to increase in coagulation. At the dose of 3 kGy and 5 kGy, the binding ability of immunoglobulins increases as compared to non-irradiated samples. By gamma irradiations, allergenicity of milk allergens like casein micelles, α-lactalbumin, β-lactoglobulins and immunoglobulins can be reduced.[Citation38]
By irradiation technique, gels with crystallinity formed and rearrangement of β-phase takes place. Gamma irradiation with heat treatment gave better conformation of β-phase as compared to the sample which only heated. When solutions irradiated, gave highly ordered films which ultimately gave better protein conformation. These films provide mechanical resistance, barrier properties and characterized by higher rate of firmness as compared to those of irradiated samples.[Citation13] Crosslinking of milk proteins is essential to form a stable structure of milk samples. Irradiation specifically γ-irradiations plays an important role in this aspect and helps to form crosslinked network among proteins. This process helps to stabilize the whole structure and also improve shelf stability of different products.
Ultraviolet irradiation
In food industries, specifically in dairy industry, non-thermal treatments are playing a significant role in the prevention of microbial load, enhancement of shelf life and to change the structure and composition of products by preserving quality. Among these non-thermal techniques, ultraviolet irradiation (UV) has important role in food industries in recent years. UV treatment of light with the wavelength range of 254 nm shows better results to inactivate the molds, bacteria, viruses and other microorganisms.[Citation68] Pathogenic microorganisms that cause unfavorable impacts on products can be deactivated by UV-irradiation treatment without affecting the overall characteristics of food products.[Citation69] Ultraviolet radiations with the wavelength range of 270–295 nm for different periods between 2–24 hours were applied on milk proteins samples. Application of the treatments depicted that, like dose and treatment time of UV irradiations increases, variations in the structure of whey proteins occurred but these changes were not seen.
When ultraviolet irradiation was applied to whey protein concentrates after heat treatment, β-lactoglobulin showed stability toward denaturation then α-lactalbumin. As compared to β-lactoglobulin, α-lactalbumin disappeared more rapidly on application of UV treatment.[Citation70] Whey proteins form aggregates with higher molecular weight when treated with UV irradiation of higher doses. At a certain level of irradiation, reduction in the size of β-lactoglobulin and α-lactalbumin occurred.[Citation71] When whey proteins are treated with UV light in the range of 280–295 nm, breakage of disulfide bond occur which ultimately causes accretion and oligomerization which result from single free thiols.[Citation72] Significant denaturation in β-lactoglobulin and α-lactalbumin did not appear when UV irradiations were applied in the range of 0.12 Jcm−2. Denaturation in α-lactalbumin takes place to certain extent when treated at higher doses as 4–12 Jcm−2 while β-lactoglobulin denatures at higher dose level. Improvement in the structure of whey proteins takes place when irradiated at UV dose of 254 nm. When considering films, modification in the properties required may attainable by denaturation and accumulation of proteins. UV treatment at higher doses causes disintegration in whey protein structure and stiffens the structure of film. Effect of UV radiation on α-lactalbumin higher than β-lactoglobulin and its denaturation occur at lower doses.[Citation73]
Ultraviolet irradiations (UV) are the best choice for the modification of milk proteins. It improves the structural configuration and characteristics of proteins. Different doses of irradiations are applied on whey proteins and the results indicated that UV with lower range did not show applicable results to β-lactoglobulin but better one for α-lactalbumin.
Sonication
Among all non-thermal techniques regarding food processing, sonication or ultrasound is the most recommended technique which not only reduces the denaturation of the proteins but also decreased the end product losses related to its attributes. This technique is environmentally friendly and less toxic as compared to other techniques. Sound waves of 20 kHz are used in ultrasound waves which are undetectable by human ear.
When sonication in the range of 20 W for 60 minutes is applied, size of casein micelles decreases up to 1–4 nm and the treatment with irradiation of 41 W with the same time duration causes the reduction in size up to 2–5 nm. A significant change in crosslinked structure of β-lactoglobulin takes place when sonicated at 20 W. The band intensity of major whey proteins (β-lactoglobulin and α-lactalbumin) takes place when sonicated at 20 W for 30 minutes. At first reduction in a band, intensity takes place but it increased after 30 min treatment. In comparison with un-sonicated sample, sonication treatment of sample for 45 to 60 minutes show lower intensity of whey proteins.[Citation15]
In all experiments, ultra-pure milk was used. Pasteurized and homogenized milk was ultra-sonicated and then ultracentrifuge at 30,000 rpm. Particle size was measured by using refractive indices of milk component, individually for fat globules, casein micelles proteins, whey protein and water. Turbidity, viscosity and pH of milk supernatant were measured by using calibrated instruments. Results showed that, as sonication time increased, size of soluble particles like whey proteins decrease. But the size of casein micelles not decreased significantly as occur in whey proteins and constituted milk. From all fractions, i.e., κ-casein, β-casein, α-casein, β -lactoglobulin, α-lactalbumin obtained from ultra-centrifuged and sonicated samples show that major changes occur in β-casein band intensities but κ-casein and α-casein remained same above sonication in first 15 minutes. It was observed that particle size of soluble proteins reduces due to shear effects. Small changes in casein micelles structure were observed. Sonication also causes turbidity and reduction in size of fat globules. Turbidity is occurred due to the variation in whey proteins and their aggregates. The Sonication process has no apparent changes in viscosity. Results showed that, among whey proteins and casein proteins, later one reduced in size with lower rate but whey proteins reduced in size and form aggregates with itself or with casein micelles on sonication, but prolonged sonication causes partial disruption from these aggregates.[Citation11] By application of ultra-sonication, ultra-centrifugation and high-pressure liquid chromatography, casein concentrate was determined.
Results indicated a reduction in particle size and increase in turbidity occur. Sonication caused the decrease in size of fat globules but further, it is not investigated that either decrease in size is due to sonication or due to alteration of casein micelles aggregates. If considered about structural stability of casein micelles, then no alteration occurs in it due to sonication. Its structure was also determined by HPLC to check its stability which did not show any denaturation of protein particles.
It is concluded that on sonication applications, whey protein solubilizes and denatures its structure which further forms an aggregate of whey proteins-whey-proteins/whey proteins-casein micelles. If considering casein micelles then its structure remains stable and did not form any aggregates on certain conditions. Ultra-sonication did not show any significant effect on casein micelles system but breakdown the whey protein aggregates.[Citation74]
Sonication treatment with different ranges of power and time duration indicates that a major change takes place in whey proteins except for treatment first 30 minutes. The reduction of whey proteins is due to the interaction of the soluble proteins with casein micelles. Denaturation of whey proteins to some extent caused the formation of aggregates. A small decrease or disruption in casein micelles may take place. Overall, sonication for a short time as 15 or 20 minutes did not show any variate results as compared to un-sonicated sample but treatment toward larger extent of time cause significant changes in structure especially in size of casein micelles and whey proteins in milk sample.
Conclusion
Milk is a perishable and nutritious natural food with a complex structure. Its structure comprises soluble (casein micelles) and insoluble proteins (whey proteins). Protein stability and their structure variate by applying different processing techniques on milk samples. Thermal processing techniques with processing conditions at lower temperature induce reversible changes in stability but high temperature range causes disintegration of whey proteins. Non-thermal processing techniques are HP, HHP and HPH. At lower pressure level casein micelles start to denature and at higher pressure, they interact with whey proteins. Gamma, UV and sonication irradiation treatments also cause such results. Overall, it is concluded that thermal and non-thermal techniques affect significantly milk proteins. At higher levels of their treatment dose, pressure and temperature, proteins started to disintegrate and formation of crosslinked network appeared. These technique affects the structure and functionality of milk proteins and also their stability.
References
- Mediwaththe, A.; Bogahawaththa, D.; Grewal, M. K.; Chandrapala, J.; Vasiljevic, T. Structural Changes of Native Milk Proteins Subjected to Controlled Shearing and Heating. Food Res. Int. 2018, 114, 151–158. DOI: https://doi.org/10.1016/j.foodres.2018.08.001.
- Van Lieshout, G. A.; Lambers, T. T.; Bragt, M. C.; Hettinga, K. A. How Processing May Affect Milk Protein Digestion and Overall Physiological Outcomes: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. DOI: https://doi.org/10.1080/10408398.2019.1646703.
- Xu, M.-F.; DAI, J.-F.; XIANG, M.-X.; CHENG, X.-F.; ZENG, X.-C.; Wan, C.-Q.; ZHOU, W.-J. Study on Differences of Milk Proteins by Liquid Chromatography-Mass Spectrometer. Chin. J. Anal. Chem. 2014, 42(4), 501–506. DOI: https://doi.org/10.1016/S1872-2040(13)60723-9.
- Vogel, K. G. Protein Amount and Milk Protein Ingredient Effects on Sensory and Physicochemical Properties of Ready-To-Drink Protein Beverages; 2019.
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Uluko, H.; Yanling, S.; Wenming, C.; Wupeng, G.; et al. Effect of Power Ultrasound Pre-treatment on the Physical and Functional Properties of Reconstituted Milk Protein Concentrate. J. Food Eng. 2014, 124, 11–18. DOI: https://doi.org/10.1016/j.jfoodeng.2013.09.013.
- Borad, S. G.; Kumar, A.; Singh, A. K. Effect of Processing on Nutritive Values of Milk Protein. Crit. Rev. Food Sci. Nutr. 2017, 57, 3690–3702. DOI: https://doi.org/10.1080/10408398.2016.1160361.
- Bogahawaththa, D.; Buckow, R.; Chandrapala, J.; Vasiljevic, T. Comparison between Thermal Pasteurization and High Pressure Processing of Bovine Skim Milk in Relation to Denaturation and Immunogenicity of Native Milk proteinsInnov. Innov. Food Sci. Emerg. Technol. 2018, 47, 301–308. DOI: https://doi.org/10.1016/j.ifset.2018.03.016.
- Douglas, F. W.; Greenberg, R.; Farrell, H. M.; Edmondson, L. F. Effects of Ultra-high-temperature Pasteurization on Milk Proteins. J. Agric. Food Chem. 1981, 29(1), 11–15. DOI: https://doi.org/10.1021/jf00103a004.
- Akkerman, M.; Johansen, L. B.; Rauh, V.; Poulsen, N. A.; Larsen, L. B. Contribution of Casein Micelle Size and Proteolysis on Protein Distribution and Sediment Formation in UHT Milk during Storage. Int. Dairy J. 2021, 104980. doi:https://doi.org/10.1016/j.idairyj.2021.104980.
- Chawla, R.; Patil, G. R.; Singh, A. K. High Hydrostatic Pressure Technology in Dairy Processing: A Review. J. Food Sci. Technol. 2011, 48(3), 260–268. DOI: https://doi.org/10.1007/s13197-010-0180-4.
- Grumezescu, A., & Holban, A. M. (Eds.). (2019). Milk-Based Beverages: Volume 9: The Science of Beverages. Woodhead Publishing. United kingdom.
- Amador-Espejo, G. G.; Suàrez-Berencia, A.; Juan, B.; Bárcenas, M. E.; Trujillo, A. J. Effect of Moderate Inlet Temperatures in Ultra-high-pressure Homogenization Treatments on Physicochemical and Sensory Characteristics of Milk. J. Dairy Sci. 2014, 97, 659–671. DOI: https://doi.org/10.3168/jds.2013-7245.
- Cieśla, K.; Salmieri, S.; Lacroix, M.; Le-Tien, C. Gamma Irradiation Influence on Physical Properties of Milk Proteins. Radiat. Phys. Chem. 2004, 71(1–2), 95–99. DOI: https://doi.org/10.1016/j.radphyschem.2004.04.068.
- Kristo, E.; Hazizaj, A.; Corredig, M. Structural Changes Imposed on Whey Proteins by UV Irradiation in a Continuous UV Light Reactor. J. Agric. Food Chem. 2012, 60, 6204–6209. DOI: https://doi.org/10.1021/jf300278k.
- Shanmugam, A.; Chandrapala, J.; Ashokkumar, M. The Effect of Ultrasound on the Physical and Functional Properties of Skim Milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 251–258. DOI: https://doi.org/10.1016/j.ifset.2012.06.005.
- Firouz, M. S.; Farahmandi, A.; Hosseinpour, S. Recent Advances in Ultrasound Application as A Novel Technique in Analysis, Processing and Quality Control of Fruits, Juices and Dairy Products Industries: A Review. Ultrason. Sonochem. 2019, 57, 73–88. DOI: https://doi.org/10.1016/j.ultsonch.2019.05.014.
- deWit, J. N.; Klarenbeek, G. Effects of Various Heat Treatments on Structure and Solubility of Whey Proteins. J. DAIRY SCI. 1984, 67, 2701–2710. DOI: https://doi.org/10.3168/jds.S0022-0302(84)81628-8.
- Scollard, P. G.; Beresford, T. P.; Needs, E. C.; Murphy, P. M.; Kelly, A. L. Plasmin Activity, β-lactoglobulin Denaturation and Proteolysis in High Pressure Treated Milk. Int. Dairy J. 2000, 10, 835–841. DOI: https://doi.org/10.1016/S0958-6946(01)00028-0.
- Needs, E. C.; Capellas, M.; Bland, A. P.; Manoj, P.; Macdougal, D.; Paul, G. Comparison of Heat and Pressure Treatments of Skim Milk, Fortified with Whey Protein Concentrate, for Set Yogurt Preparation: Effects on Milk Proteins and Gel Structure. J. Dairy Res. 2000, 67(3), 329–348. DOI: https://doi.org/10.1017/S0022029900004301.
- Garía-Risco, M. R.; Olano, A.; Ramos, M.; Lopez-Fandino, R. Micelar Changes Induced by High Pressure. Influence in the Proteolytic Activity and Organoleptic Properties of Milk. J. Dairy Sci. 2000, 83, 2184–2189. DOI: https://doi.org/10.3168/jds.S0022-0302(00)75101-0.
- Needs, E. C.; Stenning, R. A.; Gill, A. L.; Ferragut, V.; Rich, G. T. High-pressure Treatment of Milk: Effects on Casein Micelle Structure and on Enzymic Coagulation. J. Dairy Res. 2000, 67(1), 31–42. DOI: https://doi.org/10.1017/S0022029999004021.
- Huppertz, T.; Fox, P. F.; Kelly, A. L. High Pressure Treatment of Bovine Milk: Effects on Casein Micelles and Whey Proteins. J. Dairy Res. 2004, 71(1), 97. DOI: https://doi.org/10.1017/S002202990300640X.
- Pace, C. N.; Tanford, C. Thermodynamics of the Unfolding of β-lactoglobulin A in Aqueous Urea Solutions between 5 and 55°. Biochemistry. 1968, 7(1), 198–208. DOI: https://doi.org/10.1021/bi00841a025.
- Rose, D.;. 1963. Heat Stability of Bovine Milk: A Review. Dairy Sci. Abstr. 25,45-52.
- Watanabe, K.; Klostermeyer, H. Heat-induced Changes in Sulphydryl and Disulphide Levels of β-lactoglobulin A and the Formation of Polymers. J. Dairy Res. 1976, 43, 411–418. DOI: https://doi.org/10.1017/S0022029900015995.
- Anema, S. G.; Li, Y. Association of Denatured Whey Proteins with Casein Micelles in Heated Reconstituted Skim Milk and Its Effect on Casein Micelle Size. J. Dairy Res. 2003, 70(1), 73. DOI: https://doi.org/10.1017/S0022029902005903.
- Law, A. J.; Leaver, J.; Felipe, X.; Ferragut, V.; Pla, R.; Guamis, B. Comparison of the Effects of High Pressure and Thermal Treatments on the Casein Micelles in Goat’s Milk. J. Agric. Food Chem. 1998, 46, 2523–2530. DOI: https://doi.org/10.1021/jf970904c.
- Kharlamova, A.; Nicolai, T.; Chassenieux, C. Heat-induced Gelation of Mixtures of Casein Micelles with Whey Protein Aggregates. Food Hydrocolloids. 2019, 92, 198–207. DOI: https://doi.org/10.1016/j.foodhyd.2019.01.048.
- Vasbinder, A. J.; De-Kruif, C. G. Casein–whey Protein Interactions in Heated Milk: The Influence of pH. Int. Dairy J. 2003, 13, 669–677. DOI: https://doi.org/10.1016/S0958-6946(03)00120-1.
- Qi, P. X.; Ren, D.; Xiao, Y.; Tomasula, P. M. Effect of Homogenization and Pasteurization on the Structure and Stability of Whey Protein in Milk. J. Dairy Sci. 2015, 98(5), 2884–2897. DOI: https://doi.org/10.3168/jds.2014-8920.
- Viazis, S.; Farkas, B. E.; Allen, J. C. Effects of High-pressure Processing on Immunoglobulin A and Lysozyme Activity in Human Milk. J. Hum. Lact. 2007, 23, 253–261. DOI: https://doi.org/10.1177/0890334407303945.
- Cheftel, J. C. Effects of High Hydrostatic Pressure on Food Constituents: An Overview. High Press. Biotech 1992, 224, 195–209.
- Hite, B. H. The Effect of Pressure in the Preservation of Milk: A Preliminary Report; West Virginia Agricultural Experiment Station, 1899. Southern West Virginia.
- Mozhaev, V. V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the Effects of High Hydrostatic Pressure in Biotechnological Applications. Trends Biotechnol. 1994, 12(12), 493–501. DOI: https://doi.org/10.1016/0167-7799(94)90057-4.
- Hendrickx, M.; Ludikhuyze, L.; Vanden-Broeck, I.; Weemaes, C. Effects of High Pressure on Enzymes Related to Food Quality. Trends Food Sci. Technol. 1998, 9, 197–203. DOI: https://doi.org/10.1016/S0924-2244(98)00039-9.
- Gross, M.; Jaenicke, R. Proteins under Pressure. The Influence of High Hydrostatic Pressure on Structure, Function and Assembly of Proteins and Protein Complexes. Eur. J. Biochem. 1994, 221(2), 617–630. DOI: https://doi.org/10.1111/j.1432-1033.1994.tb18774.x.
- Lopez-Fandino, R.; Carrascosa, A. V.; Olano, A. The Effects of High Pressure on Whey Protein Denaturation and Cheese-making Properties of Raw Milk. J. Dairy Sci. 1996, 79, 929–936. DOI: https://doi.org/10.3168/jds.S0022-0302(96)76443-3.
- Lee, J.-W.; Kim, J.-H.; Yook, H.-S.; Kang, K.-O.; Lee, S.-Y.; Hwang, H.-J.; Byun, M.-W. Effects of Gamma Radiation on the Allergenic and Antigenic Properties of Milk Proteins. J. Food Prot. 2001, 64(2), 272–276. DOI: https://doi.org/10.4315/0362-028X-64.2.272.
- Vachon, C.; Yu, H. L.; Yefsah, R.; Alain, R.; St-Gelais, D.; Lacroix, M. Mechanical and Structural Properties of Milk Protein Edible Films Cross-linked by Heating and γ-irradiation. J. Agric. Food Chem. 2000, 48, 3202–3209. DOI: https://doi.org/10.1021/jf991055r.
- Ressouany, M.; Vachon, C.; Lacroix, M. Irradiation Dose and Calcium Effect on the Mechanical Properties of Cross-linked Caseinate Films. J. Agric. Food Chem. 1998, 46, 1618–1623.
- Gaucheron, F.; Famelart, M. H.; Mariette, F.; Raulot, K.; Michela, F.; Le Graeta, Y. Combined Effects of Temperature and High-pressure Treatments on Physicochemical Characteristics of Skim Milk. Food Chem. 1997, 59(3), 439–447. DOI: https://doi.org/10.1016/S0308-8146(96)00301-9.
- Mayayo, C.; Montserrat, M.; Ramos, S. J.; Martínez-Lorenzo, M. J.; Calvo, M.; Sánchez, L.; Pérez, M. D. Kinetic Parameters for High-pressure-induced Denaturation of Lactoferrin in Human Milk. Int. Dairy J. 2014, 39, 246–252. DOI: https://doi.org/10.1016/j.idairyj.2014.07.001.
- Kromkamp, J.; Moreira, R. M.; Langeveld, L. P.; Van-Mil, P. J. Microorganisms in Milk and Yoghurt: Selective Inactivation by High Hydrostatic Pressure. InHeat Treatments and Alternative Methods. IDF Symposium, Vienna (Austria), 6-8 Sep 1995–1996. International Dairy Federation.
- Singh, H., & Flanagan, J. (2006). Milk proteins. FOOD SCIENCE AND TECHNOLOGY-NEW YORK-MARCEL DEKKER-, 149(1), 26. Palmerston North, New Zealand.
- Makhal, S.; Vashishtha, B.; Mandal, S.; Kanawjia, S. K. High Hydrostatic in Food Preservation: Philodophy and Development. Indian Food Ind. 2003, 22, 38–45.
- Jaenicke, R.;. Enzymes under Extremes of Physical Conditions. Annu. Rev. Biophys. Bioeng. 1981, 10, 1–67. DOI: https://doi.org/10.1146/annurev.bb.10.060181.000245.
- Harte, F. M.; Gurram, S. R.; Luedecke, L. O.; Swanson, B. G.; Barbosa-Cánovas, G. V. Effect of High Hydrostatic Pressure and Whey Proteins on the Disruption of Casein Micelle Isolates. J. Dairy Res. 2007, 74(4), 452. DOI: https://doi.org/10.1017/S0022029907002762.
- Huppertz, T.; Kelly, A. L.; Fox, P. F. High Pressure-induced Changes in Ovine Milk. 2. Effects on Casein Micelles and Whey Proteins. Milchwissenschaft-Milk Sci. Int. 2006, 61, 394–397.
- Sivanandan, L.; Toledo, R. T.; Singh, R. K. Effect of Continuous Flow High‐Pressure Throttling on Rheological and Ultrastructural Properties of Soymilk. J. Food Sci. 2008, 73, 288–296. DOI: https://doi.org/10.1111/j.1750-3841.2008.00803.x.
- Chicón, R.; López-Fandiño, R.; Alonso, E.; Belloque, J. Proteolytic Pattern, Antigenicity, and Serum Immunoglobulin E Binding of β-lactoglobulin Hydrolysates Obtained by Pepsin and High-pressure Treatments. J. Dairy Sci. 2008, 91(3), 928–938. DOI: https://doi.org/10.3168/jds.2007-0657.
- Felipe, X.; Capellas, M.; Law, A. J. Comparison of the Effects of High-pressure Treatments and Heat Pasteurization on the Whey Proteins in Goat’s Milk. J. Agric. Food Chem. 1997, 45, 627–631. DOI: https://doi.org/10.1021/jf960406o.
- Barba, F. J., Tonello-Samson, C., Puértolas, E., & Lavilla, M. (2020). Present and Future of High Pressure Processing: A Tool for Developing Innovative, Sustainable, Safe and Healthy Foods: Elsevier Science. HIPERBARIC. Burgos, Spain.
- Liu, X.; Powers, J. R.; Swanson, B. G.; Hill, H. H.; Clark, S. Modification of Whey Protein Concentrate Hydrophobicity by High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2005, 6(3), 310–317. DOI: https://doi.org/10.1016/j.ifset.2005.03.006.
- Kielczewska, K.; Kruk, A.; Czerniewicz, M.; Warmińska, M.; Haponiuk, E. The Effect of High-pressure Homogenization on Changes in Milk Colloidal and Emulsifying Systems. Polish J. Food Nutr. Sci. 2003, 12, 43–46.
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of High-pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-water Emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. DOI: https://doi.org/10.1016/S1466-8564(00)00012-6.
- Paquin, P. Technological Properties of High Pressure Homogenizers: The Effect of Fat Globules, Milk Proteins, and Polysaccharides. Int. Dairy J. 1999, 9(3–6), 329–335. DOI: https://doi.org/10.1016/S0958-6946(99)00083-7.
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological Aspects and Potential Applications of (Ultra) High-pressure Homogenisation. Trends Food Sci. Tech. 2013, 31, 13–26. DOI: https://doi.org/10.1016/j.tifs.2012.03.005.
- Floury, J.; Desrumaux, A.; Legrand, J. Effect of Ultra-high-pressure Homogenization on Structure and on Rheological Properties of Soy Protein-stabilized Emulsions. J. Food Sci. 2002, 67(9), 3388–3395. DOI: https://doi.org/10.1111/j.1365-2621.2002.tb09595.x.
- Desrumaux, A.; Marcand, J. Formation of Sunflower Oil Emulsions Stabilized by Whey Proteins with High‐pressure Homogenization (Up to 350 MPa): Effect of Pressure on Emulsion Characteristics. Int. J. Food Sci. 2002, 37, 263–269. DOI: https://doi.org/10.1046/j.1365-2621.2002.00565.x.
- Iordache, M.; Jelen, P. High Pressure Microfluidization Treatment of Heat Denatured Whey Proteins for Improved Functionality. Innov. Food Sci. Emerg. Technol. 2003, 4(4), 367–376. DOI: https://doi.org/10.1016/S1466-8564(03)00061-4.
- Hayes, M. G.; Kelly, A. L. High Pressure Homogenisation of Raw Whole Bovine Milk (A) Effects on Fat Globule Size and Other Properties. J. Dairy Res. 2003, 70, 297. DOI: https://doi.org/10.1017/S0022029903006320.
- Guyomarc’h, F.; Law, A.; Dalgleish, D. G. Formation of Soluble and Micelle-bound Protein Aggregates in Heated Milk. J. Agric. Food Chem. 2003, 51(16), 4652–4660. DOI: https://doi.org/10.1021/jf0211783.
- Fairise, J. F.; Cayot, P.; Lorient, D. Characterisation of the Protein Composition of Casein Micelles after Heating. Int. Dairy J. 1999, 9, 249–254. DOI: https://doi.org/10.1016/S0958-6946(99)00070-9.
- Sandra, S.; Dalgleish, D. G. Effects of Ultra-high-pressure Homogenization and Heating on Structural Properties of Casein Micelles in Reconstituted Skim Milk Powder. Int. Dairy J. 2005, 15(11), 1095–1104. DOI: https://doi.org/10.1016/j.idairyj.2004.11.015.
- Ouattara, B.; Giroux, M.; Yefsah, R.; Smoragiewicz, W.; Saucier, L.; Borsa, J.; Lacroix, M. Microbiological and Biochemical Characteristics of Ground Beef as Affected by Gamma Irradiation, Food Additives and Edible Coating Film. Radiat. Phys. Chem. 2002, 63, 299–304. DOI: https://doi.org/10.1016/S0969-806X(01)00516-3.
- Brault, D.; D’Aprano, G.; Lacroix, M. Formation of Free-standing Sterilized Edible Films from Irradiated Caseinates. J. Agric. Food Chem. 1997, 45(8), 2964–2969. DOI: https://doi.org/10.1021/jf960955u.
- Wong, D. W.; Camirand, W. M.; Pavlath, A. E.; Parris, N.; Friedman, M. Structures and Functionalities of Milk Proteins. Crit. Rev. Food Sci. Nutr. 1996, 36, 807–844. DOI: https://doi.org/10.1080/10408399609527751.
- Buhler, S.; Solari, F.; Gasparini, A.; Montanari, R.; Sforza, S.; Tedeschi, T. UV Irradiation as a Comparable Method to Thermal Treatment for Producing High Quality Stabilized Milk Whey. Lwt. 2019, 105, 127–134. DOI: https://doi.org/10.1016/j.lwt.2019.01.051.
- Smith, W. L.; Lagunas-Solar, M. C.; Cullor, J. S. Use of Pulsed Ultraviolet Laser Light for the Cold Pasteurization of Bovine Milk. J. Food Prot. 2002, 65, 1480–1482. DOI: https://doi.org/10.4315/0362-028X-65.9.1480.
- De la Fuente, M. A.; Singh, H.; Hemar, Y. Recent Advances in the Characterisation of Heat-induced Aggregates and Intermediates of Whey Proteins. Trends Food Sci. Tech. 2002, 13(8), 262–274. DOI: https://doi.org/10.1016/S0924-2244(02)00133-4.
- Gulzar, M.; Bouhallab, S.; Jeantet, R.; Schuck, P.; Croguennec, T. Influence of pH on the Dry Heat-induced Denaturation/aggregation of Whey Proteins. Food Chem. 2011, 129, 110–116. DOI: https://doi.org/10.1016/j.foodchem.2011.04.037.
- Vanhooren, A.; Devreese, B.; Vanhee, K.; Van Beeumen, J.; Hanssens, I. Photoexcitation of Tryptophan Groups Induces Reduction of Two Disulfide Bonds in Goat α-Lactalbumin†. Biochemistry. 2002, 41(36), 11035–11043. DOI: https://doi.org/10.1021/bi0258851.
- Díaz, O.; Candia, D.; Cobos, Á. Effects of Ultraviolet Radiation on Properties of Films from Whey Protein Concentrate Treated before or after Film Formation. Food Hydrocoll. 2016, 55, 189–199. DOI: https://doi.org/10.1016/j.foodhyd.2015.11.019.
- Chandrapala, J.; Martin, G. J.; Zisu, B.; Kentish, S. E.; Ashokkumar, M. The Effect of Ultrasound on Casein Micelle Integrity. J. Dairy Sci. 2012, 95(12), 6882–6890. DOI: https://doi.org/10.3168/jds.2012-5318.