ABSTRACT
Red djulis (Chenopodium formosanum) is a native grain plant, and shows that it has anti-oxidant and anti-inflammatory effects. However, it was still unclear whether unhulled red djulis extracts promoted collagen production and anti-aging. This study aimed to explore whether the unhulled red djulis extracts had anti-aging properties. Using unhulled red djulis extracts to treat human fibroblasts for functional analysis, and used HPLC to analyze the effective components of red djulis. This study found that unhulled red djulis extracts can increase the repairing ability of the skin cells, and increased skin-barrier-related genes, antioxidant-related genes expression. In addition, it increased collagen-related genes, and inhibited AGEs and melanin formation. Finally, we isolated six active ingredients from the unshelled Red Julie extract, which can increase collagen. Thus, the unhulled red djulis extracts can affect gene expressions related to the skin barrier, antioxidation, and collagen.
Introduction
Collagen, one of the primary extracellular matrix (ECM) components of the dermis, is the most abundant protein in mammals, comprising about 30% of total protein.[Citation1] Collagens co-polymerize to form extended mechanically stiff fibrils which confer tensile strength to the tissue providing the elasticity of skin.[Citation2] However, with age, collagen content decreases by about 1% every year, resulting in loss of skin elasticity.[Citation3,Citation4] Additionally, long-term exposure to UV light can cause significant degradation of the skin’s collagen structure, leading to the formation of wrinkles in photo-aged skin.[Citation5] Advanced glycation end products (AGEs) production in skin cells was known to promote stiffness and loss of elasticity through their buildup in connective tissue.[Citation6] Therefore, novel and effective anti-aging and anti-glycation ingredients for skin care have drawn a lot of attention.
Djulis is a native grain plant in Taiwan. It has high levels of starch, dietary fiber, protein, and essential amino acids (such as lysine), making djulis a whole-nutritional grain health food.[Citation7] Due to its antioxidant capacity and anti-inflammatory properties, djulis has been identified as a potential ingredient for skin improvement.[Citation8] Some studies have shown that djulis is rich in betalain which imparts an excellent antioxidative capacity to djulis. Polyphenols, such as rutin and chlorogenic acid, are also found in djulis. In addition, treatment with red djulis extracts under UVB light exposure can induce an accumulation of betacyanins and flavonoids in the keratinocyte cells for protection against UV-induced damage.[Citation9] Red dijulis extract has antioxidant and moisturizing effects, and can stimulate the proliferation of fibroblasts and keratinocytes during wound healing and regeneration[Citation9,Citation10] In addition, previous study has shown that red djulis can delay the skin aging process and improve skin aging parameters in subjects with poor skin condition.[Citation11] Thus, red djulis has the potential to improve skin conditions. However, the anti-glycation, anti-oxidation on skin cells, and the influences of the effective components in djulis still were not clear. Therefore, most previous studies of functional compounds in djulis focused on the seed. In order to effectively utilize the abundant functional compounds of unhulled red djulis, this study used unhulled red djulis to treat human fibroblasts for functional analysis, and next used high-performance liquid chromatography (HPLC) to analyze the effective components of unhulled red djulis.
Materials and methods
Preparation of djulis extract
The djulis used in the present study (water extraction from Chenopodium formosanum) was purchased from Pingtung County, Taiwan. The whole grain djulis (unhulled) was stored at 4°C prior to use. The ratio of grounded red djulis to water was 1:10 (w/v), and the temperature used for extraction was divided into three stages, first at 25°C, then at 50°C, and finally at 70°C. The mixture was then centrifuged at 4600 × g for 20 minutes to separate solid substances. The supernatant was collected and removed debris. Finally, the unhulled djulis extract was disinfected and sterilized at 121°C for 15 minutes and stored at 4°C.[Citation12]
Cell viability
5 × 103 CCD-966SK cells were seeded into each well of a 96-well plate. After 24 h of culturing, the medium was removed and replaced with a fresh medium containing various concentrations of unhulled djulis extract. Cell viability was assessed through MTT assay. Briefly, 15 µL of MTT (Sigma; 4 mg/mL) was added to the cells and incubated for 4 hours. The medium was removed and 50 µL of DMSO was added to resolve the formazan crystal. The plate was placed on a shaker and incubated for 10 min and the absorbance was obtained at 570 nm. Cell viability in response to treatment was calculated as:
Cell viability (%) = (OD sample/OD control) × 100%.[Citation13]
Quantification of gene expressions by real-time PCR
The treated CCD-966SK fibroblasts were harvested, and total RNA was isolated from cells using an RNA purification kit (Geneaid, Taiwan). DNA-free total RNA was reversely transcribed to cDNA using a SuperScript™ Reverse Transcriptase kit (Invitrogen, Life Technologies Co., CA, USA). Quantitative real-time PCR was conducted using an ABI StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Inc., CA, USA) and the SYBR Green Master Mix (KAPA Biosystems, MA, USA) for was used transcript measurements. The reaction mixture was cycled as follows: one cycle at 95°C for 20 s, followed by 40 cycles of 95°C (1 s), 60°C (20 s), and plate reading was conducted after each cycle. The melting curves of the PCR products were analyzed during the quantitative real-time PCR. The gene-specific primers used in this study are listed in . Real-time PCR reactions were performed using the ABI system. GAPDH was used as a reference gene to normalize relative expression. Data were analyzed using the ABI StepOne™ Software v2.2.3 (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA). All PCR assays were performed in duplicate three times.[Citation14]
Table 1. Real-time quantitative PCR primers used in this study
Wound healing assay
In vitro wound scratch assay was performed as described in Yarrow et al.[Citation15] with modifications. Briefly, CCD-966SK cells were grown to 80% confluence in 24-well plates. The scratching was performed by scraping with a sterile 1-ml pipette tip across the center of the well, followed by incubation with various concentrations of red djulis extract for 17 h. The wound closures were subsequently photographed under a microscope (Eclipse Ti-U, Nikon Corporation, Tokyo, Japan) using a CCD digital camera. Cell migration was analyzed by ImageJ and expressed as a percentage of wound coverage by cells moving into the scratched wound area. Cell migration data were expressed as a percentage of wound coverage.
Collagen secretion assay
The soluble collagen was determined by the method described by Aramwit et al. (2009). Human skin fibroblast cells (CCD-966SK) were seeded at an initial concentration of 4 × 104 cells per well of 24-well plate in MEM containing 10% FBS. After 24 h, the culture medium was replaced by a fresh medium. The sample extracts were diluted with various concentrations of phosphate buffer saline (PBS) and added into each well. Cells without a sample extract served as negative controls. After incubation for 48 h at 37°C with 5% CO2, the supernatants were collected. The total amount of soluble collagen type I was assayed using the Sircol® Collagen Assay Kit (Bicolor Life Science Assays, Northern Ireland, UK). Briefly, 100 μL of experimental supernatant was mixed with 1 mL of dye solution at room temperature for 30 min. The samples were then centrifuged at 15,000 × g for 10 min to form a pellet of collagen. The supernatant was removed and the soluble collagen produced was dissolved in 1 mL of alkali reagent. The resultant alkali reagent solutions were assayed by a spectrophotometer at a wavelength of 540 nm. The amount of collagen was calculated based on a standard curve of soluble collagen (bovine skin collagen type I standard from American disease free animals).[Citation16]
Immunofluorescence staining
For immunohistochemistry, antigen retrieval was performed by heating sections of 10 mM citrate buffer (pH 6.0) in a microwave oven. Reactions with endogenous peroxidases and proteins were blocked by incubation with 3% H2O2 diluted in methanol and 10% normal goat or rabbit serum, depending on the host animal for the secondary antibody. The incubation with primary antibodies was then done overnight at 4°C. The primary antibodies used were as follows: rabbit anti-collagen I (1:50; Abcam, Cambridge, UK), rabbit anti-collagen III (1:100; Abcam), mouse anti-collagen IV (1:50; Dako, Glostrup, Denmark), rabbit anti-collagen V (1:50; Abcam), and rabbit anti-collagen VI (1:50; Abcam). The EnVision labeled polymer-HRP system (Dako) was used as the secondary antibody and peroxidase activity was visualized with a liquid diaminobenzidine substrate (Dako). Nuclei were counter-stained with hematoxylin. Collagen staining of the nodular lesions was classified into three categories according to intensity compared with background staining (strongly positive, weakly positive, and negative).[Citation17]
Tyrosinase activity inhibition assay
Tyrosinase activity inhibition assay was conducted following Chan et al.[Citation18] Briefly, 100 µl of freshly prepared 3,4-dihydroxy L-phenylalanine (L-DOPA) solution, at different concentrations (2.5, 5.0, and 10.0 mM), was added for dopachrome formation. The relationship between total protein and concentration of L-DOPA for dopachrome formation was observed. A reaction mixture (200 µl/well) consisting of cell-extracted protein and L-DPA in 0.1 M sodium phosphate buffer (pH 6.8) was added into the wells of a 96-well plate in triplicate. The plate was incubated at 37°C, and absorbance was measured at 475 nm for a time course of up to 4 hours.
Advanced glycation end-products assay
AGE-modified BSA (AGE-BSA), an AGE used to study the toxicity of AGEs on a number of cells types, was prepared as described previously.[Citation19] Briefly, 1.0 g BSA was dissolved in 10 mL of 0.5 mol/L phosphate-buffered saline (PBS; pH = 7.4) with 3.0 g D-glucose. The sample was filter-sterilized using a 0.22-μm Millipore filter and incubated at 37°C for 12 weeks under sterile conditions in the dark. After incubation, the sample was dialyzed against PBS to remove unbound sugars. In the meantime, non-glycated BSA was prepared simultaneously by the same method, but without using D-glucose. AGE-BSA was identified using a fluorescence spectrophotometer. Protein concentration was measured using the BCA protein assay.
Effective components analysis
Varian 400 NMR instrument was used to record 1H NMR and 13C NMR spectrums. The chemical shifts of spectroscopic data are given in δ (ppm) and coupling constants in hertz (Hz). Bruker amaZon SL mass spectrometer equipped with an ESI ionization source (Bruker, Bremen, Germany) was used for acquiring mass data. The HPLC system was composed of Hitachi L-2310 series pump (Hitachi, Tokyo, Japan), L-2420 UV-VIS detector (Hitachi, Tokyo, Japan), and an ODS column (5 µm, 250 × 10 mm, Discovery® HS C18, Supelco Inc., Tokyo, Japan). The Medium pressure liquid chromatography (MPLC) was performed on a CombiFlash® Rf+ (Teledyne ISCO, Lincoln, USA). Sephadex LH-20 (Amersham Pharmacia Biotech AB, Sweden) was used for separation. LiChrospher® Si 60 (5 μm, 250–10, Merck, Darmstadt, Germany) and LiChrospher® 100 RP-18e (5 μm, 250–10, Merck, Darmstadt, Germany) were used for NP-HPLC and RP-HPLC (Merck, Darmstadt, Germany), respectively.[Citation20,Citation21]
The obtained extract was partitioned with ethyl acetate (EtOAc), inclusive of n-butanol (n-BuOH) to obtain EtOAc layer (12.3 g), n-BuOH layer (22.1 g), and H2O layer (108.7 g), respectively. Following the bioassay-guided fractionation isolation (BGFI), the EtOAc layer was subjected to a Sephadex LH-20 and eluted with MeOH to yield nine fractions (Fr. 1-Fr. 9). Later on, Fr. 2 was separated by MPLC with LiChroprep® RP-18 to afford five fractions (Fr. 2–1− Fr. 2–5) by elution with a linear gradient of mixtures of MeOH−H2O (from 10:90 to 90:10). Fr. 2–2 was subjected to preparative HPLC with a Discovery® HS C18 (250 × 10 mm, 5 μm) column and eluted by mixtures of MeOH−H2O (10:90), to yield compound 2 (3.2 mg). Fr. 3 was purified by preparative HPLC with isocratic solvent system MeOH-H2O (30:70), to afford compound 1 (5.2 mg). Fr. 4 was loaded onto a preparative HPLC system and eluted by mixtures of MeOH−H2O (20:80) to yield compound 4 (4.6 mg). Fr. 5 was treated similarly to yield compound 6 (10.0 mg). However, Fr. 6 was applied to an RP-18 column by MPLC eluted with a gradient solvent of mixtures of MeOH−H2O (from 10:90 to 90:10) and further purified by HPLC with mixture of MeOH-H2O (35:65) to obtain compound 3 (5.6 mg) and compound 5 (20.3 mg). The structures of all isolates were determined by the NMR analysis.
Statistical analysis
The significance of the treatment was evaluated with paired t-test with the software Statistical Product and Service Solutions (SPSS® 18.0; SPSS Inc., Chicago, IL). A difference was considered statistically significant when the p value was < 0.05. All results were expressed as mean ± standard deviation (SD).
Results and Discussion
Unhulled red djulis extracts increased repair ability in fibroblast
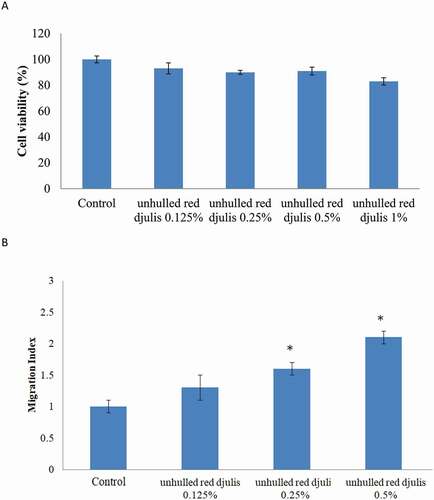
First, we treated different concentrations of unhulled red djulis extracts (0.125–1%) to observe cytotoxic effect by MTT. The results showed that unhulled red djulis extracts (0.125%-1%) not affected cell viability (), suggesting unhulled red djulis extracts did not cause cytotoxic effect. Then, we want to examine whether unhulled red djulis extracts can increase repair ability, using wound healing assay to analysis. The results showed that unhulled red djulis extracts increased cell migration in a dose-dependent manner (). Thus, unhulled red djulis extracts had low cytotoxicity and increased repair ability. Studies had shown that high concentration of red djulis extracts can generate saponin, alkaloids, phytic acid, and other components to cause cytotoxicity.[Citation22] In addition, red djulis extracts can increase superoxide dismutase (SOD), catalase, and glutathione (GSH), and then increase cell repair ability and healing speed.[Citation23]
Figure 1. The effect of unhulled red djulis on cell cytotoxicity and wound healing. (A) CCD-966SK cells were treated with unhulled djulis extract (0.125%, 0.25%, 0.5%, 1%). Then, cytotoxicity was assessed through MTT assay. (B) Scrape with a sterile 1-ml pipette tip and then CCD-966SK cells were cultured with unhulled djulis extract (0.125%, 0.25%, 0.5%, 1%), and then using Image J software to analysis. (n = 3; mean ± SD, using a paired Student’s t-test, *p < .05 compared with control group)
Unhulled red djulis extracts regulated skin-related genes, antioxidant-related genes, and collagen-related genes
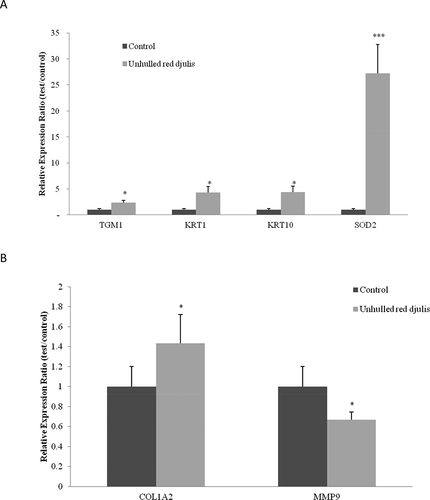
Transglutaminase 1 (TGM1) was involved in the formation of keratinocyte membranes, which are structures surrounding skin cells and help form a protective barrier between the human body and the environment.[Citation24] Keratin 1 (KRT1) and Keratin 10 (KRT10) encoded members of the cytokeratin family, respectively, and co-expressed during epithelial tissue differentiation.[Citation25] The superoxide dismutase 2 (SOD2) decomposed superoxide radicals into hydrogen peroxide and oxygen.[Citation26] The collagen type I alpha 2 chain (COL1A2) encoded type I collagen, and is found in most connective tissues. The matrix metallopeptidase 9 (MMP9) promoted reactive oxygen species (ROS) in ultraviolet light, causing collagen degradation and loss of function.[Citation19] Then, we want to know whether unhulled red djulis extracts can regulate skin-barrier-related genes (TGM1, KRT1, KRT10), antioxidant-related genes (SOD2), collagen-related genes (COL1A1 and MMP9), using qPCR to analyze gene expression. After treatment with unhulled red djulis extracts (0.5%), TGM1, KRT1, KRT10, and SOD2 genes were upregulated significantly 2.3, 4.3, 4.4, and 27.3 times, respectively, compared to those of the control group (). Additionally, the extract can increase COL1A2 gene expression 43% and decrease MMP9 gene expression 33% (). Studies had shown that the high-pressure-assisted extraction of red djulis had strong antioxidant capacity and anti-tyrosinase activity, thereby increasing the antioxidant genes expression and inhibiting MMP expression.[Citation8,Citation27] Clinical trials have indicated that the intake of red djulis can increase collagen and skin hydration by improving the adhesion of keratinocytes.[Citation11,Citation28] Therefore, unhulled red djulis extracts affected the gene expressions related to skin barrier, antioxidation, and collagen, and showed positive effects on skin barrier integrity, endogenous antioxidant activity, and skin collagen preservation.
Figure 2. Unhulled red djulis extracts regulated TGM1, KRT1, KRT10, SOD2, COL1A2 and MMP9 expression. CCD-966SK cells were treated with unhulled djulis extract (0.5%), and then used qPCR analysis. (A) Skin related genes: TGM1, KRT1, KRT10, antioxidant-related genes: SOD2. (B) Collagen-related genes: COL1A2 and MMP9. (n = 3; mean ± SD, using a paired Student’s t-test, *p < .05 compared with control group)
Unhulled red djulis extracts decreased AGEs, melanin, and increased collagen
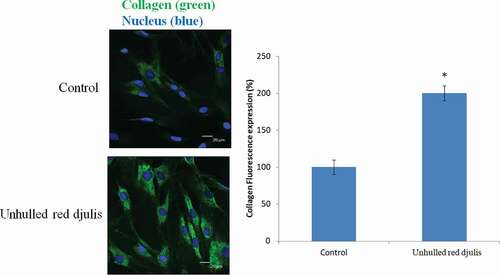
The accumulation of AGEs in the skin was related to skin aging. Inhibition of glycosylation of extracellular matrix proteins can help skin texture and appearance.[Citation29] The AGEs accumulation increased melanin content.[Citation30,Citation31] Then observe whether the unhulled red djulis extracts can decrease the AGEs and melanin content, using tyrosinase activity inhibition and advanced glycation end-products assay to analysis. The result showed that unhulled red djulis extracts decreased the AGEs compared with control group (). Unhulled red djulis extracts also significantly decreased melanin by about 30% compared to the control group (), suggesting unhulled red djulis extracts had a whitening effect by reducing the activity of tyrosinase, a key enzyme in melanin synthesis, and inhibiting melanin synthesis. Red djulis is rich in polyphenols, such as rutin. Its strong antioxidant activity can inhibit the production of tyrosinase in the skin and block melanin precipitation.[Citation32] In addition, AGEs promoted melanogenesis through receptor for AGEs, and caused collagen degradation.[Citation30] Next, we want to examine whether the unhulled red djulis extracts can increase collagen, using fluorescent staining to analyze, and collagen was green fluorescent dye, nucleus was blue dye. The result revealed that unhulled red djulis extracts can promote collagen secretion from skin fibroblast cells (). Lysine in red djulis can help calcium absorption and promote collagen formation.[Citation33] Thus, unhulled red djulis extracts can decrease AGEs, melanin, and increase collagen.
Figure 3. The effect of unhulled red djulis extracts on formation of AGEs and melanin. CCD-966SK cells were treated with unhulled djulis extract (0.5%), and then (A) analyzed the AGEs through advanced glycation end-products assay. (B) Analyzed the melanin through tyrosinase activity inhibition assay. (n = 3; mean ± SD, using a paired Student’s t-test, *p < .05 compared with control group)
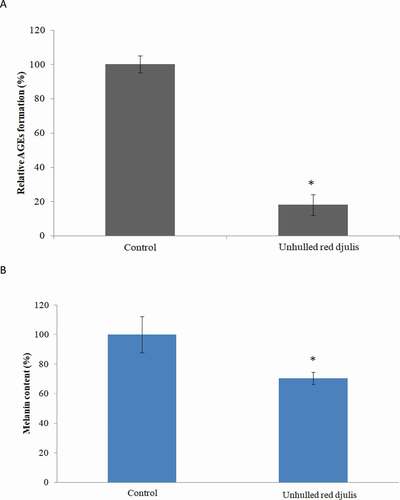
Figure 4. Effects of unhulled red djulis extracts on collagen content. CCD-966SK cells were treated with unhulled djulis extract (0.5%), and then analyzed the collagen by immunofluorescence staining. Green color: collagen. Blue color: Nucleus. (n = 3; mean ± SD, using a paired Student’s t-test, *p < .05 compared with control group)
Effective components of unhulled red djulis
Previous studies have shown that unhulled red djulis contains numerous antioxidant compounds and may be used to develop functional foods.[Citation10,Citation34] The amount of total flavones and total phenol of the unhulled red djulis groups was higher than those of the hulled red djulis (). In order to explore more clearly the possible effective components, we used unhulled red djulis extracts for HPLC and NMR. The result showed that the six effective components were identified (), which were caffeic acid (TCI-CF-01), 3,4-dihydroxyphenylacetate (TCI-CF-02), quercetin 7-O-α-L-rhamnoside 3-O-rutinoside (TCI-CF-03), guanosine (TCI-CF-04), 20-Hydroxyecdysone (TCI-CF-05), and adenine (TCI-CF-06).
Table 2. Antioxidant capacity of djulis extract
Figure 5. Effective components of unhulled red djulis extracts increased collagen content. (A) Profiles of unhulled red djulis extracts and compound structures. (B) CCD-966SK cells treated with 0.2 mg/mL of compounds 1–6, and then analyzed by collagen secretion assay. (n = 3; mean ± SD, using a paired Student’s t-test, *p < .05 compared with control group)
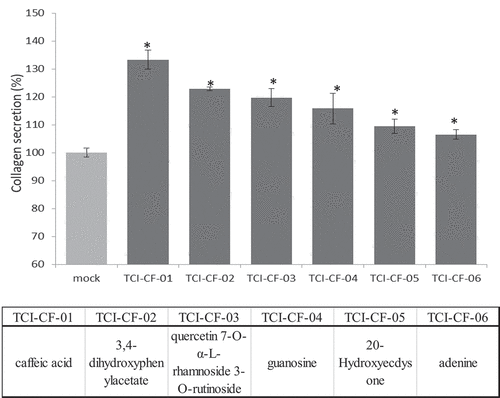
Effective components from unhulled red djulis increased the collagen content
Finally, we used TCI-CF-01-06 (0.2 mg/mL) to treat CCD-966SK cells to evaluate collagen production. The results showed that compounds 1–6 significantly increased the collagen production compared with the control group (), suggesting caffeic acid, 3,4-dihydroxyphenylacetate, quercetin 7-O-α-L-rhamnoside 3-O-rutinoside, guanosine, 20-Hydroxyecdysone, and adenine increased collagen production. Some studies have shown that caffeic acid inhibited TGF-β1-induced differentiation of fibroblasts into myofibroblasts and collagen production by regulating ROS.[Citation35] 3,4-Dihydroxybenzoate was used to inhibit collagen breakdown.[Citation36] Quercetin was considered to be an antioxidant, which can inactivate tyrosinase and has anti-oxidation and anti-melanogenesis effects.[Citation37] Synthetic guanosine and adenine regulated skin tissue regeneration and collagen production.[Citation38] Hydroxyecdysone inhibited the activation of phenoloxidase in the melanin granules to achieve the whitening effect.[Citation39] The unhulled red djulis enhanced the repairing ability of skin cells, and increased the TGM1, KRT1, KRT10, SOD2, COL1A2 expression, and decreased the MMP9 expression. In addition, unhulled red djulis can increase collagen and inhibit AGEs formation and melanin.
Conclusion
This study was also the first to discover the six effective components of unhulled red djulis. These effective components are rich in flavonoids and phenol derivatives can increase collagen production. Therefore, it was demonstrated that unhulled red djulis affected gene expressions related to the skin barrier, antioxidation, and collagen. The results showed positive effects on skin barrier integrity, endogenous antioxidant activity, and skin collagen-preservation. Finally, unhulled red djulis extract may be used as anti-aging skin care products, cosmetic ingredients, or functional beverages to provide flexible formulations in the future.
Acknowledgments
The authors would like to thank TCI gene group for their full technical and funding support. There are no conflicts to declare.
References
- Hennet, T.;. Collagen Glycosylation. Curr. Opin. Struct. Biol. 2019, 56, 131–138. DOI: https://doi.org/10.1016/j.sbi.2019.01.015.
- Yamauchi, M.; Sricholpech, M.; Scott, I. Lysine Post-translational Modifications of Collagen. Essays. Biochem. 2012, 52, 113–133. DOI: https://doi.org/10.1042/bse0520113.
- Shuster, S. A. M.; Black, M. M.; McVitie, E. V. A. The Influence of Age and Sex on Skin Thickness, Skin Collagen and Density. Br. J. Dermatol. 1975, 93(6), 639–643. DOI: https://doi.org/10.1111/j.1365-2133.1975.tb05113.x.
- Naylor, E. C.; Watson, R. E. B.; Sherratt, M. J. Molecular Aspects of Skin Ageing. Maturitas. 2011, 69(3), 249–256. doi: https://doi.org/10.1016/j.maturitas.2011.04.011.
- Bailey, A. J.;. Molecular Mechanisms of Ageing in Connective Tissues. Mech. Ageing. Dev. 2001, 1227, 735–755. DOI:https://doi.org/10.1016/S0047-6374(01)00225-1.
- Franca, R. A., Esteves, A. B. A., Borges, C. M., Quadros, K., Falcao, L. C. N., Caramori, J. C. T., & Oliveira, R. B. Advanced glycation end-products (AGEs) accumulation in skin: relations with chronic kidney disease-mineral and bone disorder. J Bras Nefrol. 2017, 39(3), 253-260. doi:https://doi.org/10.5935/0101-2800.20170042.
- Lu, W. C.; Chan, Y. J.; Tseng, F. Y.; Chiang, P. Y.; Li, P. H. Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium Formosanum). Foods. 2019, 8(11), 551. DOI: https://doi.org/10.3390/foods8110551.
- Huang, H. W.; Cheng, M. C.; Chen, B. Y.; Wang, C. Y. Effects of High Pressure Extraction on the Extraction Yield, Phenolic Compounds, Antioxidant and Anti-tyrosinase Activity of Djulis Hull. J. Food Sci. Technol. 2019, 56(9), 4016–4024. DOI: https://doi.org/10.1007/s13197-019-03870-y.
- Hong, Y. H.; Huang, Y. L.; Liu, Y. C.; Tsai, P. J. Djulis (Chenopodium Formosanum Koidz.) Water Extract and Its Bioactive Components Ameliorate Dermal Damage in UVB-Irradiated Skin Models. Biomed. Res. Int. 2016, 2016, 7368797. DOI: https://doi.org/10.1155/2016/7368797.
- Huang, C. Y.; Chu, Y. L.; Sridhar, K.; Tsai, P. J. Analysis and Determination of Phytosterols and Triterpenes in Different Inbred Lines of Djulis (Chenopodium Formosanum Koidz.) Hull: A Potential Source of Novel Bioactive Ingredients. Food Chem. 2019, 297, 124948. DOI: https://doi.org/10.1016/j.foodchem.2019.06.015.
- Lin, P., Alexander, R. A., Liang, C. H., Liu, C., Lin, Y. H., Lin, Y. H., Kuan, C. M. Collagen formula with Djulis for improvement of skin hydration, brightness, texture, crow's feet, and collagen content: A double-blind, randomized, placebo-controlled trial. J Cosmet Dermatol. 2021, 20(1), 188-194. doi:https://doi.org/10.1111/jocd.13500.
- Chen, M. N.; Chan, C. F.; Huang, S. L.; Lin, Y. S. Green Biosynthesis of Gold Nanoparticles Using Chenopodium Formosanum Shell Extract and Analysis of the Particles’ Antibacterial Properties. J. Sci. Food Agric. 2019, 99(7), 3693–3702. DOI: https://doi.org/10.1002/jsfa.9600.
- Kumar, P.; Nagarajan, A.; Uchil, P. D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, 6. doi:https://doi.org/10.1101/pdb.prot095505
- Tesena, P.; Korchunjit, W.; Taylor, J.; Wongtawan, T. Comparison of Commercial RNA Extraction Kits and qPCR Master Mixes for Studying Gene Expression in Small Biopsy Tissue Samples from the Equine Gastric Epithelium. J. Equine. Sci. 2017, 284, 135–141. DOI:https://doi.org/10.1294/jes.28.135.
- Yarrow, J. C.; Perlman, Z. E.; Westwood, N. J.; Mitchison, T. J. A High-throughput Cell Migration Assay Using Scratch Wound Healing, A Comparison of Image-based Readout Methods. BMC Biotechnol. 2004, 4, 21. DOI: https://doi.org/10.1186/1472-6750-4-21.
- Chu, C., Deng, J., Hou, Y., Xiang, L., Wu, Y., Qu, Y., & Man, Y. Application of PEG and EGCG modified collagen-base membrane to promote osteoblasts proliferation. Mater Sci Eng C Mater Biol Appl. 2017, 76, 31-36. doi:https://doi.org/10.1016/j.msec.2017.02.157.
- Donaldson, J. G.;. Immunofluorescence Staining. Curr. Protoc. Cell. Biol. 2015, 69, 43 1–4 3 7. DOI: https://doi.org/10.1002/0471143030.cb0403s69.
- Chan, Y. Y.; Kim, K. H.; Cheah, S. H. Optimization and Validation of a Cell-based Tyrosinase Assay for Screening of Tyrosinase Inhibitors. J. Health Transl. Med. 2011, 14(2), 1–4.
- Choi, S. J.; Lee, S. N.; Kim, K.; Joo, D. H.; Shin, S.; Lee, J.; Lee, H. K.; Kim, J.; Kwon, S. B.; Kim, M. J.;; et al. Biological Effects of Rutin on Skin Aging. Int. J. Mol. Med. 2016, 38(1), 357–363. DOI: https://doi.org/10.3892/ijmm.2016.2604.
- Liu, S., Gao, W., Wang, Y., He, Z., Feng, X., Liu, B. F., & Liu, X. Comprehensive N-Glycan Profiling of Cetuximab Biosimilar Candidate by NP-HPLC and MALDI-MS. PLoS One. 2017, 12(1), e0170013. doi:https://doi.org/10.1371/journal.pone.0170013.
- Mandrioli, M.; Tura, M.; Scotti, S.; Gallina Toschi, T. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis Sativa L. Molecules. 2019, 24(11), 2113. DOI: https://doi.org/10.3390/molecules24112113.
- Ashraf, M. F.; Abd Aziz, M.; Stanslas, J.; Ismail, I.; Abdul Kadir, M. Assessment of Antioxidant and Cytotoxicity Activities of Saponin and Crude Extracts of Chlorophytum Borivilianum. Sci. World J. 2013, 2013, 216894. DOI: https://doi.org/10.1155/2013/216894.
- Telorack, M.; Meyer, M.; Ingold, I.; Conrad, M.; Bloch, W.; Werner, S.; Yuspa, S. H. A Glutathione-Nrf2-Thioredoxin Cross-Talk Ensures Keratinocyte Survival and Efficient Wound Repair. PLoS Genet. 2016, 12(1), e1005800. DOI: https://doi.org/10.1371/journal.pgen.1005800.
- Shimada, K.; Ochiai, T.; Hasegawa, H. Ectopic Transglutaminase 1 and 3 Expression Accelerating Keratinization in Oral Lichen Planus. J. Int. Med. Res. 2018, 46(11), 4722–4730. DOI: https://doi.org/10.1177/0300060518798261.
- Eldirany, S. A.; Ho, M.; Hinbest, A. J.; Lomakin, I. B.; Bunick, C. G. Human Keratin 1/10-1B Tetramer Structures Reveal a Knob-pocket Mechanism in Intermediate Filament Assembly. EMBO J. 2019, 38(11). DOI: https://doi.org/10.15252/embj.2018100741.
- Treiber, N.; Maity, P.; Singh, K.; Ferchiu, F.; Wlaschek, M.; Scharffetter-Kochanek, K. The Role of Manganese Superoxide Dismutase in Skin Aging. Dermatoendocrinol. 2012, 4(3), 232–235. DOI: https://doi.org/10.4161/derm.21819.
- Jung, H. J., Lee, A. K., Park, Y. J., Lee, S., Kang, D., Jung, Y. S., Moon, H. R.(2E,5E)-2,5-Bis(3-hydroxy-4-methoxybenzylidene) cyclopentanone Exerts Anti-Melanogenesis and Anti-Wrinkle Activities in B16F10 Melanoma and Hs27 Fibroblast Cells. Molecules. 2018, 23(6). doi:https://doi.org/10.3390/molecules23061415.
- Sumigray, K. D.; Lechler, T. Cell Adhesion in Epidermal Development and Barrier Formation. Curr. Top. Dev. Biol. 2015, 112, 383–414. DOI: https://doi.org/10.1016/bs.ctdb.2014.11.027.
- Gkogkolou, P.; Bohm, M. Advanced Glycation End Products: Key Players in Skin Aging? Dermatoendocrinol. 2012, 4(3), 259–270. DOI: https://doi.org/10.4161/derm.22028.
- Lee, E. J.; Kim, J. Y.; Oh, S. H. Advanced Glycation End Products (Ages) Promote Melanogenesis through Receptor for AGEs. Sci. Rep. 2016, 6, 27848. DOI: https://doi.org/10.1038/srep27848.
- Ohshima, H., Oyobikawa, M., Tada, A., Maeda, T., Takiwaki, H., Itoh, M., & Kanto, H. Melanin and facial skin fluorescence as markers of yellowish discoloration with aging. Skin Res Technol. 2009, 15(4), 496-502. doi:https://doi.org/10.1111/j.1600-0846.2009.00396.x.
- De Freitas, M. M.; Fontes, P. R.; Souza, P. M.; William Fagg, C.; Neves Silva Guerra, E.; De Medeiros Nóbrega, Y. K.; Silveira, D.; Fonseca-Bazzo, Y.; Simeoni, L. A.; Homem-de-mello, M.;; et al. Extracts of Morus Nigra L. Leaves Standardized in Chlorogenic Acid, Rutin and Isoquercitrin: Tyrosinase Inhibition and Cytotoxicity. PLoS One.2016, 11(9), e0163130. DOI: https://doi.org/10.1371/journal.pone.0163130.
- Yamauchi, M.; Terajima, M.; Shiiba, M. Lysine Hydroxylation and Cross-Linking of Collagen. Methods Mol. Biol. 2019, 1934, 309–324. DOI: https://doi.org/10.1007/978-1-4939-9055-9_19.
- Chuang, K. J.; Chen, Z. J.; Cheng, C. L.; Hong, G. B. Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium Formosanum Seed Extract. Int. J. Mol. Sci. 2018, 19(9), 2726. DOI: https://doi.org/10.3390/ijms19092726.
- Chung, S. W., Park, I. H., Hong, S. M., Cho, J. S., Moon, J. H., Kim, T. H., & Lee, H. M. Role of caffeic Acid on collagen production in nasal polyp-derived fibroblasts. Clin Exp Otorhinolaryngol. 2014, 7(4), 295-301. doi:https://doi.org/10.3342/ceo.2014.7.4.295.
- Musselmann, K.; Kane, B.; Alexandrou, B.; Hassell, J. R. Stimulation of Collagen Synthesis by Insulin and Proteoglycan Accumulation by Ascorbate in Bovine Keratocytes In Vitro. Investig. Ophthalmol. Vis. Sci. 2006, 4712, 5260–5266. DOI:https://doi.org/10.1167/iovs.06-0612.
- Lee, S. G.; Karadeniz, F.; Seo, Y.; Kong, C. S. Anti-Melanogenic Effects of Flavonoid Glycosides from Limonium Tetragonum (Thunb.) Bullock via Inhibition of Tyrosinase and Tyrosinase-Related Proteins. Molecules. 2017, 22(9), 1480. DOI: https://doi.org/10.3390/molecules22091480.
- Xiao, M.; Gao, L.; Chandrasekaran, A. R.; Zhao, J.; Tang, Q.; Qu, Z.; Wang, F.; Li, L.; Yang, Y.; Zhang, X.;; et al. Bio-functional G-molecular Hydrogels for Accelerated Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110067. DOI: https://doi.org/10.1016/j.msec.2019.110067.
- Jing, Y. P.; Wang, D.; Han, X. L.; Dong, D. J.; Wang, J. X.; Zhao, X. F. The Steroid Hormone 20-Hydroxyecdysone Enhances Gene Transcription through the cAMP Response Element-binding Protein (CREB) Signaling Pathway. J. Biol. Chem. 2016, 291(24), 12771–12785. DOI: https://doi.org/10.1074/jbc.M115.706028.
