 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This work aimed to investigate the effect of five drying methods, namely hot-air drying (HD), microwave drying (MD), vacuum drying (VD), vacuum microwave drying (VMD), and vacuum freeze-drying (VFD), on the bioactive compounds, antioxidant activity, color, sensory attributes, and microstructure of purple cabbage. Different drying methods considerably affected the quality factors of the products. The highest remaining total phenolic content (TPC), total flavonoid content (TFC), and total anthocyanin content (TAC) concentrations were observed in the VFD cabbage at 10.57 mg GAE/g, 7.01 mg RE/g, 6.54 C-3-G mg eqiv/g, respectively. This was followed by the VMD samples at a TPC at 9.06 GAE mg/g, TFC level of 5.84 mg RE/g, and TAC at 3.80 C 3 – G mg eqiv/g. Furthermore, the VFD samples displayed the best performance in antioxidant activity. The antioxidant activity of the VMD samples was lower than the VD and MD samples, but higher than the HD samples. In terms of color and flavor, the VMD samples displayed a low ∆E (P < .05) value, while the flavor resembled that of fresh purple cabbage, compared to the VFD sample. According to these chemical and physical indicators and drying time. VMD is better for drying treatment of purple cabbage.
Graphical Abstract
INTRODUCTION
Purple cabbage (Brassica oleracea L. var. capitata L. f. rubra) is one of the most important vegetables grown worldwide .[Citation1] Purple cabbage belongs to the Cruciferae family, featuring thick, plied purple leaves. For many years, it has been used for therapeutic purposes, [Citation2] and studies show the possibility of applying red cabbage extract as a dietary supplement for treating inflammatory bowel disease. Furthermore, Dominguze and Moreno have indicated that purple cabbage has numerous beneficial properties and can be used as an alternative treatment to relieve edema, and heal burns and skin lesions, while also improving digestion and mitigating arthritic pain .[Citation3] Several scientific studies have confirmed that purple cabbage displays excellent antioxidant properties, [Citation1,Citation4] since it is rich in a variety of anthocyanin .[Citation5] Furthermore, purple cabbage is known for its high nutritional value since it is rich in minerals, vitamins, oligosaccharides, and several bioactive substances, such as flavonoid, and glucosinolates, having a positive impact on human health .[Citation4]
Drying as an old and efficient preservation technique in food processing to prevent the development of microorganisms, reducing the weight and volume of food to minimize transportation and storage cost .[Citation6] Various drying techniques are currently utilized for fruits and vegetables, each with its advantages and disadvantages. The most common drying method is hot-air drying (HD). However, HD takes long time to reduce the moisture in materials, significantly impacting color, associated flavor, and bioactive compounds .[Citation7] During vacuum drying (VD), the mass transfer occurs under low pressure, reducing the boiling temperature of the water, preserving and improving the quality of the processed product. Therefore, this technique is commonly used for heat-sensitive products .[Citation8] Microwave drying (MD) displays a high heating efficiency, as well as better aroma and color retention. However, the disparity in the absorption of microwave energy caused by the unequal internal moisture of the material during the late drying stage is likely to lead to local scorching and low quality of the dried material .[Citation9,Citation10] Vacuum microwave drying (VMD) creates volumetric heating in foods, allowing the drying process to be performed in a shorter time with a combined system that provides rapid mass and energy transfer at a low temperature under vacuum .[Citation11] Samples obtained via VMD displayed good sensory and nutritional properties .[Citation12] Furthermore, the vacuum freeze-drying (VFD) method retains the color, aroma, taste, shape, and nutritional components of the product to the maximum extent .[Citation13] This method also maintains its inherent form without hardening the surface of the material while extending the storage period .[Citation14] However, VFD requires an extended processing time, expensive equipment and high energy consumption.
Fruits and vegetables are the most important products in the agricultural sector and play a vital role in human health .[Citation15,Citation16] However, despite their nutritional and health benefits, many fruits and vegetables are seasonal and highly perishable .[Citation17] Therefore, proper preservation methods are essential. Drying is the most common and cheapest way of food preservation, significantly extending shelf life and further processing in the food industry .[Citation18,Citation19] Many researchers have studied the effect of drying methods on the quality of fruits and vegetables. Li examined the change in surface wrinkling of shiitake mushrooms during HD .[Citation20] Chen explored the moisture kinetics and microstructure evolution in apples during the high powdered MD process .[Citation21] Bozkir studied the effect of VMD on the quality of orange slices .[Citation11] VFD is a new drying technology with several advantages .[Citation7,Citation14] Zhang studied the effect of VFD on the quality of strawberry slices .[Citation13] This highlights the importance of utilizing drying technology for fruit and vegetable processing. However, minimal studies are available regarding the drying of purple cabbage.
This study examined the influence of different drying techniques (HD, MD, VD, VMD, and VFD) on the physical and chemical properties of purple cabbage. The effect of high and low temperatures combined with atmospheric pressure and vacuum techniques on the drying characteristics and dried product quality is also investigated regarding the feasibility of industrial drying via mechanical cabinet HD, MD, VD, VMD, and VFD. The results are assessed in terms of microstructure, antioxidant activity, as well as physical and chemical profiles. It is expected to generate useful information for the broader application for the purple cabbage while providing a valuable foundation for extending the processing and production of functional food.
MATERIALS AND METHODS
Raw materials
Fresh purple cabbage, uniform in size and color (initial moisture content of 93.0 ± 1.0 water g/100 g, measured by drying at 105°C), were purchased from a Walmart Store (Sichuan, China). The cabbage (12 ± 2 cm in diameter) was kept at (4 ± 1) °C for the subsequent drying experiment.
Drying process
The purple cabbage was shredded thoroughly before performing the drying process. Specifically, the stems were discarded, and the leaves were cut into 1 × 5 cm strips. The following drying methods were used for the preparation of the samples until the dry base moisture content was below 10%. Each drying method was performed at least three times, and the sample closest to the end point with a dry base moisture content of 10% was selected for subsequent analysis:
HD: The purple cabbage strips were dried in an electro-thermostatic blast oven (BPG-9240A, Shanghai Yiheng Technology Co., Ltd. Shanghai, China) at 70°C. The actual dry base moisture content at the end point was 9.93%;
MD: The purple cabbage strips were placed in an experimental microwave oven (EM7KCGW3-NR, Guangdong Midea Kitchen Electric Appliance Manufacturing Co. Ltd. Guangdong, China) and dried at 350 W power. The actual dry base moisture content at the end point was 9.89%.
VD: The purple cabbage strips were placed in the chamber of a vacuum dryer (BZF-50, Shanghai Boxun Industrial Co., Ltd. Shanghai, China) and dried at 70°C combined with vacuum degree ≤ – 0.09 MPa. The actual dry base moisture content at the end point was 9.76%;
VMD: The purple cabbage strips were placed on a tray in an experimental VMD chamber (JDH-3GZ, Guangzhou Yongze Microwave energy Equipment Co., Ltd. Guangzhou, China) and dried at 1 kW power. The actual dry base moisture content at the end point was 10.18%;
VFD: The purple cabbage strips (which was pre-cooled at −80 ± 2 °Cin an ultra-low temperature refrigerator (ULTS1368, Thermo Fisher Suzhou Co., Ltd, Suzhou, China) for 12 h) were placed in a vacuum freeze-dryer (FDU-1200, Shanghai Ailang Instrument Co., Ltd. Shanghai, China) for about 42 h at a cold trap temperature of – 48 ± 2°C and 40 ± 4 Pa. The actual dry base moisture content at the end point was 10.21%.
Scanning Electron Microscopy (SEM)
The effect of different drying methods on the surfaces of the purple cabbage slices were investigated via field-emission SEM analysis using the ZEISS EVO 15 (Carl ZEISS Microscopy GmbH, Oberkochen, Germany) according to a procedure described in a previous study .[Citation6] Cabbage pieces from different sections were gently cut into 3 × 3 mm pieces using a razor blade. The cut samples were then fixed on an SEM support and coated with gold using an ion sputter coater under vacuum conditions for 4– 5 min. After processing, the samples were observed with SEM in high-vacuum mode at an accelerating voltage of 3.0 kV. The samples were examined at a magnification of 100×, 500×, and 1 K× to identify the surface characteristics.
Total flavonoids and total phenolic acids in purple cabbage
A weight of 1.0 g of the pulverized dried purple cabbage (five different treatments) was added to 40 mL of 90% methanol solution in a beaker. Then, ultrasonically-assisted extraction was conducted for 30 min at 40°C. The reaction mixture was left to stand for 0.5 h, after which it was centrifuged at 10,000 rpm. The supernatants were transferred to a 100 mL beaker. The residue was reextracted in a 90% methanol solution, following the same procedure as before. The two supernatants were combined, and the methanol was removed at 50°C in a rotary evaporator. The obtained concentrated extract was diluted to 10 mL with water for subsequent analyses .[Citation22]
Total Flavonoid Content (TFC)
The TFC was determined using the colorimetric method described by Chumroenphat et al .[Citation7] A milliliter of the diluted extract was placed in a 10 mL glass tube, after which 1 mL of the 5% NaNO2 solution was added. After 6 min interval, 1 mL of 10% Al (NO3)3 and 4 mL of 4% NaOH solutions were added. Then, 3 mL of 70% ethanol was added to the reaction mixture, which was left to stand for 10 min before the absorbance was read at 510 nm using a spectrophotometer (Shanghai Sainty Hengping Scientific Instrument Co. Ltd., Shanghai, China). A blank was prepared by replacing the sample with 80% methanol. Results were expressed as mg rutin equivalents (RE) per g dry basis (mg RE/g DW).
Total Phenolic Content (TPC)
Folin-Ciocalteu’s reagent was used to determine the TPC according to a method described by Siriamornpun .[Citation23] Briefly, 2 mL of the diluted extract (diluted 20 times with pure water) was placed into test tubes, followed by 1.0 mL of Folin-Ciocalteu phenol reagents. After 5 min, 2.0 mL of sodium carbonate (20% w/v) solution was added to the mixture. The reaction systems were kept in dark for 30 min after constant volume of distilled water to 10 ml, followed by reading the absorbance at 760 nm using a UV 2400 spectrophotometer (Shanghai Sainty Hengping Scientific Instrument Co. Ltd., Shanghai, China). A blank was prepared by replacing the sample with 80% methanol. The equation obtained for the calibration curve of gallic acid (0.5–100 mg/L) was y = 0.8349x + 0.0243 (R2 = 0.9967). The TPC was expressed as gallic acid equivalents (GAE) per g dry basis (mg GAE/g DW).
Antioxidant capacity of purple cabbage
Free Radical Scavenging Capacity Using the DPPH Method: Purple cabbage extracts at concentrations 1.0 mg/mL were prepared by diluting the stock extract. Then, 2 mL of the extracts were mixed with 2 mL of 0.2 mmol/L DPPH dissolved in absolute ethanol. Two control groups were simultaneously prepared by using an equal volume of absolute ethanol instead of the DPPH solution (control 1) or the sample extract (control 2). The reaction mixture (sample, control 1 and control 2) was kept in the dark at room temperature for 30 min and after which the absorbance was read at 517 nm, using a spectrophotometer (UV2400, Shanghai sainty hengping scientific instrument Co. Ltd., Shanghai, China). Ethanol was used to adjust the zero absorbance. The DPPH radical scavenging ability was calculated using Eq:
where S is the DPPH radical scavenging ability (%), Ai is the absorbance of the sample, Aj is the absorbance of control 1 and A0 is the absorbance of control 2. Trolox was used as a positive control in this experiment and the results are expressed as the equivalent of Trolox .[Citation24,Citation25]
ABTS+ Antioxidant Activity: The ABTS+ cation radical scavenging assay was performed according to a method described by Jorjong with some modifications .[Citation26] The fresh 2ʹ,2ʹ-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS+) radical cation stock solution was prepared using a 1:1 (v/v) proportion of 2.6 mmol L−1 potassium persulfate and 7.4 mmol L−1 aqueous stock solution of ABTS+. The solution was combined using a vortex mixture and incubated for 12 h at room temperature before use. Trolox, the antioxidant standard in this method, was used in a range of 0–1000 μg/mL. The 0.8 mL extract (which was diluted twice) was added to 3.2 mL of ABTS+ radical cation solution and mixed via vortexing. After incubation for 0.5 h, the absorbance of the samples was measured using an ultraviolet and visible spectrophotometer (UV2400, Shanghai sainty hengping scientific instrument Co. Ltd., Shanghai, China) at 734 nm. The samples were assayed in triplicate. The TEAC values were calculated from the Trolox standard curve and expressed in millimol Trolox equivalent antioxidative capacity per gram of dry weight (mmol TEAC/g DW).
Ferric Reducing Antioxidant Power (FRAP) Assay: The antioxidant activity of the extract was also evaluated using a FRAP assay with some modifications to the method described by Chumroenphat .[Citation7] The FRAP reagent was prepared by mixing 100 mL of acetate buffer (0.3 moL−1, pH 3.6), 10 mL of TPTZ solution dissolved in 10 mL of 40 mmoL−1 HCl, and 10 mL of 20 mmoL−1 FeCl3 at a ratio of 10:1:1 (v/v/v), respectively. The FRAP reagent was freshly prepared daily and warmed to 37°C in an oven before use. Briefly, 200 μL of the sample extract was added to 4.8 mL of the FRAP reagent, mixed well, and incubated for 30 min at 37°C. The absorbance was read at 595 nm using a UV 2400 spectrophotometer (Shanghai Sainty Hengping Scientific Instrument Co. Ltd., Shanghai, China) against a control. The samples were measured in triplicate. The standard curve of the iron (II) sulfate solution (0–1200 µM) was y = 0.9536x – 0.06 (R2 = 0.9919). The results were expressed as mg FeSO4 per g dry weight (mg FeSO4/g DW).
Hydroxyl Radical Scavenging Ability (·OH): The hydroxyl radical scavenging ability (·OH) of the samples was detected as previously described by Li .[Citation27] A spectrophotometer was used to read the absorbance at 510 nm. The hydroxyl radical scavenging activity was calculated using the following equation:
where A0, Ax and Ax0 refer to the light-absorption values of the distilled water, the sample extract, and the control, respectively.
Total Anthocyanin Content (TAC)
The TAC of the purple cabbage was determined using the differential pH method described by Zhang[Citation13] and was expressed as cyanidin 3-glucoside equation (C – 3 – G eqiv).
Color analysis
During this experiment, the color was measured using a precision colorimeter (WF-32, Hangzhou Caipu Technology Co., Ltd, Hangzhou, China). The color parameters of the samples at different pretreatments were measured according to the method described by Xu and Zhang .[Citation28] The measuring head was first calibrated with a white calibration plate. After standardization, the brightness (L*), red/green (a*), yellow/blue (b*) and hue (h)values of fresh purple cabbage homogenate and dry product powder were measured. At least nine measurements were performed for each sample, after which the average was recorded. The total color difference (ΔE) and hue value (h) was calculated by using the following formula:
where L0*, a0* and b0* are the color parameters of fresh purple cabbage homogenate, and L*, a*, and b* are the color parameters of dried purple cabbage powder.
Electronic nose (E-nose) analysis
An E-nose (Pen III, Airsense, Schwerin, Germany) with ten different metal oxide semiconductor (MOS) sensors were used for the test, according to the method described by Feng et al.[Citation14] and Zhang et al.[Citation29] Briefly, 3.00 g of purple cabbage stripes, subjected to different drying methods, were coarsely ground into pieces and placed in a 20 mL headspace vial. The vial was sealed with polytetrafluoroethylene/silicone and then incubated at 40°C for 20 min. The headspace gas in the vial was extracted using a pump in the E-nose system at a flow rate of 150 mL/min and evaluated with the MOS sensors. The assessment time and flush time were 180s and 120 s, respectively. A signal was recorded every second during the sample inspection
Statistical analysis
The analysis was performed at least three times. Data were statistically analyzed using SPSS 22.0 statistical software (SPSS Inc., USA). One-way analysis of variance (ANOVA) was used to compare the groups, and the difference between the mean values was considered significant at P < .05.
RESULTS AND DISCUSSION
Effect of different drying conditions on the microstructure of purple cabbage
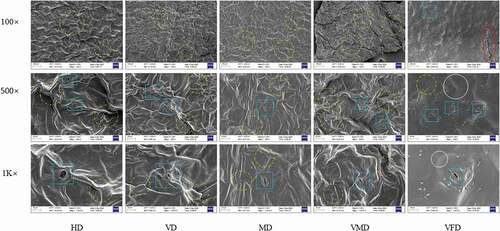
Changes in the microstructure are closely associated with the properties of a dried product .[Citation30] SEM was used to determine the microstructures to explain the effect of different drying methods on the quality properties of purple cabbage, as illustrated by the micrographs in . Distinct differences were evident in the microstructure of the cabbage subjected to different drying methods. shows that the skeleton structure of the cabbage was retained during VFD, where the removal of water occurred via sublimation from frozen substances in conjunction with the effect of the vacuum. Therefore, the texture of the VFD samples was less dense with a relative complete cell structure, corresponding with the observation of An .[Citation31] Furthermore, the VFD samples displayed a loose, brittle texture, with a complete and original structure, which was consistent with the observation of Huang .[Citation32] In addition, the semicircular concave shapes were distinctly visible, indicating that the original shape of the sample was retained. However, Chumroenphat[Citation7] reported the formation of a temporary heat and moisture gradient during any thermal drying process, resulting in degradation, deformation, and folding. This sufficiently explains the shrinkage and folding in the other four samples (HD, MD, VD, and VMD). MD caused severe shrinkage in the product (), displaying a compact structure with many dense, high lines due to the contraction and compression of the tissue. Due to the higher diffusion rate of microwave power and drying vacuum conditions, the VMD sample also had a poor effect on the microstructure (). These phenomena should be related to what An said, [Citation31] during the microwave drying, due to the rapid conversion of microwave radiation, the inner moisture was difficult to evaporate outside. Therefore, the internal accumulation of moisture led to crosslinking of the cellular structures, causing the surface to exhibit strong puckering. Furthermore, the interpretation by Feng[Citation14] can also explain this problem. A study by Bozkir showed that the bulk density of dried carrot slices exposed to VMD was higher than dried samples using tray drying, indicating that compressional folding was more likely to lead to wrinkling .[Citation11] The HD and VD were exposed to high temperature (70°C), severely damaging the skeleton structure of cabbages. Similar results have been reported by An[Citation31] and Huang, [Citation32] who found that dehydration collapsed the parenchyma cell, leading to severe surface shrinkage. The microstructure and surface shrinkage of dried products are related to the water migration mechanisms and external pressure changes. In addition, moisture loss and heating cause pressure in the cellular structure of the food leading to shrinkage and changes in the microstructure .[Citation18,Citation33]
Figure 1. Morphology (100×, 500×, and 1 K× magnification) of cabbage slices treated by HD, VD, MD, VMD, VFD. Scale bar represents 100 μm
Drying methods on the TPC, TFC, and TAC of the purple cabbage
Different drying treatments affected the TPC, TFC, and TAC of purple cabbage samples. shows that the TPC, TFC and TAC in VFD samples were significantly higher (P < .05) than in the other four treatment groups, reaching up to 10.57 ± 0.11 mg GAE/g, 7.01 ± 0.14 mg RE/g, and 6.54 ± 0.15 C-3-G mg eqiv/g, respectively. According to An, [Citation31] the increased extraction efficiency promoted the extraction of active ingredients in dried samples after VFD. The samples were fluffy and porous, exhibiting better extraction efficiency than other treatment groups, increasing the TPC, TFC and TAC detected in VFD samples.
Table 1. Drying time, the total content of phenolic, flavonoids and anthocyanin of purple cabbage treated by different drying methods
The HD, MD, VD, and VMD processes resulted in TPC losses of 21.38%, 15.80%, 24.12%, and 14.29%, respectively, compared with VFD (10.57 ± 0.11 mg GAE/g). The VMD and the MD samples losses less, all of them have high total phenol content, and there was no significant difference between these two groups. This may be related to MD mechanism. According to Calin-Sanchez[Citation18] and Lim, [Citation34] the heat generated by MD was intense and rapid since the process involves using a vacuum, ensuring a quick mass transfer and a low temperature. This process is combined with the microwave heating, guaranteeing an accelerated energy transfer. Kubra and Rao[Citation35] observed an increase in TPC of MW-dried ginger, which could be ascribed to MW energy causing the breakdown of cellular constituents, resulting in a higher release of polyphenols from the matrices. Moreover, according to An, [Citation31] the drying process resulted in high or low TPC levels depending on the type of plant material and the location of the phenolic compounds in the cell. The VD samples (8.02 ± 0.11 mg GAE/g) displayed lower TPC content than the HD samples (8.31 ± 0.09 mg GAE/g). This may be due to the VD being dried at high temperatures (70°C) for extended periods (38.00 ± 2.00 h), which is significantly longer than HD (7.00 ± 1.00 h). The TPC of dried purple cabbage decreased due to thermal degradation during the drying processing .[Citation11] Previous studies have shown that the TPC of mango, kiwi, and eggplant decreased due to the increasing temperature during the drying processes .[Citation36–39]
Compared with the TPC values, the TFC values showed a similar overall trend (). The highest content was evident in the VFD samples at 7.01 ± 0.14 mg RE/g, followed by VMD (5.84 ± 0.09 mg RE/g), MD (5.68 ± 0.12 mg RE/g), HD (5.61 ± 0.26 mg RE/g), and VD (5.44 ± 0.13 mg RE/g). VD caused the highest loss, with the drying process taking about 38.00 ± 2.00 h to transfer the moisture at 70°C, confirming that the loss of these macromolecules might be caused by a combination of the duration and temperature .[Citation31] Compared to VD, the MD samples exhibited a relatively high TFC content, possibly due to the significant reduction in drying time (0.20 ± 0.05a h < 38.00 ± 2.00 c h). Furthermore, microwave radiation penetration caused the degradation of cellular constituents, making flavonoids more accessible during the extraction .[Citation40] This can also explain why the MD samples display higher TFC than HD. The VMD sample exhibited higher TFC content than the MD sample and this could be caused by two different drying conditions. VMD creates volumetric heating in foods, and drying was performed in a shorter time with a combined system that provides rapid mass and energy transfer at low temperatures under vacuum .[Citation11] Nevertheless, the fixed MD exhibits burning, browning, and other phenomena during the middle or later drying stages due to the uneven heating of materials .[Citation41] The above mechanism may be the reason for the differences in TFC content.
TAC is responsible for the characteristic bright red color of purple cabbage .[Citation13] As shown in , significant differences were observed in the TAC of five purple cabbage treatment samples (P < .05). Compared to the VD sample (1.87 mg C 3 – G eqiv/g), the TAC of the VFD sample was significantly enhanced, reaching up to 6.54 mg C – 3 – G eqiv/g. This was consistent with research conducted by Zhang, [Citation29] who indicated that vacuum treatment significantly reduces the oxygen content during drying, inhibiting anthocyanin decomposition through oxidation. Moreover, compared to MD (2.26 ± 0.07 C – 3 – G mg eqiv/g), the TAC of VMD reached up to 3.80 C – 3 – G eqiv mg/g, also proving this phenomenon. The VD samples displayed the lowest TAC. This was consistent with a study by Lagnika, [Citation42] who showed that anthocyanins are sensitive to temperature, although their degradation can be effectively inhibited under vacuum conditions, but the VD samples has been dried for too long time, exceed 38.00 h. The order of the TAC after exposure to the different drying methods from high to low was VFD, VMD, HD, MD, and VD. The possible reason is that high temperatures and atmospheric pressure accelerate the oxidative breakdown of anthocyanins, [Citation43] while the MD energy intensity is too high, [Citation10] leading to the thermal oxidation of anthocyanins in the oxygen-containing environment at atmospheric pressure.
Drying methods on the antioxidant activity of the purple cabbage
Fruits and vegetables are rich sources of antioxidant groups, displaying antioxidant activity by acting as free radical scavengers or metal chelators .[Citation44] Previous research indicated that the antioxidant activity of fruit and vegetables might be ascribed to the presence of specific antioxidant groups, such as ascorbic acid, carotenoids, flavonoids, and phenolic acids .[Citation45–47] In this study, the scavenging ability of DPPH, hydroxyl radicals, ABTS, and FRAP, as well as the TFC, TPC, and TAC values, were used to analyze the influence of drying methods on the antioxidant activity of purple cabbage. The scavenging ability of DPPH, hydroxyl radicals, ABTS, and FRAP for cabbage dried using different methods are presented in . The VFD samples exhibited the highest antioxidant activity, including DPPH (21.37 ± 0.12e TEAC mg/g DW), hydroxyl radicals (55.37 ± 0.37 c AEAC mg/g DW), ABTS (21.67 ± 0.21e TEAC mg/g DW), and FRAP (326.38 ± 5.74e mg Fe2+/g DW), respectively. Combined with the TFC, TPC, and TAC mentioned above, the results of this study demonstrated that when exposed to the non-thermal VFD method, the scavenging ability of DPPH, hydroxyl radicals, ABTS, and FRAP, as well as the TPC, TFC, and TAC values were significantly higher than the thermal VMD, HD, MD and VD techniques.
Table 2. Effect of different drying methods on the antioxidant activity of purple cabbage
This is consistent with the study conducted by Hamid, [Citation48] who indicated that the higher antioxidant properties of the dried VFD samples might be due to the high redox potential of polyphenols, allowing them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. Similar results were reported in previous studies, indicating that intense heat extended heat treatment during the drying process usually cause severe losses of in antioxidant activity and the degradation of active components .[Citation27] The antioxidant activity values of the thermal drying methods, namely VMD, MD, HD, and VD, declined in turns, as shown in . This study demonstrated that when using the non-thermal VFD method and the rapid thermal VMD and MD techniques, the scavenging ability of DPPH, ABTS, hydroxyl radicals, and FRAP were higher as compared to the extended, high heat VD and HD methods. An[Citation31] indicated that MD had the most adverse effect on the antioxidant properties of the samples. The MD and VMD samples exhibited a lower antioxidant ability than VFD but were higher than VD and HD. Furthermore, the antioxidant activity of the HD sample was the lowest, which could be attributed to the adverse conditions of this method, such as an extended drying time at high temperature, as well as high oxygen and high airflow environment. Similar results were reported in previous studies. Intense heat and extended heat treatment during the drying process typically cause more severe losses in antioxidant activity and the degradation of active components .[Citation27,Citation49,Citation50]
Different drying methods on the color of the purple cabbage
Color is one of the most important quality evaluation indicators of dried purple cabbage. It is also a key quality factor affecting consumer acceptance and product market value .[Citation22] The L*, a*, b*, h, ΔE color parameters of the cabbage samples are summarized in . shows the surface color parameters of the HD, MD, VD, VMD, and VFD purple cabbage samples. Compared to fresh samples, dried cabbage samples exhibited higher L* values (except for the MD samples). In terms of the a* values and compared to fresh samples (0.71 ± 0.07b), no significant difference was evident between the fresh and MD (0.68 ± 0.08b) samples, while the VD sample displayed a lower value (0.50 ± 0.04a), and the VMD, HD, and VFD exhibited higher values at 0.82 ± 0.11 c, 1.04 ± 0.07d, and 1.38 ± 0.06e, respectively. In trems of hua value, there was no significant difference among Fresh (−0.36 ± 0.04b), HD (−0.20 ± 0.20b), MD (−0.46 ± 0.12b) and VMD (−0.43 ± 0.07b) samples (P < .05). The VD (−0.85 ± 0.08a) and VFD (−0.83 ± 0.69a) samples exhibited lower value. However, the b* values of all the groups were lower than the fresh sample (−0.26 ± 0.04b), except for HD (−0.22 ± 0.20b), but no significant relationship was evident between these two groups. In addition, a significant difference (P < .05) were observed in the ∆E values of the five different drying methods. The VFD product had the highest value of 4.49 ± 0.21e, indicating the most substantial difference of ∆E between this sample and the fresh sample. This was followed by VD, VMD, HD, and MD. The MD sample exhibited the smallest ∆E value at 0.16 ± 0.09a, indicating that the color of this sample was the closest to the fresh purple cabbage.
Table 3. Effect of different drying methods on the color of purple cabbage
The L*, a* and b* values of the dried purple cabbages were found to be significantly far from than the fresh one. This can be attributed to an increase in the H+ concentration during drying, [Citation51] as well as an increase in pigment concentrations due to water reduction .[Citation50] These observations can also be explained by reducing oxygen and a lower temperature present in a VD chamber under low pressure. In these circumstances, the enzymatic browning reaction, which is the leading cause of the color degradation of the dried samples, is relatively weak .[Citation52] Another key factor is drying time, since low-temperature drying methods prolongs the drying time, often causing poor color .[Citation53] In this study, VD and VFD were time-consuming drying methods with high ∆E values (2.06 ± 0.49d and 4.49 ± 0.21e) and low h value (−0.85 ± 0.08a and −0.83 ± 0.69a), respectively.
Different drying methods on the flavor of the purple cabbage
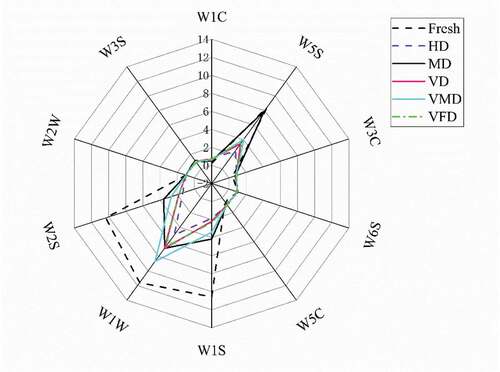
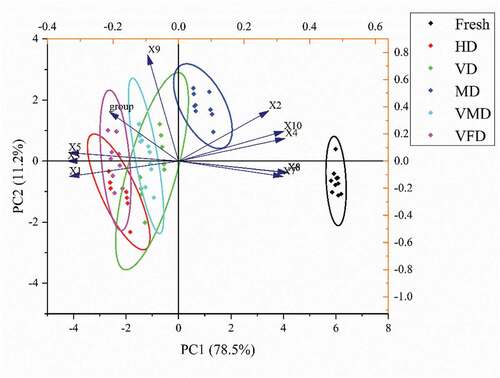
The flavor profile is an important factor effecting fruit quality .[Citation54] The principal component analysis (PCA) of electronic nose can quickly distinguish volatile compounds from different samples. As can be seen from , the first and second principal component contribution rates (PC1 and PC2) are respectively 78.50% and 11.20% and the total contribution rate is 89.70%, exceeding 85%, therefore it can reflect the principal component data. The dried samples are on the left side of the fresh sample, suggesting a significant difference in the main components. Furthermore, the fresh sample and MD sample were located in the right-hand areas of the PCA figure and far away from the other samples, indicating that the odor profiles of these two samples exhibited similar odor profiles.
Figure 2. Principal component analysis (PCA) (a) and Radar map (b) of purple cabbage with different drying methodsNote: Fresh: Fresh purple cabbage; HD: hot air drying; MD: microwave drying; VD: vacuum drying; VMD: vacuum microwave drying; VFD: vacuum freeze drying. W1C: aromatic compounds; W5S: broad range; W3C: ammonia used as an aromatic compound sensor; W6S: hydrogen; W5C: aromatic-aliph; W1S: broad methane; W1W: sulfate-organic; W2S: broad alcohol; W2W: sulfate-chloride; W3S: methane-aliph
The response data from ten sensors are shown in . The substances that could be detected by the W5S (broad range), W1W (sulfate-organic), W1S (broad methane), and W2S (broad alcohol) sensors likely contributed most to the odor profiles of the dried purple cabbage strips. The HD samples showed the lowest levels of these substances. Except for the fresh purple cabbage, the MD, VMD, and VFD samples showed the best odor performance. This is consistent with results obtained by Feng et al. who indicating that a combination of low temperatures and pressures led to the excellent preservation of the shape, color, and flavor of the dried product .[Citation14]
Conclusion
This study investigated the influence of different drying techniques on the physical (color and flavor), and chemical (TPC, TFC, TAC, and antioxidant ability) properties and microstructure of purple cabbage. The results show that VFD represent the best method for drying the samples regarding its physical, chemical and microstructure performance. However, it takes a long time and is relatively expensive to achieve high-quality dried samples. Furthermore, The VMD samples also perform well regarding antioxidant ability, TFC, TPC, TAC, color, odor as well as microstructure. The performance of the samples obtained using the other three drying methods is relatively low. Therefore, considering the physical and chemical properties, drying time and energy consumption performance, VMD is the most suitable drying method for the industry.
Acknowledgments
This work were supported by the Science and technology support program of Sichuan (2019YFN0174, 2018NZ0090, 2019NZZJ0028 and 2016FZ0019), Science and technology support program of Yibin (2018ZSF002), Chengdu Science and Technology Project key research and development program (2018-YF05-00213-SN), Sichuan Provincial Key-Laboratory Open Fund Project (GR-2018-E-01), and Innovation Team Construction Program of Sichuan Education Department (15TD0017). All authors declare no conflict of interest. Ethics approval was not required for this research. Authors’ contributions are as follows: conceptualization: [Yage Xing]; Methodology: [Tianyi Yue]; Formal analysis and investigation: [Tianyi Yue]; Writing – original draft: [Tianyi Yue]; Writing – Reviewing & Editing: [Qinglian Xu]; Resources: [Shuang Yang]; Supervision: [Xiaomin Wang, Ping Yang].
Additional information
Funding
References
- Mizgier, P.; Kucharska, A. Z.; Sokol-Letowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of Phenolic Compounds and Antioxidant and Anti-inflammatory Properties of Red Cabbage and Purple Carrot Extracts. J. Funct. Foods. 2016, 21, 133–146. DOI: https://doi.org/10.1016/j.jff.2015.12.004.
- Zielinska, M.; Lewandowska, U.; Podsędek, A.; Cygankiewicz, A. I.; Jacenik, D.; Salaga, M.; Kordek, R.; Krajewska, W. M.; Fichna, J. Orally Available Extract from Brassica Oleracea Var. Capitata Rubra Attenuates Experimental Colitis in Mouse Models of Inflammatory Bowel Diseases. J. Funct. Foods. 2015, 17, 587–599. DOI: https://doi.org/10.1016/j.jff.2015.05.046.
- Dominguez-Perles, R.; Mena, P.; Garcia-Viguera, C.; Moreno, D. A. Brassica Foods as A Dietary Source of Vitamin C: A Review. Crit. Rev. Food Sci. 2014, 54(8), 1076–1091. DOI: https://doi.org/10.1080/10408398.2011.626873.
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red Cabbage Anthocyanins: Profile, Isolation, Identification, and Antioxidant Activity. Food Res. Int. 2013, 51(1), 303–309. DOI: https://doi.org/10.1016/j.foodres.2012.12.015.
- Bowen-Forbes, C. S.; Zhang, Y. J.; Nair, M. G. Anthocyanin Content, Antioxidant, Anti-inflammatory and Anticancer Properties of Blackberry and Raspberry Fruits. J. Food Compos. Anal. 2010, 23(6), 554–560. DOI: https://doi.org/10.1016/j.jfca.2009.08.012.
- Bao, T.; Hao, X.; Shishir, M. R. I.; Karim, N.; Chen, W. Cold Plasma: An Emerging Pretreatment Technology for the Drying of Jujube Slices. Food Chem. 2021, 337, 127783. DOI: https://doi.org/10.1016/j.foodchem.2020.127783.
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in Curcuminoids and Chemical Components of Turmeric (Curcuma Longa L.) Under Freeze-drying and Low-temperature Drying Methods. Food Chem. 2021, 339, 128121. DOI: https://doi.org/10.1016/j.foodchem.2020.128121.
- Liu, C. Y.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Impact of Pulsed Electric Fields on Vacuum Drying Kinetics and Physicochemical Properties of Carrot. Food Res. Int. 2020, 137, 109658. DOI: https://doi.org/10.1016/j.foodres.2020.109658.
- Guclu, G.; Keser, D.; Kelebek, H.; Keskin, M.; Sekerli, Y. E.; Soysal, Y.; Selli, S. Impact of Production and Drying Methods on the Volatile and Phenolic Characteristics of Fresh and Powdered Sweet Red Peppers. Food Chem. 2021, 338, 128129. DOI: https://doi.org/10.1016/j.foodchem.2020.128129.
- Xu, X.; Zhang, L.; Feng, Y. B.; Zhou, C. S.; Yagoub, A. A.; Wahia, H.; Ma, H. L.; Sun, Y. H.; Sun, Y. Ultrasound Freeze-thawing Style Pretreatment to Improve the Efficiency of the Vacuum Freeze-drying of Okra (Abelmoschus Esculentus (L.) Moench) and the Quality Characteristics of the Dried Product. Ultrason. Sonochem. 2021, 70, 105300. DOI: https://doi.org/10.1016/j.ultsonch.2020.105300.
- Bozkir, H.;. Effects of Hot Air, Vacuum Infrared, and Vacuum Microwave Dryers on the Drying Kinetics and Quality Characteristics of Orange Slices. J. Food Process Eng. 2020, 43(10), e13485. DOI: https://doi.org/10.1111/jfpe.13485.
- Figiel, A.; Michalska, A. Overall Quality of Fruits and Vegetables Products Affected by the Drying Processes with the Assistance of Vacuum-microwaves. Int. J. Mol. Sci. 2016, 18(1), 71. DOI: https://doi.org/10.3390/ijms18010071.
- Zhang, L. H.; Qiao, Y.; Wang, C.; Liao, L.; Shi, D. F.; An, K. J.; Hu, J. Z.; Shi, L.; Shi, L. Influence of High Hydrostatic Pressure Pretreatment on Properties of Vacuum-freeze Dried Strawberry Slices. Food Chem. 2020, 331, 127203. DOI: https://doi.org/10.1016/j.foodchem.2020.127203.
- Feng, Y. B.; Tan, C. P.; Zhou, C. S.; Yagoub, A. A.; Xu, B. G.; Sun, Y. H.; Yu, X. J.; Xu, X.; Yu, X. Effect of Freeze-thaw Cycles Pretreatment on the Vacuum Freeze-drying Process and Physicochemical Properties of the Dried Garlic Slices. Food Chem. 2020, 324, 126883. DOI: https://doi.org/10.1016/j.foodchem.2020.126883.
- Vidinamo, F.; Fawzia, S.; Karim, M. A. Effect of Drying Methods and Storage with Agro-ecological Conditions on Phytochemicals and Antioxidant Activity of Fruits: A Review. Crit. Rev. Food Sci. 2020, 1–9. doi:https://doi.org/10.1080/10408398.2020.1816891.
- Bose, S. K.; Howlader, P.; Wang, W. X.; Yin, H. Oligosaccharide Is A Promising Natural Preservative for Improving Postharvest Preservation of Fruit: A Review. Food Chem. 2021, 341, 128178. DOI: https://doi.org/10.1016/j.foodchem.2020.128178.
- Joardder, M. U. H.; Kumar, C.; Brown, R. J.; Karim, M. A. A Micro-level Investigation of the Solid Displacement Method for Porosity Determination of Dried Food. J. Food Eng. 2015, 166, 156–164. DOI: https://doi.org/10.1016/j.jfoodeng.2015.05.034.
- Calin-Sanchez, A.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A. A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods. 2020, 9(9), 1261.
- Ul Hasan, M.; Malik, A. U.; Ali, S.; Imtiaz, A.; Munir, A.; Amjad, W.; Anwar, R. Modern Drying Techniques in Fruits and Vegetables to Overcome Postharvest Losses: A Review. J. Food Process. Pres. 2019, 43(12), e14280.
- Li, X. Y.; Liu, Y. H.; Gao, Z. J.; Xie, Y. K.; Wang, H. Computer Vision Online Measurement of Shiitake Mushroom (Lentinus Edodes) Surface Wrinkling and Shrinkage during Hot Air Drying with Humidity Control. J. Food Eng. 2021, 292, 110253.
- Chen, A. Q.; Achkar, G. E. L.; Liu, B.; Bennacer, R. Experimental Study on Moisture Kinetics and Microstructure Evolution in Apples during High Power Microwave Drying Process. J. Food Eng. 2021, 292, 110362.
- Jiang, N.; Liu, C. Q.; Li, D. J.; Zhang, Z. Y.; Liu, C. J.; Wang, D.; Niu, L. Y.; Zhang, M. Evaluation of Freeze Drying Combined with Microwave Vacuum Drying for Functional Okra Snacks: Antioxidant Properties, Sensory Quality, and Energy Consumption. LWT-Food Sci. Technol. 2017, 82, 216–226. DOI: https://doi.org/10.1016/j.lwt.2017.04.015.
- Siriamornpun, S.; Kaewseejan, N. Quality, Bioactive Compounds and Antioxidant Capacity of Selected Climacteric Fruits with Relation to Their Maturity. Sci. Hortic. 2017, 221, 33–42. DOI: https://doi.org/10.1016/j.scienta.2017.04.020.
- Tomczyk, M.; Zagula, G.; Dzugan, M. A Simple Method of Enrichment of Honey Powder with Phytochemicals and Its Potential Application in Isotonic Drink Industry. LWT-Food Sci. Technol. 2020, 125, 109204.
- Maduwanthi, S. D. T.; Marapana, R. A. U. J. Total Phenolics, Flavonoids and Antioxidant Activity following Simulated Gastro-intestinal Digestion and Dialysis of Banana (Musa Acuminata, AAB) as Affected by Induced Ripening Agents. Food Chem. 2021, 339, 127909. DOI: https://doi.org/10.1016/j.foodchem.2020.127909.
- Jorjong, S.; Butkhup, L.; Samappito, S. Phytochemicals and Antioxidant Capacities of Mao-Luang (Antidesma Bunius L.) Cultivars from Northeastern Thailand. Food Chem. 2015, 181, 248–255.
- Li, J.; Li, Z. F.; Li, L. L.; Song, C. F.; Raghavan, G. S. V.; He, F. J. Microwave Drying of Balsam Pear with Online Aroma Detection and Control. J. Food Eng. 2021, 288, 110139.
- Xu, X.; Zhang, L.; Feng, Y. B.; Yagoub., A. A.; Sun, Y. H.; Ma, H. L.; Zhou, C. S. Vacuum Pulsation Drying of Okra (Abelmoschus Esculentus (L.) Moench): Better Retention of the Quality Characteristics by Flat Sweep Frequency and Pulsed Ultrasound Pretreatment. Food Chem. 2020, 326, 127026. DOI: https://doi.org/10.1016/j.foodchem.2020.127026.
- Zhang, L. H.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D. F.; An, K. J.; Hu, J. Z. Effects of Ultrahigh Pressure and Ultrasound Pretreatments on Properties of Strawberry Chips Prepared by Vacuum-freeze Drying. Food Chem. 2020, 303, 125386. DOI: https://doi.org/10.1016/j.foodchem.2019.125386.
- Truong, T.; Truong, V.; Fukai, S.; Bhandari, B. Changes in Physicochemical Properties of Rice in Response to High-temperature Fluidized Bed Drying and Tempering. Drying Technol. 2019, 37(3), 331–340. DOI: https://doi.org/10.1080/07373937.2018.1452031.
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of Different Drying Methods on Chinese Ginger (Zingiber Officinale Roscoe): Changes in Volatiles, Chemical Profile, Antioxidant Properties, and Microstructure. Food Chem. 2016, 197, 1292–1300. DOI: https://doi.org/10.1016/j.foodchem.2015.11.033.
- Huang, T. C.; Chung, C. C.; Wang, H. Y.; Law, C. L.; Chen, H. H. Formation of 6-Shogaol of Ginger Oil under Different Drying Conditions. Dry. Technol. 2011, 29(16), 1884–1889.
- Wang, X. Y.; Gao, Y. N.; Zhao, Y. T.; Li, X. H.; Fan, J. M.; Wang, L. Y. Effect of Different Drying Methods on the Quality and Microstructure of Fresh Jujube Crisp Slices. J. Food Process. Pres. 2021, 45(2), e15162. DOI: https://doi.org/10.1111/jfpp.15162.
- Lim, Y. Y.; Murtijaya, J. Antioxidant Properties of Phyllanthus Amarus Extracts as Affected by Different Drying Methods. LWT - Food Sci. Technol. 2007, 40(9), 1664–1669.
- Kubra, I. R.; Rao, L. J. M. Microwave Drying of Ginger (Zingiber officinaleRoscoe) and Its Effects on Polyphenolic Content and Antioxidant Activity. Int. J. Food Sci. Technol. 2012, 47, 2311–2317. DOI: https://doi.org/10.1111/j.1365-2621.2012.03104.x.
- Yao, L. Y.; Fan, L. P.; Duan, Z. H. Effect of Different Pretreatments Followed by Hot-air and Far-infrared Drying on the Bioactive Compounds, Physicochemical Property and Microstructure of Mango Slices. Food Chem. 2020, 305, 125477. DOI: https://doi.org/10.1016/j.foodchem.2019.125477.
- Ozcan, M. M.; Al Juhaimi, F.; Ahmed, I. A. M.; Uslu, N.; Babiker, E. E.; Ghafoor, K. Effect of Microwave and Oven Drying Processes on Antioxidant Activity, Total Phenol and Phenolic Compounds of Kiwi and Pepino Fruits. J. Food Sci. Technol. 2020, 57(1), 233–242. DOI: https://doi.org/10.1007/s13197-019-04052-6.
- Akar, G.; Mazi, I. B. Color Change, Ascorbic Acid Degradation Kinetics, and Rehydration Behavior of Kiwifruit as Affected by Different Drying Methods. J. Food Process Eng. 2019, 42(3), e13011. DOI: https://doi.org/10.1111/jfpe.13011.
- Rodriguez-Jimenez, J. R.; Amaya-Guerra, C. A.; Baez-Gonzalez, J. G.; Aguilera-Gonzalez, C.; Urias-Orona, V.; Nino-Medina, G. Physicochemical, Functional, and Nutraceutical Properties of Eggplant Flours Obtained by Different Drying Methods. Molecules. 2018, 23(12), 3210. DOI: https://doi.org/10.3390/molecules23123210.
- Toor, R. K.; Savage, G. P. Effect of Semi-drying on the Antioxidant Components of Tomatoes. Food Chem. 2006, 94(1), 90–97. DOI: https://doi.org/10.1016/j.foodchem.2004.10.054.
- Ma, X. T.; Luo, G. Y.; Li, Z. F.; Raghavan, G. S. V.; Chen, H. Y.; Song, C. F. Microwave Power Control Scheme for Potatoes Based on Dielectric Loss Factor Feedback. J. Food Eng. 2021, 288, 110134. DOI: https://doi.org/10.1016/j.jfoodeng.2020.110134.
- Lagnika, C.; Jiang, N.; Song, J. F.; Li, D. J.; Liu, C. Q.; Huang, J. P.; Wei, Q. Y.; Zhang, M. Effects of Pretreatments on Properties of Microwave-vacuum Drying of Sweet Potato Slices. Drying Technol. 2019, 37(15), 1901–1914.
- Marsol-Vall, A.; Kelanne, N.; Nuutinen, A.; Yang, B. R.; Laaksonen, O. Influence of Enzymatic Treatment on the Chemical Composition of Lingonberry (Vaccinium Vitis-idaea) Juice. Food Chem. 2021, 339, 128052. DOI: https://doi.org/10.1016/j.foodchem.2020.128052.
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R. D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. 2016, 56, 110–129. DOI: https://doi.org/10.1080/10408398.2015.1045969.
- Song, C. F.; Ma, X. T.; Li, Z. F.; Wu, T.; Raghavan, G. S. V.; Chen, H. Y. Mass Transfer during Osmotic Dehydration and Its Effect on Anthocyanin Retention of Microwave Vacuum-dried Blackberries. J. Sci. Food Agr. 2020, 100(1), 102–109. DOI: https://doi.org/10.1002/jsfa.9999.
- Brito, T. B. N.; Lima, R. S. L.; Santos, M. C. B.; Moreira, R. F. A.; Cameron, L. C.; Fai, A. E. C.; Ferreira, M. S. L. Antimicrobial, Antioxidant, Volatile and Phenolic Profiles of Cabbage-stalk and Pineapple-crown Flour Revealed by GC-MS and UPLC-MSE. Food Chem. 2021, 339, 127882.
- Li, T. L.; Jiang, T.; Liu, N.; Wu, C. Y.; Xu, H. D.; Lei, H. J. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859. DOI: https://doi.org/10.1016/j.foodchem.2020.127859.
- Hamid,; Thakur, N. S.; Thakur, A.; Kumar, P. Effect of Different Drying Modes on Phenolics and Antioxidant Potential of Different Parts of Wild Pomegranate Fruits. Sci. Hortic. 2020, 274, 109656. DOI: https://doi.org/10.1016/j.scienta.2020.109656.
- Chan, E. W. C.; Lim, Y. Y.; Wong, S. K.; Lim, K. K.; Tan, S. P.; Lianto, F. S.; Yong, M. Y. Effects of Different Drying Methods on the Antioxidant Properties of Leaves and Tea of Ginger Species. Food Chem. 2009, 113(1), 166–172. DOI: https://doi.org/10.1016/j.foodchem.2008.07.090.
- Chen, Q. Q.; Li, Z. L.; Bi, J. F.; Zhou, L. Y.; Yi, J. Y.; Wu, X. Y. Effect of Hybrid Drying Methods on Physicochemical, Nutritional and Antioxidant Properties of Dried Black Mulberry. LWT-Food Sci. Technol. 2017, 80, 178–184. DOI: https://doi.org/10.1016/j.lwt.2017.02.017.
- Wojdylo, A.; Figiel, F.; Oszmianski, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agr. Food Chem. 2009, 57(4), 1337–1343. DOI: https://doi.org/10.1021/jf802507j.
- Artnaseaw, A.; Theerakulpisut, S.; Benjapiyaporn, C. Drying Characteristics of Shiitake Mushroom and Jinda Chili during Vacuum Heat Pump Drying. Food Bioprod. Process. 2010, 88(2–3), 105–114. DOI: https://doi.org/10.1016/j.fbp.2009.09.006.
- Hu, Q. G.; Zhang, M.; Mujumdar, A. S.; Xiao, G. N.; Sun, J. C. Drying of Edamames by Hot Air and Vacuum Microwave Combination. J. Food Eng. 2006, 77(4), 977–982. DOI: https://doi.org/10.1016/j.jfoodeng.2005.08.025.
- Xiong, Y.; Zhang, P. Z.; Johnson, S.; Luo, J. Q.; Fang, Z. X. Comparison of the Phenolic Contents, Antioxidant Activity and Volatile Compounds of Different Sorghum Varieties during Tea Processing. J. Sci. Food Agr. 2020, 100(3), 978–985. DOI: https://doi.org/10.1002/jsfa.10090.
