ABSTRACT
Herbaceous peony (Paeonia lactiflora Pall.) is a traditional Chinese medicinal herb with roots; however, the flowers, specifically the stamens have not been exploited. In this study, the main active components of P. lactiflora stamens (PLS) were identified, and in vitro antioxidant, in vivo anti-aging and stress resistance properties were examined using the nematode Caenorhabditis elegans. Our results showed that PLS contains 350 active components including flavonoids, phenolic acids, terpenoids, alkaloids, and others. PLS extracts at concentrations of 0.2–1.2 mg·mL−1 had strong antioxidant activity in vitro and showed a linear increase of concentration. The value of absorbance, scavenging activity of ·OH, DPPH and ABTS was 0.84, 31.55%, 84.67%, and 99.49% at 1.2 mg·mL−1, respectively. Moreover, PLS extracted at the same concentrations had no toxic effects on C. elegans, and the treatment with 1.2 mg·mL−1 PLS extract significantly extended C. elegans lifespan that was not at the expense of reducing its reproduction and Escherichia coli OP50 growth. Furthermore, the same treatment extract enhanced their locomotion behavior and decreased their intestinal autofluorescence during the aging process. Additionally, the stress resistance of C. elegans under both heat and oxidative stress conditions were significantly induced by 1.2 mg·mL−1 PLS extract treatment. Collectively, these results demonstrated that PLS extract exerted both in vitro antioxidant and in vivo anti-aging together with stress resistance properties, which could provide the theoretical basis for its production and popularization in China.
INTRODUCTION
Herbaceous peony (Paeonia lactiflora Pall.) Paeoniaceae is a famous flower with strong growth adaptability and high ornamental value in China, which has been an excellent plant used in gardening and for the production of cut flowers, and was cultivated widely in northern China, including Shandong, Henan, Jiangsu, Gansu, Anhui, Qinghai, and so on. Moreover, P. lactiflora is also a traditional Chinese medicinal herb that has been used in China for more than 2000 years.[Citation1] P. lactiflora root contains a variety of biologically active substances, such as paeoniflorin, paeonol, albiflorin, gallic acid, benzoic acid, and catechin,[Citation2] and it can be used to decrease the blood fat level of the rheumatoid arthritis patients,[Citation3] suppresses cancer cachexia,[Citation4] has anti-allergic activity[Citation5] and anti-proliferative activities against cancer cells.[Citation6] On the other hand, P. lactiflora flower is nontoxic and can be eaten fresh, and Ogawa et al.[Citation7] had isolated lactifloraoside I together with 28 known compounds from P. lactiflora flowers, which had significant inhibitory effect on Cu2+-induced low-density lipoprotein oxidation. Moreover, P. lactiflora sarcocarp and seeds also had antioxidant activities.[Citation8] Although P. lactiflora has high medicinal value, and studies on its use have made certain achievements, it is still far from the product development of the same family plant P. suffruticosa. For example, P. suffruticosa stamen extract is a newly emerged product on the market in recent years, and it is extremely valuable due to its high health nutritional value, short harvest time, and high manufacturing process requirements.[Citation9] Similarly, there are also numerous single-petal varieties with lots of stamens in P. lactiflora, but they are discarded because of their lower ornamental value. For this reason, we want to know whether PLS has toxic effects and they would be developed into stamen extracts like P. suffruticosa, and what the health and nutritional values are, to provide the theoretical basis for better use of P. lactiflora resources.
Free radicals, which have high chemical activity, are generated in the human body through the aerobic respiration or from various exogenous sources, which causes damage at their higher concentration.[Citation10] There are many kinds of free radicals in the human body. The free radicals used to illustrate the mechanism of aging should include hydroxyl radical (·OH-), superoxide anion radical (O2−) and lipid hydrogen peroxide radical (H2O2).[Citation11] Excessive free radicals can damage the structure and function of normal cells, reduce cell vitality, accelerate the decline of tissues and organs, and lead to the aging of the body.[Citation12] Free radicals react with various biomolecules such as lipids, proteins, and DNA, resulting in an imbalance between oxidants and antioxidants. This imbalance leads to the so-called oxidative stress phenomenon.[Citation13] Due to the presence of several antioxidants, medicinal plants can scavenge these free radicals and protect human health against a variety of diseases.[Citation10] Compared with synthetic antioxidants, medicinal plants have high flavonoids and flavanols that have strong scavenging capacity against free radicals. In addition, the synthetic antioxidants must be used under strict regulations due to their potential hazards.[Citation14] So, many researches about the use of the natural antioxidants from various natural sources were carried out. The main aim of this research was to reduce the application of synthetic antioxidants, since they may have negative health effects and are the results of consumer demand.[Citation15,Citation16] At present, there are various methods to evaluate the antioxidant activity of natural antioxidants, among which DPPH, ·OH, ABTS, and reducing capacity are the most common methods to evaluate the antioxidant activity.[Citation17,Citation18] In addition, it is one-sided to use a single evaluation method, and more than two methods should be selected to comprehensively evaluate the antioxidant activity of an antioxidant.[Citation18]
Animal experiments are currently used to evaluate the toxicity and function of natural products, which is costly and has a long cycle, so a simple, fast, and reliable method is needed. Caenorhabditis elegans is regarded as a good model organism for food nutritional evaluation because it has a short life cycle, a similar aging process with humans, relatively conservative genetic information, signal pathways, low cost of cultivation and easy experimental operation.[Citation19] In recent years, the C. elegans model was used to evaluate the anti-aging effects of natural products and their active substances in many studies. For example, Fei et al.[Citation20] found that puer tea, black tea, and green tea all increased the lifespan of C. elegans, postponed Aβ-induced progressive paralysis in Alzheimer’s disease, and improved the tolerance to the oxidative stress induced by heavy metal Cr6+. Additionally, withanolide A, one of the major withanolidal active compounds isolated from Withania somnifera, was found to extend the lifespan in human EGFR-driven cancerous C. elegans by more than 20%.[Citation21] Moreover, the extract of Lonicera japonica,[Citation22] Glycyrrhizae radix,[Citation23] and Anacardium occidentale[Citation24] also had the similar results, and the lifespan of C. elegans had been extended with 21.87%–49.15%. In this study, we first used C. elegans model to evaluate the toxicity of PLS extract, and then we expected that stamen extract of herbaceous peony would exert both in vitro antioxidant and in vivo anti-aging with stress resistance on C. elegans. These results would initiate further development and application of PLS extraction in the future.
MATERIALS AND METHODS
Plant materials and treatment
P. lactiflora single-petal cultivar “Hangshao” was used as the plant material, which was cultivated in the peony germplasm resource garden of Yangzhou University, China (32°39′N, 119°,2′E) for 7 years and identified by Yang-Qian Cao, a senior agronomist from Heze Peony Research Institute in Shandong Province of China. From 20th April to 10th May of 2017, the herbaceous peony stamens were collected from the same plant coded HS33 at the initiating bloom stage, and the filaments were removed. Based on the preliminary experiment, the stamens were fixed-dried with 800 W microwave power for 1 min, and then dried at 80°C to constant weight. The treated stamens were used for further analysis ().
Identification of active components in stamens
The freeze-dried PLS was crushed using a mixer mill (MM 400, Retsch) with a zirconia bead for 1.5 min at 30 Hz. 100 mg powder was weighted and extracted overnight at 4°C with 1.0 mL 70% aqueous methanol. Following centrifugation at 10, 000 g for 10 min, the extracts were absorbed (CNWBOND Carbon-GCB SPE Cartridge, 250 mg, 3 mL; ANPEL, Shanghai, China) and filtrated (SCAA-104, 0.22 μm pore size; ANPEL, Shanghai, China) before liquid chromatography-mass spectrometry (LC-MS) analysis.
The sample extracts were analyzed using an LC-ESI-MS/MS system (HPLC, Shim-pack UFLC SHIMADZU CBM30A system; MS, Applied Biosystems 4500 Q TRAP).[Citation25] The analytical conditions were as follows, HPLC: column, Waters ACQUITY UPLC HSS T3 C18 (1.8 µm, 2.1 mm*100 mm); solvent system, water (0.04% acetic acid): acetonitrile (0.04% acetic acid); gradient program,100:0 V/V at 0 min, 5:95 V/V at 11.0 min, 5:95 V/V at 12.0 min, 95:5 V/V at 12.1 min, 95:5 V/V at 15.0 min; flow rate, 0.40 mL/min; temperature, 40°C; injection volume: 5 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS. LIT and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (Q TRAP), API 4500 Q TRAP LC/MS/MS System equipped with an ESI Turbo Ion-Spray interface, operating in a positive ion mode and controlled by Analyst 1.6.3 software (AB Sciex). The ESI source operation parameters were as follows: ion source, turbo spray; source temperature 550°C; ion spray voltage (IS) 5500 V; ion source gas I (GSI), gas II (GSII), curtain gas (CUR) were set at 55, 60, and 25.0 psi, respectively; the collision gas (CAD) was high. Instrument tuning and mass calibration were performed with 10 and 100 μmol·L−1 polypropylene glycol solutions in QQQ and LIT modes, respectively. QQQ scans were acquired as MRM experiments with a collision gas (nitrogen) set to 5 psi. DP and CE for individual MRM transitions were done with further DP and CE optimization. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period.
Preparation of stamen extract
Treated PLS (0.6 g) were placed in a 250 mL flask, 100 mL of boiling water at 90°C was added to brew for 20 minutes, and then was vacuum filtered.[Citation9] The obtained residue was repeated in the above steps, and the filtrates were combined to rotary evaporation under reduced pressure. The volume was adjusted to 50 mL, and sterile filtration was performed using a needle filter to obtain 12 mg·mL−1 PLS extract mother liquor, which was gradually diluted with distilled water to 1.2 mg·mL−1, 1.0 mg·mL−1, 0.8 mg·mL−1, 0.6 mg·mL−1, 0.4 mg·mL,−1 and 0.2 mg·mL−1. The diluted stamen extracts were stored at 4°C.
Reducing capacity
The method of Song et al.[Citation26] was adopted and slightly modified to evaluate the reducing capacity of PLS tea. PLS extract (1 mL) and 0.2 mL phosphate buffer (0.2 M, pH = 6.6) were placed in a tube, and 0.5 mL of 1% K3Fe(CN)6 solution was added and mixed well, the tube was placed in a water bath at 50°C for 20 min. For the control and blank, K3Fe(CN)6 solution and stamen extract were replaced by distilled water, respectively. After cooling, 2 mL of 10% trichloroacetic acid was added, and centrifuged at 3000 rpm for 10 min. Then, 2.5 mL of distilled water and 0.5 mL of 1% FeCl3 were added to 2.5 mL supernatants. Finally, the absorbance was read at 700 nm with distilled water used as zero adjustment. The reducing capacity was calculated according to the following equation: reducing capacity = Abs of sample – Abs of control – Abs of blank.
Hydroxyl free radical (·OH) scavenging activity
The ·OH assay was performed by using the method described by Jing et al.,[Citation27] but with slight modifications. PLS extract (0.5 mL) was placed in a tube, and 1 mL of 2 mM FeSO4 solution, 1 mL of 8 mM H2O2 and 0.5 mL of 6 mM salicylic acid solution were added, respectively, the tube was placed in a water bath at 37°C for 1 h. For the control and blank, H2O2 and stamen extract were replaced by distilled water, respectively. After cooling, the absorbance was read at 510 nm with distilled water used for zero adjustment. Then, ·OH scavenging activity was calculated according to the following equation: ·OH scavenging activity (%) = (Abs of blank – Abs of sample – Abs of control)/Abs of blank × 100. The antioxidant capacity was expressed as an IC50 value.
DPPH free radical scavenging activity
In the DPPH assay, we used the method described by Zeng et al.[Citation28] with slight modification. PLS extract (0.5 mL) was placed in a tube, and 2.5 mL of 0.1 mM DPPH solution was added, the tube was placed in a water bath at 28°C for 1 h. For the control and blank, DPPH solution and stamen extract were replaced by 50% ethanol and distilled water, respectively. After cooling, the absorbance was read at 517 nm with distilled water used as zero adjustment. The DPPH scavenging activity was calculated according to the following equation: DPPH scavenging activity (%) = (Abs of blank – Abs of sample – Abs of control)/Abs of blank × 100. The antioxidant capacity was expressed as an IC50 value.
ABTS free radical scavenging activity
ABTS free radical scavenging activity was determined following the method described by Hwang et al.[Citation29] with slight modification. PLS extract (0.5 mL) was placed in a tube, and 3 mL of ABTS working solution was added, the tube was left to react under dark condition at room temperature for 1 h. For the control and blank, ABTS working solution and stamen extract were replaced by distilled water, respectively. The absorbance was read at 734 nm with distilled water used as zero adjustment. The ABTS scavenging activity was calculated according to the following equation: ABTS scavenging activity (%) = (Abs of blank – Abs of sample – Abs of control)/Abs of blank × 100. The antioxidant capacity was expressed as an IC50 value.
C. elegans strain and culture conditions
Wild-type C. elegans N2 obtained from Central China Normal University was maintained on C. elegans growth medium (NGM, the components includes 3 g·L−1 NaCl, 17 g·L−1 agarp, 2.5 g·L−1 peptone, 1 mL 1 mol·L−1 CaCl2, 1 mL 1 mol·L−1 MgSO4, 25 mL 1 mol·L−1 PBS buffer solution, 1 mL 5 mg·mL−1 cholesterol, and distilled water) plates seeded with Escherichia coli OP50 at 20°C as described by Brenner.[Citation30] Age synchronous populations of L4-larvae C. elegans were obtained as described previously.[Citation31] The specific steps are following: the culture plates containing a large number of gestational C. elegans were washed with 1.5 mL M9 solutions (6 g·L−1 Na2HPO4, 3 g·L−1 KH2PO4 and 5 g·L−1 NaCl), and collected to 2 mL centrifuge tube and then centrifuged, and the supernatant was discarded. An additional 1 mL M9 solution was added, and the supernatant was discarded after centrifugation. Next, 1 mL Bleach solution (1 mL 5 mol·L−1 NaOH and 2 mL 10% NaClO was measured and metered to 10 mL with distilled water) was added and then centrifuged to discard the supernatant. Then, we added a 1 mL S-buffer solution (1.47 g·L−1 K2HPO4, 5.93 g L−1 KH2PO4 and 5.85 g·L−1 NaCl), centrifuge to discard the supernatant. Repeat the previous step twice. Finally, the mixture was applied to the NGM culture plate with E. coli OP50 and incubated at 20°C at constant temperature. After about 48 h, C. elegans grew into L4-larvae. Stock stamen extract (12 mg·mL−1) was added to the NGM plates to a final concentration of 1.2 mg·mL−1, 1.0 mg·mL−1, 0.8 mg·mL−1, 0.6 mg·mL−1, 0.4 mg·mL,−1 and 0.2 mg·mL−1. E. coli OP50 was spread on the NGM plates as the food for C. elegans. For the safety assessment, lifespan, growth, locomotion behavior, reproduction, and intestinal autofluorescence assays were used as endpoints.
Safety evaluation of stamen extract on C. elegans
Methods were performed as described previously.[Citation32] The PLS extraction treatment (1.2 mg·mL−1, 1.0 mg·mL,−1 and 0.8 mg·mL−1) was performed for 24-hr from the stage of L4-larvae for safety evaluation. C. elegans was judged to be dead if they did not respond to stimulus using a metal wire, and their body length was measured depending on the flat surface area of C. elegans using Olympus SZX2-RFA16 microscope. And head thrashes, defined as a change from one direction to another and back again, were counted for 1 min. Body bends, defined as a change in the direction of the part of C. elegans corresponding to the posterior bulb of the pharynx along the y-axis, assuming that C. elegans was traveling along the x-axis, were counted for 20 sec. Brood size, defined as the number of offspring at all stages beyond the egg, was counted. Intestinal autofluorescence caused by lysosomal deposits of lipofuscin can accumulate over time in aging C. elegans.[Citation33] For the intestinal autofluorescence assay, images were collected for fluorescence in the endogenous intestine using a 525-nm bandpass filter and without automatic gain control to preserve the relative fluorescence intensity in animals. Fluorescence was recorded and color images were taken to document the results with Olympus SZX2-RFA16 microscope. Lipofuscin levels were measured using Image J Software (NIH Image, Bethesda, MD, USA) by determining the mean pixel intensity in the intestines.
Assays of lifespan, growth, locomotion behavior, and intestinal autofluorescence
PLS extract treatment (1.2 mg·mL−1, 1.0 mg·mL,−1 and 0.8 mg·mL−1) was performed throughout the lifespan from the stage of L4-larvae. C. elegans was checked every day and scored as dead when they did not respond to stimulus using a metal wire. The mean lifespan of the last 10% C. elegans was defined as the maximal lifespan, and their body length, head thrashes, body bends, brood size, and intestinal autofluorescence were measured as described previously.[Citation32]
Evaluation of growth inhibition of E. coli OP50 by stamen extract
Methods were performed according to Zhang et al.[Citation34] PLS extract was added to 100 mL LB liquid medium with a final concentration of 1.2 mg·mL−1. For the control, the corresponding amount of sterile distilled water was added. Subsequently, E. coli OP50 was cultured overnight according to 1% inoculum, and then they were inoculated into an LB liquid medium. After mixing, the absorbance was measured at 595 nm, and this value was taken as the zero reference point. The LB liquid medium was shaken and cultured at 37°C in a shaker at 200 rpm. The absorbance was measured at 595 nm every 1 h and continuously measured for 5 h to draw the growth curve of E. coli OP50.
Assays of heat stress and oxidative stress resistance
PLS extract treatment was performed throughout the heat stress and oxidative stress from the stage of L4-larvae. For this, C. elegans pre-treated with 1.2 mg·mL−1 stamen extract for 3 days were transferred to growth at 35°C to induce heat stress or in a medium with 200 μM paraquat to induce oxidative stress.[Citation35] The mean lifespan and maximal lifespan were measured as described above.
Statistical analysis
All the data were by means of three replicates with standard deviation. Data were subjected to an analysis of variance procedure (ANOVA) using SAS/STAT statistical analysis package (version 6.12, SAS Institute, Cary, NC, USA). Means were separated using Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05.
RESULTS
Active components of stamens
LC-MS were used for the active component analysis of stamens, which produced 350 peaks (Additional file 1: Figure S1) and their details were shown in Additional file 2: Table S1. Based on the mass-to-charge-ratio values, retention times, and fragmentation patterns with standards,[Citation25] 350 active components were detected based on the metabolome database of Wuhan Maiteville Biotechnology Co., Led. (Wuhan, China), and the public mass spectrometry databases of MassBank, KNAPSAck, HMDB, MoTo DB, and METLIN. And these active components could be identified as eight main categories, including 159 flavonoids, 71 phenolic acids, 21 terpenoids, 20 alkaloids, 13 lignans and coumarins, 12 tannins, 2 quinones, and 52 others. In flavonoids, the main compounds are isorhamnetin-3,7-O-diglucoside, Naringenin-4ʹ-O-glucoside, dihydrokaempferol-7-O-glucoside, 6-hydroxykaempferol-3,6-O-diglucoside, spiraeoside, and so on. In phenolic acids, 6-O-Caffeoylarbutin, monogalloyl-diglucose and dibutyl phthalate are the main components. In addition, oxypaeoniflorin, paeoniflorin, and 8-debenzoylpaeoniflorin are the main components in terpenoids. And 10-formyltetrahydrofuran, N-p-coumaroyl-N’-feruloylputrescine and trigonelline are the main components belonging to alkaloids. Moreover, secoisolariciresinol 4-O-glucoside and matairesinol-4ʹ-O-glucoside are the main components in lignans and coumarins. And 6-O-galloyl-glucose, 3-O-galloyl-glucose and digallic acid are the main components belonging to tannins. The two quinones are hydroxyAloe-emodin-8-O-glucoside and aloeemodin-8-O-D-glucoside. Besides, 2,3-dihydroxy-3-methylbutanoic acid, D-xylonic acid and citric acid are the main components belonging to other classes.
In vitro antioxidant activities of stamen extract
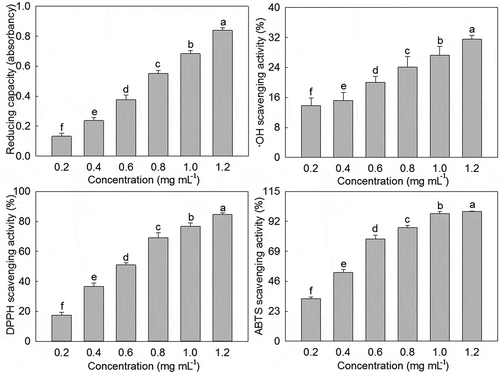
Reducing capacity was first used to evaluate the in vitro antioxidant activities of PLS extract. As shown in , PLS extract displayed a reducing ability in the range of 0.2–1.2 mg·mL−1, and an increasing linear trend with increasing concentration was presented, and the absorbance values increased from 0.62 to 0.84. Moreover, ·OH scavenging activity, DPPH scavenging activity, and ABTS scavenging activity were also used to evaluate the in vitro antioxidant activities of PLS extract. Similarly, PLS extract had a certain ·OH scavenging activity, DPPH scavenging activity, and ABTS scavenging activity within the range of 0.2–1.2 mg·mL−1, and a certain linear relationship also existed between their scavenging activity and concentration. And when the concentration was increased from 0.2 mg·mL−1 to 1.2 mg·mL−1, the scavenging activity of ·OH, DPPH, and ABTS increased from 13.88%, 17.60%, and 32.73% to 31.55%, 84.67%, and 99.49%, respectively. On this basis, the IC50 values were further calculated according to the curve fitting regression equation of PLS extract scavenging activity of ·OH, DPPH, and ABTS, and their values were 2.22 mg mL−1, 0.61 mg mL,−1 and 0.34 mg mL−1, respectively.
Stamen extract on safety of C. elegans
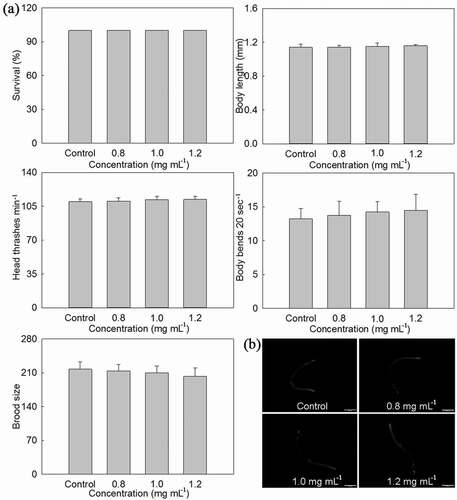
In order to determine whether PLS extract had toxic effects on C. elegans, its survival, body length, head thrashes, body bends, brood size, and intestinal autofluorescence were measured under NGM medium with different concentrations of stamen extract (, Additional file 2: Table S2). The survival in Control and treated C. elegans was all 100%. When compared with control, body length, head thrashes and intestinal autofluorescence of treated C. elegans were basically unchanged, while body bends increased and brood size decreased with the stamen extract concentration increase from 0.8 mg·mL−1 to 1.2 mg·mL−1, but significant difference was not obtained between them.
Figure 3. Effects of P. lactiflora stamen extract on safety of C. elegans. A: Effects of stamen extract on lethality, growth, locomotion behavior and reproduction of nematodes; B: Effects of stamen extract on intestinal autofluorescence of nematodes. The values represented the mean ± SD, and different letters indicate significant differences according to Duncan’s multiple range test (P < .05)
Stamen extract on lifespan of C. elegans
In order to determine whether PLS extract could extend the lifespan of C. elegans, it was treated with 0.8 mg·mL−1, 1.0 mg·mL,−1 and 1.2 mg·mL−1 stamen extract. It could be seen from ) that PLS extract of three concentrations could shift the lifespan curve of C. elegans to the right to some extent, but the right shift of C. elegans lifespan curve treated with 0.8 mg mL−1 stamen extract had no obvious effect, and the other two were significantly shifted to the right. According to Log-rank (mantel-cox) test analysis, when the effects of stamen extract of three concentrations on C. elegans lifespan were compared with Control, their P-values were 0.1649, 0.0092, and <0.0001, respectively. Therefore, the light-shifting effect of the C. elegans lifespan curve treated with 1.2 mg·mL−1 PLS extract was the most obvious.
Figure 4. Effects of P. lactiflora stamen extract on lifespan of C. elegans. A: Lifespan curves of nematodes treated with stamen extract; B: Comparison of mean lifespan and maximal lifespan in nematodes treated with stamen extract. The values represented the mean ± SD, and different letters indicate significant differences according to Duncan’s multiple range test (P < .05)
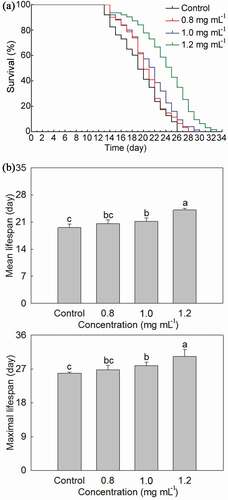
As far as the mean lifespan of C. elegans was concerned, it gradually increased with the increase of treated PLS extract concentration ()). When the treated stamen extract concentration was 0.8 mg·mL−1, the mean lifespan of C. elegans was slightly higher than that of Control, but the difference was not significant. While the mean lifespan of the C. elegans treated with 1.0 mg·mL−1 and 1.2 mg·mL−1 stamen extract was 21.21 days and 24.10 days, respectively, which was significantly higher than that of Control with about 1.65 days and 4.54 days, respectively. As far as the maximal lifespan of C. elegans was concerned, it also gradually increased with the increase of treated PLS extract concentration ()). Although the maximal lifespan of C. elegans treated with 0.8 mg·mL−1 stamen extract was higher than that of Control, the difference between the two was not significant. Whereas the maximal lifespan of C. elegans treated with 1.0 mg·mL−1 and 1.2 mg·mL−1 stamen extract was also significantly higher than that of Control, and the highest maximal lifespan of C. elegans was reached by 1.2 mg·mL−1 stamen extract treatment with 30.38 days, which was higher than the control with approximate 4.50 days. The above results indicated that 1.2 mg·mL−1 PLS extract had the best effects on extending the lifespan of C. elegans; therefore, this concentration would only be used in further study.
Stamen extract on locomotion behavior of C. elegans
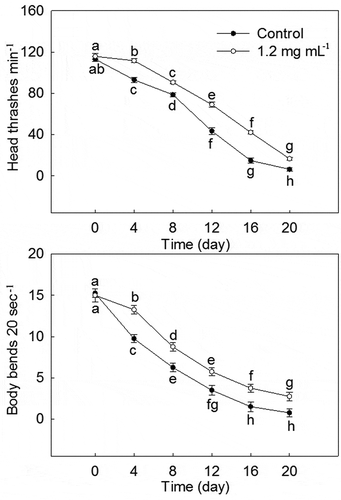
As shown in , 1.2 mg·mL−1 PLS extract had beneficial effects on the locomotion behavior of C. elegans. With the increase in C. elegans lifespan, head thrashes, and body bends in Control and 1.2 mg·mL−1 stamen extract treatment all gradually decreased. Starting from the 4th day, head thrashes and body bends of 1.2 mg·mL−1 stamen extract treatment were significantly higher than those of Control at the same time. On the 20th day, head thrashes and body bends of C. elegans treated with 1.2 mg·mL−1 stamen extract was 164.00% and 266.67% of Control.
Stamen extract on intestinal autofluorescence of C. elegans
With the increase of C. elegans lifespan, its intestinal autofluorescence in Control, and 1.2 mg·mL−1 PLS extract treatment gradually increased ()). Starting from the 4th day, the intestinal autofluorescence of C. elegans treated with 1.2 mg·mL−1 stamen extract was always significantly lower than that of Control at the same time. After measuring the intestinal autofluorescence intensity of C. elegans, the results were found to be consistent with the above observed results ()). The intestinal autofluorescence intensity of C. elegans treated with 1.2 mg·mL−1 stamen extract was always significantly lower than that of Control at the same time, and the 20th day, its intestinal autofluorescence intensity treated with 1.2 mg·mL−1 stamen extract decreased by 16.81% when compared with Control.
Figure 6. Effects of P. lactiflora stamen extract on intestinal autofluorescence and reproduction of C. elegans. A: Effects of stamen extract on intestinal autofluorescence of nematodes; B: Fluorescence intensity of intestinal autofluorescence; C Effects of stamen extract on reproduction of nematodes. The values represented the mean ± SD, and different letters indicate significant differences according to Duncan’s multiple range test (P < .05)
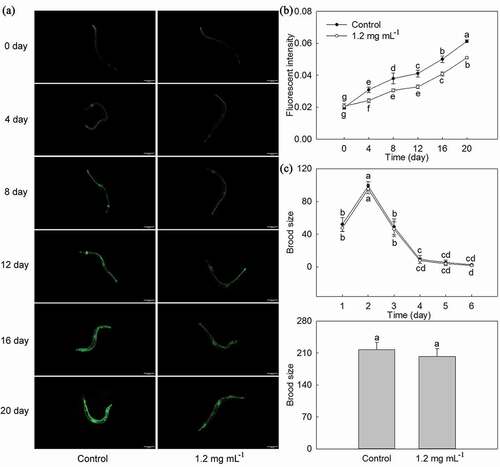
Stamen extract on reproduction of C. elegans and growth of E. coli OP50
The effects of PLS extract on reproduction of C. elegans and growth of E. coli OP50 might affect the lifespan of C. elegans; therefore, we observed the reproduction of C. elegans and growth of E. coli OP50. As shown in ), the spawning cycle of C. elegans in Control and 1.2 mg·mL−1 PLS extract treatment was all 6 days, its sexual maturity and spawning began on the first day, spawning was terminated on the 6th day, and the brood size all increased firstly and then decreased, and its peak period was concentrated in the first 3 days, the brood size produced on the second day was the highest, and the brood size produced on the 4th to the 6th day was significantly reduced, and there was no significant difference between the two. In terms of the total brood size of C. elegans, it was 217.33 and 202.50 in Control, and 1.2 mg·mL−1 PLS extract treatment, respectively, and there was no significant difference between them. Moreover, the growth trend of E. coli OP50 in Control and 1.2 mg·mL−1 PLS extract treatment was basically the same, all of which had been increasing with passage of time but there was no difference between the two (Additional file 4: Table S3).
Stamen extract on stress resistance of C. elegans
In order to clarify the effects of PLS extract on heat stress resistance of C. elegans, C. elegans in Control, and 1.2 mg·mL−1 stamen extract treatment was transferred to culture under 35°C condition. As shown in ), 1.2 mg·mL−1 stamen extract treatment could significantly shift the lifespan curve of C. elegans to the right under heat stress, and its P-value was <0.0001, which was significantly different from Control. Moreover, the mean lifespan of C. elegans treated with 1.2 mg·mL−1 stamen extract was 15.82 days, which was significantly higher than that of Control with 23.50%. Similarly, the maximal lifespan of C. elegans treated with 1.2 mg·mL−1 stamen extract was also significantly higher than that of Control with 22.52%.
Figure 7. Effects of P. lactiflora stamen extract on stress resistance of C. elegans. A: Lifespan curves of C. elegans treated with stamen extract and their comparison of mean lifespan and maximal lifespan under heat stress; B: Lifespan curves of C. elegans treated with stamen extract and their comparison of mean lifespan and maximal lifespan under oxidative stress. The values represented the mean ± SD, and different letters indicate significant differences according to Duncan’s multiple range test (P < .05)
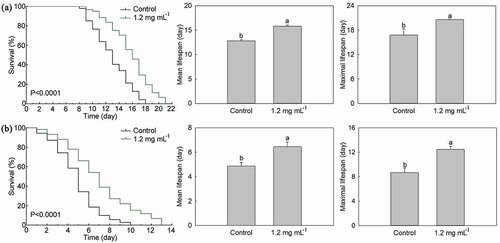
Similarly, C. elegans in Control and 1.2 mg·mL−1 PLS extract treatment were cultured in a medium containing 200 μM paraquat to that clarify the effect of PLS extract on oxidative stress resistance of C. elegans. As shown in ), 1.2 mg·mL−1 PLS extract treatment could significantly shift the lifespan curve of C. elegans to the right under oxidative stress, and its P-value was <0.0001, which was significantly different from Control. Under oxidative stress, the mean lifespan of C. elegans in 1.2 mg·mL−1 PLS extract treatment was 6.44 days, which was significantly higher than that of Control with 32.11%. And the maximal lifespan of C. elegans in 1.2 mg·mL−1 PLS extract treatment was 12.44 days, which was significantly higher than that of Control with 43.59%.
DISCUSSION
As a traditional Chinese medicine, P. lactiflora contains numerous active components. Jin et al.[Citation36] used a reagent color-development method to identify active components of P. lactiflora petals, and polysaccharides, glycosides, organic acids, flavonoids, coumarins, polyphenols, steroids, three terpenes, and anthraquinone were found. Similarly, this study also identified numerous active components: 159 flavonoids, 71 phenolic acids, 21 terpenoids, 20 alkaloids, 13 lignans and coumarins, 12 tannins, 2 quinones, and 52 others were found in PLS. Flavonoids are ubiquitously distributed in many dietary plants, including fruits, vegetables, flowers, and teas.[Citation37] They have been reported to provide health benefits for humans such as protecting the cardiovascular system, having antidiabetic, antiobesity, and anticancer effect, having antiinflammatory and antioxidant properties in vitro and in vivo.[Citation38] Phenolic acids are the most prominent group of bioactive compounds present in various plant sources.[Citation39] Phenolic acids contribute to overall health improvement, primarily because of antioxidant and antiinflammatroy actions, help in the prevention of cardiovascular diseases and various cancers, protect against oxidative damage diseases; and exhibit antimicrobial, antimutagenic, hypoglycemic and antiplatelet aggregating activities.[Citation40] In this study, 159 flavonoids and 71 phenolic acids accounted for 65.7% of the total active components of PLS, which indicated that PLS could be used for development and utilization.
Different methods for the evaluation of antioxidant activity have different detection principles, which may lead to different results when evaluating the same antioxidant. Therefore, two or more antioxidant evaluation methods should be selected to comprehensively compare the antioxidant activity of substance.[Citation41] There are many methods for evaluating antioxidant activity, but they are mainly divided into two categories according to their principles: one is reducing ability, and the other is free radical scavenging ability.[Citation42] Among them, reducing ability is an important manifestation of antioxidant activity, and at present, the most commonly used measurement method is the potassium ferricyanide reduction method.[Citation43] Song et al.[Citation26] studied the antioxidant capacity of Bletilla striata phenolic extracts and found that the reducing ability was concentration-dependent, but when the concentration reached 0.2 mg·mL−1, its absorbance no longer increased. In this study, we also found that the absorbance of PLS extract gradually increased within the range of its concentration. When the stamen extract concentration was 1.2 mg·mL−1, the absorption reached the highest value, and its reducing capacity was stronger than that of Bletilla striata phenolic extracts.[Citation26] Moreover, the free radical scavenging ability is a direct indicator for evaluating the antioxidant activity of a substance in vitro, the ·OH scavenging activity, DPPH scavenging activity, and ABTS scavenging activity are all common indicators.[Citation44] These three measurement principles are different, and the IC50 value can be used to represent the radical scavenging ability, the lower the IC50 value, the stronger the scavenging ability.[Citation45–47] Cvetanović et al.[Citation48] used ·OH scavenging activity to evaluate the antioxidant activity of chamomile water extract and found that its IC50 value was 38.1 mg·mL−1. This study found that ·OH scavenging activity of PLS extract increased with the increasing concentration, and its IC50 value was 2.22 mg·mL−1, which was lower than that of chamomile water extract. Soleimanifar et al.[Citation49] used DPPH scavenging activity to evaluate the antioxidant activity of black cumin seed oil, and found it increased with the increasing concentration in the range of 50–200 mg·mL−1, and the IC50 value was 104.76 mg·mL−1. And Somawathi et al.[Citation50] found that the different skin colored brinjal had certain DPPH scavenging activity, and the IC50 value was between 3.51 and 4.87 mg·mL−1. In this study, the DPPH scavenging activity of PLS extract increased with the increasing concentration, and the IC50 value was 0.61 mg mL−1, which was lower than that of black cumin seed oil and brinjal extract. Moreover, Li et al.[Citation51] studied the antioxidant activity of blueberry anthocyanin extracts and found that they had ABTS scavenging activity, their IC50 values were between 0.58 and 2.05 mg·mL−1. In this study, the ABTS scavenging activity and PLS extract concentration showed a certain linear relationship, and the IC50 value was 0.34 mg·mL−1, which was lower than that of blueberry anthocyanin extracts. In this study, based on the reduction ability, ·OH scavenging activity, DPPH scavenging activity, and ABTS scavenging activity, PLS extract had strong antioxidant activity with a concentration dependence, which was higher than that of common plants.
Because C. elegans have related genes that are homologous to mammals and have high similarities to human genes, they have been widely used in toxicity screening studies.[Citation50,Citation51] Nontoxic effects are a prerequisite for edible, therefore, C. elegans were used as a model to study the toxicity of different concentrations of PLS extract in this study, and we found that PLS extract had no significant effects on the lethality, growth, locomotion behavior, reproduction, and intestinal autofluorescence of C. elegans, indicating no toxicity. Moreover, during the aging process, the organ function of the body gradually declines, eventually leading to the end of life. Therefore, lifespan can be used as a quantitative indicator of aging in the anti-aging study.[Citation52] In this study, 0.8–1.2 mg·mL−1 PLS extract could extend the lifespan of C. elegans to some extent, and the treated effect of 1.2 mg·mL−1 stamen extract was the most significant. Some scholars believed that the reproductive ability was closely related to aging, and there was a trade-off relationship between reproduction and aging.[Citation53] In this study, 1.2 mg·mL−1 PLS extract could not affect the reproduction of C. elegans, which was consistent with previous results,[Citation54] indicating that stamen extract did not extend the lifespan of C. elegans by sacrificing reproduction. And 1.2 mg·mL−1 PLS extract could significantly improve the locomotion behavior of C. elegans, suggesting that stamen extract did not harm the health of C. elegans while extending its lifespan. Moreover, 1.2 mg·mL−1 PLS extract did not inhibit the growth of E. coli OP50, indicating that the anti-aging effect of stamen extract was not caused by dietary restriction. In addition, the theory of aging free radicals believes that as the age increases, the body’s ability of removing ROS decreases, the accumulation of ROS is considered to be one of the internal factors leading to aging.[Citation55] Therefore, the oxidative stress experiments are often used in the study of aging. In this study, 200 μM paraquat was used to treat C. elegans, and the results showed that the lifespan of C. elegans treated with 1.2 mg·mL−1 PLS extract was significantly longer than that of Control, indicating that stamen extracts obvious induced oxidative stress resistance. Besides oxidative stress, heat stress is also a common experiment in the study of aging. Our study showed that the lifespan of C. elegans treated with 1.2 mg·mL−1 PLS extract was significantly longer than that of Control under heat stress, indicating that stamen extracts obvious induced heat stress resistance. There was a positive correlation between the extension of C. elegans lifespan and the improvement of stress resistance, which might be one of the anti-aging mechanisms of PLS extract.
CONCLUSION
In summary, PLS contained a number of active components, and stamen extract at concentrations of 0.2–1.2 mg·mL−1 had strong antioxidant activity in vitro. Moreover, stamen extract at concentrations of 0.8–1.2 mg·mL−1 had no toxic effects on C. elegans, and 1.2 mg·mL−1 stamen extract had significant anti-aging activity in vivo by improving stress resistance. These results could provide a theoretical basis for the production and popularization of PLS extract.
ABBREVIATIONS
P. lactiflora: Paeonia lactiflora; C. elegans: Caenorhabditis elegans; PLS: P. lactiflora stamens; E. coli: Escherichia coli; ·OH: hydroxyl radical, DPPH: 2,2-diphenyl-picryl-hydrazyl radical; ABTS: 2, 2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonc acid); IC50: 50% inhibitory concentration; NGM: C. elegans growth medium.
Supplemental Material
Download Zip (372.3 KB)Acknowledgments
This work was supported by the National Natural Science Foundation of China (32071813, 31772341), the Qing Lan Project of Jiangsu Province and the High-Level Talent Support Program of Yangzhou University. The author declares that there is no competing interest toward the publication of this manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, 2015. (In Chinese).
- Zhu, S.; Yu, X.; Wu, Y.; Shiraishi, F.; Kawahara, N.; Komatsu, K. Genetic and Chemical Characterization of White and Red Peony Root Derived from Paeonia lactiflora. J. Nat. Med. 2015, 69, 35–45. DOI: https://doi.org/10.1007/s11418-014-0857-5.
- Hou, X. J.; Zhu, Y. L.; Hou, X. J. Effects of Root of Herbaceous Peony on Lipid Metabolism of Rheumatoid Arthritis Patients. Heart 2012, 98(Suppl 2), E289. DOI: https://doi.org/10.1136/heartjnl-2012-302920ac.2.
- Bae, T.; Jang, J.; Lee, H.; Song, J.; Chae, S.; Park, M.; Son, C. G.; Yoon, S.; Yoon, Y. Paeonia lactiflora Root Extract Suppresses Cancer Cachexia by Down-regulating Muscular NF-κB Signalling and Muscle-specific E3 Ubiquitin Ligases in Cancer-bearing Mice. J. Ethnopharmacol. 2020, 246, 112222. DOI: https://doi.org/10.1016/j.jep.2019.112222.
- Shi, Y. H.; Zhu, S.; Tamura, T.; Kadowaki, M.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Chemical Constituents with Anti-allergic Activity from the Root of Edulis Superba, a Horticultural Cultivar of Paeonia lactiflora. J. Nat. Med. 2016, 70, 234–240. DOI: https://doi.org/10.1007/s11418-016-0966-4.
- Li, P.; Zhang, Z.; Li, T.; Zhang, Y. B.; Sze, S. C. W.; Wang, G. C.; Li, Y. L.; Ye, W. C. Monoterpene Derivatives from the Roots of Paeonia lactiflora and Their Anti-proliferative Activity. Fitoterapia 2014, 98, 124–129. DOI: https://doi.org/10.1016/j.fitote.2014.07.017.
- Ogawa, K.; Nakamura, S.; Sugimoto, S.; Tsukioka, J.; Hinomaru, F.; Nakashima, S.; Matsumoto, T.; Ohta, T.; Fujimoto, K.; Yoshikawa, M.; et al. Constituents of Flowers of Paeoniaceae Plants, Paeonia suffruticosa and Paeonia lactiflora. Phytochem. Lett. 2015, 12, 98–104. DOI: https://doi.org/10.1016/j.phytol.2015.03.002.
- Jin, Y.; Li, C.; Jin, Y.; Piao, J.; Chen, J.; Tao, J. In Vitro Free Radical Scavenging Activities and Total Phenolic Content of the Paeonia lactiflora Pall. Sarcocarp and Seeds. J. Yangzhou Univ. (Agr. Life Sci. Editi.) 2015, 36, 100–103. (In Chinese).
- Sun, K.; Zhang, Y.; Jiang, H.; Li, B. Study on the Technology of Making Tree Peony Stamen Tea. Shandong Forest Sci. Tech. 2014, 213, 48. (In Chinese).
- Daniel, G.; Kumari, S. K. Free Radical Scavenging Activity of Aqueous (Hot) Extract of Eugenia uniflora (L.) Leaves. J. Plant Biochem. Physiol. 2019, 7, 232. DOI: https://doi.org/10.4172/2329-9029.1000232.
- Li, Y.; Gu, S. M.; Zhang, S. Q.; Wang, J. F.; Bi, Y. T.; Guo, T. B.; Zhou, Y. Research Progress on the Role of Free Radicals in the Aging Process of Leukemia Stem Cells. J. Med. Health 2021, 37, 1498–1501. (In Chinese).
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect.Disord.-Drug Target. 2020, 20, 16–26. DOI: https://doi.org/10.2174/1871526518666180628124323.
- Castro-Conzález, L. M.; Galano, A.; Alvrez-Idaboy, J. R. Free Radical Scavenging Activity of Newly Designed Sesamol Dirivatives. New J. Chem. 2021, 45, 11960. DOI: https://doi.org/10.1039/d1nj02225c.
- Zeng, M.; Xiao, F.; Zhao, Y.; Liu, Z.; Li, B.; Dong, S. Study on the Free Radical Scavenging Activity of Sea Cucumber (Paracaudina Chinensvar.) Gelatin Hydrolysate. J. Oce. Univ. China 2007, 6(3), 255–258. DOI: https://doi.org/10.1007/s11802-007-0255-7.
- Taghvaei, M.; Jafari, S. M. Application and Stability of Natural Antioxidants in Edible Oils in Order to Substitute Synthetic Additives. J. Food Sci. Tech. 2015, 52, 1272–1282. DOI: https://doi.org/10.1007/s13197-013-1080-1.
- Mitterer-Daltoé, M.; Bordim, J.; Lise, C.; Breda, L.; Casagrande, M.; Lima, V. Consumer Awareness of Food Antioxidants, Synthetic vs. Natural. Food Sci. Technol. (Campinas) 2021, 41(suppl 1), 201–208. DOI: https://doi.org/10.1590/fst.15120.
- Badarinath, A. V.; Rao, K. M.; Chetty, C. M. S.; Ramkanth, V.; Rajan, T. V. S.; Gnanaprakash, K. A Review on In-vitro Antioxidant Methods: Comparisions, Correlations and Considerations. Int. J. Pharm. Tech. Res. 2010, 2(2), 1276–1285.
- Alam, M. N.; Bristi, N. J.; Rafiquzzaman, M.. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. DOI: https://doi.org/10.1016/j.jsps.2012.05.002.
- Yang, F.; Xia, C. C.; Zhong, X. L.; Li, Q.; Li, X.; Zhang, Z. Y.; Shi, W. B.; Xu, N.; Wu, Q.; Hu, Y.; et al. Food Nutritional Evaluation: Caenorhabditis elegans as a Model Organism. Food Sci. 2019, 40, 268–276. (In Chinese).
- Fei, T.; Fei, J.; Huang, F.; Xie, T.; Xu, J.; Zhou, Y.; Yang, P. The Anti-aging and Antioxidation Effects of Tea Water Extract in Caenorhabditis elegans. Exp. Gerontol. 2017, 97, 89–96. doi:https://doi.org/10.1016/j.exger.2017.07.015.
- Akhoon, B. A.; Rathor, L.; Pandey, R. Withanolide A Extends the Lifespan in Human EGFR-driven Cancerous Caenorhabditis elegans. Exp. Gerontol. 2018, 104, 113–117. doi:https://doi.org/10.1016/j.exger.2018.02.004.
- Yang, Z. Z.; Yu, Y. T.; Lin, H. R.; Liao, D. C.; Cui, X. H.; Wang, H. B. Lonicera japonica Extends Lifespan and Healthspan in Caenorhabditis elegans. Free Radical Bio. Med. 2018, 129, 310–322. DOI: https://doi.org/10.1016/j.freeradbiomed.2018.09.035.
- Ruan, Q.; Qiao, Y.; Zhao, Y.; Xu, Y.; Wang, M.; Duan, J.; Wang, D. Beneficial Effects of Glycyrrhizae radix Extract in Preventing Oxidative Damage and Extending the Lifespan of Caenorhabditis elegans. J. Ethnopharmacol. 2016, 177, 101–110. DOI: https://doi.org/10.1016/j.jep.2015.10.008.
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan Extending and Oxidative Stress Resistance Properties of a Leaf Extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 9012396. DOI: https://doi.org/10.1155/2019/9012396.
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. DOI: https://doi.org/10.1093/mp/sst080.
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K. G.; Ren, X.; Qu, Y. In Vivo Wound Healing and in Vitro Antioxidant Activities of Bletilla striata Phenolic Extracts. Biomed. Pharmacot. 2017, 93, 451–461. doi:https://doi.org/10.1016/j.biopha.2017.06.079.
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant Potential, Total Phenolic and Total Flavonoid Contents of Rhododendron anthopogonoides and Its Protective Effect on Hypoxia-induced Injury in PC12 Cells. BMC Complement. Altern. Med. 2015, 15, 287. DOI: https://doi.org/10.1186/s12906-015-0820-3.
- Zeng, Y.; Deng, M.; Lv, Z.; Peng, Y. Evaluation of Antioxidant Activities of Extracts from 19 Chinese Edible Flowers. SpringPlus 2014, 3, 315. DOI: https://doi.org/10.1186/2193-1801-3-315.
- Hwang, S. J.; Yoon, W. B.; Lee, O. H.; Cha, S. J.; Lim, J. D. Radical-scavenging-linked Antioxidant Activities of Extracts from Black Chokeberry and Blueberry Cultivated in Korea. Food Chem. 2014, 146, 71–77. DOI: https://doi.org/10.1016/j.foodchem.2013.09.035.
- Brenner, S. The Genetic of Caenorhabditis elegans. Genetics 1974, 77(1), 71–94. DOI: https://doi.org/10.1093/genetics/77.1.71.
- Donkin, S.; Williams, P. L. Influence of Developmental Stage, Salts and Food Presence on Various End Points Using Caenorhabditis elegans for Aquatic Toxicity Testing. Environ. Toxicol Chem. 1995, 14, 2139–2147. DOI: https://doi.org/10.1002/etc.5620141218.
- Zhang, W.; Lv, T.; Li, M.; Wu, Q.; Yang, L.; Liu, H.; Sun, D.; Sun, L.; Zhuang, Z.; Wang, D. Beneficial Effects of Wheat Gluten Hydrolysate to Extend Lifespan and Induce Stress Resistance in Nematode Caenorhabditis elegans. PLoS ONE 2013, 8(9), e74553. DOI: https://doi.org/10.1371/journal.pone.0074553.
- Shen, L. L.; Wang, Y.; Wang, D. Y. Involvement of Genes Required for Synaptic Function in Aging Control in C. elegans. Neurosci. Bull. 2007, 23, 21–29. doi:https://doi.org/10.1007/s12264-007-0003-4.
- Zhang, Y.; Lv, T.; Li, M.; Xue, T.; Liu, H.; Zhang, W.; Ding, X.; Zhuang, Z. Anti-aging Effect of Polysaccharide from Bletilla striata on Nematode Caenorhabditis elegans. Pharmacogn. Mag. 2015, 11(43), 449–454. DOI: https://doi.org/10.4103/0973-1296.160447.
- Fan, D.; Hodges, D. M.; Zhang, J.; Kirby, C.; Ji, X.; Locke, S. J.; Critchley, A. T.; Prithiviraj, B. Commercial Extract of the Brown Seaweed Ascophyllum nodosum Enhances Phenolic Antioxidant Content of Spinach (Spinacia oleracea L.) Which Protects Caenorhabditis elegans against Oxidative Stress and Thermal Stress. Food Chem. 2011, 124, 195–202. DOI: https://doi.org/10.1016/j.foodchem.2010.06.008.
- Jin, Y.; Chen, M.; Jin, Y.; Tao, J. In Vitro Free Radical Scavenging Activities and Active Constituents from Paeonia lactiflora Flowers. J. Yangzhou Univ. (Agr. Life Sci. Editi.) 2012, 33, 86–90. (In Chinese).
- Yan, S.; Xie, M.; Wang, Y.; Xiao, Q.; Ding, N.; Li, Y. Semi-synthesis of a Series Natural Flavonoids and Flavonoid Glycosides from Scutellarin. Tetrahedron 2020, 75(8), 130950. DOI: https://doi.org/10.1016/j.tet.2020.130950.
- Ballard, C. R.; Junior, M. R. M. Chapter 10: Health Benefit of Flavonoids. Bioactive Compounds: Health Benefits and Potential Applications 2019, 10, 185–201. DOI: https://doi.org/10.1016/B978-0-12-814774-0.00010-4.
- Heleno, S. A.; Martins, A.; Queiroz, M. J. R. P.; Ferreira, I. C. F. R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. DOI: https://doi.org/10.1016/j.foodchem.2014.10.057.
- Rashmi, H. B.; Negi, P. S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. DOI: https://doi.org/10.1016/j.foodres.2020.109298.
- Gupta, D. Methods for Determination of Antioxidant Capacity: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 546. DOI: https://doi.org/10.13040/IJPSR.0975-8232.6(2).546-66.
- Fu, C. M.; Jiao, B. N.; Kan, J. Q. Indirect Methods and Influence Factors to Determine Total Antioxidant Activity of Fruit and Vegetable. Food Sci. 2008, 29, 457–460. (In Chinese).
- Kim, S. J.; Matsushita, Y.; Fukushima, K.; Aoki, D.; Yagami, S.; Yuk, H. G.; Lee, S. C. Antioxidant Activity of a Hydrothermal Extract from Watermelons. LWT-Food Sci. Technol. 2014, 59, 361–368. DOI: https://doi.org/10.1016/j.lwt.2014.04.041.
- Pisoschi, A. M.; Pop, A.; Cimpeanu, C.; Gabriel, P. Antioxidant Capacity Determination in Plants and Plant-derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. DOI: https://doi.org/10.1155/2016/9130976.
- Liu, J. Research on a New Method of Determination and Elimination of Free Radical. J. Wuhan Polytechnic Univ. 2005, 24, 53–55. (In Chinese).
- Gülçin, İ. Antioxidant Properties of Resveratrol: A Structure-activity Insight. Innov. Food Sci. Emerg. Tech. 2010, 11, 210–218. DOI: https://doi.org/10.1016/j.ifset.2009.07.002.
- Zhang, Y.; Sun, W.; Zhao, M.; Su, G.; Ning, Z.; Sun-Waterhouse, D. Improvement of the ACE-inhibitory and DPPH Radical Scavenging Activities of Soya Protein Hydrolysates through Pepsin Pretreatment. Int. J. Food Sci. Technol. 2015, 50, 2175–2182. DOI: https://doi.org/10.1111/ijfs.12856.
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerkoivć, J.; Zengin, G.; Gašić, Ž.; Mašković, P.; Soares, C.; Barroso, M. F.; Delerue-Matos, C.; et al. The Influence of the Extraction Temperature on Polyphenolic Profiles and Bioactivity of Chamomile (Matricaria Chamomilla L.) Subcritical Water Extracts. Food Chem. 2019, 271, 328–337. DOI: https://doi.org/10.1016/j.foodchem.2018.07.154.
- Soleimanifar, M.; Niazmand, R.; Jafari, S. M. Evaluation of Oxidative Stability, Fatty Acid Profile, and Antioxidant Properties of Black Cumin Seed Oil and Extract. J. Food Meas. Charact. 2019, 13, 383–389. DOI: https://doi.org/10.1007/s11694-018-9953-7.
- Somawathi, K. M.; Rizliya, V.; Wijesinghe, D. G. N. G.; Madhujith, W. M. T. Antioxidant Activity and Total Phenolic Content of Different Skin Coloured Brinjal (Solanum melongena). Trop. Agr. Res. 2014, 26, 152–161. doi:https://doi.org/10.4038/tar.v26i1.8080.
- Li, X.; Liu, H.; Lv, L.; Yan, H.; Yuan, Y. Antioxidant Activity of Blueberry Anthocyanin Extracts and Their Protective Effects against Acrylamide-induced Toxicity in HepG2 Cells. Int. J. Food Sci. Tech. 2018, 53, 147–155. doi:https://doi.org/10.1111/ijfs.13568.
- Wang, Q.; Yang, F.; Guo, W.; Zhang, J.; Xiao, L.; Li, H.; Jia, W.; Huang, Z. Caenorhabditis elegans in Chinese Medicinal Studies: Making the Case for Aging and Neurodegeneration. Rejuv. Res. 2014, 17, 205–208. doi:https://doi.org/10.1089/rej.2013.1512.
- Wan, Q. L.; Shi, X.; Liu, J.; Ding, A. J.; Pu, Y. Z.; Li, Z.; Wu, G. S.; Luo, H. R. Metabolomic Signature Associated with Reproduction-regulated Aging in Caenorhabditis elegans. Aging 2017, 9, 447–463. DOI: https://doi.org/10.18632/aging.101170.
- Wang, Y. J.; Ma, J. W.; Wang, X. Z.; Zhou, F.; Zhang, B. L.; Wang, L. F. Potential Anti-aging Effects of Grape Seed Procyanidins on Caenorhabditis elegans. Sci. Tech. Food Ind. 2014, 35, 369–373. (In Chinese).
- Fu, X.; Tang, Y.; Dickinson, B. C.; Chang, C. J.; Chang, Z. An Oxidative Fluctuation Hypothesis of Aging Generated by Imaging H2O2 Levels in Live Caenorhabditis elegans with Altered Lifespans. Biochem. Bioph. Res. Co. 2015, 458, 896–900. doi:https://doi.org/10.1016/j.bbrc.2015.02.055.