ABSTRACT
Cardiovascular diseases are the leading cause of death in the world and scientists pay a lot of attention to identify and reveal the mechanisms of their occurrence. Recently, the attention of scientists is focused increasingly on plant derivatives, such as flavonoids and polyphenols, due to their specific biological effects. One of these compounds is curcumin, which has many biological properties. Numerous studies have been performed to understand the molecular basis of the therapeutic properties of curcumin. As a result of these studies, there is considerable evidence to suggest that curcumin may affect signaling pathways associated with the cell growth, proliferation, survival, inflammation, and gene transcription. Antioxidant and anti-inflammatory mechanisms are the two basic mechanisms to which many of the effects of curcumin in various conditions are attributed. Many factors influence the development of heart disease, but one of the main culprits in their occurrence is the inflammatory process. According to recent research, curcumin is an ingredient that could be used in the prevention or treatment of cardiovascular disease. Also, some studies have shown that it has beneficial effects in preventing vascular damage and ischemia. Despite its beneficial and biological properties, it has been proven that curcumin has relatively low bioavailability and low stability in the human body, which limits its therapeutic application. In this regard, several attempts have been made to synthesize curcumin derivatives with improved bioavailability. In this paper, we review the potential and possibilities of using curcumin and its derivatives in the treatment of cardiovascular disease, which would significantly reduce the mortality rate in the population. Based on all of the above, it can be concluded that further studies of animal models and humans are needed to verify current knowledge about the application of curcumin and its derivatives in the treatment of cardiovascular diseases. In this way through these studies more reliable data will be generated concerning the effects of curcumin and its analogs on cellular and subcellular/molecular levels. Prospectively, we believe that this will ground the basis for further improved synthesis of curcumin-analogs, appropriate for the treatment of cardiovascular diseases.
Introduction
The term “cardiovascular disease” refers to a group of diseases characterized by damage to the heart and blood vessels, [Citation1] which, in most cases, are caused by atherosclerosis, i.e., the formation of plaque and damage to the blood vessels walls. Cardiovascular diseases are the leading cause of death in most developed countries, as well as in many developing countries .[Citation2] In developing countries, young people are dying increasingly from these diseases. These diseases are a significant cause of low work capacity, high costs of health care, and premature mortality. As a result of the preventive measures, there has been a reduction in the death rate in developed countries, but despite the decrease, the absolute number of deaths is still high, due to the aging population in most countries. Therefore, it is necessary to develop appropriate therapeutic strategies, which will act in terms of prevention and timely treatment of the first visible traces of diseases of the cardiovascular system .[Citation3]
Recently, the attention of scientists is focused increasingly on herbal medicines, trying to find remedies that are effective and inexpensive, without causing side effects, [Citation4] such as those associated with conventional medicines, used so far in the treatment of cardiovascular disease .[Citation5] The use of herbal medicines as adjunctive therapy dates a long time ago in the treatment of human diseases, [Citation6] however, in highly developed countries they have become relevant in the last twenty years. Hence, in recent years, more and more pharmacological tests have been performed on several plant derivatives, such as flavonoids and polyphenols, due to their specific biological effects, to discover new and effective drugs for these diseases .[Citation7] One of these compounds is curcumin (CUR), which has many biological properties. CUR is a light yellow pigment found in the rhizomes of turmeric (Curcuma Longa) which is a member of the ginger family (Zingiberaceae), [Citation8] with a wide range of pharmacological activities .[Citation9,Citation10] In traditional Chinese and Indian medicine, CUR has been used to treat a variety of diseases. A large body of evidence suggests that CUR has a diverse range of molecular targets, including transcription factors, growth factors, and their receptors, cytokines, enzymes, and genes regulating cell proliferation and apoptosis .[Citation11]
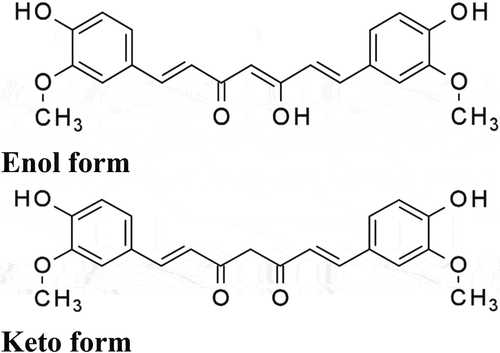
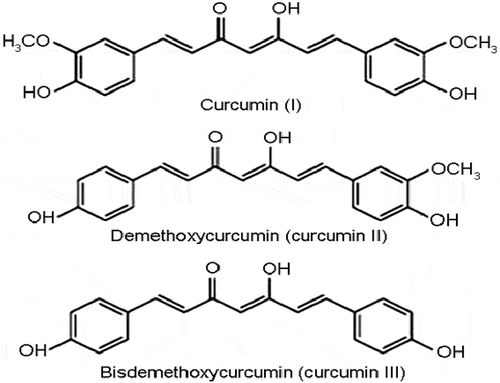
CUR in the form of (1E,6E) −1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione), also known as diferuloylmethane, is the primary polyphenol derived from turmeric through the extraction process .[Citation12] It is the main representative of the curcuminoids found in the spice turmeric, and the other two curcuminoids are: dimethoxycurcumin and bis-dimethoxycurcumin (). Curcuminoids are natural phenols that give turmeric its yellow color. Among these, curcumin is the most active and present component of turmeric and it is estimated that approximately 2 to 5% of turmeric is composed of CUR, [Citation13] while the rest is composed of minerals, carbohydrates, proteins, fats, and moisture. Isolation of pure CUR is quite difficult, therefore, commercially available CUR consists of a combination of the three curcuminoids, with CUR as the main ingredient (75–81%). CUR has several tautomeric forms, including the 1,3-diketo form and two enol forms (). The enol form is more stable in the solid phase and it is the dominant form .[Citation14,Citation15] Several studies have shown that CUR has significant biological properties, including anti-inflammatory and antioxidant, [Citation16] antimicrobial, [Citation17] and hepato-protective effects .[Citation18] Antioxidant and anti-inflammatory mechanisms are the two main mechanisms to which many of the effects of CUR in various conditions are attributed .[Citation19,Citation20] But despite its beneficial properties, reflected through its antioxidant and anti-inflammatory mechanisms, its rapid metabolism and rapid elimination from the body, as well as its chemical instability[Citation21] have been the subject of concern among researchers. In this regard, several attempts have been made to synthesize CUR derivatives with improved bioavailability. However, despite its low bioavailability, several studies have shown that CUR has a protective role in suppressing the development of atherosclerosis, heart hypertrophy, heart failure, drug-induced cardiotoxicity, myocardial infarction, aortic aneurysm, and stroke .[Citation22,Citation23]
Figure 1. Chemical structure of curcumin (diferuloylmethane), dimethoxycurcumin and bis-dimethoxycurcumin
The strong antioxidant effects of CUR at the level of the heart have already been reported in a large number of preclinical studies .[Citation24–26] Actaully, Qin (2017) in their meta-analysis devoted to the protective role of CUR in cardiovascular diseases, reported the protective effects of CUR on the endogenous glutathione status as well as upon the enzymes involved in the regulation of the same status. The same review also reported an important role of CUR in the inhibition of histone acetyltransferases and its inducible roles in the increasing of manganese superoxide dismutase activity .[Citation27] From all above, it is more than clear that therapeutic significance can be attributed to CUR, although this requires additional clinical studies with the human population as its main target. At the same time, such studies should address broad research that will potentiate the beneficial effects of CUR at several levels in the body.
Cardiovascular diseases
Cardiovascular diseases can be divided into diseases that affect: 1) the heart and blood vessels, 2) the brain and the cerebrovascular system, or 3) the lower extremities (occlusive disease of the peripheral arteries) .[Citation23,Citation28] The basis of all these diseases is most often atherosclerosis. According to the latest research, vascular inflammation plays an important role in all stages of the development of the atherosclerotic process .[Citation29,Citation30] In the beginning, inflammation has a protective effect, while later inflammatory cells and mediators have harmful effects, encouraging the onset and progression of the disease, as well as the development of its complications .[Citation30] Given that one of the main mechanisms in the occurrence of cardiovascular disease is inflammation and oxidative stress, it is necessary to discover therapeutic agents that will act to reduce these processes, and thus the occurrence of cardiovascular disease.
Cardiovascular activities of curcumin
Anti-atherosclerotic effects of curcumin
Atherosclerosis is a major cause of cardiovascular diseases, such as myocardial infarction, heart failure, stroke, and claudication.[Citation31] Therefore, in this paper, we will first give a systematic review of the causes of atherosclerosis and a systematic review of research and achievements from a molecular standpoint, regarding the use of curcumin as a therapeutic tool in the treatment of atherosclerosis.
Atherosclerosis is a chronic inflammatory response of the vessel wall or vascular epithelial dysfunction.[Citation30] Over the last few decades, preclinical and clinical studies of the cardiovascular system have significantly improved the understanding of the pathophysiological process leading to the formation, progression, and complications of atherosclerotic plaques.[Citation31] Atherosclerosis begins with an innate immune response that takes place through the recruitment of monocytes/macrophages that respond to the excessive accumulation of modified lipids in the arterial wall. Monocyte/macrophage recruitment is achieved through expressed adhesion molecules (selectins, ICAM-1, VCAM, ELAM) of activated endothelial cells. Atherosclerosis then proceeds with an acquired immune response involving the activation of antigen-specific T lymphocytes.[Citation32] Atherosclerotic changes are rich not only in monocytes/macrophages but also in T cells, primarily the Th1 subtype, which may play a role in plaque formation through a cytokine cascade initiated by their activation.[Citation33]
The endothelium, blood vessels, and smooth muscle cells are targets of proinflammatory cytokines that, when stimulated, can produce other cytokines. Cytokines further stimulate the expression of endothelial cell adhesion molecules, attracting new monocytes to sub-endothelial tissue, transforming them into macrophages and foam cells; they release even more cytokines which, by a positive feedback mechanism, lead to further progression of atherosclerosis.[Citation31]
Atherosclerotic plaque contains at least two stimuli for Th1 differentiation. Interleukin-12 (IL-12) from macrophages, smooth muscle cells, and endothelial cells is important for this differentiation. Elevated levels of IL-12 have been found in atherosclerotic plaques, while inhibition of IL-12 by the techniques of vaccination (mechanism for a complete block of IL-12 production) has been shown to reduce atherosclerosis in mice .[Citation30] IL-12 production is similarly regulated in monocytes exposed to oxidized low-density lipoprotein (ox-LDL) .[Citation34]
Interferon-I (INF-I) has also been detected in atherosclerotic plaques .[Citation32] It is astrong inhibitor of smooth muscle cell growth and collagen production, thus promoting plaque instability. In addition, IFN-I causes the expression of secretory phospholipase A2, leading to the formation of inflammatory lipid mediators such as lysophosphatidylcholine (LPC), platelet-activating factor (PAF), and eicosanoids .[Citation34] INF-I also improves antigen presentation and leads to increased production of tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), which contributes to the formation of atheromatous plaques. Mice lacking INF-I have been shown to reduce atherosclerosis by 60% and to accelerate the administration of INF-I to the atherosclerosis .[Citation34,Citation35]
Experimental atherosclerosis in animals is an important research tool, but a careful extrapolation of the results to the human population is necessary. Knowledge of the inflammatory components in the process of developing atherosclerosis offers the opportunity to develop new therapeutic strategies.
CUR has been shown to have anti-atherosclerotic activity through its anti-inflammatory and anti-oxidant mechanisms .[Citation31] According to a study conducted on smooth muscle cells isolated from the thoracic aorta of rats (Sprague‑Dawley) and stimulated for 24 hours with Angiotensin II (Ang-II), which plays a significant role in the development of atherosclerotic plaques, treatment with CUR causes decrease in Ang-II induced production of proinflammatory cytokines (IL-6 and TNF-α) in a concentration-dependent manner .[Citation36] It also reduces the production of nitric oxide (NO) .[Citation37]
Abnormal proliferation of vascular smooth muscle cells and mononuclear cells, including macrophages, [Citation38] monocytes, and lymphocytes, [Citation39,Citation40] which are among the most common immune cells in atherosclerotic lesions, play a key role in atherosclerotic lesions, inflammation and the development of atherosclerosis. According to studies conducted on mouse models, the protective effect of curcumin against atherosclerosis is probably due to its modulation by macrophages, vascular smooth muscle cells, and endothelial cells .[Citation19] In a study investigating the anti-proliferative effects of curcumin on mononuclear cells in human blood, it was shown to inhibit neutrophil activation .[Citation41] The same study also examined the anti-proliferative effect of curcumin on vascular smooth muscle cells in rabbits and shown CUR-induced inhibition in both cases serum-induced proliferation and platelet-derived growth factor (PDGF)-dependent proliferation of vascular smooth muscle cells .[Citation41] According to another study conducted in rats, the anti-proliferative effect of CUR upon vascular smooth muscle cells is due to its stimulation of peroxisome proliferator-activated receptor γ (PPAR-γ) activity, and suppression of NADPH oxidase-mediated intracellular production of reactive oxygen species (ROS) .[Citation41] In a study of mouse fibroblast cells, CUR suppressed c-Jun/AP-1 activity, [Citation42] which is known to activate variety of mitogens .[Citation43] The potential preventive role of CUR in the process of atherosclerosis is further supported by its inhibitory effect upon the migration of aortic smooth muscle cells .[Citation44] This inhibitory effect is associated with decreased TNF-α stimulated production of ROS and inhibition of matrix metallopeptidase-9 (MMP-9) expression and activation of nuclear factor-КB (NF-КB) .[Citation44]
By using “arterial balloon injury” (an important methodology in studying the molecular and cellular mechanisms involved in vascular smooth muscle cell differentiation, neo-intima formation, and vascular remodeling) in male Sprague-Dawley rat models, it was found that CUR significantly inhibits all mentioned processes as key elements in the pathogenesis of atherosclerotic processes .[Citation45,Citation46]
The vascular anti-proliferative effect of CUR was additionally demonstrated by using a model of LDL knockout mice. In this model CUR caused heme oxygenase-1 activation (HO-1), [Citation47] an enzyme that is important regulator in reducing the growth of vascular smooth muscle cells .[Citation48] Induction of HO-1 results in a reduction in atherosclerotic lesions in the LDL receptor of knockout mice .[Citation48] CUR is thought to induce HO-1 by activating the Nrf2-dependent antioxidant in various cells of the cardiovascular system (such as vascular endothelial cells, vascular smooth muscle cells, and human aortic smooth muscle cells) .[Citation47–49] Hence, the anti-proliferative effect of CUR is significantly related to its ability to induce HO-1 .[Citation49]
According to some in vitro studies, CUR caused inhibition of the platelet activating factor (PAF), and adenosine diphosphate-induced platelet aggregation .[Citation50–53] In another study conducted on a mouse model with thrombosis, it was reported that CUR-induced inhibition of platelet activation .[Citation53] These studies suggest that the antiplatelet properties of CUR may be partly responsible for some of its anti-atherosclerotic activities, but further research is needed to be confirmed .[Citation46]
Low-density cholesterol (LDL) oxidation, also plays an important role in the development of atherosclerosis. CUR has also been shown to be an effective antioxidant by preventing the oxidation and modification of LDL .[Citation54] Using a rabbit model on a high-fat diet, it was found that CUR effectively prevented LDL oxidation and reduced cholesterol and triglyceride levels .[Citation55] Actually, the last study has shown that CUR-supplementation, caused significant reduction in the level of oxidative stress and the development of atherosclerotic lesions in the thoracic and abdominal aorta .[Citation56]
In knockout mice for apolipoprotein E (apoE) and LDL receptor (apoE/LDLR), on oral CUR-administration, reduced formation of atherosclerotic lesions was reported .[Citation57] In vascular smooth muscle cells isolated from the same kind of mice, treatment with CUR causes inhibition of ox-LDL cholesterol-induced accumulation, probably via regulation of the sterol-responsive-element-binding protein (SREBP-1/caveolin-1) signaling pathway .[Citation58] According to recent research, based on the fact that atherosclerosis is a chronic inflammatory disease associated with increased oxidative stress in vascular smooth muscle cells, researchers believe that the anti-atherogenic effects of the CUR may be due to its antioxidant and anti-inflammatory properties, by suppressing the NF-КB, [Citation44] and reducing the production of pro-inflammatory cytokines such as IL-6 and TNF-α .[Citation35] In addition to anti-inflammatory effects, recent investigations suggest that it also has anti-proliferative effects on vascular smooth muscle cells through its ability to inhibit proliferative and migratory signaling pathways .[Citation46] Based on its anti-platelet properties, it is proposed that CUR can be used as a supplementary agent for patients with atherosclerosis. From the analysis of the research done so far, it can be concluded that it is necessary to conduct additional preclinical and clinical research in animal models and humans in direction of structuring new CUR-analogs, that even could surpass its supplementary role in the condition of atherosclerotic processes .[Citation48]
Anti-Hypertensive effect of curcumin
Arterial hypertension is a complex syndrome in which etiopathogenesis is interwoven with numerous interactions of various endogenous and exogenous factors. Hypertension is a risk factor for some cardiovascular diseases and organ damage .[Citation59] Patients with arterial hypertension are classified according to the etiology of primary or essential hypertension and secondary hypertension. Arterial pressure homeostasis and arterial disorders are influenced by genes that regulate the reabsorption of salt and water in the renal tubules. Essential hypertension is most often caused by inherited genetic defects in salt homeostasis. The greatest influence on blood pressure values was observed in the angiotensinogen gene polymorphism .[Citation59] The renin-angiotensin-aldosterone (RAA) system is the major regulator of salt and water metabolism in the body and the most important endocrine system in controlling blood pressure .[Citation60] The disturbed balance of this system, as a result of the factors for the reabsorption of water and salts in the organism, [Citation61] leads to vasospasm, thrombosis, atherosclerosis, and restenosis after invasive procedures. Increased hypercoagulability has been reported in patients with essential hypertension, which is one of the most important risk factors for cardiovascular disease. Obesity and insulin resistance are important hypertensive factors that increase the risk of cardiovascular complications .[Citation59]
There is clear evidence that polymorphisms in renin, angiotensinogen, and angiotensin-converting enzyme loci affect both blood pressure and hypertension .[Citation61] Because pathological increases in blood pressure occur due to abnormal expression of multiple genes, the molecular basis of hypertension is relatively unknown yet .[Citation62]
Some researchers believe that oxidative stress plays a role in the development of hypertension .[Citation63] Excessive production of free radicals causes reduced NO bioavailability. Eventually, this causes an increase in total peripheral resistance and leads to endothelial dysfunction .[Citation63] Recent research has shown that inflammation also plays a role in the development of hypertension, [Citation64] but it is not clear yet, how activated immune cells lead to the development of hypertension. With the development of new experimental methods, progress has been made in the mechanisms by which inflammation leads to hypertension and cardiovascular disease, but further research is needed .[Citation64] Changes in humoral and cellular immunity have been reported in both hypertensive patients and animal models .[Citation65] In some studies, inflammation has been associated with decreased endothelial-dependent relaxation, a process associated with changes in NO bioavailability .[Citation66,Citation67] In this direction there is considerable evidence of an association between endothelial dysfunction and essential and gestational hypertension as well .[Citation68] In addition, some studies have shown that oxidative stress, inflammation, and the immune system are involved in the development of hypertension .[Citation69]
The effect of CUR on hypertension has not been studied in details yet. In one study, the protective effect of CUR on endothelial dysfunction, vascular remodeling, and oxidative stress in hypertensive male rats was demonstrated .[Citation70] In the same study, CUR was shown to reduce oxidative stress and vascular remodeling by increasing plasma endotracheal encephalitis, endothelial NO synthase (eNOS), decreased expression of p47 f-oxide NADPH oxidase and decreased production of superoxide anion in the vascular tissues .[Citation70] The overall findings from the literature suggests that the mechanisms responsible for the antihypertensive effect of CUR are due to improve NO bioavailability and reduced oxidative stress .[Citation70] In another study, CUR was found to significantly reduce hypertension, and cause a reversible vascular response to Nω-nitro-L-arginine methyl ester (L-NAME)-induced hypertension in rats .[Citation46,Citation71] Improvement of vascular dysfunction has been associated with increased eNOS expression in aortic tissue and plasma nitrate/nitrite .[Citation71] In addition, CUR has been found to reduce the level of vascular superoxide anion production, thereby reducing the level of oxidative stress.
The beneficial effect of CUR has been demonstrated in a clinical trial in patients with renal disease – lupus nephritis, [Citation72] but according to another animal study in rat hypertrophy with renal failure, the CUR did not affect the nephritis-induced increase in systolic blood pressure .[Citation46,Citation73] Hence, we can summarize that more research is needed to shed light on the molecular mechanisms that lead to hypertension, to find an appropriate therapeutic agent (CUR-based analog) that will be used in the therapy of patients with hypertension, as well as the implementation of further studies in animal models and patients regarding the antihypertensive effects of CUR .[Citation66]
Antidiabetic effects of CUR in relation to diabetic cardiovascular complication
Diabetes is a major risk factor for cardiovascular disease, and the association between them is complex and multifactorial .[Citation74] A large percentage of patients with diabetes have dyslipidemia, which leads to atherosclerosis .[Citation75] People with diabetes have lower levels of high-density lipoprotein (HDL) cholesterol and higher levels of triglycerides and LDL cholesterol. Oxidized-LDL has pro-atherogenic properties, and as such, causes abnormal biological responses such as the attraction of leucocytes to the intima of vessels, stimulation of leucocytes to ingest lipids and differentiate into foam cells, stimulation of cell proliferation, and stimulation of leukocyte proliferation and smooth muscle cells, leading to the formation of atherosclerotic plaques .[Citation76] People with diabetes have been found to have decreased bioavailability of NO, (a potent vasodilator), and increased secretion of vasoconstrictor endothelin-1 .[Citation77] Decreased NO bioavailability in diabetics occurs due to insulin deficiency or defective insulin signaling (insulin resistance) in endothelial cells .[Citation66,Citation67] Hyperglycemia also acutely inhibits NO production in arterial endothelial cells .[Citation78] Decreased NO production and increased secretion of endothelin-1 are associated with vasoconstriction and the release of proinflammatory cytokines .[Citation79] Proinflammatory cytokines increase vascular permeability, cause programmed cell death (apoptosis), recruit invasive leucocytes, and cause increased production of ROS. Oxidative stress, in turn, plays a key role in the pathophysiology of cardiovascular complications in diabetic patients .[Citation74]
Research concerning the antidiabetic effects of CUR began after the publication of the first study in 1972, which described the effect of CUR on lowering blood glucose in only one diabetic person .[Citation80] The studies that were conducted subsequently were mostly on animal models, and only a few of them were clinically oriented .[Citation81] One of the most widely used animal model for examining the effects of CUR, is the model for study the effects upon blood glucose levels .[Citation82] In alloxan-induced diabetic rats, streptozotocin (STZ)-induced diabetic rats and STZ-nicotinamide-induced diabetic rats, [Citation83] oral administration of different doses of CUR has been shown to prevent weight loss, glucose, hemoglobin, and glycosylated hemoglobin (HbA1C) increase, [Citation84] and improved insulin sensitivity .[Citation85] Also, CUR has been shown to lower blood glucose levels in diabetic models of KK-Ay mice .[Citation86] CUR, was also effective in lowering blood glucose levels of hemoglobin and hemoglobin A1C (HbA1C) in models of STZ-induced diabetic mice .[Citation87] In some studies, the effect of CUR on lowering blood glucose has been linked to an increase in NO bioavailability, [Citation88] (which is reduced by diabetes), suggesting that CUR play pivotal role in the prevention of cardiovascular complications from diabetes .[Citation88]
According to recent studies, one of the functions of CUR in the process of hyperglycemia is the reduced levels of TNF-α[Citation89] and NF-КB, [Citation90] (ie CUR treatment reduces their expression, activation, or function) .[Citation91] The treatment with CUR also has been associated with decreased levels of IL-6 and monocyte chemoattractant protein-1 (MCP-1) in streptozotocin-diabetic mice and in monocytes treated with high glucose concentration .[Citation92] CUR has been shown also to have the ability to activate peroxisome proliferator-activated receptor γ (PPAR-γ) activity in diabetic models of KK-Ay mice .[Citation86] Some studies have shown that CUR also can increase plasma insulin levels and increase lipoprotein lipase (LPL) activity .[Citation93] Using models of streptozotocin-induced diabetic mice, CUR cause reduced expression of protein kinase C (PKC) and lead to reduced ROS production .[Citation94] However, according to some studies, CUR had no significant effects on blood glucose levels .[Citation95,Citation96] The reason for the opposite results from these studies may be due to the use of different models of induced diabetic rodents or different administration of CUR .[Citation93]
From the published studies, we can conclude that the protective effect of CUR on the cardiovascular system in diabetic cases is due to its ability to reduce oxidative stress and inflammatory processes, [Citation46] which occur in diabetes. It needs to be taken that most of these results have been obtained by experiments performed on animal models, so further clinical trials are needed, [Citation82] to confirm the potential of CUR in the treatment of diabetes induced cardiovascular complications.
Cardio-protective effects of CUR on myocardial infarction
Myocardial infarction is considered to be one of the major cardiovascular diseases with a high rate of morbidity and mortality worldwide .[Citation97] The most common cause of myocardial infarction is narrowing of the coronary arteries, which leads to the complete cessation of blood flow to the heart .[Citation67] It usually occurs suddenly, due to blockage of the coronary artery or its branches by a thrombus that gradually forms at the base of the blood vessels damaged by atherosclerosis. Atherosclerotic plaques narrow the diameter of the coronary artery, creating a base on which a clot can easily form and clogs the lumen (cavity) of the coronary artery .[Citation98]
The inflammatory process plays a key role in the formation of atherosclerotic plaque and in the progression of plaque to an unstable state, resulting in myocardial infarction .[Citation98] Myocyte death due to myocardial infarction triggers a temporary inflammatory response by activating toll-like receptors .[Citation99] In some rodent models of myocardial infarction, increased expression of pro-inflammatory cytokines has been observed: TNF-α, IL-1β, and IL-6, after only a few hours to a day after the occurrence of myocardial infarction .[Citation100] An increased role in the activation of inflammatory responses is played by the increased production of ROS due to ischemia of the heart muscle .[Citation99]
Risk factors for the onset and development of atherosclerosis include hypertension, diabetes mellitus, and dyslipidemia .[Citation101] Recent findings suggest that atherosclerosis is a partially inflammatory condition .[Citation102] Currently available methods for relieving myocardial infarction are surgical approach and antithrombotic therapy .[Citation103] Scientists today are increasingly examining herbal remedies as an alternative approach to treating various diseases, associated to the occurrence of myocardial infarction .[Citation104]
Herbal medicine has been examining CUR extensively, as alternative medicine in the treatment of myocardial infarction. According to some studies, CUR causes a significant reduction in plasma levels of cardiac enzyme markers: lactate dehydrogenase – LDH and creatine kinase – CK in rat models of infarction, treated with CUR at doses of 50 mg/kg body weight, few days after infarction, indicating its potential to reduce infarct injury at the onset of infarction .[Citation105] Hong et al. demonstrated the same scenario with these enzymes in the rat models treated with 75 mg/kg/day CUR for 3 days .[Citation106] They also reported that CUR caused different expressions of different genes compared to the control non-CUR-treated groups. These genes play a role in the inflammatory processes, proving the anti-inflammatory effect of CUR and its protective role against myocardial infarction .[Citation106,Citation107] Garvin et al. found that CUR has a modulating effect on the rate of immune cell filtration and cause improvement in the mitochondrial function in injured cardiomyocytes, thereby reducing the infarct rate .[Citation108]
Numerous studies have shown that the nuclear factor (NF), play a key role in the pathophysiology of myocardial infarction, ischemic remodeling, apoptosis, and heart failure .[Citation109] NF is thought to contribute significantly to the occurrence of heart injury by generating cardiac inflammatory processes .[Citation110] Lv et al. in a rat model of infarction, in which they ligated the anterior left descending artery, found that treatment with 150 mg/kg CUR per day after ligation significantly reduced NF-expression and increased PPAR-γ expression. The potentiation in PPAR-γ expression is very important in induction of the anti-inflammatory signaling, thus contributing to the reduction of the infarct size .[Citation111]
According to some studies, CUR is also effective in the prevention of the ischemic-reperfusion injury in the heart, which can lead to cardiomyocyte death .[Citation112] Liu et al. demonstrated that CUR induces prevention of the myocardial ischemic-reperfusion injury in a rat model by reducing serum LDH and CK enzymes and inhibiting the NF-signaling pathway .[Citation113] In a rabbit model of myocardial ischemic reperfusion injury during cardiopulmonary bypass, Saeidinia et al. reported that CUR inhibited NF-activation in the cardiomyocyte nucleus, thereby reducing levels of TNF-α and IL-6 in blood, which is the reason for inhibition of monocyte apoptosis .[Citation114] Yeh et al., found that CUR can also inhibit cardiomyocyte apoptosis by reducing NF-activation, leading to decreased pro-inflammatory cytokines[Citation35] and impaired neutrophil activation in the heart .[Citation115]
Other studies have shown that CUR activates the JAK2/STAT3 signaling pathway, leading to inhibition of reperfusion injury .[Citation113,Citation116] Initial growth response protein-1 (EGR-1) also plays a key role in the pathophysiology of acute and chronic cardiovascular disease and is associated with induction of TNF-α and IL-6 expression .[Citation117] The same group have shown that preventive treatment with CUR (150 mg/kg/day, for 5 days) improves ischemic reperfusion injury of the heart, inducing down-regulation of the EGR-1 and inflammatory factors such as TNF-α, IL-6, P-selectin, and intercellular adhesion molecule 1 (ICAM-1), and causing reduction of the size of infarction .[Citation117] In respect to data from all previously mentioned studies for the use of CUR in the prevention and treatment of myocardial infarction, its cytotoxic effects and toxicological data from preclinical and clinical studies should be considered to determine the exact dose and time of drug administration .[Citation105]
Toxicological data about cur
An important feature of CUR is that although it has been consumed for centuries, it has not been shown to cause toxicity in the body .[Citation118] The results of preclinical and clinical studies showed that CUR is extremely well-tolerated. Most preliminary toxicological data about CUR have been obtained from cancer patients in whom CUR has been tested as an anticancer or chemo-preventive agent .[Citation119] According to these studies, CUR is found to be well tolerated in humans when administered daily (even in doses up to 8 g per day; doses over 8 g are unpleasant) over several months (up to 3 years in small studies) and no dose-limiting toxicity was observed .[Citation120]
However, there have been occasional reports of gastrointestinal side effects of CUR, including nausea and diarrhea, during the first month of use, and rare abnormal increases in serum alkaline phosphatase and lactate dehydrogenase levels have been reported .[Citation120] Another study of older people (mean age 73.5 years) with Alzheimer’s disease who were given 4 g of CUR per day for 24 weeks showed that CUR is generally well tolerated, although three of them withdrew due to gastrointestinal symptoms .[Citation121]
Despite extensive preclinical data on CUR, the pharmacokinetic properties of CUR in humans are not yet fully understood .[Citation120] In a clinical study in which healthy subjects took 2 g of pure CUR powder, serum levels of CUR were almost undetectable .[Citation122] In another study, analyses of serum and urine samples, in which subjects were administered with 3.6 g CUR per day, showed a definite presence of CUR and its conjugates in both types of samples .[Citation120] Also, some studies suggested that at higher concentrations, CUR may show antioxidant, anti-inflammatory, and anti-lipid properties .[Citation119] Co-administration of CUR with piperine has also been shown to increase the bioavailability of CUR in humans by 2000% .[Citation120]
On the other hand, several studies have shown that CUR has a relatively low bioavailability, [Citation123] which occurs primarily due to poor gastrointestinal absorption, insolubility in water, [Citation124–126] rapid metabolism, and rapid elimination from the body, as well as its molecular instability. Therefore, a thorough evaluation of the potential side effects of CUR when used in high concentrations for chronic treatments is essential. Because of this need, researchers’ efforts are focused on developing CUR analogs and CUR conjugates with greater therapeutic potential, better solubility and intestinal absorption.
Newly synthesized cur derivatives in the treatment of cardiovascular disease
Due to the low bioavailability of CUR, researchers are focused on improving its pharmacological activities. Recently, enormous approach was made in direction of generation of CUR derivatives with improved bioavailability, and it has been found that reducing the functional dicarboxylic group to monocarboxylic acid, significantly improves its bioavailability and stability .[Citation127] These so-called monocarbonate CUR analogs (MACs) have been studied extensively and possess a variety of beneficial biological properties. The different types of CUR derivatives are already tabulated in different research studies .[Citation31,Citation35,Citation67] We will list the newly synthesized ones, which are intended for cardiovascular applications.
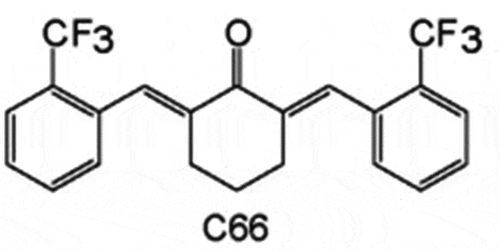
Among the monocarbonyl CUR analogs tested for cardiovascular applications is compound C66 (2E,6E)-2,6-bis [(2-trifluoromethyl) benzylidene]-cyclohexanone which has shown significantly better chemical stability and pharmacokinetic characteristics .[Citation128] According to several studies, [Citation127–129] on the use of compound C66, it has been established that this compound has a favorable pharmacological effect on complications that occur in diabetes due to its anti-inflammatory action .[Citation35] C66 has also been found to have a protective effect on pathogenic aortic changes caused by diabetes in experimental animal models of male mice aged 6–8 weeks. Treatment with C66 prevented the progression of diabetes-induced aortic inflammation, oxidative damage, apoptosis, and fibrosis .[Citation130] The researchers also found that C66 had a beneficial effect on diabetic nephropathy through mitogen-activated protein kinase (MAPK)-dependent anti-inflammatory or anti-ACE (angiotensin-converting enzyme) mechanism .[Citation131] Furthermore, the same authors have shown that C66 at a relatively low dose of 5 mg/kg/day protects against diabetic heart damage by inhibiting Janus N-terminal kinase (JNK kinase) .[Citation129] According to researchers Ren and Sowers, this compound can also be used in diabetic cardiomyopathy .[Citation21] shows the possible mechanisms of action of C66, reporting for the beneficial effect of this CUR analog in diabetic/hyperglycemic abnormalities and cardiac function retention. C66 inhibits JNK activation to stop inflammation and apoptosis during the early and late stages of diabetes/hyperglycemia. In addition, C66 preserves the level of metallothionein (heavy metal scavenger) by inhibiting JNK kinase. IКBα (nuclear factor kappa light polypeptide gene enhancer in B-cells, inhibitor alpha) is a regulatory protein that inhibits NF-КB by complexing it and trapping it in the cytoplasm .[Citation21] However, compound C66 has been shown to have poor antioxidant activity in both in vitro and in vivo conditions .[Citation132]
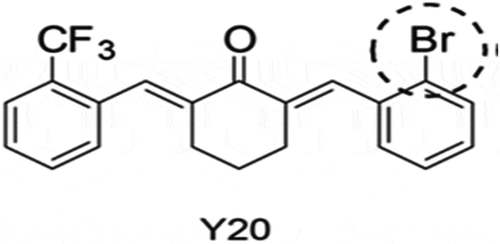
The structure of C66 () has vertical symmetry and contains two trifluoromethyl groups that may contribute to its anti-inflammatory activity. Recent research has confirmed that the introduction of bromine may enhance the antioxidant activity of the compound .[Citation132] Thus, to modify the antioxidant activity of C66, one of the trifluoromethyl groups was substituted with bromine to produce a new compound (2E, 6E) −2- (2-bromobenzylidene) −6-((2-trifluoromethyl)-benzylidene) cyclohexanone (so-called Y-20) (). Additional research has shown that Y-20 has better antioxidant properties compared to C66 and is safe for use, without side effects .[Citation133] According to Cooper and colleagues, the beneficial properties of Y20 are related to the ability of this compound to increase Nrf2 expression and inhibit NF-КB activation. Research has shown that this compound has the potential to treat overweight-induced heart disease by using Nrf2 and NF-КB as targets for the treatment of obesity-related diseases .[Citation132]
Figure 4. Schematic diagram of the mechanisms of C66 that have a beneficial effect on diabetic/hyperglycemic induced cardiac abnormalities
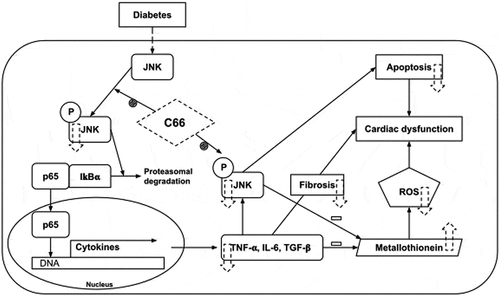
Figure 6. Curcumin and monocarbonyl curcumin derivatives with strong biological properties (antioxidant and anti-inflammatory)
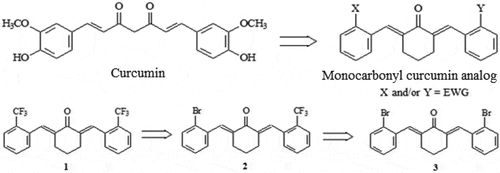
Encouraged by these studies, it can be logically concluded that the replacement of other trifluoromethyl groups of Y20 can further improve the antioxidant properties of this compound, without reducing its anti-inflammatory properties, resulting in a new compound (2E,6E)-2,6-bis (2-bromobenzylidene) cyclohexanone B2BrBC. Our research[Citation134] about the antioxidant properties of noncarbonated CUR analogs C66 and B2BrBC in isoproterenol-induced cardiac damage in rats, showed that both compounds have maximum unique antioxidant properties and the ability to withdraw electrons (i.e., activation of β-olefinic carbon), and on the other hand, the ability to release electrons necessary for their antioxidant activity .[Citation66,Citation67] In this study, reliable results were obtained for the preventive effects of the newly synthesized compound B2BrBC and its role in preventing oxidative damage at three levels (enzymatic, protein and lipid) in early myocardial hypertrophy .[Citation134] Several other studies testing the antioxidant and anti-inflammatory effects of C66 and B2BrBC, in conditions of cardiac hypertrophy in rats, support the potential of these compounds in the treatment of the progression of cardiac hypertrophy .[Citation135] In general, B2BrBC has shown significant antioxidant and anti-inflammatory effects, while C66 deficiencies are associated with poor antioxidant regulation .[Citation135] Hence, it can be summarized that by structural change in CUR, researchers have synthesized new CUR derivatives with better bioavailability and comparable or better pharmacodynamic properties than CUR; however, toxicological data on these derivatives are lacking. Therefore, further preclinical and clinical trials, with larger sample sizes and optimized doses, are needed to verify current knowledge of CUR and its derivatives in the treatment of cardiovascular disease.
Conclusion
According to research conducted so far, scientists believe that the main mechanisms for the occurrence of cardiovascular disease are increased oxidative stress and the occurrence of inflammatory responses and that the cardioprotective properties of CUR are due to its antioxidant and anti-inflammatory properties [. The mechanisms by which CUR exerts its anti-inflammatory effects are by suppressing the activation of NF-КB and reducing the increased production of proinflammatory cytokines, such as IL-6 and TNF-α. CUR also enhances the bioavailability of NO to improve endothelial function. In addition to anti-inflammatory effects, recently published data suggests that it has antiproliferative effects on vascular smooth muscle cells through its ability to inhibit proliferative and migratory signaling pathways. Along with its antioxidant effects, by preventing the oxidation and modification of LDL, reducing the production of superoxide, increasing the catalase activity, reducing the NADPH oxidative activity and its anti-platelet properties, researchers have shown that it has the potential to be used as a therapy in treating of cardiovascular disease. However, based on clinical trials conducted mainly in patients receiving anticancer therapy, it can be concluded that the efficacy of CUR in the treatment of cardiovascular disease in humans has not been well studied yet, nor has the exact dose needs to be administered to patients to achieve its cardio-protective effects been determined. In addition, the use of CUR is limited in clinical practice due to its low bioavailability, which is likely to be caused by poor absorption and rapid metabolism. With the structural modification of CUR, the researchers designed CUR analogs with better bioavailability, some of which have comparable and better pharmacodynamic properties than CUR, but toxicological investigations are still lacking. Based on all of the above, it can be concluded that further studies of animal models and humans are needed to verify current knowledge about the application of CUR and its derivatives in the treatment of cardiovascular diseases. In this way through these studies more reliable data will be generated concerning the effects of CUR and its analogs on cellular and subcellular/molecular levels. Prospectively, we believe that this will ground the basis for further improved synthesis of CUR-analogs, appropriate for the treatment of cardiovascular diseases.
Abbreviations
(Ang-II) - Angiotensin II); (CK) - Creatine kinase; (CUR) - Curcumin; (HO-1) - Heme oxygenase-1; (HDL) - High-density lipoprotein; (ICAM-1) - Intercellular adhesion molecule 1; (IL) - Interleukin; (IКBα) - nuclear factor kappa light polypeptide gene enhancer in B-cells, inhibitor alpha; (LDH - Lactate dehydrogenase; (LPL) - Lipoprotein lipase; (LDL) - Low-density lipoprotein; (MMP-9) - Matrix metallopeptidase-9; (MCP-1) - Monocyte chemoattractant protein-1; (NO) - Nitric oxide; (NF-КB) - Nuclear factor-КB; (PPAR-γ) - Peroxisome proliferator-activated receptor γ; (PAF) - Platelet activation factor; (ROS) - Reactive oxygen species; (RAA) - Renin-angiotensin-aldosterone; (SREBP) - Sterol-responsive-element-binding protein; (TNF-α) - Tumor necrosis factor alpha;
Acknowledgments
All authors contributed toward drafting, writing, and revising the paper and agree to be accountable for all aspects of the work. The authors declare that they have no conflicts of interest.
References
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control. In World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization, editors: Shanthi Mendis, Pekka Puska, and Bo Norrving. 2011; pp 3–18.
- Sans, S.; Kesteloot, H.; Kromhout, D. The Burden of Cardiovascular Diseases Mortality in Europe: Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur. Heart J. 1997, 18(8), 1231–1248. DOI: https://doi.org/10.1093/oxfordjournals.eurheartj.a015434.
- Campbell, M. S.; Fleenor, B. S. The Emerging Role of Curcumin for Improving Vascular Dysfunction: A Review. Crit. Rev. Food Sci. Nutr. 2017, 58(16), 2790–2799. DOI: https://doi.org/10.1080/10408398.2017.1341865.
- Kulevanova, S. Sovremena herbalna medicina. Fitoterapija. 2014, 1, 1–18.
- Jahan, N.; Rehman, K.; Doggar, Z. H.; Hameed, M.; Khan, Z. I.; Ahmad, K.; Mukhtar, K.; Valeem, E. E. Cardioprotective Effect of Gemmotherapeutically Treated Withania Somnifera against Chemically Induced Myocardial Injury. Pak. J. Bot. 2010, 42(2), 1487–1499.
- Wu, B. N.; Huang, Y. C.; Wu, H. M.; Hong, S. J.; Chiang, L. C.; Chen, J. A Highly Selective β1-adrenergic Blocker with Partial β2-agonist Activity Derived from Ferulic Acid, an Active Component of Ligusticum Wallichii Franch. J. Cardiovasc. Pharmacol. 1998, 31(5), 750–757. DOI: https://doi.org/10.1097/00005344-199805000-00014.
- Karamati, S. A.; Hassanzadazar, H.; Bahmani, M.; Rafieian-Kopaei, M. Herbal and Chemical Drugs Effective on Malaria. Asian Pacific J Tropical Dis. 2014, 4(2), 599–601. DOI: https://doi.org/10.1016/S2222-1808(14)60686-1.
- Priyadarsini, K. I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules. 2014, 19(12), 20091–20112. DOI: https://doi.org/10.3390/molecules191220091.
- Aggarwal, B.; Kumar, A.; Bharti, A. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23(1), 363–398.
- Ammon, W. M.; Wahl, M. Pharmacology of Curcuma Longa. Planta Med. 1991, 57(1), 1–7. DOI: https://doi.org/10.1055/s-2006-960004.
- Gabrielová, E.; Bartošíková, L.; Nečas, J.; Martin Modrianský, M. Cardioprotective Effect of 2,3-dehydrosilybin Preconditioning in Isolated Rat Heart. Fitoterapia. 2019, 132, 12–21. DOI: https://doi.org/10.1016/j.fitote.2018.10.028.
- Kumar, D. P.; Shirisha, S. D. S. N. Curcumin and Its Biological Importance. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7(2), 1100–1105. DOI: https://doi.org/10.20546/ijcmas.2018.702.137.
- Aggarwal, B. B.; Sundaram, C.; Malani, N.; Curcumin:, I. H. The Indian Solid Gold. Adv. Exp. Med. Biol. 2007, 595, 1–75.
- Kolev, T. M.; Velcheva, E. A.; Stamboliyska, B. A.; Spiteller, M. Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102(6), 1069–1079. DOI: https://doi.org/10.1002/qua.20469.
- Јankun, Ј.; Wyganowska-Świątkowska, M.; Dettlaff, K.; Jelińska, A.; Surdacka, A.; Wątróbska-Świetlikowska, D.; Skrzypczak-Jankun, E. Determining whether Curcumin Degradation/condensation Is Actually Bioactivation. Int.J. Mol. Med. 2016, 37(5), 1151–1158. DOI: https://doi.org/10.3892/ijmm.2016.2524.
- Chainani-Wu, N. Safety and Anti-inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma Longa). J. Altern. Complement. Med. 2003, 9(1), 161–168. DOI: https://doi.org/10.1089/107555303321223035.
- Han, S.; Yang, Y. Antimicrobial Activity of Wool Fabric Treated with Curcumin. Dyes Pigm. 2005, 64(2), 157–161. DOI: https://doi.org/10.1016/j.dyepig.2004.05.008.
- Park, E.; Jeon, C.; Ko, G.; Kim, J.; Sohn, D. Protective Effect of Curcumin in Rat Liver Injury Induced by Carbon Tetrachloride. J. Pharm. Pharmacol. 2000, 52(4), 437–440. DOI: https://doi.org/10.1211/0022357001774048.
- Lin, K.; Chen, H.; Chen, X.; Qian, J.; Huang, S.; Huang, W. Efficacy of Curcumin on Aortic Atherosclerosis: A Systematic Review and Meta-analysis in Mouse Studies and Insights into Possible Mechanisms. Oxid. Med. Cell. Longev. 2020, 1520747. DOI: https://doi.org/10.1155/2020/1520747.
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21(2), 204–222. DOI: https://doi.org/10.2174/092986732102131206115810.
- Ren, J.; Sowers, J. R. Application of a Novel Curcumin Analog in the Management of Diabetic Cardiomyopathy. Diabetes. 2014, 63(10), 3166–3168. DOI: https://doi.org/10.2337/db14-0863.
- Farkhondeh, T.; Samarghandian, S. Antidotal Effects of Curcumin against Agents-induced Cardiovascular Toxicity. Cardiovasc Hematological Disord Drug Targets. 2016, 16(1), 30–33. DOI: https://doi.org/10.2174/1871529X16666160802144510.
- Karuppagounder, V.; Arumugam, S.; Giridharan, V. V.; Sreedhar, R.; Bose, R. J.; Vanama, J.; Palaniyandi, S. S.; Konishi, T.; Watanabe, K.; Thandavarayan, R. A. Tiny Molecule, Big Power: Multi-target Approach for Curcumin in Diabetic Cardiomyopathy. Nutrition. 2017, 34, 47–54. DOI: https://doi.org/10.1016/j.nut.2016.09.005.
- González-Ortega, L. A.; Acosta-Osorio, A. A.; Grube-Pagola, P.; Palmeros-Exsome, C.; Cano-Sarmiento, C.; García-Varela, R.; García, H. S. Anti-inflammatory Activity of Curcumin in Gel Carriers on Mice with Atrial Edema. J. Oleo Sci. 2020, 69(2), 123–131. DOI: https://doi.org/10.5650/jos.ess19212.
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Efficacy, H. H. Safety of Turmeric and Curcumin in Lowering Blood Lipid Levels in Patients with Cardiovascular Risk Factors: A Meta-analysis of Randomized Controlled Trials. Nutr. J. 2017, 16(1), 68. DOI: https://doi.org/10.1186/s12937-017-0293-y.
- Song, H. C.; Chen, Y.; Chen, Y.; Park, J.; Zheng, M.; Surh, Y. J. et al. GSK-3β Inhibition by Curcumin Mitigates Amyloidogenesis via TFEB Activation and Anti-oxidative Activity in Human Neuroblastoma Cells. Free Radic Res. 2020, 54(11-12), 918-930. doi:https://doi.org/10.1080/10715762.2020.1791843.
- Gaze, D. The Cardiovascular System Physiology. Diagnostics and Clinical Implications. Intech Open. 2012, 123–131. DOI: https://doi.org/10.5772/2266
- Bennett, A.; Ruben, J. Endothermy and Activity in Vertebrates. Science. 1979, 206(4419), 649–654. DOI: https://doi.org/10.1126/science.493968.
- Guyton, A. C.; Hall, J. Textbook of Medical Physiology. Elsevier Inc. Philadelphia, Pennsylvania. 2006, 644-768.
- Kojić, Z.; Stojanović, D.; Ristić, S. Holesterol, Citokini I Ateroskleroza. Srce I Krvni Sudovi. 2012, 31(1), 2–6. DOI: https://doi.org/10.5937/siks1201002K.
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as A Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules. 2021, 26(13), 4036. DOI: https://doi.org/10.3390/molecules26134036.
- Frostegård, J. Immunity, Atherosclerosis and Cardiovascular Disease. BMC Med. 2013, 11(1), 117. DOI: https://doi.org/10.1186/1741-7015-11-117.
- Kojić, Z. Animal Models in the Study of Atherosclerosis. Srp Arh Celok Lek. 2003, 131(5–6), 266–270.
- Virchow, R. Cellular Pathology. As Based upon Physiological and Pathological Histology. Lecture XVI-Atheromatous Affection of Arteries. Nutr. Rev. 1989, 47(1), 23–25. DOI: https://doi.org/10.1111/j.1753-4887.1989.tb02747.x.
- Li, C.; Miao, X.; Li, F.; Adhikari, B. K.; Liu, Y.; Sun, J.; Zhang, R.; Cai, L.; Liu, Q.; Wang, Y. et al. Curcuminoids: Implication for Inflammation and Oxidative Stress in Cardiovascular Diseases. Phytother Res. 2019, 33(5), 1302–1317. DOI: https://doi.org/10.1002/ptr.6324.
- Hansson, G. K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352(16), 1685–1695. DOI: https://doi.org/10.1056/NEJMra043430.
- Li, H. Y.; Yang, M.; Li, Z.; Meng, Z. Curcumin Inhibits Angiotensin II-induced Inflammation and Proliferation of Rat Vascular Smooth Muscle Cells by Elevating PPAR-γ Activity and Reducing Oxidative Stress. Int.J. Mol. Med. 2017, 39(5), 1307–1316. DOI: https://doi.org/10.3892/ijmm.2017.2924.
- Cochain, C.; Zernecke, A. Macrophages in Vascular Inflammation and Atherosclerosis”. Pflügers Archiv - Eur J Physiol. 2017, 469(3–4), 485–499. DOI: https://doi.org/10.1007/s00424-017-1941-y.
- Jonasson, L.; Holm, J.; Hansson, G. K. Cyclosporin A Inhibits Smooth Muscle Proliferation in the Vascular Response to Injury.Proc. National Academy Sci. 1988.85;7.2303–2306.https://doi.org/10.1073/pnas.85.7.2303.
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G. K. Regional Accumulations of T Cells, Macrophages, and Smooth Muscle Cells in the Human Atherosclerotic Plaque. Arterioscler., Thromb., Vasc. Biol. 1986, 6(2), 131–138.
- Huang, H. C.; Jan, T. R.; Yeh, S. F. Inhibitory Effect of Curcumin, an Anti-inflammatory Agent, on Vascular Smooth Muscle Cell Proliferation. Eur. J. Pharmacol. 1992, 221(2–3), 381–384.
- Huang, T. S.; Lee, S. C.; Lin, J. K. Suppression of c-Jun/AP-1 Activation by an Inhibitor of Tumor Promotion in Mouse Fibroblast Cells.Proc. National Academy Sci. 1991.88;12.5292–5296.https://doi.org/10.1073/pnas.88.12.5292.
- Huei-Chen, H.; Tong-Rong, J.; Sheau-Farn, Y. Inhibitory Effect of Curcumin, an Anti-inflammatory Agent, on Vascular Smooth Muscle Cell Proliferation. Eur. J. Pharmacol. 1992, 221(2–3), 381–384. DOI: https://doi.org/10.1016/0014-2999(92)90727-L.
- Yu, Y. M.; Lin, H. C. Curcumin Prevents Human Aortic Smooth Muscle Cells Migration by Inhibiting of MMP-9 Expression”. Nutr. Metab. Cardiovasc. Dis. 2010, 20(2), 125–132. DOI: https://doi.org/10.1016/j.numecd.2009.03.001.
- Yang, X.; Thomas, D. P.; Zhang, X.; Culver, B. W.; Alexander, B. M.; Murdoch, W. J.; Rao, M. N. A.; Tulis, D. A.; Ren, J.; Sreejayan, N. Curcumin Inhibits Platelet-derived Growth Factor-stimulated Vascular Smooth Muscle Cell Function and Injury-induced Neointima Formation. Arterioscler Thromb. Vasc. Biol. 2006, 26(1), 85–90. DOI: https://doi.org/10.1161/01.ATV.0000191635.00744.b6.
- Kapakos, G.; Youreva, V.; Srivastava, A. K. Cardiovascular Protection by Curcumin: Molecular Aspects. Indian J. Biochem. Biophys. 2012, 49(5), 306–315.
- Pae, H.; Jeong, G.; Jeong, S.; Kim, H. S.; Kim, S. A.; Kim, Y. C.; Yoo, S. J.; Kim, H. D.; Chung, H. T. Roles of Heme Oxygenase-1 Incurcumin-induced Growth Inhibition in Rat Smooth Muscle Cells. Exp. Mol. Med. 2007, 39(3), 267–277. DOI: https://doi.org/10.1038/emm.2007.30.
- Wongcharoen, W.; Phrommintikul, A. The Protective Role of Curcumin in Cardiovascular Diseases. Int. J. Cardiol. 2009, 133(2), 145–151. DOI: https://doi.org/10.1016/j.ijcard.2009.01.073.
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C. J. Curcumin, an Antioxidant and Anti-inflammatory Agent, Induces Heme Oxygenase-1 and Protects Endothelial Cells against Oxidative Stress. Free Radical Biol. Med. 2000, 28(8), 1303–1312. DOI: https://doi.org/10.1016/S0891-5849(00)00294-X.
- Srivastava, R.; Dikshit, M.; Srimal, R. C.; Dhawan, B. N. Anti-thrombotic Effect of Curcumin. Thromb. Res. 1985, 40(3), 413–417. DOI: https://doi.org/10.1016/0049-3848(85)90276-2.
- Shah, B. H.; Nawaz, Z.; Pertani, S. A.; Roomi, A.; Mahmood, H.; Saeed, S. A.; Gilani, A. H. Inhibitory Effect of Curcumin a Food Spice from Turmeric, on Platelet-activating Factor- and Arachidonic Acid-mediated Platelet Aggregation through Inhibition of Thromboxane Formation and Ca2+ Signaling. Biochem Pharmacol. 1999, 58(7), 1167–1172. DOI: https://doi.org/10.1016/S0006-2952(99)00206-3.
- Mayanglambam, A.; Dangelmaier, C. A.; Thomas, D.; Reddy, D. C.; Daniel, J. L.; Kunapuli, S. P. Curcumin Inhibits GPVI-mediated Platelet Activation by Interfering with the Kinase Activity of Syk and the Subsequent Activation of PLC-gamma-2. Platelets. 2010, 21(3), 211–220. DOI: https://doi.org/10.3109/09537100903528269.
- Prakash, P.; Misra, A.; Surin, W. R.; Jain, M.; Bhatta, R. S.; Pal, R.; Raj, K.; Barthwal, M. K.; Dikshit, M. Anti-platelet Effects of Curcuma Oil in Experimental Models of Myocardial Ischemia-reperfusion and Thrombosis. Thromb. Res. 2011, 127(2), 11–18. DOI: https://doi.org/10.1016/j.thromres.2010.11.007.
- Mahfouz, M. M.; Zhou, S. Q.; Kummerow, F. A. Curcumin Prevents the Oxidation and Lipid Modification of LDL and Its Inhibition of Prostacyclin Generation by Endothelial Cells in Culture. Prostaglandins Other Lipid Mediat. 2009, 90(1–2), 13–20. DOI: https://doi.org/10.1016/j.prostaglandins.2009.06.005.
- Ramirez-Tortosa, M. C.; Mesa, M. D.; Aguilera, M. C.; Quiles, J. L.; Baro, L.; Ramirez-Tortosa, C. L.; Victoria, E. M.; Gil, A. Oral Administration of a Turmeric Extract Inhibits LDL Oxidation and Has Hypocholesterolemic Effects in Rabbits with Experimental Atherosclerosis. Atherosclerosis. 1999, 147(2), 371–378. DOI: https://doi.org/10.1016/S0021-9150(99)00207-5.
- Quiles, J. L.; Mesa, M. D.; Ramirez-Tortosa, C. L.; Aguilera, C. M.; Battino, M.; Gil, A.; Ramrez-Tortosa, M. C. Curcuma Longa Extract Supplementation Reduces Oxidative Stress and Attenuates Aortic Fatty Streak Development in Rabbits. Arterioscler Thromb. Vasc. Biol. 2002, 22(7), 1225–1231.
- Olszanecki, R.; Jawien, J.; Gajda, M.; Mateuszuk, L.; Gebska, A.; Korabiowska, M.; Chłopicki, S.; Korbut, R. Effect of Curcumin on Atherosclerosis in apoE/LDLR-double Knockout Mice. J Phys Pharmacol. 2005, 56(4), 627–635.
- Yuan, H. Y.; Kuang, S. Y.; Zheng, X.; Ling, H. Y.; Yang, Y. B.; Yan, P. K.; Li, P. K. L.; Liao, D. F. Curcumin Inhibits Cellular Cholesterol Accumulation by Regulating SREBP-1/caveolin-1 Signaling Pathway in Vascular Smooth Muscle Cells. Acta Pharmacol. Sin. 2008, 29(5), 555–563. DOI: https://doi.org/10.1111/j.1745-7254.2008.00783.x.
- Vrhovac, B.; Jakšić, B.; Reiner, Ž.; Vucelić, B. Interna medicina. Naklada Ljevak, Zagreb. 2008, 84-116. pp.124.
- Lee, Y.; Im, E. Regulation of miRNAs by Natural Antioxidants in Cardiovascular Diseases: Focus on SIRT1 and eNOS. Antioxidants (Basel). 2021, 10(3), 377. DOI: https://doi.org/10.3390/antiox10030377.
- Crews, D. E.; Williams, S. R. Molecular Aspects of Blood Pressure Regulation. Hum Biol. 1999, 71(4), 475–503.
- Garbers, D. L.; Dubois, S. K. The Molecular Basis of Hypertension. Annu. Rev. Biochem. 1999, 68(1), 127–155. DOI: https://doi.org/10.1146/annurev.biochem.68.1.127.
- Leong, X. F. The Spice for Hypertension: Protective Role Of. Curcuma Longa. Biomed Pharmacol J. 2018, 11(4), 1829–1840. DOI: https://doi.org/10.13005/bpj/1555.
- De Miguel, C.; Rudemiller, N. P.; Abais, J. M.; Mattson, D. L. Inflammation and Hypertension: New Understandings and Potential Therapeutic Targets. Curr. Hypertens. Rep. 2014, 17(1), 501. DOI: https://doi.org/10.1007/s11906-014-0507-z.
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R. J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97(3), 1127–1164. DOI: https://doi.org/10.1152/physrev.00031.2016.
- Brush, J.; Faxon, D.; Salmon, S.; Jacobs, A.; Rugan, T. Abnormal Endothelium-dependent Coronary Vasomotion in Hypertensive Patients. J. Am. Coll. Cardiol. 1992, 19(4), 809–815. DOI: https://doi.org/10.1016/0735-1097(92)90522-O.
- Li, H.; Sureda, A.; Devkota, P. H.; Pittalà, V.; Barreca, D.; Silva, S. A.; Tewari, D.; Xu, S.; Nabavi, M. S. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. DOI: https://doi.org/10.1016/j.biotechadv.2019.01.01067.
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules. 2016, 21(3), 264. DOI: https://doi.org/10.3390/molecules21030264.
- Fuente, M. D. L.; Hernanz, A.; Vallejo, M. C. The Immune System in the Oxidative Stress Conditions of Aging and Hypertension: Favorable Effects of Antioxidants and Physical Exercise. Antioxid. Redox Signaling. 2005, 7(9–10), 1356–1366. DOI: https://doi.org/10.1089/ars.2005.7.1356.
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S. E. Curcumin Improves Endothelial Dysfunction and Vascular Remodeling in 2K-1C Hypertensive Rats by Raising Nitric Oxide Availability and Reducing Oxidative Stress. Nitric Oxide. 2014, 42, 44–53. DOI: https://doi.org/10.1016/j.niox.2014.09.001.
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and Vascular Protective Effects of Curcumin and Tetrahydrocurcumin in Rats with L-NAME-induced Hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011, 383(5), 519–529. DOI: https://doi.org/10.1007/s00210-011-0624-z.
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.R. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria and Systolic Blood Pressure in Patients Suffering from Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-controlled Study. J. Ren. Nutr. 2011, 22(1), 50–57.
- Ghosh, S. S.; Salloum, F. N.; Abbate, A.; Krieg, R.; Sica, D. A.; Gehr, T. W.; Kukreja, R. C. Curcumin Prevents Cardiac Remodeling Secondary to Chronic Renal Failure through Deactivation of Hypertrophic Signaling in Rats. Am. J. Physiol. Heart. Circ. Physiol. 2010, 299(4), 975–984. DOI: https://doi.org/10.1152/ajpheart.00154.2010.
- Dokken, B. B. The Pathophysiology of Cardiovascular Disease and Diabetes: Beyond Blood Pressure and Lipids. Diabetes Spectr. 2008, 21(3), 160–165. DOI: https://doi.org/10.2337/diaspect.21.3.160.
- Fagot-Campagna, A.; Pettitt, D. J.; Engelgau, M. M.; Burrows, N. R.; Geiss, L. S.; Valdez, R.; Beckles, G. L. A.; Saaddine, J.; Gregg, E. W.; Williamson, D. F. et al. Type 2 Diabetes among North Adolescents: An Epidemiologic Health Perspective. J. Pediatr. 2000, 136(5), 664–672. DOI: https://doi.org/10.1067/mpd.2000.105141.
- Chan, A. C. Vitamin E and Atherosclerosis. J. Nutr. 1998, 128(10), 1593–1596. DOI: https://doi.org/10.1093/jn/128.10.1593.
- Koh, K. K.; Han, S. H.; Quon, M. J. Inflammatory Markers and the Metabolic Syndrome. J. Am. Coll. Cardiol. 2005, 46(11), 1978–1985. DOI: https://doi.org/10.1016/j.jacc.2005.06.082.
- Williams, S. B.; Goldfine, A. B.; Timimi, F. K.; Ting, H. H.; Roddy, M. A.; Simonson, D. C.; Creager, M. A. Acute Hyperglycemia Attenuates Endothelium-dependent Vasodilation in Humans. In Vivo. Circulation. 1998, 97(17), 1695–1701. DOI: https://doi.org/10.1161/01.CIR.97.17.1695.
- Woods, М.; Mitchell, Ј. А.; Wood, Е. G.; Barker, S.; Walcot, N. R.; Rees, G. M.; Warner, T. D. Endothelin-1 Is Induced by Cytokines in Human Vascular Smooth Muscle Cells: Evidence for Intracellular Endothelin-converting Enzyme. Mol. Pharmaco. 1999, 55(5), 902–909.
- Srinivasan, M. Effect of Curcumin on Blood Sugar as Seen in a Diabetic Subject. Indian J. Med. Sci. 1972, 26(4), 269–270.
- Sahebkar, А. “Why It Is Necessary to Translate Curcumin Intoclinical Practice for the Prevention and Treatment of Metabolic Syndrome. BioFactors. 2013, 39(2), 197–208. DOI: https://doi.org/10.1002/biof.1062.
- Zhang, D.; Fu, M.; Gao, S. H.; Liu, J. L. Curcumin and Diabetes: Evidence-Based Complementary and Alternative Medicine, 2013, 636053. https://doi.org/10.1155/2013/636053.
- Pari, L.; Murugan, P. Tetrahydrocurcumin Prevents Brain Lipid Peroxidation in Streptozotocin-induced Diabetic Rats. J. Med. Food. 2007, 10(2), 323–329. DOI: https://doi.org/10.1089/jmf.2006.058.
- Arun, N.; Nalini, N. “Efficacy of Turmeric on Blood Sugar and Polyol Pathway in Diabetic Albino Rats. Plant Foods Human Nutr. 2002, 57(1), 41–52. DOI: https://doi.org/10.1023/A:1013106527829.
- Murugan, P.; Pari, L. Influence of Tetrahydrocurcumin Onhepatic and Renal Functional Markers and Protein Levels Inexperimental Type 2 Diabetic Rats. Basic Clin Pharma-cology Toxicol. 2007, 101(4), 241–245. DOI: https://doi.org/10.1111/j.1742-7843.2007.00109.x.
- Nishiyama, T.; Tatsumasa, M.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K. et al. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma Longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Amice. J. Agric. Food Chem. 2005, 53(4), 959–963. DOI: https://doi.org/10.1021/jf0483873.
- Patumraj, S.; Wongeakin, N.; Sridulyakul, P.; Jariyapongskul, A.; Futrakul, N.; Bunnag, S. Combined Effects of Curcumin and Vitamin C to Protect Endothelial Dysfunction in the Iris Tissue of STZ-induced Diabetic Rats. Clin. Hemorheol. Microcirc. 2006, 35(4), 481–489.
- Farhangkhoee, H.; Khan, Z. A.; Chen, S.; Chakrabarti, S. Differential Effects of Curcumin on Vasoactive Factors in the Diabetic Rat Heart. Nutr. Metab. 2006, 3(1), 27. DOI: https://doi.org/10.1186/1743-7075-3-27.
- El-Azab, M. F.; Attia, F. M.; El-Mowafy, A. M. Novel Role of Curcumin Combined with Bone Marrow Transplantation in Reversing Experimental Diabetes: Effects on Pancreatic Islet Regeneration, Oxidative Stress, and Inflammatory Cytokines. Eur. J. Pharmacol. 2011, 658(1), 41–48. DOI: https://doi.org/10.1016/j.ejphar.2011.02.010.
- Soetikno, V.; Sari, F. R.; Veeraveedu, P. T.; Thandavarayan, R. A.; Harima, M.; Sukumaran, V.; Lakshmanan, A. P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin Ameliorates Macrophage Infiltration by Inhibiting NF-κB Activation and Proinflammatory Cytokines in Streptozotocin Induced-diabetic Nephropathy. Nutr. Metab. 2011, 8(1), 35. DOI: https://doi.org/10.1186/1743-7075-8-35.
- Chan, M. M. Inhibition of Tumor Necrosis Factor by Curcumin, a Phytochemical. Biochem Pharmacol. 1995, 49(11), 1551–1556. DOI: https://doi.org/10.1016/0006-2952(95)00171-U.
- Jain, S. K.; Rains, J.; Croad, J.; Larson, B.; Jones, B. Curcumin Supplementation Lowers THF-α, IL-6, IL-8, and MCP-1 Secretion in High Glucose-treated Cultured Monocytes and Blood Levels of THF-α, IL-6, MCP-1, Glucose, and Glycosylated Haemoglobin in Diabetic Rats. Antioxid. Redox Signal. 2009, 11(2), 241–249. DOI: https://doi.org/10.1089/ars.2008.2140.
- Seo, K. I.; Choi, M. S.; Jung, U. J.; Kim, H. J.; Yeo, J.; Jeon, S. M.; Lee, M. K. Effect of Curcumin Supplementation on Blood Glucose, Plasma Insulin, and Glucose Homeostasis Related Enzyme Activities in Diabetic Db/db Mice. Mol. Nutr. Food Res. 2008, 52(9), 995–1004. DOI: https://doi.org/10.1002/mnfr.200700184.
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin Supplementation Could Improve Diabetes-induced Endothelial Dysfunction Associated with Decreased Vascular Superoxide Production and PKC Inhibition. BMC Complement. Altern. Med. 2010, 10(1), 57. DOI: https://doi.org/10.1186/1472-6882-10-57.
- Nishizono, S.; Hayami, T.; Ikeda, I.; Imaizumi, K. Protection against the Diabetogenic Effect of Feeding Tert-butylhydro-quinone to Rats Prior to the Administration of Streptozotocin. Biosci. Biotechnol. Biochem. 2000, 64(6), 1153–1158. DOI: https://doi.org/10.1271/bbb.64.1153.
- Majithiya, J. B.; Balaraman, R. Time-dependent Changes in Antioxidant Enzymes and Vascular Reactivity of Aorta in Streptozotocin-induced Diabetic Rats Treated with Curcumin. J. Cardiovasc. Pharmacol. 2005, 46(5), 697–705. DOI: https://doi.org/10.1097/01.fjc.0000183720.85014.24.
- Chatterjee, P.; Joynt Maddox, K. E. U. S. National Trends in Mortality from Acute Myocardial Infarction and Heart Failure. JAMA Cardiol. 2018, 3(4), 336–340. DOI: https://doi.org/10.1001/jamacardio.2018.0218.
- Jefferson, B.; Topol, E. Molecular Mechanisms of Myocardial Infarction. Curr. Problems Cardiol. 2005, 30(7), 333–374. DOI: https://doi.org/10.1016/j.cpcardiol.2005.02.002.
- Frangogiannis, N. G. The Inflammatory Response in Myocardial Injury, Repair and Remodeling. Nat. Rev. Cardiol. 2014, 11(5), 255–265. DOI: https://doi.org/10.1038/nrcardio.2014.28.
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory Cytokines and Postmyocardial Infarction Remodeling. Circ. Res. 2004, 94(12), 1543–1553. DOI: https://doi.org/10.1161/01.RES.0000130526.20854.fa.
- Sacco, R. L.; Adams, R.; Albers, G.; Alberts, M. J.; Benavente, O.; Furie, K.; Goldstein, L. B.; Gorelick, P.; Halperin, J.; Harbaugh, R.; et al. Guidelines for Prevention of Stroke in Patients with Ischemic Stroke or Transient Ischemic Attack: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-sponsored by the Council on Cardiovascular Radiology and Intervention: The American Academy of Neurology Affirms the Value of This Guideline. Stroke. 2006, 37(2), 577–617.
- Atherosclerosis, R. R.; Epstein, F. H. - an Inflammatory Disease. N. Engl. J. Med. 1999, 340(2), 115–126. DOI: https://doi.org/10.1056/NEJM199901143400207.
- Rossini, R.; Tarantini, G.; Musumeci, G.; Masiero, G.; Barbato, E.; Calabrò, P.; Capodanno, D.; Leonardi, S.; Lettino, M.; Limbruno, U. et al. A Multidisciplinary Approach on the Perioperative Antithrombotic Management of Patients with Coronary Stents Undergoing Surgery. JACC: Cardiovascular Interventions. 2018, 11(5), 417–434.
- Rabito, M. J.; Kaye, A. D. Complementary and Alternative Medicine and Cardiovascular Disease: An Evidence-based Review. Evid. Based Complement. Altern. Med. 2013, 10, 672097. DOI: https://doi.org/10.1155/2013/672097.
- Rahnavard, M.; Hassanpour, M.; Ahmadi, M.; Heidarzadeh, M.; Amini, H.; Javanmard, M.; Nouri, M.; Rahbarghazi, R.; Safaie, N. Curcumin Ameliorated Myocardial Infarction by Inhibition of Cardiotoxicity in the Rat Model. J. Cell. Biochem. 2019, 120(7), 11965–11972. DOI: https://doi.org/10.1002/jcb.28480.
- Hong, D.; Zeng, X.; Xu, W.; Ma, J.; Tong, Y.; Chen, Y. Altered Profiles of Gene Expression in Curcumin-treated Rats with Experimentally Induced Myocardial Infarction. Pharmacol. Res. 2010, 61(2), 142–148. DOI: https://doi.org/10.1016/j.phrs.2009.08.009.
- Lv, F.; Yin, H.; He, Y.; Wu, H. M.; Kong, J.; Chai, X. Y.; Zhang, S. R. Effects of Curcumin on the Apoptosis of Cardiomyocytes and the Expression of NF-κB, PPAR-γ and Bcl-2 in Rats with Myocardial Infarction Injury. Exp. Ther. Med. 2016, 12(6), 3877–3884. DOI: https://doi.org/10.3892/etm.2016.3858.
- Garvin, A.; Jackson, M.; Korzick, D. Inhibition of Programmed Necrosis Limits Infarct Size through Altered Mitochondrial and Immune Responses in the Aged Female Rat Heart. Am. J. Physiol. Heart. Circ. Physiol. 2018, 315(5), 1434‐1442. DOI: https://doi.org/10.1152/ajpheart.00595.2017.
- Valen, G.; Yan, Z. Q.; Hansson, G. K. Nuclear Factor kappa-B and the Heart. J. Am. Coll. Cardiol. 2001, 38(2), 307–314. DOI: https://doi.org/10.1016/S0735-1097(01)01377-8.
- Mariappan, N.; Elks, C. M.; Sriramula, S.; Guggilam, A.; Liu, Z.; Borkhsenious, O.; Francis, J. NF-κB-induced Oxidative Stress Contributes to Mitochondrial and Cardiac Dysfunction in Type II Diabetes. Cardiovasc. Res. 2010, 85(3), 473–483. DOI: https://doi.org/10.1093/cvr/cvp305.
- Lv, Y. C.; Tang, Y. Y.; Zhang, P.; Wan, W.; Yao, F.; He, P. P.; Xie, W.; Mo, Z.-C.; Shi, J.-F.; Wu, J.-F. et al. Histone Methyltransferase Enhancer of Zeste Homolog 2-mediated ABCA1 Promoter DNA Methylation Contributes to the Progression of Atherosclerosis. PloS One.2016, 11(6), e0157265. DOI: https://doi.org/10.1371/journal.pone.0157265.
- Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Novel Therapeutic Approaches to Post-infarction Remodeling. Cardiovasc. Res. 2012, 94(2), 293–303. DOI: https://doi.org/10.1093/cvr/cvs109.
- Liu, K.; Chen, H.; You, Q. S.; Ye, Q.; Wang, F.; Wang, S.; Zhang, S. L.; Yu, K. J.; Lu, Q. (2017). Curcumin Attenuates Myocardial Ischemia-reperfusion Injury. Oncotarget. 2017, 8(67), 112051–112059.
- Saeidinia, A.; Keihanian, F.; Butler, A. E.; Bagheri, R. K.; Atkin, S. L.; Sahebkar, A. Curcumin in Heart Failure: A Choice for Complementary Therapy. Pharmacol. Res. 2018, 131, 112–119. DOI: https://doi.org/10.1016/j.phrs.2018.03.009.
- Yeh, C. H.; Chen, T. P.; Wu, Y. C.; Lin, Y. M.; Lin, P. J. Inhibition of NF-kappa-B Activation with Curcumin Attenuates Plasma Inflammatory Cytokines Surge and Cardiomyocytic Apoptosis following Cardiac Ischemia/reperfusion1. J. Surg. Res. 2005, 125(1), 109–116. DOI: https://doi.org/10.1016/j.jss.2004.11.009.
- Kim, Y. S.; Kwon, J. S.; Cho, Y. K.; Jeong, M. H.; Cho, J. G.; Park, J. C.; Kang, J. C.; Ahn, Y. Curcumin Reduces the Cardiac Ischemia-reperfusion Injury: Involvement of the Toll-like Receptor 2 in Cardiomyocytes. J. Nutr. Biochem. 2012, 23(11), 1514–1523. DOI: https://doi.org/10.1016/j.jnutbio.2011.10.004.
- Wang, N. P.; Pang, X. F.; Zhang, L. H.; Tootle, S.; Harmouche, S.; Zhao, Z. Q. Attenuation of Inflammatory Response and Reduction in Infarct Size by Postconditioning are Associated with Downregulation of Early Growth Response 1 during Reperfusion in Rat Heart. Shock. 2014, 41(4), 346–354. DOI: https://doi.org/10.1097/SHK.0000000000000112.
- Epstein, J.; Sanderson, I. R.; Macdonald, T. T. Curcumin as a Therapeutic Agent: The Evidence from in Vitro, Animal and Human Studies. Br. J. Nutr. 2010, 103(11), 1545–1557. DOI: https://doi.org/10.1017/S0007114509993667.
- Ovbiagele, B. Potential Role of Curcumin in Stroke Prevention. Expert Rev. Neurother. 2008, 8(8), 1175–1176. DOI: https://doi.org/10.1586/14737175.8.8.1175.
- Strimpakos, A.; Sharma, R. A. Curcumin: Preventive and Therapeutic Properties in Laboratory Studies and Clinical Trials. Antioxid. Redox. Signal. 2008, 10(3), 511–545. DOI: https://doi.org/10.1089/ars.2007.1769.
- Ringman, J. M.; Frautschy, S. A.; Teng, E.; Begum, A. N.; Bardens, J.; Beigi, M.; Gylys, K. H.; Badmaev, V.; Heath, D. D.; Apostolova, L. G. et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-week Randomized, Double Blind, Placebo-controlled Study. Alzheimers Res. Ther.2012, 4(5), 43. DOI: https://doi.org/10.1186/alzrt146.
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64(4), 353–356. DOI: https://doi.org/10.1055/s-2006-957450.
- Anand, P.; Kunnumakkara, A. B.; Newman, R. A.; Aggarwal, B. B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4(6), 807–818. DOI: https://doi.org/10.1021/mp700113r.
- Payton, F.; Sandusky, P.; Alworth, W. L. NMR Study of the Solution Structure of Curcumin. J. Nat. Prod. 2007, 70(2), 143–146. DOI: https://doi.org/10.1021/np060263s.
- Kidd, P. M. Bioavailability and Activity of Phytosome Complexes from Botanical Polyphenols: The Silymarin, Curcumin, Green Tea, and Grape Seed Extracts. Altern. Med. Rev. 2009, 14, 226–246.
- Jurenka, J. S. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14(2), 141–153.
- Zhao, C.; Liu, Z.; Liang, G. Promising Curcumin-based Drug Design: Mono-carbonyl Analogues of Curcumin (Macs). Curr. Pharm. Des. 2013, 19(11), 2114–2135.
- Liang, G.; Shao, L.; Wang, Y.; Zhao, Y.; Chu, J.; Xiao, Y.; Zhao, Y.; Li, X.; Yang, S. Yang., S.; Exploration and Synthesis of Curcumin Analogues with Improved Structural Stability Both in Vitro and in Vivo as Cytotoxic Agents. Bioorg. Med. Chem. 2009, 17(6), 2623–2631. DOI: https://doi.org/10.1016/j.bmc.2008.10.044.
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X. et al. Inhibition of High Glucose-induced Inflammatory Response and Macrophage Infiltration by a Novel Curcumin Derivative Prevents Renal Injury in Diabetic Rats. Br. J. Pharmacol. 2012, 166(3), 1169–1182. DOI: https://doi.org/10.1111/j.1476-5381.2012.01854.x.
- Li, C.; Miao, X.; Wang, S.; Adhikari, B. K.; Wang, X.; Sun, J.; Wang, Y. Novel Curcumin C66 that Protects Diabetes-induced Aortic Damage Was Associated with Suppressing JNK2 and Upregulating Nrf2 Expression and Function. Oxid. Med. Cell. Longev. 2018, 5783239. DOI: https://doi.org/10.1155/2018/5783239.
- Pan, Y.; Huang, Y.; Wang, Z.; Fang, Q.; Sun, Y.; Tong, C.; Peng, K.; Wang, Y.; Miao, L.; Cai, L. et al. Inhibition of MAPK-mediated ACE Expression by Compound C66 Prevents STZ-induced Diabetic Nephropathy. J. Cell Mol. Med. 2014, 18(2), 231–234. DOI: https://doi.org/10.1111/jcmm.12175.
- Qian, Y.; Zhong, P.; Liang, D.; Xu, Z.; Skibba, M.; Zeng, C.; Li, X.; Wei, T.; Wu, L.; Liang, G. A Newly Designed Curcumin Analog Y20 Mitigates Cardiac Injury via Anti-inflammatory and Anti-oxidant Actions in Obese Rats. PLOS ONE. 2015, 10(3), 1–16. DOI: https://doi.org/10.1371/journal.pone.0120215.
- Kupper, F. C.; Carpenter, L. J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B. H.; Robinson, J.; Verhaeghe, E. F.; Malin, G.; Luther, G. W.;; et al. In Vivo Speciation Studies and Antioxidant Properties of Bromine in Laminaria Digitata Reinforce the Significance of Iodine Accumulation for Kelps. J. Exp. Bot. 2013, 64(10), 2653–2664.
- Hadzi-Petrushev, N.; Bogdanov, J.; Krajoska, J.; Ilievska, J.; Bogdanova-Popov, B.; Gjorgievska, E.; Mitrokhin, V.; Sopi, R.; Gagov, H.; Kamkin, A.;; et al. Comparative Study of the Antioxidant Properties of Monocarbonyl Curcumin Analogues C66 and B2BrBC in Isoproteranol Induced Cardiac Damage. Life Sci. 2018, 197, 10–18. DOI: https://doi.org/10.1016/j.lfs.2018.01.028.
- Hadzi-Petrushev, N.; Angelovski, M.; Rebok, K.; Mitrokhin, V.; Kamkin, A.; Mladenov, M. Protective Effects of the Monocarbonyl Curcumin Analogues B2BRBC and C66 in Monocrotaline-induced Right Ventricular Hypertrophy. J. Biochem. Mol. Toxicol. 2019, 33(8), e22353. DOI: https://doi.org/10.1002/jbt.22353.