ABSTRACT
A simultaneous analysis method using LC-MS/MS and GC-MS/MS was developed for improving the analysis accuracy of various pesticide residues. Samples spiked with 247 and 34 pesticide residues were analyzed by LC-MS/MS and GC-MS/MS, respectively. The method was verified by measuring sensitivity, linearity, selectivity, precision, and accuracy. LC-MS/MS LOD and LOQ values were determined to be 0.03–0.5 μg/kg, and 0.6–1.5 μg/kg, respectively, while the corresponding values for GC-MS/MS were found to be 0.9–2.0 μg/kg and from 3.0–5.7 μg/kg, respectively. Most of the 34 common residues had recovery rates of 70–120%. The results showed that the developed method can be a reliable and more accurate than the general multi component analysis method.
Introduction
The use of pesticides in agriculture has become unavoidable as technology becomes increasingly specialized, as they increase productivity. However, the increasing use of pesticides threatens the environment and human and animal health when directly exposed.[Citation1] Pesticides enter the body through various channels, such as oral intake through food and water, as well as absorption through the skin. The degree of the adverse impact of exposure to pesticides depends on the exposure duration and quantity.[Citation2] Long-term exposure to pesticides has been associated with diseases, such as multiple sclerosis and cancer, as well as various chronic conditions.[Citation3] In addition, when the pesticide involved is highly complex, the associated toxicity may be much worse owing to interactions between substances.[Citation4] Hence, methods for analyzing pesticides should be continuously researched and developed to ensure that agricultural products are safe.
Sweet pepper (Capsicum annuum) contains a large quantity of beneficial compounds, including carotenoids and phenolics. Sweet pepper has recently been reported to show anti-cancer effects against some tumors and antioxidant effects against some specific chronic conditions.[Citation5] Various factors, such as the pH and lipid contents of vegetables and fruits, can affect the analysis of pesticide residues. Analysis is also affected by the matrix effect of pigments, such as the green chlorophyll pigment.[Citation6] Therefore, fruits and vegetables need to be suitably pretreated before being analyzed.
QuEChERS is one such pretreatment method; it is a simple and rapid pretreatment method that uses a highly sensitive mass spectrometer and is mainly used for multiclass and multi residue analyses.[Citation7–9] Contrary to the existing pesticide residue analysis methods, the QuEChERS method can easily be developed and used to analyze pesticide metabolites.[Citation10–12] Many global studies are directed toward the development of further methods for the analysis of pesticide multi residues by the simultaneous application of GC/MS/MS and LC/MS/MS.[Citation13]
Various methods can be used to analyze pesticide residues, such as thin-layer chromatography (TLC)[Citation14] and mass spectrometry[Citation15];however, simultaneous multi residue analysis is chiefly conducted using LC and GC coupled with mass spectrometry.[Citation16,Citation17] Interestingly, more than 200 types of multi residue can be analyzed using a single instrument. GC-MS/MS is mainly used for such analysis because it advantageously minimizes signal interference from a complex sample matrix[Citation18] and can separate highly lipophilic substances, such as chlorothalonil and endosulfan.[Citation13] Some recently introduced pesticides have polar components; in addition, they are thermolabile and difficult to volatilize. Consequently, LC-MS/MS is used for such pesticides because it is difficult to accurately analyze them by high-temperature GC.[Citation19] However, very few studies have investigated simultaneous analysis by considering the characteristics of both types of instrument. Therefore, a simultaneous analysis method that uses both types of instrument needs to be developed.
We hypothesized that the simultaneous use of LC/MS/MS and GC/MS/MS can be used more accurately analyze various pesticide residues. In this study, we established efficient qualitative and quantitative methods for analyzing pesticide residues in sweet pepper. The method involved first pretreating the sweet pepper by QuEChERS and then comparing the results obtained by each analysis method. Effectiveness was also verified by applying and monitoring the relevant experimental method.
Materials and methods
Samples
Sweet pepper was purchased directly from a local market in Busan (South Korea). Forty samples of sweet pepper were used. All samples were stored at −4°C.
Chemicals and reagents
A total of 247 pesticide residues, used as analysis standards, were purchased from AccuStandard (New Haven, USA). Formic acid (98%) and ammonium formate (99.995%) were purchased from Sigma–Aldrich (Steinheim, Germany). Acetonitrile, used for sample extraction and purification, and Lichrosolv®, used as a solvent, were purchased from Merck KGaA(Darmstadt, Germany).The QuEChERS Extraction Kit (magnesium sulfate: 98.5–101.5%; sodium chloride: ≥99.5%; sodium citrate: 99.9%; disodium citrate sesquihydrate: 99%) and 2 mL of QuEChERS dispersive SPE (primary secondary amine (PSA), octadecysilane end-capped, magnesium sulfate; 98.5–101.5%) used for purification was obtained from Agilent (Boblingen, Germany).
Pretreatment method (sample preparation)
The sample pretreatment method is shown in Fig. S1. The sample was first homogenized using a grinder (T 25 digital ULTRA-TURRAX®, IKA), and the resulting sample was used as the blank sample. After weighing 10 g of the pulverized sample, 10 mL of acetonitrile was added to each weighed sample and shaken for 1 min. Thereafter, 4 g of anhydrous magnesium sulfate, 1 g of sodium chloride, 1 g of sodium citrate, and 0.5 g of disodium citrate sesquihydrate were added to the sample solution, followed by vigorous shaking for 1 min using a rotary mixer (DE/VIVA, Collomix). Subsequently, centrifugation was performed for 5 min at 3,700 rpm using a 5920 R centrifuge (Eppendorf). For LC-MS/MS analysis, the centrifuged supernatant was filtered through a 0.2-μm syringe filter (Whatman, PTFE) and used as the test solution. For GC-MS/MS analysis, 1 mL of the supernatant was used with 25 mg PSA and 150 mg MgSO4. The supernatant was placed in a dispersive tube filled with SPE and shaken for 1 min, after which it was centrifuged at 12,000 rpm for 1 min and filtered through a 0.2-μm syringe filter (Whatman, PTFE).
Standard solution preparation
Individual standard solutions of 247 pesticide residues were prepared in ACN at a concentration of 1000–10000 μg/L. Working solutions (10–100 μg/L) were prepared by diluting the stock solution with a blank sample. The matrix matching calibration standard solution was prepared by mixing the matrix matching working standard solution with an additional blank sample extract to reach a multi compound concentration of 0.005–1 mg/L. All standard solutions were stored in glass bottles at −20°C.
The method used to prepare calibration curves for standard substances matched with metrics. Concentrations of 1–20 μg/L were used, and each calibration curve was prepared using five points. The interference effect was reduced by diluting the concentration by a factor of 10 for sample analysis. The preparation of GC-matched standard Calibration curves were obtained using five points (5–100 μg/L). The medium tends to interfere less in GC than LC; therefore, GC experiments were conducted without dilution.
Instrument analysis conditions
The analysis conditions for the LC-MS/MS and GC-MS/MS instruments are shown in . A Triple Quad 4500 instrument (AB Sciex) was used for LC-MS/MS, with Multiquiant 3.0.2 software used for data processing. A GCMS-TQ8050 instrument (Shimazu) was used for GC-MS/MS, with Lab Solution Insight software used for data processing.
Table 1. Analytical conditions of LC-MS/MS and GC-MS/MS
Method validation
Method validation was performed following the guidelines set by the European Commission,[Citation20] the International Conference on Harmonization,[Citation21] the International Union of Pure and Applied Chemistry,[Citation22] EURACHEM guidelines,[Citation23] and the Guide to the Expression of Uncertainty in Measurement.[Citation24] Methods were validated for linearity, sensitivity, selectivity, accuracy, precision, and measurement uncertainty.
Results and discussion
Simultaneous multi component analysis
shows the LC-MS/MS multiple reaction monitoring (MRM) results for 247 pesticide residues found in sweet pepper. The MRM results for 34 residues subjected to GC analysis are shown in . Quantitative analysis of the residues was carried out within the 162.9–890.4 Da range for LC and the 121.0–354.0 m/z range for GC. Qualitative residues analysis was conducted within the 51.1–567.3 Da range for LC and the 72.10–335.0 m/z range for GC. In the case of GC, the accuracy of material analysis improved when several reference ions were used for a specific material.
Table 2. Parameters for the analysis of 247 pesticide residues using LC-MS/MS
Table 3. Parameters for the analysis of 34 pesticide residues using GC-MS/MS
Method validation
The developed LC-MS/MS and GC-MS/MS analysis methods were verified for sensitivity, linearity, selectivity, precision and accuracy to confirm their effectiveness for the analysis of pesticide residues in sweet pepper (). The matrix effect (ME), which affects recovery-rate measurements, can result from various factors, such as equipment conditions and solvent composition, and is known to interfere with the detection of specific pesticides. The ME for sweet pepper was found to be less than 20% at the 0.25 mg/kg level; hence, the ME does not significantly affect the detection of pesticide residues.[Citation19]
Table 4. Uncertainty of measurement of sample weight and final volume
Table 5. Validation parameters of the developed GC-MS/MS method
The sensitivity of the developed method was evaluated by determining the limits of detection (LOD) and quantification (LOQ). The LOD and LOQ values were determined from the response and slope of each regression equation at signal-to-noise ratios (S/N) of 3:1 and 10:1, respectively, under chromatographic conditions. The LOD values for LC-MS/MS and GC-MS/MS were found to be 0.03–0.5 μg/kg and 0.9–2.01 μg/kg, respectively, while the LOQ values were determined to be 0.6–1.5 μg/kg and 3.0–5.7 μg/kg, respectively. More details are found in .
Linearity was evaluated by constructing external calibration curves using the standard mixture of each residue. Calibration curves were obtained from analyte peak are as determined at five concentrations (LC: 1, 2.5, 5, 10, and 20 μg/L, GC: 5, 10,25, 50, and 100 μg/L). The respective concentration of each mixed standard solution was injected in triplicate, with values of the regression parameters calculated from the results. Coefficients of determination (R2 > 0.99) were obtained for all the compounds studied. These results reveal that external standard calibration can be used for quantitative purposes.
Selectivity was determined by the presence or absence of interfering peaks in the chromatography window. As shown in , 247 pesticide residues were observed within 30 min of sample injection. All identified peaks showed satisfactory selectivities and were successfully separated. Thus, we confirmed LC-MS/MS and GC-MS/MS selectivity. Method precision was then determined by measuring intra- and interday precision values. For intraday precision, solutions of pesticide residues were analyzed three times within one day, while for interday precision, solutions were examined in triplicate on three consecutive days. Precision is expressed as the percentage of the relative standard deviation (%RSD). The overall LC-MS/MS %RSD and GC-MS/MS %RSD values for intraday precision were found to be <4.9% and <5.1%, respectively, while the interday values are <5.3% and <4% (, respectively). Accuracy was evaluated by adding mixed standard solutions at two concentrations (high: 20.0 μg/kg and low: 5.0 μg/kg) (). All tests were performed in triplicate. Recovery rates were found to be70.0–120.0%, which indicate that the methods are highly accurate. Based on the validation data discussed so far, we conclude that the developed method shows excellent linearity, sensitivity, selectivity, accuracy, and precision for the simultaneous analysis of pesticide residues.
Comparing the LC-MS/MS and GC-MS/MS results
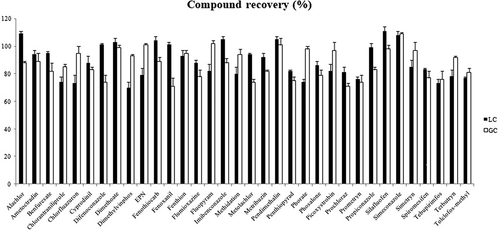
The recovery rates of 34 pesticide residues were obtained for cross-checking purposes (). The recovery rates of dimethylvinphos and tolclofos-methyl by LC and prochloraz and penoxanil by GC were relatively low. However, the test method discussed above is considered appropriate because all 34 ingredients showed recovery rates of between 70% and 120%, which meets the guidelines of the International Food Standards Committee (according to which recovery rates should be 60–120%).[Citation25,Citation26]
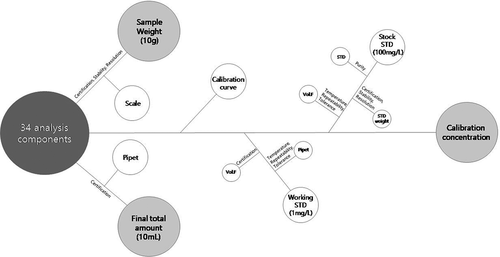
In addition, in order to compare the analysis results obtained using the two devices, measurement uncertainty based on a 20 μg/kg addition recovery-rate experiment was evaluated, the results of which are shown in . There are three main parameters to consider, namely sample amount, final total amount, and calibration curve concentration. Since the same sample pretreatment method was used, the sample volume and final total volume uncertainty are the same in each method, with differences between measurement uncertainties arising from calibration curve concentration uncertainties, as shown in Tables S1, S2 andS3. After each relative standard uncertainty was calculated to obtain the relative composite standard uncertainty, the expanded uncertainty was calculated at the 95% confidence level by multiplying the final result of each test method by the k value. The total final results and expanded uncertainties are summarized in Table S4. Expanded uncertainties of 5.2–15.1%, based on LC, and 5.2–30.4%, based on GC, were determined, which satisfy the 44% limit of the international standard [26]. As shown in Fig. S2, when the same pesticide residues were analyzed at the same concentration in both instruments, GC provided a more symmetrical peak for benfuresatethan LC, and chlorantraniliprole and flumioxazin showed higher sensitivities by LC than GC. In addition, pendimethalin exhibited tailing by LC, which, however, was not observed by GC. Because each device showed different pesticide residue characteristics, we believe that the increased accuracy obtained by comparing results through cross-checking will help to improve reliability.
Monitoring
Pesticide residues in 40 sweet pepper samples purchased from the market (Busan) were cross-checked using the analysis method described above. Out of 247 pesticide residues, 29, including acetamiprid and azoxystrobin, were found to be chief residues. An average of 4.5, a minimum of 0, and a maximum of 11 pesticide residues were detected in the 40 sweet pepper samples, with the total amounts of the detected components ranging between 0.028and 13.075 ppm. Fluopyram showed the lowest detection frequency and content, at 0.028 ppm, which was qualified and quantified through cross-checking in this study. In addition, sample number 37contained 0.01 ppm of chlorantraniliprole, the lowest concentration in a single-component sample. Importantly, we efficiently and accurately detected chlorantraniliprole through cross-checking. Because cross-checking enables the quantification and qualification of residues frequently found in sweet pepper, such as flonicamid and spirotetramat, the developed experimental method can be used for the simultaneous multi component analysis of pesticide residues in sweet pepper.
Conclusion
This study cross-checked pesticide residues generally found in sweet pepper through simultaneous analysis and by comparison using LC-MS/MS and GC-MS/MS. The sweet pepper samples were pretreated using the QuEChERS method. A total of 247 pesticide residues were analyzed by LC-MS/MS.GC-MS/MS. In addition, 34pesticide residues were qualitatively and quantitatively identified, and analyzed to determine their recovery rates and measurement uncertainties. The results reveal that the simultaneous analysis method can effectively detect pesticide residues. Analysis of the 34 residues revealed recovery rates that differ, depending on the device type, although the data are still within the appropriate range. Hence, the cross-checking analysis method can be used to effectively detect a number of pesticides. We expect that pesticide-residues analysis accuracy will be improved by applying the method to a wider range of pesticides.
Supplemental Material
Download Zip (264.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing Pesticide Use while Preserving Crop Productivity and Profitability on Arable Farms. Nature Plants. 2017, 3(3), 17008. DOI: https://doi.org/10.1038/nplants.2017.8.
- Hernandez, A. F.; Parron, T.; Tsatsakis, A. M.; Requena, M.; Alarcon, R.; Lopez-Guarnido, O. Toxic Effects of Pesticide Mixtures at a Molecular Level: Their Relevance to Human Health. Toxicology. 2013, 307, 136–145. DOI: https://doi.org/10.1016/j.tox.2012.06.009.
- Hernández, A. F.; Gil, F.; Pla, A.; Gómez, A.; Lozano, D.; Parrón, T.; Requena, M., and Alarcón, R. Emerging Human Health Concerns from Chronic Exposure to Pesticide Mixtures. In Toxicology Letters,205S, 2011; pp S2–S18 https://doi.org/https://doi.org/10.1016/j.toxlet.2011.05.020
- Damalas, C. A.; Koutroubas, S. D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics. 2016, 4(1), 1. DOI: https://doi.org/10.3390/toxics4010001.
- Kim, J. S.; Ahn, J.; Lee, S. J.; Moon, B.; Ha, T. Y.; Kim, S. Phytochemicals and Antioxidant Activity of Fruits and Leaves of Paprika (Capsicum Annuum L., Var. Special) Cultivated in Korea. J. Food Sci. 2011, 76(2), C193–C198. DOI: https://doi.org/10.1111/j.1750-3841.2010.01891.x.
- González-Curbelo, M. Á.; Socas-Rodríguez, B.; Herrera-Herrera, A. V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M. Á. Evolution and Applications of the QuEChERS Method. TrAC Trends Anal. Chem. 2015, 71, 169–185. DOI: https://doi.org/10.1016/j.trac.2015.04.012.
- Guan, H.; Brewer, W. E.; Garris, S. T.; Morgan, S. L. Disposable Pipette Extraction for the Analysis of Pesticides in Fruit and Vegetables Using Gas Chromatography/mass Spectrometry. J. Chromatogr. A. 2010, 1217(12), 1867–1874. DOI: https://doi.org/10.1016/j.chroma.2010.01.047.
- Ju, O. J.; Kwon, H. Y.; Park, B. J.; Kim, C. S.; Jin, Y. D.; Lee, J. B.; Im, G. J. Analysis of 236 Pesticides in Apple for Validation of Multiresidue Method Using QuEChERSsample Preparation and PTV-GC/TOFMS Analysis. The Korean Journal of Pesticide Science. 2011, 15, 401–416.
- Kwon, H. Y.; Kim, C. S.; Park, B. J.; Jin, Y. D.; Son, K.; Hong, S. M.; Im., G. J. Multiresidue Analysis of 240 Pesticides in Apple and Lettuce by QuEChERS Sample Preparation and HPLC-MS/MS Analysis. The Korean Journal of Pesticide Science. 2011, 15, 417–433.
- Koesukwiwat, U.; Lehotay, S. J.; Mastovska, K.; Dorweiler, K. J.; Leepipatpiboon, N. Extension of the QuEChERS Method for Pesticide Residues in Cereals to Flaxseeds, Peanuts, and Doughs†. J. Agric. Food Chem. 2010, 58(10), 5950–5958. DOI: https://doi.org/10.1021/jf902988b.
- Seo, E. K.; Kim, T. K.; Hong, S. M.; Kwon, H. Y.; Kwon, J. H.; Son,&, K.; Kim, D. H.; Analysis of Systemic Pesticide Imidacloprid and Its Metabolites in Pepper Using QuEChERS and LC-MS/MS. The Korean Journal of Pesticide Science. 2013, 174, 264–270. DOI:https://doi.org/10.7585/kjps.2013.17.4.264.
- Hernandez, A. F., Parron, T., Tsatsakis, A. M., Requena, M., Alarcon, R., & Lopez-Guarnido, O. (2013). Toxic Effects of Pesticide Mixtures at a Molecular Level: Their Relevance to Human Health. Toxicology, 307, 136–145.https://doi.org/10.1016/j.tox.2012.06.009
- Chamkasem, N.; Ollis, L. W.; Harmon, T.; Lee, S.; Mercer, G. Analysis of 136 Pesticides in Avocado Using a Modified QuEChERS Method with LC-MS/MS and GC-MS/MS. J. Agric. Food Chem. 2013, 61(10), 2315–2329. DOI: https://doi.org/10.1021/jf304191c.
- Sherma, J.; Review of Advances in the Thin Layer Chromatography of Pesticides: 2008–2010. Journal of Environmental Scienceand Health, Part B. 2011, 467, 557–568. DOI:https://doi.org/10.1080/03601234.2011.586589.
- Cai, T.; Zhang, L.; Wang, H.; Zhang, J.; Guo, Y. Assisted Inhibition Effect of Acetylcholinesterase with N-octyl Phosphonic Acid and Application in High Sensitive Detection of Organophosphorous Pesticides by Matrix-assisted Laser Desorption/ionization Fourier Transform Mass Spectrometry. Anal. Chim. Acta. 2011, 706(2), 291–296. DOI: https://doi.org/10.1016/j.aca.2011.08.035.
- Lu, D.; Yang, Y.; Luo, X.; Sun, C. A Fast and Easy GC-MS/MS Method for Simultaneous Analysis of 73 Pesticide Residues in Vegetables and Fruits. Anal. Methods. 2013, 5(7), 1721–1732. DOI: https://doi.org/10.1039/C3AY26425D.
- Sadowska-Rociek, A.; Surma, M.; Cieslik, E. Application of QuEChERS Method for Simultaneous Determination of Pesticide Residues and PAHs in Fresh Herbs. Bull. Environ. Contam. Toxicol. 2013, 90(4), 508–513. DOI: https://doi.org/10.1007/s00128-012-0951-x.
- Zhang, F.; Yu, C.; Wang, W.; Fan, R.; Zhang, Z.; Guo, Y. Rapid Simultaneous Screening and Identification of Multiple Pesticide Residues in Vegetables. Anal. Chim. Acta. 2012, 757, 39–47. DOI: https://doi.org/10.1016/j.aca.2012.10.048.
- Lee, S. W.; Choi, J. H.; Cho, S. K.; Yu, H. A.; Abd El-Aty, A. M.; Shim, J. H. Development of a New QuEChERS Method Based on Dry Ice for the Determination of 168 Pesticides in Paprika Using Tandem Mass Spectrometry. J. Chromatogr. A. 2011, 1218(28), 4366–4377. DOI: https://doi.org/10.1016/j.chroma.2011.05.021.
- European Commission. (2017). Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. 21–22 November 2017. Rev.0
- Branch, Sarah K.; (2005). Guidelines from the International Conference on Harmonisation (ICH). Journal of Pharmaceutical and Biomedical Analysis, 38(5), 798–805.https://doi.org/10.1016/j.jpba.2005.02.037.
- Thompson, M.; Ellison, S. L.; Wood, R. (2002). Harmonized Guidelines for Single-laboratory Validation of Methods of Analysis (IUPAC Technical Report). Pure and Applied Chemistry, 74, 835–855.https://doi.org/10.1351/pac200274050835.
- EURACHEM. Quantifying Uncertainty in Analytical Measurement 3rd; EURACHEM: London, UK, 2012.
- ISO. Uncertainty of Measurement – Part 3: Guide to the Expression of Uncertainty in Measurement (GUM: 1995); Geneva: Switzerland, 2008.
- Codex Alimentarius Commission. (2003). Guidelines on Good Laboratory Practice in Residue Analysis. PP. 25 CAC/GL 40-1993. Rev.1.
- Codex Alimentarius Commission. Guidelines on Measurement Uncertainty. In Pp. 8 Cac/gl 54-2004, 2017.