ABSTRACT
The high-performance liquid chromatography (HPLC) fingerprint technique is an effective method to evaluate the quality, identify species, ensure consistency and stability of traditional Chinese medicine (TCM). In this study, determination was performed on an Agilent ZORBAX SB-C18 (4.6 × 250 mm, 5 µm) column with methanol-water as mobile phase in gradient elution at the flow rate of 0.8 mL/min, the column temperature was 30°C, the detection wavelength was set to 260 nm. The HPLC fingerprints of 10 batches of extract of the aerial parts from Atractylodes lancea were evaluated by similarity analysis and chemometrics including hierarchical cluster analysis, principal component analysis. Meanwhile, the antibacterial activities of 10 batches of extract of the aerial parts from Atractylodes lancea were estimated on Escherichia coli (E. coli). The correlation coefficient of similarity between 10 batches of extract was ≥ 0.998, and the extract of the aerial parts from Atractylodes lancea have stable effects on inhibiting the proliferation of E. coli. Besides, bivariate correlation analysis (BCA) and partial least squares regression (PLSR) were applied to construct a spectrum-effect relationship, which proved the 5 main marker compounds were more relevant to anti-bacteria. Therefore, in this work, the results were successfully built an agile and efficient method that can ensure the intra-assay stability of biological activity of the extract of the aerial parts from Atractylodes lancea.
Introduction
Traditional Chinese medicines (TCMs) are in the rapidly growing market of the demand, which is attributed to the function of strengthening physical constitutions of human beings and animals as well as preventing and treating diseases.[Citation1,Citation2] Besides, the complexity of components in traditional Chinese medicine (TCM) not only brings a great change but also promotes the development of technology and method in quality control and evaluating the efficacy of TCM.[Citation3] Fingerprint analysis symbolizes a recognized and effective pathway for the quality control of TCM.[Citation4] The US Food and Drug Administration (FDA) allows chromatographic fingerprints in herbal supplements.[Citation5] In 1998, the World Health Organization (WHO) also stipulated guidelines for the evaluation of herbal medicines that if the active ingredient of the herb is unclear, chromatographic fingerprints spectrum can be provided to demonstrate the consistency of product quality.[Citation6,Citation7] At present, China has made the requirement TCM injections must be detected by fingerprint and put forward specific technical requirements. Fingerprint analysis of TCM has also been widely used in the process of standardized production of Chinese medicinal materials.[Citation8,Citation9] The quality standards of TCM formulated by the Chinese Pharmacopoeia is conformed to the characteristics of TCM.[Citation10,Citation11] At the same time, the HPLC plays a vital role in the qualitative and quantitative determination of active components of Chinese herbal medicine for the advantages of high instrumentation, fast analysis speed, and reuse of chromatographic columns, etc.[Citation4,Citation12]
Atractylodes lancea (Thunb.) DC. is named “CangZhu” in China, which belongs to the Asteraceae family.[Citation13] The pharmacological research showed that the medicinal part “root” of Atractylodes lancea is widely used due to its anti-ulcer, anti-arrhythmia, anti-inflammatory, anti-bacteria, liver protection, lowering blood pressure, and other functions.[Citation14,Citation15] In practice, the aerial parts of Atractylodes lancea are generally discarded or incinerated as garbage, which not only pollute the environment but also seriously waste resources. In our previous study, we found that the aerial parts of Atractylodes lancea have antibacterial, antioxidant, and anti-inflammatory effects.[Citation16] Therefore, it is of immediate significance to study the aerial parts of Atractylodes lancea.
As is well-known, in the study of TCM, the intra-assay stability of sample quality affects the intra-assay stability of sample bioactivity. Therefore, it is obliged to study the quality control method of the intra-assay extract of the aerial parts from Atractylodes lancea. In this paper, we used HPLC to establish the chromatographic fingerprints for 10 batches of the intra-assay extract of the aerial parts from Atractylodes lancea. This method, helpful for obtaining more information about the quality control of the intra-assay extract of the aerial parts from Atractylodes lancea, has been proved to be simple, rapid, practical, and effective. Used similarity analysis (SA) and chemometrics to evaluate the spectral effect relationship between fingerprints and anti-bacterial activities that are measured by MTT assay. We built the method to provide a foundation for improving the quality control system of the intra-assay extract of the aerial parts from Atractylodes lancea.
Materials and methods
Reagents
High-purity water was obtained with the Millipore Milli-Q water purification system (Billerica, MA, USA). Analytical grade ethanol was supplied by Beijing Chemical Works (Beijing, China). Analytical grade acetic, formic acid, and trifluoroacetic acid were supplied by Agela Technologies (Tianjin, China). The methanol and acetonitrile, used in the chromatographic process should be of non-confusable chromatographic grade, were supplied by Thermo Fisher Scientific (Waltham, MA, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2 H- tetrazolium bromide (MTT) and LB medium were supplied by Solarbio (Beijing, China). Dimethyl sulfoxide was supplied by Sigma (Darmstadt, Germany).
Plant material
All samples of the aerial parts of Atractylodes lancea were gathered from Inner Mongolia, P.R. China, in September 2020. A voucher specimen was deposited at the Institute of Feed Research (Specimen number 20200901)
Extract preparation
The dry aerial parts of Atractylodes lancea were ground into powder and passed through a 40-mesh sieve. Accurately weigh 20 g of the powdery sample that was added to 800 mL of 50% (v/v) ethanol-water solution (the ratio of material to liquid is 1:40). The solution was ultrasonically extracted at 500 W for 30 min. After centrifugation at 6000 rpm for 10 min, the supernatant was collected and concentrated by rotary evaporation at 45 ± 2°C. Then use vacuum freeze-drying technology to turn the concentrated liquid into dry powder extract, and extract was stored at 4°C.
Sample solution preparation
Accurately weigh 100 mg of the powdery extract of the aerial parts of Atractylodes lancea into 1 mL of high-purity water and vortex to dissolve, the solubility of the powdery is up to 91%. After filtering through a 0.22 µm membrane filter, take the filtrate for subsequent chromatographic analysis.
Instrumental conditions
All chromatographic analyses adopted Shimadzu HPLC system, which consists of LC-20 AD pump, autosampler (SIL-20A), system controller (CBM-20A), column temperature chamber (CT0-20A), and UV Visible diode array detector (SPD-M20A 230 V). An Agilent ZORBAX SB-C18 column (4.6 × 250 mm, 5 µm; Agilent Technologies, Santa Clara, CA, USA) was used in the study. The microplate reader (MultiskanTM SkyHigh, ThermoFisher Scientific, MA, USA) was used in the MTT assay.
Method validation
The precision of the instrument was verified by evaluating six injections of the same sample solution. The stability was analyzed by the same sample at 0, 2, 4, 6, 8, 10, 12 hours. The repeatability was assessed by the 6 batches of the aerial parts from Atractylodes lancea at once time.[Citation17] The relative standard deviations (RSDs) were calculated based on the relative peak areas (RPA) and relative retention times (RRT) of each characteristic peak, and peak 10 was selected as the main peak to calculate the RSDs.[Citation18]
Chemometrics analysis
Recently, fingerprint combined with chemometrics for analysis of TCM represents a comprehensive and popular qualitative approach.[Citation19] In this paper, chemometrics analysis named principal component analysis (PCA), hierarchical cluster analysis (HCA), bivariate correlation analysis (BCA), and partial least squares regression (PLSR) were used for data of fingerprints and spectrum effect relationship. The relative peak areas of 19 common peaks from 10 batches of extract of the aerial parts from Atractylodes lancea were imported into SPSS software (version 23.0) and SIMCA software (version 14.1) for data processing.[Citation3,Citation20]
MTT assay
According to the MTT assay to measure the proliferation of E. coli (1 × 109), that E. coli (100 μL) was co-cultured with the prepared solutions of 10 batches of extract of the aerial parts from Atractylodes lancea (10 mg/mL)[Citation16] in triplicate in a 96 well plate at 37°C for 12 hours, shook the plate intermittently. Next, add the 10 μL of the MTT (5 mg/mL) reagent to each well and incubated at 37°C. After 1 hour, dimethyl sulfoxide (100 μL) was added to stop the reaction, and the plate was shaken gently to redissolve the crystals formed. The absorbance was measured at 490 nm with a microplate reader. The blank control that no extract solutions were added into the plate was set up in the entire experiment. The inhibition rate of E. coli was calculated as [1 + (B – A)/A] 100%, where A and B are the average relative absorbances of the control and samples, respectively.[Citation21]
Data analysis
A professional software similarity evaluation system, Chromatographic Fingerprint of Traditional Chines Medicine (version 2004A), was used for data analysis. By calculating the correlation coefficients of total chromatographic profiles between samples, the simulated average chromatogram was generated. The inhibition rate of 10 batches of extract shows as mean ± SD of three independent experiments. Using the ANOVA with Tukey post hoc in statistical comparison and P < .05 is considered statistically significant.
Results and discussion
Optimization of HPLC conditions
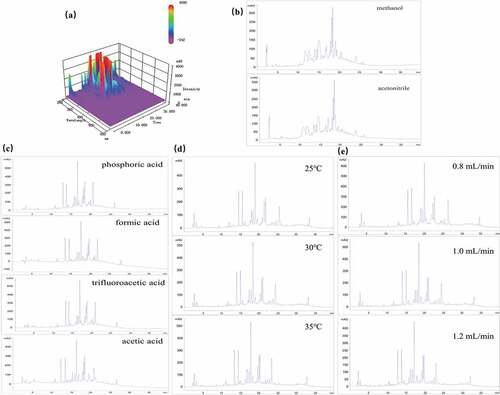
Based on the separation efficiency of Chinese herbal medicine, samples were affected by detection wavelength, mobile phase, flow rate, column temperature, and chromatographic analysis time, the separation conditions of the aerial parts from Atractylodes lancea were optimized.[Citation22,Citation23] To obtain the more characteristic peaks, the sample solution was scanned at 200 ~ 600 nm () and selected 260 nm as the best detection wavelength. Methanol and acetonitrile were compared as different mobile phases () that displayed that the methanol had better distinguishability and shorter analysis time. So, chose the methanol (A)-water (B) as the mobile phase. Besides, a better separation effect can be achieved by adding acid as a modifier via the literature.[Citation21] Thus, compared with the addition of (0.1%) phosphoric acid, (0.1%) formic acid, (0.1%) trifluoroacetic acid, and (0.1%) acetic acid in water (), it was found that the (0.1%) trifluoroacetic acid was the best choice to further improve the separation effect and peak shape. In addition, column temperature plays an equally important part in the separation of extract.[Citation24] The final results showed that the best separation and most chromatographic peaks could be obtained when column temperature set at 30°C instead of 25°C or 35°C (). For purpose of furthering the improvement of the separation, the optimized flow rate was 0.8 mL/min (). In all HPLC experiments, the injection volume was set to 10 µL. Gradient elution procedures of HPLC of the aerial parts from Atractylodes lancea were established as follows: Methanol (A)-0.1% trifluoroacetic acid (B): 0–15 min, mobile phase A followed from 5% to 35%; 15–25 min, mobile phase A followed from 35% to 45%; 25–30 min, mobile phase A followed from 45% to 60%, 30–40 min, mobile phase A followed from 60% to 95%.
Method validation
HPLC fingerprint is a chromatogram to characterize the chemical composition of TCM and has become the main research method to evaluate the quality of TCM.[Citation19] HPLC fingerprint with high stability and specificity can be used for qualitative determination and effective evaluation of TCM.[Citation25,Citation26] Taking advantage of HPLC, samples were analyzed under the optimal chromatographic conditions. Simultaneously, the average chromatogram of the samples obtained by the similarity evaluation system was used as the standard characteristic fingerprint of the aerial parts from Atractylodes lancea. Peaks with great resolution and reasonable heights that were regarded as “common peaks” for qualification of the samples. We surveyed 19 common peaks occurring within 40 min in all samples, with fingerprint parameter assessment on account of chromatographic intensity, trajectory, and stability (). Among 19 common peaks, peak 10 (marked with “S” in ) showed the steady peak time and content, which was selected to calculate the RRT and the RPA. The formulas of RRT and RPA were RRT = RTpeak/RTpeak10 and RPA = PApeak/PApeak10, respectively. Furthermore, the other 18 peaks were also selected as characteristic peaks for assessment of the aerial parts from Atractylodes lancea owing to relatively high absorption intensities and correlative differences in the field of retention time.
Figure 2. A characteristics fingerprint of the extracts of the aerial parts from Atractylodes lancea. Peaks 1–19 were used as common peaks.
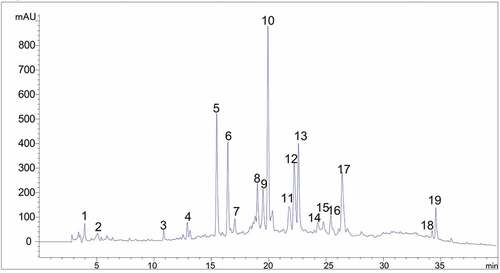
Table 1. The data of precision, stability and repeatability of the method
After preparing the same solution of extract, the solution was repeated to measure six times in one day under the optimal chromatographic conditions. The RSDs of RRT and RPA of 19 common peaks were < 0.57% and < 2.57%, respectively. The result said that the precision of the instrument is well. Under the optimal chromatographic conditions, the extract solution that was kept at room temperature was injected at 0, 1, 2, 4, 6, 8, 10, 12 hours, respectively. The RSDs of RRT and RPA of 19 common peaks were < 0.87% and < 3.90%, respectively. The result said that the solution was stable in 12 hours. Produced 6 batches of the aerial parts from Atractylodes lancea at one time and evaluate them according to the optimal chromatographic conditions. The RSDs of RRT and RPA of 19 common peaks were < 0.34% and < 4.33%, respectively. The result said that the repeatability of the method is effective. The data of precision, stability, and repeatability of the suggested chromatography method are listed in . By using this method, we can obtain relatively stable extract of the aerial parts from Atractylodes lancea, which can be used as a basis for subsequent functional experiments.
Similarity analysis of the extract of the aerial parts from Atractylodes lancea
Similarity has been widely recognized as a legal evaluation index of fingerprint.[Citation27,Citation28] Our study built the binary linear gradient elution method of HPLC fingerprint of the extract of the aerial parts from Atractylodes lancea at the first time. In order to determine the consistency of each batch of medicinal materials, HPLC fingerprint similarity analysis was conducted on 10 batches of extract of the aerial parts from Atractylodes lancea under the optimal chromatographic conditions. Import the chromatographic data at 260 nm into the “Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System (2004A Edition)” provided by the Chinese Pharmacopoeia Committee, and perform multi-point calibration and chromatographic peak matching. After all peaks were matched, the superimposed spectrum and the control spectrum , calculated the similarity value between the fingerprints of each sample (). It’s easy to discover that the 10 batches of samples in a high degree of similarity. Our next step is to determine the compositions represented by each peak.
Figure 3. The overlay HPLC fingerprints of 10 batches extracts of the aerial parts from Atractylodes lancea (1–10) by a similarity evaluation system that analyses sample similarity against a generated reference chromatogram (R).
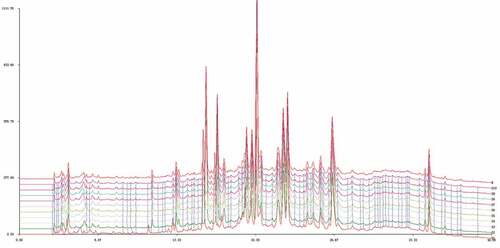
Table 2. Similarities and MTT assay inhibition rate of the 10 batches of extract of aerial parts from Atractylodes lancea.
Inhibition effect
The MTT assay was used to assess the inhibition effect on E. coli. The inhibition effect (10 mg/mL) of 10 batches of extract of the aerial parts from Atractylodes lancea on E. coli was detected. As shown in , the inhibition rates of 10 batches of extract of the aerial parts from Atractylodes lancea on E. coli were relatively stable without statistically significant difference. The results suggested that 10 batches of extract of the aerial parts from Atractylodes lancea have a stable inhibition effect on E. coli, indicating that good quality control could guarantee the batch stability of the inhibition effect of the extract of the aerial parts from Atractylodes lancea.
Chemometric analysis
Hierarchical cluster analysis (HCA)
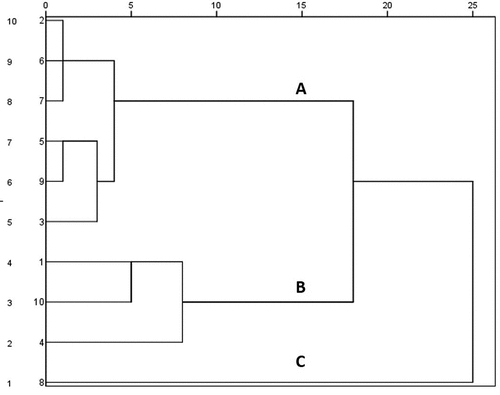
Import the peak areas of 19 common peaks from the 10 batches of extract of the aerial parts from Atractylodes lancea into the SPSS software (version 23.0) as variables to obtain a 10 × 19-order original data matrix, using the inter-group connection method, and using the Euclidean square distance as the metric to perform clustering. The analysis results are shown in . 10 batches of extract of the aerial parts from Atractylodes lancea can be grouped into 3 categories, When the classification distance is 15. Categories included subgroup A (S2, S3, S5~ S7, S9), subgroup B (S1, S4, S10) and subgroup C (S8). The results indicate that the samples may not be collected at the same time.
Principal component analysis (PCA)
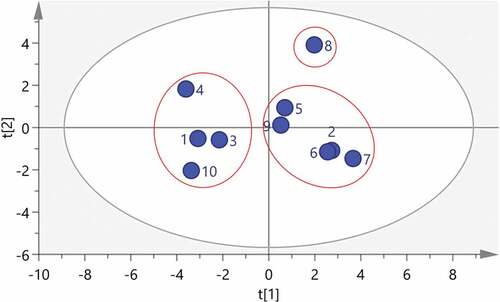
PCA can transform multiple variables into a few comprehensive variables, principal components.[Citation29] It is an effective statistical method of dimensionality reduction that the method eliminated the correlation between evaluation indicators position of samples more objectively.[Citation30] It is significant to applicate the PCA of the quality of the extract of the aerial parts from Atractylodes lancea. After factoring the common peak areas data of 10 batches of extract of the aerial parts from Atractylodes lancea through SPSS software (version 23.0) and 6 principal components were gathered. The eigenvalues and variance contribution rates obtained by PCA are shown in that the eigenvalues of the first six principal components are > 1, indicating that the first six principal components play a leading role in the quality evaluation of the extract of the aerial parts from Atractylodes lancea. Six principal components are the symbolic components for the classification differences of the samples. The accumulation of the six principal components reached 93.546% (>90%), which can objectively reflect the comprehensive quality of the extract of the aerial parts from Atractylodes lancea. According to that the loading coefficient of each factor contained in each principal component comprehensively reflects the influence of the measured component on each principal component. In addition, the common peak areas were imported into the SIMCA software (version 14.1) to draw the principal component analysis score as shown in . The 10 batches of extract of the aerial parts from Atractylodes lancea were divided into 3 categories, which were basically consistent with the results of the HCA.
Table 3. Total variance interpretation of PCA
Table 4. Matrix score coefficient chemical constituents of the extracts of the aerial parts from Atractylodes lancea.
Spectrum-effect relationship
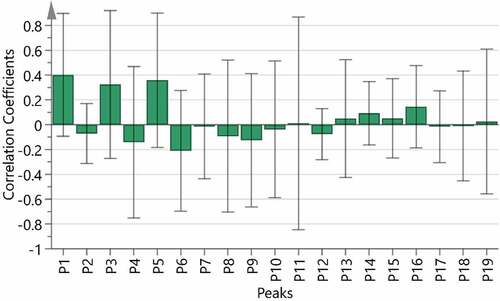
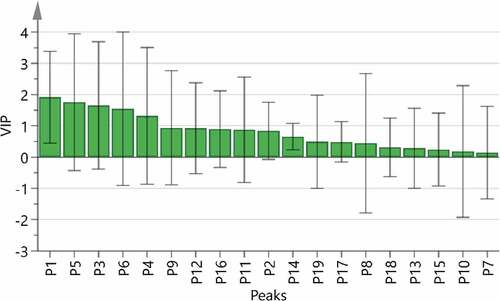
It is possible to find and identify the relevant components of the TCM efficacy when the combination of chromatographic fingerprint and drug efficacy evaluation. The spectrum-effect relationship’s research turns the “visual” quality of TCM to the consistency of “spectrum” and “effect.”[Citation3,Citation17,Citation19,Citation31] Used BCA model to establish the spectral effect relationship between the data of the 19 common peak areas and inhibitions of E. coli and the analysis results are shown in . The result of the correlation coefficients showed that the P1, P3, P5, P11, P13, P14, P15, P16 and P19 were positively correlated with the inhibition effect on E. coli (). In the PLSR model, the peak areas of 19 common peaks were defined as independent variables, inhibitions were selected as the dependent variables. The variable importance in projection (VIP) scores results were obtained from SIMCA software (version 14.1). As is shown in , the VIP>1 was used as the screening criterion, P1, P5, P3, P6 and P4 are the main marker components that affect the inhibition on E. coli.
Conclusion
In this study, the HPLC fingerprint was successfully established to fully reflect the species and number of chemical components of the extract of the aerial parts from Atractylodes lancea. The chromatographic fingerprints of 10 batches of extract not only had a similarity as high as 0.998, but also had a stable and consistent distribution among peaks, which suggested the samples from the same location had sufficient similarity in chemical composition to demonstrate the stability of quality of the extract of aerial parts from Atractylodes lancea. Meanwhile, combining chemometric methods to evaluate the spectrum-effect relationship between quality control and antibacterial effect of the extract of the aerial parts from Atractylodes lancea. It means that our study should focus on the 6 principal components extracted and the 5 iconic components with significant positive impact on the antibacterial effect in the process of follow-up experiments. In summary, we established the quality evaluation method of HPLC fingerprint chromatography combined with the chemometric which has reference significance for further improving the quality standards of the extract of the aerial parts from Atractylodes lancea.
Acknowledgments
The aerial parts of Atractylodes Lancea were supplied by Inner Mongolia Jiuhe Agricultural Science and Technology Development Co. Ltd (Inner Mongolia, PR China). This work was supported by the Agricultural Science and Technology Innovation Program (ASTIP) (Grant No. CAAS-ZDRW202111).The authors declare no conflict of interest. The data that support the findings of this study are available on request from the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liang, J.; Zhou, Y. S.; Xin, C. X.; Yang, C. C.; Wei, J. Y.; Huang, D. F., and Zhao, L.C. Discussion on the Quality Evaluation Method of Traditional Chinese Medicine Compound Preparations. Med. Plant. 2020, 1 , 11–16.
- Yang, A. X.; Wang, X. X.; Xiong, C. M.; Chen, J., and Ruan, J. L. Quality Control of tuirejieduling Granules Using High-performance Liquid Chromatography Fingerprint Method and Simultaneous Determination of Four Main Active Ingredients. Pharmacogn. Mag. 2013, 9(34), 162–166. DOI: 10.4103/0973-1296.111284.
- Liu, S.; Liang, Y. Z., and Liu, H. T. Chemometrics Applied to Quality Control and Metabolomics for Traditional Chinese Medicines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1015-1016, 82–91. 1015-1016 DOI: 10.1016/j.jchromb.2016.02.011.
- Liang, Y. Z.; Xie, P. S., and Chan, K. Quality Control of Herbal Medicines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812(1–2), 53–70. DOI: 10.1016/S1570-0232(04)00676-2.
- Jiang, Y.; Xia, J. P.; Yang, J. H.; Zhang, Z. F.; Hu, C. Q., and Zhang, Z. R. Guidelines and strategy of the International Conference of Harmonization (ICH) and Its Member States to Overcome Existing Impurity Control Problems for Antibiotics in China Chin. J. Nat. Med. . 2015, 13 (07) ,0498–0506.
- Li, X. M.; Liu, J.; Pan, F. F., and Yang, P. L. Effect of quality Control on the Antiproliferative Activity of the Extract from Portulaca Oleracea L. In Aspergillus Flavus. Biomed. Chromatogr. 2018, 32(12), e4354. DOI: 10.1002/bmc.4354.
- WHO. Quality control methods for medicinal plant material (Geneva (Swizerland): WHO). 1998, 2301–2315 9789241545105 .
- Ni, L. J.; Zhang, L. G., and Li, P. Consideration on Research and application of Fingerprint of Traditional Chinese Medicine. Nat. Prod. R & Da. 2002, 14 (05) , 60–64.
- Zou, C. C., and Yan, H. Y. Research Progress on Chromatographic Fingerprint Similarity Evaluation Method for Traditional Chinese Medicine in the past 30 Years (1988-2017) and Its Prospect (In Chinese). China J. Chin. Mater. Med. 2018, 43 (10) , 1967–1977.
- Liu, C. X.; Xiao, P. G.; Peng, Y., and Song, N.N. Challenges in Research and development of Traditional Chinese Medicines. Chin. Herb. Med. 2009, 1 (01) , 1–28.
- Zhao, J. Q.; Shi, T. N.; Zhu, W. F.; Chen, L. H.; Guan, Y. M., and Chen, J. Quality control Method of Sterols in Fermented Cordyceps Sinensis Based on Combined Fingerprint and Quantitative Analysis of Multicomponents by Single marker. J. Food Sci. 2020, 85(10), 2994–3002. DOI: 10.1111/1750-3841.15412.
- Huang, L.; Wu, X. M.; Ji, Y., and Wang, Y. Fingerprint analysis of Placenta Polypeptide Injection by High Performance Liquid Chromatography. J. Pharm. Anal. 2012, 2(1), 71–75. DOI: 10.1016/j.jpha.2011.10.003.
- Committee, N. P. Pharmacopoeia of People’s Republic of China (Chem. Indus.Press). 978-7-5067-7539-7. 2005.
- Jiang, J. S.; Xu, K.; Feng, Z. M.; Yang, Y. N., and Zhang, P. C. Four New Sesquiterpenes from Atractylodes Lancea. Phytochem. Lett. 2018, 26, 88–92. DOI: 10.1016/j.phytol.2018.05.023.
- Tsusaka, T.; Makino, B.; Ohsawa, R. H.; Ezura, H. Evaluation of Heritability of β-eudesmol/hinesol Content Ratio in Atractylodes Lancea de Candolle. Hereditas. 2020, 157(1), 7. DOI: 10.1186/s41065-020-00123-3.
- Li, X. M.; Yang, P. L.; Liu, J.; Yang, J.; Shi, D. D.; Man, C.; Wang, Y. D., and Pan, F. F. Application of Stem and Leaf Extract from Atractylodes Lancea. China patent publ. date 2020.
- Nijat, D.; Lu, C. F.; Lu, J. J.; Abdulla, R.; Hasan, A.; Aidarhan, N., and Aisa, H.A. Spectrum-effect relationship between UPLC Fingerprints and Antidiabetic and Antioxidant Activities of Rosa Rugosa. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1179, 122843. DOI: 10.1016/j.jchromb.2021.122843.
- Pan, F. F.; Zhang, H. Y.; Li, X. M.; Yang, P. L.; Zhang, T. C.; Luo, X. G., and Ma, W. J. Effect of Quality control on the Total Antioxidant Capacity of the Extract from Sonchus Brachyotus DC. Int. J. Food Prop. 2018, 21(1), 1362–1370. DOI: 10.1080/10942912.2018.1489840.
- Liang, Y. Z.; Yi, L. Z.; Xu, Q. S. Chemometrics and Modernization of Traditional Chinese Medicine. Sci. China Ser. B: Chem. 2008, 51(8), 718–728. DOI: 10.1007/s11426-008-0084-6.
- Fayeulle, N.; Meudec, E.; Boulet, J. C.; Vallverdu-Queralt, A.; Hue, C.; Boulanger, R.; Cheynier, V., and Sommerer, N. Fast discrimination of Chocolate Quality Based on Average-mass-spectra Fingerprints of Cocoa Polyphenols. J. Agric. Food. Chem. 2019, 67 (9) , 2723–2731. DOI: 10.1021/acs.jafc.8b06456.
- Liu, J.; Li, X. M.; Shi, D. D.; Wen, Z. G., and Yang, P. L. Effect of quality Control on the Proliferation of the Extract from Taraxacum Mongolicum Hand.-Mazz. In Lactobacillus Plantarum. Biomed. Chromatogr. 2019, 33 (12) , e4687.
- Li, L.; Xie, F. F.; Yan, P. H.; Zeng, X. Y., and Cai, Y. On the HPLC Fingerprint of Zhuang Medicine Spilanthes Paniculata Wall. Ex DC. Med. Plant. 2019, 10 (3) , 19–23.
- Wang, B.; Shen, L.; Cong, W. J.; Lin, X.; Feng, Y.; Zhu, Y. Y., and Wang, Q. A Simple HPLC Method for Simultaneous Analysis of 7 Bioactive Constituents for Liuwei Dihuang Pill and Its Application in quality Consistency Evaluation. Anal. Methods. 2013, 5(9), 2384–2390. DOI: 10.1039/c3ay40125a.
- Qin, K. M.; Wang, B.; Cai, H.; Li, W. D.; Yao, Z. Q.; Zhang, X. D.; Lu, T. L., and Cai, B. C. Simultaneous Determination of Five Marker Compounds in Xuanfu Daizhe Tang by High-performance Liquid Chromatography Coupled with Diode Array Detection for Quality Control. Pharmacogn. Mag. 2012, 8(32), 250–255. DOI: 10.4103/0973-1296.103647.
- Zhao, L.; Wen, E.; Upur, H., and Tian, S. G. High Performance Liquid Chromatography-diode Array Detector Method for the simultaneous Determination of Five Compounds in the Pulp and Seed of Sea Buckthorn. Pharmacogn. Mag. 2017, 13(49), 136–140. DOI: 10.4103/0973-1296.197656.
- Liu, Y. S.; Cao, M.; Wang, Y. M., and Luo, G. A. Similarity System Theory to Evaluate the Similarity of high Performance Liquid Chromatography Fingerprint of Traditional Chinese Medicine Quantitatively. Chin. J. Anal. Chem. 2006, 34, 333–337.
- Lan, L. L.; Zhang, Y. J.; Zhang, M. T.; Sun, G. X. Evaluation of the Quality of Compound Liquorice Tablets by DSC and HPLC Fingerprints Assisted with Dissolution. J. Pharm. Biomed. Anal. 2019, 175, 112715. DOI: 10.1016/j.jpba.2019.06.012.
- Zhou, J.; Fang, X.; Zhang, T.; Zhao, Z.; Zhu, R.; Xiang, F.; Qiao, J. Quantitative Similarity Assessment of Non-linear Chemical Fingerprint of Traditional Chinese Medicine by Similarity System Theory. J. Cent. South Univ. Technol. 2011, 18(2), 343–352. DOI: 10.1007/s11771-011-0702-x.
- Wang, F. K., and Yang, C. W. Applying Principal Component Analysis to a Gr&R Study. J. Chin. Inst. Indus. Eng. 2007, 24(2), 182–189. DOI: 10.1080/10170660709509032.
- Zhang, K.; Zhang, Y. T.; Qu, P. P. Comprehensive Multivariate Grey Incidence Degree Based on Principal Component Analysis. J. Syst. Eng. Elect. 2014, 25(5), 840–847. DOI: 10.1109/JSEE.2014.00097.
- Zhang, S. J.; Zhang, Y. G.; Li, D. H.; Wu, H. W.; Niu, J. T.; Si, X. L., and Li, Y. F. [Prediction of Q-markers of Astragali Radix Based on Network Pharmacology and Fingerprint]. China J. Chinese Materia Medica. 2021, 46(11), 2691–2698. DOI: 10.19540/j.cnki.cjcmm.20200925.201.