ABSTRACT
Ischemia reperfusion (I/R) injuries occurred in many pathological and surgical processes (e.g. thrombolytic therapy, organ transplantation, aortic cross-clamping, coronary angioplasty and cardiopulmonary bypass) and harmed multiple organs and tissues. Vitamin D is a well-known sterol hormone and a nutritional ingredient able to promote the deposit of calcium and regulate phosphorus metabolism in the body. In addition, vitamin D has therapeutic effects on some diseases (e.g. cardiovascular disease, diabetes, cancer, neurological diseases, multiple sclerosis and inflammation). Studies showed that vitamin D3 was closely related with I/R injury occurrences in heart, brain, spine, liver, kidney, and ovary. The literature searching was conducted in PubMed, Embase, Cochrane Library, Web of Science, and SCOPUS from inception to 20 September 2021. Data showed that supplements with vitamin D3 can remarkably attenuate I/R injuries. This paper reviewed recent progresses of vitamin D3 preventing I/R injuries in clinical investigations and animal tests to enlighten future studies.
Introduction
Ischemia reperfusion (I/R) refers to the process of restored perfusions after blood decreases or occlusions in tissues and organs. I/R usually occurs in some clinical and pathological processes, such as thrombolytic therapy, [Citation1] organt ransplantation, [Citation2] aortic cross-clamping, [Citation3] coronary angioplasty, [Citation4] cardiopulmonary bypass.[Citation5] Although the reperfusion can timely supply oxygen and nutrients to reduce tissue necrosis, further injury (I/R injury) often occurs due to inflammatory reactions and oxidative stress. I/R injury included molecule damages, cell deaths induced by apoptosis, necrosis, and autophagy as well as tissue and organ dysfunctions.[Citation6] Free radicals and inflammation play vital roles in the process of I/R injury.[Citation7] Due to the short therapeutic window period for reperfusion during and after ischemia, preconditioning owned optimized effects against I/R injury for safety, easy application and cost-effectiveness.[Citation8] In contrast to the side effects of traditional drugs in preconditioning, functional ingredients were the better choice to prevent I/R injury (e.g. resveratrol, [Citation9] GABA, [Citation10] n-3 polyunsaturated fatty acids [Citation11]). Vitamin D, as an endogenous and foodborne substance, owns numerous biological functions and recently had been found able to attenuate I/R injury in many studies. Reviewing corresponding research progress would enlighten the application of vitamin D in preventing I/R injury in future.
Sources, biosynthesis, and metabolism of vitamin D
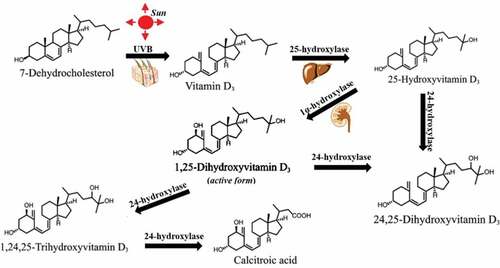
Vitamin D is a type of fat-soluble sterol hormone that can increase the deposit of calcium and regulating phosphorus metabolism in our body. Its main forms include vitamin D2 and vitamin D3, [Citation12] which were chemically characterized in 1931 and 1935, respectively. Natural vitamin D3 extensively exists in fish liver oils and the flesh of fatty fish (e.g. trout, salmon).[Citation13] There are also lower contents of vitamin D3 in beef, liver, cheese, egg yolks, etc. For human, endogenous vitamin D3 is synthesized from 7-dehydrocholesterol in skin through ultraviolet B (UVB) light from the sun.[Citation14] Only 1, 25-hydroxyvitamin D3 (its active form) plays physiological actions through hydroxylations of vitamin D3 in position 25 (25-hydroxyvitamin D3) and 1 (1,25- hydroxyvitamin D3) in sequence by 25-hydroxylase in liver and 1-hydroxylasein kidney (). Serum 1,25- hydroxyvitamin D3 level can be strictly regulated by parathyroid hormone, calcium, and phosphate in serum.[Citation15] And, 25-hydroxyvitamin D3 and 1,25-hydroxyvitamin D3 can be further inactivated by 24-hydroxylase.[Citation16] However, 25-hydroxyvitamin D3 level more well reflects the vitamin D status because 25-hydroxyvitamin D3 or 25(OH)D3 has a longer half-life (15 days) compared with 1,25-hydroxyvitamin D3 with the shorter half-life (several hours).[Citation15] In contrast, vitamin D2 in nature is formed from ergosterol in mushrooms under ultraviolet light .[Citation17] Although 1,25-dihydroxyergocalciferol (the active form of vitamin D2) can also be activated by 25-hydroxylase in liver and 1-hydroxylase in kidney, [Citation18] related studies are less for its less sources and less active.
Figure 1. Vitamin D3 structure and the pathway of its activation and inactivation in body. Vitamin D3 is synthesized from 7-dehydrocholesterol through UVB irradiation in skin. 25-hydroxylase enzyme converts vitamin D3 to 25-hydroxyvitamin D3 in liver. Then, 1α-hydroxylation produces the active vitamin D3(1,25-hydroxyvitamin D3). 25-hydroxyvitamin D3 and 1,25-hydroxyvitamin D3 can be further inactivated into 24,25-hydroxyvitamin D3 and 1,24,25-hydroxyvitamin D3.
Biological functions and receptors of vitamin D
It is well-known that vitamin D promotes calcium absorption in the gut and enables bone mineralization and growth.[Citation14] Besides, lots of clinical surveys showed that vitamin D supplements can attenuate many diseases, such as cardiovascular disease, [Citation19] multiple sclerosis, [Citation20] type 2 diabetes, [Citation21] cancers, [Citation22] inflammation, [Citation15] neurologic diseases (e.g. Parkinson’s disease and cognitive loss).[Citation23] It also provided suggestions about these actions of vitamin D from the findings of positive relationships between low vitamin D and these disease occurrences.[Citation24] Recently, vitamin D had been found being able to ameliorate the pathological process of COVID-19.[Citation25] Constant findings of new vitamin D functions expanded potential applications of vitamin D.
1, 25-hydroxyvitamin D3 plays its biological actions via binding two types of vitamin D receptors (VDR), which were called as the membrane-located VDR, and the nuclear-located VDR, separately.[Citation26] The former mainly mediates non-genomic actions, such as channel responses, adipocyte metabolism, insulinotropic effects, antiapoptotic pathways via second messengers (phospholipase C, phospholipase A2, phosphatidylinositol-3 kinase, Ca2+, cyclic AMP, etc.). The latter produced the genomic effects via its heterodimer with retinoid X receptor binding to vitamin D response element .[Citation27] Meanwhile, on-genomic action also mediates the genomic function of vitamin D and nuclear-located VDR also mediated the non-genomic action.[Citation28] It hinted that there may be overlapping between the actions of membrane-located VDR and the nuclear-located VDR in different circumstances. VDR distributed in almost every organs (e.g. intestine, kidney, pancreas, bronchial epithelial cells, skin, brain, heart, bone, immune cells, reproductive tissues [Citation29,Citation30]). Therefore, vitamin D possibly owns extensive actions in the whole body.
Dietary requirement for vitamin d and its deficiency and excess
In order to meet normally physiological needs of vitamin D, a certain amount of fortified dietary intake is essential because the dietary intake of vitamin D generally is inadequate.[Citation27] Although different countries and institutions proposed many recommended levels of dietary intakes, the differences in these standards generally can be omitted. In contrast, USA made out more comprehensive standards, including daily recommended dietary allowances (RDA) and tolerable upper intake levels (UL).[Citation31] For instance, RDA of vitamin D is 400IU under 1 year old and 600 IU above 1 year old. UL is about 2–5 times over RDA. Serum levels of vitamin D (mainly 25-hydroxyvitamin D3) are often used to reflect the real-time status of vitamin D and are often used to indicate the disease occurrences.[Citation32] However, the standards about levels of 25-hydroxyvitamin D3 deficiency were different. For example, National Academies of Sciences, Engineering, and Medicine concluded that serum 25-hydroxyvitamin D3 level less than 30 nmol/L (12 ng/mL) is deficiency.[Citation15] In contrast, Endocrine Society stated that the threshold is 75 nmol/L (30 ng/mL).[Citation33] The discrepancy may originate from multiple factors, such as age, [Citation34] obesity [Citation15] and skin reason, [Citation35] which all disturb clinical diagnosis.
The deficiency and excess of vitamin D is closely related to some diseases. First, vitamin D deficiency not only causes well-known rickets and osteomalacia in the skeletal system, but also is associated with some extraskeletal actions (e.g. cell proliferation, immune and muscle function, skin, and reproduction, vascular and metabolic properties [Citation36]), which accounting for high risks of diseases and pathologies stated above. In another hand, excessive vitamin D also causes toxicity, characterized by hypervitaminosis D, hypercalcemia, renal dysfunction and hypercalcemia-related pathologies (nausea, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, polyuria, excessive thirst, kidney stone, etc).[Citation37,Citation38] The intake under 4000IU/day (100 µg/day) is considered safe.[Citation39] Therefore, vitamin D toxicity is infrequent in daily life.
Vitamin D and myocardial I/R injury
Ischemic heart disease is a prevalent human killer. Myocardial ischemia is decreased blood flow unable to meet the demand of heart for oxygen and nutrients. It resulted from coronary stenosis, thrombosis, and hyperconstriction of the coronary arteries.[Citation40] Then, it causes many symptoms, such as angina, unstable angina, and shortness of breath. Even, more serious consequences often occurred, such as arrhythmias, myocardial infarction, sudden death.[Citation41] The timely reperfusion of blood flow can reduce myocardial ischemia injury and is considered as the first-line therapeutic strategy.[Citation42] However, the reperfusion of myocardial blood supply after ischemia causes further injury. Some independent factors (e.g. metabolic disorders, oxidative stress, calcium overload, inflammation, apoptosis, necrosis, autophagy, and pyroptosis [Citation43,Citation44]) may be responsible for the development of myocardial I/R injury. Recently, vitamin D deficiency was linked with the occurrence of myocardial I/R injury and it was supplemented for reducing myocardial I/R injury in clinical and animal studies.
Clinical evidence for the role of vitamin D in myocardial I/R injury
Many clinical data associated vitamin D with the occurrence of cardiovascular diseases, such as myocardial infarction, coronary artery disease, hypertrophy, cardiomyopathy, cardiac fibrosis, heart failure, aneurysm, and atherosclerosis.[Citation45,Citation46] For example, there exist vitamin D deficiency and its function abnormalities in cardiovascular diseases.[Citation47,Citation48] Although it is not fully confirmed that vitamin D deficiency can directly induce cardiovascular diseases due to short of nutritional experimental studies about how vitamin D deficiency causing cardiovascular diseases according to present data, vitamin D supplements produced beneficial effects on them.[Citation49,Citation50] Consequently, its deficiency or declined function is usually considered as an important risk factor for coronary artery disease.[Citation51]
Timely reperfusion after myocardial ischemia referred to acute spontaneous reperfusion (SR), or was carried out clinically via thrombolytic treatment and primary percutaneous coronary intervention (PCI).[Citation52] However, myocardial reperfusion injury follows. Notably, the abnormal statuses of the vitamin D system in body also contributed a lot for the pathology of myocardial I/R injury according to a series of clinical investigations. A recent study [Citation53] showed that various degrees of vitamin D deficiencies (25(OH)D: <12.7 ng/ml, n = 250; 12.7–21.59 ng/ml, n = 235; ≥21.6 ng/ml, n = 220) may predict different risks of myocardial I/R injury and less deficiency of vitamin D is associated with less necessity of coronary artery bypass grafting (CABG) (16.2%; 8.1%; 7.9%). Not only that, overall mortality (7.6%;2.9%; 0.4%) was significantly related to different degrees of vitamin D deficiencies at the 996.5 day of median follow-ups after PCI to the patients with coronary artery disease. However, the criterion of vitamin D insufficiency, which is defined as less than 30 ng/mL in blood[Citation54] may be suitable for heart, in contrast with the standard of 25(OH)D insufficiency with the serum levels of 10–20 ng/ml, and 25(OH)D deficiency with serum levels under 10 ng/ml as a previous research [Citation55] had defined. For instance, Fatih Sen et al. [Citation56] (2015) found that higher vitamin D levels (averaging 36.2 ± 10.7 ng/mL) in blood increased the patency rate of saphenous vein grafts (SVGs) in patients. In contrast, patients with occlusion of SVGs only had 21.1 ± 10.4 ng/ml of mean blood vitamin D levels. On the other hand, high vitamin D levels was positively related with the improved 10-year survival time of patients [Citation57] and decreased atherosclerosis occurrences in aorta of patients after CABG surgeries [Citation58]). However, there were also few reports showing that exorbitant levels of vitamin D (e.g. ≥89 ng/ml) for a long time in human body also increased the risk of coronary artery disease.[Citation59,Citation60] And, there was higher prevalence in clinical patients with CABG surgery due to high levels of solar exposure or high diet intakes of vitamin D in some regions (e.g. Kerala [Citation61]). Therefore, it is notable that vitamin D may own two-edge effects on myocardial I/R injury.
In view of the connection between vitamin D and myocardial I/R injury discussed above, some studies had carried out treatments with vitamin D to attenuate myocardial I/R injury. In a randomized, double-blind, placebo-controlled study, [Citation62] vitamin D treatments (150,000 IU daily for 3 days) before cardiopulmonary bypass significantly attenuated myocardial apoptosis and the inflammatory status in patients with vitamin D deficiencies (< 20 ng/mL). However, treatments with different doses of vitamin D produced dose-dependent effects in protecting myocardial I/R injury. A study [Citation63] had showed that vitamin D supplements (50,000 IU) at 48 hours before CABG surgery can prevent postoperative atrial fibrillation occurrences (a common arrhythmia) of patients with the mild deficiency of vitamin D (20–29 ng/mL). However, the treatment did not significantly hold back the development of postoperative atrial fibrillations of patients with the severe deficiency of vitamin D (< 20 ng/mL). In contrast, another study [Citation64] carried out different treatments for patients with different shortages of vitamin D (300,000 IU oral vitamin D for patients with vitamin D deficiency ( < 21 ng/mL) and 150,000 IU for those with vitamin D insufficiency (21–29 ng/mL) 48 h before CABG surgery. The both treatments significantly prevented postoperative atrial fibrillation occurrences. Therefore, appropriate doses of vitamin D should be considered in order to attenuate myocardial I/R injury for different degrees of vitamin D deficiencies.
Animal tests investigating vitamin D attenuating myocardial I/R injury
In addition to clinical studies, some animal tests in vivo and in vitro showed the potential effects of vitamin D protecting heart from I/R injury. A study [Citation65] showed that mRNA levels of myocardial VDR were unaltered after 30 minutes of myocardial ischemia in mice and upregulated following 24-hour reperfusion. The treatment of a VDR enhancer (paricalcitol at 1 μg/kg i.p at 15 min before reperfusion) restored myocardial VDR levels and reduced apoptosis through inhibiting autophagy dysfunction-mediated cell death. It had also been demonstrated in another study, [Citation66] in which VDRs and the cardiac muscle cell apoptosis of myocardial I/R mice were increased under low free vitamin D level while vitamin D binding protein was overexpressed. However, in another model of myocardial I/R based on obstructive nephropathy, [Citation67] paricalcitol pretreatments (at 30 ng/kg/d, i.p, for 15 days) improved heart remodeling and arrhythmias of rat myocardial I/R model. Meanwhile, the treatment restored reduced VDR levels, which may aggravate myocardial I/R injury. Even, the treatment with a vitamin D analog (22-oxacalcitriol, 20 µg/kg) after I/R (30 minute/3 hour) significantly inhibited inflammatory response in the myocardium of model rats.[Citation68] In addition, a recent study found that vitamin D treatment attenuated myocardial I/R injury via rectifying VDR .[Citation69] Another study [Citation70] showed that vitamin D also attenuated myocardial I/R injury via inhibiting inflammations. And, vitamin D in vitro produced protective effects against myocardial I/R injury by protecting mitochondrial structural and functional integrity and mitophagy apart from inhibiting inflammation .[Citation71]
In addition, combining vitamin D in a lower dose (0.1 μg/kg/day) and other substances (e.g. resveratrol, 1 mg/kg/day) can produce synergistic effects in ameliorating ventricular ectopic beats in myocardial I/R injury via increasing antioxidase levels (e.g. catalase) .[Citation72] In contrast, the single therapy of either vitamin D or resveratrol with the same doses did not decrease incidence of arrhythmias. Therefore, vitamin D had a potential prospective in preventing myocardial I/R injury.
Vitamin D and renal I/R injury
Renal I/R injury was induced by multiple conditions, such as renal transplantation, [Citation73] shock and sepsis.[Citation74] The damage of renal blood vessels and glomeruli further induced acute kidney injury, [Citation75] leading to renal failure and increased deaths. Oxidative stress, inflammation and mitochondrial dysfunction mainly accounted for the pathological process .[Citation76] Recent studies showed that vitamin D played a vital role in the process of renal I/R. It had been demonstrated that vitamin D synthesis (synthetic enzyme) in kidney was reduced while renal blood perfusion was decreased (as showed in patients with renovascular hypertension caused by unilateral renal artery stenosis [Citation77]), though vitamin D levels were unchanged in acute kidney I/R (27 min/18 h). Further, the deficiency of vitamin D or the dysfunction of its receptor pathway aggregated renal I/R injury. In a 35-day experiment, [Citation78] vitamin D-free treatment can further aggregate the vascular damage in arat model of renal I/R injury (45 min /7 d after the 28-day feeding). The numbers of activated CD4+ and CD8+ cells that infiltrated as well as h17/T-regulatory cell ratio were enhanced by vitamin D deficiency. It indicated that inflammation occurred. Another study [Citation79] using the same animal model showed that vitamin D-free treatment also increased renal cell proliferation and cell injury (e.g. lower renal aquaporin 2 expression) in renal I/R injury. And, vitamin D deficiency can also reduce the protein levels of VDRs in kidneys of model rats, which further aggregated renal I/R injury. It was because the VDR pathways were a vital factor to protect renal I/R injury according to a latest research.[Citation80] Conversely, renal I/R injury can damage VDR pathways (e.g. mRNA and protein levels of calcium ion transporter expressions) despite of unchanged vitamin D levels in plasma according to a research.[Citation81] In chronic renal injury after renal I/R in rats (45 min/62 d), vitamin D-free treatment (from 28 d before I/R to the end of the experiment) also contributed to renal pathologies (fibrosis, inflammatory reaction, tubular dilation, atrophy, etc).[Citation82] Decreased Klotho protein expressions further aggregated chronic renal I/R injury possibly due to less vitamin D production because the protein helped the synthesis of vitamin D.[Citation83] Therefore, supplementation with vitamin D would had double beneficial effects on improving renal I/R injury.
Thus far, many studies had been carried out to explore the protectiverole of vitamin D or its analogs on renal I/R injury. Most focused on animal I/R and cell models (rat or mouse) (shown in ) in addition to a clinical study that the high-dose administration of vitamin D improved the anti-inflammatory state and acute kidney injury induced by renal hypoperfusion before and after the cardiopulmonary bypass surgery.[Citation99] Preconditioning with vitamin D and its analogs can reduce renal I/R injury and restore renal function regardless of bilateral and unilateral renal I/R models. These effects of vitamin D were related to increasing renal cell proliferation, reducing apoptosis, inhibiting inflammatory reactions, and inhibiting oxidative stress. In addition, the protective role of vitamin D on renal I/R injury required a certain dose (e.g. vitamin D at 0.5 μg/kg dose affording the maximum protection [Citation93]). Meanwhile, the pretreatment for a certain period of time is necessary. For instance, the pretreatment of vitamin D at 6 hours and 1 hour before renal I/R (60 min/7 d) had no effects in protection against renal I/R injury even at high dose (2 mg/kg).[Citation100] Besides, vitamin D had also synergistic action with endogenous active substances (melatonin [Citation86]) in attenuating renal I/R injury. However, more clinical studies needed to be carried out for future applications.
Table 1. The protective effects of vitamin D and its analogs on renal I/R injury
Vitamin D and hepatic I/R injury
Hepatic I/R injury is caused by pathological and surgical factors (hemorrhage, [Citation101] liver resections [Citation102] and transplantation [Citation103]). Severe hepatic I/R injury induces systematic dysfunctions of multiple organs and even deaths.[Citation104] Therefore, attenuating hepatic I/R injury is always one of the hot study topics. The relationship of vitamin D with liver had been widely explored. Liver is not only one place in the process of vitamin D synthesis, but also the target of vitamin D action. First, vitamin D deficiency or dysfunctions aggregated occurrences of some liver diseases (e.g. liver cirrhosis, [Citation105] non-alcoholic fatty liver disease, [Citation106] liver transplant complications [Citation107]). Recent studies shown that administering vitamin D or its analogs can attenuate hepatic I/R injury. Ansam and Doaa [Citation108] (2014) found that the oral administration of vitamin D (500 IU/kg/d for 2 w before I/R) ameliorated oxidative injuries, inflammation and apoptosis in livers of a partial I/R rats (the left lateral and median lobes of the liver, 70%, 45 min/1 h), and the mesenteric venous congestion was also avoided. Then, Jinghui Yang et al. [Citation109] (2015) explored the effects of vitamin D pretreatment (500 IU/kg/d for 4 w) on the mouse hepatic I/R injury (60 min/6 h) at different time points after the ischemia treatment. The results showed that vitamin D protected hepatic function and reduced histological damage, oxidative stress, apoptosis, and inflammatory activation. And, the protective effect of vitamin D was best at 6 h after reperfusion. In another study, [Citation110] the pretreatment with a vitamin D analog (paricalcitol) (20 μg/kg,i.p injection 24 h) also significantly attenuated hepatic I/R (60 min/6 h) injury and histological damage. However, this dose of vitamin D treatment caused the pro-inflammatory reaction in the paricalcitol + sham group. It reminded that the dose selection should be noticed in later investigations, though vitamin D was a prospective nutritional therapeutic agent for attenuating hepatic I/R injury.
Vitamin D and cerebral I/R injury
Cerebral I/R injury resulted from rapid reperfusion after cerebral ischemia. Ischemic stroke, cardiac arrest, trauma and perinatal hypoxic ischemic injury were the common reasons.[Citation111] Cerebral I/R injury was presented with inflammatory activation, oxidative stress, blood–brain barrier destruction, and neuronal death.[Citation112] In view of restoring blood supply or reperfusion being still the most effective treatment, ameliorating cerebral I/R injury was one of the most active research fields. Brain barrier is passable to vitamin D, [Citation113] making the effects of vitamin D on brain got lots of attentions. It had been confirmed that vitamin D modulates multiple cerebral functions (e.g. neural stem cell proliferation and differentiation, neuroprotection, anti-inflammation, repairing brain barrier, etc.) [Citation114] through genomic and non-genomic mechanisms. The role of vitamin D in cerebral I/R injury originated from the finding of the positive relationship between low vitamin D levels and high cerebral I/R injuries. A study reported that blood–brain barrier dysfunction was significantly aggregated in middle cerebral artery I/R (90 min/72 h) rat model when vitamin D was in the insufficient status (15.56 ± 1.25 ng/mL in serum).[Citation115] Another study showed that vitamin D deficiency in diet with one-fifth of normal plasma vitamin D levels for 8 weeks exacerbated the stroke severity in intracerebral transient I/R rats (16 h/24 h, 3, 7 and 14 days) via dysregulating inflammatory response and suppressing neuroprotectant levels (insulin-like growth factor I).[Citation116] In this condition, even acute injections of vitamin D (10 μg/kg every 24 h from 4 h after stroke) did not attenuate the injuries. Therefore, it should be noted that long-term higher levels of vitamin D as early as possible may be needed to decrease cerebral I/R injury while vitamin D in deficiency. In clinic cases, low 25(OH)D levels were associated with worse outcomes at 3 months in patients treated with intravenous thrombolysis (using tissue plasminogen activators [Citation117] and alteplases [Citation118]) after acute ischemic stroke. And, low vitamin D had been associated with early adverse outcomes in hypoxic-ischemic encephalopathy [Citation119] and the infarct severity in acute ischemic stroke.[Citation120] Therefore, it promoted researchers to investigate different therapeutic strategies in supplying vitamin D to attenuate cerebral I/R injury.
Recently, increasing data had shown that vitamin D supplements can attenuate cerebral I/R injury and restore neuronal functions (in ). Both pretreatments and administrations after I/R of vitamin D produced remarkable protective effects (maintaining blood–brain barrier, and attenuating neuronal functional damages) against cerebral I/R injuries in different animal models. Antioxidative actions, inhibiting apoptosis and promoting proliferation, and anti-inflammation were involved in these effects.
Table 2. The protective effects of vitamin D on cerebral I/R injury
Moreover, some studies found that vitamin D together with other physiological substances played synergistic effects in attenuating cerebral I/R injury. For example, the co-treatment of vitamin D and other steroid hormones (e.g. progesterone [Citation127]) remarkably produced the most synergical effects on attenuating cerebral I/R injury in functional outcomes and apoptosis preventions. It was made under the lower dose of vitamin D and normal doses of progesterone whenever in vivo and in vitro. In another study, [Citation132] although both vitamin D and dehydroascorbic acid also produced significantly synergical effects on middle cerebral artery I/R injury (90 min/2 h) via preventing free radical generating, the monotherapy with vitamin D3 or dehydroascorbic acid had no effects on the I/R injury. It hinted that the summation effect via the antioxidative abilities partly contributed to the neurological protection. However, clinical investigations needed to be carried out for further applications.
Vitamin D and spinal I/R injury
Spinal I/R injury followed the unsuccessful surgery in the spinal cord (e.g. thoracoabdominal aortic intervention [Citation133]). Nerve damages can cause dysfunctions of sensations and movements. The therapeutic effects with previous measures (e.g. pharmacologic administrations, [Citation134] hyperbaric oxygen, [Citation135] ischemia [Citation136] and stem cells [Citation137]) were unsatisfied. Recently, vitamin D had been used to prevent spinal I/R injury. Calcitriol pretreatment (0.5 μg/kg, i.p) for 7 days before spinal I/R (20 min/24 h) of rabbits, remarkably improved histopathological, ultrastructural, and neurological scores.[Citation138] Inhibited oxidative stress and neurotic apoptosis accounted for the effects. However, more investigations needed to be carried before clinic applications.
Vitamin D and ovarian I/R injury
Ovarian ischemia resulted from some conditions, such as surgery, pregnancy, ovarian diseases, etc. The symptom of acute pains needs the timely therapy for blood perfusions.[Citation139] Still, ovary edema, bleeding and necrosis in females occurred after reperfusion.[Citation140] Even, early reperfusion after ischemia cannot restore ovarian functions. Thus, other interventions attenuating ovarian I/R injury need constantly to be developed. It had been demonstrated that vitamin D was correlated with ovarian functions (ovarian reserve, polycystic ovarian syndrome, and endometriosis).[Citation141] Thus far, limited data about the effects of vitamin D on ovarian I/R (3 h/3 h) injury were presented. A single study [Citation142] conducted with rat ovarian I/R model showed that the pretreatment with vitamin D at 30 min before I/R remarkably attenuated oxidative stress and histopathologic injury in ovarium. Further animal tests and clinical investigations were indispensable for clarify the potential protective effects of vitamin on ovarian I/R injury.
Vitamin D and ischemia infarction
Ischemia infarction in I/R is caused by prolonged ischemia (artery blockages, rupture, mechanical compression, or vasoconstriction).[Citation143] It occurs in different organs (heart, [Citation144] brain, [Citation145] bowels, [Citation146] etc.) and further aggregates I/R injury. Present studies of vitamin D affecting ischemia infarction mainly focused on heart and brain. For heart, clinical investigations showed that lower vitamin D levels in serum were associated with triggered initial phase or the outcome of myocardial ischemic infarction [Citation51] and higher 25(OH)D3 levels in serums of patients with ST-elevation myocardial infarction were associated with decreased I/R injury as well as increased acute SR, early revascularization, thrombolysis before PCI and PCI effects.[Citation147] And, there were no significant fluctuations about vitamin D levels and (or) its functions within short term in myocardial ischemia (e.g. in the first 48 hours after onset of acute myocardial infarction).[Citation148,Citation149] Therefore, the vitamin D deficiency before myocardial I/R is the key factor aggravating I/R injury. It was the same results under different degrees of vitamin D deficiencies (<10.2 ng/ml; 10.2–18.7 ng/ml; ≥18.8 ng/ml in serums).[Citation150] It can be provided evidence in another study that pretreatments of vitamin D (300,000 IU orally 12 h before PCI) to patients significantly lowered hs-CRP levels (an inflammatory marker) in contrast to no effects while administering vitamin D to patients after elective PCI.[Citation151]
Different from clinical data in the term of myocardial infarction, the effects of vitamin D on brain infarction were observed in animal model. Vitamin D treatments before and after brain ischemia infarction alleviated infarction and promoted proliferation of vascular endothelial cells in a rat model of middle cerebral artery I/R.[Citation152,Citation153] However, the role of vitamin D in ischemia infarctions of other organs needed further studies.
Mechanism of vitamin D attenuating I/R injury
Rectifying VDR dysfunctions in I/R injury
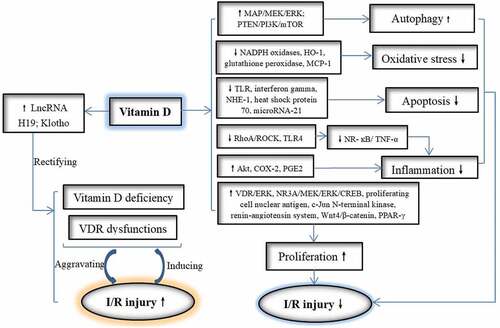
Vitamin D had been used to attenuate I/R injury for a long time in clinical investigations or animal studies. Related mechanisms are summarized in . First, vitamin D treatments reduced I/R injury through rectifying VDR dysfunctions (e.g. rectifying the increase [Citation66,Citation67] or the decrease [Citation69] of VDR levels in myocardial I/R). And, with the finding of lncRNA H19 [Citation69] and Klotho [Citation83] mediating VDR activation by vitamin D, it hinted that there may be more mechanisms of vitamin D regulating VDR needed to be revealed.
Figure 2. The pathways of vitamin D attenuating I/R injuries. Vitamin Dattenuating I/R injuries by suppressing oxidative stress, inflammation, and apoptosis as well as improving proliferation and autophagy. Exogenous vitamin D alsorectified its deficiency and VDRs by improving LncRNA and Klotho levels, which also reduced vicious cycle between I/R injuries and the dysfunctions of vitamin D and VDRs.
Attenuating oxidative stress, inflammation, autophagy, and necrosis in I/R injury
Oxidative stress and inflammatory reactions are vital factors damaging many organs in I/R, which can be decreased significantly by vitamin D administrations. In decreasing oxidative stress, suppressed NADPH oxidases, heme oxygenase-1 (HO-1), glutathione peroxidase [Citation94] and monocytechemoattractant protein-1 (MCP-1) [Citation96] are involved. Anti-inflammatory effects of vitamin D were performed via inhibiting Ras homolog family member A (RhoA)/ Rho kinase (ROCK)/nuclear factor kappa-B (NF-κB), [Citation70] NF-κB/tumor necrosis factor-α (TNF-α), [Citation68] toll-like receptors 4 (TLR4)/NF-Κb [Citation88] and increasing protein kinase B (Akt), [Citation90] cyclooxygenase 2 (COX-2) and Prostaglandin E2 (PGE2) .[Citation85] In addition, vitamin D can attenuate I/R injury through preventing apoptosis, inducing autophagy and promoting proliferation. Signal pathways related to anti-apoptosis include repressed TLR, interferon gamma, sodium–hydrogen exchanger-1 (NHE-1), [Citation91] heat shock protein 70 and microRNA-21.[Citation97] And, vitamin D promotes proliferation by activating VDR/extracellular-regulated kinase 1/2 (ERK), [Citation129] N-methyl-D-aspartate receptor subunit 3A (NR3A)/ERK kinase (MEK)/ ERK/ cyclic AMP responsive element-binding protein (CREB), [Citation126] proliferating cell nuclear antigen, [Citation84] renin–angiotensin system, wingless-related MMTV integration site 4 (Wnt4)/β-catenin, [Citation95] peroxisome proliferator-activated receptor gamma (PPAR-γ), [Citation93] brain-derived neurotrophic factor (BDNF), [Citation130] glial derived neurotrophic factor (GDNF), [Citation152] and inhibiting c-Jun N-terminal kinase.[Citation84] Vitamin D also induces autophagy through activating mitogen-activated protein (MAP)/MEK/ERK and phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) .[Citation109]
Conclusion
I/R injury haunted human health in multiple pathological and medical processes. Recently, vitamin D was connected with I/R injuries of different organs and tissues. Both pretreatments and administrations after I/R generally showed protective effects on I/R injury. Effects of the former were superior to those of the latter. On the other hand, different standard of vitamin D deficiency possibly affected next treatments. In addition, due to the studies about vitamin D improving I/R injuries mostly confined in animal models, further clinical investigations and mechanism explorations needed to be carried out before clinical recommendations.
Abbreviations
Akt: protein kinase B; BDNF: brain-derived neurotrophic factor; CABG: coronary artery bypass grafting; COX2: cyclooxygenase 2; CREB: cyclic AMP responsive element-binding protein; ERK: extracellular-regulated kinase 1/2; GDNF: glial-derived neurotrophic factor; HO-1: heme oxygenase-1; I/R: ischemia reperfusion; lncRNAH19:long non-coding RNA H19; MAP: mitogen-activated protein; MCP-1: monocytechemoattractant protein-1; MEK: mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa-B; NHE-1: sodium–hydrogen exchanger-1; NR3A: N-methyl-D-aspartate receptor subunit 3A; PCI: percutaneous coronary intervention; PGE2: prostaglandin E2; PI3K: phosphatidylinositol 3-kinase; PPAR-γ: peroxisome proliferator-activated receptor gamma; PTEN: phosphatase and tensin homolog deleted on chromosome 10; RAS: renin-angiotensin system; RDA: recommended dietary allowances; RhoA: Ras homolog family member A; ROCK: Rho kinase; SR: spontaneous reperfusion; aphenous vein grafts (SVGs); TLR: Toll-like receptor; TNF: tumor necrosis factor; TRKB: tyrosine kinase receptor B; VDR: vitamin D receptors; UL: upper intake levels; Wnt4: Wingless-related MMTV integration site 4.
Acknowledgments
This research was supported by research grants from Henan philosophy and social science planning project “Research on the reform path of medical humanities education based on Blended Learning” (Project No.: 2019BJY006). Jia Shang, Xiao Wan, Zhenxing Xie, Wunong Zhang, Chaoran Chen collected the data and composed the manuscript. All authors have reviewed and agreed to the publication of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Burak, A.; Cenk, C.; Fahrettin, K.; Kamil, G.; Fatih, A.; Ertuğrul, O.; Murat, B. Barıs O.The Relationship between Grade of Ischemia, Success of Reperfusion, and Type of Thrombolytic Regimen. Med. Sci. Monit. 2015, 21(3), 716–721. DOI: 10.12659/MSM.892645.
- Christina, M. ; Lukas, G.; Peter, S.; Bettina, L.; Philipp, S. Combating Ischemia-Reperfusion Injury with Micronutrients and Natural Compounds during Solid Organ Transplantation: Data of Clinical Trials and Lessons of Preclinical Findings. Int. J. Mol. Sci. 2021, 22(19), 10675. DOI: 10.3390/ijms221910675.
- Georgios, M; Alkistis, K; Michael, P; Anna, P; Dimitris, A; Anastasios, M; Theodoros, L; Konstantinos, M; Spyros, V; Andreas, M. L. Remote Ischemic Preconditioning Decreases the Magnitude of Hepatic Ischemia-Reperfusion Injury on a Swine Model of Supraceliac Aortic Cross-Clamping. Ann. Vasc. Surg. 2018, 48(4), 241–250. DOI: 10.1016/j.avsg.2017.08.006.
- Efstathios, K. I.; Ioannis, A. P.; Katerina, F.; Dimitrios, F.; Ioanna, A.; Aias, A.; Ignatios, I.; Dionyssios, L.; Dimitrios, T. K. Staccato Reperfusion Prevents Reperfusion Injury in Patients Undergoing Coronary Angioplasty: A 1-year Follow-up Pilot Study. Atherosclerosis. 2009, 204(2), 497–502. DOI: 10.1016/j.atherosclerosis.2008.09.037.
- Eltzschig, H. K; Collard, C. D. Vascular Ischaemia and Reperfusion Injury. Br. Med.Bull. 2004, 70, 71–86. DOI: 10.1093/bmb/ldh025.
- Eltzschig, H. K.; Eckle, T. Ischemia and Reperfusion–from Mechanism to Translation. Nat. Med. 2011, 17(11), 1391–1401. DOI: 10.1038/nm.2507.
- Stephenson, D.; Yin, T.; Smalstig, E. B.; Hsu, M. A.; Panetta, J.; Little, S.; Clemens, J. Transcription Factor Nuclear Factor-kappa B Is Activated in Neurons after Focal Cerebralischemia. J. Cerebr. Blood. F. Met. 2000, 20(3), 592–603. DOI: 10.1097/00004647-200003000-00017.
- Ravingerova, T; Farkasova, V; Griecsova, L; Carnicka, S; Murarikova, M; Barlaka, E; Kolar, F; Bartekova, M; Lonek, L; Slezak, J, et al. Remote Preconditioning as a Novel“Conditioning” Approach to Repair the Broken Heart: Potential Mechanisms and ClinicalApplications. Physiological Research. 2016, 65, S55–S64. DOI: 10.33549/physiolres.933392.
- Liao, Z; Liu, D; Tang, L; Yin, D; Yin, S; Lai, S; Yao, J; He, M. Long-term Oral Resveratrol in Take Provides Nutritional Preconditioning against Myocardial Ischemia /Reperfusion Injury:involvement of VDAC1 Downregulation. Mol. Nutr. Food. Res. 2015, 59(3), 454–464. DOI: 10.1002/mnfr.201400730.
- Chen, C; Zhou, X; He, J; Xie, Z; Xia, S; Lu, G. The Roles of GABA in Ischemia-Injury in the Central Nervous System and Peripheral Organs. Oxid.Med.Cell.Longev. 2019, 2019, 4028394. DOI: 10.1155/2019/4028394.
- Zuniga, J.; Cancino, M.; Medina, F.; Varela, P.; Vargas, R.; Tapia, G.; Videla, L. A.; Fernandez, V. N-3 PUFA Supplementation Triggers PPAR-alpha Activation and PPAR-alpha/ NF-kappaBinteraction:anti-inflammatory Implications in Liver Ischemia-reperfusion Injury. PLoS One. 2011, 6(12), e28502. DOI: 10.1371/journal.pone.0028502.
- Dorland’s Illustrated Medical Dictionary uVToV. 2011, 32 edition.
- Roseland, J. M. P. K; Patterson, K. Y; Pehrsson, P. R, and Taylor, C. L. Vitamin D in Foods: Anevolution of Knowledge. In Vitamin D, Volume 2: Health, Disease and Therapeutics, FourthEdition; Feldman, D., Pike, J. W., Bouillon, R., Giovannucci, E., Goltzman, D., Hewison, M., Eds.; British: Elsevier, 2018; pp 41–78.
- Jones, G. Vitamin D. In ModernNutrition in Health and Disease 11th; CB, R. A. C., Cousins, R. J., Tucker, K. L., Ziegler, T. R. Eds.; Lippincott Williams & Wilkins: Philadelphia, 2014.
- Institute of Medicine. National Academy Press: FaNBDRIfCaVDW, DC, 2010.
- Adams, J. S.; Hewison, M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010, 95(2), 471–478. DOI: 10.1210/jc.2009-1773.
- Wang, T.; Bengtsson, G.; Kärnefelt, I.; L.o, B. Provitamins and Vitamins D₂ and D₃ in Cladina Spp. Over a Latitudinal Gradient: Possible Correlation with UV Levels. J. Photochem. Photobiol. B: Biol. 2001, 62(1–2), 118–122. DOI: 10.1016/S1011-1344(01)00160-9.
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of Vitamin D. Recommendations 1981”. Eur.J. Biochem. 1982, 124(2), 223–227.
- Barbarawi, M; Kheiri, B; Zayed, Y; Barbarawi, O; Dhillon, H; Swaid, B; Yelangi, A; Sundus, S; Bachuwa, G; Alkotob, M. L. :., et al. Vitamin D Supplementation andCardiovascular Disease Risks inMore than 83000 Individuals in 21 Randomized ClinicalTrials: A Meta-analysis. JAMA. Cardiol. 2019, 4(8), 765–776. DOI: 10.1001/jamacardio.2019.1870.
- Sintzel, M. B.; Rametta, M.; Reder, A. T. Vitamin D and Multiple Sclerosis: A ComprehensiveReview. Neurol. Ther. 2018, 7(1), 59–85. DOI: 10.1007/s40120-017-0086-4.
- Seida, J. C.; Mitri, J.; Colmers, I. N.; Majumdar, S. R.; Davidson, M. B.; Edwards, A. L.; Hanley, D. A.; Pittas, A. G.; Tjosvold, L.; Johnson, J. A. Clinical Review: Effect of Vitamin D3supplementation on Improving Glucose Homeostasis and Preventing Diabetes: A Systematicreview and Meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99(10), 3551–3560. DOI: 10.1210/jc.2014-2136.
- Byers, T. Anticancer Vitamins du Jour–The ABCED’s so Far. Am.J .Epidemiol. 2010, 172(1), 1–3. DOI: 10.1093/aje/kwq112.
- Bivona, G; Gambino, C. M; Iacolino, G; Ciaccio, M. Vitamin D and the Nervoussystem. Neurol.Res. 2019, 41(9), 827–835. DOI: 10.1080/01616412.2019.1622872.
- Holick, M. F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis,treatmentand Prevention. Rev. Endocr. Metab. Disord. 2017, 18(2), 153–165. DOI: 10.1007/s11154-017-9424-1.
- Raisi-Estabragh, Z; Martineau, A. R; Curtis, E. M; Moon, R. J; Darling, A; Lanham-New, S; Ward, K. A; Cooper, C; Munroe, P. B; Petersen, S. E, et al. Vitamin D Andcoronavirusdisease2019 (COVID-19): Rapid Evidence Review. Aging. Clin. Exp. Res. 2021, 33(7), 2031–2041. DOI: 10.1007/s40520-021-01894-z.
- Tecilazich, F.; Formenti, A. M.; Giustina, A. Role of Vitamin D in Diabetic retinopathy:Pathophysiologicaland Clinical Aspects. Rev. Endocr. Metab. Disord. 2020, 10, 1–13.
- Bouillon, R; Carmeliet, G; Verlinden, L; van Etten, E; Verstuyf, A; Luderer, H. F; Lieben, L; Mathieu, C; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptornull Mice. Endocr. Rev. 2008, 29(6), 726–776. DOI: 10.1210/er.2008-0004.
- Hii, C. S.; Ferrante, A. The Non-Genomic Actions of Vitamin. D.Nutrients. 2016, 8(3), 135. DOI: 10.3390/nu8030135.
- Wang, Y. J; Zhu, J. G; DeLuca, H. F. Where Is the Vitamin D Receptor? Archives ofBiochemistryandBiophysics. 2012, 523(1), 123–133.
- Marino, R; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients. 2019, 11(7), 1460. DOI: 10.3390/nu11071460.
- Ross, A. C.; Taylor, C. L.; Yaktine, A. L., and Del Valle, H. B. In Dietary ReferenceIntakes forCalcium and Vitamin D. The National Academies Collection: Reports Funded by NationalInstitutes of Health. 2011. Eds. Washington, (DC): The National Press.
- Giustina, A.; Adler, R. A.; Binkley, N.; Bouillon, R.; Ebeling, P. R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C. T.; Bilezikian, J. P. Controversies in Vitamin D:Summary Statement from an International Conference. J. Clin.Endocrinol. Metab. 2019, 104(2), 234–240. DOI: 10.1210/jc.2018-01414.
- Rosen, C. J.; Abrams, S. A.; Aloia, J. F.; Brannon, P. M.; Clinton, S. K.; Durazo-Arvizu, R. A.; Gallagher, J. C.; Gallo, R. L.; Jones, G.; Kovacs, C. S., et al. IOM Committee Members Respond to Endocrine Societyvitamin D Guideline. J. Clin. Endocrinol. Metab. 2012, 97(4), 1146–1152. DOI: 10.1210/jc.2011-2218.
- Chalcraft, J. R.; Cardinal, L. M.; Wechsler, P. J.; Hollis, B. W.; Gerow, K. G.; Alexander, B. M.; Keith, J. F.; Larson-Meyer, D. E. Vitamin D Synthesis following a SingleBoutof SunExposure in Older and Younger Men and Women. Nutrients. 2020, 12(8), 2237. DOI: 10.3390/nu12082237.
- Brown, L. L; Cohen, B; Tabor, D; Zappala, G; Maruvada, P; Coates, P. M. The Vitamin Dparadox in Black Americans: A Systems-based Approach to Investigating Clinical Practice,research, and Public Health - Expert Panel Meeting Report. BMC.Proc. 2018, 12(Suppl 6), 6. DOI: 10.1186/s12919-018-0102-4.
- Bouillon, R; Marcocci, C.; Carmeliet, G.; Bikle, D; White, J. H; Dawson-Hughes, B.; Lips, P.; Munns, C. F.; Lazaretti-Castro, M.; Giustina, A., et al. Skeletal and ExtraskeletalActions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40(4), 1109–1151. DOI: 10.1210/er.2018-00126.
- Galior, K; Grebe, S; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients. 2018, 10(8), 953. DOI: 10.3390/nu10080953.
- Vogiatzi, M. G; Jacobson-Dickman, E; DeBoer, M. D. Drugs, Therapeutics Committee ofThe Pediatric Endocrine S: Vitamin D Supplementation and Risk of Toxicity in PediatricS: Areview of Current Literature. J.Clin. Endocrinol.Metab. 2014, 99(4), 1132–1141. DOI: 10.1210/jc.2013-3655.
- Ross, A. C.; Manson, J. E.; Abrams, S. A.; Aloia, J. F.; Brannon, P. M.; Clinton, S. K.; Durazo-Arvizu, R. A.; Gallagher, J. C.; Gallo, R. L.; Jones, G., et al. The 2011 Report on Dietary Referenceintakes for Calcium and Vitamin D from theInstitute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96(1), 3–58. DOI: 10.1210/jc.2010-2704.
- Shimokawa, H.; Yasuda, S. Myocardial Ischemia: Current Concepts and Future Perspectives. J. Cardiol. 2008, 52(2), 67–78. DOI: 10.1016/j.jjcc.2008.07.016.
- Fishbeina, G. A.; Fishbeina, M. C., and Buja, L. M. Cardiovascular Pathology. 4th. Academic Press; 2016. Chapter 7 - Myocardial Ischemia and Its Complications; pp. 239–270., 2016.
- Basalay, M. V.; Yellon, D. M.; Davidson, S. M. Targeting Myocardial Ischaemic Injuryin the Absence of Reperfusion. Basic. Res. Cardiol. 2020, 115(5), 63. DOI: 10.1007/s00395-020-00825-9.
- Heusch, G. Myocardial Ischaemia-reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020, 17(12), 773–789. DOI: 10.1038/s41569-020-0403-y.
- Zhang, C; He, M; N,i, L.; He, K; Su, K; Deng, Y; Li, Y; Xia, H. The Role of ArachidonicAcid Metabolism in Myocardial Ischemia-Reperfusion Injury. Cell. Biochem. Biophys. 2020, 78(3), 255–265. DOI: 10.1007/s12013-020-00928-z.
- Muscogiuri, G; Annweiler, C; Duval, G; Karras, S; Tirabassi, G; Salvio, G; Balercia, G.; Kimball, S.; Kotsa, K.; Mascitelli, L., et al. Vitamin D Andcardiovascular Disease: From Atherosclerosis to Myocardial Infarction and Stroke. Int. J.Cardiol. 2017, 230, 577–584. DOI: 10.1016/j.ijcard.2016.12.053.
- Rai, V; Agrawal, D. K. Role of Vitamin D in Cardiovascular Diseases. Endocrinol. Metab.Clin. North. Am. 2017, 46(4), 1039–1059. DOI: 10.1016/j.ecl.2017.07.009.
- Bouillon, R. Vitamin D and Cardiovascular Disorders. Osteoporos. Int. 2019, 30(11), 2167–2181. DOI: 10.1007/s00198-019-05098-0.
- Legarth, C; Grimm, D; Kruger, M; Infanger, M; Wehland, M. Potential Beneficial Effectsof Vitamin D in Coronary Artery Disease. Nutrients. 2019, 12(1), 99. DOI: 10.3390/nu12010099.
- Saponaro, F.; Marcocci, C.; Zucchi, R. Vitamin D Status and Cardiovascular Outcome. J. Endocrinol. Invest. 2019, 42(11), 1285–1290. DOI: 10.1007/s40618-019-01057-y.
- Cioffi, G.; Gatti, D.; Adami, S. Vitamin D Deficiency, Left Ventricular Dysfunction and Heartfailure. G. Ital. Cardiol (Rome). 2010, 11(9), 645–653.
- Amen, S. O.; Baban, S. T. Association of Vitamin D Deficiency with Acute MyocardialInfarction in Iraqi Patients. Eur. Cardiol. 2020, 15, e29. DOI: 10.15420/ecr.2020.15.1.PO6.
- Hausenloy, D. J; Yellon, D. M. Myocardial Ischemia-reperfusion Injury: A Neglected Therapeutictarget. J. Clin. Invest. 2013, 123(1), 92–100. DOI: 10.1172/JCI62874.
- Verdoia, M.; Nardin, M.; Rolla, R.; Negro, F.; Gioscia, R.; Afifeh, A. M. S.; Viglione, F.; Suryapranata, H.; Marcolongo, M.; De Luca, G. Novara Atherosclerosis Study G: Prognostic Impact of Vitamin D Deficiency in Patients with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Eur. J. Intern. Med. 2020, 83(1), 62–67.
- Bischoff-Ferrari, H. A.; Dawson-Hughes, B.; Staehelin, H. B.; Orav, J. E.; Stuck, A. E.; Theiler, R.; Wong, J. B.; Egli, A.; Kiel, D. P.; Henschkowski, J. Fall Prevention with Supplementaland Active Forms of Vitamin D: A Meta-analysis of Randomised Controlled Trials. BMJ. 2009, 339, b3692.
- Braun, L. A; Spitzer, O; Levkovich, B; Bailey, M; Stanguts, C; Hose, L.; Rosenfeldt, F. Prevalence of Vitamin D Deficiency Prior to Cardiothoracic Surgery. Heart. Lung. Circ. 2014, 23(10), 978–980. DOI: 10.1016/j.hlc.2014.03.014.
- Sen, F; Yilmaz, S; Balci, K. G; Sen, O; Gul, M; Cay, S; Topaloglu, S; Aydogdu, S. Therelationship between Vitamin D Levels and Saphenous Vein Graft Patency. Coron. Artery.Dis. 2015, 26(4), 328–332. DOI: 10.1097/MCA.0000000000000240.
- Zarei, M; Najafi, M; Movahedi, E; Javanbakht, M. H; Choi, Y. H; Yaseri, M; Shirvani, A; Sellke, F. W; Stranges, S. The Predictive Role of Circulating Telomerase and Vitamin Dforlong-term Survival in Patients Undergoing Coronary Artery Bypass Grafting Surgery (CABG). PLoS One. 2020, 15(8), e0237477. DOI: 10.1371/journal.pone.0237477.
- Sheane, B. J; Smyth, P; Scott, K; Aziz, R; Buckley, M; Lodge, E; Kiely, N; Kingston, M; McGovern, E; Healy, M, et al. An Association betweenMicroRNA-21 Expression and Vitamin D Deficiency in Coronary Artery Disease. Microrna. 2015, 4(1), 57–63. DOI: 10.2174/2211536604666150414203919.
- Rajasree, S.; Rajpal, K.; Kartha, C. C.; Sarma, P. S.; Kutty, V. R.; Iyer, C. S.; Girija, G. Serum25-hydroxyvitamin D3 Levels are Elevated in South Indian Patients with Ischemic Heartdisease. Eur. J. Epidemiol. 2001, 17(6), 567–571. DOI: 10.1023/A:1014559600042.
- Linden, V. Vitamin D and Myocardial Infarction. Br. Med. J. 1974, 3(5932), 647–650. DOI: 10.1136/bmj.3.5932.647.
- Kutty, V. R; Balakrishnan, K. G; Jayasree, A. K; Thomas, J. Prevalence of Coronary Heartdisease in the Rural Population of Thiruvananthapuram District, Kerala, India. Int.J. Cardiol. 1993, 39(1), 59–70. DOI: 10.1016/0167-5273(93)90297-T.
- Tasdighi, E.; Hekmat, M.; Beheshti, M.; Baghaei, R.; Mirhosseini, S. M.; Torbati, P.; Pourmotahari, F.; Foroughi, M. Vitamin D Treatment Attenuates Heart ApoptosisAfter Coronary Artery Bypass Surgery: A Double-Blind, Randomized, Placebo-ControlledClinical Trial. J. Cardiovasc. Pharmacol. Ther. 2020, 25(4), 338–345. DOI: 10.1177/1074248420920495.
- Cerit, L.; Ozcem, B.; Cerit, Z.; Duygu, H. Preventive Effect of Preoperative Vitamin DSupplementation on Postoperative Atrial Fibrillation. Braz. J. Cardiovasc. Surg. 2018, 33(1), 347–352. DOI: 10.21470/1678-9741-2018-0014.
- Kara, H; Yasim, A. Effects of High-dose Vitamin D Supplementation on the Occurrence Ofpost-operative Atrial Fibrillation after Coronary Artery Bypass Grafting: Randomized Controlledtrial. Gen. Thorac. Cardiovasc.Surg. 2020, 68(5), 477–484. DOI: 10.1007/s11748-019-01209-0.
- Yao, T. B; Ying, X. Y; Zhao, Y. C; Yuan, A. C; He, Q; Tong, H; Ding, S; Liu, J. L.; Peng, X.; Gao, E. H., et al. Vitamin D Receptor Activation Protects against MyocardialReperfusionInjury through Inhibition of Apoptosis and Modulation of Autophagy. Antioxid. Redox Signaling. 2015, 22(8), 633–650. DOI: 10.1089/ars.2014.5887.
- Wu, Y.; Liu, F.; Ma, X.; Adi, D.; Gai, M. T.; Jin, X.; Yang, Y. N.; Huang, Y.; Xie, X.; Li, X. M., et al. iTRAQ Analysis of a Mouse Acute Myocardial Infarction Model Reveals thatvitaminD Binding Protein Promotes Cardiomyocyte Apoptosis after Hypoxia. Oncotarget. 2018, 9(2), 1969–1979.
- Diez, E. R; Altamirano, L. B; Garcia, I. M; Mazzei, L; Prado, N. J; Fornes, M. W; Carrion, F. D; Zumino, A. Z; Ferder, L; Manucha, W. Heart Remodeling Andischemia-reperfusion Arrhythmias Linked to Myocardial Vitamin D Receptorsdeficiency in Obstructive Nephropathy are Reversed by Paricalcitol. J. CardiovascPharmacol. Ther. 2015, 20(2), 211–220.
- Zhou, C. N.; Yao, W.; Gong, Y. N.; Li, Y.; Wang, C. H.; Huo, Y. F. 22-oxacalcitriolprotects Myocardial Ischemia-reperfusion Injury by Suppressing NF-kappaB/TNF-alpha Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23(12), 5495–5502. DOI: 10.26355/eurrev_201906_18219.
- Hobuss, L; Foinquinos, A; Jung, M; Kenneweg, F; Xiao, K; Wang, Y; Zimmer, K.; Remke, J.; Just, A.; Nowak, J., et al. Pleiotropic Cardiac Functions Controlled by Ischemia-induced lncRNA H19. J. Mol.Cell. Cardiol. 2020, 146, 43–59. DOI: 10.1016/j.yjmcc.2020.07.001.
- Qian, X; Zhu, M; Qian, W; Song, J. Vitamin D Attenuates Myocardial Ischemia-reperfusion Injury by Inhibiting Inflammation via Suppressing the RhoA/ROCK/NF-kB Pathway. Biotechnol. Appl. Biochem. 2019, 66(5), 850–857. DOI: 10.1002/bab.1797.
- Lee, T. L; Lee, M. H; Chen, Y. C; Lee, Y. C; Lai, T. C; Lin, H. Y; Hsu, L. F; Sung, H. C.; Lee, C. W.; Chen, Y. L. Vitamin D Attenuates Ischemia/Reperfusion-InducedCardiac Injury by Reducing Mitochondrial Fission and Mitophagy. Front.Pharmacol. 2020, 11, 604700.
- Safari, F; Zarei, F; Shekarforoush, S; Fekri, A; Klishadi, M. S; Hekmati- Moghaddam, S. Combined 1,25-Dihydroxy-vitamin D and Resveratrol: A Novel Therapeutic Approacto Ameliorate Ischemia Reperfusion-Induced Myocardial Injury. Int. J. Vitam. Nutr. Res. 2015, 85, 174–184. DOI: 10.1024/0300-9831/a000236.
- Salvadori, M; Rosso, G; Bertoni, E. Update on Ischemia-reperfusion Injury in Kidneytransplantation: Pathogenesis and Treatment. World. J. Transplant. 2015, 5(2), 52–67. DOI: 10.5500/wjt.v5.i2.52.
- Le Clef, N.; Verhulst, A.; D’Haese, P. C.; Vervaet, B. A. Unilateral Renal Ischemia-Reperfusionas a Robust Model for Acute to Chronic Kidney Injury in Mice. PLoS One. 2016, 11(3), e0152153. DOI: 10.1371/journal.pone.0152153.
- Chertow, G. M; Burdick, E; Honour, M; Bonventre, J. V; Bates, D. W. Acute Kidney Injury,mortality, Length of Stay, and Costs in Hospitalized Patients. J. Am. Soc. Nephrol. 2005, 16(11), 3365–3370. DOI: 10.1681/ASN.2004090740.
- Bonventre, J. V; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin.Invest. 2011, 121(11), 4210–4221. DOI: 10.1172/JCI45161.
- Kiersztejn, M.; Wiecek, A.; Kokot, F.; Schmidt-Gayk, H.; Wystrychowski, A.; Kuczera, M. Levels of 1,25-dihydroxyvitamin D3 Concentration in Renal Venous Blood Serum of Patientswith Renovascular Hypertension Caused by Unilateral Renal Artery Stenosis. Pol. Arch. Med.Wewn. 1998, 99(4), 281–286.
- de Braganca, A. C.; Volpini, R. A.; Mehrotra, P.; Andrade, L.; Basile, D. P. Vitamin D Deficiencycontributes to Vascular Damage in Sustained Ischemic Acute Kidney Injury. Physiol. Rep. 2016, 4(13), e12829. DOI: 10.14814/phy2.12829.
- de Braganca, A. C.; Volpini, R. A.; Canale, D.; Goncalves, J. G.; Shimizu, M. H.; Sanches, T. R.; Seguro, A. C.; Andrade, L. Vitamin D Deficiency Aggravates Ischemic Acute Kidney Injury Inrats. Physiol. Rep. 2015, 3(3), e12331. DOI: 10.14814/phy2.12331.
- Silva Barbosa, A. C.; Zhou, D.; Xie, Y.; Choi, Y. J.; Tung, H. C.; Chen, X.; Xu, M.; Gibbs, R. B.; Poloyac, S. M.; Liu, S., et al. Inhibition of EstrogenSulfotransferase (SULT1E1/EST) Ameliorates Ischemic Acute Kidney Injury in Mice. J. Am.Soc. Nephrol. 2020, 31(7), 1496–1508. DOI: 10.1681/ASN.2019080767.
- Meurer, M; Hocherl, K. Renal Ischemia-reperfusion Injury Impairs Renal Calcium, Magnesium,and Phosphate Handling in Mice. Pflugers. Arch. 2019, 471(6), 901–914. DOI: 10.1007/s00424-019-02255-6.
- Goncalves, J. G; de Braganca, A. C; Canale, D; Shimizu, M. H; Sanches, T. R; Moyses, R. M; Andrade, L; Seguro, A. C; Volpini, R. A. Vitamin D Deficiency Aggravates Chronic Kidneydisease Progression after Ischemic Acute Kidney Injury. PLoS One. 2014, 9(9), e107228. DOI: 10.1371/journal.pone.0107228.
- Kuro-o, M. Klotho, Phosphate and FGF-23 in Ageing and Disturbed Mineral Metabolism. Nat.Rev. Nephrol. 2013, 9(11), 650–660. DOI: 10.1038/nrneph.2013.111.
- Kim, Y. O; Li, C; Sun, B. K; Kim, J. S; Lim, S. W; Choi, B. S; Kim, Y. S; Kim, J; Bang, B. K; Yang, C. W. Preconditioning with 1,25-dihydroxyvitamin D3 Protects against Subsequentischemia-reperfusion Injury in the Rat Kidney. Nephron. Exp. Nephrol. 2005, 100(2), e85–94. DOI: 10.1159/000084574.
- Hwang, H. S; Yang, K. J; Park, K. C; Choi, H. S; Kim, S. H; Hong, S. Y; Jeon, B. H; Chang, Y. K.; Park, C. W.; Kim, S. Y., et al. Pretreatment with Paricalcitol Attenuatesinflammation in Ischemia-reperfusion Injury via the Up-regulation of Cyclooxygenase-2 Andprostaglandin E2. Nephrol. Dial. Transplant. 2013, 28(5), 1156–1166. DOI: 10.1093/ndt/gfs540.
- Sinanoglu, O; Sezgin, G; Ozturk, G; Tuncdemir, M; Guney, S; Aksungar, F. B; Yener, N. Melatonin with 1,25-dihydroxyvitamin D3 Protects against Apoptotic Ischemia-reperfusion Injury in the Rat Kidney. Ren. Fail. 2012, 34(8), 1021–1026. DOI: 10.3109/0886022X.2012.700887.
- Sezgin, G; Ozturk, G; Guney, S; Sinanoglu, O; Tuncdemir, M. Protective Effect of Melatoninand 1,25-dihydroxyvitamin D3 on Renal Ischemia-reperfusion Injury in Rats. Ren. Fail. 2013, 35(3), 374–379. DOI: 10.3109/0886022X.2012.760409.
- Lee, J. W.; Kim, S. C.; Ko, Y. S.; Lee, H. Y.; Cho, E.; Kim, M. G.; Jo, S. K.; Cho, W. Y.; Kim, H. K. Renoprotective Effect of Paricalcitol via a Modulation of the TLR4-NF-kappaB Pathway Inischemia/reperfusion-induced Acute Kidney Injury. Biochem. Biophys. Res. Commun. 2014, 444(2), 121–127. DOI: 10.1016/j.bbrc.2014.01.005.
- Ersan, S; Celik, A; Tanrisev, M; Kose, I; Cavdar, Z; Unlu, M; Kocak, A; Ural, C; Yilmaz, B.; Kose, T. Pretreatment with Paricalcitol Attenuates Level and Expression of Matrixmetalloproteinases in a Rat Model of Renal Ischemia-reperfusion Injury. Clin. Nephrol. 2017, 88(11), 231–238. DOI: 10.5414/CN109121.
- Hong, Y. A.; Yang, K. J.; Jung, S. Y.; Park, K. C.; Choi, H.; Oh, J. M.; Lee, S. J.; Chang, Y. K.; Park, C. W.; Yang, C. W., et al. Paricalcitol Pretreatment Attenuates RenalIschemia-Reperfusion Injury via Prostaglandin E2 Receptor EP4 Pathway. Oxid. Med. Cell.Longev. 2017, 2017, 5031926. DOI: 10.1155/2017/5031926.
- Hamzawy, M; Gouda, S. A. A; Rashed, L; Morcos, M. A; Shoukry, H; Sharawy, N. 22-oxacalcitriol Prevents Acute Kidney Injury via Inhibition of Apoptosis and Enhancement Ofautophagy. Clin. Exp. Nephrol. 2019, 23(1), 43–55. DOI: 10.1007/s10157-018-1614-y.
- Azak, A; Huddam, B; Haberal, N; Kocak, G; Ortabozkoyun, L; Senes, M; Akdogan, M. F; Denizli, N; Duranay, M. Effect of Novel Vitamin D Receptor Activator Paricalcitol on Renalischaemia/reperfusion Injury in Rats. Ann. R. Coll. Surg. Engl. 2013, 95(7), 489–494. DOI: 10.1308/003588413X13629960049117.
- Kapil, A.; Singh, J. P.; Kaur, T.; Singh, B.; Singh, A. P. Involvement of Peroxisome Proliferator-activated Receptor Gamma in Vitamin D-mediated Protection against Acute Kidney Injury in Rats. J. Surg. Res. 2013, 185(2), 774–783. DOI: 10.1016/j.jss.2013.07.017.
- Li, J; Xu, S; Zhu, J. B; Song, J; Luo, B; Song, Y. P; Zhang, Z. H; Chen, Y. H; Zhang, Z. Q; Xie, D. D, et al. Pretreatment with Cholecalciferol Alleviates Renal CellularStress Response during Ischemia/Reperfusion-Induced Acute Kidney Injury. OxidativeMedicine and Cellular Longevity .2019, 2019, 1897316.
- Ali, R. M; Al-Shorbagy, M. Y; Helmy, M. W; El-Abhar, H. S. Role of Wnt4/beta-catenin, AngII/TGFbeta, ACE2, NF-kappaB, and IL-18 in Attenuating Renal Ischemia/reperfusion-inducedinjury in Rats Treated with Vit D and Pioglitazone. Eur. J. Pharmacol. 2018, 831, 68–76. DOI: 10.1016/j.ejphar.2018.04.032.
- Arfian, N; Budiharjo, S; Wibisono, D. P; Setyaningsih, W. A. W; Romi, M. M; Saputri, R; Rofiah, E. K; Rahmanti, T; Agustin, M; Sari, D. C. R. Vitamin D Ameliorates Kidney IschemiaReperfusion Injury via Reduction of Inflammation and Myofibroblast Expansion.Kobe. J.Med. Sci. 2020, 65(4), E138–E143.
- Golmohammadi, M. G.; Banaei, S.; Nejati, K.; Chinifroush-Asl, M. M. Vitamin D3 Anderythropoietin Protect against Renal Ischemia-reperfusion Injury via Heat Shock Protein 70 andmicroRNA-21 Expression. Sci. Rep. 2020, 10(1), 20906. DOI: 10.1038/s41598-020-78045-3.
- Dos Santos, M. S.; Canale, D.; Bernardo, D. R. D.; Shimizu, M. H. M.; Seguro, A. C.; Volpini, R. A.; de Braganca, A. C. The Restoration of Vitamin D Levels Slows the Progression of Renalschemic Injury in Rats Previously Deficient in Vitamin D.Front. Med (Lausanne). 2021, 8, 625647.
- Pegah, E; Manouchehr, H; Mahmoud, B; Ramin, B; Seyed, M. M; Fatemeh, P; Seyed, A. Z; Mahnoosh, F. A. Randomized,Double-Blind,Placebo-Controlled, Clinical Trial of High-Dose, Short-Term Vitamin D Administration in the Prevention of Acute Kidney Injury after Cardiac Surgery. Cardiorenal Med. 2021, 11(1), 52–58. DOI: 10.1159/000511058.
- Parra, C; Salas, P; Dominguez, J. Effects of Immunosuppressive Drugs on Rat Renal Ischemiareperfusion Injury. Transplant. Proc. 2010, 42(1), 245–247. DOI: 10.1016/j.transproceed.2009.11.018.
- Douzinas, E. E; Livaditi, O; Tasoulis, M. K; Prigouris, P; Bakos, D; Goutas, N; Vlachodimitropoulos, D; Andrianakis, I; Betrosian, A; Tsoukalas, G. D. Nitrosative Andoxidative Stresses Contribute to Post-ischemic Liver Injury following Severe Hemorrhagic Shock:the Role of Hypoxemic Resuscitation. PLoS One. 2012, 7(3), e32968. DOI: 10.1371/journal.pone.0032968.
- Arkadopoulos, N.; Defterevos, G.; Nastos, C.; Papalois, A.; Kalimeris, K.; Papoutsidakis, N.; Kampouroglou, G.; Kypriotis, D.; Pafiti, A.; Kostopanagiotou, G., et al. Development of a Porcine Model of Post-hepatectomy Liver Failure. J. Surg. Res. 2011, 170(2), e233–242. DOI: 10.1016/j.jss.2011.06.006.
- Jaeschke, H. Molecular Mechanisms of Hepatic Ischemia-reperfusion Injury Andpreconditioning. Am J Physiol-Gastrointestinal Liver Physiol. 2003, 284(1), G15–G26. DOI: 10.1152/ajpgi.00342.2002.
- Cannistra, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic Ischemia Reperfusion Injury: A Systematic Review of Literatureand the Role of Current Drugs and Biomarkers. Int. J. Surg. 2016, 33(Suppl 1), S57–70. DOI: 10.1016/j.ijsu.2016.05.050.
- Bjelakovic, G.; Nikolova, D.; Bjelakovic, M.; Gluud, C. Vitamin D Supplementation Forchronic Liver Diseases in adults.Cochrane. Database. Syst. Rev. 2017, 11, CD011564.
- Barchetta, I; Cimini, F. A; Cavallo, M. G. Vitamin D Supplementation and Non-AlcoholicFatty Liver Disease: Present and Future. Nutrients. 2017, 9(9), 1015. DOI: 10.3390/nu9091015.
- Ferrándiz-Pulido, C.; Torres, I. B.; Juárez-Dobjanschi, C.; Zarzoso-Muñoz, I.; Berastegui, C.; Castells, L.; García-Patos, V.; Moreso, F. Vitamin D Deficiency in Solid-organ Transplant Recipientsfrom a Spanish Mediterranean Population. Clin. Exp. Dermatol. 2019, 44(4), e103–e109. DOI: 10.1111/ced.13915.
- Seif, A. A; Abdelwahed, D. M. Vitamin D Ameliorates Hepatic Ischemic/reperfusion Injury Inrats. J. Physiol. Biochem. 2014, 70(3), 659–666. DOI: 10.1007/s13105-014-0335-2.
- Yang, J.; Chen, Q.; Tian, S.; Song, S.; Liu, F.; Wang, Q.; Fu, Z. The Role of 1,25-dyhydroxyvitamin D3 in Mouse Liver Ischemia Reperfusion Injury: Regulation of Autophagythrough Activation of MEK/ERK Signaling and PTEN/PI3K/Akt/mTORC1 Signaling. Am. J.Transl. Res. 2015, 7(12), 2630–2645.
- Kim, M. S; Lee, S; Jung, N; Lee, K; Choi, J; Kim, S. H; Jun, J; Lee, W. M; Chang, Y; Kim, D. The Vitamin D Analogue Paricalcitol Attenuates Hepatic Ischemia/reperfusion Injurythrough Down-regulation of Toll-like Receptor 4 Signaling in Rats. Archives of Medical Science. 2017, 13(2), 459–469. DOI: 10.5114/aoms.2016.60650.
- Galkin, A. Brain Ischemia/Reperfusion Injury and Mitochondrial Complex I Damage. Biochemistry (Mosc). 2019, 84(11), 1411–1423. DOI: 10.1134/S0006297919110154.
- Liang, T. Y; Peng, S. Y; Ma, M.; Li, H. Y; Wang, Z; Chen, G. Protective Effects of Sevofluranein Cerebral Ischemia Reperfusion Injury: A Narrative Review. Med. Gas. Res. 2021, 11(4), 152–154. DOI: 10.4103/2045-9912.318860.
- Molinari, C; Morsanuto, V; Ghirlanda, S; Ruga, S; Notte, F; Gaetano, L; Uberti, F. Role ofCombined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell. Longev. 2019, 2019, 2843121. DOI: 10.1155/2019/2843121.
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J. J.; Eyles, D. Vitamin D and the Brain: Genomic Andnon-genomic Actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. DOI: 10.1016/j.mce.2017.05.035.
- Sayeed, I; Turan, N; Stein, D. G; Wali, B. Vitamin D Deficiency Increases Blood-brain Barrierdysfunction after Ischemic Stroke in Male Rats. Exp. Neurol. 2019, 312, 63–71. DOI: 10.1016/j.expneurol.2018.11.005.
- Balden, R; Selvamani, A; Sohrabji, F. Vitamin D Deficiency Exacerbates Experimental Strokeinjury and Dysregulates Ischemia-induced Inflammation in Adult Rats. Endocrinolog. 2012, 153(5), 2420–2435. DOI: 10.1210/en.2011-1783.
- Daumas, A; Daubail, B; Legris, N; Jacquin-Piques, A; Sensenbrenner, B; Denimal, D; Lemaire-Ewing, S; Duvillard, L; Giroud, M; Bejot, Y. Association between AdmissionSerum 25-Hydroxyvitamin D Levels and Functional Outcome of Thrombolyzed StrokePatients. J. Stroke. Cerebrovasc. Dis. 2016, 25(4), 907–913. DOI: 10.1016/j.jstrokecerebrovasdis.2016.01.005.
- Wei, L; Chen, C; Dai, Y. Q.; Ding, L.; Li, H. Y.; Lin, Y. J.; Wu, H. T.; Wu, Z.; Lu, Z. Q. Serum25-hydroxyvitamin D Deficiency Predicts Poor Outcomes among Acute Ischemic Strokepatients Receiving Intravenous Thrombolysis. Chinese Med. J. 2019, 132(4), 491–494. DOI: 10.1097/CM9.0000000000000084.
- McGinn, E. A; Powers, A; Galas, M; Lyden, E; Peeples, E. S. Neonatal Vitamin D Status IsAssociated with the Severity of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy:A Pilot Study. Neuropediatrics. 2020, 51(104), 251–258. DOI: 10.1055/s-0040-1708535.
- Li, Y. Y; Wang, Y. S; Chen, Y; Hu, Y. H; Cui, W; Shi, X. Y; Jiang, W; Zhang, J. M. Association of Serum 25(OH)D Levels with Infarct Volumes and Stroke Severity inAcute Ischemic Stroke. Journal of Nutrition Health & Aging. 2018, 22(1), 97–102. DOI: 10.1007/s12603-017-0926-z.
- Won, S; Sayeed, I; Peterson, B. L; Wali, B; Kahn, J. S; Stein, D. G. Vitamin D PreventsHypoxia/Reoxygenation-Induced Blood-Brain Barrier Disruption via Vitamin DReceptor-Mediated NF-kB Signaling Pathways. Plos One. 2015, 10(3), e0122821. DOI: 10.1371/journal.pone.0122821.
- Atif, F; Yousuf, S; Espinosa-Garcia, C; Harris, W. A. C; Stein, D. G. Post-ischemic Strokesystemic Inflammation: Immunomodulation by Progesterone and Vitamin Dhormone. Neuropharmacology. 2020, 181, 108327. DOI: 10.1016/j.neuropharm.2020.108327.
- Li, Y. R; Li, H. Protective Effects of Exogenous Vitamin D on Nerve Injury in Mice Withcerebral Ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019, 35(4), 300–303. DOI: 10.12047/j.cjap.5794.2019.063.
- Evans, M. A.; Kim, H. A.; Ling, Y. H.; Uong, S.; Vinh, A.; De Silva, T. M.; Arumugam, T. V.; Clarkson, A. N.; Zosky, G. R.; Drummond, G. R., et al. Vitamin D3Supplementation Reduces Subsequent Brain Injury and Inflammation Associated withIschemic Stroke. Neuromolecular. Med. 2018, 20(1), 147–159. DOI: 10.1007/s12017-018-8484-z.
- Yuan, J; Guo, X; Liu, Z; Zhao, X; Feng, Y; Song, S; Cui, C; Jiang, P. Vitamin D Receptoractivation Influences the ERK Pathway and Protects against Neurological Deficits Andneuronal Death. Int. J. Mol. Med. 2018, 41(1), 364–372. DOI: 10.3892/ijmm.2017.3249.
- Fu, J.; Xue, R.; Gu, J.; Xiao, Y.; Zhong, H.; Pan, X.; Ran, R. Neuroprotective Effect Ofcalcitriol on Ischemic/reperfusion Injury through the NR3A/CREB Pathways in the Rathippocampus. Mol. Med. Rep. 2013, 8(6), 1708–1714. DOI: 10.3892/mmr.2013.1734.
- Atif, F; Yousuf, S; Sayeed, I; Ishrat, T; Hua, F; Stein, D. G. Combination Treatment Withprogesterone and Vitamin D Hormone Is More Effective than Monotherapy in Ischemic Stroke:the Role of BDNF/TrkB/Erk1/2 Signaling in Neuroprotection. Neuropharmacology. 2013, 67, 78–87. DOI: 10.1016/j.neuropharm.2012.10.004.
- Kim, S. W.; Oh, J. S.; Park, J.; Jeong, H. H.; Oh, Y. M.; Choi, S.; Choi, K. H. Neuroprotectiveeffect of Paricalcitol in a Rat Model of Transient Global Cerebral Ischemia. Int. J. Emerg.Med. 2020, 13(1), 30. DOI: 10.1186/s12245-020-00289-7.
- Guo, X; Yuan, J; Wang, J; Cui, C; Jiang, P. Calcitriol Alleviates Global Cerebral Ischemia-induced Cognitive Impairment by Reducing Apoptosis Regulated by VDR/ERK Signalingpathwayin Rat Hippocampus. Brain. Res. 2019, 1724, 146430. DOI: 10.1016/j.brainres.2019.146430.
- Sadeghian, N.; Shadman, J.; Moradi, A.; Ghasem Golmohammadi, M.; Panahpour, H. Calcitriol Protects the Blood-Brain Barrier Integrity against Ischemic Stroke and Reducesvasogenic Brain Edema via Antioxidant and Antiapoptotic Actions in Rats. Brain. Res.Bull. 2019, 150, 281–289. DOI: 10.1016/j.brainresbull.2019.06.010.
- Velimirovic, M.; Jevtic Dozudic, G.; Selakovic, V.; Stojkovic, T.; Puskas, N.; Zaletel, I.; Zivkovic, M.; Dragutinovic, V.; Nikolic, T.; Jelenkovic, A., et al. Effects of Vitamin D3 on the NADPH Oxidase and Matrix Metalloproteinase9 in an Animal Model of Global Cerebral Ischemia. Oxid. Med. Cell. Longev. 2018, 2018, 3273654. DOI: 10.1155/2018/3273654.
- Ekici, F.; Ozyurt, B.; Erdogan, H. The Combination of Vitamin D3 and Dehydroascorbic Acidadministration Attenuates Brain Damage in Focal Ischemia. Neurol. Sci. 2009, 30(3), 207–212. DOI: 10.1007/s10072-009-0038-6.
- Coselli, J. S; LeMaire, S. A; Conklin, L. D; Koksoy, C; Schmittling, Z. C. Morbidity Andmortality after Extent II Thoracoabdominal Aortic Aneurysm Repair. Ann. Thorac. Surg. 2002, 73(4), 1107–1115. discussion 1115-1106. DOI: 10.1016/S0003-4975(02)03370-2.
- Faheem, H; Mansour, A; Elkordy, A; Rashad, S; Shebl, M; Madi, M; Elwy, S; Niizuma, K.; Tominaga, T. Neuroprotective Effects of Minocycline and Progesterone on White Mattinjury after Focal Cerebral Ischemia. J.Clin. Neurosci. 2019, 64, 206–213. DOI: 10.1016/j.jocn.2019.04.012.
- Hentia, C; Rizzato, A; Camporesi, E; Yang, Z; Muntean, D. M; Sandesc, D; Bosco, G. Anoverview of Protective Strategies against Ischemia/reperfusion Injury: The Role of Hyperbaricoxygen Preconditioning. Brain.Behav. 2018, 8(5), e00959. DOI: 10.1002/brb3.959.
- Karimipour, M; Farjah, G. H; Molazadeh, F; Ansari, M; Pourheidar, B. Protective Effect ofContralateral, Ipsilateral, and Bilateral Remote Ischemic Preconditioning on Spinal Cord Ischemia Reperfusion Injury in Rats. Turk. Neurosurg. 2019, 29(6), 933–939. DOI: 10.5137/1019-5149.JTN.26237-19.3.
- Jia, X. F; Kowalski, R. G; Sciubba, D. M; Geocadin, R. G. Critical Care of Traumatic SpinalCord Injury. Journal of Intensive Care Medicine. 2013, 28(1), 12–23. DOI: 10.1177/0885066611403270.
- Gurer, B; Karakoc, A; Bektasoglu, P. K; Kertmen, H; Kanat, M. A; Arikok, A. T; Erguder, B. I.; Sargon, M. F.; Ozturk, O. C.; Celikoglu, E. Comparative Effects of Vitamin D Andmethylprednisolone against Ischemia/reperfusion Injury of Rabbit Spinal Cords. Eur.J .Pharmacol. 2017, 813, 50–60. DOI: 10.1016/j.ejphar.2017.07.028.
- Martin, C. M. K. No Ovarian Torsion in a 20-Year-Old Patient. C.j.e.m. 2006, 8, 126–129.
- Yildirim, N.; Simsek, D.; Kose, S.; Yildirim, A. G. S.; Guven, C.; Yigitturk, G.; Erbas, O. Theprotective Effect of Gingko Biloba in a Rat Model of Ovarian Ischemia/reperfusion injury:Improvement in Histological and Biochemical Parameters. Adv. Clin. Exp. Med. 2018, 27(5), 591–597. DOI: 10.17219/acem/68896.
- Chen, Y.; Zhi, X. Roles of Vitamin D in Reproductive Systems and Assisted ReproductiveTechnology. Endocrinology. 2020, 161(4), bqaa023. DOI: 10.1210/endocr/bqaa023.
- Tokgoz, V. Y.; Sipahi, M.; Keskin, O.; Guvendi, G. F.; Takir, S. Protective Effects of Vitamin Don Ischemia-reperfusion Injury of the Ovary in a Rat Model. Iran. J. Basic. Med. Sci. 2018, 21(6), 593–599. DOI: 10.22038/IJBMS.2018.26914.6581.
- Definition of Infarction. In MedicineNet. April 27 2011. WebMD, (Charles P.D).
- Nikolaos, G. F. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5(4), 1841–1875. DOI: 10.1002/cphy.c150006.
- Suresh, S; Michael, D. H. Massive Cerebral Infarction. Neurologist. 2005, 11(3), 150–160. DOI: 10.1097/01.nrl.0000159987.70461.d7.
- David, A. T. Acute Intestinal Ischemia and Infarction Semin . Gastrointest Dis. 2003, 14(2), 66–76.
- Akkus, O.; Topuz, M.; Oz, F.; Harbalioglu, H.; Koca, H.; Kaplan, M.; Sen, O.; Bulut, A.; Gur, M. Impact of 25(OH)D3 on Spontaneous Reperfusion and SYNTAX Score Inpatients withST-elevation Myocardial Infarction. Turk. Kardiyol. Dern. Ars. 2018, 46(4), 268–275.
- Scragg, R.; Jackson, R.; Holdaway, I.; Woollard, G.; Woollard, D. Changes in Plasmavitamin Levels in the First 48 Hours after Onset of Acute Myocardial Infarction. Am. J. Cardiol. 1989, 64(16), 971–974. DOI: 10.1016/0002-9149(89)90792-3.
- Barth, J. H.; Field, H. P.; Mather, A. N.; Plein, S. Serum 25 Hydroxy-vitamin D Does Not Exhibitan Acute Phase Reaction after Acute Myocardial Infarction. Ann. Clin.Biochem. 2012, 49(Pt4), 399–401. DOI: 10.1258/acb.2011.011195.
- Verdoia, M.; Ceccon, C.; Nardin, M.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosisstudy, G. Vitamin D Deficiency and Periprocedural Myocardial Infarction in Patients Undergoingpercutaneous Coronary Interventions. Cardiovasc. Revasc. Med. 2018, 19(7 Pt A), 744–750. DOI: 10.1016/j.carrev.2018.03.002.
- Aslanabadi, N; Jafaripor, I; Sadeghi, S; Hamishehkar, H; Ghaffari, S; Toluey, M.; Azizi, H.; Entezari-Maleki, T. Effect of Vitamin D in the Prevention of Myocardial InjuryFollowingElective Percutaneous Coronary Intervention: A Pilot Randomized Clinical Trial. J. Clin.Pharmacol. 2018, 58(2), 144–151. DOI: 10.1002/jcph.989.
- Wang, Y; Chiang, Y. H; Su, T. P; Hayashi, T; Morales, M; Hoffer, B. J; Lin, S. Z. VitaminD(3) Attenuates Cortical Infarction Induced by Middle Cerebral Arterial Ligation in Rats. Neuropharmacology. 2000, 39(5), 873–880. DOI: 10.1016/S0028-3908(99)00255-5.
- Bao, G. Q.; Yu, J. Y. Vitamin D3 Promotes Cerebral Angiogenesis after Cerebral Infarction Inrats by Activating Shh Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22(20), 7069–7077. DOI: 10.26355/eurrev_201810_16179.
