ABSTRACT
Mei (Prunus mume) is widely planted in Eastern Asian countries. To evaluate the effect of cultivar and producing origin on the quality of Mei fruit, we studied the phytochemical properties and antioxidant activities of the 16 Mei fruit cultivars from 6 provinces in China, as well as their profiles of 121 volatile compounds, 5 organic acids, 13 minerals, 24 amino acids and 3 active peptides. Multivariate analysis revealed that the four Mei fruit cultivars with a drier climate in Yunnan province had the characteristics of low water moistures and proteins contents, high total acids, soluble solids, flavones, and phenols contents, and high antioxidant activities, which could be distinguished from fruits of other producing areas. Further component analysis showed that Mei fruits were rich in volatile compounds, organic acids (such as citric acid and malic acid), minerals (such as K, Ca, Mg, P, and Fe), and amino acids (such as Orn, Asn, and Asp), and the nutrient composition ratio in different cultivars was different. In conclusion, Mei fruits can be used as nutritious health food and different cultivars focus on different nutritional compositions.
Introduction
Vegetables and fruits are generally rich in nutrients and other bioactive ingredients such as sugar, protein, mineral, polyphenol, organic acid, and vitamins. In addition to being general foods, some of them are often developed as functional foods to prevent disease and play the role of health care. There are many studies on the nutritional components of some common fruits such as citrus, apple, grape, blueberry, strawberry, and so on,[Citation1–5] however, there were fewer studies on Mei fruit (the fruit of Prunus mume Siebold & Zucc., also called Mei fruit in China and Japanese apricot in Japan).
Mei belongs to the family Rosaceae,[Citation6] and its fruit has recently been recognized as a healthy food in consideration of its beneficial effects on the body. The organic acids in Mei fruit have antibacterial activity,[Citation7] dietary fibers extracted from Mei fruit reduce blood lipid levels and regulate intestinal flora,[Citation8] and the flavones and phenols present in Mei fruit have antioxidant, antibacterial and anticancer activities, and protect against cardiovascular diseases, diabetes, osteoporosis, and the nervous system diseases in the body.[Citation9–12] Other nutrients, such as amino acids which are indispensable nutrients for body growth to regulate gene expression and protein function[Citation13–15] and minerals that play an important role in the metabolism of the body,[Citation16] were rarely reported in Mei fruit.
Mei is naturally planted in 18 provinces (autonomous regions), mainly in Yunnan, Guangdong, Zhejiang, Sichuan, Fujian, Jiangsu provinces of China. Mei has been cultivated in China for over 3000 years, and southwest Yunnan province is recognized as its place of native biogeographic origin .[Citation6] Plants of different areas have specific characteristics due to the influence of geological and environmental factors (such as, precipitation, temperature and sunlight).[Citation17] Different Mei fruit cultivars are formed in suitable origins during long-term cultivation.[Citation18] Previous studies have indicated that the morphology and partial nutrients like total sugars, acids and proteins of Mei fruit were associated with the cultivars and producing areas.[Citation18,Citation19] In recent years, consumers’ growing interest in high-quality food requires more attention to the impact of cultivars and their origin on nutrients in Mei fruits.
In this study, we collected 16 Mei fruit cultivars from Yunnan, Sichuan, Guangdong, Fujian, Zhejiang, and Jiangsu provinces for phytochemical properties and antioxidant activities analysis. Subsequently, multivariate statistical analyses were performed to reveal the influence of producing region and cultivar on Mei fruit quality and the intrinsic relationship among numerous measured nutrients. In addition, the profiles of volatiles, organic acids, minerals, and amino acids of different Mei fruit cultivars were studied. This study could be used as a reference for the raw material selection of Mei fruit deep-processing products.
Materials and methods
Samples
Sixteen Mei fruits cultivars in similar medium maturity were collected from June to July in 6 provinces (Yunnan, Guangdong, Zhejiang, Sichuan, Fujian, and Jiangsu) of China during the 2017–2018 period to avoid the influence of sampling time. Information about these Mei fruit samples, including cultivated varieties, provinces, locality, longitude, latitude, average yearly rainfall, and collection time is shown in and the specific geographical areas are visualized in Fig. S1. Mei fruit samples were picked randomly from twenty to thirty trees at each site, each batch contained 10 kg of fruit. After harvest, the fruits were fresh transported to the laboratory within one day and frozen at −20°C immediately. The fruit was identified as Prunus mume Siebold & Zucc by Dr. Xiaobo Li of Shanghai Jiao Tong University. Only healthy fruit without any kind of infection or physical damage was randomly picked, freeze-dried, and ground into powder.
Table 1. Samples of the fruit of P. mume. collected from 2017–2018
Chemical reagents
Standards (glucose, gallic acid, rutin, oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid, bovine serum albumin, and 2-octanol) and ABTS (Batch No. k1420001) were purchased from Aladdin Industrial Inc. (Shanghai, China). Folin-Ciocalteu’s phenol reagent (Lot No. bcbk7037v), DPPH (Lot No. stbd4146v), Trolox (Lot No. bcbn9262v), tryptophan (Trp), phenylalanine (Phe), leucine (Leu), isoleucine (Ile), γ-aminobutyric acid (GABA), methionine (Met), valine (Val), proline (Pro), tyrosine (Tyr), cysteine (Cys), alanine (Ala), hydroxyproline (Hpro), threonine (Thr), glycine (Gly), glutamic acid (Glu), glutamine (Gln), serine (Ser), glutathione (GSH), asparagine (Asn), L-alanyl-L-glutamine (Ala-Gln), citrulline (Cit), aspartic acid (Asp), arginine (Arg), histidine (Hit), lysine (Lys), ornithine (Orn) and cystine (Cyst) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA) and were of greater than 98% purity. HPLC-grade acetonitrile and ammonium acetate were purchased from Merck (Darmstadt, Germany). Distilled water was prepared by a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other reagents and chemicals used were of analytical grade.
Physical properties
The diameter of the widest part of the fruit along the direction of the middle suture was measured by using a Vernier calliper (Shanghai Huiyi Scale Industry Co., Ltd., China). The fruit and kernel were weighed using an electronic balance after the separation of the pulp and kernel. The edible rate was calculated as the proportion of pulp weight to the whole fruit weight.
Proximate composition analysis
The water moisture of the fruit (fresh basis, fw) was determined by the direct hot-air drying method.[Citation20] The content of soluble solids (fresh basis, fw), total ash, and acid-insoluble ash (dry basis, dw) of the collected Mei fruits were measured as previously described.[Citation20] The soluble solids were measured by using a hand-held saccharometer (WZS 80, Brix 0–80%, Shanghai Instrument Electrical Physical Optical Instrument Co., Ltd., China).
Crude protein (dry basis, dw) was determined according to the Coomassie brilliant blue method[Citation21] with bovine serum albumin as the reference, and absorption was measured at 595 nm by using a UV-vis spectrophotometer. Total sugar (dry basis, dw) was determined as reported previously[Citation22] using glucose as the standard, and the absorbance was measured at 490 nm. Reducing sugar content (dry basis, dw) was determined according to reference[Citation23] with glucose as the standard, and the absorbance was measured at 540 nm. Total phenol content (dry basis, dw) was determined according to reference[Citation24] with gallic acid as the standard, and the absorbance was measured at 700 nm. Determination of total flavones (dry basis, dw) was performed according to reference[Citation25] with rutin as the standard, and the absorption was measured at 500 nm. The optimal conditions for extraction of total phenols and flavones in Mei fruit samples were determined by employing response surface methodology (RSM) using the Design-Expert 8.5 software (Trial version, State-Ease Inc., Minneapolis, MN). The ethanol aqueous solution of 30%-90%, a solid-liquid ratio of 80–120 mL/g, and the extraction time of 30–50 min were optimized. Detailed extraction conditions of flavone and total phenol with response surface methodology are shown in supplementary materials (Table S1a, S1b).
Volatile component
The volatile compounds present in freeze-dried Mei powder (0.5 g) were extracted by headspace solid-phase microextraction (SPME). The desorbed volatile compounds from SPME were separated and analyzed on an Agilent 7890A-5975C gas chromatography (GC-MS) system coupled to a DB wax (30 m × 0.25 mm × 0.25 µm) chromatographic column with 2-octanol (100 ng) as the internal standard. The injection, interface, ion source, and four-stage rod temperatures were maintained at 260°C, 260°C, 230°C and 150°C, respectively. The carrier gas (He) was supplied at a flow rate of 1.0 ml/min with no shunt. The injection volume was 1.00 µL. The column temperature was held at 40°C for 5 min, increased to 250°C at a rate of 4°C/min, and then held for 5 min. The mass spectrometer was operated in EST + mode at 70 eV, scanning the range m/z 20–400 in a 1 s cycle in full scan mode. The detector voltage was 2200 V. Identification of volatile compounds was performed by comparing the obtained mass spectra with those in the NIST 2011 spectral library. The relative content of each volatile compound was calculated by dividing each GC peak area by the sum of the areas of the peaks for all compounds.
Organic acids
The composition and contents of the organic acid of Mei fruit were determined by using an Agilent 1200 high-performance liquid chromatography system equipped with a DAD detector (HPLC-DAD) as described,[Citation26] with some modifications. Several performance parameters were studied, including linearity, LOD, LOQ, precision, repeatability, and stability.[Citation27] (Details of the extraction and analysis conditions of organic acid are shown in supplementary materials)
Mineral composition
The minerals present in the fruit were determined by inductively coupled plasma spectrometry (ICP-OES, iCAP™ 7600, Thermo Scientific, USA) and inductively coupled plasma mass spectrometry (ICP-MS, iCAP™ Q, Thermo Scientific, USA). Mei fruit samples were accurately weighed and digested with HCl-HNO3 according to a previously described method.[Citation28] Ca, Cu, Fe, K, Mg, Mn, Na, P, and Zn were determined by ICP-OES spectrometry, and As, Cd, Hg, and Pb were determined by ICP-MS spectrometry. Each batch of fruit was analyzed three times.
Amino acids and short peptides
The extraction of amino acids and short active peptides was using water as a solvent under ultrasonic extraction. The ultrasonic extraction parameters to obtain the maximum yield of the total contents of 27 target compounds from Mei fruit were optimized by employing RSM and fixed as ultrasonic power of 26 kHz, extraction time of 40 min, and the solid-liquid ratio of 300 ml/g (Details are shown in supplementary Table S2). The 24 amino acids and 3 short active peptides were analyzed by using ultra-performance liquid chromatography coupled with triple-quadrupole linear ion-trap tandem mass spectrometry (UHPLC-QTRAP-MS2), according to the method previously established in our laboratory.[Citation29] The Waters ACQUITY UPLC system was coupled to a 5500 QTRAP® quadrupole mass spectrometer (ABSCIEX, USA). Matrix effects and linearity of the method were reexamined complying with the International Conference on Harmonization (ICH) regulations for confirmation analysis.[Citation27] Details of linearity are shown in supplementary materials (Table S3).
Antioxidant capacity test
DPPH free radical scavenging ability,[Citation30] ABTS• + radical scavenging ability,[Citation31] and ferric ion reducing ability (IRA)[Citation32] were measured according to the relevant literature. Vitamin C (VC) was used as a positive control for DPPH free radical scavenging and ABTS• + free radical scavenging. The IC50s of scavenging rates were calculated by polynomial regression. DPPH free radical scavenging ability reflected the antioxidant activity to supply hydrogen. Unlike DPPH free radical scavenging, the scavenging of ABTS• + is an electron transfer process. In the presence of electron-donating antioxidants such as phenols, colored ABTS • + reacts to form achromatic ABTS. Antioxidants can also reduce the ferric (Fe3 +)-TPTZ complex to the ferrous (Fe2 +)-TPTZ form, resulting in the development of intense blue color with an absorption maximum at 593 nm; hence, in the IRA assay, color formation reflects the reducing ability of the samples. The IRA of each sample was compared with that of Trolox (μg sample/μg Trolox). The assay was repeated 3 times for each batch of samples.
Statistical analysis
SPSS 22.0 (SPSS, Chicago, IL, U.S.A.) was used for one-way ANOVA analysis, with P < .05 indicating that the difference was statistically significant. Simca-p software (Umetrics, Umea, Sweden) was used for cluster analysis (HCA) and the principal component analysis (PCA).
Results and discussion
Physical and geometric properties
The diameter, weight (single fruit weight, kernel weight), and edible rate of Mei fruits are shown in . The diameters of Mei fruits ranged from 2.83 to 4.04 cm; DY-BM fruits (Sichuan) were the smallest, and ZS-M (Yunnan) fruits were the largest. Similarly, the fruit weight ranged from 11.76–33.43 g, the weight of DY-BM fruits was only 35% that of ZS-M fruits. The edible rate of YF-QM (Guangdong) was the lowest (77.45%), and that of WH-HHM (Sichuan) was the highest (90.29%). The physical properties (diameter, fruit weight, and edible rate) of the Mei fruits investigated in this paper are consistent with the ranges reported in the literature.[Citation18]
Table 2. Physicochemical properties of Mei fruit commonly grown in China
Proximate composition
The chemical compositions (water moisture, soluble solids, total acids, total ashes, acid-insoluble ashes, crude proteins, total sugars, total reducing sugars, total phenols, and flavones) of Mei fruits of varying origins are shown in . The proximate chemical contents of fruits from different regions were significantly different (P < .05). The results of the significance tests are presented in the supplementary materials (Table S4). The water moisture of the Mei fruit ranged from 86% to 92%; only cultivated varieties from Yunnan province (Yan-M, Ku-M, ZS-M, and LJ-HM) contained less than 90% moisture. Considering the least average rainfall of Yunnan compared with other regions, the climate is drier than the other areas. Water shortage in the growing environment could increase the accumulation of permeable components such as soluble solids, organic acids, and sugars, which help maintain the water moisture of the fruit, the accumulation of permeable components was found to depend on the severity of the water shortage.[Citation33] Similar results were obtained in our study that Mei fruit of Yunnan fruits contained higher organic acids and soluble solids than that from other regions. The total sugar content ranged from 47.18 to 213.86 mg/g dw; the total sugar contents of Yunnan samples were low, equivalent to 60% of the total sugar content of Sichuan Mei fruit (WH-HHM, DY-BM, and PW-DQM). The main sugars in Mei fruit are reducing sugars such as fructose and glucose and non-reducing sugars such as sucrose .[Citation34] The content of reducing sugar which makes the fruit soft and easy to absorb water ranged from 16.99 to 75.42 mg/g dw, and the content of reducing sugar in Yan-M and Ku-M from a drier climate was nearly 5 times that of SY-DQM. The total acid content ranged from 1.88 to 3.79 g/100 g fw. The total acid content was ranked in the order of Yunnan > Sichuan and Fujian > Guangdong, Zhejiang, and Jiangsu. The total ash content of Mei fruits was 48.7–87.8 mg/g dw, and the acid-insoluble ash content was 0–2.1 mg/g dw (the acid-insoluble ash content value of 0 was caused by low content and weighing error). The total ash content of fruits ranked as follows: Zhejiang and Jiangsu > Guangdong and Fujian > Yunnan and Sichuan. The crude protein content of Mei fruit varied from 22.9 to 74.3 mg/g dw, with Yan-M and Ku-M the lowest contents, indicating the opposite trend in sugar and protein content. Phenols and flavones are secondary metabolites of plants and protect plants from radical damage and microbiotic invasion. Phenols and flavones act as natural antioxidants and must be obtained from fruits and vegetables. As shown in , the total flavone content of Mei fruit ranged from 6.39 to 138.69 mg/g dw with rutin as the standard; the flavone content of Yan-M fruits (Dali, Yunnan) was the highest at 138.69 mg/g dw. The total phenol content of the fruits (calculated based on gallic acid) ranged from 4.91 to 27.53 mg/g dw; Ku-M fruits (Dali, Yunnan) had the highest phenol content, with a value of 27.53 mg/g dw. The flavone and total phenol contents of fruits from Yunnan, especially Dali (Yan-M, Ku-M, and ZS-M), were higher than those of fruits of other origins (P < .05). The phenols present in Mei fruit consisted mainly of derivatives of hydroxycinnamic acid.[Citation35] As Mei fruit grow and mature, the content of phenols relative to mass gradually decreases, but the total amount remains unchanged. It was reported that the total phenol content of Prunus mume cv. Backaha fruit from Korea detected with the same method was approximately 160.73 μg/g dw.[Citation36] Our research showed that the total phenol content of Mei fruit grown in Yunnan was 21.91–27.45 mg/g dw, higher than that of Mei fruits from other regions of China, and much higher than that of the fruit of Prunus mume cv. Backaha. Phenols and flavones in nectarine, peach, and plum, some other Prunus species of the family Rosaceae, are regarded as the main contributors to their antioxidant activity.[Citation37]
Antioxidant activity
Free radicals are the major factors responsible for causing many diseases. To evaluate the antioxidant activity of Mei fruit, multiple antioxidant activity assays based on DPPH and ABTS• + free radical scavenging ability, and IRA assay were assessed in this experiment. As shown in , the IC50 of DPPH free radical scavenging ability assay ranked in the order of Zhejiang and Jiangsu > Guangdong and Fujian > Sichuan > Yunnan, from the highest to the lowest, thus the antioxidant capacity of Mei fruit due to its hydrogen supplementation, was ranked as Yunnan > Sichuan > Guangdong and Fujian > Zhejiang and Jiangsu. The ABTS• + free radical scavenging ability and IRA assay were also consistent with this trend. The antioxidant activity of Mei fruits from Yunnan (Yan-M, Ku-M, ZS-M, and LJ-HM) was 2–10 times higher than that of fruits of other origins (P < .05), indicating that these Mei fruits cultivars might contain higher antioxidant components.
Influence of origin on physicochemical and antioxidant properties and internal relationship of detected items
The PCA analysis of proximate physicochemical properties and antioxidant activities of Mei fruits was conducted to differentiate the differences of cultivars. As shown in (), There were two principal components obtained: a first principal component PC 1 (47.4%) and a second principal component PC 2 (14.2%). PC 1 integrated water moisture, soluble solids, total acids, crude proteins, reducing sugars, phenols, flavones, DPPH scavenging ability, and IRA, and PC 2 integrated diameter, and weight. The cumulative contribution rate of PC 1 and PC 2 is 61.6%, a value that fully reflects the physical and chemical properties of Mei fruit. According to the PCA analysis (), the 36 batches of 16 Mei fruit cultivars could be clustered into 2 groups. The Yan-M, Ku-M, ZS-M, and LJ-HM fruit (Yunnan) were clustered in group 1 (red circle); with higher soluble solids, total acid, reducing sugar, total phenol, total flavone content, and antioxidant capacity; and lower water moisture and crude protein content than the remaining samples clustered in group 2 (blue circle). Although Mei fruit is widely planted in large areas of China, the native biogeographic origin of Mei fruit is thought to be Yunnan Province,[Citation6] and cultivars from Yunnan Province clustered together. The most likely explanation is that climate difference plays a role in Mei fruit quality. Recently, one similar study shows that the characteristics of honey produced in different biogeographical areas of Algeria are significantly affected by climate .[Citation38]
Figure 1. (a) Loading scatter plot and (b) Score scatter plot of PCA analysis of Mei fruit, and (c) Heat map of different detection indexes in Mei fruit with Pearson correlation analysis. (D, diameter; W, weight; ER, edible rate; WM, water moisture; TAsh, total ash; AIAsh, acid-insoluble ash; TA, total acid; SS, soluble solids; CP, crude protein; TS, total sugar; TRS, total reducing sugar; TF, total flavones; TP, total phenols; DPPH, DPPH scavenging ability; ABTS, ABTS scavenging ability; IRA, ferric ion reducing ability. *: P < .05, **: P < .01).
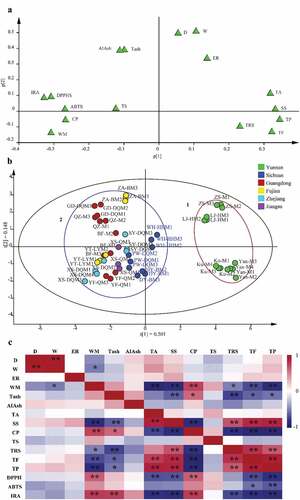
Furthermore, the relationship between physicochemical properties was studied by performing correlation analysis. As shown in , the quality parameters of water moisture, total ash, and crude protein were in a positive correlation, and they were negatively correlated with the contents of total acids, soluble solids, reducing sugars, total phenols, and total flavones. This finding was of great practical value: we could compare the water moisture of fruits from different producing areas to infer the nutrient content of total acids, proteins, mineral elements, soluble solids, reducing sugar, total phenols, and flavones. The relationship between physicochemical properties and antioxidant activities is visualized in . The IC50 of DPPH free radicals scavenged by Mei fruit was negatively correlated with the fruit’s content of total acids, soluble solids, total phenols, and total flavones (P < .01) and positively correlated with water moisture and crude protein (P < .01). The IC50 of ABTS• + free radical-scavenging ability showed a negative correlation with total flavone content (P < .05) and total phenol content (P < .01). The reduction ability of ferric ions exhibited a negative correlation with total acids, soluble solids, reducing sugars, total phenol, and total flavone content (P < .01) and a significant positive correlation with water moisture, crude protein, and total ash (P < .01). In summary, antioxidant capacity was positively associated with total phenols, total flavones, total acids, and soluble solids and negatively associated with water moisture and crude protein. While previous studies have only shown that phenolic components have antioxidant effects,[Citation9] this study broadens the understanding of the antioxidants.
Profile and multivariate analysis of volatiles, organic acids, amino acids, short peptides, and mineral components
Influence of origin on volatiles profile
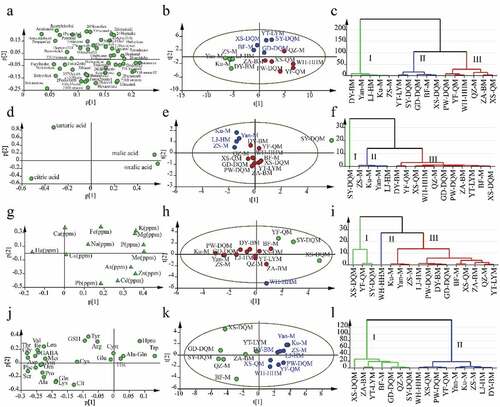
Volatiles of unripe and ripe Mei fruit cultivar has been reported in a previous study .[Citation39] However, the odor of Mei fruit cultivars from the different producing areas also smells different. The amounts of various volatile components present in Mei fruits of different origins are shown in the supplemsentary materials (Table S5). One hundred and twenty-one kinds of volatile compounds were identified in Mei fruits; these accounted for 94.70% – 96.08% of the total volatile compounds present. PW-DQM fruit (Sichuan) contained the largest number of volatile compounds kind (90), and SY-DQM fruit (Zhejiang) contained the fewest (60). The fruits contained almost all types of volatile organic compounds, including alkanes, olefins, aldehydes, ketones, fatty acids, alcohols, and fatty acid esters. The most abundant volatile compounds present in Mei fruit were benzaldehyde, acetic acid, 2-pentyl-furan, 2-methyl-butanoic acid, 3-furaldehyde, hexanal, and ethylene acetate. The representative total ion chromatogram of volatile oils in Mei fruit detected with GC-MS is shown in Fig. S2.
Fifty-three of the common volatile components (content > 0.1%) were chosen for HCA and PCA analysis (), thereby reducing the dimensionality of the data and making it possible to best distinguish the differences between samples. 16 Mei fruit cultivars were divided into three groups. Yan-M, Ku-M, ZS-M, and LJ-HM from Yunnan and DY-BM (Sichuan) clustered together due to their lower contents of 2-hexenal, benzaldehyde, furfural, benzyl alcohol, pentanoic acid, (E)-heptanal and (E, E)-2,4-heptadienal. XS-DQM and SY-DQM from Zhejiang, BF-M and GD-DQM from Guangdong, and YT-LYM (Fujian) were clustered into another group possessing higher pentanal content. The remaining QZ-M, YF-QM, and ZA-BM from Guangdong, WH-HHM and PW-DQM from Sichuan, and XS-QM (Jiangsu) were clustered into a third group due to their high content of 1-butanol and ethyl acetate. Volatile compounds are the source of the fruit’s aroma, the flavor of Mei fruit of different origins depended on the volatile composition of the fruit. The aroma of Mei fruit resembled the mixed aroma of various fruits due to the occurrence of apricot-like benzaldehyde, peach-like (E)-2-hexen-al, milk-like (2,3-butanedione), acetoin, lemon-like D-limonene, and other fruit-like aromatic compounds such as ethyl acetate.
Influence of origin on organic acid profile
Only four organic acids (citric acid, malic acid, tartaric acid, and oxalic acid) were detected of the six tested organic acids (oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid). As shown in , the content of citric acid was the highest, accounting for more than 90% of the total acids (Except SY-DQM fruits), followed by malic acid (contents of more than 8%), tartaric acid, and oxalic acid. Citric acid and malic acid were the main organic acids present in Mei fruit, consistent with a previous study, which studied six cultivars of Mei fruit.[Citation7] More cultivars were compared in this study; the HCA and PCA analysis showed that the organic acid profiles of 16 Mei fruit cultivars could be divided into three groups (). The SY-DQM (Zhejiang) fruits were in an independent group due to their higher content of malic acid and oxalic acid and their lower content of citric acid compared to fruits of other origins. The Yan-M, Ku-M, ZS-M, and LJ-HM fruits from Yunnan province, which displayed higher tartaric acid content and lower oxalic acid content, were clustered into a second group; and the other samples were clustered into a third group.
Table 3. Profile of organic acids and mineral components of Mei fruit commonly grown in China
Influence of origin on minerals profile
Our results showed that Mei fruit was rich in beneficial mineral elements K, Ca, Mg, Na, P, Fe, Zn, Mn, and Cu, while containing trace content of harmful mineral elements including As, Cd, Hg, and Pb (). It thus provides a good source of dietary supplementation of minerals for human health. Of these minerals, K was present in the highest concentration in all Mei fruit cultivars, followed by P, Ca, Mg, Na, and Fe. Compared to blueberry, which is currently popular as health fruits, the content of K in Mei fruit was similar, while the contents of Ca and Mg were almost 10 times that of blueberry, and the content of Fe was almost 5 times that of blueberry,[Citation40] indicating that Mei fruit possesses abundant mineral content. HCA and PCA analysis showed that Mei fruit cultivars could be divided into three main groups according to their mineral content (). The XS-DQM and SY-DQM fruits from Zhejiang and YF-QM from Guangdong had higher K (ppm), Mg (ppm), and P (ppm) content and could be clustered into one group; WH-HHM fruits from Sichuan separated independently due to their higher content of Cu and Zn ions. The remaining samples could be clustered together, which were further separated into several branches according to the mineral contents of the samples. The members of the clusters were relatively scattered from different cultivars of the fruits, indicating that minerals were sensitive to their cultivars and producing areas.
Influence of origin on amino acids and short peptides profile
The representative extracted ion chromatogram of amino acids with quadrupole mass spectrometry is shown in Fig. S3. The quantified results of 24 Free amino acids (including 8 essential protein amino acids, 12 non-essential protein amino acids, and 4 non-protein amino acids, GABA, Hpro, Cit, Orn) and 3 small peptides (fewer than 3 amino acids, including 2 active dipeptides Ala-Gln and Cyst and 1 active tripeptide GSH) in Mei fruit are presented in . The contents of Orn, Asn, and Asp from all origins were abundant in Mei fruits. It was reported that Asn and Asp could inhibit fatigue, while Orn can promote protein synthesis and metabolism of sugar and fat,[Citation41] and a combination of Orn and Asp has been used to treat cirrhosis, hyperammonemia, and hepatic encephalopathy.[Citation42] Besides, the content of GABA, which promotes nerve development and maintains osmotic pressure balance,[Citation43] in Mei fruit was also very rich. The total amino contents of Mei fruit are higher than that of medicinal and edible homologous fruits such as Lycium barbarum and Siraitia grosvenorii.[Citation29,Citation44] Amino acid content variated in fruits of different regions and cultivars. The content of the total amino acids in the SY-DQM (Zhejiang) was approximately 25 and 18 times higher than that of the DY-BM (Sichuan) and PW-DQM (Sichuan), respectively. The total content of the amino acid compounds in three Mei fruit cultivars in the same region of Yunnan province ranked in the order of Yan-M > ZS-M> Ku-M. The relationship between the 21 co-existed amino acids and cultivars were analyzed by HCA and PCA (). Mei fruit samples were clustered into two groups; fruits of BF-M, QZ-M, GD-DQM from Guangdong, ZA-BM, YT-LYM from Fujian, and XS-DQM, SY-DQM from Zhejiang appeared in group I, due to their high content of Thr, Ser, Gly, GABA, Phe, Val, Ile, Asp, Orn, Asn, Pro, Ala, Leu, and Met, especially Asn, Asp, and Orn. Fruits cultivars of Yan-M, Ku-M, ZS-M, and LJ-HM of Yunnan, WH-HHM and DY-BM of Sichuan, XS-DQM of Jiangsu, and YF-QM of Guangdong were clustered in group II, in which the overall amino acid content was lower than that of the fruits in group I. These results were consistent with the previous crude protein results.
Table 4. Profile of amino acids and short peptides of Mei fruit commonly grown in China
Conclusion
In summary, the properties of Mei fruit cultivars from different regions were compared. Cultivars and growing areas played a crucial role in Mei fruit quality, from the result that the 4 cultivars in Yunnan province with a drier climate were differentiated from that of the 12 cultivars in other regions. The drier climate could lead to lower water moisture and proteins, higher content of soluble solids, total acids, reducing sugars, total phenols, and total flavones content, and higher antioxidant activities. Mei fruit is a good source of nutrient components of organic acids, amino acids, and minerals. The consumer could choose satisfied Mei fruit cultivars by their unique nutrient proportions, and this work could also provide a reference for the selection of Mei fruit cultivars as raw materials for processing downstream products.
Supplemental Material
Download ()Acknowledgments
The authors acknowledge the analysis support from Yu Gao and Jing Lai at the Instrumental Analysis Center of Shanghai Jiao Tong University for the measurement of volatile components and mineral components, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Arpita, B.; Angel, N.; Betts, N. M.; Lyons, T. J. Strawberry as a Functional Food: An Evidence-based Review. Crit. Rev. Food Sci. Nutr. 2014, 54(6), 790–806.
- Hyson, D. A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2(5), 408.
- Çetin, A., and Sağdıç, O. A Concise Review: Antioxidant Effects and Bioactive Constituents of Grape. Erciyes Med. J. 31(4), 369–375 . 2009.
- Manganaris, G. A.; Goulas, V.; Vicente, A. R.; Terry, L. A. Berry Antioxidants: Small Fruits Providing Large Benefits. J. Sci. Food Agric. 2014, 94(5), 825–833.
- Lado, J.; Gambetta, G.; Zacarias, L. Key Determinants of Citrus Fruit Quality: Metabolites and Main Changes during Maturation. Sci. Hortic. 2018, 233, 238–248.
- Z-l, L. Deciduous Fruit Production in China. Deciduous Fruit Prod. Asia Pac, 10, 1999, 30 .
- Gao, Z. Evaluation of Different Kinds of Organic Acids and Their Antibacterial Activity in Japanese Apricot Fruits. Afr. J. Agric. Res. 2012, 7(35), 4911–4918.
- Tamura, M.; Ohnishi, Y.; Kotani, T.; Gato, N. Effects of New Dietary Fiber from Japanese Apricot (Prunus Mume Sieb. Et Zucc.) On Gut Function and Intestinal Microflora in Adult Mice. Int. J. Mol. Sci. 2011, 12(4), 2088–2099.
- Bhooshan, P. K.; Ibrahim, R. S. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longevity. 2009, 2(5), 270.
- Shin, E. J.; Hur, H. J.; Sung, M. J.; Park, J. H.; Yang, H. J.; Kim, M. S.; Kwon, D. Y.; Hwang, J.-T. Ethanol Extract of the Prunus Mume Fruits Stimulates Glucose Uptake by Regulating PPAR-γ in C2C12 Myotubes and Ameliorates Glucose Intolerance and Fat Accumulation in Mice Fed a High-fat Diet. Food Chem. 2013, 141(4), 4115–4121.
- Yan, X. T.; Li, W.; Sun, Y. N.; Yang, S. Y.; Lee, S. H.; Chen, J. B.; Jang, H. D.; Kim, Y. H. Identification and Biological Evaluation of Flavonoids from the Fruits of Prunus Mume. Bioorg. Med. Chem. Lett. 2014, 24(5), 1397–1402.
- Xia, D.; Wu, X.; Shi, J.; Yang, Q.; Zhang, Y. Phenolic Compounds from the Edible Seeds Extract of Chinese Mei (Prunus Mume Sieb. Et Zucc) and Their Antimicrobial Activity. LWT Food Sci. Technol. 2011, 44(1), 347–349.
- Sarmadi, B. H.; Ismail, A. Antioxidative Peptides from Food Proteins: A Review. Peptides. 2010, 31(10), 1949–1956.
- Mcgaha, T. L.; Huang, L.; Lemos, H.; Metz, R.; Mautino, M.; Prendergast, G. C.; Mellor, A. L. Amino Acid Catabolism: A Pivotal Regulator of Innate and Adaptive Immunity. Immunol. Rev. 2012, 249(1), 135.
- Akram, M.; Asif, H. M.; Uzair, M.; Akhtar, N.; Madni, A.; Shah, S. M. A.; Hasan, Z. U.; Ullah, A. Amino Acids: A Review Article. J. med. plant res. 2011, 5(17), 3997–4000.
- Soetan, K. O.; Olaiya, C. O.; Oyewole, O. E. The Importance of Mineral Elements for Humans, Domestic Animals and Plants - a Review. Afr. J. Food Sci. 2010, 4(5), 200–222.
- Zhao, H.; Guo, B.; Wei, Y. Determining the Geographic Origin of Wheat Using Multielement Analysis and Multivariate Statistics. J. Agric Food Chemi. 2011, 59(9), 4397.
- Fang, J.; Qiao, Y.; Zhang, Z.; Mengyuan, C. Study on the Quantitative Distribution of Some Fruit Qualitative Characters of J Apanese Apricot(Armeniaca Mume Sieb.)Cultivars and Their Evaluation Criteria. J. Fruit Sci. 2002, 19(3), 175–179.
- Wang, Y. X.; Zhang, C.; Fan, D. X. Analysis of Green Plum Fruit Quality from Different Regions. Southwest China J. Agric. Sci. 2014, 27(3), 1248–1251.
- Latimer, G. W., Jr. Official Methods of Analysis of AOAC International. AOAC International. 1995, 6, 1–6.
- Sedmak, J. J.; Grossberg, S. E. A Rapid, Sensitive, and Versatile Assay for Protein Using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 79(1–2), 544–552.
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y. C. Carbohydrate Analysis by a Phenol-sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339(1), 69–72.
- Miller, G. L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Biochem. 1959, 31(3), 426–428.
- Spanos, G. A.; Wrolstad, R. E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric Food Chemi. 1990, 38(7), 285–294.
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64(4), 555–559.
- Lin, Y. S.; Liu, X. M.; Zhong, W. X.; Wang, S. Y.; Yang, C. Y. Tang QS: Chromatographic Characterization of Organic Acids in Prunus Mume and Its Application. Mod. Food Sci. Technol. 2014, 30(9), 280–285.
- Rambla-Alegre, M.; Esteve-Romero, J.; Carda-Broch, S. Is It Really Necessary to Validate an Analytical Method or Not? that Is the Question. J. Chromatogr. A. 2012, 1232, 101–109.
- Ali, S.; Masud, T.; Abbasi, K. S. Physico-chemical Characteristics of Apricot (Prunus Armeniaca L.) Grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130(2), 386–392.
- Zhou, G.; Wang, M.; Li, Y.; Peng, Y.; Li, X. Rapid and Sensitive Analysis of 27 Underivatized Free Amino Acids, Dipeptides, and Tripeptides in Fruits of Siraitia Grosvenorii Swingle Using HILIC-UHPLC-QTRAP(®)/MS (2) Combined with Chemometrics Methods. Amino Acids. 2015, 47(8), 1589–1603.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Sci. Technol. 1995, 28(1), 25–30.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9–10), 1231.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239(1), 70–76.
- Yakushiji, H.; Morinaga, K.; Nonami, H. Sugar Accumulation and Partitioning in Satsuma Mandarin Tree Tissues and Fruit in Response to Drought Stress. J. Am. Soc. Hortic. Sci. 1998, 123(4), 719–726.
- Nakamura, A. Organic Acids, Free Amino Acids and Sugars Compositions in Ume (Prunus Mume) Extract, and Change of Their Component during Preparation Process of Ume Extract. Eiyo to Shokuryo. 1995, 48(3), 232–235.
- Mitani, T.; Horinishi, A.; Kishida, K.; Kawabata, T.; Yano, F.; Mimura, H.; Inaba, N.; Yamanishi, H.; Oe, T.; Negoro, K. Phenolics Profile of Mume, Japanese Apricot (Prunus Mume Sieb. Et Zucc.) Fruit. Biosci. Biotechnol. Biochem. 2013, 77(8), 1623.
- Kim, H. R.; Kim, I. D.; Dhungana, S. K.; Kim, M. O.; Shin, D. H. Comparative Assessment of Physicochemical Properties of Unripe Peach (Prunus Persica) and Japanese Apricot (Prunus Mume). Asian Pac. J. Trop. Biomed. 2014, 4(2), 97.
- Gil, M. I.; Tomás-Barberán, F. A.; Hess-Pierce, B.; Kader, A. A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric Food Chemi. 2002, 50(17), 4976–4982.
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M. S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M. C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods. 2020, 9(7), 938.
- Miyazawa, M.; Shirakawa, N.; Utsunomiya, H.; Inada, K. I.; Yamada, T. Comparision of the Volatile Components of Unripe and Ripe Japanese Apricot (Prunus Mume Sieb. Et Zucc.). Nat. Prod. Res. 2009, 23(17), 1567–1571.
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Mineral Composition of Wild and Cultivated Blueberries. Biol. Trace Elem. Res. 2017, 181(1), 173–177.
- Marquezi, M. L.; Roschel, H. A.; Dos, S. C. A.; Sawada, L. A., Jr. Effect of Aspartate and Asparagine Supplementation on Fatigue Determinants in Intense Exercise. Int. J. Sport Nutr. Exercise Metab. 2003, 13(1), 65.
- Kircheis, G.; Nilius, R.; Held, C.; Berndt, H.; Buchner, M.; Görtelmeyer, R.; Hendricks, R.; Krüger, B.; Kuklinski, B.; Meister, H. Therapeutic Efficacy of L-ornithine-L-aspartate Infusions in Patients with Cirrhosis and Hepatic Encephalopathy: Results of a Placebo-controlled, Double-blind Study. Hepatology. 1997, 25(6), 1351–1360.
- Cesetti, T.; Ciccolini, F.; Li, Y. GABA Not Only a Neurotransmitter: Osmotic Regulation by GABA(A)R Signaling. Front. Cell. Neurosci. 2012, 6(2), 3.
- Chen, X.; You, J.; Suo, Y.; Fan, B. Sensitive Determination of Taurine, γ-Aminobutyric Acid and Ornithine in Wolfberry Fruit and Cortex Lycii by HPLC with Fluorescence Detection and Online Mass Spectrometry Identification. J. Chromatogr. Sci. 2014, 53(4), 492–497.