ABSTRACT
Although recent studies show the hypolipidemic effect of Rosa roxburghii, however reported findings are contradictory. Using meta-analysis, we aimed to explore the effect of R. roxburghii on blood lipid levels. An extensive literature search was performed to investigate the effect of R. roxburghii on various lipid indexes. The results showed that R. roxburghii fruit can significantly reduce total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels and increase high-density lipoprotein cholesterol levels. R. roxburghii fruit can therefore be used to regulate blood lipid concentrations and it can be considered a valuable adjuvant therapy for blood lipid control. However, this needs confirmation through additional research.
INTRODUCTION
Dyslipidemia is considered a serious factor for human health because of its strong correlation with various common and life-threatening diseases. Many studies have reported that hyperlipidemia is the most crucial inducer of cardiovascular disease (CVD) .[Citation1,Citation2] Levels of plasma total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) are closely associated with the incidence of CVD, and abnormal levels of these blood lipid indexes could rapidly induce CVDs,[Citation3] posing a serious threat to the individual’s health.[Citation4,Citation5] In addition to CVD, abnormal lipid profiles are also closely associated with obstructive sleep apnea,[Citation6,Citation7] coronary artery disease (CAD),[Citation8] diabetes,[Citation9] cancer,[Citation10,Citation11] and other critical diseases, which are often accompanied by abnormal lipid index concentrations. Moreover, many researchers have currently considered this change a marker for predicting the risk of related diseases. For example, the lipid ratios formed by these factors of the lipid spectra, atherogenic index of plasma (logTGS/HDL-C), Castelli’s risk index (CRI-I) (TC/HDL-C), CRI-II (LDL-C/HDL-C), atherogenic coefficient (AC) (<TC-HDL-C>/HDL-C), and Cholindex (LDL-C-HDL-C) have a significant positive correlation with CVD.[Citation12] TG/HDL and TG/HDL-C were also considered effective indicators for predicting insulin resistance.[Citation13] CRI-I and CRI-II were found to be strong predictors of CAD.[Citation14] TC and HDL-C have also been reported to be significantly associated with cancer survival.[Citation15] These results showed that the abnormal blood lipids levels have a significant correlation with these diseases and may even be an essential factor in inducing or promoting the occurrence and development of diseases and their complications. The comprehensive application of exercise, dietary adjustment, and medication is the standard treatment for hyperlipidemia.[Citation16–19] However, drugs that regulate blood lipids are often accompanied by severe side effects and drug resistance. Because of the limitations of lipid-lowering drugs, naturally occurring nutritious food rich in bioactive substances has attracted people’s attention as a promising adjuvant or as an alternative therapy.
Rosa roxburghii Tratt (RRT) is a Rosaceae plant that grows in mountainous areas at an altitude of 500–2500 m in Southwest China, is widely used as a kind of functional food in China. An in-depth research on RRT in recent years has gradually drawn people’s attention to its various bioactive properties such as reducing blood glucose levels,[Citation20] anti-oxidation,[Citation21,Citation22] anti-renal fibrosis,[Citation23] anti-aging,[Citation24] anti-radiation,[Citation25,Citation26] and anti-tumor.[Citation27] Another useful property is its ability to reduce blood lipid.[Citation28,Citation29] Some clinical studies have shown that RRT reduces blood lipid, thereby offering a protective effect in hyperlipidemia. However, findings on this effect were still divergent in the literature. We aimed to systematically evaluate published human and animal studies to determine the effect of RRT on blood lipid.
Materials and methods
Search strategy
In order to obtain relevant research in a comprehensive manner, literature searches were executed using the unitary keyword “Rosa roxburghii” in the PubMed, Web of Science, Wiley on Line Library, Springer Link, and Science Direct databases. Additionally, searches were conducted on the China National Knowledge Internet (CNKI), Wanfang Database, and VIP database using “Ci Li” (RRT in Chinese) as the subject terms. No language limitations were employed during the searches, and all pieces of literature published up to November 16, 2021, were searched. Detailed search strategies of these databases are provided in Supplementary Table 1.
Inclusion criteria
The types of studies to be included should be randomized controlled trials (RCTs). Studies on hyperlipidemia-related diseases were selected, regardless of the subjects’ species, gender, age, and region. The studies had to contain the following two groups: an intervention group (taking RRT) and a control group (not taking RRT) and had to contain measured relevant lipid indexes of TC, TG, LDL-C, and HDL-C. The meta-analysis excluded articles on blood lipids that were irrelevant for the objectives of the review, did not provide accurate data (for example, data was in the form of histograms), or were repeat publications. Those with incomplete data or reporting findings from in vitro experiments or studies with no control groups were also excluded.
Data extraction and quality assessment
Two investigators conducted independent screenings and data extractions from all the obtained literature. The two investigators cross-checked the selected studies between them, for compliance with the predefined inclusion criteria. Any disagreements were discussed with the third researcher and resolved by consensus. All included studies were assessed for bias risk using Cochrane Collaboration Group tools.[Citation30]
Statistical analysis
Stata 15 and Review Manager software (version 5.3, Copenhagen, Denmark) were used for the statistical analysis. When the data were heterogeneous, the random effect model was used. Otherwise, a fixed-effect model was used. A probability value p ≤ .05 was considered to be statistically significant. Funnel plots and Begg’s and Egger’s tests were used to detect potential publication bias.
Results
Search results
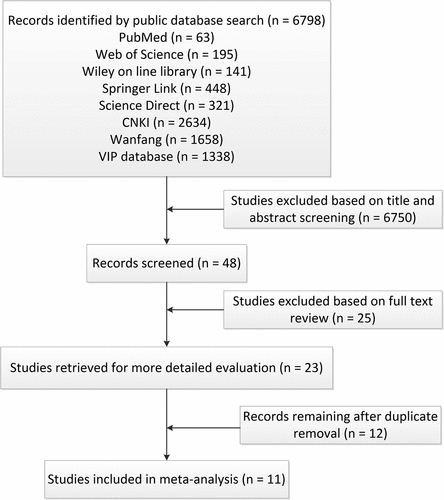
A total of 6798 related articles were obtained through retrieval. After excluding the irrelevant articles by reading the titles and abstracts, a further full-text review was performed and many articles were discarded for the following reasons: data was unavailable, in-vitro experiments were used, no control groups were included in the trial, and repetitive articles. Finally, 11 articles were selected, which were in the Chinese language. The articles yielded 448 experimental samples for the study, comprising 235 cases in the study group and 213 cases in the control group. The flow chart of literature screening is shown in , and the overall characteristics of included studies are shown in .
Table 1. Characteristics of the eleven included studies
Risk of bias
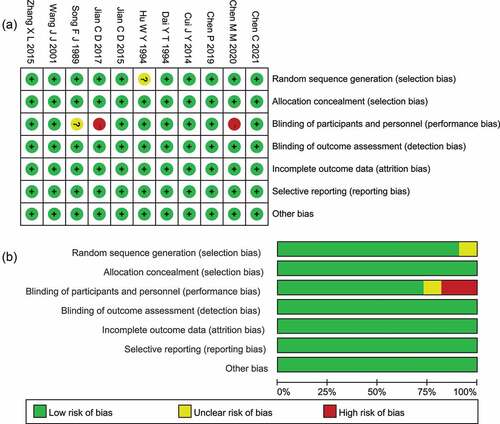
The final 11 studies selected were assessed for risk of bias. One study[Citation31] was presented as ambiguous in the “random sequence generation” for the inadequately described randomization, two[Citation32,Citation38] studies were at high risk, and one[Citation39] study appeared ambiguous in the performance bias for lacking or undefining of blinding. All studies were at low risk in other bias assessments, and the results are summarized in .
Meta-analysis
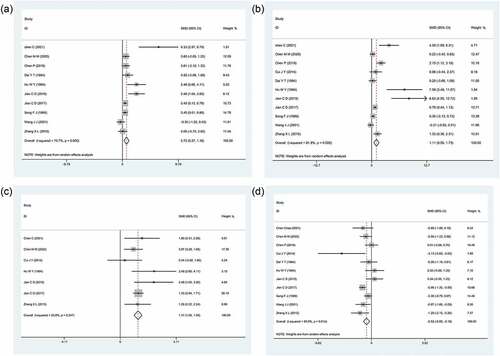
Effects on TG and HDL-C were measured in all 11 studies.[Citation31–41] A total of 10[Citation31–33,Citation35–41] and seven[Citation31,Citation32,Citation34,Citation36–38,Citation41] studies measured TC and LDL-C, respectively. The fixed-effect model was used for LDL-C meta-analysis and the random-effect model was applied for other (TC, TG, and LDL-C) analyses. The results () showed that RRT has distinct therapeutic effects by reducing TC, TG, and LDL-C and significantly increasing HDL-C.
Publication bias
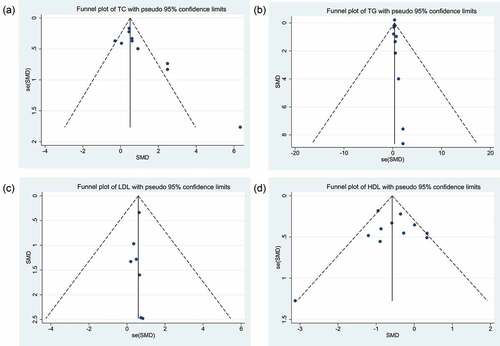
Funnel plots were used to detect possible publication bias. For TC and TG, the funnel plots presented a skewed distribution. For TC, p = .032 was obtained with the Egger’s test and p = .020 with the Begg’s test; and for TG, p = .021 was obtained with the Egger’s test and p = .013 with Begg’s test. These results indicated that there might be some publication bias for TC and TG (). The funnel plots of LDL-C and HDL-C seemed to be symmetrical, and p = .535 was obtained for LDL-C with the Egger’s test and p = .230 with the Begg’s test. For HDL-C, p = .857 was obtained with the Egger’s test and p = 1.000 was obtained with the Begg’s test. The p-values for LDL-C and HDL-C suggested there was no publication bias for LDL-C and HDL-C ().
Discussion
Dyslipidemia can pose a severe threat to human health. Finding substitutes that can effectively control blood lipids while avoiding the toxic side effects of drugs can provide significant benefits for treating cardiovascular and cerebrovascular diseases. The current systematic review and meta-analysis found that RRT preparations showed significant beneficial effects in regulating blood lipid levels. Supplementation of RRT can reduce the plasma concentrations of TC, TG, and LDL-C and increase the plasma concentration of HDL-C.
Although the data are not included in this analysis, some studies have reported the beneficial effects of RRT extract on dyslipidemia. After eight weeks of ingesting the ethanol extract of RRT fruit, TC, TG, and LDL-C levels in the serum of rats with hyperlipidemia can be significantly reduced, and HDL-C levels can be increased.[Citation28] The findings are consistent with those obtained by Song et al. after feeding RRT juice to Kunming mice for 30 days.[Citation42] In a study by Wang et al., 900 mg/kg/day RRT fruit polysaccharide was orally fed to db/db mice for eight weeks, and the total serum cholesterol, TG, and LDL-C levels of the model mice decreased significantly, whereas the HDL-C level increased.[Citation29]
Abnormal blood lipid parameters may lead to other diseases. For example, the increase of serum cholesterol will promote the production of reactive oxygen species,[Citation43] and the increase of TC, TG, and LDL-C will lead to the injury of arterial endothelium[Citation44] – both of which are major causes of CVDs. Wu et al. showed that the ethanol extract of RRT fruit can significantly downregulate the expression of the sterol regulatory-element binding protein-1 gene (srebp-1) and its target acetyl-CoA carboxylase-1 gene (acc-1) in the mRNA and protein levels of mouse liver tissue, and upregulate the expression of LDLR and peroxisome proliferator-activated receptor (PPAR)-α. These four genes are closely related to the biosynthesis of TG, TC, very-low-density lipoprotein, and LDL-C concentrations. Song et al.[Citation42] fed hyperlipidemic mice with RRT juice and after 30 days performed comparative protein group analysis. The results showed that 15 differentially expressed essential proteins (Cyp7a1, Cyp3a11, Tm7sf2, COAT2, CSAD, RBP3, Lpin1, Dhrs4, Aldh1b1, GK, Acot 4, TSC22D1, PGFS, and EH) were identified. These changes in protein expression indicated that RRT juice has a role in regulating lipid metabolism by regulating fatty acid metabolism, biosynthesis of bile acids and steroids, and production of lipid peroxides. Because hyperlipidemia will lead to the generation of active oxygen and the increase of lipid peroxidation, the intense antioxidant activity of RRT may be one of its mechanisms for lowering blood lipid. RRT fruit is rich in bioactive products such as L- ascorbic acid, superoxide dismutase (SOD), polysaccharide, quercetin, kaempferol, triterpenes, procyanidins, and vanillin.[Citation45,Citation46] These natural ingredients may play an essential role in the hypolipidemic activity of RRT, although the specific compounds and mechanism of action need to be confirmed by further research. Because RRT is rich in bioactive substances, it is likely to target multiple molecules or molecular pathways in the fat metabolism system as part of beneficial regulation.
The aforementioned gene expression regulation and signaling molecules have associated RRT with lipid metabolism. The effect of RRT through gut microbiota is another mechanism by which it regulates blood lipid levels. Some functional foods can improve dyslipidemia by regulating gastrointestinal flora.[Citation47] A close relationship exists between the species of flora and lipid levels.[Citation48] In the gastrointestinal tract of mice with lipid metabolism disorder, the number of Scleroderma and Bacteroides was significantly increased compared with that of the normal host.[Citation49,Citation50] R. roxburghii polysaccharide has a significant regulatory effect on the dominant species present in the intestinal flora. It can significantly reduce the proportion of Scleroderma/Bacteroides in mice with lipid metabolism disorders, reverse the increased abundance of Clostridium and Desulfovibrio, and decrease the abundance of Bacteroides and Lactobacillus.[Citation29,Citation51] Some anaerobes ferment the host’s non-digestible carbohydrates in the intestinal tract and produce various metabolites, which is one of the important ways by which the microbiota affects the host’s lipid metabolism. For example, short-chain fatty acids (SCFAs) can activate PPAR[Citation52] and promote gluconeogenesis,[Citation53] thereby playing a beneficial role in promoting the metabolism of lipids and cholesterol of the host. R. roxburghii polysaccharide can significantly increase the content of SCFAs in the intestinal tract of mice, which is one of the mechanisms by which it regulates blood lipids. The effect of RRT on other microbial metabolites that are closely related to lipid disorders, such as conjugated linoleic acids,[Citation54] nuclear receptor farnesol X,[Citation55] and bile acids,[Citation56] may become the direction of its research on the regulation of lipid metabolism in the future. In addition to the positive effects of microflora metabolites, some microbial components played a negative role in lipid metabolism. The release of lipopolysaccharides (LPS) into the circulatory system through the intestinal tract with increased permeability can induce chronic inflammation and blood lipid-metabolism disorder.[Citation48,Citation57] Polysaccharide intake from RRT significantly reduced the abundance of LPS-producing Enterocaccaceae and Desulfovibrionaceae in the gastrointestinal tract,[Citation29] and a decrease in LPS levels was also detected.[Citation58] The detailed mechanism of these effects induced by RRT is still unclear, but the promoted effects play a positive and beneficial role in the lipid metabolism of an individual. However, this effect needs to be confirmed by more research, especially in human clinical trials.
Previous research reports have examined the safety of RRT. As a fruit widely used for the dual purposes of medicine and food, no acute toxicity, long-term toxicity, mutagenicity, teratogenicity, or other adverse reactions have been found in toxicological studies.[Citation23,Citation29,Citation59,Citation60] Therefore, RRT can be an effective and safe candidate product for reducing blood lipid levels.
Although our research results are interesting, some limiting factors are needed to be considered. The diverse populations and various disease models included in the study contributed toward increasing the heterogeneity in the research results. In addition, the differences in dosage, dosage form, and measurements must also be considered. At the same time, consideration must be given to the fact that the number of included studies was small. Another limitation of this study is that the protocol is not registered on any website. The lack of methodological safeguards of this review is a disadvantage, although the analysis was designed and performed according to the Cochrane guidelines. These abovementioned factors may also be the source of publication bias and heterogeneity in the study.
Conclusion
This meta-analysis confirmed the effectiveness of RRT fruit administration for hyperlipidemia-related diseases. However, the lipid-lowering components and pharmacological mechanism remain to be identified, and more high-quality randomized clinical trials are required to verify this benefit. The findings from our study progress the research and development of diet-based therapy and health products for reducing blood lipids. It also indirectly supports the continued use of this fruit for its medicinal value.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Ma, Y.; Wang, W.;, Zhang, J.; Lu, Y.; Wu, W.; Yan, H.; Wang, Y. Hyperlipidemia and Atherosclerotic Lesion Development in Ldlr-deficient Mice on a Long-term High-fat Diet. PloS One. 2012, 7(4), article no. e35835.
- Poss, J.; Custodis, F.; Werner, C.; Weingartner, O.; Bohm, M.; Laufs, U. Cardiovascular Disease and Dyslipidemia: Beyond LDL. Curr. Pharm. Des. 2011, 17(9), 861–870.
- Brischetto, C. S.; Connor, W. E.; Connor, S. L.; Matarazzo, J. D. Plasma Lipid and Lipoprotein Profiles of Cigarette Smokers from Randomly Selected Families: Enhancement of Hyperlipidemia and Depression of High-density Lipoprotein. Am. J. Cardiol. 1983, 52(7), 675–680.
- Virani, S. S.; Alonso, A.; Benjamin, E. J.; Bittencourt, M. S.; Callaway, C. W.; Carson, A. P.; Chamberlain, A. M.; Chang, A. R.; Cheng, S.; Delling, F. N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation. 2020, 141(9), e139–e596.
- Chamberlain, J. J.; Johnson, E. J.; Leal, S.; Rhinehart, A. S.; Shubrook, J. H.; Peterson, L. Cardiovascular Disease and Risk Management: Review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann. Intern. Med. 2018, 168(9), 640–650.
- Susanto, A. D.; Harahap, R. A.; Antariksa, B. The Prevalence and Related Risk Factors of Obstructive Sleep Apnea in Heart Failure Patients at the Indonesian Referral Hospital for Respiratory Diseases. J. Nat. Sci. Biol. Med. 2020, 11(2), 164–168.
- David, B.; Francisco, G. R. Obstructive Sleep Apnea and Dyslipidemia: From Animal Models to Clinical Evidence. Sleep. 2019, 42(3), zsy236. DOI: 10.1093/sleep/zsy236.
- Patil, V. C.; Avhad, A. B.; Kulkarni, A. R.; Pandere, K. High-sensitive C-reactive Protein in Patients with Coronary Artery Disease. J. Nat. Sci. Biol. Med. 2020, 11(1), 39–44.
- Athyros, V. G.; Doumas, M.; Imprialos, K. P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and Lipid Metabolism. Hormones (Athens). 2018, 17(1), 61–67.
- Khan, W.; Augustine, D.; Rao, R. S.; Patil, S.; Awan, K.; Sowmya, S.; Haragannavar, V.; Prasad, K. Lipid Metabolism in Cancer: A Systematic Review. J. Carcinog. 2021, 20(1), 4.
- Zhang, X.; Zhao, X. W.; Liu, D. B.; Han, C. Z.; Du, L. L.; Jing, J. X. Lipid Levels in Serum and Cancerous Tissues of Colorectal Cancer Patients. World J. Gastroenterol. 2014, 20(26), 8646–8652.
- Abid, H. A.; Abid, Z. H.; Abid, S. A. Atherogenic Indices in Clinical Practice and Biomedical Research: A Short Review. Atherogenic Indices and Cardiovascular Diseases. Baghdad J Biochem. Appl Biol Sci. 2021, 2(2), 59–69. DOI: 10.47419/bjbabs.v2i02.52.
- Sowndarya, K.; Joseph, J. A.; Shenoy, A.; Hegde, A. Evaluation of Triglyceride/high-density Lipoprotein Ratio as a Surrogate Marker for Insulin Resistance in Healthy Young Males. J. Nat. Sci. Biol. Med. 2021, 12(2), 213–217.
- Rader, D. J.; Davidson, M. H.; Caplan, R. J.; Pears, J. S. Lipid and Apolipoprotein Ratios: Association with Coronary Artery Disease and Effects of Rosuvastatin Compared with Atorvastatin, Pravastatin, and Simvastatin. Am. J. Cardiol. 2003, 91(5A), 20C–24C.
- Zhou, P.; Li, B.; Liu, B.; Chen, T.; Xiao, J. Prognostic Role of Serum Total Cholesterol and High-density Lipoprotein Cholesterol in Cancer Survivors: A Systematic Review and Meta-analysis. Clin. Chim. Acta. 2018, 477, 94–104. DOI: 10.1016/j.cca.2017.11.039.
- Davignon, J. Beneficial Cardiovascu Larpleiotropic Effects of Statins. Circulation. 2004, 109(23 Suppl 1), III39–43. DOI: 10.1161/01.CIR.0000131517.20177.5a.
- Ridker, P. M.; Cannon, C. P.; Morrow, D.; Rifai, N.; Rose, L. M.; McCabe, C. H.; Pfeffer, M. A.; Braunwald, E. C-reactive Protein Levels and Outcomes after Statin Therapy. N. Engl. J. Med. 2005, 352(1), 20–28.
- Ganji, S. H.; Tavintharan, S.; Zhu, D. M.; Xing, Y.; Kamanna, V. S.; Kashyap, M. L. Niacinnoncom Petitively Inhibits DGAT2 but Not DGAT1 Activity in HepG2 Cells. J. Lipid Res. 2004, 45(10), 1835–1845. DOI: 10.1194/jlr.M300403-JLR200.
- Pike, N. B.; Wise, A. Identification of a Nicotinic Acid Receptor: Is This the Molecular Target for the Oldest Lipid-lowering Drug? Curr. Opin. Investig. Drugs. 2004, 5(3), 271–275.
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, Functional, and Biological Properties of Water-soluble Polysaccharides from Rosa Roxburghii Tratt Fruit. Food Chem. 2018, 249, 127–135. DOI: 10.1016/j.foodchem.2018.01.011.
- Rensburg, C. J.; Erasmus, E.; Loots, D. T.; Oosthuizen, W.; Jerling, J. C.; Kruger, H. S.; Louw, R.; Brits, M.; van der Westhuizen, F. H. Rosa Roxburghii Supplementation in a Controlled Feeding Study Increases Plasma Antioxidant Capacity and Glutathione Redox State. Eur. J. Nutr. 2005, 44(7), 452–457.
- Chen, G. J.; Kan, J. Q. Characterization of a Novel Polysaccharide Isolated from Rosa Roxburghii Tratt Fruit and Assessment of Its Antioxidant in Vitro and in Vivo. Int. J. Biol. Macromol. 2018, 107(Pt A), 166–174. DOI: 10.1016/j.ijbiomac.2017.08.160.
- Zhan, J. H.; Liu, M. J.; Pan, L. J.; He, L.; Guo, Y. Oxidative Stress and TGF- β 1/Smads Signaling are Involved in Rosa Roxburghii Fruit Extract Alleviating Renal Fibrosis. Evid Based Complementary and Altern Med. 2019, 2019, 4946580. DOI: 10.1155/2019/4946580.
- Ma, Y. X.; Zhu, Y.; Wang, C. F.; Wang, Z. S.; Chen, S. Y.; Shen, M. H.; Gan, J. M.; Zhang, J. G.; Gu, Q.; He, L. The Aging Retarding Effect of ‘Long-life CiLi.’ Mech. Ageing Dev. 1997, 96(1–3), 171–180.
- Xu, S. J.; Wang, X.; Wang, T. Y.; Lin, Z. Z.; Hu, Y. J.; Huang, Z. L.; Yang, X. J.; Xu, P. Flavonoids from Rosa Roxburghii Tratt Prevent Reactive Oxygen Species-mediated DNA Damage in Thymus Cells Both Combined with and without PARP-1 Expression after Exposure to Radiation in Vivo. Aging (Albany NY). 2020, 12(16), 16368–16389.
- Xu, S. J.; Zhang, F.; Wang, L. J.; Hao, M. H.; Yang, X. J.; Li, N. N.; Ji, H. L.; Xu, P. Flavonoids of Rosa Roxburghii Tratt Offers Protection against Radiation Induced Apoptosis and Inflammation in Mouse Thymus. Apoptosis. 2018, 23(9–10), 470–483.
- Chen, Y.; Liu, Z. J.; Liu, J.; Liu, L. K.; Zhang, E. S.; Li, W. L. Inhibition of Metastasis and Invasion of Ovarian Cancer Cells by Crude Polysaccharides from Rosa Roxburghii Tratt in Vitro. Asian Pac. J. Cancer Prev. 2014, 15(23), 10351–10354.
- Wu, P. H.;, Han, S. C. H.; Wu, M. H. Beneficial Effects of Hydroalcoholic Extract from Rosa Roxburghii Tratt Fruit on Hyperlipidemia in High-Fat-Fed Rats. Acta. Cardiol. Sin. 2020, 36(2), 148–159.
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic Db/db Mice. J. Agric. Food Chem. 2020, 68(1), 147–159.
- Higgins, J. P.; Altman, D. G.; Gotzsche, P. C.; Juni, P.; Moher, D.; Oxman, A. D.; Savovic, J.; Schulz, K. F.; Weeks, L.; Sterne, J. A. C., et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2011, 343(oct18 2), d5928.
- Chen, C.; Tan, S. M.; Wang, H.; Yang, S.; Dai, X. T. Effects of Rosa Roxburghii Tratt and Its Active Ingredients on Glucose and Lipid Metabolism in Type 2 Diabetic Mice. Food Sci. 2021, 2021, 1–14. http://kns.cnki.net/kcms/detail/11.2206.ts.20210723.1740.028.html
- Chen, M. M.; Zhan, J. H. Clinical Study of Rosa Roxburghii Freeze-dried Powder on the Treatment of CKD3 Stage 3 Dyslipidemia. Electron. J. Clin. Med. Lit. 2020, 7(61), 53–54.
- Chen, P.; Tan, S. M.; Chen, X. M.; Huang, Y.; Song, C. J. Study on Hypolipidemic Activity of Rosa Roxburghii Tratt, Propolis and Crataegus Oral Liquid. Mod. Food Sci. Technol. 2019, 35(8), 78–83.
- Cui, J. Y.; Gan, L.; Wan, W. R.; Xiong, R. B.; Zhang, Z.; Luo, B. D. Experimental Study of Rosa Roxburghii Juice’s Antilipidemic Effect in the Different Groups of Hyperlipidemia Model Mice. J. Health Prev. Med. 2014, 25(1), 7–10.
- Dai, Y. T.; Zhang, Z.; Gao, Z. F.; Zhao, G. X.; Wang, Y.; Li, F. R. Effect of Ci-Li (Rosa Voxburghii Tratt) on Experimental Hyperlipidemia and Atherosclerosis in Quails. Acta Nutrimenta Sinica. 1994, 02, 200–203.
- Hu, W. Y.; Bai, Y.; Han, X. F.; Zeng, Q.; Zhong, F. S.; He, W. F. Anti-atherosclerosis Effect of Rosa Roxburghii Tratt. Chin. Pharm. J. 2015, 11(8), 10–11.
- Jian, C. D.; Li, X. B.; Hang, J. M.; Meng, L. Q.; Yuan, S. S. Relationship between Anti-atherosclerosis Effect of Rosa Roxburghii Tratt Juice and Superoxide Dismutase. Inner Mongolia J. Tradit. Chin. Med. 2015, 34(6), 108.
- Jian, C. D.; Tang, X. L.; Huang, X. H.; Chen, H. Y.; Tang, H. D. Clinical Study on Anti-atherosclerosis Effect of Rosa Roxburghii Juice in Patients with Cerebral Infarction. Asia. Pac. Tradit. Med. 2017, 13(3), 136–137.
- Song, F. J.; Xu, Y. Z.; Liu, Y. Effect of Compound Rosa Roxburghii Juice on Lowering Blood Lipid. Beijing Med. J. 1989, 2, 72–75.
- Wang, J. J.; Liu, X. Z.; Liu, X. L.; Zhuang, Y. Y.; Li, L. Y. Effect of Rose Roxburghii Tratt Juice on Atherosclerosis in Hypercholesterolemic Hamsters. Chin. J. Arterioscler. 2001, 9(1), 17–20.
- Zhang, X. L.;. Investigation on Flavonoid from Rosa Roxburghii Tratt and Its Biological Activity; East China Normal University: Shanghai, 2005.
- Song, P. P.; Shen, X. C. Proteomic Analysis of Liver in Diet-induced Hyperlipidemic Mice under Fructus Rosa Roxburghii Action. J. Proteomics. 2021, 230, 103982. DOI: 10.1016/j.jprot.2020.103982.
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A.; Virani, S. S.; Alonso, A.; Benjamin, E. J.; Bittencourt, M. S.; Callaway, C. W.; Carson, A. P.; Chamberlain, A. M. Nasturtium Officinale Reduces Oxidative Stress and Enhances Antioxidant Capacity in Hyper-cholesterolaemic Rats. Chem. Biol. Interact. 2008, 172(3), 176–184. DOI: 10.1016/j.cbi.2008.01.006.
- McBride, P. Triglycerides and Risk for Coronary Artery Disease. Curr. Atheroscler. Rep. 2008, 10(5), 386–390. DOI: 10.1007/s11883-008-0060-9.
- Xu, J.; Vidyarthi, S. K.; Bai, W.; Pan, Z. Nutritional Constituents, Health Benefits and Processing of Rosa Roxburghii: A Review. J. Funct. Foods. 2019, 60, 103456. DOI: 10.1016/j.jff.2019.103456.
- Burke, D. S.; Smidt, C. R.; Vuong, L. T. Momordica Cochinchinensis, Rosa Roxburghii, Wolfberry, and Sea Buckthorn-highly Nutritional Fruits Supported by Tradition and Science. Curr. Top. Nutraceutical Res. 2005, 3(4), 259–266.
- Chen, J. J.; Xie, J.; Zeng, B. H.; Li, W. W.; Bai, S. J.; Zhou, C.; Chen, W.; Wei, H.; Xie, P. Absence of Gut Microbiota Affects Lipid Metabolism in the Prefrontal Cortex of Mice. Neurol. Res. 2019, 41(12), 1104–1112.
- Isabel, M. V.; Monica, S. T.; Noriega, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M. L.; Tovar, A. R.; Torres, N. A Dietary Intervention with Functional Foods Reduces Metabolic Endotoxaemia and Attenuates Biochemical Abnormalities by Modifying Faecal Microbiota in People with Type 2 Diabetes. Diabetes Metab. 2019, 45(2), 122–131.
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F., Mansourian, R.; Chou, C. J. Germ-free C57BL/6J Mice are Resistant to High-fat- Diet-induced Insulin Resistance and Have Altered Cholesterol Metabolism. FASEB J. 2010, 24(12), 4948–4959.
- Allayee, H.; Hazen, S. L. Contribution of Gut Bacteria to Lipid Levels: Another Metabolic Role for Microbes? Circ. Res. 2015, 117(9), 750–754. DOI: 10.1161/CIRCRESAHA.115.307409.
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates High-fat Diet-induced Intestinal Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Food Funct. 2021. DOI: 10.1039/d1fo03190b.
- Canfora, E. E.; Jocken, J. W.; Blaak, E. E. Short-chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11(10), 577–591. DOI: 10.1038/nrendo.2015.128.
- Segain, J. P.; Bletiere, R. D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H. M.; Galmiche, J. P. Butyrate Inhibits Inflammatory Responses through NF-kappaB Inhibition: Implications for Crohn’s Disease. Gut. 2000, 47(3), 397–403.
- Chaplin, A.; Parra, P.; Serra, F.; Palou, A. Conjugated Linoleic Acid Supplementation under a High-fat Diet Modulates Stomach Protein Expression and Intestinal Microbiota in Adult Mice. PLoS One. 2015, 10(4), e0125091.
- Pathak, P.; Xie, C.; Nichols, R. G.; Ferrell, J. M.; Boehme, S.; Krausz, K. W.; Patterson, A. D., Gonzalez, F. J.; Chiang, J. Y. L. Intestine Farnesoid X Receptor Agonist and the Gut Microbiota Activate G-protein Bile Acid Receptor-1 Signaling to Improve Metabolism. Hepatology. 2018, 68(4), 1574–1588.
- Oscar, C. T.; Anne, T.; Philippe, L.; Bart, S. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017, 152(7), 1679–1694.
- Harada, N.; Kusuyama, A.; Morishima, M.; Okada, K.; Takahashi, A.; Nakaya, Y.; Pathak, P.; Xie, C.; Nichols, R. G.; Ferrell, J. M., et al. Bezafibrate Improves Bacterial Lipopolysaccharide-induced Dyslipidemia and Anorexia in Rats. Metabolism. 2007, 56(4), 517–522.
- Liu, M. H.; Zhang, Q.; Zhang, Y. H.; Lu, X. Y.; Fu, W. M.; He, J. Y. Chemical Analysis of Dietary Constituents in Rosa Roxburghii and Rosa Sterilis Fruits. Molecules. 2016, 21(9), 1204.
- Wang, L.-T.; Lv, M.-J.; An, J.-Y.; Fan, X.-H.; Dong, M.-Z.; Zhang, S.-D.; Wang, J.-D.; Wang, Y.-Q.; Cai, Z.-H.; Fu, Y.-J., et al. Botanical Characteristics, Phytochemistry and Related Biological Activities of Rosa Roxburghii Tratt Fruit, and Its Potential Use in Functional Foods: A Review. Food Funct. 2021, 12(4), 1432–1451.
- Westhuizen, F. H.; Rensburg, C. S. J.; Rautenbach, G. S.; Marnewick, J. L.; Loots, D. T.; Huysamen, C.; Louw, R.; Pretorius P. J.; Erasmus, E. In Vitro Antioxidant, Antimutagenic and Genoprotective Activity ofRosa Roxburghii Fruit Extract. Phytother Res. 2008, 22(3), 376–383.