?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study focused on evaluating the influence of ultrasonic-assisted pasteurization (UAP) on the quality and shelf stability of jambul squash. Squash was analyzed for physicochemical characteristics during a storage period of 4 months. There was a significant effect (p < .05) of storage period on the acidity, DPPH radical scavenging activity, and flavonoids of jambul squash with a significant increase in acidity and DPPH radical scavenging activity and decrease in flavonoids with the corresponding increases in sonication time and pasteurization temperature during storage. The microbial content of jambul squash during storage remained lower than untreated samples. Microstructure evaluation of jambul squash showed de-shaped middle lamella and cell wall after UAP treatment. Outcomes of the current study revealed that squash samples with increased sonication time and pasteurization temperature had less microbial content than untreated samples.
Introduction
Jambul (Syzygium cumini L.) is an important fruit from the family Myrtaceae. The well-known names of S. cumini are Jambul, Indian blackberry, Java plum, Jamun, etc. Ra Jamun and Kaatha varieties are commonly grown in Pakistan.[Citation1] Jambul is rich in carbohydrates, such as glucose and fructose, and minerals like manganese, zinc, iron, calcium, sodium, and potassium. The major phytochemicals found in edible pulp are vitamin C, vitamin A, riboflavin, nicotinic acid, anthocyanins, and in seeds are glycoside jamboline; in bark and stem are tannins, phytosterols, and gallic acid .[Citation2]
The demand for jambul and its value-added products is rising due to the presence of plenty of natural antioxidants (i.e., anthocyanins) and therapeutic benefits, including anti-inflammatory, anti-diabetic,[Citation2] anti-cancer, anti-microbial, gastrointestinal health-promoting properties.[Citation3,Citation4] Jambul fruit is widely available and beneficial; however, it is still an underutilized fruit that is not commercially processed in Pakistan. Jambul fruit is degraded because of microbial spoilage and deteriorative quality losses. The best strategy to minimize these losses and render jambul fruit all-season available is to prepare some valuable products from its fruit.[Citation5] Fermented beverages, for example, vinegar, juice, ready-to-serve drinks, and squashes (non-aged), are the potential processed products of jambul. Jambul is also suitable for making sherbets and syrups .[Citation1]
Consumers demand more nutrient-rich, fresh, and healthy products with improved storage life and the least flavor and vitamin losses that compel the food processor to introduce new processing and preservation techniques.[Citation6,Citation7] Heat treatment is the most widely used strategy for increasing the shelf life of foods due to its ability to inactivate microbes and enzymes. Since heat treatment can spoil various organoleptic properties of foods and reduce nutrients’ bioavailability, there is an upcoming trend for developing new techniques involving low-intensity thermal treatments required for food preservation.[Citation8] Most of the non-thermal innovative food processing techniques as alternatives to conventional thermal processing include pulse electric field,[Citation9] ozonation,[Citation10] high-pressure processing,[Citation11] and sonication,[Citation12,Citation13] which exhibit a variety of benefits, such as improving nutritional value, maintaining the quality of food products by retaining their unique properties, preventing spoilage, extending storage life, minimizing food poisoning and economic losses.[Citation14,Citation15]
Ultrasound is making more strides in the food industry than conventional methods.[Citation14,Citation15] Ultrasound is a power generated by sound waves of frequencies greater than the standard frequency range perceived by the human ear, i.e., above 16 kHz.[Citation16] An ultrasound, when propagated, induces compressions and depressions of intermediate particles due to the cavitation phenomenon, which leads to massive amounts of energy production. In food processing, high-intensity ultrasound at frequencies of 20 to 100 kHz is beneficial for microbial inactivation.[Citation17] Compared to conventional thermal processes, the temperature during processing is considerably reduced when heat and ultrasound are used simultaneously, rendering it economically viable with the least energy consumption and high-cost effectiveness.[Citation8]
In the food industry, ultrasound has become an innovative methodology as a non-thermal and eco-friendly alternative to conventional thermal processing. Ultrasound has proved to be particularly useful in sterilization, extraction, freezing, and filtration, providing minimum processing times and increased efficiency. It was reported that ultrasound treatments are inexpensive, simple, reliable, and effective to conventional extraction techniques.[Citation18] This study aimed to evaluate the effect of ultrasonic-assisted pasteurization (UAP) on the quality and shelf stability of jambul squash.
Materials and methods
Raw materials procurement
Good quality, fresh, and fully ripe jambul (Syzgium cumini L.) variety Ra Jaman and other raw materials like sugar, preservative, color, flavor, and plastic bottles were purchased from the local market Sargodha, Pakistan.
Preparation of jambul squash
Fruits were washed, destoned to remove the pit, blanched with steam for 5 min, and then sugar, citric acid, and potassium metabisulfite were mixed through a blender (Cambridge Blender with Mill BL-2046) to prepare squash.
Ultrasonic-assisted pasteurization (UAP) treatment
Sonication of squash samples was performed in a sonicator (UP400S, Hielscher Ultrasonics GmbH Hielscher Inc., USA) of 750 W with probe 0.5 inches. 250 mL sample was taken in a 500 mL jacketed vessel of sonicator, and sonication was done at 70% amplitude. Samples were treated at 5, 10, and 15 min with pulse duration of 5s on and 5 s off and 20 kHz frequency. The depth of the probe was kept 5 cm in the juice samples. Then, the samples were pasteurized by placing them in a water bath for 30 min at 60, 70, and 80°C, respectively. Sample preparation and treatments were carried out in triplicate. Fresh untreated jambul squash was selected as a control (T0). All experiments were performed in the dark.[Citation19] Potassium-meta bisulfite (KMS) was used as a chemical preservative in treatment T0 + to compare the squash with control and other treatments (sonication). The treatment plan presented in was followed for further study.
Table 1. Treatments plan of the study
Physico-chemical properties
Jambul squash was subjected to physicochemical analyses to assess the physical properties and chemical composition of squash. Total soluble solids in jambul squash were determined by hand refractometer (Atago Corp, Tokyo, Japan) as per AOAC Method No. 932.12.[Citation20] pH of squash was measured with pH meter (Model: HI 2211, HANNA instruments) by following method No. 981.12 described in AOAC.[Citation20] Acidity was determined by method No. 942.15 described in AOAC.[Citation20] The acidity was calculated using the following formula:
Citric acid meq factor = 0.064
Total antioxidant activity (TAA)
To determine TAA, squash samples of all treatments were tested using the method described by .[Citation21] Ascorbic acid was used for making standard calibration curves, and the results were expressed as microgram ascorbic acid equivalent (μg AAE)/mL sample. All determinations were performed in triplicates from triplicate experimental trials.
Total flavonoids contents (TFC)
Flavonoid contents were determined by the method described by.[Citation19] Catechin (in ethanol) was used as a standard, and the results were expressed as μg of (+)-catechin equivalent (CE)/100 mL sample. All calculations were recorded in triplicates.
Total phenolic contents (TPC)
The TP contents were determined using Folin-Ciocalteu reagent with some modifications in the method, as explained by.[Citation19] Gallic acid (in ethanol) was used as a standard, and the results of total phenolics were expressed as µg gallic acid equivalents (GAE)/ g samples. All determinations were carried out in triplicates.
DPPH radical scavenging activity
Squash treatments were assessed for their DPPH radical scavenging activity by[Citation22] with minor changes. After diluting, 1 mL extract was taken, and 1 mL of DPPH (60 μmol in ethanol) solution was added. The solution was then placed in the dark for 30 min, and then absorbance was measured at 517 nm using a spectrophotometer. A decrease in absorbance was calculated, and inhibition of radical scavenging activity (RSA) was calculated as μmol AAE/mL.
Microbiological analysis
Total plate counts (TPC) of all samples were carried out according to ISO 4833–1:2013.[Citation23] A serially diluted sample (1 mL) was plated on plate count agar media (BioWorld, Ohio, Germany). The total aerobic plate counts (Log10 CFU/g) were determined for 2 days at 30°C. For yeast & mold (Y & M) counts (Log10 CFU/g), the AOAC method[Citation20] was used in the same manner (after 4 days at 25°C) using potato dextrose agar (BioWorld, Ohio, Germany).
Microstructure evaluation of jambul squash after sonication
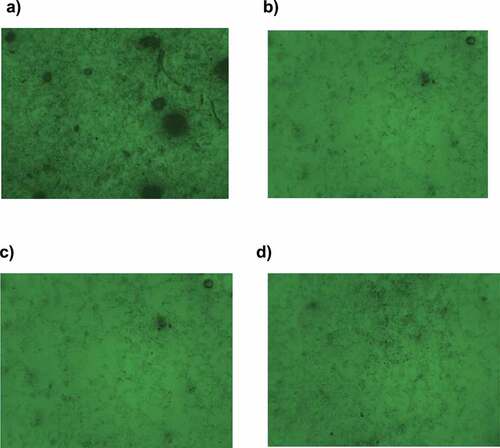
The microstructure evaluation of UAP treated fresh Jambul squash samples was taken to assess Jambul squash tissues’ homogeneity using a compound microscope with permissible green channel (OPTIKA Microscope, 4083.B3, Optikam B3 Digital Camera, Italy,) equipped with Image focus. A small drop of homogenized Jambul squash was placed on a glass slide and covered with a coverslip with no bubbles. The same method was adopted for the samples without UAP treatment. The prepared slides were examined under 40X magnification, and images were taken to evaluate the proper sonication time for a well-homogenized Jambul squash sample with excellent antioxidant potential.
Statistical analysis
The statistical analysis of data obtained from each parameter was conducted with the ANOVA technique at a significance level of p ≤ .05, and significant differences between mean values were determined by Tukey HSD pairwise comparison test. The statistical analyses were performed using Statistix 8.1 software (Analytical Software, Tallahassee, FL, USA).
Results and discussion
UAP effects on total soluble solids (0Brix)
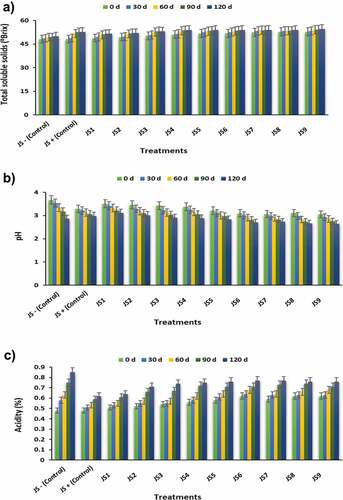
An increase in the °Brix was observed within the treatment throughout the study. The highest °Brix was found in JS9 (53.76 ± 0.78°Brix), and the lowest 0Brix was observed in JS0- (49.08 ± 0.68°Brix) (). The impact of storage duration on °Brix of jambul squash illustrated that TSS gradually increased with the corresponding rise in storage period. At 120 days of storage, the highest °Brix was observed with the mean value of 53.07 ± 1.43°Brix, as shown in . Similar results were determined by[Citation24] when they applied sonication on carrot grape blend juice and noticed that TSS increased from 12.7 to 13 °B with an increase in sonication time. As extraction efficiency improves, total sugars and soluble solids may be increased. Islam et al.[Citation25] noticed that TSS increased with increasing storage duration, which could be attributed to polysaccharide breakdown into monosaccharides and oligosaccharides.
UAP effects on pH
The results of the effects of UAP on the pH of squashes are shown in . The maximum pH was observed in JS9 (2.84 ± 0.03) and the lowest in JS0- (3.31 ± 0.05) and JS1 (3.32 ± 0.03). The storage period delineated a momentous effect on the pH of jambul squash as it decreased from 3.30 ± 0.04 to 2.85 ± 0.04 (). Nadeem et al. [Citation24] prepared sonicated carrot-grapes juice blend and observed a significant effect of sonication on the pH of blended juice. Abid et al. [Citation26] observed similar results for pH in sonicated grapefruit juice. They observed non-significant variation (p > .05) in the pH of grapefruit juice upon sonication; however, slight increase in pH was observed on account of different phytochemicals entities like mineral elements or vitamins released from fruit tissues. Due to the destructive impact of sonication on the cell structure, it releases vitamins from their bound form, thus enhancing their concentration and increasing the pH.
UAP effects on titratable acidity
The more pronounced increasing trend in acidity () was found in JS0 (0.48 ± 0.01 to 0.85 ± 0.03%) followed by JS1 to JS5, and the lowest increasing trend was noticed in JS6 to JS9 (0.62 ± 0.05 to 0.77 ± 0.02%). The effect of the storage period on the acidity of jambul squash showed that the acidity gradually increased with the progression in storage time.[Citation27] determined[Citation28]*** similar results, who observed that apple juice’s acidity significantly rose with corresponding increases in ultrasonic treatment time due to ultrasound-induced heat generation (in that study, the temperature was constant 25 ± 1°C). Another study by[Citation26] revealed similar results in which apple juice was subjected to ultrasound treatment at 30, 60, and 90% amplitude level for 3 min and resulting samples were stored at 40°C for 30 days, and it was concluded that storage study increased the titratable acidity of apple juice from 0.23 ± 0.10 to 0.26 ± 0.02%.
UAP effects on total phenolic (TPC) and flavonoids contents (TFC)
TPC of jambul squash was observed in range of 55.20 ± 0.52 µg/mL gallic acid equivalent in JS0 to 488.80 ± 10.62 µg/mL GAE in JS6 at the start of the study (). The TPC values of all the samples were decreased from 426.26 ± 5.42 to 77.80 ± 3.95 µg/mL GAE during storage of 120 days. The content of phenolic may be depleted due to the production of free radicals.[Citation29] TFC of jambul squash was the minimum in JS0 control samples (264.40 ± 2.59 µg CE/mL) and the maximum (469.00 ± 18.00 µg CE/mL) in JS6 (). TFC in all samples decreased during 4 months storage period. Nadeem et al. [Citation24] reported a significant increase in total phenols and flavonoids content in a sonicated carrot-grapes juice blend. Abid et al. [Citation30] determined the impact of sonication on the flavonoid content of apple juice, and it was observed that sonicated samples showed more improvement in TFC than non-sonicated sample control samples. These investigations recommended that increases in sonication time led to enhancement of flavonoids and bioactive compounds in the juice.
Table 2. Effects of UAP on total phenolic content (µg/mL GAE) of jambul squash during storage
Table 3. Effects of UAP on total flavonoid content (µg/mL catechin equivalent) of jambul squash during storage
UAP effects on total antioxidant activity and DPPH-radical scavenging activity
The antioxidant values of all jambul squash samples ranged from 814.0 ± 45.0 to 1306.7 ± 56.9 µg/mL AAE at the start of the storage period (). The highest antioxidants value was examined in JS6, while the minimum value was observed in JS0. A decreasing trend was observed during storage.[Citation31] narrated similar results that antioxidant capacity and phenolic contents of grape fruit juice were increased with a corresponding increase in sonication time, i.e., 290 µg/g to 820 µg/g at 90 min. In both cases, treatment sonicated for 90 min in a bath-type sonicator was noticed more stability during 28 days of storage. The DPPH radical scavenging activity (RSA) of squash samples ranged from 3846.7 ± 20.2 to 4703.3 ± 58.6 µmol/mL AAE (). The highest value was observed in JS6 (4703.3 ± 58.6 µmol AAE/mL) followed by JS5 (4553.3 ± 56.2 µmol/mL AAE), while the minimum value was recorded in JS0- (3846.7 ± 20.2 µmol AAE/mL).[Citation26] determined the impact of sonication on the DPPH-RSA of apple juice and concluded that sonicated samples showed significant improvement in DPPH-RSA than the non-sonicated control sample. It was further observed that sonication treatment enabled the accelerated release of antioxidants from the tissue matrices resulting in the scavenging of free radicals. These investigations recommended that an increase in sonication time enhances the flavonoids and bioactive compounds in juice.
Table 4. Effects of UAP on total anti-oxidant activity (µg/mL AAE) of jambul squash during storage
Table 5. Effects of UAP on DPPH radical scavenging activity (µmol/mL AAE) of jambul squash during storage
Impact of UAP on microbial content
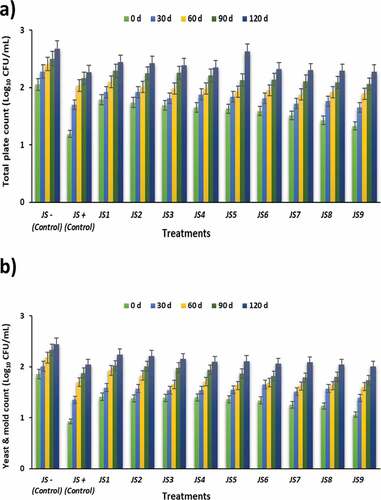
Results for the microbiological status of jambul squash during the storage period showed a significant (p < .05) influence of temperature and treatment time on microbial count (). The outcomes of the current study revealed that squash samples subjected to increased sonication time and pasteurization temperature had less microbial content than untreated samples. The maximum total plate count was observed in JS0- (2.05 ± 0.01 CFU/mL) at the start of the storage study and increased up to 2.68 ± 0.01 CFU/mL after 120 days, and the minimum total plate count was noticed in JS9 (1.33 ± 0.04 CFU/mL) (). The maximum mold/yeast (Y&M) count was observed in JS0- (1.86 ± 0.02 CFU/mL), and the minimum Y&M count was observed in JS9 (1.06 ± 0.07 CFU/mL) (). Nadeem et al. [Citation24] reported a significant decrease in microbial count in sonicated carrot-grapes juice blend. Zou and Jiang [25] examined the impact of ultrasound on microbial load in carrot juice and examined a significant decrease in the total plate and mold counts in all samples that were sonicated for a time duration of 20, 40, and 60 min in comparison with non-sonicated juice samples. Abid et al. [Citation26] claimed that the decrease in microbial content was due to increased biocides produced by sonication-induced cavitations. These cavitations increase pressure and generate free radicals, which inactivate bacteria.
UAP effects on microstructure
Microstructure evaluation of jambul squash showed de-shaped middle lamella and cell wall after UAP treatment. elucidates the microstructure evaluation of the control sample in which middle lamella and cell wall are seen intact; however, a progressive uniformity in microstructure profile on account of structural disintegration is seen in ), illuminating the effect of JS1, JS6, and JS9 respectively on the microstructure of jumbul squash. Moreover, a gradual improvement in the chemical and antioxidant profile was also observed in these treatments might be due to structural integration releasing bound compounds after sonication treatment. These results are in agreement with the findings of,[Citation32] who earlier reported a similar cell de-shape (middle lamella & cell wall) effect in mangoes when observed under a compound microscope.
Conclusion
This study demonstrated the influence of ultrasonic-assisted pasteurization (UAP) on the functional properties of jambul squash during storage. The results showed a significant (p < .05) effect of UAP on total phenolic contents, flavonoid contents, DPPH radical scavenging activity, and antioxidant potential. Squash samples with the increased sonication time and pasteurization temperature had less microbial load than untreated samples. When heat and ultrasound were used together, the temperature during the process was considerably reduced compared to the conventional heating process, making it an ideal technique to preserve bioactive components and enhance nutritional profile. Sonication is an effective method that preserves fruit beverages quality by retaining a significant amount of bioactive compounds and reducing pathogenic microorganisms.
Additionally, it contributes to the economy by requiring less energy. It could be concluded that UAP, as an environmentally friendly technique, can be effectively used to produce minimally processed foods, thereby preserving nutritional entities in foods and enhancing the dietetic perspective on foods with the least energy consumption and the highest cost-effectiveness.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Singh, C. S.; Paswan, V. K.; Rai, D. C. Process Optimization of Spray Dried Jamun (Syzygium Cumini L.) Pulp Powder. LWT. 2019, 109, 1–6. DOI: 10.1016/j.lwt.2019.04.011.
- Panghal, A.; Kaur, R.; Janghu, S.; Sharma, P.; Sharma, P.; Chhikara, N. Nutritional, Phytochemical, Functional and Sensorial Attributes of Syzygium Cumini L. Pulp Incorporated Pasta. Food Chem. 2019, 289, 723–728. DOI: 10.1016/j.foodchem.2019.03.081.
- Ayyanar, M.; Subash-Babu, P. Syzygium Cumini (L.) Skeels: A Review of Its Phytochemical Constituents and Traditional Uses. Asian Pac. J. Trop. Biomed. 2012, 2(3), 240–246. DOI: 10.1016/S2221-1691(12)60050-1.
- Chattopadhyay, P.; Chatterjee, S.; Sen, S. K. Biotechnological Potential of Natural Food Grade Biocolorants. Afr. J. Biotechnol. 2008, 7(17), 2972–2985.
- Shahnawaz, M.; Sheikh, S. A.; Nizamani, S. M.; Bhanger, M. I.; Imran, A.; Ejaz, A. A Study on the Determination of Mineral Elements in Jamun Fruit (Eugenia Jambolana) Products. Pak. J. Nutr. 2012, 11(2), 181–186. DOI: 10.3923/pjn.2012.181.186.
- Barba, F. J.; Gavahian, M.; Es, I.; Zhu, Z.; Chemat, F.; Lorenzo, J. M.; Khaneghah, A. M. Solar Radiation as a Prospective Energy Source for Green and Economic Processes in the Food Industry: From Waste Biomass Valorization to Dehydration, Cooking, and Baking. J. Cleaner Prod. 2019, 220, 1121–1130. DOI: 10.1016/j.jclepro.2019.02.175.
- Ranjha, M. M. A. N.; Irfan, S.; Nadeem, M.; Mahmood, S. A Comprehensive Review on Nutritional Value, Medicinal Uses, and Processing of Banana. Food Rev. Int. 2020. DOI: 10.1080/87559129.2020.1725890.
- Hilali, S.; Fabiano-Tixier, A.-S.; Ruiz, K.; Hejjaj, A.; Ait Nouh, F.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green Extraction of Essential Oils, Polyphenols, and Pectins from Orange Peel Employing Solar Energy: Toward a Zero-Waste Biorefinery. ACS Sustainable Chem. Eng. 2019, 7(13), 11815–11822. DOI: 10.1021/acssuschemeng.9b02281.
- Ranjha, M. M. A. N.; Kanwal, R.; Shafique, B.; Arshad, R. N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P. Ł.; Irfan, M.; Khalid, M. Z., and Roobab, U., et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules. 2021, 26(16), 1–26.
- Li, Z.; Sun, Y.; Jin, H.; Wang, Q.; Jin, Y.; Huang, X.; Sheng, L. Improvement and Mechanism of Emulsifying Properties of Liquid Egg Yolk by Ozonation Technology. LWT. 2022, 156, 113038. DOI: 10.1016/j.lwt.2021.113038.
- Roobab, U.; Afzal, R.; Ranjha, M. M. A. N.; Zeng, X.-A.; Ahmed, Z.; Aadil, R. M. High Pressure-Based Hurdle Interventions for Raw and Processed Meat: A Clean-Label Prospective. Int. J. Food Sci. Technol. 2022, 57(2), 816–826. DOI: 10.1111/ijfs.15499.
- Nadeem, M.; Ghaffar, A.; Hashim, M. M.; Murtaza, M. A.; Ranjha, M. M. A. N.; Mehmood, A.; Riaz, M. N. Sonication and Microwave Processing of Phalsa Drink: A Synergistic Approach. Int. J. Fruit Sci. 2021, 21(1), 993–1007. DOI: 10.1080/15538362.2021.1965942.
- Ranjha, M. M. A. N.; Irfan, S.; Lorenzo, J. M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R. N.; Wang, L.; Nayik, G. A., and Roobab, U., et al. Sonication, A Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes. 2021, 9(8), 1–21.
- Ranjha, M. M. A. N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M. N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M. A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020. DOI: 10.1080/15538362.2020.1782804.
- São José, J. F. B. D.; de Andrade, N. J.; Ramos, A. M.; Vanetti, M. C. D.; Stringheta, P. C.; Chaves, J. B. P. Decontamination by Ultrasound Application in Fresh Fruits and Vegetables. Food Control. 2014, 45, 36–50. DOI: 10.1016/j.foodcont.2014.04.015.
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of Ultrasound for Green Extraction of Proteins from Spirulina. Mechanism, Optimization, Modeling, and Industrial Prospects. Ultrason. Sonochem. 2019, 54, 48–60. DOI: 10.1016/j.ultsonch.2019.02.016.
- Chen, F.; Zhang, M.; Yang, C.-H. Application of Ultrasound Technology in Processing of Ready-to-Eat Fresh Food: A Review. Ultrason. Sonochem. 2020, 63, 104953. DOI: 10.1016/j.ultsonch.2019.104953.
- Firouz, S.; Mahmoud, A. F.; Hosseinpour, S. Recent Advances in Ultrasound Application as A Novel Technique in Analysis, Processing and Quality Control of Fruits, Juices and Dairy Products Industries: A Review. Ultrason. Sonochem. 2019, 57, 73–88. DOI: 10.1016/j.ultsonch.2019.05.014.
- Jabbar, S.; Abid, M.; Wu, T.; Muhammad Hashim, M.; Hu, B.; Lei, S.; Zhu, X.; Zeng, X. Study on Combined Effects of Blanching and Sonication on Different Quality Parameters of Carrot Juice. Int. J. Food Sci. Nutr. 2014, 65(1), 28–33. DOI: 10.3109/09637486.2013.836735.
- AOAC. “Official Methods of Analysis of AOAC International.” 2016.
- Wang, J.; Kranthi Vanga, S.; Raghavan, V. High-Intensity Ultrasound Processing of Kiwifruit Juice: Effects on the Ascorbic Acid, Total Phenolics, Flavonoids and Antioxidant Capacity. LWT. 2019, 107, 299–307. DOI: 10.1016/j.lwt.2019.03.024.
- Ismail, T.; Suleman, R.; Akram, K.; Hameed, A.; Llah, I.-U.; Amir, M.; Akhtar, S. Pomegranate (Punica Granatum L.) Peel Extracts Inhibit Microbial Growth and Lipid Oxidation in Minced Shrimps Stored at 4°C. J. Aquat. Food Prod. Technol. 2019, 28(1), 84–92. DOI: 10.1080/10498850.2018.1561571.
- Mulyono, T. P.; Hartati, F. K.; Djauhari, A. B. Sanitary Test of Penyetan Vendors’ Plates Using Swab Test Method of Total Plate Count and Escherichia Coli in Tambaksari District of Surabaya. Food Science and Technology Journal (Foodscitech). 2020, 22 SE–Articles, 8–17. DOI:10.25139/fst.v0i0.2057.
- Nadeem, M.; Ubaid, N.; Qureshi, T. M.; Munir, M.; Mehmood, A. Effect of Ultrasound and Chemical Treatment on Total Phenol, Flavonoids and Antioxidant Properties on Carrot-Grape Juice Blend during Storage. Ultrason. Sonochem. 2018, 45, 1–6. DOI: 10.1016/j.ultsonch.2018.02.034.
- Islam, M. A.; Ahmad, I.; Ahmed, S.; Sarker, A. Biochemical Composition and Shelf Life Study of Mixed Fruit Juice from Orange & Pineapple. Journal of Environmental Science and Natural Resources. 2015, 71 SE–Articles, 227–232. DOI:10.3329/jesnr.v7i1.22175.
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M. M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of Ultrasound on Different Quality Parameters of Apple Juice. Ultrason. Sonochem. 2013, 20(5), 1182–1187. DOI: 10.1016/j.ultsonch.2013.02.010.
- Yuan, Y.; Hu, Y.; Yue, T.; Chen, T.; Lo, Y. M. Effect of Ultrasonic Treatments on Thermoacidophilic Alicyclobacillus Acidoterrestris in Apple Juice. J. Food Process. Preserv. 2009, 33(3), 370–383. DOI: 10.1111/j.1745-4549.2009.00407.x.
- Zou, Y.; Jiang, A. Effect of Ultrasound Treatment on Quality and Microbial Load of Carrot Juice. Food Sci. Technol. 2016, 36(1), 111–115. DOI: 10.1590/1678-457X.0061.
- Rajashri, K.; Roopa, B. S.; Negi, P. S.; Rastogi, N. K. Effect of Ozone and Ultrasound Treatments on Polyphenol Content, Browning Enzyme Activities, and Shelf Life of Tender Coconut Water. J. Food Process. Preserv. 2020, 44(3), e14363. DOI: 10.1111/jfpp.14363.
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M. M.; Bing, H.; Saeeduddin, M.; Zeng, X. Qualitative Assessment of Sonicated Apple Juice during Storage. J. Food Process. Preserv. 2015, 39(6), 1299–1308. DOI: 10.1111/jfpp.12348.
- Aadil, R. M.; Zeng, X.-A.; Abbasi, A. M.; Khan, M. S.; Khalid, S.; Jabbar, S.; Abid, M. Influence of Power Ultrasound on the Quality Parameters of Grapefruit Juice during Storage. Sci. Lett. 2015, 3, 6–12.
- Rimkeeree, K.; Charoenrein, S. Effect of Cultivar and Ripening Stage on Quality and Microstructure of Frozen Mangoes (Mangifera Indica Linn.). Int. J. Food Prop. 2014, 17(5), 1093–1108. DOI: 10.1080/10942912.2012.698342.