?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study identified the quality, physicochemical properties, and oxidation stability of tengkawang butter (TB) from indigenous plants in various areas of Kalimantan, Indonesia. The fatty acid profile was dominated by palmitic acid, stearic acid, and oleic acid. Acidity ranged from 6.88 to 15.94%, while the peroxide number was 0.41 and 8.27 meq O2/kg. TB had a melting point of 36–37°C. The solid fat content (SFC) varied from 0.03 to 3%. The Oxidation Stability Index (OSI22) was more than 10,000 h. The composition and properties show the potential of TB to become a cocoa butter equivalent (CBE).
Introduction
The requirement for vegetable fats and oils in Indonesia is relatively high. Statistical data indicate that Indonesia imported 15,294,525 kg of such fats and oils in 2021 to fulfil the domestic needs for butter.[Citation1] Tengkawang is an indigenous plant in Kalimantan, Indonesia that can meet the need for vegetable fat. It belongs to the Dipterocarpaceae plant family and is divided into several species, such as Shorea stenoptera, S. pinanga, S. mecisopteryx, and S. macrophylla.[Citation2] Tengkawang fruit is processed to produce tengkawang butter (TB), which has a high fatty acid content and thus potential as a source of vegetable butter.[Citation3]
Tengkawang butter is still produced in a traditional manner in the community, using a tool called the apit which has a butter production capacity of 4–5 kg.[Citation4] Previous studies have reported that the quality of traditional tengkawang fat is lower than that required under the Indonesian National Standard (SNI).[Citation3,Citation5,Citation6] The low quality is due to the traditional use of high heating and curing processes to increase the acidity and peroxide values (PVs).[Citation3] Previous studies carried out the purification process using chemical solvents and adsorbents such as activated carbon,[Citation5] thermal-activated bentonite,[Citation6] and acid-activated bentonite[Citation3] to increase the quality of tengkawang butter. However, no research comprehensively discusses tengkawang butter as a food source such as cocoa butter equivalent (CBE).
Tengkawang butter has potential as a cocoa butter substitute (CBS)/cocoa butter equivalent (CBE) because it is vegetable fat. CBE/CBS are fats that fulfil the function of cocoa butter (CB), in whole or in part, as they share a similar fatty acid composition.[Citation7,Citation8] Among the requirements for fats to become CBE are that they have a similar fatty acid composition, melting point, and physicochemical properties to those of CB.[Citation9,Citation10] Oxidation stability, another requirement for fat quality to become a CBE, can be estimated using both normal and accelerated oxidation stability methods. The normal oxidation stability process is carried out on fat samples stored for a specific time, through an oxidation analysis such as peroxide number, acidity, and anisidine value. The accelerated oxidation stability process is carried out by the rancimat method at a specific temperature (100–140°C), and the induction time data are.[Citation11,Citation12] The induction time value calculates the estimated shelf life of a fat/oil that becomes CBE.
In addition to quality and physicochemical data, it is necessary to know the stability of TB to determine whether it can be part of a CBE. However, no research has identified the physicochemical properties and oxidation stability of traditional tengkawang fat from several areas in Kalimantan. This study used tengkawang from four regions producing TB, namely Bengkayang, Nanga Yen, Sintang, and Kapuas Hulu, to determine the quality, physicochemical properties, and oxidation stability. The data obtained by this study can be used as a reference regarding the quality of conventional TB from several producing areas. Knowledge of quality and oxidation stability data enables the operating conditions of the purification process and the potential of TB as a CBE to be determined.
Materials and methods
Preparation materials
Tengkawang butter were obtained from several villages in West Kalimantan, Indonesia, namely Nanga Yen, Sintang, Kapuas Hulu, and Bengkayang. Pro analysis chemicals such as Na2S2O3, NaOH, KI, Ethanol, H3PO4 were obtained from Merck Millipore (Germany).
Fatty acid profile analysis
Fatty acid profile analysis was done using AOCS Ce 2–66 method.[Citation13] Tengkawang butter was analyzed and determined using gas chromatography (GC) with a flame ionization detector (FID). The GC column used was a DB FastFame Capillary column 60 m × 0.25 mm, id 0.25 m, with Helium gas as the carrier. The injection volume and temperature were 1 μL and 240°C, respectively.
Analysis of SNI 2903:2016 quality
The quality parameters defined in SNI 2903:2016 and SNI 01–3555:1998 are acidity, peroxide number, iodine number, saponification number, and water content.[Citation14,Citation15] In this study, acidity analysis and saponification number were based on the acid-base titration method with 0.1 N KOH and 0.5 N HCl as titrant for acidity and saponification number. The iodine number analysis was performed with wijs solution as the reagent, natrium thiosulfate (Na2S2O3) as the titrant, and starch as an indicator in an iodometric titration. Iodometric titration with natrium thiosulfate (Na2S2O3) solution as titrant and starch as indicator was used to determine the peroxide number. Water content analysis in Tengkawang samples was carried out using the Karl Fischer titration (KFT) method based on ASTM D6304.[Citation16] The KFT instrument used was the Aqua 40.00 model with an oil module.
Tengkawang butter thermal analysis
A differential scanning calorimetry (DSC) 214 Polyma, NETZCH equipment was used to conduct a thermal investigation of tengkawang butter samples. The samples were heated from room temperature to 60°C at a 1°C/min rate and kept for 10 minutes to record the heating phenomena. To evaluate thermal characteristics changes, the thermogram data was compared with several butter sources.
Solid fat content (SFC) of tengkawang butter
A solid fat content (SFC) analysis was performed with the NMR instrument. The fat was heated until it melted and was homogeneous. Twelve tubes of oil were put into SFC tubes of about 4 ± 1 cm (two tubes for each temperature). The SFC tube was transferred to a water bath at 60°C to melt the tengkawang butter, then to a water bath with temperature variations of 15, 20, 25, 30, 35, and 40°C and left for 30–35 minutes. SFC was measured by inserting the tube into the holder on the NMR device, which had been set according to the Non-Stab AOCS method.
Oxidation stability index (OSI) of tengkawang butter
The oxidation stability index (OSI) analysis of tengkawang butter was performed using the 893 Professional Rancimat instrument, Metrohm, and the ISO 6886 method.[Citation17] A sample of 3.0 ± 0.01 g was heated in a holder with a constant gas flow of 10 L/h with variations in temperature (100, 110, 120, and 130°C). The induction time value was calculated as the OSI value.
Tengkawang butter shelf-life prediction, temperature coefficient, and Q10 number calculation
The value logarithm of OSI was associated with variations in temperature (100, 110, 120, and 130°C) through linear regression. The regression equation of each type of tengkawang butter was applied to calculate the value of the temperature coefficient as the slopes of the line. The linear equation of the OSI is as follows:
where y is the natural logarithm of OSI, A is the temperature coefficient, x is the temperature, B is the intercept. The shelf-lives were estimated as OSIs at 22°C (OSI22) using the appropriate Equationequations 6(6)
(6) at x = 22. The following equation was used to calculate the Q10 numbers.
Statistical data analysis
This investigation used an analysis of variance (ANOVA) test. If the quality data for tengkawang butter changed, further testing was carried out at the 5% level using Duncan’s Multiple Range Test (DMRT).[Citation18] All experiments were performed in triplicate or duplicate.
Result and discussion
Tengkawang butter’s fatty acid profile
The fatty acid content of tengkawang butter samples from Sintang, Nanga Yen, Bengkayang, and Kapuas Hulu was determined using the fatty acid profile analysis technique. Instrument gas chromatography (GC) with a mass detector (MS) or flame ionization detector (FID) is a widely used technique for the analysis of fatty acids contained in oil and butter in foods.[Citation19,Citation20]
As can be seen from the fatty acid profile data shown in , in general, the dominant types of fatty acids in the four TB samples are the same: stearic acid, oleic acid, and palmitic acid, whose composition was 40.05%, 32.34%, and 24.05%, respectively, for Bengkayang TB; 43.12%, 31.71%, and 22.04%, respectively, for Nanga Yen TB; 45.77%, 31.14%, and 17.23%, respectively, for Sintang TB; and 46.98%, 32.74%, and 16.37%, respectively, for Kapuas Hulu TB. The DMRT analysis indicated that the composition of stearic acid, oleic acid, and palmitic acid of the four tengkawang butters was differed significantly. The difference in the composition of the four TB samples was how the tengkawang tree grows and the different ways TB was extracted.[Citation21] Traditional methods and differences in extraction methods can also affect the composition and quality of tengkawang butter. In the Nanga Yen region, the extraction process was carried out by steaming at a high temperature (100–150°C), while in Sintang, was carried out by curing.[Citation3] Meanwhile, in Bengkayang and Kapuas Hulu, heat pressure extracted oil from tengkawang fruit.
Figure 1. Tengkawang butter fatty acid profile from different regions. There is no significant difference in the same letters, according to DMRT 5%.
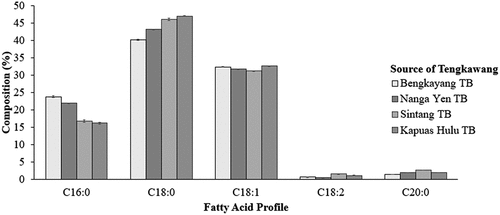
The composition of tengkawang butter in this research was similar to that of cocoa butter (CB) and cocoa butter equivalent (CBE) composition, due to stearic acid, oleic acid, and palmitic acid in the latter two. Palmitic acid was estimated to constitute 25% of CB and 27% of CBE; stearic acid 33% of CB and 36% of CBE; and oleic acid 32% of CB and 35% of CBE.[Citation22,Citation23] CBE is a vegetable butter with composition, properties, and characteristics close to cocoa fat.[Citation8,Citation9,Citation24,Citation25] TB can be used as a CBE because it has a fatty acid composition similar to that of CB
Physicochemical quality of tengkawang butter
Acidity, peroxide number, iodine number, saponification number, water content, and melting point are physicochemical characteristics of tengkawang butter. shows the quality of tengkawang butter against Indonesia national standard (SNI) standards and cocoa butter standards. The four tengkawang have melting points based on the standard melting point. The melting points (o C) of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB are 36.8, 36.7, 36.6 and 36.7, respectively. DMRT analysis indicates that the melting point values of the four TB samples are not significantly different Butter used as food must have a melting point in the body temperature range, around 36–37°C.[Citation27] If fat has a solid content at room temperature and melts below body temperature, it will cause a smooth cooling sensation in the mouth because it absorbs heat from the walls of the mouth.[Citation28] Tengkawang butter has a slightly lower melting point than the human body temperature (37°C), so it can be melted in the mouth if consumed.
Table 1. Physicochemical properties of tengkawang butter. There is significant difference in the different letters, according to DMRT 5% in same parameters
The water content of the four tengkawang butter is very low, below 1%, according to the standards of tengkawang (SNI) and cocoa butter (FAO). The water content (%) of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB are 0.23, 0.25, 0.11, 0.08, respectively. The DMRT analysis indicates that the water content value of Kapuas Hulu TB and Sintang TB was significantly different from that of Bengkayang TB and Nanga Yen TB. Water content is an important indicator of butter and oil storage because it can cause a decrease in fat quality in certain compositions. The water content can increase the free fatty acids (FFA) composition and the acid number value because the fat hydrolysis process occurs under certain conditions.[Citation29] If the water content is higher, the FFA content will be higher, causing the fat rancidity process to be faster. Therefore, in the fat extraction process, the water content must be considered to not accelerate the process of fat damage due to rancidity.
Acidity is a value equivalent to the amount of FFA in fat. The acidity values in the four butter are enormous, being far above the maximum limits for tengkawang (SNI) and cocoa butter (FAO) standards. The acidity values (%) of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB were 6.88, 9.68, 10.71, and 15.94 respectively. DMRT analysis indicates that the acidity values of the four TB samples were significantly different The high acidity value indicates the high content of FFA in traditional tengkawang butter. The traditional fat extraction process, such as using a high temperature steaming process and hot air drying, can increase the FFA value due to the hydrolysis process[Citation3,Citation30]; however, no other purification processes yet exist to improve the quality of TB. Therefore, a treatment process according to industry standards is needed in the extraction and purification process to improve the quality of traditional TB.
Peroxide value (PV) /number (PN) is an indicator for monitoring the oxidative process of oils and fats.[Citation31] A high peroxide value indicates an increase in the hydroperoxide composition or decomposition of fats/oils. The peroxide value correlates with the rancidity of the fat/oil.[Citation32] In general, the peroxide value should not be above 10–20 meq/kg fat to avoid the rancidity of the fat.[Citation33] The peroxide value in tengkawang butter samples was below the maximum standard for tengkawang butter (SNI) and cocoa butter (FAO). The peroxide values (meq O2/kg) of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB were 0.41, 0.56, 4.33, and 8.27, respectively. DMRT analysis indicates that the PVs of the four TB samples were significantly different A low peroxide value indicates that tengkawang butter is more resistant to oxidation processes that cause rancidity. However, because the acidity value of tengkawang was still high, a rancidity correlation occurs due to the amount of FFA that can undergo an oxidation process that causes rancidity. Therefore, the purification process must still be carried out even though the PV is in accordance with the standard.
Iodine value (IV)/number (IN) is the relative degree of unsaturation in fats/oils determined by reactions with halogens.[Citation34] Since the melting point and oxidative stability depend on unsaturation, the iodine value helps estimate the quality of fats/oils.[Citation35] The iodine number in the four tengkawang has an iodine value according to the standard for tengkawang fat (SNI) and cocoa butter (FAO). The Iodine values (I2/100 g) of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB were 21.72, 29.33, 32.46, 31.38, respectively. DMRT analysis indicates the INs of the four TB samples were significantly different.
The saponification number calculates the level of ester bonds in fats/oils through an acid-base titration process. The molecular weight and concentration of fatty acids in the fat/oil determine the saponification number.[Citation36] Therefore, an adequate saponification number was used to calculate the average relative molecular mass of a fat/oil. The saponification number of tengkawang fat follows the standards of tengkawang (SNI) and cocoa butter (FAO). The saponification numbers of Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB were 200.99, 201.88, 198.74, 196.51, respectively. DMRT analysis indicates that the saponification number of Kapuas Hulu TB and Sintang TB were significantly different from those of Bengkayang TB and Nanga Yen TB.
The data shown in indicate that several parameters are follow the SNI tengkawang standard and CB standard, such as water content, IN, saponification number, melting point, and peroxide number. However, one parameter has not been achieved, namely acidity, which has a value above the standard. Therefore, a purification process is needed to improve the quality of traditional TB to meet SNI and CB standards.
Thermal properties of tengkawang butter
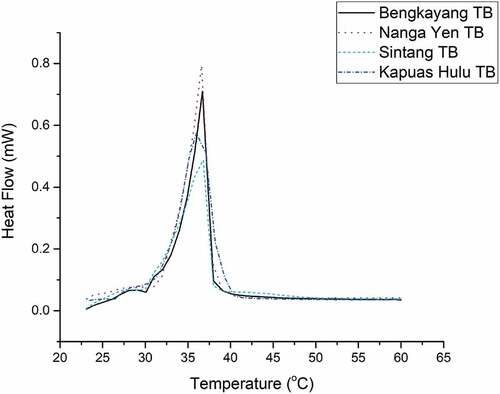
Thermal properties analysis was carried out to determine the thermal phenomena of tengkawang butter. shows the DSC thermogram of the four tengkawang butter samples studied. The fourth DSC peak, tengkawang butter, was at 36°C and showed the optimum melting point of tengkawang butter. However, the onset value (temperature begins to melt) of the four tengkawang butter was different, due to the variations in the fatty acid composition of each.
Sintang TB has the lowest onset of 32.40°C and offset of 37.80°C, while Kapuas Hulu TB has the largest onset of 35.30°C and offset of 38.90°C. The onset and offset values were 34.90°C and 37.80°C for Nanga Yen TB and 33.10°C and 37.80°C for Bengkayang TB. Tengkawang butter samples from Bengkayang, Nanga Yen, Sintang, and Kapuas Hulu had enthalpy values (J/g) of 113.80, 138.80, 96.09, and 153.50, respectively. The enthalpy values of the four tengkawang butters were notably varied, according to DMRT analysis. Because Kapuas Hulu TB has the largest saturated C18 fatty acid content of four tengkawang butters, it has the highest onset value. Furthermore, because long-chain saturated fatty acids (SFA) need more energy to melt, Kapuas Hulu TB has the highest enthalpy value. Some research has shown TB to have a similar melting temperature and enthalpy to CB and dark chocolate. Dark chocolate has an enthalpy of 121.52 J/g and onset and offset temperature of 12.54°C and 32.2°C.[Citation37] The onset and offset temperatures of cocoa butter were 25.97°C and 37.70°C, and the enthalpy is 116.20 (J/g).[Citation23] The dominant fatty acid composition is similar to tengkawang butter in cocoa butter and dark chocolate.
Solid fat content (SFC) of tengkawang butter
Solid fat content (SFC) in fats and oils at a specific temperature is the ratio of the proton magnetic signal in the solid phase to the liquid phase.[Citation38] SFCs in fats and oils were responsible for several characteristics of fatty foods, such as physical properties, organoleptic properties, and spreadability, affecting the plasticity of oil/fat products.[Citation39] SFC affects the structure and sensory in vegetable fats related to the composition, unsaturation content, and fatty acid chain length.[Citation40–42]
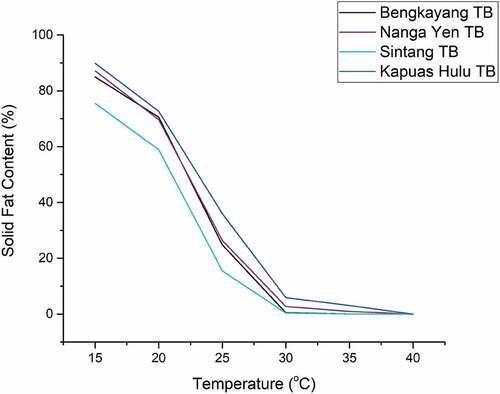
presents the SFC graph of the four tengkawang butter samples. After the temperature increase, the SFC value of tengkawang butter decreased because there was a change in the crystalline phase of fatty acids.[Citation43] DMRT analysis indicates that the SFC value of Kapuas Hulu was significantly different from that of the other three TB samples. Tengkawang butter has an SFC value of 75–89% when heated at 15°C. The SFC value of tengkawang butter was roughly 58–70% at a temperature of 20°C was similar to cocoa butter.[Citation44] The SFC value of three tengkawang butters (Nanga Yen, Sintang, and Bengkayang) was less than 1% at 35°C, whereas Kapuas Hulu tengkawang is 3.11%. The content of saturated fatty acids (SFA) and long-chain (C18) in Kapuas Hulu TB was greater than the other three samples. The higher the composition of SFA and long-chain fatty acids, the greater the value of SFC.[Citation40,Citation41] At room temperature, the value of SFC at 20–25°C determines product stability and resistance to fat/oil; a value of not less than 10% was crucial for preventing the oxidation process.[Citation45] SFC values between 35–37°C indicate fat’s thickness and flavor release properties in the mouth. Butter without a waxy mouthfeel should have an SFC value below 3.5% at 33.3°C and thoroughly melt at body temperature.[Citation46]
The SFC value of tengkawang butter at a temperature of 20–25°C was above 10%, so tengkawang butter has good product stability based on the SFC value. In addition, the SFC value of tengkawang butter at a temperature of 35°C was between 0.03 to 3.11% to minimize a waxy mouthfeel. In addition, tengkawang melts at a body temperature of 36–37°C. The SFC value of tengkawang butter at a temperature of 35°C was close to the content of cocoa butter and CBE. At 20°C, cocoa butter has n SFC value of 75–78%, 72–74% at 25 degrees oC, 55–57% at 30°C, and 0% at 35°C.[Citation44] While at 20°C, the CBE has an SFC value of 74–76%, 62–64% at 25°C, 45–46% at 30°C, and 2.66% at 35°C. Therefore, tengkawang butter has great potential to become CBE, both pure and mixed.
Oxidation stability of tengkawang butter
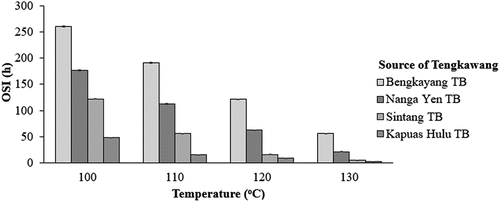
The OSI of TB was analyzed using the Rancimat method. presents the OSI value (h) of Bengkayang TB, Nanga Yen TB, Sintang TB, and Kapuas Hulu TB, which were 260.41, 176.54, 122.26, and 48.135, respectively, at a temperature of 100°C; 191.38, 112. 59, 55.83, and 15.67, respectively, at a temperature of 110°C; 121.21, 62.36, 16.24, and 8.77, respectively, at 120°C; and 55.85, 21.37, 5.62, and 2.62, respectively, at a temperature of 130°C.
DMRT analysis indicates that the OSI values of the four TB samples were significantly different. The OSI value of Bengkayang TB was greater than that of the other three samples, indicating that Bengkayang TB was more resistant to the oxidation process. This resistance can be seen from the acidity and peroxide values of Bengkayang TB, which are lower than those of the other tengkawang samples. The OSI value decreases with increasing temperature, because with a higher temperature, the fat oxidation process, namely the reaction of hydroperoxide formation (primary oxidation) and ketones/aldehydes (secondary oxidation), is formed more quickly.[Citation47,Citation48] The OSI value has a relationship with the acidity and peroxide number of tengkawang butter. These results are consistent with various types of oils and fats such as peanut oil,[Citation49] palm stearin,[Citation50] olive oil,[Citation51] hazelnut oil[Citation52] and peanut butter.[Citation53] The higher the acidity and peroxide number, the smaller of the OSI value, indicating a faster oxidation process.
presents calculations between the OSI logarithm and temperature variations. The regression between the logarithm of OSI and temperature estimates the value of OSI at temperatures of 22°C and Q10; OSI22 was the oxidative stability of the sample at temperatures of 22°C. This was the estimated time required for the sample to reach oxidation stability given in-store storage conditions.[Citation54] The oxidative stability index (OSI) values at a temperature of 22°C from Bengkayang TB, Nanga Yen TB, Sintang TB and Kapuas Hulu TB were 15,208; 44,926; 473,696; and 66,420, respectively. DMRT analysis indicates that the OSI22 values of the four TB samples were significantly different. Based on the OSI22 value, the four tengkawang samples have large OSI values, of above 10,000 h. This indicates that tengkawang butter can last up to 416 days if stored at a temperature of 22°C, the temperature in a food store. When the temperature is raised by ten degrees, the oxidation rate rises by the factor Q10.[Citation54] The Q10 value of Bengkayang TB, Nanga Yen TB, Sintang TB, and Kapuas Hulu TB were 1.702, 2.097, 2.836, and 2.735, respectively. DMRT analysis indicates that the Q10 values of the four TB samples were significantly different. The Q10 value of almost all food was approximately 1.5–3.0.[Citation55,Citation56] According to these results, the Q10 value of tengkawang butter matches the Q10 value of most foods’s Q10 value. When the analysis temperature was raised by 10°C, accelerated oxidative reactions and OSI were reduced by half.
Table 2. The results were calculated using a linear connection between the OSI logarithm and temperature
Tengkawang butter has a large OSI value because it has a large SFA content (65–67%), causing it to be more resistant to the oxidation process. In addition, the low peroxide value of tengkawang butter (PV < 10%) increases the oxidation resistance. Tengkawang butter has a better OSI value than other types of fats and oils, including cocoa butter. Three tengkawang butters, namely Sintang TB, Bengkayang TB, and Nanga Yen TB, had an OSI (h) value larger than dark chocolate, which had only a value of 5.35 at 130°C.[Citation57] Furthermore, at a temperature of 120°C, tengkawang butter has a higher OSI (h) value than mango butter as CBE, which only has a value of 14.32.[Citation58] Therefore, tengkawang butter has potential as CBE in terms of oxidation resistance.
Conclusion
Because it has a fatty acid composition similar to cocoa butter, tengkawang butter (TB) has promise as a cocoa butter equivalent (CBE). The fatty acid profile of the four tengkawang butter samples was dominated by palmitic acid (16–24%), stearic acid (40–47%), and oleic acid (31–33%). The fat content in TB was above 99%, with energy from the fat of 3,755–3,763 J/100 g. The acidity (%), peroxide number (meq O2/kg), and iodine number (I2/100 g) values were 6.88, 0.41, 21.72 for Bengkayang TB, 9.68, 0.56, 29.33 for Nanga Yen TB, 10.71, 4.33, 32.46 for Sintang TB, and 15.94, 8.27, 31.38 for Kapuas Hulu TB. Melting points (oC) and SFC at 35°C (%) of Bengkayang, Nanga Yen, Sintang and Kapuas Hulu TB were 36.8 and 0.03, 36.7 and 0.97, 36.6 and 0.03, and 36.7 and 3.11, respectively. The oxidative stability index (OSI) values at a temperature of 22°C from Bengkayang, Nanga Yen, Sintang and Kapuas Hulu TB were 15,208; 44,926; 473,696; and 66,420, respectively. The data obtained by this study can be used as a reference regarding the quality of traditional TB from several regions in Kalimantan. Knowing the quality and oxidation stability data enables operating conditions of the purification process and the potential of TB as a CBE to be determined.
Acknowledgments
The Ministry of Research and Higher Education of Indonesia provided financial assistance for this study through the research grant “Hibah Program Menuju Doktor Mahasiswa Unggul” (PMDSU) 2021 (NKB-354/UN2.RST/HKP.05.00/2021). The authors also gratefully acknowledged the financial support for publication by Universitas Indonesia through the scheme of Hibah Riset Kolaborasi Internasional WCU 2021 (NKB-643/UN2.RST/HKP.05.00/2021).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- BPS-Statistics Indonesia. (2021). FOREIGN TRADE STATISTICAL BULLETIN December 2020. [Online]: https://www.bps.go.id/publication/2021/03/02/2414ea722c9406ee86506b88/buletin-statistik-perdagangan-luar-negeri-impor-desember-2020.html. 20 September 2021
- Gusti, R. E. P.; Zulnely, Z.; Kusmiyati, E. Sifat Fisika-kimia Lemak Tengkawang Dari Empat Jenis Pohon Induk. Jurnal Penelitian Hasil Hutan. 2012, 30(4), 254–260. DOI: 10.20886/jphh.2012.30.4.254-260.
- Darmawan, M. A.; Muhammad, B. Z.; Harahap, A. F. P.; Ramadhan, M. Y. A.; Sahlan, M.; Haryuni, ; Supriyadi, T., Abd-Aziz, S. Gozan, M., et al Reduction of the Acidity and Peroxide Numbers of Tengkawang Butter (Shorea Stenoptera) Using Thermal and Acid Activated Bentonites. Heliyon. 2020, 6(12), e05742. December/01. DOI: 10.1016/j.heliyon.2020.e05742.
- Maharani, R.; Fernandes, A., and Pujiarti, R. 2016. “Comparison of Tengkawang Fat Processing and Its Effect on Tengkawang Fat Quality from Sahan and Nanga Yen Villages, West Kalimantan, Indonesia.” in AIP Conference Proceedings, 18-19 September 2015, Yogyakarta, Indonesia. vol. 1744, no. 1: AIP Publishing LLC, p. 020051.
- Hidayat, N.; Darmawan, M.; Intan, N., and Gozan, M. 2019. “Refining and Physicochemical Test of Tengkawang Oil Shorea Stenoptera Origin Sintang District West Kalimantan.” in IOP Conference Series: Materials Science and Engineering, 4 - 6 October 2018, West Sumatra, Indonesia, vol. 543, no. 1: IOP Publishing, p. 012011.
- Muhammad, B. Z.; Darmawan, M. A., and Gozan, M. 2019. “Reduction of Beta-carotene with Thermal Activated Bentonite in Illipe Butter from Nanga Yen, Kalimantan Barat.” in AIP Conference Proceedings, 23 - 24 October 2019, Tangerang, Indonesia, vol. 2175, no. 1: AIP Publishing LLC, p. 020046.
- Bahari, A.; Akoh, C. C. Texture, Rheology and Fat Bloom Study of ‘Chocolates’ Made from Cocoa Butter Equivalent Synthesized from Illipe Butter and Palm Mid-fraction. LWT. 2018, November/01/, 97, 349–354. DOI: 10.1016/j.lwt.2018.07.013.
- Wang, H.-X.; Wu, H.; Ho, C.-T.; Weng, X.-C. Cocoa Butter Equivalent from Enzymatic Interesterification of Tea Seed Oil and Fatty Acid Methyl Esters. Food Chem. 2006, 974, 661–665. DOI: 10.1016/j.foodchem.2005.04.029 August/01/
- Bootello, M. A.; Hartel, R. W.; Garcés, R.; Martínez-Force, E.; Salas, J. J. Evaluation of High Oleic-high Stearic Sunflower Hard Stearins for Cocoa Butter Equivalent Formulation. Food Chem. 2012, 1343, 1409–1417. DOI: 10.1016/j.foodchem.2012.03.040 October/01/
- Simoneau, C.; Hannaert, P.; Anklam, E. Detection and Quantification of Cocoa Butter Equivalents in Chocolate Model Systems: Analysis of Triglyceride Profiles by High Resolution GC. Food Chem. 1999, 651, 111–116. April/01/. DOI: 10.1016/S0308-8146(98)00106-X
- Ghosh, M.; Upadhyay, R.; Mahato, D. K.; Mishra, H. N. Kinetics of Lipid Oxidation in Omega Fatty Acids Rich Blends of Sunflower and Sesame Oils Using Rancimat. Food Chem. 2019, January/30/, 272, 471–477. DOI: 10.1016/j.foodchem.2018.08.072.
- N. Velasco, J.; Andersen, M. L.; Skibsted, L. H. Evaluation of Oxidative Stability of Vegetable Oils by Monitoring the Tendency to Radical Formation. A Comparison of Electron Spin Resonance Spectroscopy with the Rancimat Method and Differential Scanning Calorimetry. Food Chem. 2004, 854, 623–632. DOI: 10.1016/j.foodchem.2003.07.020 May/01/
- AOCS, 1993. “Preparation of Methyl Esters of Long-Chain Fatty Acid.” AOCS Ce 2-66.
- BSN. (2016). SNI 2903:2016. Lemak Tengkawang Sebagai Bahan Baku.
- BSN. “SNI 01-3555-1998: Cara Uji Minyak Dan Lemak.” BSN, Jakarta 01 1–31 . 1998.
- ASTM. “Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration.” ASTM D6304 – 07, p. 6, 2007.
- ISO. (1997). ISO 6886:1997. Animal and Vegetable Fats and Oils—determination of Oxidation Stability (Accelerated Oxidation Test).
- Gomez, K. A., and Gomez, A. A. Statistical Procedures for Agricultural Research; New Jersey, U.S: John Wiley & Sons, 1984.
- Basconcillo, L. S.; Zaheer, R.; Finan, T. M.; McCarry, B. E. A Shotgun Lipidomics Approach in Sinorhizobium Meliloti as A Tool in Functional Genomics. J. Lipid Res. 2009, 50(6), 1120–1132. DOI: 10.1194/jlr.M800443-JLR200.
- Christie, W.; Han, X. Oily Press Bridgewater; UK: ed, 2010.
- Ketaren. Pengantar Teknologi Minyak Dan Lemak Pangan; UI Press: Jakarta, 2008.
- Wang, H.; Maleky, F. Effects of Cocoa Butter Triacylglycerides and Minor Compounds on Oil Migration. Food Res. Int. 2018, 106, 213–224. DOI: 10.1016/j.foodres.2017.12.057.
- Jia, C.-H.; Shin, J.-A.; Lee, K.-T. Evaluation Model for Cocoa Butter Equivalents Based on Fatty Acid Compositions and Triacylglycerol Patterns. Food Sci. Biotechnol. 2019, 28(6), 1649–1658. DOI: 10.1007/s10068-019-00630-8.
- Bresson, S.; Lecuelle, A.; Bougrioua, F.; El Hadri, M.; Baeten, V.; Courty, M.; Pilard, S.; Rigaud, S., Faivre, V., et al. Comparative Structural and Vibrational Investigations between Cocoa Butter (CB) and Cocoa Butter Equivalent (CBE) by ESI/MALDI-HRMS, XRD, DSC, MIR and Raman Spectroscopy. Food Chem. 2021, 363, November/30/ 130319. DOI: 10.1016/j.foodchem.2021.130319.
- Kadivar, S.; De Clercq, N.; Mokbul, M.; Dewettinck, K. Influence of Enzymatically Produced Sunflower Oil Based Cocoa Butter Equivalents on the Phase Behavior of Cocoa Butter and Quality of Dark Chocolate. LWT - Food Sci. Technol. 2016, March/01/, 66, 48–55. DOI: 10.1016/j.lwt.2015.10.006.
- FAO. “Codex Standard for Cocoa Butters,” 2016. [Online]. Available: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B86-1981%252FCXS_086e.pdf 5 September 2021.
- Galindo‐Cuspinera, V.; de Sousa, J. V.; Knoop, M. Sensory and Analytical Characterization of the “Cool‐melting” Perception of Commercial Spreads. J. Texture Stud. 2017, 48(4), 302–312. DOI: 10.1111/jtxs.12256.
- Kodali, D. R. Trans Fats—chemistry, Occurrence, Functional Need in Foods and Potential Solutions. Trans Fats Alternatives 01. 2005, 1–25.
- Zhang, Z., Jin, H., Suo, J., Yu, W., Zhou, M., Dai, W., Song, L., Hu, Y., Wu, J., et al. Effect of Temperature and Humidity on Oil Quality of Harvested Torreya Grandis Cv. Merrillii Nuts during the After-Ripening Stage,” (In English. Frontiers in Plant Science, Original Research.2020, 11(1646), 2020-October-23. DOI: 10.3389/fpls.2020.573681.
- Bai, X.; Zhang, M.-L.; Zhang, Y.; Zhang, J.; Zhang, Y.; Wang, C.; Liu, R., et al. Effects of Steaming, Microwaving, and Hot-Air Drying on the Physicochemical Properties and Storage Stability of Oat Bran. J. Food Qual. 2021, 2021, July/01 4058645. DOI: 10.1155/2021/4058645.
- Steele, R. Understanding and Measuring the Shelf-life of Food; Sawston, Cambridge: Woodhead Publishing, 2004.
- Irwin, J. W., and Hedges, N. 13 - Measuring Lipid Oxidation. In Understanding and Measuring the Shelf-Life of Food; Steele, R., Ed.; Sawston, Cambridge: Woodhead Publishing, 2004; pp 289–316.
- Kong, F., and Singh, R. P. 12 - Advances in Instrumental Methods to Determine Food Quality Deterioration. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Sawston, Cambridge: Woodhead Publishing, 2011; pp 381–404.
- Sanders, T. H. Ground Nut Oil. In Encyclopedia of Food Sciences and Nutrition (Second Edition); Caballero, B., Ed. Academic Press: Oxford, 2003; pp 2967–2974.
- Patterson, H. B. W. Chapter 12 - Quality and Control. In Hydrogenation of Fats and Oils, Second Edition) ed.; List, G. R., King, J. W., Eds. Urbana, USA: AOCS Press, 2011; pp 329–350.
- Bart, J. C. J.; Palmeri, N., and Cavallaro, S. 6 - Emerging New Energy Crops for Biodiesel Production. In Biodiesel Science and Technology; Bart, J. C. J., Palmeri, N., Cavallaro, S., Eds.; Sawston, Cambridge: Woodhead Publishing, 2010; pp 226–284.
- Afoakwa, E. O.; Paterson, A.; Fowler, M.; Vieira, J. Characterization of Melting Properties in Dark Chocolates from Varying Particle Size Distribution and Composition Using Differential Scanning Calorimetry. Food Res. Int. 2008, 41(7), 751–757. DOI: 10.1016/j.foodres.2008.05.009.
- Fiebig, H.-J.; Lüttke, J. Solid Fat Content in Fats and Oils - Determination by Pulsed Nuclear Magnetic Resonance Spectroscopy [C-IV 3g (2003)]. Eur. J. Lipid Sci. Technol. 2003, 105(7), 377–380. DOI: 10.1002/ejlt.200390076.
- Dos Santos, M. T.; Gerbaud, V.; Le Roux, G. A. C. Solid Fat Content of Vegetable Oils and Simulation of Interesterification Reaction: Predictions from Thermodynamic Approach. J. Food Eng. 2014, 126, 198–205. DOI: 10.1016/j.jfoodeng.2013.11.012.
- Jahurul, M.; Zaidul, I. S. M.; Nik Norulaini, N. A.; Sahena, F.; Abedin, M. Z.; Mohamed, A.; Mohd Omar, A. K., et al. Hard Cocoa Butter Replacers from Mango Seed Fat and Palm Stearin. Food Chem. 2014, 154, 323–329. DOI: 10.1016/j.foodchem.2013.11.098.
- Ribeiro, A. P. B.; Basso, R. C.; Grimaldi, R.; Gioielli, L. A.; Goncalves, L. A. G. Effect of Chemical Interesterification on Physicochemical Properties and Industrial Applications of Canola Oil and Fully Hydrogenated Cottonseed Oil Blends. J. Food Lipids. 2009, 16(3), 362–381. DOI: 10.1111/j.1745-4522.2009.01152.x.
- Norazlina, M. R.; Jahurul, M. H. A.; Hasmadi, M.; Mansoor, A. H.; Patricia, M.; Ramlah, M. R. G. Physicochemical Properties of Bambangan Kernel Fat and Its Stearin Mixtures with Cocoa Butter. LWT. 2022, January/01/, 153, 112556. DOI: 10.1016/j.lwt.2021.112556.
- Ribeiro, A. P. B.; Masuchi, M. H.; Miyasaki, E. K.; Domingues, M. A. F.; Stroppa, V. L. Z.; de Oliveira, G. M.; Kieckbusch, T. G., et al. Crystallization Modifiers in Lipid Systems. J. Food Sci. Technol. 2015, 52(7), 3925–3946.
- Torbica, A.; Jambrec, D.; Tomić, J.; Pajin, B.; Petrović, J.; Kravić, S.; Lončarević, I., et al. Solid Fat Content, Pre-crystallization Conditions, and Sensory Quality of Chocolate with Addition of Cocoa Butter Analogues. Int. J. Food Prop. 2016, 19(5), 1029–1043.
- Karabulut, I.; Turan, S.; Ergin, G. Effects of Chemical Interesterification on Solid Fat Content and Slip Melting Point of Fat/oil Blends. Eur. Food Res. Technol. 2004, 218(3), 224–229. DOI: 10.1007/s00217-003-0847-4.
- Lida, H. M. D. N.; Ali, A. R. M. Physico-chemical Characteristics of Palm-based Oil Blends for the Production of Reduced Fat Spreads. J. Am. Oil Chem. Soc. 1998, 75(11), 1625–1631. DOI: 10.1007/s11746-998-0103-y.
- Liu, K.; Liu, Y.; Chen, F. Effect of Storage Temperature on Lipid Oxidation and Changes in Nutrient Contents in Peanuts. Food Sci. Nutr. 2019, 7(7), 2280–2290. DOI: 10.1002/fsn3.1069.
- Gertz, C.; Aladedunye, F.; Matthäus, B. Oxidation and Structural Decomposition of Fats and Oils at Elevated Temperatures. Eur. J. Lipid Sci. Technol. 2014, 11611, 1457–1466. DOI: 10.1002/ejlt.201400099 November/01
- Pattnaik, M.; Mishra, H. N. Oxidative Stability of Ternary Blends of Vegetable Oils: A Chemometric Approach. LWT. 2021, May/01/, 142, 111018. DOI: 10.1016/j.lwt.2021.111018.
- Zhu, T.-W.; Zhang, X.; Zong, M.-H.; Linhardt, R. J.; Wu, H.; Li, B. Storage Stability Studies on Interesterified Blend-based Fast-frozen Special Fats for Oxidative Stability, Crystallization Characteristics and Physical Properties. Food Chem. 2020, February/15/, 306, 125563. DOI: 10.1016/j.foodchem.2019.125563.
- Cherif, M.; Rodrigues, N.; Veloso, A. C. A.; Zaghdoudi, K.; Pereira, J. A.; Peres, A. M. Kinetic-thermodynamic Study of the Oxidative Stability of Arbequina Olive Oils Flavored with Lemon Verbena Essential Oil. LWT. 2021, April/01, 140, 110711. DOI: 10.1016/j.lwt.2020.110711.
- Gülmez, Ö.; Şahin, S. Evaluation of Oxidative Stability in Hazelnut Oil Treated with Several Antioxidants: Kinetics and Thermodynamics Studies. LWT. 2019, August/01/, 111, 478–483. DOI: 10.1016/j.lwt.2019.05.077.
- Degon, J. G.; Zheng, C.; Elkhedir, A.; Yang, B.; Zhou, Q.; Li, W. Effect of Microwave Pre-treatment on Physical Quality, Bioactive Compounds, Safety Risk Factor, and Storage Stability of Peanut Butter. Oil Crop Sci. 2021, 63, 137–144. DOI: 10.1016/j.ocsci.2021.07.006 July/01/
- Simoes Grilo, F.; Srisaard, Y.; Wang, S. C. Prediction of Walnut Deterioration Using Kernel Oxidative Stability. Foods. 2020, 9(9), 1207. DOI: 10.3390/foods9091207.
- Bravi, E.; Sileoni, V.; Perretti, G.; Marconi, O. Accelerated Shelf-life Model of Gluten-free Rusks by Using Oxidation Indices. Food Chem. 2020, October/01/, 326, 126971. DOI: 10.1016/j.foodchem.2020.126971.
- Choi, J.-Y.; Lee, H.-J.; Cho, J.-S.; Lee, Y.-M.; Woo, J.-H.; Moon, K.-D. Prediction of Shelf-life and Changes in the Quality Characteristics of Semidried Persimmons Stored at Different Temperatures. Food Sci. Biotechnol. 2017, 265, 1255–1262. DOI: 10.1007/s10068-017-0173-4 October/01
- Deus, V. L.; Resende, L. M.; Bispo, E. S.; Franca, A. S.; Gloria, M. B. A. FTIR and PLS-regression in the Evaluation of Bioactive Amines, Total Phenolic Compounds and Antioxidant Potential of Dark Chocolates. Food Chem. 2021, September/30/, 357, 129754. DOI: 10.1016/j.foodchem.2021.129754.
- Kaur, G.; Kaur, D.; Kansal, S. K.; Garg, M.; Krishania, M. Potential Cocoa Butter Substitute Derived from Mango Seed Kernel. Food Chem. 2022, March/15/, 372, 131244. DOI: 10.1016/j.foodchem.2021.131244.