ABSTRACT
The possible use of dietary components as therapeutic agents is well known and could be a tool against Coronavirus Disease 2019 (COVID-19). This review summarizes the evidence-based literature for immuno-modulation and antiviral activity of different vitamins (A, B, C, D, E), omega-3 fatty acids, selenium, zinc and flavonoids. These substances lessen the vulnerability of risk groups and retard intricate chain of events related to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) invasion. The potential roles of these substances include inhibition of SARS-CoV-2 pathogenesis, inactivation of ACE2 receptors, regulation of innate and adaptive immunity, stimulation of anti-inflammatory responses, regulation of cytotoxic cells activity, antiviral immune induction, retardation of incessant viral replications, suppression of cell signaling pathways and putative inhibition of SARS-CoV-2 major proteases. Moreover, after recovery from COVID-19, nourishing diet is needed to speed up the long-haul symptoms and lingering health issues.
Introduction
Coronavirus Disease 2019 (COVID-19) outbreak due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), [Citation1,Citation2] cataclysmically palpitate populations around the globe with continuous rise in number of infected individuals and deaths. COVID-19 can easily spread and transmit from infected individual to healthy individual during close contact situations. [Citation3,Citation4] Global uncertainty prevails around all potential future demeanors in unprecedented ways due to COVID-19 pandemic. Some countries have observed high peaks but still have a fear of reemergence of the infection [Citation5]; whereas many others are still badly hit with massive increase in number of COVID-19 patients.[Citation6]
Despite success and approval of certain vaccines, the complete eradication of COVID-19 from the whole world seems to be a long and winding road due to multiple COVID-19 variants circulating globally causing more lethal second or third wave.[Citation7] Some of these variants exhibited large scale mutations and these mutated forms have resulted in alteration in the structure of viral spike proteins that help the virus to more easily evade components of immune system and resultantly virus act more virulently at infected cells, increase the chances of viral spread and more severe outcomes. Some variants of corona virus designated as variants of concern including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617) (Organization 2021) are spreading rapidly across all geographical regions of the world and increase the number of infected individuals and deaths; while others are designated as variants of interest including Epsilon (B.1.427/B.1.429), Zeta (P.2), Eta (B.1.525), Theta (P.3), Iota (B.1.526) and Kappa (B.1.617.1).[Citation8]
These variants have high infection rate and spread more rapidly than other variants. Scientists are striving to know more about such variants and their implications. Due to emergence of different variants, and further chances of appearance of more infectious, transmissible and virulent forms, certain vaccines may show less effectiveness to produce proper immune response and may not be long-lasting. [Citation9,Citation10] These variants already enhance the risk of hospital admission and mortality rates.[Citation11] These virulent mutated forms are actually generated due to viral evolution inside an immunosuppressed and chronically infected patient.[Citation12]
The estimation of protective immunity after vaccination is uncertain.[Citation13] In such situations, no protective mechanism exists in individuals and there is possibility of reinfection and high risk of developing more mutations. The more the virus willreplicate,spread and transfer, the more mutant forms will evolve and unusual mutations adapt by SARS-CoV-2.[Citation14] Moreover, after accumulation of lot of mutations, certain variants may ultimately become more virulent and continue to threat life on earth. Some of these emerging SARS-CoV-2 strains are not detected by current diagnostic tests and may also cause reinfection or infect even fully vaccinated individuals.
At present, no registered drug treatment is available to reduce the complex symptoms incurred during COVID-19. In such pandemic circumstances, it is vital to find an alternative therapeutic way to save humanity from such lethal variants of SARS-CoV-2. The most reliable option that lowers the chances of infection and gives robust immunity in immunocompromised individuals is to choose nutrient-rich foods that actively prepare the body to fight off viral infections.[Citation15] Hence, the present review comprehensively highlights the importance of dietary measures, nutrients and bioactive moieties present in food stuffs in reducing the infection, transmissibility, severe outcomes during COVID-19 progression and their necessity after recovery from infection.
Symptoms and clinical manifestation
Coronaviruses vary significantly in risk factors. The individuals infected by SARS-CoV-2 usually show symptoms after completion of viral incubation period that takes a median of 5.2 days.[Citation16] After that period, certain individuals still remain asymptomatic while others show moderate to severe symptoms of infection. A time period of 6 to 41 days generally observed after initiation of COVID-19 symptoms to fully recover with a median time of approximately 14 days.[Citation17] A large number of factors influence the duration of infection. The age of the patient and the immune system predominantly affect on this period and severity of symptoms. The infection period is of short duration in case of individuals with age less than 70.[Citation18] The well-known symptoms after illness due to COVID-19 are fever, dry cough, difficulty in breathing and fatigue. People may also experience other symptoms including headache, diarrhea, lymphopenia, hemoptysis, dyspnea and sputum production. [Citation17,Citation19,Citation20]
Many clinical studies revealed by CT scan of chest show pneumonia with many other anomalous descriptions like acute cardiac injury, RNAaemia, incidence of grand-glass opacities and acute respiratory distress syndrome that may become lethal.[Citation19] Clinical manifestations of COVID-19 have striking similarities with past viral infections like SARS and MERS. About 23–32% COVID-19 infected individuals admitted to the intensive care unit commonly develop severe pneumonia and 17–29% acquire acute respiratory distress syndrome during disease progression. [Citation21,Citation22] The death ratio due to SARS-CoV-2 infection in symptomatic and asymptomatic individuals is surprisingly low and, in many countries, it is about 0.5–4% (95% confidence interval).[Citation23] The patients admitted to hospitals are mostly in the average age group of 49–56 years and most of these patients (32–51%) have already possessed underlying health status due to chronic maladies. Men were infected more (54–73%) than women. The infectivity numbers and severity of COVID-19 symptoms is remarkably low in children. In many cases, children remain asymptomatic or acquired only mild symptoms as is the case in previous corona viral infections.[Citation24] Among hospitalized patients, common manifestation includes fever (83–100%), dry cough (59–82%), myalgia (11–35%), headache (7–8%) and diarrhea (2–10%) respectively.
In most severe cases, the infected individuals may lead to heart failure and septic shock.[Citation25] Moreover, multi-organ failure was observed in critically ill patients during disease advances due to uncontrolled inflammatory responses that cause pulmonary tissue damages and reduction in lung capacity and functional efficacy. The tissue damage at alveolar level by SARS-CoV-2 may cause pathological changes in lung tissues which may result in reduced infiltration and hyperplasia. Besides damages to lung tissues and respiratory disorders, critically ill patients may also experience infiltration of immune cells to the site of lung injuries, thrombosis, abnormal increase in inflammatory responses and multi-organ failure.[Citation26] Furthermore, it is evident that different variants of SARS-CoV-2 produce different symptoms to the initial strain. Some hospitalized patients affected with the more virulent form Delta variant (B.1.617) showed unusual symptoms including joint pain, abdominal pain, loss of appetite, nausea, vomiting and hearing impairments . Some COVID-19 patients experienced no fever or late inception of fever that initially spotted. Due to wide variations in the symptoms among infected individuals due to different SARS-CoV-2 variants, scientists and health professionals encouraged to get tested for COVID-19 who feels unwell.[Citation27]
Dietary measures for covid-19 prevention and treatment
In the history, man often finds it difficult to cure certain maladies. In these circumstances, the most reliable option is to choose nutrient-rich foods that actively prepare the body to relegate the chances of infection, minimize severe outcomes during disease progression by enhancing functional aspects of immune cells. Healthy dietary approaches and selection of right food choices maintain proper physiological functions in the body needed against noxious agents like SARS-CoV-2.
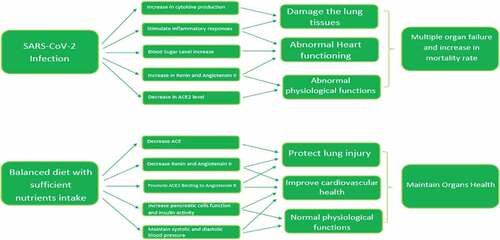
All the basic building blocks of human organization arise from the food substances that are eaten. The healthy dietary choices and proper food intake give all the essential substances that are required for normal functions of the body. Poor dietary selections result in malfunctioning of vital biochemical pathways necessary to combat diseases and ultimately individuals become more prone to infectious agents. The dietary intake of diversified foods with abundant quantity of vitamins, minerals and bioactive molecules maintains homeostasis in the body that is essentially needed for proper health maintenance. The nutrient homeostasis within the body can only be achieved by selection of foods related to all the major food groups in a right and balanced combination.[Citation28] The lack of certain nutrients in diet for prolonged period has resulted in immunocompromised health status. Such individuals have more chances of acquiring non-communicable diseases and vulnerability for COVID-19 with severe outcomes as well (). So, selection of well-balanced diet and identification of those food groups that critically play a vital role in reducing severity of respiratory illnesses, increase functional stability in immune system and health of different organs is vital during COVID-19 crises.
Prevention from diseases, avoidance from complications during disease progression and quick recovery is directly related with food components we intake. Proper nourishment is the only long-term solution to improve health necessarily in this massive upheaval time. Food choices that maintain nutrient homeostasis is such an approach that is crucial to enhance immunity against infectious agents and critically important in reducing multiple complications related to COVID-19. Foods in different regions of the world are adapted according to culture and eating behavior of that particular region. Despite of these differences, dietary selections must provide enough minerals, vitamins, antioxidants and phytochemicals needed to boost immunity against pathogens.
Dietary components are vital in reducing susceptibility to an infection, minimize complications during infection and are constantly required after recovery from diseases like COVID-19. COVID-19 badly impacts lives of people in many countries.[Citation29] These communities now try to reconsider their nutritional status and dietary patterns. It is blatant fact that healthy nutritional status plays a profound role in reducing the symptoms associated with the diseases and severity of infection urging to implement this approach during COVID-19 crises.[Citation30] Good nutritional status of populations all over the world is crucial to reduce the exponential rise in COVID-19 cases and to avoid severity of COVID-19 infection and deaths. [Citation31,Citation32]
Inhibitory role of diet for sars-cov-2 ingress
Different civilizations and cultures have adapted different food choices and dietary selections which can be considered as a major aspect in the difference of number of COVID-19 cases around different topographical regions of the world. In fact, consuming certain type of foods might have a direct relation in dropping SARS-CoV-2 infection rate and mortality.[Citation29]
The angiotensin converting enzyme 2 (ACE2) acts as input receptor and facilitates the SARS-CoV-2 pathogenesis and its ingress into the host cell. To enter the host cell, the virus needs glycoprotein S to be bound with ACE2 receptors.[Citation33] After binding, viral membrane and host cell membrane fuses to assist tranquil viral entry into host cell. On the host cell surface, Type II transmembrane serine protease enzymes are present that remove ACE2 after recognizing fusion of membranes and play important role in activation of receptor-like spike S proteins. [Citation34,Citation35] These receptor-like spike S proteins induce alterations after activation of S proteins and allow the virus to enter into the host cell.[Citation36] The peak level of ACE2 expression was mostly observed at the nasal epithelial cells and upper and lower respiratory tract.[Citation37]
After entering into the host cell, the genetic material of virus is translated inside the nucleus of the host cell. Hence, ACE2 plays a crucial role in SARS-CoV-2 entry into the host cell. This viral entry into host cell can be controlled by inactivation of ACE2 receptors on the host cell surfaces. Interestingly, the ACE2 level has direct relation and high sensitivity with the intake of food substances and dietary choices.[Citation38] As for example, prominent ACE inhibitory activity and subsequent hypotensive effects were observed after intake of broccoli protein hydrolyzate.[Citation39] Contrary to this, an increase in the level of ACE was noticed with intake of diet consisting of high saturated fats.[Citation40] A large number of compounds present in food and dietary approaches influence the ACE levels and ultimately direct enhancing and inhibitory activities.[Citation41] Hence, retaining healthy nutritional status is crucial to inhibit viral entry and COVID-19 spread. Emerging research has further revealed that the expression of genes related to ACE2 markedly influenced by intake of certain nutrients in diet. [Citation42,Citation43] An important dietary component isotretinoin (derivative of vitamin A) actively involved in downregulation of ACE2 receptors. As SARS-CoV-2 attaches to ACE2 protein receptors to facilitate viral invasion into host cells, the isotretinoin as dietary component lowers the chances of viral attachment to the receptors of host cells and ultimately minimize the chances of acquiring COVID-19.[Citation44]
Likewise, vitamin D blocks the Ang-2/Tie-2 and renin-angiotensin pathways and helps in reduction of lipopolysaccharide-induced lung injury, that undoubtedly has a relevancy to pathogenesis SARS-CoV-2.[Citation45] Moreover, vitamin D declines the chances of binding of viral particles to ACE2 by supporting the attachment of ACE2 receptors to angiotensin II type 1 receptor, hence, completely inhibits virus-host cell interactions.[Citation46] Hence, number of dietary substances have potential role to hamper the virus entry into host cell and impede dissemination of SARS-CoV-2 ().
Significance of dietary components during covid-19
Little information is available about reliable COVID diet that provides critical role in lowering severe outcomes associated with COVID-19 progression and fatality rates. A well-balanced diet and healthy nutritional status must be under consideration to maintain robust immune system at this pandemic situation () because the SARS-CoV-2 is spreading continuously around the globe and the battle of compete eradication of SARS-CoV-2 appears to remain for prolonged time that was initially prophesied. To minimize the current pandemic crises, proper diet and healthy nutritional aspects are the exclusive ways to develop prolong insusceptibility against SARS-CoV-2,[Citation47] and ultimately retards incessant viral replications and subsiding associated mutations. Following are the dietary components that are vital to minimize the COVID-19 severity and associated complications.
Vitamin A
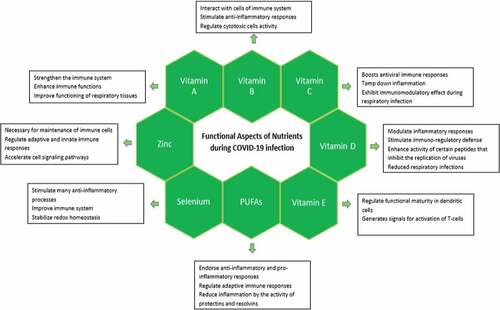
Vitamin A is obtained from its plants derived precursor β-carotene. The three active forms of this fat-soluble vitamin are retinol (alcoholic form), retinal (aldehyde form) and retinoic acid (acidic form). The retinol is present as retinyl ester with long chain fatty acid in animal tissues, retinal is obtained by oxidation of retinol and retinoic acid is produced by oxidation of retinal. The animal sources of vitamin A include meat, milk, cheese, kidney, liver, eggs and fish liver oils and plant sources include carrots, pumpkin, papaya, broccoli, spinach, apricots, mango and sweet potatoes. Vitamin A strengthens the immune system and enhances many immune functions such as increase in antibodies production, cytokine expression, lymphopoiesis, apoptosis, mucins and keratins and enhance the functional stability of B lymphocytes, T lymphocytes, neutrophils, monocytes and natural killer cells. [Citation48,Citation49] Vitamin A is essentially important in reduction of severe complexities during progression of respiratory diseases and helpful in minimizing the death rate.[Citation50] Therefore, regular intake of vitamin A by COVID-19 patients is a pragmatic approach to improve functioning of respiratory tissues.
In past research, it was observed that deficient intake of vitamin A in virally infected individuals may lead to severe respiratory complications and may result in rapid increase in inflammations in lung tissues due to poor functioning of immune responses as was reported in patients infected with respiratory syncytial virus, measles virus and influenza virus. [Citation51,Citation52] Conclusively, we can say that vitamin A is the best option for lung infection prevention and treatment during COVID-19.
B vitamins
B vitamins are water soluble vitamins that contribute as coenzymes in many biologically active pathways. The B vitamins are considered vital as they support and enhance metabolic rate essential to maintain controlled division and growth of cells. Their consumption is helpful in increasing nervous and immune system functions and reducing inflammatory responses when taken as food but not in vitamin tablet form. B vitamins are present in abundant quantity in whole grains, meat, liver, whole unprocessed food, meat products, potatoes, bananas, chili, peppers, beans, yeast and molasses.
The B vitamins dynamically interact with cells of immune system to block many pathophysiological pathways and stimulate anti-inflammatory responses necessary to generate during infectious state.[Citation53] The individuals having deficiency of B vitamins or remain deficient in infectious state may exhibit more severe outcomes due to delay in immune responses necessary to compete at this stage.[Citation54]
Each B vitamin has diversified and wide-ranging roles in maintaining various biochemical activities within the body. For instance, the titer of MERS-CoV reduced effectively in plasma products of humans by consumption of vitamin B2 in the presence of UV light and have a potential to be effective against SARS-CoV-2.[Citation55]
Vitamin B6 act as coenzyme in many metabolic pathways related to immune functions as synthesis of cytokines and formation of antibodies from amino acids is due to activity of B6 as coenzyme. The deficiency of vitamin B6 impairs growth, lymphocyte maturation, lymphocyte mitogen response, antibody production and T-cell activity.[Citation56] It is evident from past data that intake of vitamin B6 contrariwise related to individual’s inflammation status.[Citation57] Moreover, vitamin B6 is helpful in COVID-19 critically ill patients as its supplementation increase total lymphocyte cells.[Citation58] Spinach, beans, bananas, pineapple, avocados and potatoes are foods with sufficient amount of vitamin B6.
Vitamin B12 has profound impact on preservation and aggregation of healthy gut microbiome that imparts crucial role for physiological aspects of both adaptive and innate immune system. [Citation59,Citation60] Vitamin B12 is actively involved in immunomodulation particularly in regulation of cytotoxic cells activity including T Lymphocytes, CD8+ and natural killer cells.[Citation61] Therefore, Vitamin B12 plays a pivotal role in inhibition of excessive immuno-responses [Citation62] especially helpful for those COVID-19 patients that suffered from microbiota dysbiosis and enduring worst symptoms of infection.[Citation63]
The COVID-19 patients admit to hospitals have showed better health outcomes after giving a diet containing vitamin B12, magnesium and vitamin D and decrease the risk factors of respiratory failure, lessen the chances to acquire ventilators and intensive care support. Moreover, high vitamin B12 intake in COVID-19 patients substantially reduces inflammation rate.[Citation64] The dietary sources of Vitamin B12 include milk, meat, eggs, fish and shellfish etc. [Citation65] Hence, B vitamins are important in reducing COVID-19 infection rate and associated complexities appeared in patients. [Citation32]
Vitamin C
Vitamin C (ascorbic acid) is water soluble vitamin. The important dietary sources of vitamin C are citrus fruits, kiwifruit, strawberries, Brussels sprouts, broccoli, potatoes, tomatoes, green and red bell peppers and others.
Vitamin C acts as co-factor in many biochemical pathways that mediate essential physiological aspects such as collagen synthesis, iron absorption, hormones production and enhances immunity by regulating immune cells activity.[Citation66] It works as an antioxidant and is important for regulation of immune functions. Vitamin C has a fundamental role in development, growth and regular tissue repairing within the body.[Citation67] Vitamin C intake tamps down inflammation within the body. Vitamin C is remarkably vital as it exhibit immunomodulatory effect during respiratory infections. .[Citation68,Citation69] Its regular consumption reduces the asthmatic attacks and improves lung tissue damages. Vitamin C dietary intake is directly related to provide prevention from lower respiratory tract infections. [Citation67,Citation70] Vitamin C boosts production of antibodies as well as functionality of white blood cells. In diseased and stress conditions, its consumption provides supportive role in maintenance of organs health.
It also provides protection against infection related to coronaviruses.[Citation71] Moreover, in a study, it was reported that vitamin C provide resistance to tracheal organ cultures of chick embryo after infected with avian coronavirus.[Citation72] Furthermore, in another research, Vitamin C was found effective in relieving flu like symptoms (running or congested nose, sneezing and inflamed sinuses) of coronavirus.[Citation73] In a study, Hemila [Citation74] conducted three human controlled trials for probing the effect of vitamin C on pneumonia and it was found that vitamin C was associated with lowering the incidence of respiratory tract infections and pneumonia.
Vitamin C increases synthesis of interferons alpha and beta and boosts antiviral immune responses.[Citation66] Vitamin C dietary consumption in adequate amount is valuable in COVID-19 prevention due to its involvement in recovery of upper and lower respiratory tract infections and modulation of antiviral cellular and humoral responses. In micronutrient-deficient individuals, vitamin C intake is crucial because these individuals have predisposed risk of COVID-19 severe outcomes during the course of disease, although prevention and treatment of acute respiratory diseases by supplementation of vitamin C need further investigations.[Citation70,Citation75] The dietary recommendations can be fulfilled by intake of natural foods like citrus fruits, kiwi, broccoli etc.[Citation76] It is noteworthy that broccoli contains three times higher vitamin C contents as citrus fruits. Likewise, baked potatoes are a huge reservoir of this valuable nutrient. Conclusively, vitamin C has a significant role in decreasing the chances and risk of respiratory tract infections.
Vitamin D
Vitamin D is a group of fat-soluble secosteroids and contains vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). In humans, vitamin D is naturally synthesized from cholesterol by adequate exposure to sun. In pandemic restrictions, this vitamin D level reduced due to lesser outdoor activities and sun exposure. Therefore, it is necessary to increase the dietary consumption of vitamin D. The main sources of vitamin D are meat, tuna, salmon, sardines, cod liver oil, mushrooms and egg yolks .[Citation77] To strengthen the resilience against COVID-19, vitamins D supplementation is also considered valuable.[Citation18] Adequate level of vitamin D intake is essential to control inception of hypertension, cardiovascular diseases, cancer and diabetes that are high-risk groups to face severe form of illnesses and respiratory infections during COVID-19.[Citation78]
Vitamin D as valuable micronutrient plays a vital role in many functions in the body including maintenance of bones and musculoskeletal health. For innate and adaptive immunity, vitamin D acts as modulator by regulating cell signaling pathways and cytokines. [Citation79,Citation80] Vitamin D is critically important in proper functioning of immune cells and plays a crucial contribution in modulating inflammatory responses after viral infections.[Citation81] The T and B immune cells possess vitamin D receptors that executes valuable role in proliferation and differentiation of these cells.[Citation82] In experimentally induced lipopolysaccharide inflammation, direct association was observed between vitamin D and reduction of pro-inflammatory cytokine Interleukin-6 concentrations.[Citation83,Citation84]
The epidemiological data reveals importance of vitamin D to reduce infection rate, severity and complications and may play a potential role during COVID-19 due to anti-inflammatory responses.[Citation85] This impact of retardation of inflammation is particularly important in immuno-compromised individuals as they suffer from hyper-inflammatory responses after COVID-19. In people with deficiency of vitamin D, immune response may not regulate properly and hence produce more cytokines and consequently acute respiratory distress syndrome.[Citation86] The deficiency of vitamin D in blood plasma may result in high rate of respiratory infections predominantly in individuals having underlying lung conditions.[Citation87] Low levels of vitamin D increase the risk of upper respiratory tract viral infections and the severity of infection and vitamin D supplementations speed up the recovery process and minimize unendurable symptoms.[Citation88,Citation89]
Vitamin D Supplementation minimize the chances of developing acute respiratory infections and high level of vitamin D in blood results in low risk of pneumonia.[Citation90,Citation91] Moreover, intake of vitamin D supplements after influenza vaccination in pediatric patients have resulted in an increase in humeral immunity.[Citation52] Preexisting chronic conditions, obesity, older age and males are the high-risk groups to observe vitamin D deficiency and ultimately vulnerability to COVID-19 infection and related complications.[Citation92–94]
To establish a link between increase in risk of viral infections and deficiency of vitamin D, several mechanisms are proposed but presently all of these underlying mechanisms are still inconclusive. The potential mechanisms include the antiviral immune induction, induction of autophagy and apoptosis, modulation of immunoregulatory defense, genetic or epigenetic regulations.[Citation95] In addition, activity of certain peptides like cathelicidins and defensins enhance in the presence of vitamin D.[Citation96] These peptides inhibit the replication of viruses and reduce the level of pro-inflammatory cytokines and enhance the concentration of anti-inflammatory cytokines that provide rapid recovery from inflammation-related pneumonia.[Citation96] The vitamin D effectively reduces COVID-19 symptoms and adverse outcomes. On the other hand, fatality rates were remaining high in individuals with chronic diseases, comorbidities and age; all have deficient Vitamin D status.
Furthermore, it is evident that increased COVID-19 mortality in the world have relation with Northern Latitude.[Citation97] It is also noteworthy that individuals with darker skin pigmentation or those who reside at high latitudes are particularly infected with COVID-19. These populations also have high risk of preexisting chronic disease, obesity and deficiency of vitamin D. [Citation85,Citation98] The severity of disease in these individuals is due to inadequate sun exposure and hence, deficiency of vitamin D.
Vitamin E
Vitamin E is a fat-soluble vitamin and it includes tocopherols and tocotrienols. The vitamin E rich foods include broccoli, spinach, sunflower seeds, peanut butter, walnut, soaked almonds, hazelnuts, wheat germ and edible oils. Vitamin E is easily incorporated into cell membranes as it is fat-soluble and provides protection from oxidative damage.[Citation99] The intake and presence of high amount of vitamin E in body mitigates the stress on immune cells. Vitamin E possesses potential antioxidant activity and protects the host by active modulation of immune cells after viral invasion.[Citation100] The role of vitamin E to preserve immune responses is well recognized. Vitamin E modulates the concentration of nitric oxide by increasing the activity of natural killer cells.[Citation101] Vitamin E administration during infectious state increases antibody activities humoral (B cells) immune responses.[Citation102] Vitamin E regulates functional maturity in dendritic cells and related functions,[Citation103] that are crucial to orchestrate intertwining adaptive and innate immune systems for generation of subtle responses.[Citation104] Vitamin E generates signals for activation of T-cells and helps in formation of synapse in naïve T-cells.[Citation102] The deficiency of vitamin E leads to impaired cell-mediated and humoral immunity.[Citation105] The deficiency of vitamin E is relatively more pronounced in people with old age and their cell-mediated and humoral immunity can easily be improved by vitamin E as supplement. Due to its wide-ranging effects, recommendation of Vitamin E is mandatory during COVID-19 infection to overcome complexities.[Citation32]
Omega‐3 polyunsaturated fatty acids
A type of fat and vital nutrients which are not synthesized by human body is omega-3 polyunsaturated fatty acids (PUFAs) that performs many physiological aspects in human body. Their requirement can be fulfilled by direct intake from dietary sources. The food sources with rich supply of omega-3 PUFAs include seafood (sardines, salmon and tuna), fatty fish, krill, seal oil, plant oils (canola, soybean and others), flaxseeds and nuts.[Citation106] Omega-3 PUFAs predominantly involved in endorsing anti-inflammatory and pro-inflammatory responses.[Citation107]
Long-chain omega-3 PUFAs are essentially important as they regulate adaptive immune responses and resultantly reduce the inflammation. This reduction in inflammation is due to activity of important mediators like protectins and resolvins that are derived from their precursor omega-3 PUFAs.[Citation108] These mediators also activate anti-inflammatory reactions by stimulating oxygenated metabolites such as oxylipins.[Citation109]
Many PUFAs including Omega-3 PUFAs showed to have a potential of reducing viral activities.[Citation110] Another important lipid mediator, Protectin D1, derived from omega-3 PUFA could substantially attenuate influenza virus replication by RNA export machinery.[Citation111] Therefore, Omega-3 PUFAs and their derivative may have a potential to use as novel anti-viral drug and to gain prospective advantages against SARS-CoV-2. [Citation112,Citation113]
Selenium
Selenium is a fundamental trace element and is important for mammalian redox biology. Dietary selenium comes from cereals, bread, milk, meat, fishes, giblets, garlic, mushrooms, asparagus, sea foods and nuts. [Citation114–116] Selenium is found to combat various infectious diseases.[Citation117] Deficiency of selenium leads to increase in oxidative stress in the host and trigger the activity of viral pathogensity. [Citation118–120] Moreover, immune system can be improved by regular consumption of selenium in diet.[Citation121]
Selenium dietary intake generate various pleiotropic effects including stimulatory role in many anti-inflammatory processes and powerful antioxidant activities.[Citation117] To regulate enzymes in certain biochemical pathways, selenium contributes as cofactor. Surprisingly, only five molecules named selenite, selenite, selenoneine, selenomethionine, and selenocysteine are bioavailable resources via intake of food.[Citation122][Citation122]
Selenium forms a unique group of proteins, selenoproteins, from selenium-containing amino acid selenocysteine and this selenium molecular incorporation have resulted wide ranging biological activities.[Citation51] Selenium being component of many selenoproteins especially glutathione peroxidases and thioredoxin reductases has crucial role in maintaining host defense system to combat viral infections by stabilizing its redox homeostasis, redox signaling, powerful antioxidant activities; regulating leukocyte functions and activities of natural killer cells.[Citation51,Citation123] The cytolysis of natural killer cells depends upon high amount of selenium.[Citation124] In the presence of selenium T-lymphocytes proliferate actively and enhance immunoglobulin production by influencing the humoral system.[Citation123,Citation125]
The high amount of selenium dietary consumption exhibits potent antiviral effects [Citation117] while its low concentration or absence may result in improper immune responses, high rate of infections and increased risk of adverse outcomes and fatality. The deficiency of selenium accelerates the viral pathogenesis and associated risks.[Citation119] The alterations and mutations in viral genome occur more speedily in selenium deficient environment and consequently a-virulent or mild forms become more virulent. [Citation118,Citation119]
Furthermore, in selenium-deficient state, pathogenic conditions become worse due to excessive production of pro-inflammatory chemokines.[Citation119] Selenium intake regulate excessive immune responses by acting on about all components of immune system thus reduce chronic inflammation and severe outcomes during infection. In individuals with critical deficiency, selenium supplements are appropriate and reliable option to treat viral diseases like COVID-19.[Citation120] In the past, it was observed that selenium supplementation increases the immunity in individuals after influenza vaccination.[Citation126]
The deficiency of selenium among individuals around the globe is a matter of serious concern during this pandemic situation and can only be resolved by consumption of selenium-enriched food. The recommended dietary dose of selenium is range between 50 and 70 μg per day.[Citation51] Conclusively, maintenance of selenium level is helpful in reducing the COVID-19 complex symptoms during disease progression.
Zinc
Zinc is nutritionally essential trace mineral necessary for development, growth, proliferation of cells, synthesis of DNA,[Citation127] upregulation of transcriptional factors and enzymatic functions. [Citation32,Citation128] Zinc is present abundantly in red meat, poultry, fish, shellfish, lentils, beans, nuts, pumpkin seeds, sesame seeds, milk, and dairy products. Zinc is actively involved in normal cellular functions of immune system predominantly related to nonspecific immunity such as natural killer cells and neutrophils. Zinc plays an ubiquitous function in the development and maintenance of cells in immune system,[Citation129] regulate adaptive and innate immune responses and acceleration of cell signaling pathways. [Citation130,Citation131] The structural component of zinc-finger transcription regulators related to immunity is zinc.[Citation132]
The complex symptoms and pathological effects during viral infections are improved by regular dietary consumption of zinc.[Citation133] This improvement is partly due to reduction in viral replications by increasing antiviral immune responses as zinc suppresses the RNA-dependent RNA polymerase activity – a core enzyme required for replication of RNA viruses like SARS-CoV-2.[Citation134]
Zinc interacts with various organic ligands and this interaction has profound role in signal transduction, DNA and RNA metabolism and gene expression. In addition, increasing the intracellular zinc concentration along with zinc-ionophores like pyrithione impairs replication of large number of RNA viruses. Their combination was also helpful in inhibition of SARS coronavirus even when used in lower concentration.[Citation134]
In developing countries, about two billion people face zinc deficiency that increases susceptibility of these populations to infectious diseases due to dysfunction of both cell-mediated and humoral immunity.[Citation135] Despite no availability issue of zinc in modern lifestyle, its deficiency is surprisingly common and resulted severe outcomes in present pandemic crises.[Citation133] Zinc deficiency is critically important during COVID-19 as it impairs antiviral immunity by functional disability of T lymphocyte to produce and stimulate Th1 cytokines.[Citation136] Furthermore, maintaining levels of zinc in cells is vital because certain strains of viruses have developed mechanisms to alter homeostasis of cellular zinc to assist viral genetic material in speedy replications and their persistence.[Citation137] The weakened immune responses in virally infected patients can be enhanced by high doses of zinc supplementation.[Citation138] To retain resilience against SARS-CoV-2, up to 50 mg of zinc intake on daily basis is needed.[Citation132] Conclusively, zinc intake is helpful in coping with COVID-19 and its related symptoms and strengthening the immune system.
Flavonoids
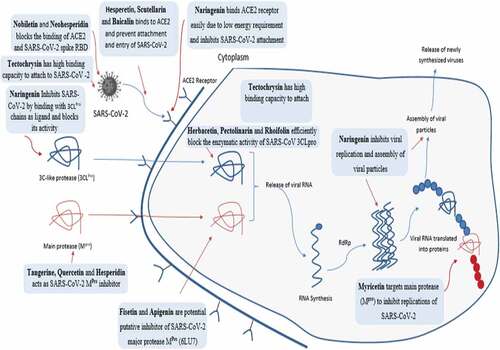
Flavonoids are the most important polyphenols and include flavones, flavonols, flavanones, isoflavones, catechins, anthocyanidins, and chalcones.[Citation139] Flavonoids are a diverse group of phytonutrients found in almost all fruits and vegetables. A wide range of biological and pharmacological actions within the body are due to different types of flavonoids. Some of these vital activities are anti-microbial (antiviral, antifungal and antibacterial), anti-inflammatory, antioxidant, anti-cancer, anti-allergic and anti-diarrheal activities. Recently, flavonoids are considered remarkably important due to their antiviral effects () and potent antioxidant potential. Literature demonstrated various studies related to the antiviral effects of flavonoids ().
Table 1. Antiviral Effects of Flavonoids
Nourishing diet for covid-19 recovered patients
After recovery from COVID-19, most people feel better after few days but post-COVID conditions may appear in some individuals. These post-COVID conditions are actually single or a combination of ongoing, new and returning health issues that people may experience four or more weeks after recovery. Some people remain asymptomatic after days or weeks after infected with SARS-CoV-2 can attain post-COVID conditions. The lengths and time for these conditions may vary depending on physiological and resilience ability of individuals.
After recovery, people commonly experience irregularities like abnormal pulmonary function, [Citation154] olfactory dysfunction,, [Citation155–157] cognitive impairments, [Citation158] Anosmia,[Citation159] long-term cardiovascular consequences,[Citation160] myalgia, gastrointestinal symptoms and dysgeusia.[Citation161] In addition, chest or stomach pain, fast heart beating rate, fever, mood changes, dizziness, change in period cycles are other common symptoms that continuously persist in some recovered patients.
In some cases, COVID-19 recovered patients tested positive for SARS-CoV-2 after hospital discharge.[Citation162] The COVID-19 discharge standards need to be improved based on more clinical evidences. In a report, persistent viral shedding was observed for 60 days after COVID-19 infection and 36 days after complete resolution of symptom suggesting that mild symptomatic, asymptomatic and recently recovered patients may require prolonged isolation.[Citation163]
Critically ill COVID-19 patients mostly experience acute respiratory distress syndrome and multi-organ failure and these adverse anomalies remain persist for long time in COVID-19 recovered patients.[Citation158] The feeling of patient to be fully recovered is a long process although officially declared COVID negative. COVID-19 recovered patients still experience multi organ effects or autoimmune conditions and become highly enfeebled and exhaust during the recovery stage hence, healthy diet and proper nutrition is essential for these individuals to be fully recovered. Healthy diet is also needed as patients could experience re-activation of a long-lasting virus carriage or might be re-infected, as well as potential long-term effects of drugs or diseases that hamper the immune response.[Citation164]
Rapid recovery of these individuals requires subtle nutrients homeostasis for effective host immune responses, accuracy in presence of metabolites, spontaneous flow of metabolic pathways and proper physiological responses. For completion of these aspects, diets rich in vitamins, minerals, phytonutrients and antioxidants are mandatory.[Citation165]
The oxidative stress increases during COVID-19 pathological events and increases ratio of oxidant species and promotes damage to cells via denaturation of protein. Many studies revealed that RNA viruses control the host antioxidant defense system, so affects certain enzymes like superoxide dismutase, catalase, and decreases the levels of antioxidant molecules such as ascorbic acid, carotenoids, and glutathione. The dietary intervention is the only approach to maintain levels of these molecules.
For proper body functions, maintaining organ health and their physiology, foods nutrients work in synergy. Hence, after recovery from COVID-19, add extra beef, chicken, tuna, vegetable dishes, soups, casseroles and salads.[Citation166] Choose higher fat milk and cheese, eggs, peanut butter, nuts as well. Some people with COVID-19 may reduce muscular mass or body weight and it makes them difficult to recover rapidly and performs routinely activities. These people need to increase calories and proteins to increase weight and bolster recovery. Sprouts are a great way to incorporate more protein in diet. A diet rich in calories is required to overcome stress after recovery and allow the consumed food substances to rebuilding your strength. Along with proper diet, sufficient intake of water and fluids is central to COVID-19 recovery.
Conclusion
In the present pandemic situation, it is vital to find an alternative therapeutic way to save humanity from lethal variants of SARS-CoV-2. The most reliable option is the regular use of dietary nutrients and bioactive moieties in reducing the infection, transmissibility, severe outcomes during COVID-19 progression and speed up the recovery process. A well-balanced diet significantly supports an ideal immune system and lowers the chances of spreading viral diseases. Various nutritional components such as vitamin A, B, C, D, E, omega-3, fatty acids, selenium, zinc and flavonoids have immuno-modulatory and antiviral properties. In a nutshell, uses of dietary components are a consistent and long-lasting pragmatic approach to eradicate SARS-CoV-2 and other such future viruses from the world.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- World Health Organization. WHO. Director-General’s Remarks at the Media Briefing on 2019-nCoV On. 11 February 2020.
- Gorbalenya, A. E.; Baker, S. C.; Baric, R. S.; De Groot, R. J.; Drosten, C.; Gulyaeva, A. A.; Haagmans, B. L.; Lauber, C.; Leontovich, A. M.; Neuman, B. W., et al. The Species Severe Acute Respiratory Syndrome-related Coronavirus, Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. https://go.nature.com/3cW9qJR
- Li, J.; Zhang, L.; Liu, B.; Song, D. Case Report: Viral Shedding for 60 Days in a Woman with COVID-19. The American Journal of Tropical Medicine and Hygiene. 2020, 102(6), 1210–1213. DOI: 10.4269/ajtmh.20-0275.
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W., et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382(10), 970–971.
- Xu, S.; Li, Y. Beware of the Second Wave of COVID-19. Lancet. 2020, 395(10233), 1321–1322. DOI: 10.1016/S0140-6736(20)30845-X.
- Ali, I. COVID-19: Are We Ready for the Second Wave? Disaster Medicine and Public Health Preparedness. 2020, 14(5), e16–e18. DOI: 10.1017/dmp.2020.149.
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S. C., and Di-Napoli, R. F. Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls. StatPearls Publishing: Treasure Island (FL), 2021. https://www.ncbi.nlm.nih.gov/books/NBK554776/
- World Health Organization. COVID-19 Weekly Epidemiological Update . edition 45. 22June 2021.https://apps.who.int/iris/handle/10665/342009
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M. M.; Schwartz, O.; Porrot, F.; Robillard, N.; Puech, J., et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature. 2021, 596(7871), 276–280.
- Wall, E. C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y., et al. Neutralising Antibody Activity against SARS-CoV-2 VOCs B.1.617.2 And B.1.351 by BNT162b2 Vaccination. Lancet. 2021, 397(10292), 2331–2333. DOI: 10.1016/S0140-6736(21)01290-3.
- Iacobucci, G. Covid-19: New UK Variant May Be Linked to Increased Death Rate, Early Data Indicate BMJ. 2021, 372(230), n230. DOI: 10.1136/bmj.n230.
- Choi, B.; Choudhary, M. C.; Regan, J.; Sparks, J. A.; Padera, R. F.; Qiu, X.; Solomon, I. H.; Kuo, H. H.; Boucau, J.; Bowman, K., et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383(23), 2291–2293. DOI: 10.1056/NEJMc2031364.
- Helweg-Larsen, J., and Benfield, T. Vaccination Immunology in SARS-CoV-2. Ugeskrift for Laeger. 2021, 183(11), PMID: 33734074.
- Moelling, K. Within-Host and Between-Host Evolution in SARS-CoV-2-New Variant’s Source Viruses. 2021, 14(1), 13. DOI: 10.3390/v14010013.
- Agarwal, S.; Saha, S.; Deb, T.; Darbar, S. Immunity Augmenting Food Supplements for Susceptible Individuals in Combating Pandemic COVID-19 (Review). Parana J. Sci. Educ. 2020, 6(4), 79–88.
- Li, Q.; Guan, X.; Wu,; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K. S. M.; Lau, K. S.; Wong, E. H., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–infected Pneumonia. N. Engl. J. Med. 2020, 382(13), 1199–1207. DOI: 10.1056/NEJMoa2001316.
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Erratum to ``A Review of the 2019 Novel Coronavirus (COVID-19) Based on Current Evidence [International Journal of Antimicrobial Agents 55/6 (2020) 105948]. Int. J. Antimicrob. Agents.2020, 56(3), 106137. DOI: 10.1016/j.ijantimicag.2020.106137.
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-ncov) in Vitro. Cell Res. 2020, 30(3), 269–271. DOI: 10.1038/s41422-020-0282-0.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X., et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet. 2020, 395(10223), 497–506. DOI: 10.1016/S0140-6736(20)30183-5.
- Ren, L. L.; Wang, Y. M.; Wu, Z. Q.; Xiang, Z. C.; Guo, L.; Xu, T.; Jiang, Y. Z.; Xiong, Y.; Li, Y. J.; Li, X. W., et al. Identification of a Novel Coronavirus Causing Severe Pneumonia in Human: A Descriptive Study. Chinese Med. J. 2020, 133(9), 1015. DOI: 10.1097/CM9.0000000000000722.
- Jensen, S.; Thomsen, A. R. Sensing of RNA Viruses: A Review of Innate Immune Receptors Involved in Recognizing RNA Virus Invasion. J. Virol. 2012, 86(6), 2900–2910. DOI: 10.1128/JVI.05738-11.
- Ma, D. Y.; Suthar, M. S. Mechanisms of Innate Immune Evasion in Re-emerging RNA Viruses. Curr. Opin. Virol. 2015, 12, 26–37. DOI: 10.1016/j.coviro.2015.02.005.
- Nelemans, T.; Kikkert, M. Viral Innate Immune Evasion and the Pathogenesis of Emerging RNA Virus Infections. Viruses. 2019, 11(10), 961. DOI: 10.3390/v11100961.
- Ivashkiv, L. B.; Donlin, L. T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14(1), 36–49. DOI: 10.1038/nri3581.
- Zhou, F.; Fan, G.; Liu, Z.; Cao, B. SARS-CoV-2 Shedding and infectivity–Authors’ Reply. Lancet. 2020, 395(10233), 1340. DOI: 10.1016/S0140-6736(20)30869-2.
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020, 12(5), 1466. DOI: 10.3390/nu12051466.
- Shob, K. New Symptoms, High Viral Load, Here’s How India’s Second Wave Of Covid-19 Looks Different From The First 2021. 2021.
- FAO. Maintaining a Healthy Diet during the COVID-19 Pandemic. 2020.
- Bousquet, J.; Anto, J. M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C. A.; Blain, H.; Canonica, G. W.; Cardona, V., et al. Is Diet Partly Responsible for Differences in COVID‐19 Death Rates between and within Countries? Clin. Transl. Allergy. 2020, 10(1), 16. DOI: 10.1186/s13601-020-00323-0.
- Beck, M. A.; Handy, J.; Levander, O. A. Host Nutritional Status: The Neglected Virulence Factor. Trends Microbiol. 2004, 12(9), 417–423. DOI: 10.1016/j.tim.2004.07.007.
- World Health Organization. Information Note on COVID-19 and NCDs. Geneva, Switzerland: WHO. 2020. Available at: https://www.who.int/who-documents-detail/covid-19-and-ncds. Accessed June, 1, 2020.
- Zhang, L.; Liu, Y. Potential Interventions for Novel Coronavirus in China: A Systematic Review. J. Med. Virol. 2020, 92(5), 479–490. DOI: 10.1002/jmv.25707.
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the Receptor-binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine. Cell. Mol. Immunol. 2020, 17(6), 613–620. DOI: 10.1038/s41423-020-0400-4.
- Rabi, F. A.; Al Zoubi, M. S.; Al-Iede, M. M.; Kasasbeh, G.; Badran, E. F. Coronaviruses in Children: A Review of Potential Mechanisms of Childhood Protection. Acta Paediatr. 2021, 110(3), 765–772. DOI: 10.1111/apa.15691.
- Rabi, F. A.; Al Zoubi, M. S.; Kasasbeh, G. A.; Salameh, D. M.; Al-Nasser, A. D.; Kasasbeh, G. A.; Salameh, D. M.; Al-Nasser, A. D.; Salameh, D. M.; Al-Nasser, A. D., et al. SARS-CoV-2 and Coronavirus Disease 2019: What We Know so Far. Pathogens. 2020, 10(1), 9. DOI: 10.3390/pathogens10010009.
- Simmons, G.; Zmora, P.; Gierer, S.; Heurich, A.; Pöhlmann, S. Proteolytic Activation of the SARS-coronavirus Spike Protein: Cutting Enzymes at the Cutting Edge of Antiviral Research. Antiviral Res. 2013, 100(3), 605–614. DOI: 10.1016/j.antiviral.2013.09.028.
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F., et al. SARS-CoV-2 Entry Factors are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26(5), 681–687. DOI: 10.1038/s41591-020-0868-6.
- Tejpal, S.; Sanghera, N.; Manoharan, V.; Planas-Iglesias, J.; Bastie, C. C., and Klein-Seetharaman, J. Angiotensin Converting Enzyme (ACE): A Marker for Personalized Feedback on Dieting. Nutrients. 2020, 12(3), 660.
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In Vitro and in Vivo Studies on the Angiotensin-Converting Enzyme Inhibitory Activity Peptides Isolated from Broccoli Protein Hydrolysate. J. Agric. Food Chem. 2019, 67(24), 6757–6764. DOI: 10.1021/acs.jafc.9b01137.
- Schüler, R.; Osterhoff, M. A.; Frahnow, T.; Seltmann, A. C.; Busjahn, A.; Kabisch, S.; Xu, L.; Mosig, A. S.; Spranger, J.; Möhlig, M., et al. High‐saturated‐fat Diet Increases Circulating Angiotensin‐converting Enzyme, Which Is Enhanced by the Rs4343 Polymorphism Defining Persons at Risk of Nutrient‐dependent Increases of Blood Pressure. J. Am. Heart Assoc. 2017, 6(1), e004465. DOI: 10.1161/JAHA.116.004465.
- Fan, H.; Liao, W.; Wu, J. Molecular Interactions, Bioavailability, and Cellular Mechanisms of Angiotensin-converting Enzyme Inhibitory Peptides. J. Food Biochem. 2019, 43(1), e12572. DOI: 10.1111/jfbc.12572.
- Oliveira-Andrade, J. M.; Paraíso, A. F.; Garcia, Z. M.; Ferreira, A. V.; Sinisterra, R. D.; Sousa, F. B.; Guimarães, A. L. S.; de Paula, A. M. B.; Campagnole-Santos, M. J.; Dos Santos, R. A., et al. Cross Talk between angiotensin-(1-7)/Mas Axis and Sirtuins in Adipose Tissue and Metabolism of High-fat Feed Mice. Peptides. 2014, 55, 158–165. DOI: 10.1016/j.peptides.2014.03.006.
- Yu, H. R.; Tain, Y. L.; Tiao, M. M.; Chen, C. C.; Sheen, J. M.; Lin, I. C.; Li, S. W.; Tsai, C. C.; Lin, Y. J.; Hsieh, K. S., et al. Prenatal Dexamethasone and Postnatal High-fat Diet Have a Synergistic Effect of Elevating Blood Pressure through a Distinct Programming Mechanism of Systemic and Adipose Renin–angiotensin Systems. Lipids Health Dis. 2018, 17(1), 1–10. DOI: 10.1186/s12944-018-0701-0.
- Sinha, S.; Cheng, K.; Aldape, K.; Schiff, E.; Ruppin, E. Systematic Cell Line-based Identification of Drugs Modifying ACE2 Expression. 2020.
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y. C. VDR Attenuates Acute Lung Injury by Blocking Ang-2-Tie-2 Pathway and Renin-angiotensin System. J. Mol. Endocrinol. 2013, 27(12), 2116–2125. DOI: 10.1210/me.2013-1146.
- Alothman, S. I. Foods as First Defense against COVID-19. In Alternative Medicine Interventions for COVID-19; Muhammad, Zia-Ul-Haq,; May Nasser, Bin-Jumah,; Sarah, I. Alothman,; Hanan, A. Henidi; Eds.; Springer: Cham, 2021; pp 153–192.
- Mattioli, A. V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 Outbreak: Changes in Diet and Physical Activity Increase the Risk of Cardiovascular Disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30(9), 1409–1417. DOI: 10.1016/j.numecd.2020.05.020.
- Jee, J.; Hoet, A. E.; Azevedo, M. P.; Vlasova, A. N.; Loerch, S. C.; Pickworth, C. L.; Hanson, J.; Saif, L. J. Effects of Dietary Vitamin A Content on Antibody Responses of Feedlot Calves Inoculated Intramuscularly with an Inactivated Bovine Coronavirus Vaccine. Am. J. Vet. Res. 2013, 74(10), 1353–1413. DOI: 10.2460/ajvr.74.10.1353.
- Kańtoch, M.; Litwińska, B.; Szkoda, M.; Siennicka, J. Importance of Vitamin A Deficiency in Pathology and Immunology of Viral Infections. Rocz. Panstw. Zakl. Hig. 2002, 53(4), 385–392.
- Glasziou, P. P.; Mackerras, D. E. Vitamin A Supplementation in Infectious Diseases: A Meta-analysis. BMJ. 1993, 306(6874), 366–370. DOI: 10.1136/bmj.306.6874.366.
- Guillin, O. M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients. 2019, 11(9), 2101. DOI: 10.3390/nu11092101.
- Patel, N.; Penkert, R. R.; Jones, B. G.; Sealy, R. E.; Surman, S. L.; Sun, Y.; Tang, L.; DeBeauchamp, J.; Webb, A.; Richardson, J., et al. Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses following Pediatric Influenza Vaccination. Viruses. 2019, 11(10), 907. DOI: 10.3390/v11100907.
- Spinas, E.; Saggini, A.; Kritas, S. K.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Frydas, A.; Tei, M.; Speziali, A., et al. Crosstalk between Vitamin B and Immunity. J. Biol. Regul. Homeost. Agents. 2015, 29(2), 283–288.
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. DOI: 10.3389/fnut.2019.00048.
- Keil, S. D.; Ragan, I.; Yonemura, S.; Hartson, L.; Dart, N. K.; Bowen, R. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 in Plasma and Platelet Products Using a Riboflavin and Ultraviolet Light-based Photochemical Treatment. Vox. Sang. 2020, 115(6), 495–501. DOI: 10.1111/vox.12937.
- Wintergerst, E. S.; Maggini, S.; Hornig, D. H. Contribution of Selected Vitamins and Trace Elements to Immune Function. Ann. Nutr. Metab. 2007, 51(4), 301–323. DOI: 10.1159/000107673.
- Morris, M. S.; Sakakeeny, L.; Jacques, P. F.; Picciano, M. F.; Selhub, J. Vitamin B-6 Intake Is Inversely Related To, and the Requirement Is Affected By, Inflammation Status. J. Nutr. 2010, 140(1), 103–110. DOI: 10.3945/jn.109.114397.
- Cheng, C. H.; Chang, S. J.; Lee, B. J.; Lin, K. L.; Huang, Y. C. Vitamin B6 Supplementation Increases Immune Responses in Critically Ill Patients. Eur. J. Clin. Nutr. 2006, 60(10), 1207–1213. DOI: 10.1038/sj.ejcn.1602439.
- Degnan, P. H.; Taga, M. E.; Goodman, A. L. Vitamin B12 as a Modulator of Gut Microbial Ecology. Cell Metab. 2014, 20(5), 769–778. DOI: 10.1016/j.cmet.2014.10.002.
- Negi, S.; Das, D. K.; Pahari, S.; Nadeem, S.; Agrewala, J. N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. DOI: 10.3389/fimmu.2019.02441.
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, M.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by Vitamin B12: Augmentation of CD8+ T Lymphocytes and Natural Killer (NK) Cell Activity in Vitamin B12‐deficient Patients by methyl‐B12 Treatment. Clinl. Exp. Immunol. 1999, 116(1), 28–32. DOI: 10.1046/j.1365-2249.1999.00870.x.
- Dhar, D.; Mohanty, A. Gut Microbiota and Covid-19- Possible Link and Implications. Virus Res. 2020, 285, 198018. DOI: 10.1016/j.virusres.2020.198018.
- Zuo, T.; Zhang, F.; Lui, G. C. Y.; Yeoh, Y. K.; Li, A. Y. L.; Zhan, H.; Wan, Y.; Chung, A. C.; Cheung, C. P.; Chen, N., et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology. 2020, 159(3), 944–955. DOI: 10.1053/j.gastro.2020.05.048.
- Tan, C. W.; Ho, L. P.; Kalimuddin, S.; Cherng, B. P. Z.; Teh, Y. E.; Thien, S. Y.; Wong, H. M.; Tern, P. J. W.; Chandran, M.; Chay, J. W. M., et al. Cohort Study to Evaluate Effect of Vitamin D, Magnesium, and Vitamin B12 in Combination on Severe Outcome Progression in Older Patients with Coronavirus (COVID-19). Nutrition. 2020, 79, 111017. DOI: 10.1016/j.nut.2020.111017.
- Watanabe, F. Vitamin B12 Sources and Bioavailability Exp. Biol. Med (Maywood). 2007, 232(10), 1266–1274. DOI: 10.3181/0703-MR-67.
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S. Y.; Kong, J. M.; Hwang, Y. I.; Kang, J. S. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13(2), 70–74.
- Carr, A. C., and Maggini, S. Vitamin C and Immune Function. Nutrients. 2017, 9(11), 1211.
- Elmadfa, I.; Meyer, A. L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets. 2019, 19(8), 1100–1115. DOI: 10.2174/1871530319666190529101816.
- Manning, J.; Mitchell, B.; Appadurai, D. A.; Shakya, A.; Pierce, L. J.; Wang, H.; Nganga, V.; Swanson, P. C.; May, J. M.; Tantin, D.; Spangrude, G. J. Vitamin C Promotes Maturation of T-cells. Antioxid. Redox Signal. 2013, 19(17), 2054–2067.
- Hemilä, H. Vitamin C and Infections Nutrients. 2017, 9(4), 339.
- Hemilä, H., and Chalker, E. Vitamin C for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2013. 2013 Cd000980: Cochran. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000980.pub4/abstract
- Atherton, J. G.; Kratzing, C. C.; Fisher, A. The Effect of Ascorbic Acid on Infection Chick-embryo Ciliated Tracheal Organ Cultures by Coronavirus. Arch. Virol. 1978, 56(3), 195–199. DOI: 10.1007/BF01317848.
- Field, C. J.; Johnson, I. R.; Schley, P. D. Nutrients and Their Role in Host Resistance to Infection. J. Leukoc. Biol. 2002, 71(1), 16–32.
- Hemilä, H. Vitamin C Intake and Susceptibility to Pneumonia Pediatr. Infect. Dis. J. 1997, 16(9), 836–837. DOI: 10.1097/00006454-199709000-00003.
- Van Driel, M. L.; Beller, E. M.; Thielemans, E.; Deckx, L.; Price‐Haywood, E.; Clark, J.; De Sutter, A. I. Oral Vitamin C Supplements to Prevent and Treat Acute Upper Respiratory Tract Infections. The Cochrane Database Systc. Rev. 2019. DOI:10.1002/14651858.CD013292.
- Kalantar-Zadeh, K.; Moore, L. W. Impact of Nutrition and Diet on COVID-19 Infection and Implications for Kidney Health and Kidney Disease Management. J. Ren. Nutr. 2020, 30(3), 179–181. DOI: 10.1053/j.jrn.2020.03.006.
- Crowe, F. L.; Steur, M.; Allen, N. E.; Appleby, P. N.; Travis, R. C.; Key, T. J. Plasma Concentrations of 25-hydroxyvitamin D in Meat Eaters, Fish Eaters, Vegetarians and Vegans: Results from the EPIC-Oxford Study. Public. Health. Nutr. 2011, 14(2), 340–346. DOI: 10.1017/S1368980010002454.
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H. B.; Boucher, B. J.; Cannell, J. J.; Foresta, C.; Grübler, M. R.; Kotsa, K.; Mascitelli, L. Vitamin D and Chronic Diseases: The Current State of the Art. Arch. Toxicol. 2017, 91(1), 97–107.
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M. A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, ZinC, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Alternat. Med. 2018, 2018, 5813095. DOI: 10.1155/2018/5813095.
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A Helpful Immuno-modulator. Immunology. 2011, 134(2), 123–139. DOI: 10.1111/j.1365-2567.2011.03482.x.
- Vanherwegen, A. S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. North Am. 2017, 46(4), 1061–1094. DOI: 10.1016/j.ecl.2017.07.010.
- Wu, D.; Lewis, E. D.; Pae, M.; Meydani, S. N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. DOI: 10.3389/fimmu.2018.03160.
- McGonagle, D. Interleukin-6 Use in COVID-19 Pneumonia Related Macrophage Activation Syndrome Autoimmunity Reviews. 2020.
- Zhang, Y.; Leung, D. Y.; Richers, B. N.; Liu, Y.; Remigio, L. K.; Riches, D. W.; Goleva, E. Vitamin D Inhibits Monocyte/macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188(5), 2127–2135.
- Laird, E.; Rhodes, J., and Kenny, R. A. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19. Ir. Med. J. 2020, 113(5), 81.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; Tai, Y. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8(4), 420–422.
- Ginde, A. A.; Mansbach, J. M.; Camargo, C. A. Jr. Association between Serum 25-hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Int. Med. 2009, 169(4), 384–390. DOI: 10.1001/archinternmed.2008.560.
- Jolliffe, D. A.; Griffiths, C. J.; Martineau, A. R. Vitamin D in the Prevention of Acute Respiratory Infection: Systematic Review of Clinical Studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329.
- Bergman, P.; Norlin, A. C.; Hansen, S.; Rekha, R. S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J. D.; Andersson, J. Vitamin D 3 Supplementation in Patients with Frequent Respiratory Tract Infections: A Randomised and Double-blind Intervention Study. BMJ Open. 2012, 2(6), e001663.
- Martineau, A. R.; Jolliffe, D. A.; Hooper, R. L.; Greenberg, L.; Aloia, J. F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A. A.; Goodall, E. C. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-analysis of Individual Participant Data. BMJ. 2017, 356, i6583. DOI: 10.1136/bmj.i6583.
- Zhou, Y. F.; Luo, B. A.; Qin, L. L. The Association between Vitamin D Deficiency and Community-acquired Pneumonia: A Meta-analysis of Observational Studies. Medicine. 2019, 98(38), e17252. DOI: 10.1097/MD.0000000000017252.
- Laird, E.; Walsh, J. B.; Lanham-New, S.; O’Sullivan, M.; Kenny, R. A.; Scully, H.; Crowley, V.; Healy, M. A. A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study. Nutrients. 2020, 12(12).
- Jia, X.; Yin, C.; Lu, S.; Chen, Y.; Liu, Q.; Bai, J.; Lu, Y. Two Things about COVID-19 Might Need Attention. 2020. Preprints. 10.20944/preprints202002.0315.v1.
- Thornton, J. Don’t Forget Chronic Lung and Immune Conditions during Covid-19, Says WHO BMJ. 2020, 368, m1192. DOI: 10.1136/bmj.m1192.
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S. M. The Interplay between Vitamin D and Viral Infections. Rev. Med. Virol. 2019, 29(2), e2032. DOI: 10.1002/rmv.2032.
- Grant, W. B.; Lahore, H.; McDonnell, S. L.; Baggerly, C. A.; French, C. B.; Aliano, J. L.; Bhattoa, H. P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020, 12(4), 988.
- Braiman, M. Latitude Dependence of the COVID-19 Mortality Rate—A Possible Relationship to Vitamin D Deficiency? 2020, Available at SSRN. 3561958.
- Farrar, M. D.; Kift, R.; Felton, S. J.; Berry, J. L.; Durkin, M. T.; Allan, D.; Vail, A.; Webb, A. R.; Rhodes, L. E. Recommended Summer Sunlight Exposure Amounts Fail to Produce Sufficient Vitamin D Status in UK Adults of South Asian Origin. Am. J. Clin. Nutr. 2011, 94(5), 1219–1224.
- Galmés, S.; Serra, F., and Palou, A. Vitamin E Metabolic Effects and Genetic Variants: A Challenge for Precision Nutrition in Obesity and Associated Disturbances. Nutrients. 2018, 10(12), 1919.
- Moriguchi, S.; Muraga, M. Vitamin E and Immunity. Vitam. Horm. 2000, 59, 305–336.
- Stiff, A.; Trikha, P.; Mundy-Bosse, B.; McMichael, E.; Mace, T. A.; Benner, B.; Kendra, K.; Campbell, A.; Gautam, S.; Abood, D.; Landi, I. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018, 24(8), 1891–1904.
- Beharka, A.; Han, S.; Adolfsson, O.; Wu, D.; Smith, D.; Lipman, R.; Cao, G.; Meydani, M.; Meydani, S. N. Long-term Dietary Antioxidant Supplementation Reduces Production of Selected Inflammatory Mediators by Murine Macrophages. Nutr. Res. 2000, 20(2), 281–296.
- Lee, G. Y.; Han, S. N. The Role of Vitamin E in Immunity. Nutrients. 2018, 11(1), 10. DOI: 10.3390/nu11010010.
- Xuan, N. T.; Trang, P. T.; Van Phong, N.; Toan, N. L.; Trung, D. M.; Bac, N. D.; Nguyen, V. L.; Hoang, N. H.; Van Hai, N. Klotho Sensitive Regulation of Dendritic Cell Functions by Vitamin E. Biol. Res. 2016, 49(1), 45.
- Han, S. N.; Meydani, S. N. Impact of Vitamin E on Immune Function and Its Clinical Implications. Expert. Rev. Clin. Immunol. 2006, 2(4), 561–567. DOI: 10.1586/1744666X.2.4.561.
- Covington, M. B. Omega-3 Fatty Acids. Am. Fam. Physician. 2004, 70(1), 133–140.
- Sansbury, B. E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119(1), 113–130. DOI: 10.1161/CIRCRESAHA.116.307308.
- Cai, C.; Koch, B.; Morikawa, K.; Suda, G.; Sakamoto, N.; Akhras, S.; Dietz, J.; Hildt, E.; Zeuzem, S.; Welsch, C. Macrophage-Derived Extracellular Vesicles Induce Long-Lasting Immunity against Hepatitis C Virus Which Is Blunted by Polyunsaturated Fatty Acids. Front. Immunol. 2018, 9, 723. DOI: 10.3389/fimmu.2018.00723.
- Serhan, C. N.; Levy, B. D. Resolvins in Inflammation: Emergence of the Pro-resolving Superfamily of Mediators. J. Clin. Invest. 2018, 128(7), 2657–2669. DOI: 10.1172/JCI97943.
- Leu, G. Z.; Lin, T. Y.; Hsu, J. T. Anti-HCV Activities of Selective Polyunsaturated Fatty Acids. Biochem. Biophys. Res. Commun. 2004, 318(1), 275–280. DOI: 10.1016/j.bbrc.2004.04.019.
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; Kadowaki, A. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell. 2013, 153(1), 112–125.
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di Nunno, N.; Di Mizio, G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; Valenzano, A. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21(9), 3104.
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashem, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; Abdolahi, M. Omega-3 Polyunsaturated Fatty Acids Supplementation Improve Clinical Symptoms in Patients with Covid-19: A Randomized Clinical Trial. Int. J. Clin. Pract. 2021, 75(12), e14854.
- Fraczek, A., and Pasternak, K. Selenium in Medicine and Treatment. J. Elem. 2013, 18(1).
- Kieliszek, M.; Błażejak, S. S. Significance, and Outlook for Supplementation. Nutrition. 2013, 29(5), 713–718. DOI: 10.1016/j.nut.2012.11.012.
- Kieliszek, M., and Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules. 2016, 21(5), 609.
- Rayman, M. P. Selenium and Human Health. Lancet. 2012, 379(9822), 1256–1268. DOI: 10.1016/S0140-6736(11)61452-9.
- Beck, M. A.; Levander, O. A.; Handy, J. Selenium Deficiency and Viral Infection. J. Nutr. 2003, 133(5), 1463s–7s. DOI: 10.1093/jn/133.5.1463S.
- Beck, M. A.; Nelson, H. K.; Shi, Q.; Van Dael, P.; Schiffrin, E. J.; Blum, S.; Barclay, D.; Levander, O. A. Selenium Deficiency Increases the Pathology of an Influenza Virus Infection. Faseb. J. 2001, 15(8), 1481–1483.
- Harthill, M. Review: Micronutrient Selenium Deficiency Influences Evolution of Some Viral Infectious Diseases. Biol. Trace Elem. Res. 2011, 143(3), 1325–1336. DOI: 10.1007/s12011-011-8977-1.
- Ma, X.; Bi, S.; Wang, Y.; Chi, X.; Hu, S. Combined Adjuvant Effect of Ginseng Stem-leaf Saponins and Selenium on Immune Responses to a Live Bivalent Vaccine of Newcastle Disease Virus and Infectious Bronchitis Virus in Chickens. Poult. Sci. 2019, 98(9), 3548–3556. DOI: 10.3382/ps/pez207.
- Vindry, C.; Ohlmann, T., and Chavatte, L. Selenium Metabolism, Regulation, and Sex Differences in Mammals. In Selenium; Sarah, Blossom; Eds.; Springer, 2018; pp 89–107.
- Saeed, F.; Nadeem, M.; Ahmed, R. S.; Tahir Nadeem, M.; Arshad, M. S.; Ullah, A. Studying the Impact of Nutritional Immunology Underlying the Modulation of Immune Responses by Nutritional Compounds–a Review. Food Agric. Immunol. 2016, 27(2), 205–229. DOI: 10.1080/09540105.2015.1079600.
- Zwolak, I.; Zaporowska, H. Selenium Interactions and Toxicity: A Review. Selenium Interactions and Toxicity. Cell Biol. Toxicol. 2012, 28(1), 31–46. DOI: 10.1007/s10565-011-9203-9.
- Alpert, P. T. The Role of Vitamins and Minerals on the Immune System Home Health Care Manag. Pract. 2017, 29(3), 199–202. DOI: 10.1177/1084822317713300.
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C. N.; Goldson, A. J.; Dainty, J. R.; Nicoletti, C. Selenium Supplementation Has Beneficial and Detrimental Effects on Immunity to Influenza Vaccine in Older Adults. Clin. Nutr. 2017, 36(2), 407–415.
- Fuhrman, J. Immunity Benefits of Zinc as We Age Retrieved from verywellhealth. com/surprising‐immunity‐benefits‐of‐zinc‐4047431. 2020. [Google Scholar].
- Calder, P. C.; Carr, A. C.; Gombart, A. F., and Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral InfectionsNutrients. 2020, 12(4), 1181.
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. DOI: 10.1016/j.abb.2016.03.022.
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18(10). DOI: 10.3390/ijms18102222.
- Wessels, I., Maywald, M., and Rink, L. Zinc as a Gatekeeper of Immune Function, Nutrients. 2017, 9(12), 1286.
- Razzaque, M. S. COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance?. Tohoku J. Exp. Med. 2020, 251(3), 175–181. DOI: 10.1620/tjem.251.175.
- Read, S. A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10(4), 696–710. DOI: 10.1093/advances/nmz013.
- te Velthuis, A. J.; van den Worm, S. H.; Sims, A. C.; Baric, R. S.; Snijder, E. J.; van Hemert, M. J. Zn(2+) Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6(11), e1001176. DOI: 10.1371/journal.ppat.1001176.
- Tuerk, M. J.; Fazel, N. Zinc Deficiency. Curr. Opin. Gastroenterol. 2009, 25(2), 136–143. DOI: 10.1097/MOG.0b013e328321b395.
- Shankar, A. H.; Prasad, A. S. Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection. Am. J. Clin. Nutr. 1998, 68(2), 447s–63s. DOI: 10.1093/ajcn/68.2.447S.
- Lazarczyk, M.; Favre, M. Role of Zn 2+ Ions in Host-Virus Interactions. J. Virol. 2008, 82(23), 11486–11494. DOI: 10.1128/JVI.01314-08.
- Iovino, L.; Mazziotta, F.; Carulli, G.; Guerrini, F.; Morganti, R.; Mazzotti, V.; Maggi, F.; Macera, L.; Orciuolo, E.; Buda, G.; Benedetti, E. High-dose Zinc Oral Supplementation after Stem Cell Transplantation Causes an Increase of TRECs and CD4+ Naïve Lymphocytes and Prevents TTV Reactivation. Leuk. Res. 2018, 70, 20–24. DOI: 10.1016/j.leukres.2018.04.016.
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. DOI: 10.1017/jns.2016.41.
- Chen, H.; Du, Q. Potential Natural Compounds for Preventing SARS-CoV-2 (2019-ncov) Infection. Preprints. 2020, 2020010358. DOI: 10.20944/preprints202001.0358.v3.
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M. J. Naringenin, A Flavanone with Antiviral and Anti-inflammatory Effects: A Promising Treatment Strategy against Covid −19. Phytother. Res. 2020, 34(12), 3137–3147. DOI: 10.1002/ptr.6781.
- Huang, J. B. S.; Zheng, W.; Huang, J. B. S.; Huang, J. B. S.; Bao, S.; Xu, Q.; Ma, Z. Citrus Fruits are Rich in Flavonoids for Immunoregulation and Potential Targeting ACE2. 2022. Nat. Prod. Bioprospect. 2022, 12(1), 4.
- Rocha, M. N. D.; Alves, D. R.; Marinho, M. M.; Morais, S. M. D.; Marinho, E. S. Virtual Screening of Citrus Flavonoid Tangeretin: A Promising Pharmacological Tool for the Treatment and Prevention of Zika Fever and COVID-19. J. Comput. Biophys. Chem. 2021, 20(3), 283–304. DOI: 10.1142/S2737416521500137.
- Zhou, J.; Huang, J. Current Findings regarding Natural Components with Potential anti-2019-nCoV Activity. Front. Cell Dev. Biol. 2020, 8, 589. DOI: 10.3389/fcell.2020.00589.
- Haggag, Y. A.; El-Ashmawy, N. E.; Okasha, K. M. Is Hesperidin Essential for Prophylaxis and Treatment of COVID-19 Infection? Med. Hypotheses. 2020, 144, 109957. DOI: 10.1016/j.mehy.2020.109957.
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A. A.; Rashad, A.A.; Kayed, A.E.; El-Shesheny, R.; Kayali, G.; Ali, M. A. Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2. Pathogens. 2021, 11(1), 10.
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J.; Li, X. Myricetin Inhibits SARS-CoV-2 Viral Replication by Targeting M(pro) and Ameliorates Pulmonary Inflammation. Front. Pharmacol. 2021, 12, 669642. DOI: 10.3389/fphar.2021.669642.
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M. R. Anti-inflammatory Potential of Quercetin in COVID-19 Treatment. J. Inflamm (Lond). 2021, 18(1), 3. DOI: 10.1186/s12950-021-00268-6.
- Ahmadian, R.; Rahimi, R.; Bahramsoltani, R. Kaempferol: An Encouraging Flavonoid for COVID-19. Bol. Latinoam. Caribe Plantas Med. Aromat. 2020, 19(5), 492–494. DOI: 10.37360/blacpma.20.19.5.33.
- Yang, C.; Yang, W.; He, Z.; He, H.; Yang, X.; Lu, Y.; Li, H. Kaempferol Improves Lung Ischemia-Reperfusion Injury via Antiinflammation and Antioxidative Stress Regulated by SIRT1/HMGB1/NF-κB Axis. Front. Pharmacol. 2019, 10, 1635. DOI: 10.3389/fphar.2019.01635.
- Oladele, J. O.; Oyeleke, O. M.; Oladele, O. T.; Olowookere, B. D.; Oso, B. J.; Oladiji, A. T. Kolaviron (Kolaflavanone), apigenin, fisetin as potential Coronavirus inhibitors: In silico investigation. Res. Square Preprints. 2020, 20, 1–13.
- Omotuyi, I. O.; Nash, O.; Ajiboye, B. O.; Olumekun, V. O.; Oyinloye, B. E.; Osuntokun, O. T.; Olonisakin, A.; Ajayi, A. O.; Olusanya, O.; Akomolafe, F. S.; Adelakun, N. Aframomum Melegueta Secondary Metabolites Exhibit Polypharmacology against SARS-CoV-2 Drug Targets: In Vitro Validation of Furin Inhibition. Phytother. Res. 2021, 35(2), 908–919.
- Jo, S.; Kim, S.; Shin, D. H.; Kim, M. S. Inhibition of SARS-CoV 3CL Protease by Flavonoids. J. Enzyme. Inhib. Med. Chem. 2020, 35(1), 145–151. DOI: 10.1080/14756366.2019.1690480.
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Lei, C.; ChenR.; Zhong, N.; Li, S. Abnormal Pulmonary Function in COVID-19 Patients at Time of Hospital Discharge. Eur. Respir. J. 2020, 55(6).
- Otte, M. S.; Eckel, H. N. C.; Poluschkin, L.; Klussmann, J. P.; Luers, J. C. Olfactory Dysfunction in Patients after Recovering from COVID-19. Acta Otolaryngol. 2020, 140(12), 1032–1035. DOI: 10.1080/00016489.2020.1811999.
- Otte, M. S.; Klussmann, J. P.; Luers, J. C. Persisting Olfactory Dysfunction in Patients after Recovering from COVID-19. J. Infect. 2020, 81(3), e58. DOI: 10.1016/j.jinf.2020.06.054.
- Whitcroft, K. L.; Hummel, T. Olfactory Dysfunction in COVID-19, Diagnosis and Management. Jama. 2020, 323(24), 2512–2514. DOI: 10.1001/jama.2020.8391.
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D., et al. The Landscape of Cognitive Function in Recovered COVID-19 Patients. J. Psychiatr. Res. 2020, 129, 98–102. DOI: 10.1016/j.jpsychires.2020.06.022.
- Hopkins, C.; Surda, P.; Whitehead, E.; Kumar, B. N. Early Recovery following New Onset Anosmia during the COVID-19 Pandemic - an Observational Cohort Study. J. Otolaryngol. Head Neck Surg. 2020, 49(1), 26. DOI: 10.1186/s40463-020-00423-8.
- Puntmann, V. O.; Carerj, M. L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-NicoteraM.; Zeiher, A. M.; Vehreschild, M. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5(11), 1265–1273.
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. Jama. 2020, 324(6), 603–605. DOI: 10.1001/jama.2020.12603.
- An, J.; Liao, X.; Xiao, T.; Qian, S.; Yuan, J.; Ye, H.; Qi, F.; Shen, C.; Wang, L.; Liu, Y.; Cheng, X. Clinical Characteristics of Recovered COVID-19 Patients with Re-detectable Positive RNA Test. Ann. Transl. Med. 2020, 8(17), 1084.
- Li, X. J.; Zhang, Z. W.; Zong, Z. Y. A Case of A Readmitted Patient Who Recovered from COVID-19 in Chengdu, China. Crit. Care. 2020, 24(1), 152. DOI: 10.1186/s13054-020-02877-8.
- Gousseff, M.; Penot, P.; Gallay, L.; Batisse, D.; Benech, N.; Bouiller, K.; Collarino, R.; Conrad, A.; Slama, D.; Joseph, C.; Lemaignen, A. Clinical Recurrences of COVID-19 Symptoms after Recovery: Viral Relapse, Reinfection or Inflammatory Rebound? J. Infect. 2020, 81(5), 816–846.
- Bohlouli, J.; Moravejolahkami, A. R.; Ganjali Dashti, M.; Balouch Zehi, Z.; Hojjati Kermani, M. A.; Borzoo-Isfahani, M.; Bahreini-Esfahani, N. COVID-19 and Fast Foods Consumption: A Review. Int. J. Food Prop. 2021, 24(1), 203–209. DOI: 10.1080/10942912.2021.1873364.
- Darbar, S.; Agarwal, S.; Saha, S. Effective Food Habits to Improve Immunity against Covid-19. J. Basic Pharmacol. Toxicol. 2021, 5(1), 1–6.