ABSTRACT
Sorghum (Sorghum bicolor L.) is ranked five cereal crop worldwide. It belongs to the Poaceae family and is formally part of plant-derived food. Nutritionally, it is composed of carbohydrates, kafirin (protein), polyunsaturated fatty acids (PUFA), fibers and resistant starch. Sorghum is used in three different fields: food, feed, and biomass production. Phenolic compounds including phenolic acid, flavonoids, stilbenes and tannins, vitamins including B-complex, A, D, E and K and the minerals (potassium, phosphorus, magnesium and zinc) were involved in the bioactivity of sorghum. The functional composition of sorghum plays an essential role in human health by inhibiting the risk of chronic diseases. Available epidemiological evidence suggests that tannin (proanthocyanidins) in sorghum acts as an antioxidant protecting from inflammation and cancer. Its fiber content can reduce blood cholesterol and glucose level, and is also helpful in celiac disease. Phytochemicals in sorghum enhance cardiovascular health in animals. These properties have not been reported in humans, which needs investigation. This review aims to describe the available information on the bioactive characteristics of sorghum, how it is used in different functional foods, and how it has the potential to combat various disorders. The literature has been collected from Google Scholar, Science Direct, PubMed and Web of Science.
Introduction
Sorghum (Sorghum bicolor L) is the world’s 5th most prominent cereal crop; with annual cereal production of approximately 5.3 metric tonnes.[Citation1] Besides being used as a staple food by the natives of Africa, Central America and South Asia; sorghum is also used worldwide as animal feed. In the United States, sorghum is utilized as a crude material for the grain ethanol measure. In China, the Philippines, and India, sweet sorghum is used as a crude material in the sugar ethanol measure. Different nations have also been investigating the utilization of sorghum as an inexhaustible material and as a possible source of high-esteem particles to create sustainable oil and other significant mechanical synthetics.[Citation2] It usually gives food and animal feed; however, as of late it additionally gives fermentable sugars to the production of inexhaustible powers and synthetic compounds.[Citation3] Secondary plant metabolites such as phenols, plant sterols, and policosanols are found in sorghum. There has been a rise in interest in phytochemicals because of their antioxidant activity, cholesterol-lowering qualities, and other possible health advantages. The phenolic acids and flavonoids found in sorghum fall into two primary types. The studies suggest that the sorghum possesses protein (4.4–21.1%), fat (2.1–7.6%), crude fiber (1.0–3.4%), total carbohydrates (57.0–80.6%), starch (55.6–75.2%) and total minerals as ash (1.3–3.5%). Additionally, it possesses phenolic compounds including antioxidants and can be consumed as an alternative meal for celiac disease victims.[Citation4] The sorghum plant is shown in .
Sorghum is classified mainly based on both appearance and total extractable phenols. White sorghums (also known as food-type) have no detectable tannins or anthocyanins and very low total extractable phenol levels; red sorghums have no tannins but have a red pericarp with significant levels of extractable phenols; black sorghums have a black pericarp and very high levels of anthocyanins; and brown sorghums have a pigmented tetracarp with varying degree.
The germ, endosperm and bran are three different parts of sorghum grain. The extent and composition of the sorghum depends on the range and conditions of development.[Citation5] Microscopic organisms are made out of fat-solvent nutrients, β-complex nutrients.[Citation6] Sorghum is perhaps the most generally utilized crops for food, animals feed, liquor formation, and biofuels. Many investigation teams have shown that sorghum contains a few beneficial components in human diseases (heftiness, diabetes, atherosclerosis, and irritation). However, sorghum is used as susceptible food to treat and counter-action of human diseases.[Citation7] This review aims to explore the nutritional composition of sorghum, its use in different functional foods, and its different disease countering characteristics in humans.
Methodology
To collect the literature about the sorghum we used Science Direct, Google Scholar, Web of Science and PubMed. For the paid articles we acquired help from Food and Nutrition Society, Gilgit Baltistan, Pakistan and the society provided us free access to the paid articles. We made an outline first to make the proper structure of the review and then made the conceptualization. By following the framework we developed the partitions of the review and discussed the overall composition of the sorghum, bioactive potential in functional foods and the potential to combat chronic diseases of sorghum.
Nutritional composition of sorghum
The nutrient content of sorghum varies according to the variety. Sorghum’s nutritional value depends on the type of food.[Citation8] Starch and non-starch polysaccharides (carbohydrates), lipids and proteins are the key components of sorghum.[Citation9] ().
Table 1. Nutritional components of sorghum
Carbohydrates
The main carbohydrate of sorghum is starch that is found as granules in endosperm. The starch content of different varieties varies greatly, ranging from per 100 g wheat: 32.1 to 72.5 g.[Citation13] Though some waxy sorghums can lack or have low levels of amylose, Amylopectin and Amylose are the two main components of sorghum starch.[Citation14] The majority of starch grains are consistently digested (30.0–66.2%),[Citation15] whereas the rest are either fast digestive (15.3–26.6%) or resistive (16.7–43.2%).[Citation16] Un-dissolvable fibers (75.0–90.0%), mostly soluble fibers, and arabinoxylans (10.0–25.0%) are made up of sorghum polysaccharides (6.0 to 15.0 g/100 g),[Citation14] and non-starch polysaccharides.[Citation17,Citation18]
The characteristics of the plant’s genetics and the conditions in which it grows sorghum affected the quality of starch and grain’s main polysaccharide content.[Citation19] In certain ranges, starch content varies between 42.1 and 62.5 g/100 g, with amylopectin (81.0–96.5%) and amylose (3.5–19.0%) accounting for most of the total.[Citation8,Citation13] Sorghum starch’s rheological properties,[Citation15] like lubricating, gelatinization, retrogradation, and digestibility, are influenced by the amount of amylose and amylopectin it contains.[Citation20] Sorghum has the most minimal starch edibility among cereals because of the close interaction between proteins, tannins and starch granules.[Citation21]
Protein (kafirin)
Kafirins and non-prolamin are two types of sorghum protein (globulins, albumins, and glutelins). The primary source of protein storage in sorghum grain with albumins and glutelins is kafirins which are responsible for 70% of the total protein in whole grain,[Citation22] and globulins are responsible for the remaining 30%. Based on molecular weight, there are four types of kafirin: α-, β-, γ- and δ- kafirin. These hydrophobic proteins are located in endosperm protein bodies that are tightly bound.[Citation23] Glutamic acid, proline, and leucine are abundant in sorghum grains.[Citation22] However, like other cereal grains, it might be lacking in lysine, an issue that sorghum breeding or food fortification may overcome.[Citation24] In the intestine, α-kafirins are considered the last digested proteins and their indigestibility limit their nutritional value due to their high abundance.[Citation25] Since Cysteine is abundant in γ-kafirins, which creates disulfide bonds, they are thought to prevent α -kafirins from being accessed by enzymes that break down water.[Citation26] As a result of expanded compound powerlessness of the essential stockpiling protein, α-kafirin,[Citation27] it reduces the development of disulfide associations among β and γ-kafirins, as well as alterations in protein body morphology,[Citation28] modified varieties have higher digestibility.[Citation29] Proteins found in sorghum are challenging to digest.[Citation30] Sorghum kafirins cannot be enzymatically assimilated in the gastrointestinal tract because of their high polymerization levels and broad disulfides; their close relationship to tannins and starch also problematic protein absorption.[Citation23] However, sorghum is a good food for people with obesity and diabetes because of its low starch and protein absorption.[Citation31]
Polyunsaturated fatty acids
The lipid content of sorghum is minimal (1.24 to 3.07 g/100 g),[Citation32] with unsaturated fatty acids accounting for 83–88% of the total.[Citation18] Unsaturated fatty acids make up most of the lipid in sorghum grain; its lipid profile is closely related to maize’s, although it is liquid at room temperature.[Citation10,Citation14]
Bioactive compounds
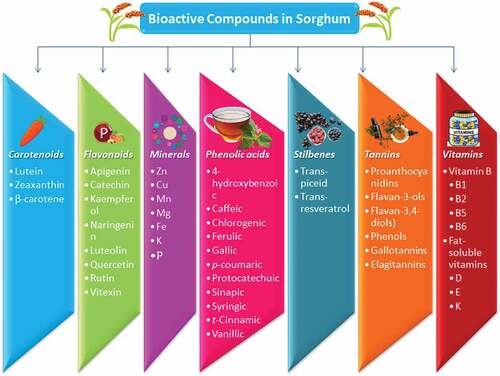
Sorghum is a good substitute for gluten and a storehouse of biologically active polyphenols and phytonutrients (). High-performance liquid chromatography identified and specified Sorghum’s phytochemicals in five genetic constitutes of sorghum.[Citation33,Citation34] shows the bioactive composition of sorghum.
Table 2. Bioactive composition of sorghum
Phenolic acids
Phenolic acids are simple substances but are rich with phenolic substances found in nearly all sorghum kernels, aggregating from 445 to 2850 μg/g.[Citation41] The unbound phenolic acids accessible by natural solvents are not bound to the cell wall and are frequently spotted in the seed case and seed coat.[Citation42] Usually are associated with glycerol and carbohydrate monomers and remain in the unbound configuration as esters (conjugated) or aldehydes (non-conjugated).[Citation43] Although bound phenolic acids adhere to the cell walls (lignin) as structural components through molecular bonds,[Citation36] and their separation demands an acidic or basic environment and elevated temperature to destroy the molecular bonding.[Citation44,Citation45] In sorghum, around 70% to 95% of phenolic acids have bound configuration. One of them, ferulic acid (200 to 600 μg/g in the kernel) is uncountable and can add about 90% of the combined phenolic acids.[Citation36] Due to substantial molecular bonding tolerant to enzymatic metabolism, phenolic acids have less absorption ability.[Citation45]
Flavonoids
Flavonoids are predominantly present in the outer layer of sorghum. The varieties and amounts are analogous to the exocarp color, density and existence of stained seed coat.[Citation46] In plants, they are an essential group of phenolic components and exhibit widespread and divergent phenolic substances in sorghum.[Citation43] Flavonoids are the key flavan skeleton linked to insaturation at C2–C3 and branches at the beginning.[Citation47] A complete class of flavonoids has been distinguished in sorghum containing phytochemicals (3-deoxyanthocyanidins), flavones, flavanones, flavan-3-ols, flavan-4-ols, flavonols, and dihydroflavonols.[Citation48] A portion of the flavonoids, 3-deoxyanthocyanidins, flavones and flavanones are the huge segments in sorghum.[Citation49]
Tannins (proanthocyanidins)
Tannins are phenolic chemicals found in many plant species that are typically used as a defensive strategy against infections and herbivores.[Citation43] Some major grains, including wheat, rice and corn do not contain these compounds, but sorghum is a rich source of these compounds.[Citation50] Sorghum controls the number of condensed tannins in genes such as S and Tannin-1.[Citation51] Different oligomers and polymers in sorghum tannins vary in composition, amount, and distribution.[Citation16] Type I (no detectable levels), type II (tannins that can only be recovered in acidic alcohol), and type three (tannins that can only be extracted in acidified methanol) are the three categories.[Citation52] Flavan-3-ols and/or flavan-3, 4-diols (catechin oligomers or polymers) make up almost all sorghum’s tannins.[Citation53] Tannins from sorghum have a high degree of crystallinity and molecular weight of more than 10 (69 to 81%). Sorghum tannin concentration ranges from 0.2 to 48.0 mg/g, with black testa sorghum having the greatest tannin level.[Citation54] On the other hand, the season can affect both the content and activity of tannins in sorghum. When selecting and breeding tannin-containing sorghum genotypes to achieve health advantages, the influence of the environment on tannins should be taken into account.[Citation32]
Stilbenes
Stilbenes are a short group of phenolic compounds that have multiple consequences for good human health and plant disorders protection through phenylpropanoid development.[Citation55] The total content of stilbenes is the granule color, and in small quantities is available in white species. Trans-piceid (equal to 0.1 mg/kg) is found in white sorghum extremely low, while trans-resveratrol is missing in red sorghum.[Citation56]
Vitamins and minerals
Various types of vitamins and minerals are present in sorghum. The important vitamins in sorghum are vitamins B-complex such as pyridoxine, riboflavin and thiamine, and fat-soluble vitamins including A, D, E & K.[Citation10] Sorghum is a good mineral source such as phosphorus, potassium, iron and zinc.[Citation32] It is identified that the availability of iron varies from 6.6% to 15.7% while zinc availability ranges from 9.7% to 17.1%.[Citation57] The study was conducted to improve iron or zinc and bioavailability through biological fortification, fortification, and the genetic transformation of sorghum.[Citation48]
Uses of sorghum
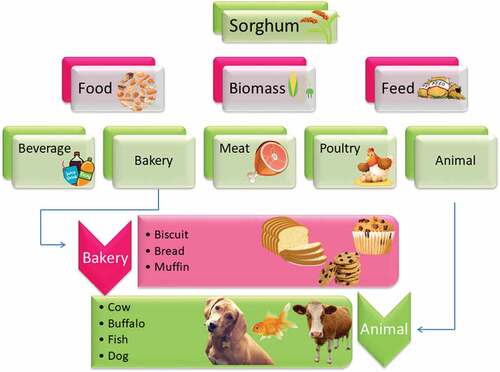
Sorghum is being used as a food item and introduced into other foods to enhance the performance, nutrition, and health functions of foods and make functional foods. Pasta, a famous international staple meal consisting of durum wheat semolina, may use sorghum as a possible health functional component.[Citation58] For example, replacing 20% to 40% of the semolina in pasta with sorghum whole grain flour significantly enhances the pasta’s complex carbohydrates, phenolic compounds, and antioxidant properties. While large quantities of sorghum meal have proven to reduce customer acceptance and sensory characteristics. It is feasible to create pasta of equivalent tactile quality while keeping up sustenance and medical advantages if sorghum flour is used at 20% – 30%. The health benefits of sorghum-based spaghetti differ depending on the sorghum crop used.[Citation59] In addition to light sorghum in phenolic content, consuming linguine made with red sorghum (high in phenolic compounds) improved people’s health by enhancing the phenolic content of plasma and the ability to antioxidize.[Citation60] Sorghum may also be used to make muffins that have been demonstrated to have significant levels of complex carbohydrates and poorly digesting carbohydrates.[Citation61] Sorghum may also be used in Chinese steamed bread to boost its health benefits. Wu et al.[Citation62] have discovered that the full-grain sorghum flour, either white or red, works well in place of wheat flour in bread, increasing phenolic compound and antioxidant properties considerably. Sorghum is poorly used and is one of the most commonly used kernels in animal feed. Some sorghums, especially tannins, can have anti-nutritional characteristics and impede improved feed productivity.[Citation63]
On the other hand, sorghum might be used as a starch substitute in pet food formulations. The scent intensity may not be as high as the commercial version, which means other components need to be included to make it even more pleasant. Nonetheless, food for dogs manufactured with sorghum fractions has a unique nutritional profile comparable to conventional food for dogs and pet and pet owner demand.[Citation63]
Sorghum might be used as an animal feed ingredient to increase animal health and productivity. The grain of sorghum distillers is an ethanol by-product that proved helpful as a food additive for pigs and rabbits.[Citation64] Sorghum distillers’ crop is a low-cost resource high in immune activators, which are produced during processing and help mammals’ immunity.[Citation65,Citation66] According to research, adding 400 to 450 g/kg sorghum distillers’ grain to the pig diet boosted back fat levels while having no detrimental influence on growth or carcass production.[Citation65] Similarly, adding 65 g/kg sorghum distillers in the rabbit diet will not harm growth or carcass quality.[Citation64] Grain decortication is a simple way to obtain bran. It may then be utilized in food items as a natural colorant and antioxidant preservative to enhance food feature and sustainability. Its capabilities in foods may be achieved at a lower rate of usage. The prepackaged pork will be supplemented with 0.15 to 0.65% high tannin sorghum bran. The bran sorghum can give a deeper color and a sorghum flavor to meat products and give them a darkened color and an aroma of sorghum. It is not necessarily an indication of poor customer consent or meat quality. The use of organic ingredients to improve food quality while preserving the sensory quality could be an exciting future field of study.[Citation67] shows the potential uses of sorghum.
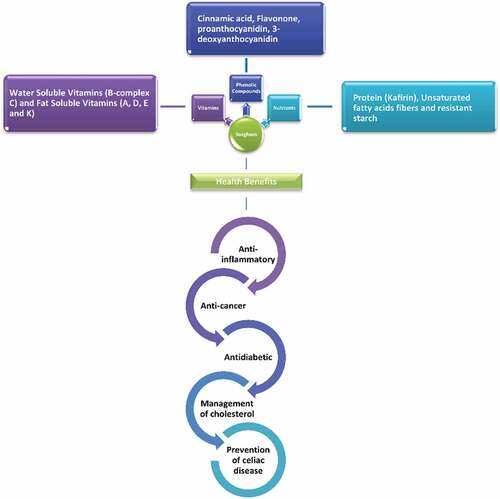
Potential impact of sorghum on the human health
Among cereals, sorghum is composed of some phytochemicals in which phenolic acids, policosanols, anthocyanins, tannins and phytosterols are involved. These phytochemicals play an essential role in human health. Sorghum has more antioxidant activity than other cereals, reducing the possibility of cancer. Sorghum-related phytochemical tannins are reduced by obesity as they reduce caloric value. The consumption of sorghum reduces one of the world’s leading cardiovascular diseases (CVDs).[Citation68] ().
Reduction of inflammation
The fundamental phenolic segments recognized in sweet sorghum syrup are phenolic acids, which incorporate high ellagic acid, erucic acid, and protocatechuic acid. Sorghum tannins can benefit human eating since they have anti-cancer effects that mitigate and decrease starch consumption.[Citation69]
Sorghum alleviation assessment is still in its earliest stages, but the results are empowering.[Citation70] Adding entire red grains that don’t contain tannins or their lipid parts to a high-fat eating routine can lessen the statement of TNF-α in rodents.[Citation71] The practical advantages of consuming whole sorghum and its derivatives are unclear, but the longer expression of adiponectin, which hampers the fiery marker, maybe somewhat expected. However, the tannin-rich sorghum decreases the development of edema in rodents, reducing cyclooxygenase 2 (COX-2), which reduces vascular permeability and the edema combined with neutrophil invasions.[Citation72] In this way, results of in-vitro studies show that the relaxing effects of sorghum are derived from their protein impact, while cytokines are mainly affected.[Citation73]
Role in cancer prevention
Cancer progression in people is subject to the effects of phase I (cytochrome P-450), and phase II catalysts that also lack endogenous and ecological carcinogens.[Citation74] Sorghum has a chemical prevention impact on phase-II proteins, as mentioned in the past, and especially the benefits of NQO reductase. However, it is difficult to deduce its effect on people because of the lack of examination. There is epidemiological evidence that it can prevent human malignancy.[Citation75] Sorghum as a staple food has expanded in dark South Africa’s rate of esophageal malignancy.[Citation76] The mechanism through which sorghum reduces the risk of human malignant esophageal growth remains unclear.[Citation77,Citation78] For malignant growth cells, sorghum 3-desocyanidins are more cytotoxic than anthocyanins in food sources (cyanide and pelargonidin).[Citation36,Citation79] Despite 3-deoxyantocyanidins, the estrogenic effect of sorghum flavonoids causes colon malignancy apoptosis.[Citation76,Citation77]
The phenolic sorghum systems intensify in-vitro cell apoptosis by the over-expression of apoptotic qualities and proteins (BAX and BAK protein and p53 quality articulation), improved enzymatic action (enactment of caspase-9 and caspase-3),[Citation80] and suppression of antiapoptosis.[Citation76] Phenolic compounds in sorghum result in repression of cell malignancies and metastasis by decreasing STAT5 and STAT3 phosphorylation, increasing insulin-like factor 1 (IGF-1 R) expression, and developing vascular endothelial growth factor (VEGF).[Citation36] In addition, sorghum contains tannins, which can behave as anti-cancer agents, despite 3-deoxyantocyanins. Various indications have shown the effect of compounds on tannins isolated from different food sources; they can activate apoptosis and block signal transduction routes. Thus, they have drawn wide consideration in malignant growth treatment.[Citation81] However, sorghum tannins should be better concentrated. A novel report found the in vitro, confined function of human aromatase (CYP 19) with tannin-rich sumac and sorghum wheat more grounded than dark sorghum-rich 3-deoxyantocyanine grain. Sumac sorghum tannins are widely inhibitors of, repressing and triggering aromatase than 3 deoxyantocyanidins found in dark sorghum.[Citation82] The effect of sorghum on the body against cancer was not investigated. In the past, cell development and metastasis of mice malignant cells (MDA-MB 231 and MCF-7) were reduced after applying subcutaneous rich sorghum methanolic extract.[Citation83] In addition, an abundance of sorghum in tannins leads to colon cell apoptosis.[Citation71]
Diabetes
Diabetes is a disease in which blood sugar level goes too high from average. A previous study showed that sorghum contains some components (phenolic compounds) that help in the glucose metabolism of animals.[Citation71] In mouse studies, intake of sorghum phenolic extracts may reduce the area under the blood sugar and glucose curve.[Citation84] Because of its strong effect on plasma glucose and insulin, researchers have shown that the hypoglycemic effect of phenolic extracts of sorghum is similar to that of the hypoglycemic agent glibenclamide used in the control group.[Citation71] The mechanism of action of phenolic compounds in sorghum involves metabolic pathways before and after carbohydrate absorption, which can help prevent and treat human blood glucose abnormalities. These extracts were recently confirmed to inhibit the in vitro activities of Bacillus stearothermophilus α-glucosidase and human pancreas and saliva α-amylase.[Citation84] Therefore, the central system of sorghum activity in human digestion may be to reduce the progression of glucose processing through chemical resistance. Results of exploration show that phenolic mixtures also influence the dependent pathways of insulin, recalling the chemical’s binding and sensitivity to the human body. Diabetic mice with extracts of phenolic compounds were shown to increase insulin concentration.[Citation85]
The development shows improved functioning of the β-cells and is of clinical importance, especially in those with type 2 diabetes with insulin action decreased. In addition, oral administration of sorghum phenolic extracts can prevent diabetes by improving insulin sensitivity and treating diabetes.[Citation71] This theory is based on how mice handle the intake of a high-fat diet, extract of sorghum phenolic compounds causing antidiabetic effects, increasing their adiponectin utilization and decrease their TNF-α by overexpression of PPAR-γ. The study’s outcomes showed that the stripped sorghum grain contains a lot of flavonoids, which are fundamental for infection control and prevention. Anti-diabetes and anti-obesity effects of these sorghum grains with flavonoids as the result of their practical components and food development are expected for further research in animal models.[Citation86]
Cardiovascular diseases and dyslipidemia inhibition
A considerable amount of bioactive phenolic compounds is found in sorghum grains. However, the protective effects of sorghum against dyslipidemia and CVDs are contributed by phenolic chemicals. Sorghum lipids (phytosterols and policosanols) have improved cardiovascular health by controlling synthesis, absorption and elimination of cholesterol.[Citation87] For instance, incorporating sorghum lipids into hamsters’ diets improved cholesterol elimination and lowered cholesterol levels in the liver.[Citation88] Similarly, the effect of these lipids on intestinal microbiota (including lowering the Coriobacteriacea family to reduce cholesterol absorption) is also evident.[Citation89]
Various studies revealed the role of sorghum in cholesterol metabolism. According to studies conducted in the past, the treatment of sorghum phenolic extract dramatically reduced plasma cholesterol and triglycerols level in hyperlipidemic or diabetic rats.[Citation84] As a result, the entire grain of sorghum and its components and isolated phenolic sorghum compounds seemed to bring different health benefits. Scientific literature concluded that sorghum has recently been linked to reducing blood pressure. Moreover, a separate sorghum α-kafirin reduced the angiotensin I converting enzyme activity in both competitive and noncompetitive ways.[Citation90]
Celiac disease
Celiac is an asymptomatic disease in individuals with wheat protein and gluten sensitivity. In general, rye and barley are dietary nutrients, and wheat contains gluten. However, gluten can harm your small intestines if you have a celiac disease. It can make it difficult for vitamins and other nutrients to assimilate.[Citation91]
Recently, the entire record of food industries is the improvement of new, biologically active and healthy foodstuffs and drinks, and several sorghum-based foods have been produced and analyzed. In contrast to other cereals such as barley and a significant quantity of gluten, sorghum is recognized as a gluten-free source. It promotes the use of a harmless substitute for those with celiac infection. After eating sorghum food, there was no sign of any gastrointestinal discomfort in people with celiac problems.[Citation92] Sorghum is a potential substitute for gluten-containing food. As a store of phenolic components and nutrients that are generally not available in many foods, it also contributes to the significant demand in the West and developing countries for good gluten-containing replacement diets.
The production of gluten-free beers for persons with celiac disease is also expected to provide sorghum as a safe source. Based on the study, white sorghum beer contained two times more phenolic compounds than barley beer, showing its higher antioxidant effects. This beer also carried remarkable quantities of γ -aminobutyric acids with viable antihypertensive actions and had α-glucosidase inhibitory effects.[Citation93–95]
Future perspectives
Sorghum’s potential involvement in the prevention and treatment of chronic diseases has to be investigated further. The lipid profile of sorghum, which is high in unsaturated fatty acids, may provide extra lipid-lowering properties. Sorghum, being a fiber-rich food, is likely to provide a feeling of fullness. It’s also worth remembering that in order for functional foods to offer their possibly subtle advantages, they must be consumed on a regular basis. To prove that sorghum foods have potentially beneficial effects on chronic lifestyle-related disease or risk factors, rigorous scientific investigation in human studies is required. Future Randomize control Trials (RCTs) should try to compare the effects of a control and sorghum-intervention diet on chronic disease biomarkers or health outcomes, allowing evidence for longer-term benefits to emerge. The influence of the complete meal reflecting the synergy between the components must be evaluated, since most of the research to date has focused on extracts and components. Background diet may confuse findings and make it difficult to assign effects to the dietary variable of interest, thus it’s equally vital to investigate the composition of the background diet. Furthermore, the influence of sorghum-based foods on chronic lifestyle-related illness must be considered in the context of the whole diet and thoroughly monitored throughout the trial for translation to practice. Unfortunately, the investigations about sorghum are restricted in their capacity to assign direct antioxidant benefits of sorghum because they do not take into consideration metabolic transformations and interactions that affect polyphenol bioavailability and biological activity in the body after intake. It’s crucial to figure out if the concentrations of sorghum grain extracts employed in these experiments can be eaten safely via dietary consumption of sorghum-based foods. Regardless, studying the impact of diet on cancer is difficult, and clinical studies are troublesome for ethical reasons. Nonetheless, information gained from many types of experimental study contributes to a broader understanding of how the sorghum food matrix may be advantageous.
Conclusion
Sorghum (Sorghum bicolor) is an underused cereal whole grain that may help avoid chronic lifestyle illnesses, especially in areas where associated morbidity and death rates are important public health costs. Sorghum has heavily resisted starch and fiber, bioactive compounds, and kafirin protein. This review identifies that sorghum is utilized in food, feed, and biomass production in separate contexts. Sorghum’s bioactivity is influenced by phenolic compounds such as phenolic acid, flavonoids, stilbenes, and tannins, vitamins such as B-complex, A, D, E, and K, and minerals such as potassium, phosphorus, magnesium, and zinc. Sorghum’s functional composition significantly impacts human health by reducing the risk of chronic illnesses like diabetes and CVDs. According to epidemiological studies, sorghum’s tannins (proanthocyanidins) have been shown to protect against inflammation and cancer. Celiac disease patients benefit from the high fiber content of this food. To expand on the positive in-vitro and animal studies undertaken to far, high-quality clinical research evaluating the effects of sorghum eating in people should be the next step. To give future directions for consumers, the food business, producers, health experts, and government-based grain advocacy groups, human proof supporting the long-term impacts of eating sorghum as part of a healthy diet is required. If proved beneficial, high-quality data from human studies might position sorghum as a key source of treatment for a variety of chronic diseases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- FAOSTAT FAO Statistics Division. http://faostat.fao.org/2020 ( accessed June 30, 2020).
- Dahlberg, J. The Role of Sorghum in Renewables and Biofuels. Sorghum. 2019, 269–277. DOI: 10.1007/978-1-4939-9039-9_19.
- Stutts, L. R.; Vermerris, W. Elucidating Anthracnose Resistance Mechanisms in Sorghum—A Review. Phytopathology®. 2020, 110(12), 1863–1876. DOI: 10.1094/PHYTO-04-20-0132-RVW.
- Ratnavathi, C. V.; Patil, J. V.; Chavan, U. D. Sorghum Biochemistry: An Industrial Perspective; Academic Press, 2016. DOI: 10.1016/B978-0-12-803157-5.00001-0.
- Waniska, R. D.; Rooney, L. W. Structure and Chemistry of the Sorghum Caryopsis. Sorghum. 2000, 2, 649–679.
- Slavin, J. Whole Grains and Human Health. Nutr. Res. Rev. 2004, 17(1), 99–110. DOI: 10.1079/NRR200374.
- Amarakoon, D.; Lou, Z.; Lee, W. J.; Smolensky, D.; Lee, S. H. A Mechanistic Review: Potential Chronic Disease‐preventive Properties of Sorghum. J. Sci. Food Agric. 2021, 101(7), 2641–2649. DOI: 10.1002/jsfa.10933.
- Hill, H.; Lee, L. S.; Henry, R. J. Variation in Sorghum Starch Synthesis Genes Associated with Differences in Starch Phenotype. Food Chem. 2012, 131(1), 175–183. DOI: 10.1016/j.foodchem.2011.08.057.
- Dicko, M. H.; Gruppen, H.; Zouzouho, O. C.; Traoré, A. S.; Van Berkel, W. J.; Voragen, A. G. Effects of Germination on the Activities of Amylases and Phenolic Enzymes in Sorghum Varieties Grouped according to Food End‐use Properties. J. Sci. Food Agric. 2006, 86(6), 953–963. DOI: 10.1002/jsfa.2443.
- USDA. National Nutrient Database for Standard Reference Legacy Release: Full Report (All Nutrients) 20067, Sorghum Grain. 2019. https://ndb.nal.usda.gov/ndb/foods/show/20067?n1=%7BQv%3D1%7D&fgcd=&man=&lfacet=&count=&max=25&sort=default&qlookup=sorghum&offset=&format=Full&new=&measureby=&Qv=1&ds=&qt=&qp=&qa=&qn=&q=&ing=
- Palavecino, P. M.; Penci, M. C.; Calderón‐Domínguez, G.; Ribotta, P. D. Chemical Composition and Physical Properties of Sorghum Flour Prepared from Different Sorghum Hybrids Grown in Argentina. Starch‐Stärke. 2016, 68(11–12), 1055–1064. DOI: 10.1002/star.201600111.
- Mohammed, N. A.; Ahmed, I. A. M.; Babiker, E. E. Nutritional Evaluation of Sorghum Flour (Sorghum Bicolor L. Moench) during Processing of Injera. 2011. http://khartoumspace.uofk.edu/123456789/22249
- Udachan, I. S.; Sahu, A. K.; Hend, F. M. Extraction and Characterization of Sorghum (Sorghum Bicolor L. Moench) Starch. Int. Food Res. J. 2012, 19(1), 315–319.
- Martino, H. S. D.; Tomaz, P. A.; Moraes, E. A.; Concei¸c~ao, L. L.; Oliveira, D. S.; Queiroz, V. A. V.; Rodrigues, J. A. S.; Pirozi, M. R.; Pinheiro-Sant’Ana, H. M.; Ribeiro, M. R. Chemical Characterization and Size Distribution of Sorghum Genotypes for Human Consumption. Rev. Inst. Adolfo. Lutz. 2012, 71, 337–344.
- Sang, Y.; Bean, S.; Seib, P. A.; Pedersen, J.; Shi, Y. C. Structure and Functional Properties of Sorghum Starches Differing in Amylose Content. J. Agric. Food Chem. 2008, 56(15), 6680–6685. DOI: 10.1021/jf800577x.
- Mkandawire, N. L.; Kaufman, R. C.; Bean, S. R.; Weller, C. L.; Jackson, D. S.; Rose, D. J. Effects of Sorghum (Sorghum Bicolor (L.) Moench) Tannins on α-amylase Activity and in Vitro Digestibility of Starch in Raw and Processed Flours. J. Agric. Food Chem. 2013, 61(18), 4448–4454. DOI: 10.1021/jf400464j.
- Taylor, J. R.; Emmambux, M. N. Developments in Our Understanding of Sorghum Polysaccharides and Their Health Benefits. Cereal Chem. 2010, 87(4), 263–271. DOI: 10.1094/CCHEM-87-4-0263.
- U.S. Department of Agriculture, A. R. S. USDA National Nutrient Database for Standard Reference. 25th ed.; Washington, DC, 2012. DOI: 10.1093/ajcn/88.2.324.
- Shegro, A.; Shargie, N. G.; van Biljon, A.; Labuschagne, M. T. Diversity in Starch, Protein and Mineral Composition of Sorghum Landrace Accessions from Ethiopia. J. Crop. Sci. Biotechnol. 2012, 15(4), 275–280. DOI: 10.1007/s12892-012-0008-z.
- Singh, H.; Sodhi, N. S.; Singh, N. Characterisation of Starches Separated from Sorghum Cultivars Grown in India. Food Chem. 2010, 119(1), 95–100. DOI: 10.1016/j.foodchem.2009.05.086.
- Barros, F.; Awika, J. M.; Rooney, L. W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in Vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60(46), 11609–11617. DOI: 10.1021/jf3034539.
- Shewry, P. R.; Tatham, A. S. The Prolamin Storage Proteins of Cereal Seeds: Structure and Evolution. J. Biochem. 1990, 267(1), 1. DOI: 10.1042/bj2670001.
- Belton, P. S.; Delgadillo, I.; Halford, N. G.; Shewry, P. R. Kafirin Structure and Functionality. J. Cereal Sci. 2006, 44(3), 272–286. DOI: 10.1016/j.jcs.2006.05.004.
- Pellett, P. L.; Ghosh, S. Lysine Fortification: Past, Present, and Future. Food Nutr. Bull. 2004, 25(2), 107–113. DOI: 10.1177/156482650402500201.
- Wu, Y.; Yuan, L.; Guo, X.; Holding, D. R.; Messing, J. Mutation in the Seed Storage Protein Kafirin Creates a High-value Food Trait in Sorghum. Nat. Commun. 2013, 4(1), 1–7. DOI: 10.1038/ncomms3217.
- Galili, G.; Amir, R. Fortifying Plants with the Essential Amino Acids Lysine and Methionine to Improve Nutritional Quality. Plant Biotechnol. J. 2013, 11(2), 211–222. DOI: 10.1111/pbi.12025.
- Henley, E. C.; Taylor, J. R. N.; Obukosia, S. D. The Importance of Dietary Protein in Human Health: Combating Protein Deficiency in sub-Saharan Africa through Transgenic Biofortified Sorghum. Adv. Food Nutr. Res. 2010, 60, 21–52. DOI: 10.1016/S1043-4526(10)60002-2.
- Oria, M. P.; Hamaker, B. R.; Axtell, J. D.; Huang, C. P. A Highly Digestible Sorghum Mutant Cultivar Exhibits A Unique Folded Structure of Endosperm Protein Bodies. PNAS. 2000, 97(10), 5065–5070. DOI: 10.1073/pnas.080076297.
- Mehlo, L.; Mbambo, Z.; Bado, S.; Lin, J.; Moagi, S. M.; Buthelezi, S.; Chikwamba, R.; Chikwamba, R. Induced Protein Polymorphisms and Nutritional Quality of Gamma Irradiation Mutants of Sorghum. Mutat. Res-Fund. Mol. M. 2013, 749(1–2), 66–72. DOI: 10.1016/j.mrfmmm.2013.05.002.
- Kumar, T.; Dweikat, I.; Sato, S.; Ge, Z.; Nersesian, N.; Chen, H.; Clemente, T.; Bean, S.; Ioerger, B. P.; Tilley, M. Modulation of Kernel Storage Proteins in Grain Sorghum (Sorghum Bicolor (L.) Moench). Plant Biotechnol. J. 2012, 10(5), 533–544. DOI: 10.1111/j.1467-7652.2012.00685.x.
- Taylor, J.; Bean, S. R.; Ioerger, B. P.; Taylor, J. R. Preferential Binding of Sorghum Tannins with γ-kafirin and the Influence of Tannin Binding on Kafirin Digestibility and Biodegradation. J. Cereal Sci. 2007, 46(1), 22–31. DOI: 10.1016/j.jcs.2006.11.001.
- Afify, A. E. M. M.; El-BELTAGI, H. S.; Abd El-Salam, S. M.; Omran, A. A. Oil and Fatty Acid Contents of White Sorghum Varieties under Soaking, Cooking, Germination and Fermentation Processing for Improving Cereal Quality. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2012, 40(1), 86–92. DOI: 10.15835/nbha4017585.
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Gill, S. C.; Tripathi, S. C.; Gupta, O. P.; Mangrauthia, S. K.; Sundaram, R. M.; Sawant, C. P. Characterization of Phenolic Compounds and Antioxidant Activity in Sorghum [Sorghum Bicolor (L.) Moench] Grains. Cereal Res. Commun. 2021, 1–11. DOI: 10.1007/s42976-020-00118-w.
- Arshad, M. S.; Khalid, W.; Ahmad, R. S.; Khan, M. K.; Ahmad, M. H.; Safdar, S.; Suleria, H. A. R. Functional Foods and Human Health: An Overview. Funct. Food. 2021, 3.
- Kang, J.; Price, W. E.; Ashton, J.; Tapsell, L. C.; Johnson, S. Identification and Characterization of Phenolic Compounds in Hydromethanolic Extracts of Sorghum Wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. DOI: 10.1016/j.foodchem.2016.05.052.
- Yang, L.; Allred, K. F.; Geera, B.; Allred, C. D.; Awika, J. M. Sorghum Phenolics Demonstrate Estrogenic Action and Induce Apoptosis in Nonmalignant Colonocytes. Nutr. Cancer. 2012, 64(3), 419–427. DOI: 10.1080/01635581.2012.657333.
- Zaroug, M.; Orhan, I. E.; Senol, F. S.; Yagi, S. Comparative Antioxidant Activity Appraisal of Traditional Sudanese Kisra Prepared from Two Sorghum Cultivars. Food Chem. 2014, 156, 110–116. DOI: 10.1016/j.foodchem.2014.01.069.
- Shen, J.; Pang, R.; Weiss, R. J.; Schuster, M.; Jaitly, N.; Yang, Z.; Wu, Y. Natural Tts Synthesis by Conditioning Wavenet on Mel Spectrogram Predictions. In 2018 IEEE international conference on acoustics, speech and signal processing (ICASSP), 2018, pp 4779–4783. IEEE.
- Xiong, Y.; Zhang, P.; Warner, R. D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18(6), 2025–2046. DOI: 10.1111/1541-4337.12506.
- Davidson, D. J. Effect of Different Methods and Timing of Nitrogen (N) Application on Sorghum (Sorghum bicolor L) Grain Yield. Doctoral dissertation, Oklahoma State University, 2019.
- Althwab, S.; Carr, T. P.; Weller, C. L.; Dweikat, I. M.; Schlegel, V. Advances in Grain Sorghum and Its Co-products as a Human Health Promoting Dietary System. Int. Food Res. 2015, 77, 349–359. DOI: 10.1016/j.foodres.2015.08.011.
- Vanamala, J. K.; Massey, A. R.; Pinnamaneni, S. R.; Reddivari, L.; Reardon, K. F. Grain and Sweet Sorghum (Sorghum Bicolor L. Moench) Serves as a Novel Source of Bioactive Compounds for Human Health. Crit. Rev. Food Sci. Nutr. 2018, 58(17), 2867–2881. DOI: 10.1080/10408398.2017.1344186.
- Dykes, L.; Rooney, L. W. Sorghum and Millet Phenols and Antioxidants. J. Cereal Sci. 2006, 44(3), 236–251. DOI: 10.1016/j.jcs.2006.06.007.
- Wu, G.; Bennett, S. J.; Bornman, J. F.; Clarke, M. W.; Fang, Z.; Johnson, S. K. Phenolic Profile and Content of Sorghum Grains under Different Irrigation Managements. Int. Food Res. J. 2017, 97, 347–355. DOI: 10.1016/j.foodres.2017.04.030.
- Chiremba, C.; Taylor, J. R.; Rooney, L. W.; Beta, T. Phenolic Acid Content of Sorghum and Maize Cultivars Varying in Hardness. Food Chem. 2012, 134(1), 81–88. DOI: 10.1016/j.foodchem.2012.02.067.
- Awika, J. M.; McDonough, C. M.; Rooney, L. W. Decorticating Sorghum to Concentrate Healthy Phytochemicals. J. Agric. Food Chem. 2005, 53(16), 6230–6234. DOI: 10.1021/jf0510384.
- Awika, J. M. Sorghum: Its Unique Nutritional and Health-promoting Attributes. Gluten-free Anci. Grains. 2017, 21–54. Wood head publishing, DOI: 10.1016/B978-0-08-100866-9.00003-0.
- de Morais Cardoso, L.; Montini, T. A.; Pinheiro, S. S.; Pinheiro-Sant’Ana, H. M.; Martino, H. S. D.; Moreira, A. V. B. Effects of Processing with Dry Heat and Wet Heat on the Antioxidant Profile of Sorghum. Food Chem. 2014, 152, 210–217. DOI: 10.1016/j.foodchem.2013.11.106.
- Buer, C. S.; Imin, N.; Djordjevic, M. A. Flavonoids: New Roles for Old Molecules. J. Integr. Plant Biol. 2010, 52(1), 98–111. DOI: 10.1111/j.1744-7909.2010.00905.x.
- Kaufman, R. C.; Herald, T. J.; Bean, S. R.; Wilson, J. D.; Tuinstra, M. R. Variability in Tannin Content, Chemistry and Activity in a Diverse Group of Tannin Containing Sorghum Cultivars. J. Sci. Food Agric. 2013, 93(5), 1233–1241. DOI: 10.1002/jsfa.5890.
- Wu, Y.; Li, X.; Xiang, W.; Zhu, C.; Lin, Z.; Wu, Y.; Yu, J.; Pandravada, S.; Ridder, D. D.; Bai, G. Presence of Tannins in Sorghum Grains Is Conditioned by Different Natural Alleles of Tannin1. PNAS. 2012, 109(26), 10281–10286. DOI: 10.1073/pnas.1201700109.
- Schons, P. F.; Ries, E. F.; Battestin, V.; Macedo, G. A. Effect of Enzymatic Treatment on Tannins and Phytate in Sorghum (Sorghum Bicolor) and Its Nutritional Study in Rats. Int. J. Food Sci. 2011, 46(6), 1253–1258. DOI: 10.1111/j.1365-2621.2011.02620.x.
- Awika, J. M.; Dykes, L.; Gu, L.; Rooney, L. W.; Prior, R. L. Processing of Sorghum (Sorghum Bicolor) and Sorghum Products Alters Procyanidin Oligomer and Polymer Distribution and Content. J. Agric. Food Chem. 2003, 51(18), 5516–5521. DOI: 10.1021/jf0343128.
- Price, M. L.; Van Scoyoc, S.; Butler, L. G. A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26(5), 1214–1218. DOI: 10.1021/jf60219a031.
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and Roles of Stilbenes in Plants. Plant Sci. 2009, 177(3), 143–155. DOI: 10.1016/j.plantsci.2009.05.012.
- Bröhan, M.; Jerkovic, V.; Collin, S. Potentiality of Red Sorghum for Producing Stilbenoid-enriched Beers with High Antioxidant Activity. J. Agric. Food Chem. 2011, 59(8), 4088–4094. DOI: 10.1021/jf1047755.
- Kumar, A. A.; Reddy, B. V.; Ramaiah, B.; Sahrawat, K. L.; Pfeiffer, W. H. Gene Effects and Heterosis for Grain Iron and Zinc Concentration in Sorghum [Sorghum Bicolor (L.) Moench]. Field Crops. Res. 2013, 146, 86–95. DOI: 10.1016/j.fcr.2013.03.001.
- Khan, I.; Yousif, A.; Johnson, S. K.; Gamlath, S. Effect of Sorghum Flour Addition on Resistant Starch Content, Phenolic Profile and Antioxidant Capacity of Durum Wheat Pasta. Int. Food Res. J. 2013, 54(1), 578–586. DOI: 10.1016/j.foodres.2013.07.059.
- Khan, I.; Yousif, A. M.; Johnson, S. K.; Gamlath, S. Effect of Sorghum Flour Addition on in Vitro Starch Digestibility, Cooking Quality, and Consumer Acceptability of Durum Wheat Pasta. J. Food Sci. 2014, 79(8), S1560–S1567. DOI: 10.1111/1750-3841.12542.
- Khan, I.; Yousif, A. M.; Johnson, S. K.; Gamlath, S. Acute Effect of Sorghum Flour-containing Pasta on Plasma Total Polyphenols, Antioxidant Capacity and Oxidative Stress Markers in Healthy Subjects: A Randomised Controlled Trial. Clin. Nutr. 2015, 34(3), 415–421. DOI: 10.1016/j.clnu.2014.08.005.
- Poquette, N. M.; Gu, X.; Lee, S. O. Grain Sorghum Muffin Reduces Glucose and Insulin Responses in Men. Food Funct. 2014, 5(5), 894–899. DOI: 10.1039/C3FO60432B.
- Wu, G.; Shen, Y.; Qi, Y.; Zhang, H.; Wang, L. I.; Qian, H.; Johnson, S. K. Improvement of in Vitro and Cellular Antioxidant Properties of Chinese Steamed Bread through Sorghum Addition. LWT. 2018, 91, 77–83. DOI: 10.1016/j.lwt.2017.12.074.
- Di Donfrancesco, B.; Koppel, K.; Aldrich, C. G. Pet and Owner Acceptance of Dry Dog Foods Manufactured with Sorghum and Sorghum Fractions. J. Cereal Sci. 2018, 83, 42–48. DOI: 10.1016/j.jcs.2018.07.011.
- García, A. C.; Hernández, V. M.; Bonet, J.; Coma, J.; Andrés, M. L. Effects of Inclusion of Sorghum Distillers Dried Grains with Solubles (DDGS) in Diets for Growing and Finishing Pigs. Span. J. Agric. Res. 2012, 4, 1016–1024.
- Yang, Y.; Shen, Y.; Pan, Y.; Xia, P.; Zhang, D.; He, Z.; Lu, J.; Li, H.; Lu, J. Effects of Dietary Sorghum Dried Distiller’s Grains with Solubles on Growth Performance, Diet Nutrient Digestibility, Carcass Characteristics and Immunity in Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103(1), 363–369. DOI: 10.1111/jpn.13008.
- Pomerenke, J. L.; Souza, L. W. O.; Shurson, G. C. Concentrations of β-glucans and Mannan Oligosaccharides in Corn Dried Distillers Grains with Soluble (DDGS) and Its Relationship to Fiber Components. J. Anim. Sci. 2004, 93(Suppl. 1), 206.
- Cabral, A. R.; Waters, C.; Laird, H. L.; Cavitt, L. C.; Miller, R. K.; Rooney, W. L.; Kerth, C. R. Sorghum Bran as an Antioxidant in Pork and Poultry Products. Muscle Biol. 2018, 2(2), 2010. DOI: 10.221751/rmc2018.073.
- Awika, J. M.; Rooney, L. W.; Waniska, R. D. Properties of 3-deoxyanthocyanins from Sorghum. J. Agric. Food Chem. 2004, 52(14), 4388–4394. DOI: 10.1021/jf049653f.
- Palacios, C. E.; Nagai, A.; Torres, P.; Rodrigues, J. A.; Salatino, A. Contents of Tannins of Cultivars of Sorghum Cultivated in Brazil, as Determined by Four Quantification Methods. Food Chem. 2021, 337, 127970. DOI: 10.1016/j.foodchem.2020.127970.
- Moraes, É. A.; Natal, D. I. G.; Queiroz, V. A. V.; Schaffert, R. E.; Cecon, P. R.; de Paula, S. O.; Martino, H. S. D.; Ribeiro, S. M. R.; Martino, H. S. D. Sorghum Genotype May Reduce Low-grade Inflammatory Response and Oxidative Stress and Maintains Jejunum Morphology of Rats Fed a Hyperlipidic Diet. Int. Food Res. J. 2012, 49(1), 553–559. DOI: 10.1016/j.foodres.2012.07.029.
- Kim, J.; Park, Y. Anti-diabetic Effect of Sorghum Extract on Hepatic Gluconeogenesis of Streptozotocin-induced Diabetic Rats. Nutr. Metab. 2012, 9(1), 1–7. DOI: 10.1186/1743-7075-9-106.
- Shim, T. J.; Kim, T. M.; Jang, K. C.; Ko, J. Y., and Kim, D. J. Toxicological Evaluation and Anti-inflammatory Activity of a Golden Gelatinous Sorghum Bran Extract. Biosci. Biotechnol. Biochem. 2013 DOI: 10.1271/bbb.120731.
- Burdette, A.; Garner, P. L.; Mayer, E. P.; Hargrove, J. L.; Hartle, D. K.; Greenspan, P. Anti-inflammatory Activity of Select Sorghum (Sorghum Bicolor) Brans. J. Med. Food. 2010, 13(4), 879–887. DOI: 10.1089/jmf.2009.0147.
- Takabe, W.; Matsukawa, N.; Kodama, T.; Tanaka, K.; Noguchi, N. Chemical Structure–dependent Gene Expression of Proteasome Subunits via Regulation of the Antioxidant Response Element. Free Radic. Res. 2006, 40(1), 21–30. DOI: 10.1080/10715760500354430.
- Awika, J. M.; Yang, L.; Browning, J. D.; Faraj, A. Comparative Antioxidant, Antiproliferative and Phase II Enzyme Inducing Potential of Sorghum (Sorghum Bicolor) Varieties. LWT. 2009, 42(6), 1041–1046. DOI: 10.1016/j.lwt.2009.02.003.
- Woo, H. J.; Oh, I. T.; Lee, J. Y.; Seu, M. C.; Woo, K. S.; Nam, M. H.; Kim, Y. H.; Kim, Y. H. Apigeninidin Induces Apoptosis through Activation of Bak and Bax and Subsequent Mediation of Mitochondrial Damage in Human Promyelocytic Leukemia HL-60 Cells. Process Biochem. 2012, 47(12), 1861–1871. DOI: 10.1016/j.procbio.2012.06.012.
- Shih, C. H.; Siu, S. O.; Ng, R.; Wong, E.; Chiu, L. C.; Chu, I. K.; Lo, C. Quantitative Analysis of Anticancer 3-deoxyanthocyanidins in Infected Sorghum Seedlings. J. Agric. Food Chem. 2007, 55(2), 254–259. DOI: 10.1021/jf062516t.
- Suganyadevia, P.; Saravanakumara, K. M.; Mohandasb, S. Identification of 3- Deoxyanthocyanins from Red Sorghum (Sorghum Bicolor) Bran and Its Biological Properties. Afr. J. Pure Appl. Chem. 2011, 5, 181–193.
- Hwang, J. M.; Choi, K. C.; Bang, S. J.; Son, Y. O.; Kim, B. T.; Kim, D. H.; Lee, J. C.; Kim, D. H.; Shi, X.; Lee, J.-C. Anti-oxidant and Anti-inflammatory Properties of Methanol Extracts from Various Crops. Food Sci. Biotechnol. 2013, 22(1), 265–272. DOI: 10.1007/s10068-013-0076-y.
- Suganyadevia, P.; Saravanakumara, K. M.; Mohandasb, S. Evaluation of Antiproliferative Activity of Red Sorghum Bran Anthocyanin on a Human Breast Cancer Cell Line (MCF-7). Int. J. Breast Cancer. 2011, 1–6.
- Huang, W. Y.; Cai, Y. Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer. 2009, 62(1), 1. DOI: 10.1080/01635580903191585.
- Dowsett, M.; Cuzick, J.; Ingle, J.; Coates, A.; Forbes, J.; Bliss, J.; Peto, R.; Baum, M.; Buzdar, A.; Colleoni, M. Meta-analysis of Breast Cancer Outcomes in Adjuvant Trials of Aromatase Inhibitors versus Tamoxifen. Am. J. Clin. Oncol. 2010, 28(3), 509–518. DOI: 10.1200/JCO.2009.23.1274.
- Lewis, J. B. Effects of bran from sorghum grains containing different classes and levels of bioactive compounds in colon carcinogenesis. Doctoral dissertation, Texas A & M University, 2010.
- Chung, I. M.; Kim, E. H.; Yeo, M. A.; Kim, S. J.; Seo, M. C.; Moon, H. I. Antidiabetic Effects of Three Korean Sorghum Phenolic Extracts in Normal and Streptozotocin-induced Diabetic Rats. Int. Food Res. J. 2011, 44(1), 127–132. DOI: 10.1016/j.foodres.2010.10.051.
- Kim, J. S.; Hyun, T. K.; Kim, M. J. The Inhibitory Effects of Ethanol Extracts from Sorghum, Foxtail Millet and Proso Millet on α-glucosidase and α-amylase Activities. Food Chem. 2011, 124(4), 1647–1651. DOI: 10.1016/j.foodchem.2010.08.020.
- Ofosu, F. K.; Elahi, F.; Daliri, E. B. M.; Yeon, S. J.; Ham, H. J.; Kim, J. H.; Oh, D. H.; Oh, D.-H. Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules. 2020, 25(12), 2854. DOI: 10.3390/molecules25122854.
- Carr, T. P.; Weller, C. L.; Schlegel, V. L.; Cuppett, S. L.; Guderian, D. M., Jr; Johnson, K. R. Grain Sorghum Lipid Extract Reduces Cholesterol Absorption and Plasma non-HDL Cholesterol Concentration in Hamsters. J. Nutr. 2005, 135(9), 2236–2240. DOI: 10.1093/jn/135.9.2236.
- Hoi, J. T.; Weller, C. L.; Schlegel, V. L.; Cuppett, S. L.; Lee, J. Y.; Carr, T. P. Sorghum Distillers Dried Grain Lipid Extract Increases Cholesterol Excretion and Decreases Plasma and Liver Cholesterol Concentration in Hamsters. J. Funct. Foods. 2009, 1(4), 381–386. DOI: 10.1016/j.jff.2009.09.005.
- Martínez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A. K.; Carr, T. P.; Walter, J.; Walter, J. Diet-induced Metabolic Improvements in a Hamster Model of Hypercholesterolemia are Strongly Linked to Alterations of the Gut Microbiota. Appl. Environ. Microbiol. 2009, 75(12), 4175–4184. DOI: 10.1128/AEM.00380-09.
- Kamath, V.; Niketh, S.; Chandrashekar, A.; Rajini, P. S. Chymotryptic Hydrolysates of α-kafirin, the Storage Protein of Sorghum (Sorghum Bicolor) Exhibited Angiotensin Converting Enzyme Inhibitory Activity. Food Chem. 2007, 100(1), 306–311. DOI: 10.1016/j.foodchem.2005.10.004.
- Green, P. H.; Lebwohl, B.; Greywoode, R. Celiac Disease. J. Allergy Clin. Immunol. 2015, 135(5), 1099–1106. DOI: 10.1016/j.jaci.2015.01.044.
- Koehler, P., and Wieser, H. Chemistry of Cereal Grains. In Handbook on Sourdough Biotechnology; Springer: Boston, MA, 2013; pp 11–45. 2013. DOI: 10.1007/978-1-4614-5425-0_2.
- Garzón, A. G.; Torres, R. L.; Drago, S. R. Changes in Phenolics, γ‐aminobutyric Acid Content and Antioxidant, Antihypertensive and Hypoglycaemic Properties during Ale White Sorghum (Sorghum Bicolor (L.) Moench) Brewing Process. Int. J. Food Sci. 2019, 54(5), 1901–1908. DOI: 10.1111/ijfs.14102
- Babar, Q.; Ali, A.; Saeed, A., and Tahir, M. F. 2021. Novel Treatment Strategy against COVID-19 through Anti-Inflammatory, Antioxidant and Immunostimulatory Properties of the B Vitamin Complex. Intechopen. DOI: 10.5772/intechopen.100251.
- Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; Ahmed, M.; Bostani, A. Bio-Molecular Characteristics of Whey Proteins with Relation to Inflammation. Intechopen. DOI: 10.5772/intechopen.99220.