ABSTRACT
‘Lady Finger’ banana is one of the most-produced bananas in Indonesia. Unfortunately, it has a short fruit shelf life, which became a major problem in post-harvest handling. Shelf life in climacteric fruits can be improved through the inhibition of physiological processes such as ethylene production, respiration, and transpiration by the application of 1-Methylcyclopropene (1-MCP) and chitosan. Physiological process inhibition will influence fruit color, firmness, sugar content, and other postharvest qualities. The potential use of 1-MCP, chitosan, and a combination of both toward postharvest life quality of ‘Lady Finger’ banana were assessed in this study. Four concentrations of 1-MCP (0.25, 0.50, 0.75, and 1.00 µL/L) and also its combination with 1.25% chitosan were applied to the ‘Lady Finger’ banana. The result showed that the combination of 1-MCP and chitosan improved the postharvest life quality of the ‘Lady Finger’ banana. The combination of 1-MCP (0.5–1.0 µL/L) and chitosan increased fruit shelf life up to 5–6 days longer compared to control and inhibited the change of fruit pH, TA, TSS, total sugar, color index, and weight loss. These results indicate that the effect of 1-MCP will be more effective in improving the fruit shelf life of bananas if it is combined with chitosan.
Introduction
Banana is one of the horticultural crops with high economic value. Demand for bananas is relatively high and constant globally, including in Indonesia. Indonesia is the seventh-largest banana producer in the world with a total production of 8.2 million tons in 2020.[Citation1] Musa Acuminate ‘Lady Finger’ is one of the local’s favorites bananas, characterized by its small fruit, sweet taste, and high in vitamins and minerals.[Citation2] ‘Lady Finger’ banana is preferred by the local people for its fragrant aroma, sweetness, nutritional value, and esthetic value that is often used as a decoration in Indonesian traditional ceremonies.[Citation3] Despite its popularity and high demand, the ‘Lady Finger’ banana is a rather uncommon product in the market mostly due to its postharvest life-quality problem.
Banana is classified as climacteric fruits,[Citation4] in which the ripening process is indicated by the increase of ethylene production. Ethylene is a gaseous plant hormone that regulates various physiological processes in plants, including seed germination, seedling growth, leaf expansion, blooming, and wilting, and fruit ripening.[Citation5–7] Ethylene accelerates fruit maturation, which contributes to changes in nutrient content and other compounds such as the change in taste, color, aroma, and texture.[Citation8,Citation9] Consumers prefer bananas with perfect shape, bright peel color, smooth texture, uniform maturity, natural freshness, firm pulp, and fragrance.[Citation10] In order to maintain the fruit quality and increase shelf life, the ethylene effect must be minimized especially during transportation and storage. Several methods can be performed in order to minimize and prevent the effect of ethylene such as the inhibition of ethylene biosynthesis and action. One of the procedures that can inhibit ethylene biosynthesis or perception is the application of ethylene inhibitors such as 1-MCP [Citation11]
1-MCP is an effective ethylene inhibitor that blocks ethylene perceptin to the receptor. In several studies, 1-MCP was effective in extending the fruit shelf life of plums, strawberries, bananas, apples, golden berry, and pears.[Citation2,Citation12–14] The effectiveness of 1-MCP depends on concentration, plant development stage, species, temperature, and application duration.[Citation15] Vilas-Boas and Kader[Citation16] showed that the application of 1 μL/L of 1-MCP for 6 hours of incubation at 10°C significantly inhibits ethylene production and maintains kiwifruit firmness.
Respiration is another factor that affects the reduction of postharvest life quality and the change in nutrient value. Respiration can be inhibited by applying fruit coating. Chitosan is a natural polymer with sealing properties that can be applied as fruit coating. Chitosan has the function to inhibit the exchange of gases that slow down the rate of respiration. Chitosan has now been widely applied to maintain the shelf life of several fruit commodities.[Citation17] The application of 2.5% chitosan is effective in maintaining vitamin C content and weight of citrus, strawberries, and bananas.[Citation18] Chitosan 1.25% is effective in inhibiting weight loss and decreasing respiration rate in California papaya.[Citation19] Other than respiration inhibition, chitosan can also inhibit transpiration, water, and weight loss, depending on the thickness of the chitosan layer.[Citation20] Chitosan and 1-MCP were known to be effective to maintain the postharvest quality of several fruits, but the study on the combined effect of both has not been evaluated. A study regarding 1-MCP and chitosan combination effect will be beneficial to prolonging postharvest life and nutritional quality of ‘Lady Finger’ banana. In this study, the combined effect of 1-MCP and chitosan application on postharvest life and the nutritional quality of ‘Lady Finger’ bananas were assessed.
Materials and methods
Experimental material preparation
‘Lady Finger’ banana samples were obtained from farmer plantations in Sumedang, West Java, Indonesia with optimum maturity and scale 1 (green) of fruit peel. The fruits were then transported to the laboratory. Each hand was separated from its bunch, and the two top and bottom fingers of the banana bunch were selected for each treatment. The selected fruits were then cleaned with water to remove dirt and other foreign material. Exposed tissue at the base of the fruit due to the separation process was treated with fungicides to prevent fungal contamination. Fruit samples were then treated without 1-MCP and chitosan as controls, with chitosan, with 1-MCP (0.25, 0.5, 0.75, and 1 µL/L), and with a combination of 1-MCP (0.25, 0.5, 0.75, and 1 µL/L) + chitosan.
1-MCP and chitosan treatment
1-MCP was applied in accordance with manufacturer instructions. 1-MCP powder was weighed to the desired concentration and placed inside a 60 L glass chamber containing fruit samples. A few drops of tap water were added to the powder formulation to release 1-MCP gas. Control and chitosan-only-treated samples were placed in 1-MCP-free glass chambers. After 20 hours of incubation at ambient temperature (22 ± 4°C; 80 ± 5% RH), the chambers were opened and ventilated. Chitosan was prepared according to the method described by Jiang and Li[Citation21] and Lustriane[Citation22] with some modifications. To prepare 1 L of 1.25% chitosan solution, 12.5 g of chitosan from crab shells (Sigma-Aldrich) was dispersed in distilled water containing 1% of glacial acetic acid to dissolve the chitosan and agitated using a stirrer until homogeneous. The solution was adjusted to pH 5.0 by adding 1 mol NaOH. Chitosan-treated samples and a combination of 1-MCP + chitosan-treated samples were then dipped in a prepared 1.25% chitosan solution for 1 minute, meanwhile, control samples and samples treated with only 1-MCP were dipped in distilled water. Afterward, all samples were strained and air-dried to ensure no water residue was left on the peel. Four banana hands from each treatment (three replicates for each treatment) were packed in a ventilated cartoon box and kept on a laboratory bench to ripen at 22 ± 4°C and 80 ± 5% RH.
Fruit firmness
Fruit firmness was evaluated over 14 days of postharvest storage and measured in accordance with the method carried out by Mubarok et al.[Citation23] by Ultrasonic Hardness Tester model FHR-5 (Nippon Optical Works Co., Ltd., Tokyo, Japan).
Weight loss
The postharvest water loss was evaluated over 14 days of postharvest storage and measured gravimetrically. Weight loss was measured according to the method described by Lustriane et al.[Citation22] Fruits were weighed at the initial condition and on each sampling day during the postharvest storage. The percentage of weight loss was calculated by calculating the differences between the initial weight and final weight of bananas divided by the initial weight.
Water content
Water content was measured by the gravimetric method. This is a method for extracting or removing a portion of water from the material by evaporating the water using heat energy. Samples were placed in an oven at 80°C until a constant mass was achieved. Constant weight was measured twice in a row with results that did not differ by more than 0.25%.[Citation24]
Shelf life evaluation
For fruit shelf-life evaluation, fruits were harvested at the same maturation stage (scale 1) characterized by green peel color. Fruit shelf life was calculated when storage began up until the fruit was damaged, indicated by the alteration of fruit peel color to yellow with a brownish spot (scale 7) based on a commercial peel color scale (Chiquitas Brands, Inc) [Citation24,Citation25]
Analysis of pH, Titratable Acidity (TA), and Total Soluble Solid (TSS)
Fruit pH was analyzed using a pH meter according to the methods described in Mubarok et al.[Citation23,Citation26] For TA analysis, samples were made from 50 mL of ground banana juice. The samples were then inserted into a 100 mL beaker glass and diluted with 500 mL of distilled water. Afterward, samples were titrated while stirred with 0.1 N of NaOH until the samples reached a pH of 8.1.[Citation23,Citation27] However, TSS was measured by PAL-J (Atago, Tokyo, Japan) as described by Mubarok et al.[Citation23,Citation26]
Analysis of total sugar
Total sugar was determined using the anthrone method[Citation2,Citation28] with some modifications as follows: 0.3 g of dry peel sample inserted into a 50 mL conical flask, diluted with 2.5 mL deionized distilled water and 10 mL of 80% ethanol. Then heated at 220°C for 15 minutes and then cooled. Peel samples were transferred to a 25 mL volumetric flask and filled with water. The mixed solution was homogenized by manual agitation. Fifty microliters of the solution were transferred into a test tube and diluted 20 times using 950 µL of deionized distilled and further mixed with 1 mL per 1000 µL of deionized distilled. Anthrone solution consisted of 0.5 g of anthrone and 50 mL of sulfuric acid was prepared. Sample and anthrone solution was inserted into a test tube and placed in boiling water for 12 minutes, then cooled. Orion AquaMate 8000 UV–vis Spectrophotometers (Thermo Scientific, USA) at 630 nm was used to measure total sugar.
Pulp to peel ratio
Pulp-to-peel ratio was measured according to the method described by Karmawan et al.[Citation29] and Dwivany et al.[Citation30] Pulp and peel were separated and weighed individually. The pulp and peel ratio was calculated by dividing pulp weight by peel weight.
Color index
The ‘Lady Finger’ banana peel color index was assumed to be the same as the color distribution of the Cavendish banana. The color index was determined based on a commercial peel color scale (Chiquitas Brands, Inc) that was divided into seven scale indexes (1–7) as follows: Scale 1: Green peel color, 2: Predominantly green with a trace of yellow peel color, 3: More green than yellow, 4: More yellow than green, 5: Yellow with green necks, 6: Entirely yellow, and 7: Predominantly yellow with brown spots.[Citation24]
Statistical analysis
The data obtained were analyzed using a one-factor analysis of variance (ANOVA). To determine the differences among the investigated data, Duncan Multiple Range Test (DMRT) at p < .05 was used. All statistical analyses were performed using SPSS 20.0 statistical software.
Results
Postharvest losses
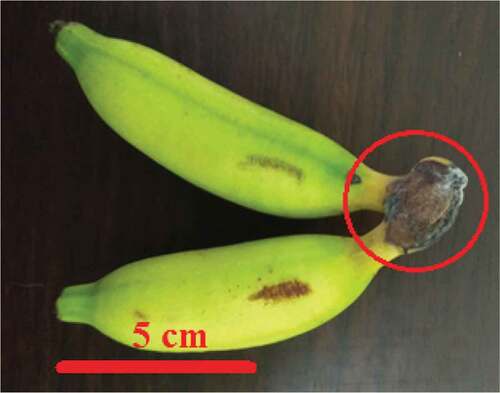
Postharvest losses of investigated bananas mostly occurred due to microorganism attacks that cause crown end rot (). Damage was first seen in control bananas resulting in decreased fruit shelf life. The symptoms appeared on the 9th day after storage (DAS), indicated by the fruit damage and growth of fungal colonies on the base of bananas ().
Fruit firmness
After 14 days of postharvest storage, the fruit firmness was decreased. Based on statistical data analysis, the result showed that chitosan can only delay fruit softening up until 7 DAS. The application of 1-MCP and the combination of 1-MCP and chitosan provided better results in softening process inhibition at 14 DAS. Samples that received these treatments had greater fruit firmness and were significantly different compared to the control ().
Table 1. The effect of 1-MCP and chitosan on fruit firmness during 14 days of postharvest storage
Water content of fruit pulp
Water content in all treated samples increased during 14 days of storage (). Based on statistical data analysis, water content at 0 DAS was not significantly different among the treatments, but after 7 and 14 DAS, differences were detected. After 7 dan 14 DAS, control samples displayed the highest water content and 1-MCP 1.00 µL/L + Chitosan treated fruits contain the lowest water content with a 5.51% lower water content than control. The application of chitosan, 1-MCP, and its combination, significantly inhibited increasing the water content, but chitosan application was ineffective after 14 DAS. At this time, the water content was comparable and did not significantly differ compared with the control fruits ().
Table 2. The effect of 1-MCP and chitosan on the water content of banana pulp during 14 days of postharvest storage
Weight loss
Weight loss is one important postharvest characteristic of horticultural crops such as bananas. This study reported that weight loss increased in all investigated fruit during 14 days of postharvest storage up to 2,5 times higher than weight loss in 7 DAS. Based on statistical data analysis, the application of 1-MCP was effective to slow down fruit weight loss at 14 DAS, but the application of chitosan was not effective. The effectiveness of chitosan in fruit weight loss prevention can be improved through the combination of 1-MCP and chitosan. The combination of 1-MCP and chitosan significantly inhibited the banana fruit weight loss up to 14 DAS compared to control, with 1-MCP 1.00 µL/L + chitosan treated fruits resulted in the lowest fruit weight loss and significantly different compared with other treatments except 1-MCP 0.75 µL/L + chitosan ()
Table 3. The effect of 1-MCP and chitosan on weight loss of banana pulp during 14 days of postharvest storage
Fruit shelf life
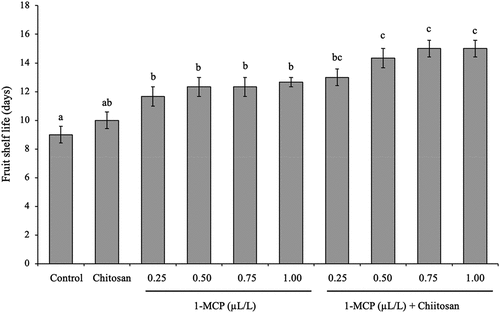
The range of fruit shelf life of banana fruit as an effect of 1-MCP, chitosan, and its combination is between 9 and 15 days (). Based on statistical data analysis, the application of chitosan was ineffective to extend fruit shelf life, but 1-MCP in all concentrations significantly extend the shelf life of banana fruit up to 3 days longer than control fruits. Our data showed that the fruit shelf life of the banana ‘Lady Finger’ can be extended even further by the application of 1-MCP combined with chitosan. The combination of 1-MCP with a concentration of more than 0.5 µL/L and chitosan gave longer fruit shelf life up to 2–3 days and was significantly different compared to 1-MCP treated fruit in all concentrations or up to 6 days longer than control fruits ().
Fruit pH
At the beginning of storage (0 days), the fruit pH was comparable among the treatment with the range from 5.65 to 5.72. The fruit pH decreased during 14 days of postharvest storage with an average value ranging from 5.39 to 5.54 at 14 DAS. The application of 1-MCP 0.25 µL/L and chitosan did not affect the change of fruit pH and was comparable with the control fruit. However, the application of 1-MCP of more than 0.5 µL/L and its combination with chitosan significantly affect the changing pH which was significantly higher compared with control. The highest fruit pH was detected from 1-MCP 0.1 µL/L + chitosan-treated fruit with the value of 5.62 and 5.53 at 7 and 14 DAS, respectively ().
Table 4. The effect of 1-MCP and chitosan on pH of banana pulp during 14 days of postharvest storage
Titratable acidity
During 14 days of postharvest storage, TA values in all investigated fruits were increased. Based on statistical data analysis, at the beginning of storage (0 days), the average TA value was comparable among treatments with a range from 0.051 to 0.056. However, the TA value was significantly different among treatments after 7 and 14 DAS. At 7 DAS, the application of 1-MCP 1 µL/L and combination of all 1-MCP concentrations with chitosan showed significantly lower TA values compared to the control fruit. However, after 14 DAS, the combination of 1-MCP and chitosan except for 1-MCP 0.75 µL/L + chitosan treated fruits showed significantly lower TA values compared to control fruit ().
Table 5. The effect of 1-MCP and chitosan on titratable acidity of banana pulp during 14 days of postharvest storage
Total Soluble Solid (TSS)
TSS in all of the investigated banana fruits was increased during 14 days of postharvest storage. At the beginning of storage, there were no significant differences in TSS among the treatments with the value between 5.51° and 6.40°Brix. TSS value from 1-MCP chitosan treated fruits was increased dramatically at 7 DAS, but it did not show in the combination of 1-MCP and chitosan treated fruits with the range of TSS between 6.32° and 16.73°Brix. Whereas, after 14 DAS, there showed a narrow rage value of TSS of all investigated fruits between 19.66 to 22.46 °Brix. A significant value of TSS was detected after 7 and 14 DAS. The application of 1-MCP in all concentrations and the combination of 1-MCP in all concentration with chitosan inhibited the increase of TSS and was lower TSS value than control and chitosan-treated fruits. On the other hand, the application of chitosan did not significantly affect the change in TSS value ().
Table 6. The effect of 1-MCP and chitosan on total soluble solid of banana pulp during 14 days of postharvest storage
Total sugar
Total sugar in all of the investigated banana fruits was increased during 14 days of postharvest storage, but it shows a different tendency toward total sugar. The application of chitosan, 1-MCP, and control fruits, resulted in the elevation of total sugar but was not detected in the combination of 1-MCP and chitosan-treated fruits. At the beginning of storage, TSS was indifferent among the treatments with a value between 1.70% and 2.20%. The application of 1-MCP combined with chitosan resulted in a small change in total sugar content from 0 DAS but gradually increased after 14 DAS. Based on the statistical data analysis, after 7 and 14 DAS, the application of 1-MCP and also 1-MCP combined with chitosan significantly inhibit the sugar formation which was indicated by the value of total sugar. Total sugar of treated fruits was lower than control with the lowest value obtained from 1-MCP 1.00 µL/L + chitosan treated fruits (13,60% lower than control) at 7 DAS and from 1-MCP 0.50 µL/L + chitosan treated fruits with the value of 11,92%. The application of chitosan did not significantly affect the total sugar at 14 days of postharvest storage ().
Table 7. The effect of 1-MCP and chitosan on total sugar of banana pulp during 14 days of postharvest storage
Pulp and peel ratio
The ratio of pulp and peel can be used as an indicator of the banana maturation process. In all investigated fruits, the pulp and peel ratio increased at 14 days of storage. At 0 DAS, the value of pulp and peel was not significantly different in all treatments with the value between 1.67 and 1.90. However, after 7 and 14 DAS, the application of 1-MCP and combination of 1-MCP + chitosan in all concentrations significantly inhibited the pulp and peel ratio increase compared with control. The application of chitosan did not affect the change of pulp and peel ratio of the banana. After 14 DAS the lowest value of pulp and peel ratio detected from 1-MCP 0.75 µL/L + chitosan treated fruits with a ratio of 41.24% lower than control ()
Table 8. The effect of 1-MCP and chitosan on pulp and peel ratio during 14 days of postharvest storage
Color index
Banana maturity can easily be detected through the shift of color from green to yellow. Bananas used in this study had the same color scale of 1. The color scale has increased during 14 days of storage, from 1 to 7. After 7 DAS, 1-MCP or a combination of 1-MCP + chitosan treated fruits had different color scales compared to control (). However, the application of 1-MCP has not been able to inhibit fruit discoloration at 14 DAS. The application of 1-MCP in all concentrations combined with chitosan showed all the treated sample colors were below scale 7. The lowest color scale was detected from the combination of 1-MCP (0.75 and 1.00 µL/L) + chitosan (). Therefore, the application of 1-MCP combined with chitosan has higher effectiveness in color change inhibition of bananas during the storage period.
Figure 3. The effect of 1-MCP and chitosan on fruit performance and the change of color of banana ‘Lady Finger’ during 14 days of postharvest storage.
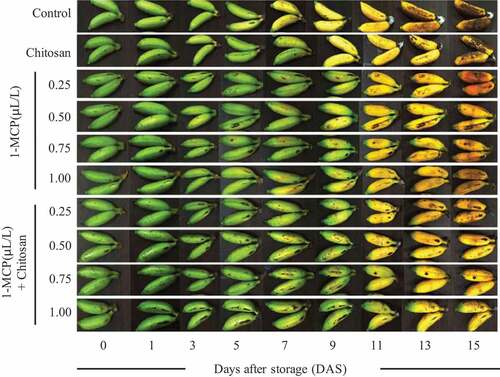
Table 9. The effect of 1-MCP and chitosan on the color index during 14 days of postharvest storage
Discussion
Good postharvest handling will inhibit the loss of postharvest banana quality. Postharvest loss in bananas can be caused by biological, physiological, and mechanical disorders. This study showed that biological disorder occurred in all investigated fruit characterized by dark brown/black color in the base of banana fruit hand () which may relate to Colletotrichum musae infection. Triest and Hendrickx[Citation31] and Jahan et al.[Citation32] reported that C. musae is one of the majority diseases in bananas causing decays at the base of a banana hand and accelerating the premature ripening of fruit. The physiological disorder is another damage in bananas during the ripening process that occurs due to respiration, transpiration, and ethylene.[Citation33] Preventing physiological disorders requires inhibition of ethylene production and action, also transpiration prevention by using ethylene inhibitors such as 1-MCP or using fruit coating as a part of effective strategies.[Citation34]
1-MCP is an ethylene antagonist that interacts with the fruit ethylene receptors and thereby prevents ethylene action.[Citation35] The prevention of ethylene action resulted in the absence of ethylene response, hence causing several physiological processes such as delay in fruit ripening. Both ethylene and 1-MCP act similarly by withdrawing electrons from metal in the receptor, causing a ligand substitution process that induces an action response. Since 1-.MCP is so highly strained, that its effect would be stronger than that of ethylene; therefore, the ethylene response can be inhibited.[Citation35]
Fruit firmness is an indicator of fruit quality. Ethylene affects the metabolic processes that change the chemical composition of the fruit. During the ripening process, there is generally an increase in soluble pectin and a decrease in insoluble pectin.[Citation36] The application of 1-MCP has been able to inhibit the decrease in fruit firmness during storage (), which is similar to previous research conducted by Mubarok et al.[Citation2] In addition, chitosan can help close air cavities on the surface of the fruit which slows the process of pectin breaking down into simple chains. Pectin acts as an inter-cell adhesive and does not dissolve in water. With the application of 1-MCP and chitosan, therefore the mechanism of ethylene action and respiration can be inhibited in the acceleration of fruit softening by inhibiting pectin degradation.[Citation19,Citation36,Citation37]
Fruit firmness also is strongly influenced by the water content in the fruit. This study showed that during storage, the water content was increased in the pulp () which causes a reduction in fruit firmness. Increasing water content in the pulp during storage is caused by the breakdown of starch from long-chain carbohydrates to short-chain and also due to the osmotic pressure between pulp and peel.[Citation38,Citation39] 1-MCP inhibits the effect of ethylene by delaying the activity of cell wall degrading enzymes that cause the cell wall to be hydrolyzed from insoluble substances to become soluble.[Citation40] When combined with chitosan, evaporation is further inhibited as chitosan inhibits gas exchange. A thicker chitosan layer can inhibit higher amounts of water vapor and a higher concentration of chitosan can inhibit higher amounts of oxygen entering the peel and further inhibits evaporation. Chitosan has been described to maintain the natural standard of fruits and vegetables by reducing respiration rates, ethylene production, and transpiration.[Citation41,Citation42]
The application of a combination of 1-MCP and chitosan can inhibit ethylene, respiration, and transpiration activity which in turn inhibits substrate conversion and water evaporation. Transpiration is a process that causes weight loss. Reduction or loss of water content can directly lead to reduced fruit shelf life.[Citation43] During storage, the ripening process occurs, causing weight loss due to physicochemical changes in the form of water release. Chitosan coating inhibits water release by closing air cavities on the surface of the fruit. As stated by Sitorus et al.[Citation20] a high concentration of chitosan that coats the surface of the fruit can prevent water loss due to transpiration to minimize weight loss.
Shelf life is a determining factor in the quality of postharvest horticultural commodities, especially bananas. It is calculated by observing fruit quality during storage, through both physical appearance and the results of fruit quality analysis. One important parameter to observe is weight loss, which is related to the respiration and transpiration levels that occur in fruit.[Citation44] Shelf life is defined as a certain time span for a product to remain fit for consumption according to the criteria set by consumers.[Citation45] 1-MCP treated fruits combined with chitosan showed a longer shelf life than control samples (). Schotsman and Prange[Citation46] showed that 1-MCP inhibits ethylene perception that affects the respiration process, softening, discoloration, aroma production, and prevent physiological damage. In addition, chitosan coating can improve the effectiveness of 1-MCP as it can prevent oxygen from entering the peel during respiration. The chitosan layer will inhibit oxygen diffusion among cells since the pores are closed which in turn will inhibit respiration and reduce physiological damage. Furthermore, chitosan is an antimicrobial that inhibits the growth of fungi and is able to extend the shelf life of bananas.[Citation47]
During the ripening process, the pH level decreases while TA content increases.[Citation48] Organic acid levels will initially increase and reach their maximum value then decrease slowly when ripe. 1-MCP blocks ethylene receptors so that organic acid breakdown and acid synthesis during the ripening process are inhibited.[Citation44] This study shows that 1-MCP can inhibit changes in pH and TA during storage (). It can inhibit acid conversion during ripening that maintain acidity levels. This can be seen in 1-MCP treated fruit that showed TA values did not drastically change during storage (). Mubarok et al.[Citation2,Citation26] stated that during storage, the amount of titrated acid increases causing a low pH value. The addition of chitosan increases the effectiveness of 1-MCP in inhibiting changes in pH and TA. This is assumed that the chitosan coating modifies the internal atmosphere of the fruit by reducing the O2 uptake and keeping CO2 at a high level,[Citation49] which inhibits respiration and acid degradation. The respiration process during ripening affects the level of titrated acid which also affects the breakdown of organic acids.[Citation35]
There was an increase in the total soluble solid during storage related to increasing in the respiration process that accelerates the fruit senescence and increases total soluble solid during the climacteric peak.[Citation3] This study revealed that 1-MCP treated fruit or 1-MCP + chitosan treated fruit have lower TSS values (). Our result was similar to Balaguera-Lopez et al.[Citation50] which shows that 1-MCP inhibits fruit metabolic activity during the ripening process and maintains TSS value. Furthermore, chitosan inhibits O2 diffusion which affects decreasing O2 in the fruit. The decreasing O2 affects in the low use of substrates such as sugar which results in a lower conversion of starch.
Starch metabolism and sugar accumulation occurred during the ripening process of bananas. An increase in total sugar content can be used as a chemical indicator that fruit maturation has occurred.[Citation51] 1-MCP works by blocking ethylene receptors that stimulate ATPase activity to provide energy for continued metabolism. Meanwhile, the chitosan layer that covers the surface of bananas inhibits O2 uptake and maintains high CO2 levels in the fruit.[Citation45] Transpiration during storage causes the decrease of water content in banana peels. In addition, water will be transported from the peel into the pulp which decreases peel weight. Patil and Shanmugasundaram[Citation39] stated that the percentage weight of banana pulp at the beginning of fruit development is very low, while peel weight is very high. The decrease in peel weight during ripening is caused by hemicellulose and cellulose breakdown into starch. The ratio of pulp and peel has a positive correlation, therefore although weight loss occurs due to transpiration, the pulp will have increased water content. Teka[Citation52] stated that ethylene can affect water content through fruit hardness by accelerating the hydrolysis of polysaccharides on cell walls and forming water in the fruit during the ripening process. Many studies revealed that 1-MCP has a function to block ethylene perception. 1-MCP inhibits cell wall degradation that maintains water content and fruit hardness. Likewise, high levels of CO2 due to chitosan coating inhibits the respiration, transpiration, and ethylene activity process that inhibits starch breakdown and sugar accumulation.[Citation19,Citation45]
Color changes that occur during ripening are caused by the degradation of chlorophyll and xanthophyll. The intensity of the yellow color will increase until the climacteric peak and subsequently changes to brown due to pheophytin.[Citation53,Citation54] Matile et al.[Citation55] reported that ethylene accelerates chlorophyll degradation by enhancing the activation of chlorophyllase in the conversion of chlorophyll a and b to chlorophyllide and phytol. Our study showed that 1-MCP can maintain green color for longer periods compared to control (). It was comparable to previous publications that dialkalyamine compounds and related derivatives of 1-MCP prevented the degradation of chlorophyll in the banana peel.[Citation56] 1-MCP was effective in preventing an ethylene-induced color change of banana peel from green to yellow (). The 1-MCP application could act as negative feedback control of ethylene production. The non-activation of the ethylene receptor would inhibit the downregulating action of ethylene. [Citation57]
Conclusion
In conclusion, the postharvest quality of tropical ‘Lady Finger’ banana can be maintained by the application of 1-MCP and chitosan. The results showed that the application alone of chitosan has not been able to improve the postharvest quality of ‘Lady Finger’ bananas as effectively as 1-MCP. The effectiveness of 1-MCP can be improved by combination with chitosan. The combination of 1-MCP in all concentrations with chitosan has a good effect on the postharvest quality of ‘Lady Finger’ banana compared to 1-MCP alone, such as in improving fruit shelf-life, preventing discoloration, maintaining fruit firmness, and inhibiting weight loss. Moreover, banana treated with a 1-MCP and chitosan combination has lower total sugar content and total soluble solids. It is concluded that 1-MCP combined with chitosan is a potential postharvest treatment to improve the postharvest life of banana fruitCitation57.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- BPS. Statistics of Annual Fruit and Vegetable Plants in Indonesia. Indonesia: Badan Pusat Statistik, 2020, cited 20th April 2022. http//bps.go.id
- Mubarok, S.; Suwali, N.; Suminar, E., and Kamaluddin, N. N. 1-Methylcyclopropene as an Effective Ethylene Inhibitor to Extend Musa Acuminata Colla ‘Muli’ Postharvest Quality. IOP Conference Series: Earth and Environmental Science. Bandung-Indonesia. 2019; 334. Doi:10.1088/1755-1315/334/1/012051
- Hartanto, R., and Sianturi, C. Perubahan Kimia, Fisika Dan Lama Simpan Buah Pisang Muli Dalam Penyimpanan Atmosfir Pasif. Prosiding Seminar Nasional Sains dan Teknologi-II 2008 Universitas Lampung, Bandar Lampung-Indonesia, 17–18 November 2008. pp. IX107–IX115
- Bleecker, A. B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Florida State University. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. DOI: 10.1146/annurev.cellbio.16.1.1.
- Iqbal, N.; Khan, N. A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M. I. R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. DOI: 10.3389/fpls.2017.00475.
- Mubarok, S.; Ezura, H.; Rostini, N.; Suminar, E.; Wiguna, G.; Suminar, E.; Wiguna, G. Impacts of Sletr1-1 and Sletr1-2 Mutations on the Hybrid Seed Quality of Tomatoes. J. Integr. Agric. 2019, 18(5), 1170–1176. DOI: 10.1016/S2095-3119(19)62614-6.
- Mubarok, S.; Maunah, M.; Rufaidah, F.; Suminar, E.; Rismayanti, A. Y.; Suminar, E.; Rismayanti, A. Y. Dataset on the Combination Effects of 1-Methylcyclopropene and Salicylic Acid on Postharvest Quality of Cut Roses ‘Peach Avalanche’ and ‘Sexy Red.’ Data Brief. 2020, 33, 106600. DOI: 10.1016/j.dib.2020.106600.
- Mubarok, S.; Okabe, Y.; Fukuda, N.; Ariizumi, T.; Ezura, H. Favorable Effects of the Weak Ethylene Receptor Mutation Sletr1-2 on Postharvest Fruit Quality Changes in Tomatoes. Postharvest. Biol. Technol. 2016, 120, 1–9. DOI: 10.1016/j.postharvbio.2016.04.022.
- Mubarok, S.; Yulianto, F.; Budiarto, R.; Rahmat, B. P. N.; Khoerunnisa, S. A. Metabolite Correlation with Antioxidant Activity in Different Fruit Maturation Stages of Physalis Peruviana. Biodiversitas J Biol Diversity. 2021, 22(5), 2743–2749. DOI: 10.13057/biodiv/d220536.
- Sholihati, S.; Abdullah, R.; Suroso, S. Kajian penundaan kematangan pisang raja (Musa paradisiaca Var. Sapientum L.) melalui penggunaan media penyerap etilen kalium permanganat. Rona Teknik Pertanian. 2015, 8(2), 76–89. DOI: 10.17969/rtp.v8i2.3005.
- Mubarok, S.; Kusumiyati, Fauzi, A. A.; Nuraini, A.; Rufaidah, F.; Qonit, M. A. H. Effect of Benzyl Amino Purine and 1-Methylcyclopropene in Maintaining Rooting Quality of Chrysanthemum (Chrysanthemum Morifolium Ramat Cv. ‘White Fiji’) Cuttings. Res. Crops. 2020, 21(1), 141–150.
- Blankenship, S. M. Ethylene Effect and Benefits of 1-MCP. Perishables Handling Q. 2001, 108, 1–4.
- Zhu, X.; Shen, L.; Fu, D.; Si, Z.; Wu, B.; Chen, W.; Li, X. Effects of the Combination Treatment of 1-MCP and Ethylene on the Ripening of Harvested Banana Fruit. Postharvest. Biol. Technol. 2015, 107, 23–32. DOI: 10.1016/j.postharvbio.2015.04.010.
- Mubarok, S.; Dahlania, S.; Suwali, N. Dataset on the Change of Postharvest Quality of Physalis Peruviana L. As an Effect of Ethylene Inhibitor. Data Brief. 2019, 24, 103849. DOI: 10.1016/j.dib.2019.103849.
- Fauzi, A. A.; Kusumiyati, Mubarak, S.; Rufaidah, F.; Rufaidah, F. Beberapa catatan pemanfaatan 1-Methylcyclopropene pada krisan (Chrysanthemum morifolium Ram.). J. Pertanian Terpadu. 2018, 6(1), 1–10. DOI: 10.36084/jpt.v6i1.137.
- Vilas-Boas, E. V. B.; Kader, A. A. Effect of 1-methylcyclopropene (1 MCP) on Softening of fresh-cut Kiwifruit, Mango and Persimmon Slices. Postharvest. Biol. Technol. 2007, 43, 238–244. DOI: 10.1016/j.postharvbio.2006.09.010.
- Ghidelli, C.; Pérez-Gago, M. B. Recent Advances in Modified Atmosphere Packaging and Edible Coatings to Maintain Quality of fresh-cut Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58(4), 662–679. DOI: 10.1080/10408398.2016.1211087.
- Trisnaningrum, Y. F.; Wahyuni, S.; Rofieq, A. Penggunaan Chitosan Cangkang Keong Mas (Pomacea Canaliculata) Untuk Bahan Pengawet Alami Dalam Mempertahankan Mutu Buah Selama Proses Penyimpanan Sebagai Media Audio Visual Pembelajaran Bioteknologi. J. Pendidikan Biologi Indonesia. 2016, 2(3), 237–247. DOI: 10.22219/jpbi.v2i3.3871.
- Firmansyah, Y.; Efendi, R.; Rahmayuni. Pemanfaatan Kitosan Untuk Memperpanjang Umur Simpan Buah Pepaya Varietas California. J. SAGU. 2016, 15(2), 11–20.
- Sitorus, R. F.; Karo, T. K.; Lubis, Z. Pengaruh Konsentrasi Kitosan Sebagai Edible Coating Dan Lama Penyimpanan Terhadap Mutu Buah Jambu Biji Merah. Jurnal Rekayasa Pangan Dan Pertanian. 2014, 2(1), 1–10.
- Jiang Y and Li Y. (2001). Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chemistry, 73(2), 139–143. DOI: 10.1016/S0308-8146(00)00246-6
- Lustriane, C.; Dwivany, F. M.; Suendo, V.; Reza, M. Effect of Chitosan and chitosan-nanoparticles on Post Harvest Quality of Banana Fruits. J. Plant Biotechnol. 2018, 45, 36–44. DOI: 10.5010/JPB.2018.45.1.036.
- Mubarok, S.; Ezura, H.; Qonit, M. A. H.; Prayudha, E.; Anas, Suwali, N.; Kusumiyati, Kurnia, D. Alteration of Nutritional and Antioxidant Level of Ethylene Receptor Tomato Mutants, Sletr1-1 and Sletr1-2. Sci. Hortic. 2019, 256, 108546. DOI: 10.1016/j.scienta.2019.108546.
- Kenkel, J. Analytical Chemistry for Technicians; 2000 N.W. Corporate Blvd., Boca Raton, Florida 33431: CRC Press, LLC, 2003; pp 543.
- Boudhrioua, N.; Giampaoli, P.; Bonazzi, C. Changes in Aromatic Components of Banana during Ripening and air-drying. Lebensm.Wiss. u.Technol. 2003, 36, 633–642. DOI: 10.1016/S0023-6438(03)00083-5.
- Mubarok, S.; Okabe, Y.; Fukuda, N.; Ariizumi, T.; Ezura, H. Potential Use of a Weak Ethylene Receptor Mutant Sletr1-2, as Breeding Material to Extend Fruit Shelf Life of Tomato. J. Agric. Food Chem. 2015, 63, 7995–8007. DOI: 10.1021/acs.jafc.5b02742.
- Mubarok, S.; Farhah, F. F.; Anas, Suwali, N.; Kurnia, D.; Kusumiyati, Suminar, E.; Ezura, H.; Suminar, E.; Ezura, H. Data on the Yield and Quality of Organically Hybrids of Tropical Tomato Fruits at Two Stages of Fruit Maturation. Data Brief. 2019, 25, 104031. DOI: 10.1016/j.dib.2019.104031.
- Trineeva, O. V. Comparative Characteristics of Sugar Determination by Different Methods in Leaves of Nettle. Drug Develop. Regis. 2020, 9(2), 91–97. DOI: 10.33380/2305-2066-2020-9-2-91-97.
- Karmawan, L. U.; Suhandono, S.; Dwivany, F. M. Isolation of MA-ACS Gene Family and Expression Study of MA-ACS1 Gene in Musa Acuminata Cultivar Pisang Ambon Lumut. J. Hayati Biosci. 2009, 16(1), 35–39. DOI: 10.4308/hjb.16.1.35.
- Dwivany, F. M.; Hermawaty, D.; Esyanti, R. R. ‘Raja Bulu’ Banana MaACS1 and MaACO1 Gene Expression during Postharvest Storage. Acta Horticul. 2016, 1120, 111–114. DOI: 10.17660/ActaHortic.2016.1120.16.
- Triest, D.; Hendrickx, M.; Hogan, D. A. Postharvest Disease of Banana Caused by Fusarium Musae: A Public Health Concern?. PLoS Pathog. 2016, 12, 11. DOI: 10.1371/journal.ppat.1005940.
- Jahan, S.; Lia, R. S.; Chowdhury, E. K.; Hasan, F.; Islam, A.; Sikdar, B.; Khalekuzzaman,; Khalekuzzaman, M. Characterization of Crown Rot Disease of Banana Fruit and eco-friendly Quality Improvement Approach during Storage. Microbiol. Res. J. Int. 2019, 27(3), 1–13. DOI: 10.9734/mrji/2019/v27i330099.
- Pathare, P. B.; Al-Dairi, M. Effect of Mechanical Damage on the Quality Characteristics of Banana Fruits during short-term Storage. Discover Food. 2022, 2(4). DOI: 10.1007/s44187-022-00007-7.
- Dias, C.; Ribeiro, T.; Rodrigues, A. C.; Ferrante, A.; Vasconcelos, M. W.; Pintado, M. Improving the Ripening Process after 1-MCP Application: Implications and Strategies. Trends Food Sci. Technol. 2021, 113, 382–396. DOI: 10.1016/j.tifs.2021.05.012.
- Sisler, E. C.; Serek, M. Inhibitors of Ethylene Responses at the Receptor Level: Recent Developments. Physiol. Plant. 1997, 100, 577–582. DOI: 10.1111/j.1399-3054.1997.tb03063.x.
- Song, L.; Wang, Z.; Wang, Z.; Meng, G.; Zhai, R.; Cai, M.; Ma, F.; Xu, L. Screening of Cell wall-related Genes that are Expressed Differentially during Ripening of Pears with Different Softening Characteristics. Postharvest Biol. Technol. 2016, 115, 1–8. DOI: 10.1016/j.postharvbio.2015.12.012.
- Bennett, A. B.; Labavitch, J. M. Ethylene and ripening-regulated Expression and Function of Fruit Cell Wall Modifying Proteins. Plant Sci. 2008, 175, 130–136. DOI: 10.1016/j.plantsci.2008.03.004.
- Brizzolara, S.; Manganaris, G. A.; Fotopoulos, V.; Watkins, C. B.; Tonutti, P. Primary Metabolism in Fresh Fruits Druing Storage. Front. Plant Sci. 2020, 11, 80. DOI: 10.3389/fpls.2020.00080.
- Patil, S. K.; Shanmugasundaram, S. Physicochemical Changes during Ripening of Monthan Banana. Int. J. Technol. Enhance. Emerg. Eng. Res. 2015, 3(2), 18–21.
- Jeong, J.; Huber, D. J.; Sargent, S. A. Influence of 1-methylcyclopropene (1-MCP) on Ripening and cell-wall Matrix Polysaccharides of Avocado (Persea Americana) Fruit. Postharvest Biol. Technol. 2002, 25(3), 241–256. DOI: 10.1016/S0925-5214(01)00184-3.
- Li, H.; Yu, T. Effects of Chitosan on Incidence of Brown Rot, Quality and Physiological Attributes of Postharvest Peach. J. Sci. Food Agric. 2000, 8, 269–274.
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal Activity of Chitosan on Two Postharvest Pathogens of Strawberry Fruits. Phytopathology. 1992, 82, 398–402. DOI: 10.1094/Phyto-82-398.
- Kitinoja, L.; Kader, A. A. Small-Scale Postharvest Handling Practices: A Manual for Horticultural Crops, 4th ed.; Postharvest Horticulture Series No. 8E. University of California: Davis, 2002.
- Nunes, C. N., and Emond, J. P. Relationship between Weight Loss and Visual Quality of Fruits and Vegetables. Proceedings of the Florida State Horticultural Society. 2007, Florida. 120: 235–245
- Olivares-Tenorio, M. L.; Dekker, M.; van Boekel, M. A. J. S.; Verkerk, R. Evaluating the Effect of Storage Conditions on the Shelflife of Cape Goosseberry (Hysalis Peruviana L). LWT Food Sci. Technol. 2017, 80, 523–530. DOI: 10.1016/j.lwt.2017.03.027.
- Schotsmans, W. C.; Prange, R. K.; Binder, B. M. 1-Methylcyclopropene: Mode of Action and Relevance in Postharvest Horticulture Research. Hortic. Rev. 2009, 35, 263–313.
- Supeni, G.; Irawan, S. Pengaruh penggunaan kitosan terhadap sifat barrier edible film tapioka termodifikasi. J. Kimia Kemasan. 2012, 34(1), 199–206. DOI: 10.24817/jkk.v34i1.1854.
- Chomchalow, S.; El Assi, N. M.; Sargent, S. A.; Brecht, J. K. Fruit Maturity and Timing of Ethylene Treatment Affect Storage Performance of Green Tomatoes at Chilling and Nonchilling Temperatures. Hort. Technol. 2002, 12(1), 104–114. DOI: 10.21273/HORTTECH.12.1.104.
- Volpe, S.; Cavella, S.; Torrieri, E. Biopolymer Coatings as Alternative to Modified Atmosphere Packaging for Shelf Life Extension of Minimally Processed Apples. Coatings. 2019, 9, 569. DOI: 10.3390/coatings9090569.
- Balaguera-Lopez, E.; Helber, C. A.; Martinez, A.; Harrera. Refrigeration Affects the Postharvest Behavior of 1-methylclopropene-treated Cape Gooseberry (Physalis Peruviana) Fruits with the Calyx. Agro.Colombiana. 2016, 33, 356–364. DOI: 10.15446/agron.colomb.v33n3.51896.
- Xiao, Y.; Kuang, J.; Qi, X.; Ye, Y.; Qu, Z.; Chen, J.; Lu, W. A Comprehensive Investigation of Starch Degradation Process and Identification of A Transcriptional Activator MabHLH6 during Banana Fruit Ripening. Plant Biotechnol. J. 2018, 16(1), 151–164. DOI: 10.1111/pbi.12756.
- Teka, T. A. Analysis of the Effect of Maturity Stage on the Postharvest Biochemical Quality Characteristics of Tomato (Lycopersicum Esculentum Mill.) Fruit. Int. Res. J. Pharm. App. Sci. 2013, 3(5), 180–186.
- Müller, T.; Kräutler, B. Chlorophyll Breakdown as Seen in Bananas: Sign of Aging and Ripening – A Mini Review. Gerontology. 2011, 57, 521–527. DOI: 10.1159/000321877.
- Yan, L.; Fernando, W. M. A. D. B.; Brennan, M.; Brennan, C. S.; Jayasena, V.; Coorey, R. Effect of Extraction Method and Ripening Stage on Banana Peel Pigments. Int. J. Food Sci. Technol. 2016, 51, 1449–1456. DOI: 10.1111/ijfs.13115.
- Matile, P.; Hoertensteiner, S.; Thomas, H.; Kraeutler, B. Chlorophyll Breakdown in Senescence Leaves. Plant Physiol. 1996, 112, 1404–1409. DOI: 10.1104/pp.112.4.1403.
- Sisler, E. C.; Goren, R.; Apelbaum, A.; Serek, M. The Effect of Dialkylamine Compounds and Related Derivates of 1-Methylcyclopropene in Counteracting Ethylene Response in Banana Fruit. Postharvest. Biol. Technol. 2009, 51, 43–48. DOI: 10.1016/j.postharvbio.2008.06.009.
- Kadner, R.; Druege, U. Role of Ethylene Action in Ethylene Production and Post Storage Leaf Senescence and Survival of Pelargonium Cutting. Plant Growth Reg. 2004, 43, 187–196. DOI: 10.1023/B:GROW.0000045999.61765.7e.