?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Consumers demand for functional foods and nutraceutical is increasing owing to their health endorsing properties. Natural bioactive compounds are getting attention due to their health promoting potential. In addition, the extraction of these bioactive compounds is a significant industrial and technological perspectives. These bioactive moieties can be extracted via various conventional and modern methods. For instance; solid-phase extraction, solid-phase micro-extraction, and liquid-liquid extraction are considered as traditional/conventional methods. In contrast, modern eco-innovative methods for extraction such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), pulsed electric field (PEF), supercritical fluid extraction (SFE), instant controlled pressure drop (DIC), etc. are more economical and environment friendly. Additionally, these are ever-increasing demands of energy-efficient methods for the recovery of valuable compounds. Moreover, these methods produced less wastewater and hazardous substances. Conclusively, this review highlighted the conventional and modern extraction technologies and the role of these eco-innovative technologies in achieving the goal of a sustainable food system.
Introduction
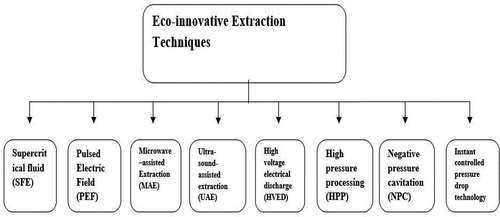
Bioactive compounds especially polyphenols and non-starch polysaccharides are abundantly present in fruits, nuts, roots, vegetables, herbs, and spices.[1] Owing to the importance of bioactive compounds, manufacturing sector is looking for eco-innovative technologies to minimize the loss of bioactive compounds.[Citation2] These compounds have anti-oxidants, anti-cancer, anti-inflammatory, anti-diabetic, anti-lipidemic and anti-depressive properties.[Citation1,Citation3] Many researchers have been extracted bioactive compounds using different modern methods. Various extraction methods are used to extract/recover these valuable bioactive compounds from different sources. Choice of extraction method depends on the type, preparation procedure and energy consumption.[Citation4] It must be chosen to minimize loss and energy consumption and maximum extraction yield of desired compounds. Many researchers, including Koçak and Pazir[Citation5] reported that almost 60% of the total time is consumed in the sample preparation step. The final analysis, i.e., the spectroscopic or chromatographic technique, needs approximately 7% of the total time.[Citation6] Failure to choose a proper extraction method could result in loss of the desired compound in addition to this misinterpretation of the conclusion or results. Current review highlighted various types of extraction techniques which can be differentiated as traditional and eco-friendly. Many traditional techniques such as solid-liquid extraction (SLE), liquid-liquid extraction (LLE), and solid-phase microextraction (SPME) are used for extraction purposes. These traditional methods are old and have many drawbacks like loss of nutrients, low extraction yield & long extraction time, higher energy consumption and not economical. In previous literature, Sasidharan et al.[Citation6] described many extraction techniques and also discussed their advantages such as less solvent utilization, less time requirement, energy-efficient, and higher extraction yield. In modern era, eco-friendly and innovative techniques are mostly used to extract bioactive compounds from different natural sources. These extraction techniques are ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), pressurized liquid extraction (PLE), membrane ultra-filtration extraction, instant controlled pressure drop (DIC) extraction, surfactant-mediated extraction and enzymatic extraction having high yield and efficiency. Furthermore, ultrasound, microwave and high-pressure technologies have been used in different food industries for the shelf-life extension.[Citation7]
Major food bioactive compounds
The major bioactive components and their sources are listed below:
Non-starch Polysaccharides i.e. cellulose, hemicellulose naturally present in cereal and cereal by-products.[Citation8] Inulin is abundantly present in chicory roots, Jerusalem artichokes, nectarine, seaweed, sugar cane bagasse, cassava waste, rice bran, rice straw, apple pomace etc.[Citation9] Polyphenols are naturally present in fruits, vegetables, teas, and spices. Tocopherols and carotenoids (vitamin A or β-carotene) are abundant in leafy green vegetables, Glucosinolates sulforaphane from broccoli, Capsaicinoids capsaicin present in peppers, alkaloids such as caffeine is present in coffee beans. Terpenoids limonene are mostly present in citrus fruits.[Citation10] Organosulfur compounds are present in allium vegetables such as garlic and onions.[Citation11] Triterpenes including squalene obtained from olive oil. Phytosterols are present in plant cell membranes. Soya bean, nuts, seeds, vegetable oil, and margarine are rich sources of phytosterols. Polyunsaturated fatty acids (PUFAs) are present in many seafoods. Bioactive peptides, including carnosine present in red meat.[Citation12]
Role of food bioactive compounds in human health
Major sources of bioactive compounds are plants, fruits & vegetables, cereals, meat and seafood. The bioactive compounds such as polysaccharides, polyphenols, carotenoids, alkaloids, etc. are abundantly present in above mentioned natural sources. Others including triterpenes and triterpenoids (a functional form of triterpenes) include about 18 different subclasses, and among these, saponins and squalene derivatives are mainly recognized. Ursane, lupine and oleanane are considered as most effective anti-cancer compounds.[Citation13] Additionally, Saeed et al.[Citation1] described that non-starch polysaccharides especially arabinoxylans act as anti-inflammatory, anti-oxidant and anti-diabetic agents.
Phytosterols are major bioactive component of plant cells.[Citation2] In various researches, the presence of phytosterols including stigmasterol, campesterol, and sitosterol in the diet help in lowering the effect of low-density lipoprotein cholesterol in the body.[Citation14] Isoprenoid is another name for terpenoids, comprises a large variety of compounds and thus belongs to the biggest group of secondary metabolites. Terpenoids are specifically present in rich amounts in various fruits and plants, and their volatility at room temperature is responsible for their specific aroma i.e. citrus fruits contain limonene. Tocopherols (such as α and γ tocopherols) and carotenoids (i.e. β- carotene) are providers of essential nutrients such as vitamin A and E. These are thus referred to as bioactive food compounds.[Citation15]
Caffeine, mainly obtained from tea and coffee, is also among the most commonly consumed alkaloid in the human diet. Glucosinolates are mainly present in cruciferous plants, typically a part of the Mediterranean diet that possesses specific odor and taste such as cauliflower, broccoli, brussels sprouts, and cabbage.[Citation16] These compounds are also proved to be responsible for many healthy activities in the body, such as anti-inflammatory and chemo-preventive effects. Sulforaphane is the compound present in broccoli and acts as a preferable anti-cancerous compound. Cellulose gives the structural framework to plant cells and glycogen and starch that provide energy reserves to animal and plant cells are the most common polysaccharides related to human health and thus termed bioactive food compounds.[Citation17] Dietary fiber inulin and its derivate fructooligosaccharides are depicted to contribute toward homeostasis of the microbiota symbiosis of the human gut and thus positively effect on human health, infant nutrition, lipid metabolism, and blood sugar level as well as reduces the risk of colon cancer and obesity.[Citation9,Citation17] Polyunsaturated fatty acids such as docosahexaenoic acid, linoleic acid, and eicosapentaenoic acid are significant food bioactive compounds and contribute to beneficial health effects.[Citation18] Overconsumption of these bioactive compounds may stimulate deterioration caused by oxidative stress mainly to the walls of blood vessels and can lead to a lethal cardiovascular disorder.[Citation19] Polyunsaturated fatty acids must be taken by diet because mammals cannot synthesize them. Common polyunsaturated fatty acids are oils and seeds of soybean, flax, sunflower, corn, walnuts, and fish, including herring, salmon, and mackerel. Peptides are major bioactive compounds and are naturally present in meat including chicken, pork, beef, fish and various seafood. These valuable compounds are also responsible for preventing or reducing the risk of many diseases such as metabolic syndrome, functional gut environment, blood pressure homeostasis, and muscle wasting disorder (i.e., sarcopenia).[Citation20] Polyphenols are bioactive compounds present in a variety of medicinal plants. The health benefits of polyphenols are determined by the amount consumed as well as their bioavailability. Therefore, polyphenols have been identified in the treatment of cardiovascular disease, osteoporosis, neurodegenerative illness, cancer, and diabetes mellitus, according to recent investigations.[Citation21]
Bioaccessibility and bioavailability of bioactive compounds
In a regular diet, polyphenol exists in various components forms. For maintenance of a healthy intake of these polyphenols is fundamental. Several complex processes are involved in the metabolism, distribution, and transport of these compounds to the target. So these processes can affect the structure and bioactivities of these compounds.[Citation22] The following two factors affect the absorption of polyphenol in gastrointestinal.
Bioaccessibility
Bioavailability is the number of compounds bioactive polyphenols that can undergo and are available for metabolization processes. The interaction of polyphenol with food components like carbohydrates, lipids, proteins, etc., may influence the metabolization of these bio-active polyphenols.[Citation23]
Bioavailability
Bioavailability refers to the ability of this bioactive polyphenol to be metabolized and distributed throughout the whole body. Generally, polyphenol has poor bioavailability. Many factors limit the polyphenols’ metabolism, including the complexity of structure, solubility, the interaction between molecules, and the degree of polymerization (DOP). Mc-Clements et al.[Citation24] illustrated bioavailability (BA) of phytochemicals depend upon four basic factors:
BA: Bioavailability
S: Stability of bioactive compounds at the time of ingestion,
B: Bioaccessibility of bioactive compounds
T: Fractions of bioactive compounds that remain biologically active form after passage through the gastro intestinal tract to the absorption site
A: Fraction of bioaccessible bioactive compounds that are actually absorbed across the epithelium cells
It is estimated that between 5 to 10% of bioactive compounds from the food matrix are absorbed and transported to the liver.[Citation16] Change in pH (gastric 2 pH) to (intestinal 6–7.5 pH) may influence flavonoid bio-accessibility. Therefore it is very obvious that polyphenols ingestion and they are in vivo bioactivities is a complex phenomenon.[Citation25] To overcome the hindrance of polyphenols metabolism and enhance their absorption, several strategies have been developed, such as the application of food processing conditions, utilization of food matrices to protect polyphenols against degradation, and modified delivery system for the transportation of phenolic compounds to targeted cells.[Citation26] Moreover, the beneficial health effects of bioactive compounds are dependent on their bioaccessibility and bioavailability.
Factors affect the extraction of bioactive compounds
Some of the factors which control mass transfer as well as solubility of polyphenol include pressure, the particle size of the sample, temperature, pH of the solution, ultrasonic power and frequency in case of (UAE), microwave power in case of (MAE), and electric field strength as well as pulse duration in case of (PEF), etc. Many experiments have been done to evaluate the impacts of these important factors. To evaluate the best conditions for maximum recovery, one of the best applications is the response surface method. It was reported by Azmir et al.[Citation4] that remarkable efforts made on modern processes could be utilized by considering research dedicated to these aspects. The selection of solvent for the extraction of polyphenols from different sources, especially plants, before choosing an extraction process is one of the most important considerations in the selection of a suitable solvent. Bart and Pilz[Citation27] reported that the selection of solvent depends upon the nature of specific bioactive compounds. Altemimi et al.[Citation28] reported that the chosen solvent must have the same polarity as the desired solute to be fully dissolved in extraction methods. Due to this fact, different types of extraction methods are required for different solutes, and their chemical nature can be polar, nonpolar, and even thermally labile.[Citation29] To extract hydrophilic bioactive compounds, polar solvents (ethanol, methanol, or ethyl acetate).
On the other hand, for the isolation of phenolic compounds, polar solvents having lower boiling points, for example, ethanol, acetone, methanol, and a mixture of acetone and water, would be the best choice. Bleakley et al.[Citation29] reported that mainly products that require water extraction include hydrophobic compounds, metals, water-soluble amino acids, peptides, sugars, ions, nucleotides, etc. Furthermore, it was reported by Tomsone et al.[Citation30] and Bouchard et al.[Citation31] that products that require ethanol extraction consist of very polar, basic, acidic and neutral compounds. Many researchers reported that ethyl acetate extraction is needed for moderately hydrophobic products, low as well moderately polar, neutral etc.[Citation32]
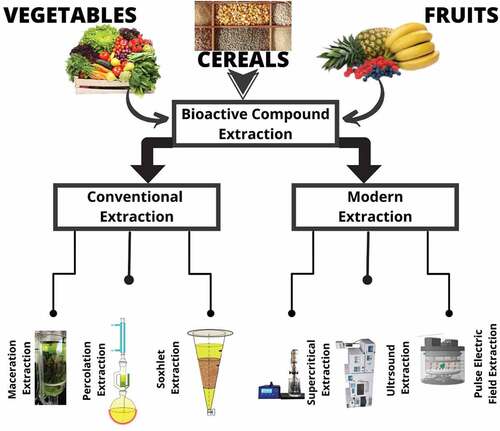
Different traditional and novel extraction methods used to extract bioactive compounds from natural sources are shown in .
Traditional extraction methods
Liquid-liquid extraction
It is also well known as solvent extraction, which comprises two immiscible liquid phases. One of the two phases is aqueous, while the second one is the organic phase. Successful extraction depends upon the dissolution of the analytic in the organic phase. Along with a plant or other material from which desired compound wants to be extracted, the organic and aqueous phases are mixed in a separator funnel. Two distinct layers of liquid as a result of shaking are generated. According to Wells,[Citation33] liquid-liquid extraction of the targeted analytic compound, the analyte distributes itself between the two immiscible liquids according to the relative solubility in each solvent.[Citation34] The liquid-liquid extraction technique is ideal for temperature-sensitive substances and an azeotropic mixture; no need to distillation process.[Citation35] Bidari et al.[Citation36] reported that this technique has many drawbacks, such as the large volume of organic solvents required, emulsion formation, difficulties related to automation, labor-intensive nature, etc.
Solid-phase extraction (SPE)
In this extraction process from a moving liquid, thorough removal of the chemical composition is ensured. The chemical constituent is retained on the solid sorbent, and finally, the desired constituent is recovered from the sorbent by the elution.[Citation37] The important requirement for successful solid-phase extraction is that a reproducible and maximum quantity of analytical solute must be taken by solid extraction. Solutes must be eluted completely from solid particles. According to Poole,[Citation38] the shortcoming of solid-phase extraction is a limitation in the capability of sorbent sorption in addition to this disruption of the analyte. To get rid of small particle solid phase extraction need a filtration process. Solid-phase extraction is a more beneficial technique than liquid-liquid extraction because it make effective use of solvent and economical.[Citation39]
Solid-phase microextraction (SPME)
A simple extraction method that involves the dispersion of solid phase in a little amount of extracting phase and for a precise period exposure to sample is done. It is reported by Merkle et al.[Citation40] two fundamental steps for SPME for a precise duration, outwardly coated fibers or sorbent are exposed to the target substance or sample, and the sorbent is moved for gas chromatography or HPLC. According to Merkle et al.[Citation40] the SPME methods have many benefits due to their simplicity and the possibility of automation. Further, SPME is an effective method for analyzing bioactive compounds occurring in very low concentrations in various foods. It can reduce troubles related to solvent clearance. Wells,[Citation33] enumerated that SPME facilitates distinctive research, such as extraction from very small samples (i.e., single cells).
Extraction of Phenolic compounds by utilizing conventional methods, such as maceration, infusion, digestion, and Soxhlet, has been done for many years.[Citation41] Most important among these methods are maceration as well as Soxhlet. Caldas et al.[Citation42] used conventional and non-conventional methods for the recovery of different phenolic compounds from the grape skin. Alara et al.[Citation43] utilized the leaves of vernonia cinerea and peel of feijoa, and also determined the total phenolic compound ranges between 48.6 − 71 mg of GAE (gallic acid equivalent) per gram. In soxhlet extraction and maceration, a high solvent to feed ratio approximately higher than 20 is usually used. In the case of maceration in a specific solvent for a specific period, raw material is extracted. For maceration, little or no agitation is required. Maceration can be done at a lower temperature as compared to soxhlet extraction. It was reported by Ji et al.[Citation44] that utilizing maceration, phenolic compounds were extracted from oil mixture at ambient temperature and optimal conditions. So maceration is beneficial because of low-temperature requirements and low-cost methods. In addition to this required equipment is easy to use. But maceration needs a longer time for extraction and gives lesser yields.
Novel techniques
Ultrasound-assisted extraction (UAE)
Ultrasound is one of the advanced technologies, based on the mechanism of sound frequency ranging between 18 to 100 kHz, which is higher than the hearing frequency of the human ear. It is reported by Jambrak et al.[Citation45] Chemat et al.[Citation46] that by enhancing the mass transfer and bursting the cellular matrix, Ultrasound releases large amounts of targeted compounds and enhances the yield for extraction. In ultrasound extraction method, acoustic cavitation activity occurs, in which microbubbles form in the liquid phase grow when the mixture is exposed to the ultrasound. Before collapsing, it oscillates rapidly owing to the changes in pressure. According to Alarcon-Rojo et al.[Citation47] high-power ultrasound is the type of ultrasonication in which intensities are excessive over 1 W.cm-2 (range from 10 to 1000 W.cm-2). Power ultrasound consists of low frequency (20 kHz) and high frequency (100 kHz), can produce cavitation and is excessively used in food industries.
In contact with the subject plant material, ultrasound waves modify both (the cavitation effect and physicochemical properties) to stimulate extractable compounds’ liberation and modify the mass transport by disrupting the cell walls.[Citation48] Ultrasounds are used successively in the area of plant extraction. Many food compounds such as plant and animal tissues (antioxidants, pigments, organic compounds, and aromas) are withdrawn and examined from various metrics. Ultrasound is a promising choice, particularly when combined with other alternatives among the various available combinations. Together with other technologies, ultrasound can be used for rapid heat and mass transfer in the extraction field.
Sumere et al.[Citation49] reported that the combination of ultrasound and PLE for the phenolic compound extraction from pomegranate peels is possible. In these technologies, the extraction of bioactive compounds was improved. Ultrasound improved extraction yields primarily when large particles were used, varying in temperature (70 to 80°C) and ultrasound power (480 and 640 W), respectively. In combination with PLE, the UAE permitted water usage as solvent extraction by reducing the extraction time. Both SFE and ultrasound-assisted methods have been widely studied. The treatment of agave bagasse antioxidants under the effect of ultrasound on SFE was evaluated by Santos-Zea et al.,[Citation50] who observed an increase in the antioxidants yield recovery when several ultrasound transducers were used. Ultrasound and SFE together cause a rise in the extraction of 1.7-folds (antioxidant) and three-folds (saponin) and also showed that the geometry of the transducer would greatly boost the intensification impact of ultrasound in SFE processes. A desirable approach to the processing of various good quality products consisting of the same matrices (phenolic compounds and essential oils), is the combination of different extraction methods. In addition to getting phenolic compounds, these integrated methods provide interesting alternatives for deriving compounds (bioactive compounds) from natural sources. Bioactive compounds extraction from the peppers was the method reported by de-Aguiar et al. .[Citation51] Firstly, to remove the nonpolar fraction, SFE was used, and in the next step, SFE extracted phenolic compounds (biquinho pepper source) were recovered by PLE.
Instant controlled pressure drop technology
According to the Regulated Pressure-Drop procedure (Détente instantannée contrôlée in French, DIC) in combination with the hydro-thermo-mechanical, the auto-vaporization thermodynamics and instantaneity are core-based concepts that cause the evolution of organic products (foods, cosmetic and pharmaceutical biopolymers). There are four key components of DIC equipment, which include (a) a Regulated pressure-drop valve in the vacuum pump extraction vessel to ensure the steam pressure release is fast and controlled; (b) A vacuum machine (vacuum pump and a tank) more significant than that of the vessel to be treated with a capacity 50 times; (c) an extraction vessel, a part of it called heating jacket autoclave is the portion where the treated sample is placed; (d) An extract collection trap for condensate recovery; the tank pressure is maintained at about 5 kPa by a water ring pump. According to Mounir et al.[Citation52] instant controlled pressure drop (DIC) treatment tends to be an excellent alternative to extending heat-sensitive food granule powder such as apples and onions. The study of Mkaouar et al.[Citation53] demonstrated the polyphenols extraction from olive leaves extracts to be rich in bioactive compounds. Retained bioactive molecules and permitting nutritional value are possible by DIC coupled to hot air drying, at optimum DIC conditions with 0.35 MPa pressure for 10 seconds. Another study by Alonzo-Macías et al.,[Citation54] reported that different methods were used for the drying of strawberries. However, pretreatment of DIC is considered to be way much better than other classical drying methods in the extraction and texturization of vegetable materials. Some of the fragile fruits need to be dry, for this purpose, drying is required for commonly used methods (classical hot air drying and freeze-drying) seem to be less effective than DIC. Furthermore, Alonzo-Macías et al.[Citation54] also reported that swell-drying treated strawberries have higher phenolic compounds, total anthocyanins and flavonoids as compared with the other dying methods.
Pulsed electric field (PEF)
PEF (electroporation or electro-permeabilization) is a non-thermal mechanism in which an external electrical field for a brief period (nanoseconds/ milliseconds) is applied to a bio cell.[Citation55] Even though the membrane permeabilization mechanism is not that well known it is recognized that electroporation consists of four distinct phases, which include; (i) Post-treatment stage of PEF with intracellular compound leakage, the input of extracellular compounds (as irreversible electroporation or pore resealing, and membrane integrity recovery, reversible electroporation). (ii) Modification of the size or the number of the formed pores (During the treatment of PEF), (iii) Enhance the trans-membrane probability of the cytoplasmic membrane by applying the external electric field that causes the charging of the cell membrane, (iv) If a threshold of trans-membrane possibility extends to 0.2–1.0 V, the formation of the small metastable hydrophilic pores.
According to the study of Toepfl et al.[Citation56] PEF unlocks a broad range of applications in food processing due to the mentioned cell membrane of enlarged permeability phenomenon or electroporation interference. The application can be classified depending on the magnitude (external electric field) and (specific energy field). In this area, PEF has become very common because it enables the solid-liquid extraction critical speed. According to Barba et al.,[Citation57] when PEF is applied, extraction technologies in different agro-industries become more selective and less energy-consuming. The application of PEF has excellent potential to substitute or regulate conventional thermal technology (e.g., extraction of sugar from sugar beets). Compounds found in plant cells, for example, colorants which include carotenoids and chlorophylls, sucrose-containing polyphenols, and other secondary metabolites through PEF combine methods, can be accelerated.[Citation58] Results from PEF Pretreatment-assisted extraction indicate a hike in sucrose concentration, better juice filterability, and a drop in colloidal impurity concentration and coloration.[Citation59] Before the maceration fermentation stage, PEF pretreatment can be used to produce wine-making, polyphenol extraction is optimized, and the resulting wine has distinct organoleptic characteristics.[Citation60] Traditional wine-making residues are subject to the same pretreatment increase in anthocyanin (colorant extraction) selectivity is also spotted. Furthermore, yields are significantly increased when mechanical expressions are applied after moderate PEF treatments, fruit juices, and vegetable oils. The electrical treatment in the oil does not cause bad tastes or flavor and yield slightly cloudy, substantially odorous, and more polyphenol components in apple juice.[Citation61,Citation62]
Enzyme-assisted extraction (EAE)
Enzyme-assisted Extraction (EAE) is another latest technique in which to improve the recovery technique, enzymes are added to the extraction medium.[Citation63] The major enzyme function is to weaken or squishy the cell walls when extracted from plant materials. This provide access to the solvent for the active ingredients. Bound phytochemicals (inside cells or on cell walls) are hard to extract with normal solvent extraction. Enzymes digested the surrounding materials that can help let out these components distinctively. However, EAE is considered favorable for the polyphenols bound to protein or carbohydrate extraction (inside or on cell walls). Lipase, α-amylase, pectinase, amyloglucosidase, laccase and protease are commonly used enzymes for enzymatic extraction.[Citation64] The size of the particle and the enzyme proportion to the sample are core control factors for maximizing the polyphenol yield. In the enzymatic hydrolysis extraction method, a sample (enzyme and solvent) mixture is incubated at low temperatures (35–50°C), together with adjusted pH. Hydrolysis is stopped when deactivating enzymes at a temperature of 80–90°C, and less energy is required to prevent degradation in low-temperature extraction. The EAE is famous for the property that it’s an environmentally friendly process. The enzyme works best in an acidic medium, and water is used being organic solvents or as a chemical alternative. Prolonged extraction time (3 hours to 48 hours) is the main drawback of EAE.[Citation65]
Pressurized liquid extraction (PLE)
To deal with high-temperature resistance polyphenols, high-pressure extraction methods are effective. Additionally, these methods boost the polyphenol’s recovery. Pressurized liquid extraction (PLE) depends upon the principle of the ratio of boiling point temperature proportional to the pressure. When the pressure of the extraction system is increased before raising the temperature, the solution remains in a liquid state. The temperature of the PLE varies between 50°C and 200°C.[Citation66,Citation67] However, the maximum extraction temperature depends both on the solvent and on the polyphenols. The chemical solubility (polyphenols in liquids) in PLE is increased by several researchers.[Citation68,Citation69] At an elevated temperature, higher concentrations of polyphenols are recovered. Since a liquid’s sensible heat is less than the vaporization heat, the process is energy saving.us, to increase temperature, less heat is needed than to produce vapor.[Citation67] In PLE, solvents are primarily water and aqueous alcohols. Hence a large part of solvents are water, solvents are low cost, nontoxic, and environmentally friendly. The extraction equipment is essential, primarily the extractor and the associated setup.
Principle supercritical fluids (SCF)
It is one of the most advanced techniques that can be used to substitute organic solvents used in several processes. SCF’s specificity depends on their physical features, which can be modulated, beyond their critical values, by increasing the temperature or/and pressure parameters. Compared to traditional methods, SCF has a liquid-like density that induces a liquid-like solvating power. Many main advantages arise from the use of SCF. By treating (i.e., applying heat and pressure) beyond its critical pressure (Pc) and critical temperature (Tc) values, a fluid is considered to be in its critical state.[Citation70] Development of solvent-free extract is obtained in case of minimal or no use of solvents (when using co-solvents). In this process, the depressurization step allows the number of unit operations to be reduced, requiring no purification or separation procedures. SCFs are adapted for the processing of heat-sensitive biomolecules by operating the entire mechanism at reduced temperatures. To enhance the extraction efficiency, supercritical fluids with combined mechanisms have been examined.[Citation71]
A method that combines pressing and using supercritical gases (Gas Assisted Mechanical Expression) has been recently estimated to enhance extraction yield. This technique has been widely applied to different seeds (cocoa, linseeds, sesame).[Citation72] Bandarra et al.[Citation73] have observed that dense gases or supercritical fluid greatly favored pressing, reduced mechanical pressure of approximately 10 MPa is needed to enhance the yield of oil extracted from 10 to 20. Modern filtration technologies in coupling with extraction technique i.e.SC-CO2 have been evaluated to purify compounds having less molecular weight, such as 1500 g mol−1. This technique has been applied to purify beta carotene extracted from carrot oil and triglycerides from fish oil. Temelli[Citation74] illustrated that development is still going on in the combination of membrane and SCF technologies for refining edible oils.
Microwave extraction
Microwave heating causes the electromagnetic waves to dissipate in an irradiated medium, and this dissipation is based on the dielectric properties and electric field intensity averaged by local time. The essential distinction between heating mechanisms i.e. conventional and microwave is the transfer of heat to the medium from that of the heating system occurs in the case of the conventional type of heating, while the dissipation of heat in irradiated medium occurs in the case of microwave heating.[Citation75] Transfer of heat in microwave heating mechanism, unlike conventional heating, is restricted to the thermal convection or conduction currents. Thus it is suggested from above that a quick temperature rise can be attained. In addition, only the rate at which power is applied and heat is lost is based on the maximum temperature values achieved by heating the substance through microwaves. Ganzler et al.[Citation76] illustrated how microwave energy can be used for extracting several food ingredients for the first time in 1986. There has been a growing appetite for new products in the last decade. Automation-ready processes, along with various other distinctive features such as less time required for extraction and minimal use of organic solvents in avoiding contamination and reducing costs required for the preparation of samples are highly fascinating. Depending on these priorities, developments in green microwave extraction mechanisms have developed two methods i.e. microwave hydrodiffusion and gravity (MHG) and microwave-assisted distillation (MAD). Fully reproducible food processes can be completed with high reproducibility in just a few seconds or may take minutes also, consuming just little energy and the short time usually required for those traditional processes that are to be heated by radiation or conduction methods. Reduced production costs, simplified handling, and work-up provided greater final product purity and removed wastewater post-treatment. The resulting benefit for food production could include efficient control of heating processes, rapid packaged food heating, reduced size of equipment, more yield, and reduced process steps. Compared to conventional methods, microwave-assisted extraction has been regarded as a valuable substitute for extracting various biologically active substances from raw plants and animals. MAE’s key benefits include: (1) increased extraction yield; (2) reduced extraction time. Instead of conventional organic solvents, most cases use water as a solvent and simplify the process. Therefore, the MAE method is used to extract bioactive compounds from several animals and plant sources.[Citation77]
Various modern extraction methods are also shown in .
Combination of modern techniques for effective extraction
MAE and PEF
According to Azmir et al.[Citation4] the idea of pulsed electric field-assisted extraction is to enhance mass transfer rates through cell membranes by denaturing its structure. Pulsed electric field and microwave-assisted extraction are efficient techniques used to extract biologically active substances from plant sources. Because of their short processing time, both techniques consume a low level of energy, as mentioned earlier. The pulsed electric fields can run in batch or continuous phases based on various treatment chamber configuration modes. While microwave-assisted extraction is currently intermittent.[Citation78] Individual unique specifications typically determine the instruments used in PEF-assisted extraction. MAE usually uses environmentally friendly and inexpensive sources of solvent (water) for extraction. However, PEF extraction methods usually use organic solvents, such as ethanol.[Citation79] Consequently, MAE equipment is cheaper and simpler to operate than PEF.
MAE and SFE
Supercritical fluid extraction technique uses solvent i.e. supercritical fluid, to extract biologically active substances from plant sources. Supercritical fluid extraction and microwave-assisted extraction are preferable techniques for being efficient and environmentally friendly for extraction purposes.[Citation80] MAE has a comparatively lower operating temperature than SFE. Equipment coupled with the high-pressure technique that is expensive and a safety hazard to operating personnel is needed by the SFE technique. It is possible to recycle and reuse supercritical fluids, which would reduce waste generation. The choice of extraction solvent for MAE is more detailed compared to SFE. Extraction solvent can be chosen based on the polarity of several targeted compounds.
EAE and MAE
Enzyme-assisted extraction is mainly concerned with applications of various enzymes capable of catalyzing reactions of exquisite specificity, and region selectivity.[Citation81] MAE, UAE, and supercritical fluid extraction are those extraction techniques that have been applied to enzyme pretreatment.[Citation82] The new powerful extraction technologies are both EAE and MAE. The solvent consumption and reduction in extraction reduce the cost of extraction. Enzyme preparations currently available do not fully hydrolyze the cell wall of plants, restricting the production of targeted substances. The price of microwave-assisted extraction is less since the enzymes are comparatively costly for processing vast quantities of raw material than solid reagents. However, the other extraction techniques, like the MAE technique, are typically non-specific techniques and can introduce variation. Moreover, microwave-assisted extraction is estimated to bear comparatively greater investment costs, including the high price of the ball mill installation.[Citation83]
NPC and MAE
Negative pressure cavitation (NPC) is a modern extraction technology that is an environmentally friendly and reliable technique. The naturally occurring fluid mechanics phenomenon is called Cavitation, divided according to the cause of formation into the hydrodynamic and acoustic cavitation.[Citation84] NPC is an economical and energy-effective system that can sustain suitable intensity and low-temperature ranges constantly. Negative pressure cavitation and microwave-assisted extraction technologies made it possible to efficiently save time and energy requirements and ensure effective performance to extract several biologically active substances from plant sources. Both these techniques can be effectively used to remove temperature-sensitive compounds that bear less operating temperatures. In general, water is mainly used in the MAE technique instead of a conventional organic solvent. Solvents that are organic such as ethanol are still required in negative pressure cavitation extraction techniques are highly costly and less environment friendly. In general, the NPC extraction technology device is not mature enough. Some researchers utilized their laboratory-designed NPC equipment. A negative pressure cavitation instrument is expensive, and thus it is more difficult to manipulate the NPC process.[Citation85]
It is not sufficient for all parameters to be expanded at the same time. We should therefore assess the necessary factors for the scale-up. EAE, MAE, SFE, negative pressure cavitation (NPC) extraction and pulsed electric field (PEF) extraction are efficient techniques for extracting compounds from plant materials are enzyme-assisted extraction (EAE), supercritical fluid extraction (SFE).[Citation86] So, the combination of innovative methods is highly effective for the extraction of bioactive compounds. The effects of different extraction techniques on the extraction yield of bioactive compounds are discussed in .
Table 1. Effect of different extraction techniques on extraction yield of bioactive compounds
Potential applications of bioactive compounds
Bioactive compounds are widely used therapeutic agents and have many health endorsing properties. These compounds are capable to improve the technical aspects of innovative products. The main reason behind its application in the development of natural and health-friendly products which are also less aggressive to the environment.[Citation99] Poly-phenols being a bioactive compound are proven to behave as a preservative in food products. Like a common synthetic antioxidant (hydroxytoluene BHT, 100 ppm), in sheep meat nuggets, 1.5% lychee pericarp extract exhibits clear prevention instead of lipid oxidation.[Citation100]
The antibacterial property of polyphenol additives inhibits the growth of certain bacteria (mesophyll, Staphylococcus aureus and coliforms) and also prevents the expansion of molds and yeast.[Citation101] Furthermore, anthocyanin polyphenols are widely used as coloring additives (additive number-E163) in food manufacturing industries. Albuquerque et al.[Citation102] described that natural colorants and stable red-purple colors can be used in many food matrices obtained from anthocyanin pigments which are extracted from berries.[Citation103] Apart from that, anthocyanin studies in fresh sausages exhibit that it can also reduce microbiological degradation and raise antioxidant activity. It can be easily observed from these examples, that the inevitable use of polyphenols in several food products is value-added to health protection by developing functional products and enriching the bioactive compounds.[Citation104] Different usage in food industries as bioactive packaging agents, for example, anthocyanin (fish freshness indicators) anti-lipidic peroxidation agents (olive oil packing),[Citation105] and tannin edible films (protein-based) have an antioxidative and antimicrobial effect on Listeria innocua and Escherichia coli.[Citation106]
Tannin water treatment is an active example of phenolic therapy, which functioned as an absorbent that can complex with protein and metal ions to eliminate the water pollutants (surfactants, heavy metals, pharmaceutical compounds, and dyes). Considering the sudden changes in consumers’ demand for natural and healthy products over synthetic additives, polyphenol compounds have become more recognized in food industries. Artificial dyes used in textile industries result in the production of wastewater full of harmful chemicals; however, Albuquerque et al.[Citation102] proved that in silk and wool fabric dyeing, polyphenols possessed colors (vary from yellow to purple) are used, which make it less toxic as compared to other artificial chemicals. Polyphenol dyes (obtained from oak barks) when applied to the silk (tussah silk) also provide antimicrobial and UV protection. It was also observed that when cotton is dyed with extract of phenolic tea, it exhibits UV protection quality.
Polyphenol compounds (phytochemicals) also prove their worth in the field of cosmetic industry. The potentialities of polyphenols play a vital role in anti-aging and skin protection (Vivo ad invitro skin).[Citation101] Due to its anti-inflammatory property, it’s highly used in a cream base, and as it blocks solar radiations, it’s also used in sunblock creams. During the chemotherapy treatment, epigallocatechin-3-gallate (obtained from green tea) promotes hair growth,[Citation102] and a polyphenol-rich balm reduces nail damage.[Citation103]
Hence, bioactive compounds have now been reported to exhibit important health effects including antimicrobial, antioxidant, anti-inflammatory, anticancer, antidiabetic and wound healing actions in humans.
Role of eco-innovative techniques in sustainable food system
United Nations’ Sustainable Development Goals (SDGs) are the foundation of a sustainable food system. To achieve the target of zero hunger, food security, and improving nutrition by 2030, in 2015, the SDGs identified the essential modification in the food and agriculture system. A sustainable food system involves economic, environmental, and social sustainability. Modern extraction techniques play an essential role in maintaining a sustainable food system by recovering valuable bioactive and other valuable substances from food by-products. It was reported by Herrero et al.[Citation107] that supercritical fluids-CO2 could be utilized for the extraction of substances such as flavor, oil, pigments, and flavors from plants and other sources. Carotenoids are complicated compounds that are easily susceptible to oxidation and be affected by light and heat. Saini and Keum,[Citation77] described that SFE-CO2 has more potential to recover carotenoids from plants rather than organic solvents.
Similarly, it was stated that for the extraction of carotenoids using SFC-CO2and ethanol as solvent from carrot peels, for the mass yield of 53.1%, the best conditions were 14.3% of ethanol, 58.5°C, 360 bar and for a mass yield of 86.1% recovery of carotenoids 15.5% ethanol, 59°C, 349 bar were observed best. According to Wu et al.[Citation108,Citation109]*** mechanochemical assisted extractions are innovative methods were found eco-friendly to recover value-added products or compounds. Sarkis et al.[Citation88] reported that pulse electric field (PEF) and high voltage electrical discharge (HVED) can lessen the requirement of high temperature as well as the organic solvent. Moreover, pre-treatments were helpful from the sesame cake for better extraction of polyphenols, proteins, and lignans. According to Rosello-Soto et al.,[Citation110] the ultrasound technique maximizes the extraction yield of bioactive compounds and minimizes extraction time. It is stated by Roohinejad et al.[Citation99] that negative pressure cavitation (NPC) for extraction of the bioactive compound is a novel technique that is proved to be economical and highly efficient. The eco-friendly extraction methods are crucial to extract bioactive compounds and are used in sustainable food systems.
Conclusion
Traditional as well as novel extraction technologies and their combinations are also the limelighted of this current article. Novel extraction technologies are used for getting maximum yield in less time, and enhancing quality as well as considered as eco-environmentally. Various researchers are now focusing on the use of these innovative extraction technologies in combination. However, these unique extraction procedures still need to be adequately developed whilst optimized conditions are needed to make a scaling process. These processes might be an essential step toward the long-term production and use of bioactive compounds from medicinal plants. Furthermore, the combination of modern extraction methods should be introduced at pilot scales. So, there is a dire need for mechanistic studies to thoroughly understand the mechanisms leading to the extraction of bioactive compounds from different extraction methods.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saeed, F.; Hussain, M.; Arshad, M. S.; Afzaal, M.; Munir, H.; Imran, M.; Tufail, T.; Anjum, F. M. Functional and Nutraceutical Properties of Maize Bran Cell Wall Non-starch Polysaccharides. Int. J. Food Prop. 2021, 24(1), 233–248. DOI: 10.1080/10942912.2020.1858864.
- Gomes-Araújo, R.; Martínez-Vázquez, D. G.; Charles-Rodríguez, A. V.; Rangel-Ortega, S.; Robledo-Olivo, A. Bioactive Compounds from Agricultural Residues, Their Obtaining Techniques, and the Antimicrobial Effect as Postharvest Additives. Int. J. Food Sci. 2021, 2021. DOI: 10.1155/2021/9936722.
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-food Wastes: Present Insights and Future Challenges. Molecules. 2020, 25(3), 510. DOI: 10.3390/molecules25030510.
- Azmir, J.; Zaidul, I. S. M.; Rahman, M. M.; Sharif, K. M.; Mohamed, A.; Sahena, F.; Jahurul, M. H. A.; Ghafoor, K.; Norulaini, N. A. N.; Omar, A. K. M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117(4), 426–436. DOI: 10.1016/j.jfoodeng.2013.01.014.
- Koçak, E.; Pazır, F. Effect of Extraction Methods on Bioactive Compounds of Plant Origin. Turkish Journal of Agriculture-Food Science and Technology (TURJAF). 2018, 6(6), 663–675. DOI: 10.24925/turjaf.v6i6.663-675.1527.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K. M.; Latha, L. Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8(1), 1–10. DOI: 10.4314/ajtcam.v8i1.60483.
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Cravotto, G.; Cravotto, G. Impact of Ultrasound, Microwaves and High-pressure Processing on Food Components and Their Interactions. Trends Food Sci. Technol. 2021, 109, 1–15. DOI: 10.1016/j.tifs.2021.01.017.
- Hussain, M.; Ullah Khan, A.; Saeed, F.; Afzaal, M.; Mushtaq, Z.; Niaz, B.; Anjum, F. M.; Mohamed, A. A.; Alamri, M. S.; Anjum, F. M. Physicochemical Characterization of Cereal Bran Cell Wall with Special Reference to Its Rheological and Functional Properties. Int. J. Food Prop. 2022, 25(1), 305–314. DOI: 10.1080/10942912.2022.2032138.
- Rahim, M. A.; Saeed, F.; Khalid, W.; Hussain, M.; Anjum, F. M. Functional and Nutraceutical Properties of Fructo-oligosaccharides Derivatives: A Review. Int. J. Food Prop. 2021, 24(1), 1588–1602. DOI: 10.1080/10942912.2021.1986520.
- Jideani, A. I.; Silungwe, H.; Takalani, T.; Omolola, A. O.; Udeh, H. O.; Anyasi, T. A. Antioxidant-rich Natural Fruit and Vegetable Products and Human Health. Int. J. Food Prop. 2021, 24(1), 41–67. DOI: 10.1080/10942912.2020.1866597.
- Afzaal, M.; Saeed, F.; Rasheed, R.; Hussain, M.; Aamir, M.; Hussain, S.; Anjum, F. M.; Alamri, M. S.; Anjum, F. M. Nutritional, Biological, and Therapeutic Properties of Black Garlic: A Critical Review. Int. J. Food Prop. 2021, 24(1), 1387–1402. DOI: 10.1080/10942912.2021.1967386.
- Carpena, M.; Pereira, R. D.; Garcia-Perez, P.; Otero, P.; Soria-Lopez, A.; Chamorro, F.;, and Simal-Gandara, J. An Overview of Food Bioactive Compounds and Their Properties. Membrane Separation of Food Bioactive Ingredients. 2021, 39–79. https://link.springer.com/book/10.1007/978-3-030-84643-5
- Hill, R. A.; Connolly, J. D. Triterpenoids. Nat. Prod. Rep. 2018, 35(12), 1294–1329. DOI: 10.1039/C8NP00029H.
- Moreau, R. A.; Nyström, L.; Whitaker, B. D.; Winkler-Moser, J. K.; Baer, D. J.; Gebauer, S. K.; Hicks, K. B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses.Prog. Lipid Res 2018, 70, 35–61. DOI: 10.1016/j.plipres.2018.04.001.
- Multari, S.; Carlin, S.; Sicari, V.; Martens, S. Differences in the Composition of Phenolic Compounds, Carotenoids, and Volatiles between Juice and Pomace of Four Citrus Fruits from Southern Italy. Eur. Food Res. Technol. 2020, 246(10), 1991–2005. DOI: 10.1007/s00217-020-03550-8.
- Câmara, J. S.; Albuquerque, B. R.; Aguiar, J.; Corrêa, R. C.; Gonçalves, J. L.; Granato, D.; Pereira, J. A.; Barros, L.; Ferreira, I. C. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods. 2021, 10(1), 37. DOI: 10.3390/foods10010037.
- Porter, N. T.; Martens, E. C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. Doi:10.1146/annurev-micro-102215-095316.
- Saini, R. K.; Keum, Y. S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and significance—A Review. Life Sci. 2018, 203, 255–267. DOI: 10.1016/j.lfs.2018.04.049.
- Calder, P. C. Polyunsaturated Fatty Acids and Inflammation. Prostag. Leukotr ESS. 2006, 75(3), 197–202. DOI: 10.1016/j.plefa.2006.05.012.
- Ferreira, I. C.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16(12), 1543–1560. DOI: 10.2174/092986709787909587.
- Abbas, M.; Saeed, F.; Anjum, F. M.; Afzaal, M.; Tufail, T.; Bashir, M. S.; Ishtiaq, A.; Hussain, S.; Suleria, H. A. R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20(8), 1689–1699. DOI: 10.1080/10942912.2016.1220393.
- Majdi, C.; Pereira, C.; Dias, M. I.; Calhelha, R. C.; Alves, M. J.; Rhourri-Frih, B.; Ferreira, I. C.; Barros, L.; Amaral, J. S.; Ferreira, I. C. F. R. Phytochemical Characterization and Bioactive Properties of Cinnamon Basil (Ocimum basilicum cv.‘cinnamon’) and Lemon Basil (Ocimum× Citriodorum). Antioxidants. 2020, 9(5), 369. DOI: 10.3390/antiox9050369.
- Rein, M. J.; Renouf, M.; Cruz‐Hernandez, C.; Actis‐Goretta, L.; Thakkar, S. K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75(3), 588–602. DOI: 10.1111/j.1365-2125.2012.04425.x.
- McClements, D. J. Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 2020, 38, 107287. DOI: 10.1016/j.biotechadv.2018.08.004.
- Kamiloglu, S. Effect of Different Freezing Methods on the Bioaccessibility of Strawberry Polyphenols. Int. J. Food Sci. 2019, 54(8), 2652–2660. DOI: 10.1111/ijfs.14249.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules. 2016, 21(7), 901. DOI: 10.3390/molecules21070901.
- Bart, H. J., and Pilz, S., Eds. Industrial Scale Natural Products Extraction; Wiley Online Library: John Wiley & Sons, 2011.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D. G.; Lightfoot, D. A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017, 6(4), 42. DOI: 10.3390/plants6040042.
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods. 2017, 6(5), 33. DOI: 10.3390/foods6050033.
- Tomsone, L.; Kruma, Z.; Galoburda, R. Comparison of Different Solvents and Extraction Methods for Isolation of Phenolic Compounds from Horseradish Roots (Armoracia rusticana). World Acad. Sci. Eng. Technol. 2012, 64(4), 903–908. https://www.researchgate.net/publication/285076719.
- Bouchard, A.; Jovanović, N.; Jiskoot, W.; Mendes, E.; Witkamp, G. J.; Crommelin, D. J.; Hofland, G. W. Lysozyme Particle Formation during Supercritical Fluid Drying: Particle Morphology and Molecular Integrity. J. Supercrit. Fluids. 2007, 40(2), 293–307. DOI: 10.1016/j.supflu.2006.07.005.
- Cañadas, R.; Gonzalez-Miquel, M.; González, E. J.; Díaz, I.; Rodriguez, M. Overview of Neoteric Solvents as Extractants in Food Industry: A Focus on Phenolic Compounds Separation from Liquid Streams. Int. Food Res. J. 2020, 136, 109558. DOI: 10.1016/j.foodres.2020.109558.
- Wells, M. J. Principles of Extraction and the Extraction of SemivolatileOrganics from Liquids. CHEMICAL ANALYSIS-NEW YORK-INTERSCIENCE THEN JOHN WILEY-.2003, 37–138. https://books.google.com.pk/books
- Yahya, N. A.; Attan, N.; Wahab, R. A. An Overview of CosmeceuticallyRelevant Plant Extracts and Strategies for Extraction of Plant-Based Bioactive Compounds. Processing Food Bioprod. Process 2018, 112, 69–85. DOI: 10.1016/j.fbp.2018.09.002.
- Conde-Hernández, L. A.; Botello-Ojeda, A. G.; Alonso-Calderón, A. A.; Osorio-Lama, M. A.; Bernabé-Loranca, M. B.; Chavez-Bravo, E. Optimization of Extraction of Essential Oils Using Response Surface Methodology: A Review. Essent. Oil-Bear. Plants. 2021, 24(5), 937–982. DOI: 10.1080/0972060X.2021.1976286.
- Bidari, A.; Ganjali, M. R.; Norouzi, P.; Hosseini, M. R. M.; Assadi, Y. Sample Preparation Method for the Analysis of Some Organophosphorus Pesticides Residues in Tomato by Ultrasound-Assisted Solvent Extraction Followed by Dispersive Liquid–Liquid Microextraction. Food Chem. 2011, 126(4), 1840–1844. DOI: 10.1016/j.foodchem.2010.11.142.
- Murakami, H.; Omiya, M.; Miki, Y.; Umemura, T.; Esaka, Y.; Inoue, Y.; Teshima, N. Evaluation of the Adsorption Properties of Nucleobase-modified Sorbents for a Solid-phase Extraction of Water-soluble Compounds. Talanta. 2020, 217, 121052. DOI: 10.1016/j.talanta.2020.121052.
- Poole, C. F. New Trends in Solid-Phase Extraction. Trends Analyt Che 2003, 22(6), 362–373. DOI: 10.1016/S0165-9936(03)00605-8.
- Tan, S. C.; Leow, J. W. S.; Lee, H. K. Emulsification-assisted Micro-solid-phase Extraction Using a Metal-organic Framework as Sorbent for the Liquid Chromatography-tandem Mass Spectrometric Analysis of Polar Herbicides from Aqueous Samples. Talanta. 2020, 216, 120962. DOI: 10.1016/j.talanta.2020.120962.
- Merkle, S.; Kleeberg, K. K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography. 2015, 2(3), 293–381. DOI: 10.3390/chromatography2030293.
- Osorio-Tobón, J. F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. DOI: 10.1007/s13197-020-04433-2.
- Caldas, T. W.; Mazza, K. E.; Teles, A. S.; Mattos, G. N.; Brígida, A. I. S.; Conte-Junior, C. A.; Tonon, R. V.; Godoy, R. L. O.; Cabral, L. M. C.; Tonon, R. V. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops Prod. 2018, 111, 86–91. DOI: 10.1016/j.indcrop.2017.10.012.
- Alara, O. R.; Abdurahman, N. H.; Ukaegbu, C. I. Soxhlet Extraction of Phenolic Compounds from Vernonia cinerea Leaves and Its Antioxidant Activity. J. Appl. Res. Med. Aromat. Plants. 2018, 11, 12–17. DOI: 10.1016/j.jarmap.2018.07.003.
- Ji, Y.; Hou, Y.; Ren, S.; Yao, C.; Wu, W. Highly Efficient Extraction of Phenolic Compounds from Oil Mixtures by Trimethylamine-Based DicationicIonic Liquids via Forming Deep Eutectic Solvents. Fuel Process. Technol. 2018, 171, 183–191. DOI: 10.1016/j.fuproc.2017.11.015.
- Jambrak, A. R.; Mason, T. J.; Lelas, V.; Herceg, Z.; Herceg, I. L. Effect of Ultrasound Treatment on Solubility and Foaming Properties of Whey Protein Suspensions. J. Food Eng. 2008, 86(2), 281–287. DOI: 10.1016/j.jfoodeng.2007.10.004.
- Chemat, F.; Tomao, V., and Virot, M. Ultrasound-Assisted Extraction in Food Analysis. Semih, Otles Eds. Handbook of Food Analysis Instruments. 2008, 85–103; Routledge. https://d1wqtxts1xzle7.cloudfront.net/54933605/Semih_Otles-Handbook_of_food_analysis_instruments-CRC_Press_2009-with-cover-page-
- Alarcon-Rojo, A. D.; Janacua, H.; Rodriguez, J. C.; Paniwnyk, L.; Mason, T. J. Power Ultrasound in Meat Processing. Meat Sci. 2015, 107, 86–93. DOI: 10.1016/j.meatsci.2015.04.015.
- Li, S.; Lei, D.; Zhu, Z.; Cai, J.; Manzoli, M.; Jicsinszky, L.; Grillo, G.; Cravotto, G. Complexation of Maltodextrin-based Inulin and Green Tea Polyphenols via Different Ultrasonic Pretreatment. Ultrason. Sonochem. 2021, 74, 105568. DOI: 10.1016/j.ultsonch.2021.105568.
- Sumere, B. R.; de Souza, M. C.; Dos Santos, M. P.; Bezerra, R. M. N.; da Cunha, D. T.; Martinez, J.; Rostagno, M. A. Combining Pressurized Liquids with Ultrasound Improves the Extraction of Phenolic Compounds from Pomegranate Peel (Punica granatum L.). UltrasonSonochem. 2018, 48, 151–162. DOI: 10.1016/j.ultsonch.2018.05.028.
- Santos-Zea, L.; Gutiérrez-Uribe, J. A.; Benedito, J. Effect of Ultrasound Intensification on the Supercritical Fluid Extraction of Phytochemicals from Agave Salmiana Bagasse. J. Supercrit. Fluids. 2019, 144, 98–107. DOI: 10.1016/j.supflu.2018.10.013.
- de Aguiar, A. C.; da Fonseca Machado, A. P.; Angolini, C. F. F.; de Morais, D. R.; Baseggio, A. M.; Eberlin, M. N.; Martinez, J.; Martínez, J. Sequential High-Pressure Extraction to Obtain Capsinoids and Phenolic Compounds from Biquinho Pepper (Capsicum Chinense). J. Supercrit. Fluids. 2019, 150, 112–121. DOI: 10.1016/j.supflu.2019.04.016.
- Mounir, S.; Besombes, C.; Al-Bitar, N.; Allaf, K. Study of Instant Controlled Pressure Drop DIC Treatment in Manufacturing Snack and Expanded Granule Powder of Apple and Onion. Dry. 2011, 29(3), 331–341. DOI: 10.1080/07373937.2010.491585.
- Mkaouar, S.; Bahloul, N.; Gelicus, A.; Allaf, K.; Kechaou, N. Instant Controlled Pressure Drop Texturing for Intensifying Ethanol Solvent Extraction of Olive (Oleaeuropaea) Leaf Polyphenols. Sep. Purif. Technol. 2015, 145, 139–146. DOI: 10.1016/j.seppur.2015.03.014.
- Alonzo-Macías, M.; Cardador-Martínez, A.; Mounir, S.; Montejano-Gaitán, G.; Allaf, K. Comparative Study of the Effects of Drying Methods on Antioxidant Activity of Dried Strawberry (Fragaria Var. Camarosa). J. Food Res. 2013, 2(2), 92–107. DOI: 10.5539/jfr.v2n2p92.
- Saulis, G. Electroporation of Cell Membranes: The Fundamental Effects of Pulsed Electric Fields in Food Processing. Food Eng. Rev. 2010, 2(2), 52–73. Accessed 16 May 2010. https://link.springer.com/article/10.1007/s12393-010-9023-3
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22(4), 405–423. DOI: 10.1080/87559120600865164.
- Barba, F. J.; Parniakov, O.; Pereira, S. A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Vorobiev, E. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Int. Food Res. J. 2015, 77, 773–798. DOI: 10.1016/j.foodres.2015.09.015.
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving Mass Transfer to Soften Tissues by Pulsed Electric Fields: Fundamentals and Applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. DOI: 10.1146/annurev-food-022811-101208.
- Loginova, K. V.; Vorobiev, E.; Bals, O.; Lebovka, N. I. Pilot Study of Countercurrent Cold and Mild Heat Extraction of Sugar from Sugar Beets, Assisted by Pulsed Electric Fields. J. Food Eng. 2011, 102(4), 340–347. DOI: 10.1016/j.jfoodeng.2010.09.010.
- El Darra, N.; Turk, M. F.; Ducasse, M. A.; Grimi, N.; Maroun, R. G.; Louka, N.; Vorobiev, E. Changes in Polyphenol Profiles and Color Composition of Freshly Fermented Model Wine Due to Pulsed Electric Field, Enzymes, and Thermovinification Pretreatments. Food Chem. 2016, 194, 944–950. DOI: 10.1016/j.jfoodeng.2010.09.010.
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J., and Sánchez-Gimeno, A. C. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioproc. Tech. 2013, 6(6), 1367–1373. Accessed 14 March 2012. https://link.springer.com/article/10.1007/s11947-012-0817-6
- Turk, M. F.; Vorobiev, E.; Baron, A. Improving Apple Juice Expression and Quality by Pulsed Electric Field on an Industrial Scale. Food Sci. Technol. 2012, 49(2), 245–250. DOI: 10.1016/j.lwt.2012.07.024.
- Nadar, S. S.; Rao, P.; Rathod, V. K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. DOI: 10.1016/j.foodres.2018.03.006.
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I. C. Enzyme-assisted Extractions of polyphenols–A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. DOI: 10.1016/j.tifs.2019.03.029.
- Malik, J., and Mandal, S. C. Extraction of Herbal Biomolecules. In Subhash, C., Mandal, Amit Kumar Nayak, Amal Kumar Dhara, Eds. Herbal Biomolecules in Healthcare Applications; Academic Press, 2022; pp 21–46.
- Pasrija, D., and Anandharamakrishnan, C. Techniques for Extraction of Green Tea Polyphenols: A Review. Food Bioproc. Tech. 2015, 8(5), 935–950. Accessed 10 February 2015. https://link.springer.com/article/10.1007/s11947-015-1479-y
- Hossain, M. B.; Rawson, A.; Aguiló-Aguayo, I.; Brunton, N. P.; Rai, D. K. Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction. Molecules. 2015, 20(5), 8560–8573. DOI: 10.3390/molecules20058560.
- de Sousa Sabino, L. B.; Alves Filho, E. G.; Fernandes, F. A. N.; de Brito, E. S.; da Silva Júnior, I. J. Optimization of Pressurized Liquid Extraction and Ultrasound Methods for Recovery of Anthocyanins Present in JambolanFruit (Syzygium cumini L.). Food Bioprod. Process.2021 2021, 127, 77–89. DOI: 10.1016/j.fbp.2021.02.012.
- Mukhopadhyay, M.; Panja, P. Pressurized Hot Water as a Novel Extractant of Natural Products: AReview. Indian Chem. Eng 2010, 51(4), 311–324. DOI: 10.1080/00194500903430655.
- Brunner, G. Supercritical Fluids: Technology and Application to Food Processing. J. Food Eng. 2005, 67(1–2), 21–33. DOI: 10.1016/j.jfoodeng.2004.05.060.
- Pawliszyn, J.;. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Academic Press, 2012. Accessed 1 June 2012.
- Willems, P.; Kuipers, N. J. M.; De Haan, A. B. Hydraulic Pressing of Oilseeds: Experimental Determination and Modeling of Yield and Pressing Rates. J. Food Eng. 2008, 89(1), 8–16. DOI: 10.1016/j.jfoodeng.2008.03.023.
- Bandarra, N. M.; Batista, I.; Bispo, P.; Nunes, M. L.; Venegas-Venegas, E.; Rincón-Cervera, M. A.; Guil-Guerrero, J. Fish Oil: Production, Consumption and Health Benefits. Fish Oil: Production, Consumption and Health Benefits. 2012, 1–39.
- Temelli, F. Perspectives on Supercritical Fluid Processing of Fats and Oils. J. Supercrit. Fluids. 2009, 47(3), 583–590. DOI: 10.1016/j.supflu.2008.10.014.
- Pérez-Martínez, B. T.; Aboudzadeh, M. A.; Schubert, U. S.; Leiza, J. R.; Tomovska, R. Microwave Irradiation versus Conventional Heating Assisted Free-radical Copolymerization in Solution. Chem. Eng. J. 2020, 399, 125761. DOI: 10.1016/j.cej.2020.125761.
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave Extraction: A Novel Sample Preparation Method for Chromatography. J. Chromatogr. A. 1986, 371, 299–306. DOI: 10.1016/S0021-9673(01)94714-4.
- Saini, R. K.; Keum, Y. S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. DOI: 10.1016/j.foodchem.2017.07.099.
- Cardoso-Ugarte, G. A.; Sosa-Morales, M. E.; Ballard, T.; Liceaga, A.; San Martín-González, M. F. Microwave-Assisted Extraction of Betalains from Red Beet (Beta Vulgaris). Food Sci. Technol. 2014, 59(1), 276–282. DOI: 10.1016/j.lwt.2014.05.025.
- Chemat, F., and Cravotto, G. Eds. Microwave-assisted Extraction for Bioactive Compounds: Theory and Practice; New York: Springer Science & Business Media: Vol. 4, 2012.
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G. P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent Developments in Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: Role of Key Parameters, Technological Achievements and Challenges. J. CO2 Util. 2020, 36, 196–209. DOI: 10.1016/j.jcou.2019.11.014.
- Gardossi, L.; Poulsen, P. B.; Ballesteros, A.; Hult, K.; Švedas, V. K.; Vasić-Rački, Đ.; Halling, P. J.; Magnusson, A.; Schmid, A.; Wohlgemuth, R. Guidelines for Reporting of Biocatalytic Reactions. Trends Biotechnol. 2010, 28(4), 171–180. DOI: 10.1016/j.tibtech.2010.01.001.
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S. S. Enzyme-Assisted Supercritical Fluid Extraction of Phenolic Antioxidants from Pomegranate Peel. J. Supercrit. Fluids. 2015, 104, 122–131. DOI: 10.1016/j.supflu.2015.05.020.
- Chemat, F.; Vian, M. A.; Fabiano-Tixier, A. S.; Nutrizio, M.; Jambrak, A. R.; Munekata, P. E.; Lorenzo, J. M.; Barba, F. J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22(8), 2325–2353. DOI: 10.1039/C9GC03878G.
- Yao, X. H.; Zhang, D. Y.; Luo, M.; Jin, S.; Zu, Y. G.; Efferth, T.; Fu, Y. J. Negative Pressure Cavitation-Microwave Assisted Preparation of Extract of Pyrola Incarnata Fisch. Rich in Hyperin, 2′-O-Galloylhyperin and Chimaphilin and Evaluation of Its Antioxidant Activity. Food Chem. 2015, 169, 270–276. DOI: 10.1016/j.foodchem.2014.07.115.
- Panda, D.; Manickam, S. Cavitation technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9(4), 766. DOI: 10.3390/app9040766.
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes. 2020, 8(10), 1222. DOI: 10.3390/pr8101222.
- Jun, X.; Deji, S.; Shou, Z.; Bingbing, L.; Ye, L.; Rui, Z. Characterization of Polyphenols from Green Tea Leaves Using a High Hydrostatic Pressure Extraction. Int. J. Pharm. 2009, 382, 139–143. DOI: 10.1016/j.ijpharm.2009.08.023.
- Sarkis, J. R.; Boussetta, N.; Tessaro, I. C.; Marczak, L. D. F.; Vorobiev, E. Application of Pulsed Electric Fields and High Voltage Electrical Discharges for Oil Extraction from Sesame Seeds. J. Food Eng. 2015, 153, 20–27. DOI: 10.1016/j.jfoodeng.2014.12.003.
- Cardoso, C. L.; Serrano, C. M.; Quintero, E. T.; Lopez, C. P.; Antezana, R. M.; Martinez de la Ossa, E. J. High Pressure Extraction of Antioxidants from Solanum Stenotomun Peel. Molecules. 2013, 18, 3137–3151. DOI: 10.3390/molecules18033137.
- Phongthai, S.; Lim, S. T.; Rawdkuen, S. Optimization of Microwave-Assisted Extraction of Rice Bran Protein and Its Hydrolysates Properties. J. Cereal Sci. 2016, 70, 146–154. DOI: 10.1016/j.jcs.2016.06.001.
- Kannan, V. Extraction of Bioactive Compounds from Whole Red Cabbage and Beetroot Using Pulsed Electric Fields and Evaluation of Their Functionality. Dissertations and Theses in Food Science and Technology. Paper. 2011. 11. 147 pages. 67–95. Available at; http://digitalcommons.unl.edu/foodscidiss/11 [accessed on 08 Dec 2017]
- Sun, M.; Temelli, F. Supercritical Carbon Dioxide Extraction of Carotenoids from Carrot Using Canola Oil as a Continuous Co-solvent. J. Supercrit. Fluids. 2006, 37(3), 397–408. DOI: 10.1016/j.supflu.2006.01.008.
- Jun, X. Application of High Hydrostatic Pressure Processing of Food to Extracting Lycopene from Tomato Paste Waste. High Press. Res. 2006, 26(1), 33–41. DOI: 10.1080/08957950600608741.
- Adil, I.; Bayındırlı, A. Pressurized Liquid Extraction of Phenolic Compounds from Fruit Pomaces. Natural and Applied Sciences, Middle East Technical University. Ph. D. Thesis, 2006, 122 pp, Ankara, TURKEY. http://etd.lib.metu.edu.tr/upload/3/12607525/index.pdf
- Luthria, D. L.; Biswas, R.; Natarajan, S. Comparison of Extraction Solvents and Techniques Used for the Assay of Isoflavones from Soybean. Food Chem. 2007, 105, 325–333. DOI: 10.1016/j.foodchem.2006.11.047.
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á. D.; García-Martinez, J. B.; L’Abbate, P. Optimization of Enzyme-assisted Extraction of Flavonoids from Corn Husks. Processes. 2019, 7(11), 804. DOI: 10.3390/pr7110804.
- Min, J. Y.; Kang, S. M.; Park, D. J.; Kim, Y. D.; Jung, H. N.; Yang, J. K.; Seo, W. T.; Kim, S. W.; Karigar, C. S., and Choi, M. S. Enzymatic Release of Ferulic Acid from LpomoeaBatatas (Sweet Potato) Stem. Biotech. Bioprocessing. 2006, 11(August 2006), 372–376. https://link.springer.com/content/pdf/10.1007/BF03026256.pdf
- Hussain, M.; Qamar, A.; Saeed, F.; Rasheed, R.; Niaz, B.; Afzaal, M.; Mushtaq, Z.; Anjum, F. Biochemical Properties of Maize Bran with Special Reference to Different Phenolic Acids. Int. J. Food Prop. 2021, 24(1), 1468–1478. DOI: 10.1080/10942912.2021.1973026.
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent Advances in Extracting Phenolic Compounds from Food and Their Use in Disease Prevention and as Cosmetics. Crit. Rev Food Sci. Nutr. 2020, 1–22. DOI: 10.1080/10408398.2020.1754162.
- Das, A. K.; Rajkumar, V.; Nanda, P. K.; Chauhan, P.; Pradhan, S. R.; Biswas, S. Antioxidant Efficacy of Litchi (Litchi chinensisSonn.) Pericarp Extract in Sheep Meat Nuggets. Antioxidants. 2016, 5(2), 16. DOI: 10.3390/antiox5020016.
- Martillanes, S.; Rocha-Pimienta, J.; Gil, M. V.; Ayuso-Yuste, M. C.; Delgado-Adámez, J. Antioxidant and Antimicrobial Evaluation of Rice Bran (Oryza Sativa L.) Extracts in a Mayonnaise-Type Emulsion. Food Chem. 2020, 308, 125633. DOI: 10.1016/j.foodchem.2019.125633.
- Albuquerque, B. R.; Oliveira, M. B. P.; Barros, L.; Ferreira, I. C. Could Fruits Be a Reliable Source of Food Colorants? Pros and Cons of These Natural Additives. Crit. Rev Food Sci. Nutr. 2020, 1–31. DOI: 10.1080/10408398.2020.1746904.
- Baldin, J. C.; Michelin, E. C.; Polizer, Y. J.; Rodrigues, I.; de Godoy, S. H. S.; Fregonesi, R. P.; Trindade, M. A.; Carvalho, L. T.; Fávaro-Trindade, C. S.; de Lima, C. G. Microencapsulated Jabuticaba (Myrciaria cauliflora) Extract Added to Fresh Sausage as Natural Dye with Antioxidant and Antimicrobial Activity. Meat Sci. 2016, 118, 15–21. DOI: 10.1016/j.meatsci.2016.03.016.
- Caleja, C.; Ribeiro, A.; Filomena Barreiro, M.; CFR Ferreira, I. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23(19), 2787–2806. DOI: 10.2174/1381612822666161227153906.
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging Films Formulated with Gelatin and Anthocyanins Nanocomplexes: Physical Properties, Antioxidant Activity and Its Application for Olive Oil Protection. Food Hydrocoll. 2019, 96, 617–624. DOI: 10.1016/j.foodhyd.2019.06.004.
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of Tannins to Enhance the Functional Properties of Protein-Based Films. Food Hydrocoll. 2020, 100, 105443. DOI: 10.1016/j.foodhyd.2019.105443.
- Herrero, M.; Del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, Seaweeds, Microalgae, and Food By-products as Natural Sources of Functional Ingredients Obtained Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. TrAC - Trends Anal. Chem. 2015, 71, 26–38. DOI: 10.1016/j.trac.2015.01.018.
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical Assisted Extraction: A Novel, Efficient, Eco-friendly Technology. Trends Food Sci. Technol. 2017, 66, 166–175. DOI: 10.1016/j.tifs.2017.06.011.
- Roselló-Soto, E.; Galanakis, C. M.; Brnčić, M.; Orlien, V.; Trujillo, F. J.; Mawson, R.; Knoerzer, K.; Tiwari, B. K.; Barba, F. J. Clean Recovery of Antioxidant Compounds from Plant Foods, By-products and Algae Assisted by Ultrasounds Processing. Modeling Approaches to Optimize Processing Conditions. Trends Food Sci. Technol. 2015, 42(2), 134–149. DOI: 10.1016/j.tifs.2015.01.002.
- Roohinejad, S.; Koubaa, M.; Barba, F. J.; Greiner, R.; Orlien, V.; Lebovka, N. I. Negative Pressure Cavitation Extraction: A Novel Method for Extraction of Food Bioactive Compounds from Plant Materials. Trends Food Sci. Technol. 2016, 52, 98–108. DOI: 10.1016/j.tifs.2016.04.005.