ABSTRACT
This study aimed to quantitatively assess the physicochemical properties and antioxidant activities of seven jujube cultivars (NYDZ, MZ, TZ, HZ, HTDZ, GTZ, and JSXZ) before and after the blackening process and to select the most suitable cultivar for blackening. Red jujubes were blackened by aging at high temperature (75°C) and humidity (80%) conditions for 6 days. The contents of soluble solids, total phenolics, total flavonoids, polysaccharides, cyclic adenosine monophosphate, 5-hydroxymethylfurfural, and antioxidant activities were analyzed using the spectrophotometric method and high-performance liquid chromatography (HPLC). Results showed that the antioxidant activities of all cultivars were significantly enhanced (p < .05) and the contents of selected bioactive compounds in all cultivars increased after blackening, indicating that blackened jujube had higher nutritional value than red jujube. Correlation analysis showed that the total phenolic compounds were the primary source of jujube antioxidant activities. The heat map showed that HTDZ could be selected as the most suitable cultivar for future production of blackened jujube. Red jujube cultivar with high total phenolic content was the best choice for blackening.
Introduction
Red jujubes (Ziziphus jujuba Mill.) have more than 4,000 years of cultivation history in China and have been widely consumed as food or traditional herb medicines.[Citation1,Citation2] China produces more than 98% of the red jujubes in the world, which accounted for nearly 100% of the red jujube trade. The red jujube industry creates about 6 billion dollars in revenue for the nation’s economy. It is rich in bioactive compounds, such as phenolics,[Citation3,Citation4] polysaccharides,[Citation5] and cyclic adenosine monophosphate (cAMP),[Citation6,Citation7] and shows good antioxidant activities.
Blackened jujube is a new product, which is prepared through a ‘blackening’ process.[Citation8] The process is similar to the processing method of black garlic.[Citation9,Citation10] It has been reported that blackened jujubes had a lower content of sucrose but higher contents of reducing sugars, such as glucose and fructose, compared with regular red jujubes. The contents of functional substances in the blackened jujubes, such as phenolics, flavonoids, and 5-hydroxymethylfurfural (5-HMF), were also higher compared to those in red jujubes.[Citation11] Although 5-HMF was considered harmful,[Citation12] its antioxidant activities have aroused more research interests.[Citation13] Studies have shown that the antioxidant activities of red jujubes were enhanced after blackening,[Citation14] and the antioxidant activities of different jujube cultivars were different.[Citation15,Citation16] However, there were few studies on the quantitative comparison of physicochemical properties and antioxidant activities of blackened jujubes of different cultivars, and on the selection of jujube cultivars suitable for the blackening processing.
Therefore, the main objectives of this study were as follows: (i) to study and compare the physicochemical properties and antioxidant activities of different jujube cultivars; (ii) to study the correlation between different bioactive compounds and the antioxidant activities of jujubes; and (iii) to select the suitable jujube cultivars for blackening processing. The research findings provided important information for the selection of suitable jujube cultivars for the production of value-added blackened jujube products.
Materials and methods
Chemicals
2,2’-Diphenyl-1-picrylhydrazyl (DPPH•) was obtained from Shanghai Hualan Chemical Technology Co. Ltd. (Shanghai, China). 2,2’-azinobis (3-ethylbenzothia zone-6-sulfonic acid) diammonium salt (ABTS•+) was from Hefei Bomei Biological Technology Co. LTD (Anhui, China). Ascorbic acid (VC), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), trifluoroacetic acid (TFA), and 3’,5’-cyclic AMP were from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). 5-HMF was from Shanghai Ruiyong Biological Technology Co. Ltd. (Shanghai, China). Acetonitrile and methanol were both of HPLC grade and were purchased from Shandong Yu Wang He Tianxia New Material Co. Ltd. (Yucheng, China). In this study, unless otherwise specified, all other chemicals used were analytical grade.
Materials
Red jujubes of seven cultivars (the water content was about 21% on a wet basis) were obtained from different places in China as follows: Jun Zao (JZ) from Xinjiang Hotan Jujube Industrial Base, Xinjiang; Hui Zao (HZ) from Xinjiang Ruoqiang Jujube Industrial Base, Xinjiang; Gou Tou Zao (GTZ) from Yanchuan County, Shaanxi; Tan Zao (TZ) and Mu Zao (MZ) from Jia County Hongrun Co., Shaanxi; Jin Si Xiao Zao (JSXZ) from Laoling Hundred Jujube Garden, Shandong; and Ning Yang Da Zao (NYDZ) from Haoyun Orchard, Shandong. The purchasing date of the samples was November 19, 2019. Before experiments, the samples were stored at −10°C.
Blackening processing
A sample of 500.0 g red jujubes from each cultivar was soaked in 300 mL water for 1 h in sealed and airtight bags to reach 80% relative humidity (RH) in the bags. Then, the bags containing the jujubes were placed in an incubator (101–2ES, Beijing Yongguangming Medical Instrument Factory, China) for blackening at 75°C and 80% RH for 6 days. After pitting, the sample was dried with lyophilizer (ALPHA2-4, Henan Brother Instrument Equipment Co., China.) and ground to powder.
Extraction
1.00 g jujube powder was extracted with 16 mL of 60% ethanol aqueous solution with ultrasound (KQ2200DE, Jiangsu Kunshan Co., China) at 99 W for 30 min. The solution was centrifuged (TG16, Changsha Yingtai Co., China) at 66.7 Hz for 10 min, and the supernatant was separated. The extraction was repeated twice, and the extracts were used for determining the total phenolics, total flavonoids, and antioxidant activities.
Color index
A colorimeter (WSC-2B, Shanghai Optical Instrument Co., China) was used to measure the L (lightness), a (redness), and b (yellowness) values of jujube peel. Each sample was measured nine times.
Total phenolic contents of jujube
The Folin–Ciocalteu method was used to determine the total phenolic content of jujube with some modifications.[Citation17] 0.5 mL of the standard gallic acid solution (0.04–0.20 mg/mL) or extracts were mixed with 0.5 mL forinol-phenol solution (0.50 N). After 5 min, 2.5 mL of Na2CO3 (10% w/v) were added. Then, the volume was adjusted to 10 mL with distilled water. The absorbances were measured at 760 nm after 60 min. The contents were shown as mg gallic acid equivalents (mg GAE/g DW).
Total flavonoid contents of jujube
The method for determining the total flavonoid content was referred to Wang et al. with some modifications.[Citation18] 1 mL of the standard rutin solution (0.10–0.50 mg/mL) or extracts were mixed with 0.3 mL of 5% NaNO2 and 0.3 mL of 10% Al(NO3)3. After 6 min, 4.0 mL of NaOH (4% w/v) were added. Finally, the volume was adjusted to 10 mL with 70% ethanol. The absorbances were measured at 510 nm after 15 min. The contents were shown as mg rutin equivalents/g DW (mg RE/g DW).
Analysis of 5-HMF
The extraction and determination methods of 5-HMF were referred to Sun et al. with slight modification.[Citation11] 2.50 g jujube powder was mixed with 10.0 mL of 50% methanol. Then, the sample was homogenized with a magnetic stirrer (79–1, Changzhou Guohua Instrument Co., China) for 10 min. Then, the volume was adjusted to 25 mL with distilled water. Then, the samples were extracted in an ultrasonic for 30 min. The supernatant was filtered through a 0.45-μm PTFE filter for testing. 5-HMF analysis was performed using an LC-20A system Shimadzu (Kyoto, Japan) high-performance liquid chromatography (HPLC) system with a UV–Vis detector. HPLC conditions were as follows: the compounds were eluted with a mobile phase of a methanol-water mixture (5:95, v/v) and flowed through an Inertsil ODS-3 column (250 × 4.6 mm; 5 µm). The flow rate was 1 mL/min, and the detection temperature was 35°C. The samples were detected at a wavelength of 282 nm. The injection volume was 10 µL.
Analysis of cAMP
The cAMP content was measured with a method referred to Chen et al. with some modifications.[Citation19] Specifically, 0.50 g of jujube powder was mixed with 25 mL distilled water and extracted in an ultrasonic bath for 30 min. Then, the supernatant was separated and filtered through a 0.45-μm PTFE filter for testing. HPLC conditions were as follows: the compounds were eluted with a mobile phase of a methanol-potassium dihydrogen phosphate solution (0.05 mol/L) mixture (10:90, v/v) and flow through an Inertsil ODS-3 column (250 × 4.6 mm; 5 µm). The flow rate was 1 mL/min, and the detection temperature was 30°C. The sample was detected at a wavelength of 282 nm. The injection volume was 10 µL.
Analysis of polysaccharides
The method of extraction and determination of polysaccharides was referred to Yang et al. and was slightly modified.[Citation20] Specifically, 0.50 g jujube powder was mixed with 12 mL 80% ethanol in an ultrasonic bath for 30 min and repeated twice. 0.5 mL standard glucose solution (0.04–0.20 mg/mL) or sample solution was mixed with 1 mL phenol and 5 mL concentrated sulfuric acid at 30°C for 30 min. The absorbance was then measured at 490 nm.
Soluble solids content (SSC)
1.00 g jujube powder was mixed with 6 mL distilled water, and then, the supernatant was separated. The extracts were collected and detected with a saccharimeter (PAL-1, ATAGO Co. Japan) for the soluble solid content.
Analysis of monosaccharides
Referred to Lin et al.,[Citation21] 0.50 g jujube powder was hydrolyzed with 2.00 mol/L trifluoroacetic acid (TFA) at 110°C for 8 h, then derivatized with water. Then, 250 µL sample solution, 250 µL 0.60 mol/L NaOH, and 500 µL 0.40 mol/L PMP-methanol were added into 5 mL EP tubes, and the mixture was heated at 70°C. After 1 h, the solution was placed in 15°C water for 10 min. After cooling, 500 µL of 0.30 mol/L HCl were added. The supernatant was separated and then filtered through a 0.45-μm PTFE filter for testing. HPLC conditions were as follows: the compounds were eluted with a mobile phase of a 0.05 mol/L potassium dihydrogen phosphate (pH was adjusted with sodium hydroxide solution to 6.70)–acetonitrile mixture (83:17, v/v) and flow through an Inertsil ODS-3 column (250 × 4.6 mm; 5 µm). The flow rate was 1 mL/min, and the detection temperature was 30°C. The sample was detected at a wavelength of 250 nm. The injection volume was 20 µL.
Determination of DPPH• scavenging activity
The determination method of DPPH• scavenging activity referred to Wang et al. with some modifications.[Citation22] 0.1 mL of the standard VC solution (0.00–0.30 mg/mL) or extracts were mixed with 5 mL of 0.10 mmol/L DPPH•, and then, the mixture was placed in dark and measured at 517 nm after 30 min. The contents were shown as mg ascorbic acid/g DW (mg VC/g DW).
Determination of ABTS•+ radical cation
According to Re et al.,[Citation23] ABTS•+ solution was prepared by mixing 10 mL of 7.00 mmol/L ABTS•+ solution with 10 mL of 2.45 mmol/L K2S2O8 solution. The mixture was left at room temperature in the dark for 12–16 h until a stable oxidation state was reached. Then, the ABTS•+ solution was diluted in 80% ethanol to an absorbance of 0.700 ± 0.050 at 734 nm. 5 mL of the ABTS•+ solution and 0.2 mL of the standard Trolox solution (0.00–0.10 mg/mL) or extracts were mixed. Then, the sample was placed in the dark and measured at 734 nm after 8 min. The contents were shown as mg Trolox equivalent/g DW (mg TE/g DW).
Determination of reducing power
The determination method of ferric ion reducing antioxidant activity referred to Feng et al. with some modifications.[Citation24] 0.6 ml sample solution or the Trolox standard solution (0.00–0.50 mg/mL), 2.5 ml 0.20 mol/L phosphate buffer solution, and 2.5 ml potassium ferricyanide solution (1% W/V) mixed together. The mixture was then extracted with an ultrasound at 50°C for 20 min. Then, 2.5 mL trichloroacetic acid (10% W/V) solution was added to the mixture followed by shaking. 2.5 mL of the supernatant was added with 2.5 mL of distilled water and 2.5 mL of ferric chloride solution (0.1% W/V). Then, the sample was measured at 700 nm after 10 min. The contents were shown as mg Trolox equivalent/g DW (mg TE/g DW).
Statistical analysis
Data were shown as means values ± standard deviation. SPSS Statistics 25.0 (IBM, New York, USA) was used for data analysis. Duncan’s multiple-comparison tests were conducted at significance levels of p < .05 or p < .01. Correlations were performed using a standard Pearson correlation method. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were used to analyze the differences among the different cultivars. The heat map analysis was used to select the most suitable jujube cultivars for blackening.
Results and discussion
Color of jujube peel
The color indexes of the seven jujube cultivars before and after the blackening process are summarized in , and the appearances of HZ before and after blackening are shown in ) as an example. It was found that the redness (a), yellowness (b), and lightness (L) of jujubes decreased significantly after blackening. The color difference (ΔE) of JSXZ was the highest among the seven cultivars after blackening, and MZ had the lowest value of the color difference ()). Most of the jujubes had significant differences in terms of lightness and redness after blackening. Among them, it can be noticed that HTDZ had the highest lightness than the other cultivars both before and after blackening. However, red NYDZ had the highest redness but the lowest redness after blackening. The change of color after blackening may be due to the generation of substances such as melanoidin Yuan et al.[Citation25] The difference in color may be due to the difference in physicochemical properties of different cultivars.
Figure 1. (a) The picture of the HZ before and after blackening. (b) Color differences (ΔE) of seven jujube cultivars before and after blackening process (different letters in each column represent significant differences (p < .05)).
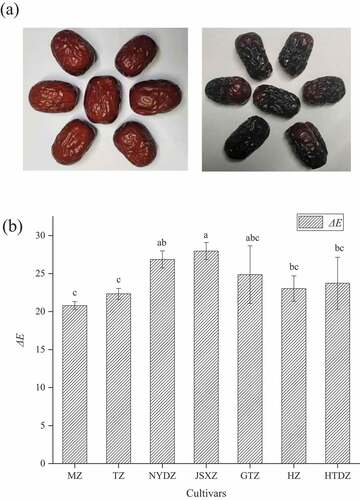
Table 1. Color index of jujube peel.
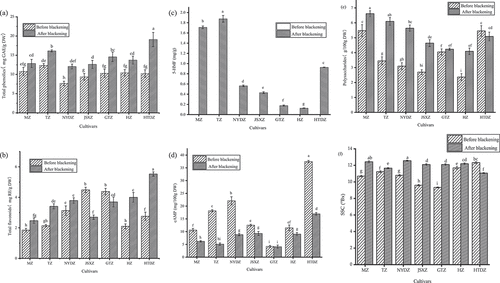
Total phenolic contents (TPC)
) shows that the TPC of the seven red jujube cultivars ranged from 7.67 mg GAE/g DW to 12.34 mg GAE/g DW, which were higher than the result of five red jujube cultivars (5.18 to 8.53 mg GAE/g) measured by Li et al.[Citation26] The TPC in all of the cultivars increased significantly (p < .05) with a range from 12.08 mg GAE/g DW to 19.09 mg GAE/g DW after the blackening process. A reported study also showed that the TPC in black garlic was increased after blackening Lu et al.[Citation27] These results showed that the blackening process was an effective way to increase the TPC. Among the seven cultivars, blackened HTDZ had the highest TPC, which was almost twice as high as in the red HTDZ. The TPC in MZ increased the least after blackening. The above results indicated that there were differences among different cultivars.
Total flavonoid contents (TFC)
As shown in ), the TFC of the seven cultivars ranged from 1.85 mg RE/g DW to 4.49 mg RE/g DW, which were higher than the results (1.22 to 3.19 mg RE/g DW) measured by Zhao et al.[Citation28] The TFC in most cultivars increased significantly after the blackening process (p < .05) except for the JSXZ and GTZ (2.47 to 5.53 mg RE/g DW). The increase in TFC was due to the production of reduced ketones and dehydrogenated reduced ketones by 2,3- evolution reaction under alkaline conditions in the middle stage of the Maillard reaction. It was worth mentioning that blackened HTDZ also had the highest TFC. The TFC in blackened HTDZ was 2.77 times that before blackening. In addition, the TFC in blackened HZ jujubes increased by 1.90 times as much as before blackening. However, the TFC of blackened JSXZ decreased the most, and the reason for this phenomenon was remained to be studied.
Contents of 5-HMF
5-HMF was produced by 1,2- evolution reaction under alkaline conditions. As shown in ), 5-HMF did not exist in the red jujubes. However, the content of 5-HMF in the jujubes was significantly higher (p < .05) after the blackening process, which increased from 0.13 to 1.88 mg/g DW. As the blackening went on, the content of 5-HMF had a tendency to continue to increase in jujubes, as shown in Park et al.[Citation29] Therefore, it is necessary to choose an appropriate blackening time. The 5-HMF content was the highest in blackened MZ and the lowest in blackened HZ.
Contents of cAMP
) shows that the contents of cAMP in the blackened jujubes decreased from 4.24–37.47 mg/100 g DW to 4.09–16.96 mg/100 g DW after the blackening process. It was also reported that as the blackening went on, the content of cAMP tended to continue to decrease in jujubes (after 96 h of blackening, corresponding to 65.85% relative decrease), as shown in Sun et al.[Citation11] Therefore, selecting an optimal blackening time can effectively avoid the loss of cAMP. Among the seven cultivars, the cAMP content in the blackened GTZ decreased the least, which was 3.53%. The cAMP content in HTDZ was the highest both before and after blackening.
Contents of polysaccharides
As shown in ), the polysaccharide contents in six jujube cultivars increased significantly after the blackening (p < .05), but the polysaccharide content of blackened HTDZ had a small reduction of 7.27%. The results generally suggested that blackening increased the content of polysaccharides. The difference in the polysaccharide contents in different cultivars might relate to the difference in their producing areas. Among them, the polysaccharide content in blackened NYDZ increased the most (81.94%). The polysaccharide content in MZ was the highest both before (5.48 g/100 g DW) and after blackening (6.61 g/100 g DW). Therefore, from the perspective of obtaining high polysaccharide content, NYDZ and MZ were the better choices for the blackening process.
Analysis of SSC
As shown in ), the SSC in six jujube cultivars significantly increased after the blackening (p < .05), but the SSC of HTDZ had a small reduction of 10.22%. The SSC in GTZ showed the largest increase after blackening (29.69%). The blackened NYDZ had the highest SSC, which was 12.57°Bx.
Analysis of monosaccharides
As shown in , 10 kinds of monosaccharides were separated and identified in both the red and blackened jujubes. shows that the contents of 10 monosaccharides in each of the cultivars were significantly different (p < .05). After blackening, the monosaccharide contents of different cultivars changed in different trends. Among the 10 monosaccharides, the content of glucose was the highest, followed by arabinose. The glucose contents of GTZ, HZ, and HTDZ decreased by 50.77%, 74.04%, and 7.81%, respectively, after blackening (). This trend may be beneficial to the development of food for diabetic patients. Among the seven cultivars, the contents of mannose, ribose, rhamnose, galacturonic acid, glucose, galactose, xylose, arabinose, and fucose in the JSXZ were the highest and increased after blackening.
Figure 3. HPLC chromatographs of monosaccharides in JSXZ before and after blackening (a) enlarged and (b) original.
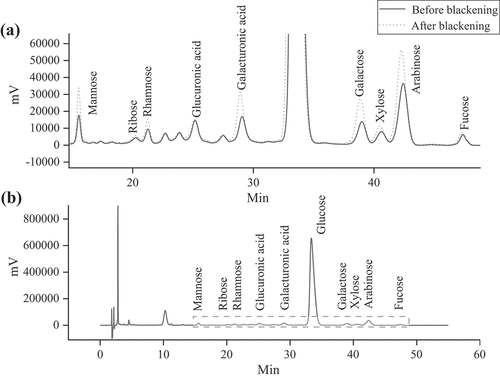
Table 2. Mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, arabinose, and fucose contents of seven jujube cultivars before and after blackening process.
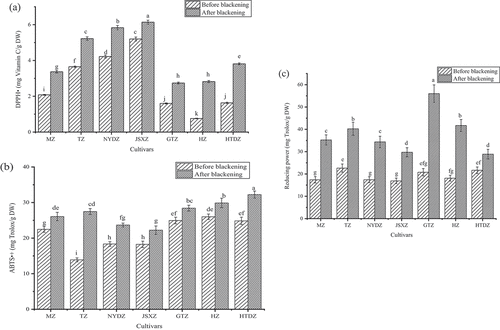
Antioxidant activities
As shown in , the DPPH• and ABTS•+ scavenging activities and reducing power varied for different jujube cultivars. After blackening, the DPPH• scavenging, ABTS•+ scavenging activities, and reducing power in the jujubes increased by 18.77–217.05%, 13.86–97.63%, and 33.33–169.49%, respectively. shows that the antioxidant activities of the jujube were enhanced significantly after the blackening process (p < .05). Among them, HTDZ showed the strongest ABTS•+ scavenging capacity, GTZ showed the strongest reducing power and JSXZ showed the strongest DPPH• scavenging capacity after blackening. The higher levels of radical scavenging activities of the blackened jujubes should be attributed to the increased contents of phenolics and flavonoids, and the melanoidin generated in the blackening process Kim et al.[Citation30] Therefore, the blackening process can improve the antioxidant activities of the jujubes.
Correlation analysis
Pearson correlation coefficients are calculated and summarized in . The ABTS•+ scavenging activity had a significant positive correlation with SSC, TFC, TPC, polysaccharides, and 5-HMF; the reducing power positively correlated to the SSC, TPC, polysaccharides, and 5-HMF significantly; the DPPH• scavenging activity had a significant positive correlation with ribose, rhamnose, and galacturonic acid. In general, the results suggested that from the aspect of antioxidant activity enhancement, the high TPC in jujube made the antioxidant activities stronger after blackening.
Table 3. Correlation coefficients (r) between the antioxidant activities and the physicochemical compounds of jujube before and after blackening process.
It was reported that the increase of TPC during blackening might be due to the conversion of fruit pulp into soluble polyphenols.[Citation14] It can be seen that TPC was positively correlated with SSC. The change of the TPC might be related to the transformation of insoluble substances into soluble solids during blackening. Correlation analysis showed that the content of cAMP was negatively correlated with 5-HMF, and so it was speculated that cAMP might be involved in the reaction of generating 5-HMF. It was worth mentioning that a positive correlation between polysaccharides and SSC was observed. The reported research also found that the increase in SSC during heat treatment was due to the conversion of insoluble carbohydrates to dextrins or monosaccharides.[Citation31] Since dextrin belongs to polysaccharides, which might be the reason for the increase in polysaccharide contents after blackening. Then, there were the relatively small amounts of monosaccharides, including galacturonic acid, ribose, and rhamnose that had a positive correlation with DPPH• ().
Correlation analysis between the contents of bioactive compounds in red jujubes and the increase in antioxidant activities after blackening showed that TPC was positively correlated with the increase in antioxidant activities (ABTS•+ R2 = 0.476, p < .05). Therefore, red jujube cultivars with higher total phenolic content can be selected for blackening.
Principal component analysis (PCA) and hierarchical cluster analysis (HCA)
PCA and HCA were performed to evaluate the differences between the seven jujube cultivars in terms of the bioactive compounds and antioxidant activities. Sixteen physicochemical indexes of blackened jujube were selected for the analysis. shows that the three main components explained 90.56% of the whole variation (). The first component (PC1) accounted for 59.84% of the whole variation and was positively correlated with the contents of mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, galactose, glucose, xylose, arabinose, and fucose. Meanwhile, it was negatively correlated with ABTS•+ scavenging activity and reducing power in the blackened jujubes. PC2 explained that 22.38% of the whole variation was positively correlated with the ABTS•+ scavenging activity, TPC, TFC, and ribose, while it was negatively correlated with SSC. PC3 explained that 8.34% of the whole variation, was positively correlated with the contents of polysaccharides (). ) shows that based on the PC1 and PC2, the seven blackened jujube cultivars were classified as shown, demonstrating that there were significant differences in the subgroups of jujube cultivars. Meanwhile, the boundaries of different jujube cultivars could be found and used to identify among them. It can be seen from ) that based on their physicochemical indexes, the different blackened jujube cultivars could be completely distinguished from the left, and relatively similar jujube cultivars can be gradually clustered based on their physicochemical indexes.
Figure 5. (a) Principal component analysis (PC1 and PC2) of blackened jujube, (b) Dendrogram of 7 jujube cultivars before and after blackening, and (c) Heat map of 7 jujube cultivars before and after blackening process.
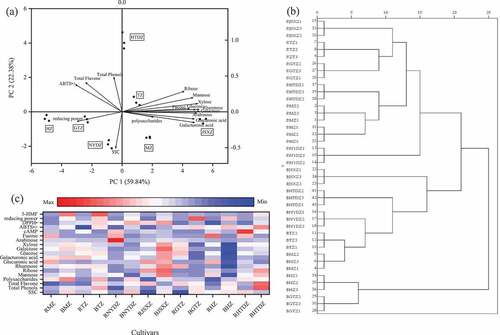
Table 4. Eigenvalues, proportion of variation, and eigenvectors of the three principal components for the blackened jujubes.
Heat map analysis
According to the results of PCA and HCA, there were differences among the seven cultivars of blackened jujubes in terms of physicochemical properties and antioxidant activities. Therefore, it was necessary to select the best cultivar suitable for blackening, which was performed through the heat map analysis. As shown in ), the redder the color, the higher the value of physicochemical properties and antioxidant activities, the bluer the color, the lower the value. It can be directly seen that the antioxidant activities of blackened HTDZ were increased with the increase of TPC, TFC, and ribose. From the aspect of antioxidant activities, combined with the results of correlation analysis, HTDZ with the highest TPC after blackening was the best cultivar.
Conclusion
The experimental results revealed that the antioxidant activities of all seven red jujube cultivars were enhanced significantly after the blackening process. Meanwhile, the contents of monosaccharides, polysaccharides, soluble solids, total phenolics, and total flavonoids in most cultivars increased significantly after the blackening process. Correlation analysis between before and after blackening showed that the TPC, TFC, polysaccharides, 5-HMF, SSC, ribose, rhamnose, and galacturonic acid were positively correlated with antioxidant activities. Correlation analysis between the content of bioactive compounds in red jujubes and the increase in antioxidant activities after blackening showed that red jujube cultivars with higher total phenolic content can be selected for blackening. The PCA and HCA results suggested that the seven blackened jujube cultivars could be distinguished in terms of physicochemical properties and antioxidant activities. Through heat map analysis, HTDZ, which had the highest TPC after blackening, was selected as the best cultivars for future production of blackened jujube.
Funding
Projects funded by the central government to guide local Scientific and Technological Development (YDZX2021071), Shandong Province Key Research and Development Plan (the rural revitalization scientific and technological innovation improving action) (2021TZXD011), Dezhou Health Food Industry Innovation and Entrepreneurship Community and Shandong Land Development Group Co. LTD Project “High efficient green production and high-value utilization of the whole industrial chain key technology integration and industrialization demonstration of red jujube.”
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bao, T.; Hao, X.; Shishir, M. R. I.; Karim, N.; Chen, W. Cold Plasma: An Emerging Pretreatment Technology for the Drying of Jujube Slices. Food Chem. 2021, 337, 127783. DOI: 10.1016/j.foodchem.2020.127783.
- Rashwan, A. K.; Karim, N.; Shishir, M. R. I.; Bao, T.; Lu, Y.; Chen, W. Jujube Fruit: A Potential Nutritious Fruit for the Development of Functional Food Products. J. Funct. Foods. 2020, 75. DOI: 10.1016/j.jff.2020.104205.
- Nikkhah, M.; Hashemi, M. Boosting Antifungal Effect of Essential Oils Using Combination Approach as an Efficient Strategy to Control Postharvest Spoilage and Preserving the Jujube Fruit Quality. Postharvest. Biol. Technol. 2020, 164. DOI: 10.1016/j.postharvbio.2020.111159.
- Song, L.; Liu, P.; Yan, Y.; Huang, Y.; Bai, B.; Hou, X.; Zhang, L., et al. Supercritical CO2 Fluid Extraction of Flavonoid Compounds from Xinjiang Jujube (Ziziphus jujuba Mill.) Leaves and Associated Biological Activities and Flavonoid Compositions. Ind. Crops Prod. 2019, 139, 111508. DOI: 10.1016/j.indcrop.2019.111508.
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of Structural Characterization and Antioxidant Activity of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. DOI: 10.1016/j.ijbiomac.2020.02.018.
- Jiang, T.; He, F.; Han, S.; Chen, C.; Zhang, Y.; Che, H. Characterization of cAMP as an anti-allergic Functional Factor in Chinese Jujube (Ziziphus jujuba Mill.). J. Funct. Foods. 2019, 60, 103414. DOI: 10.1016/j.jff.2019.06.016.
- Reche, J.; Hernández, F.; Almansa, M. S.; Carbonell-Barrachina, Á. A.; Legua, P., and Amorós, A. Physicochemical and Nutritional Composition, Volatile Profile and Antioxidant Activity Differences in Spanish Jujube Fruits. LWT . 2018, 98, 1–8.
- Gao, L.; Gu, D.; Sun, X.; Zhang, R. Investigation of Processing Technology for Aged Black Jujube. Food Sci. Nutr. Stud. 2019, 34, 107. DOI: 10.22158/fsns.v3n4p107.
- Zhang, R.; Sun, X.; Zhang, K.; Zhang, Y.; Song, Y.; Wang, F. Fatty Acid Composition of 21 Cultivars of Chinese Jujube Fruits (Ziziphus jujuba Mill.). J. Food Meas. Charact. 2020. DOI: 10.1007/s11694-020-00718-4.
- Zhao, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Effect of High Hydrostatic Pressure Conditions on the Composition, Morphology, Rheology, Thermal Behavior, Color, and Stability of Black Garlic Melanoidins. Food Chem. 2021, 337, 127790. DOI: 10.1016/j.foodchem.2020.127790.
- Sun, X.; Gu, D.; Fu, Q.; Gao, L.; Shi, C.; Zhang, R.; Qiao, X., et al. Content Variations in Compositions and Volatile Component in Jujube Fruits during the Blacking Process. Food Sci. Nutr. 2019, 7(4), 1387–1395. DOI: 10.1002/fsn3.973.
- Kanar, Y.; Mazı, B. G. HMF Formation, Diastase Activity and Proline Content Changes in Bee Pollen Dried by Different Drying Methods. LWT. 2019, 113, 108273. DOI: 10.1016/j.lwt.2019.108273.
- Olivares-Tenorio, M.-L.; Verkerk, R.; van Boekel, M. A. J. S.; Dekker, M. Thermal Stability of Phytochemicals, HMF and Antioxidant Activity in Cape Gooseberry (Physalis peruviana L.). J. Funct. Foods. 2017, 32, 46–57. DOI: 10.1016/j.jff.2017.02.021.
- Kim, J.-E.; Kim, M.-A.; Kim, J.-S.; Park, D.-C.; Lee, S.-P. Enhancing the Organoleptic and Functional Properties of Jujube by a Quick Aging Process. Preventive Nutr. Food Sci. 2013 1, 18(1), 50–59. DOI:10.3746/pnf.2013.18.1.050.
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á. A.; Hernández, F. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Jujube Fruits as Affected by Cultivar and Drying Method. Food Chem. 2016, 207, 170–179. DOI: 10.1016/j.foodchem.2016.03.099.
- Gao, Q.-H.; Wu, P.-T.; Liu, J.-R.; Wu, C.-S.; Parry, J. W.; Wang, M. Physico-chemical Properties and Antioxidant Capacity of Different Jujube (Ziziphus jujuba Mill.) Cultivars Grown in Loess Plateau of China. Sci. Hortic. 2011, 130(1), 67–72. DOI: 10.1016/j.scienta.2011.06.005.
- Kou, X.; Chen, Q.; Li, X.; Li, M.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z., et al. Quantitative Assessment of Bioactive Compounds and the Antioxidant Activity of 15 Jujube Cultivars. Food Chem. 2015, 173, 1037–1044. DOI: 10.1016/j.foodchem.2014.10.110.
- Bini, W.; Longgang, L.; Qingyuan, H., and Ying, L . Quantitative Assessment of Phenolic Acids, Flavonoids and Antioxidant Activities of Sixteen Jujube Cultivars from China. Plant Foods Human Nutr. 75,154–160. 2020.
- Chen, C.; Li, H.; Lv, X.; Tang, J.; Chen, C.; Zheng, X. Application of near Infrared Spectroscopy Combined with SVR Algorithm in Rapid Detection of cAMP Content in Red Jujube. Optik. 2019, 194, 163063. DOI: 10.1016/j.ijleo.2019.163063.
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S. K.; Zheng, Z.; Zhang, R. Structural Characterization and Antioxidant Activities of One Neutral Polysaccharide and Three Acid Polysaccharides from Ziziphus Jujuba Cv. Hamidazao: A Comparison. Carbohydr. Polym. 2021, 261, 117879. DOI: 10.1016/j.carbpol.2021.117879.
- Lin, T.; Liu, Y.; Lai, C.; Yang, T.; Xie, J.; Zhang, Y. The Effect of Ultrasound Assisted Extraction on Structural Composition, Antioxidant Activity and Immunoregulation of Polysaccharides from Ziziphus jujuba Mill Var. Spinosa Seeds. Ind. Crops Prod. 2018, 125, 150–159. DOI: 10.1016/j.indcrop.2018.08.078.
- Wang, B.; Huang, Q.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.; Cao, W.; Lv, X.; Pan, Z., et al. Changes in Phenolic Compounds and Their Antioxidant Capacities in Jujube (Ziziphus jujuba Miller) during Three Edible Maturity Stages. LWT - Food Sci. Technol. 2016, 66, 56–62. DOI: 10.1016/j.lwt.2015.10.005.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9), 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y.; Wang, Y.; Cao, B.; Zhang, F., et al. Quality Characteristics and Antioxidant Activities of Goat Milk Yogurt with Added Jujube Pulp. Food Chem. 2019, 277, 238–245. DOI: 10.1016/j.foodchem.2018.10.104.
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An Analysis of the Changes on Intermediate Products during the Thermal Processing of Black Garlic. Food Chem. 2018, 239, 56–61. DOI: 10.1016/j.foodchem.2017.06.079.
- Li, J.-W.; Fan, L.-P.; Ding, S.-D.; Ding, X.-L. Nutritional Composition of Five Cultivars of Chinese Jujube. Food Chem. 2007, 103(2), 454–460. DOI: 10.1016/j.foodchem.2006.08.016.
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition Analysis and Antioxidant Properties of Black Garlic Extract. J. Food Drug Anal. 2017, 25(2), 340–349. DOI: 10.1016/j.jfda.2016.05.011.
- Zhao, H.-X.; Zhang, H.-S.; Yang, S.-F. Phenolic Compounds and Its Antioxidant Activities in Ethanolic Extracts from Seven Cultivars of Chinese Jujube. Food Sci. Hum. Wellness. 2014, 3(3), 183–190. DOI: 10.1016/j.fshw.2014.12.005.
- Park, H. J.; Lee, S. H.; Kim, H. Y.; Jang, G. Y.; Hwang, I. G.; Woo, K. S.; Kwon, O.-S.; Lee, J.-S.; Jeong, H.-S., et al. Changes in Chemical Components and Antioxidant Activity of Dried Jujube with Different Aging Temperatures and durations(Article). J. Korean Soc. Food Sci. Nutr. 5, 2012, 41(5), 591–597. DOI: 10.3746/jkfn.2012.41.5.591.
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of Phenolic Acids and Flavonoids in Black Garlic at Different Thermal Processing Steps. J. Funct. Foods. 2013, 5(1), 80–86. DOI: 10.1016/j.jff.2012.08.006.
- Shin, S. R.; Lee, S. H.; Yoon, K. Y., and Kim, K. S. Changes in the Physical Characteristics and Components of the Jujube Fruits by Drying Methods. Korean J. Food Preserv. 1998, 5(4), 346–349.