?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai] is a cucurbit with high value in Burkina Faso. It is appreciated for its fruits, which are widely consumed. In the literature, watermelon is described as an important source of bioactive compounds beneficial to health. There is very little scientific data on this supposed nutraceutical potential of the watermelon. This study aimed to characterize the nutraceutical and antioxidant potential of the pulp of five watermelon cultivars grown in Burkina Faso to identify the best cultivars for consumers. The study focused on the physicochemical, nutraceutical, and antioxidant activity characterization of the cultivars using standard methods. This study revealed a significant variation in total phenolics, flavonoids, β-carotene, and lycopene contents between the five cultivars while the color did not show significant variation. The color parameters with ΔE ranging from 40.62 ± 4.87 (SB cultivar) to 48.90 ± 15.81 (KK cultivar) determined did not reveal any significant difference between cultivars. For the phytochemical parameters: total phenolic compounds, total flavonoids, β-carotene, and lycopene, the cultivars KK and KA showed the highest content. The statistical analysis of the results shows positive correlations between phytochemical composition and pulp color. The color could thus, depending on the cultivar and its maturity, constitute a preliminary indicator of choice for the estimation of the content of bioactive compounds.
Introduction
Plant products have always been used to meet the basic needs of humans including food and medicine. Indeed, plants are the primary source of nutritional and bioactive compounds capable of preventing diseases.[Citation1] These bioactive compounds such as phenolics have shown anti-inflammatory, anti-hypertensive, anti-diabetic, and anti-obesity properties.[Citation2] Among these plants, fruits and vegetables are known to be important sources of bioactive compounds and thus abound in functional properties, useful in the fight against diseases.[Citation3,Citation4] Watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai] belongs to this category of plants and is of both therapeutic and nutritional interest. Citrullus lanatus is an annual herbaceous in the Cucurbitaceae plants family and native to Africa.[Citation5] It is cultivated worldwide and in most sub-Saharan African countries including Burkina Faso for its edible fleshy pulp fruits.[Citation6] Its production has been booming in recent years and is now increasingly important among cash crops. Watermelon production and marketing are a growing business at the national level and are accessible to all socio-economic.[Citation7] Reports indicate that watermelon is rich in phenolic compounds and antioxidants (polyphenols, flavonoids, lycopene, citrulline …) that have a positive effect on health by reducing the risk of cardiovascular, chronic, and neurodegenerative diseases.[Citation4,Citation8] Moreover, its low energy value and low-fat content make it a recommended food for overweight and hypertensive people.[Citation3,Citation9]
Because of its nutritional profile, watermelon could contribute to the fight against malnutrition by improving the diet of households and against diseases caused by oxidative stress.[Citation4,Citation8] This fruit could therefore constitute a serious alternative to reduce the prevalence of malnutrition (23% of the population), particularly linked to the scarcity of fruit in the diet.[Citation10,Citation11] However, research work indicates that the biochemical profile of plants can be influenced by genetic factors, maturity, and ecology.[Citation12] Unfortunately, there is no study on the phytochemical and antioxidant profile of watermelon cultivars in Burkina Faso. This study aimed to document the nutraceutical potential of five watermelon cultivars grown in Burkina Faso. This literature can contribute to the food and pharmaceutical valorization of watermelon, to the conservation of the best genetic heritage, and especially to the achievement of food security which remains a priority in developing countries, particularly in sub-Saharan Africa.
Materials and methods
Plant material
The plant materials consisted of fruits of five watermelon cultivars collected from producers and retailers in four regions of Burkina. The sampling was done from September to December 2020. The cultivars selected are listed in . Only the pulp taken from the heart of the ripe fruit was used for this study. Part of the samples was dried in an electric dryer (Dreyer 1100 VISMARA) for 2 weeks at 37°C and the other part was frozen at −20°C until use.
Table 1. Watermelon cultivars selected for this study.
Determination of pulp color
The color of the fruit pulp was determined by the colorimetry method.[Citation13] Cubic slices of pulp (4 × 4 × 4 cm thick) taken from the center of three fruits of each sample were placed on white paper and then flashed with a colorimeter (PCE-CMS 1). The parameters L*, a*, b*,ΔE, C, and H were determined according to the “CIE-LAB” colorimetric system.
pH and Titratable acid determination
The determination of pH of watermelon juice has been made by using a digital glass electrode pH meter, PHS-25 w.[Citation14] Juice extraction was done by pressing.[Citation15] The titratable acidity was determined by an acid-base assay with a 0.1 N sodium hydroxide solution, in the presence of phenolphthalein as a colored indicator according to the AOAC methods.[Citation16]
Determination of Phenolic Compounds Content
The extraction of phenolic compounds consisted in dispersing 500 mg of the dry ground pulp of each sample in 15 mL of 80% (v/v) methanol under continual agitation at 200 revolutions per minute (rpm) for 4 hours at 25°C. After centrifugation at 4000 rpm. The collected supernatant constituted the crude extract that was used for analyses. The determination of total phenolic content (TPC) was performed by the method of using Folin & Ciocalteu’s reagent with slight modification.[Citation17] The TPC was expressed as mg gallic acid equivalent (mg GAE) per 100 mg of dry matter (DM). Determination of total flavonoid content was performed according to the colorimetric method with aluminum trichloride (AlCl3) of Dowd adapted.[Citation18] Results were expressed as mg quercetin equivalent (mg QE) per 100 mg of dry matter (DM).
Determination of Tannins Content
The determination of hydrolyzable tannins content was performed according to the ferric trichloride (FeCl3) method.[Citation19] Each extract (5 mg/mL) was treated with a solution of ferric trichloride (FeCl3) in hydrochloric acid (HCl) and the absorbance of the mixture was read after 15s at 660 nm. Hydrolyzable tannins contents were expressed as mg gallic acid equivalent per g dry extract (mg GAE/g DM).
Determination of condensed tannins was performed by the acidified vanillin method with slight modification.[Citation20] Each extract (5 mg/mL) was treated with a 1% vanillin solution in 70% sulfuric acid. The absorbance of the mixture was read at 500 nm after 15 min of incubation in a water bath at 20°C in the dark. Condensed tannins content was calculated and expressed as mg catechin equivalent per g dry extract (mg Cat. E/g DM) according to the formula (1):
where TC: condensed tannins; 5.2 x 10−2: constant expressed in Catechin equivalent; A: absorbance; V: volume of extract used; P: weight of samples.
Determination of Anthocyanins
Total Anthocyanin Pigment content (APC) was performed by the pH differential method.[Citation21] The anthocyanin pigment concentration was calculated and expressed as cyanidin-3-glucoside equivalents (CE), as follows (2):
Where APC = Anthocyanin pigment (CE mg/L); A = (A520 nm – A700 nm) pH 1.0 – (A520 nm – A700 nm) pH 4.5; MW (molecular weight) = 449.2 g/mol for cyanidin-3-glucoside (cyd-3-glu); DF = dilution factor established in D; l = pathlength in cm; ε = 26,900 molar extinction coefficients, in L*mol – 1 * cm – 1, for cyd-3-glu, and 103 = factor for conversion from g to mg.
Determination of β-Carotene and Lycopene Content
Determination of β-carotene and lycopene content was performed with the spectrometric method.[Citation22] For this purpose, the samples were extracted with the acetone-water mixture (70:30 v/v). Then the absorbance of the supernatant was measured with a UV-visible spectrophotometer (HELIOS EPSILON, THERMO Scientific) at 453; 505; 645 and 663 nm. The contents of beta carotene and lycopene expressed respectively in mg of β-carotene/g and mg of Lycopene/g of dry materials and were determined according to the Equationequations (3(3)
(3) ;4):
Where: A = absorbance of the sample measured.
Evaluation of antioxidant activity
Evaluation of antioxidant activity by the Ferric Reducing Antioxidant Power (FRAP) test: The evaluation of antioxidant activity by the iron reduction method is based on the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+) by reducing compounds (antioxidants), which is accompanied by the appearance of an intense blue coloration measurable at 700 nm.[Citation23] The concentration of reducing compounds (antioxidants) in the extract is expressed in mg ascorbic acid equivalent (AAE) per 100 mg of dry matter.
Evaluation of Antioxidant Activity by the ABTS Test: The evaluation of the antiradical activity by ABTS assay is based on the discoloration of the stable radical cation ABTS.+ [2.2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] to ABTS, in the presence of antiradical compounds.[Citation24] The chromophore radical cation ABTS.+ involved, with a blue-green color is followed has its λmax at 734 nm in a spectrophotometer (Epoch; BioTeK). The content of compounds with a reducing effect on the radical cation ABTS.+ (antiradical compounds) is expressed in mmol Ascorbic Acid Equivalent per 100 mg dry matter (mM AAE/100 mg DM).
Evaluation of the Antioxidant Activity by the DPPH Reduction Test: Evaluation of antioxidant activity was performed by 2.2-diphenyl-picrylhydrazyl (DPPH) free radical scavenging method.[Citation25] When the stable DPPH free radical (dark purple hue) reacts with an antiradical, it produces discoloration of the solution, a reduction in absorbance measurable at 517 nm with a spectrophotometer (Epoch; BioTeK). The antioxidant activity of the samples was expressed as a percentage of inhibition (% inh) according to the formula (5):
Where % Inh = antiradical activity in the percentage of inhibition; Abs (blank) = Absorbance of the blank; Abs (sample) = Absorbance of the sample.
Statistical analysis
The statistical analysis of the data was performed using XLSTAT software (Addinsoft, 2021). It consisted of the analysis of variances (ANOVA) by Tukey’s test at the 5% threshold for the comparison of means and principal component analysis (PCA). The results were expressed as mean ± standard error (SD) of the mean.
Results and discussion
Results
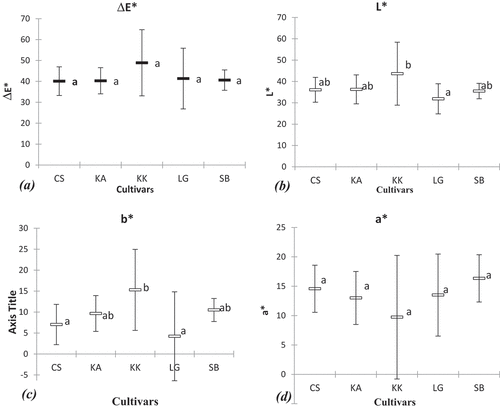
The color of the pulp ranges from orange-red to red. Only the L*and b* parameters of the color show a significant difference according to the cultivar and the harvest area. The other parameters a*, ΔE, H, and C didn’t show significant differences depending on the cultivar (). All cultivars show positive values for the color parameters (L*, a*, b > 0). Even so, the cultivar KK presents strong values, whether for the ΔE, L*, b* and C* translating a more vivid color.
Figure 1. Averages of pulp color parameters of the five watermelon cultivars studied. (a): mean of total color, E; (b): mean of brightness, L*; (c): mean of b*; (d): mean of a*.
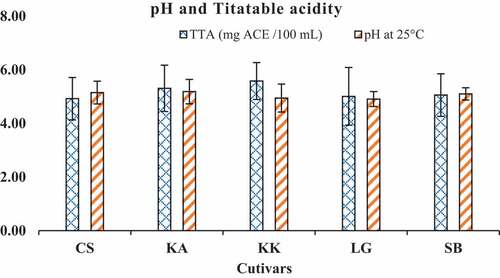
The total titratable acidity (TTA) and pH of watermelon juice samples range from 4.92 to 5.58 mg CAE/100 mL of juice and 4.90 to 5.18 respectively ().
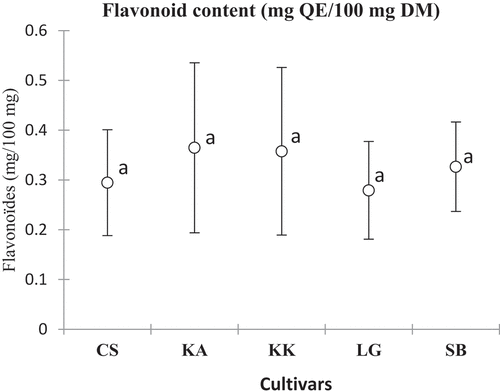
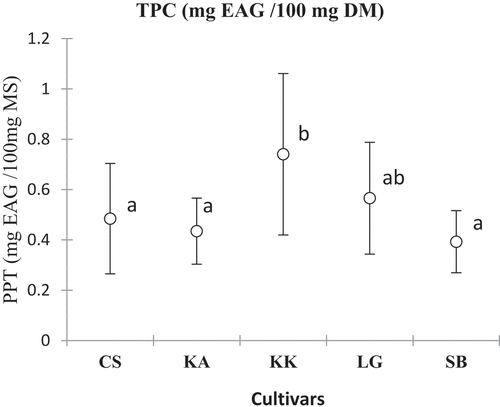
The total phenolic content ranged from 0.39 ± 0.12 (SB) to 0.74 ± 0.32 mg GAE/100 mg DM (KK) with a significant difference depending on the cultivar and the harvest area. As for total flavonoids, the levels varied from 0.27 ± 0.09 (LG) to 0.36 ± 0.17 mg TE/100 mg DM (KA). The variations observed were not significant according to cultivars ().
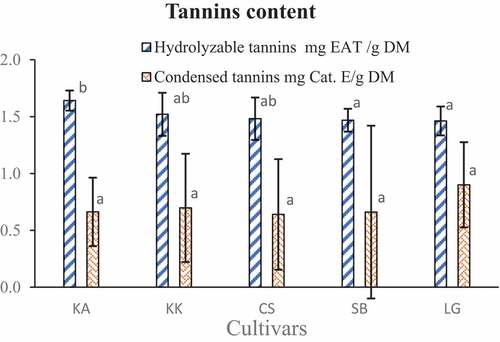
The hydrolyzable tannins content ranges from 1.46 ± 0.10 (LG) to 1.64 ± 0.19 mg GAE/g DM. The condensed tannins content also ranges from 0.64 ± 0.30 to 0.90 ± 0.76 Cat. E/g DM (). The variation of hydrolyzable tannins content shows singulative difference according to the cultivars but it was not the same for condensed tannins which don’t show singulative difference according to the cultivars.
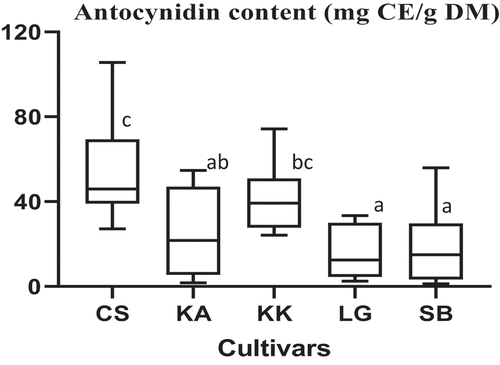
The anthocyanidin content of the cultivars ranges from 15.93 (LG) to 54.16 mg CE/g DM (CS). This variation was significantly dependent on the cultivar. CS and KK Cultivars show the highest anthocyanidin content ().
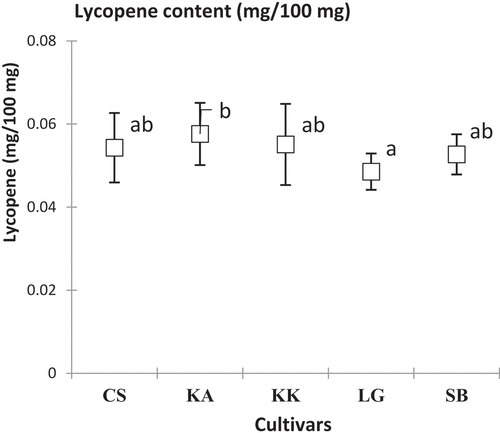
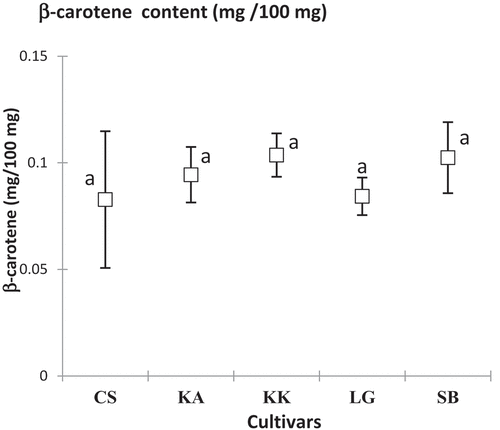
The contents of carotenoids, β-carotene and lycopene varied respectively from 0.083 ± 0.03 (CS) to 0.104 ± 0.01 mg/100 mg DM (KK) and from 0.049 ± 0.00 (LG) to 0.058 ± 0.00 mg/100 mg DM (KA). These assay levels were significantly different (p = .00; F = 16.30 and p = .00; F = 22.99) depending on the cultivar and harvest area. KK and KA cultivars showed the highest β-carotene and lycopene contents ().
The antioxidant activities evaluated by DPPH and ABTS methods are summarized in . According to the ABTS method, the cultivar KK showed the highest activity (61.54 ± 14.32 mmol AAE/g DM). The antioxidant activity according to the ABTS method is positively correlated with their total phenolic and carotenoid content. According to the DPPH method, the highest percentage of inhibition (89.60% ± 1.91%) at a 1 mg/mL sample concentration was observed with the LG cultivar. KK and LG cultivars showed the highest activities by both methods ().
Table 2. Antioxidant activity of watermelon cultivars studied.
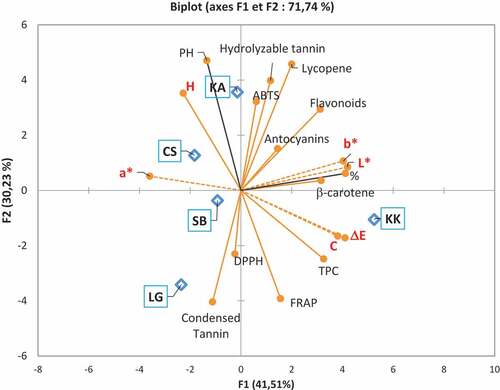
Statistical analysis revealed correlations between the different parameters studied. The Pearson correlation matrix of the different variables studied showed positive relationships between the different variables in the study (). Thus, a positive correlation between the color parameters (a* and H) and the secondary metabolite contents were observed. It should also be noted that the content of phenolic compounds and carotenoids and antioxidant activity (ABTS) are positively correlated. Principal component analysis was used to graphically illustrate the correlations between the variables and the proximity of the cultivars (). It revealed, indeed, proximity between the cultivars LG, CS, and SB. The axis F1 (carrying 41.51% of the information) carries the variables TPC, Flavonoids β-carotene, and Lycopene to the variables ΔE, L* and b*. It should be noted that the different phenolic and carotenoid contents are also positively related to the L*, b* of the pulp as well as the chromaticity, and C of the color.
Table 3. Correlation matrix (Pearson).
Discussion
The color parameters analysis of the five cultivars studied shows that the L* and b* parameters were significantly different according to the cultivar. Therefore, the pulp color alone would not be sufficient to differentiate the cultivars. This difficulty could be explained by the fact that the color of the pulp depends on several factors, in particular the degree of maturity of the fruit. Indeed, the color of the fruit varies greatly due to the accumulation of pigments in the fruit during ripening.[Citation26,Citation27] However, the color of the pulp is an important sensory parameter for consumers. Indeed, along with fruit size and sugar content, it is the main trait underlying consumers’ choice.[Citation28] Studies on sensory traits have revealed that bright red color, consistency, and taste are the main attributes determining consumer acceptance of the product.[Citation29] The statistical analysis reveals positive correlations noted between the parameters (a and H*) of color and the contents of β-carotene and lycopene. There is also a negative correlation between L* and lycopene content as reported.[Citation30] Indeed, according to the literature, the red color of watermelon pulp is mainly related to its carotenoid composition, especially lycopene.[Citation31]
The titratable acidity and the pH are some parameters with show the different organic acid content of watermelon samples. The study didn’t reveal a significant difference between the five cultivars for the pH and titratable acidity parameters. However, by comparison with other studies, the results show that the different cultivars studied are low acidity and hydronium potential.[Citation32] This trait is interesting for a kind of consumption that would not appreciate too acidic fruits.
The pH and the Total titratable acidity of Citrullus lanatus cultivars fruits samples recorded were generally low on account of their pH which was slightly acidic. But these results were slightly higher than the pH of the pulp (5.07 ± 0.08) recorded previously.[Citation9] The pH and the TTA are some important parameters that custom the quality of fruit. Furthermore, titratable acidity and pH are parameters linked to physiological processes, in particular fruit ripening. They can be made up of indicators of the state of maturation of the fruit.[Citation33] The effects of pH and on the stability of polyphenols and antioxidant activity of extracts was shown.[Citation34,Citation35]
The total phenolic contents observed varied significantly depending on the cultivar but are comparable to those reported in the literature 16.30–19.30 mg GAE/g of watermelon extracts.[Citation36] They were quite interesting for all cultivars in this study (3.9 ± 1.2 to 7.4 ± 3.2 mg GAE/g DM) with the cultivar KK in pole position. These levels of phenolic compounds make watermelon an important dietary source of phenolic compounds. Indeed, the high content of phenolic compounds could constitute an interesting therapeutic potential due to their contribution to biological activities.[Citation37,Citation38] This composition is even more interesting when watermelon is commonly consumed raw. The flavonoid content also varied depending on the cultivar. The studied cultivars showed total flavonoid contents comparable to those (3.51–7.76 mg QE/g) reported previously.[Citation39] The cultivars KK and KA with the highest contents can be recommended to consumers. The high levels of phenolic compounds could be a nutritional advantage, as these compounds are the source of watermelon’s biological properties. It has been shown, for example, that constituents such as flavonoids, tannins, and polyphenols are responsible for the laxative activity of Citrullus lanatus. Indeed, work conducted on rats showed that aqueous extracts of watermelon pulp had significant laxative and antiulcerogenic activity.[Citation38,Citation40]
In addition, the five watermelon cultivars studied showed quite interesting carotenoid contents. Indeed, β-carotene contents are similar to those found in previous studies (10.8 ± 1.6 g/g) on watermelon.[Citation27] Indeed, lycopene levels found in this study ranged from 30 mg/kg to 120 mg/kg of fresh materials (FM) as reported by others authors.[Citation41] These fairly high lycopene levels corroborate with the literature that cites watermelon as an important source of lycopene.[Citation38] It is worth noting a positive correlation (0.78) between β-carotene and lycopene contents which could be explained by the lycopene bioaccumulation process. Indeed, lycopene accumulation is related to the low expression of a transcription factor, an enzyme (lycopene β-cyclase) that converts lycopene to β-carotene.[Citation26]
The cultivars show low tannins content compared to the tannins level on the leaf.[Citation42] But, even though the tannins are known as antinutritional compounds, some studies showed that they are a lot of biological activities that improve the defense against bacterial infections.[Citation43] These phytochemicals especially flavonoids and tannins have been reported to have anti-diarrhea properties.[Citation44] The survey shows that the cultivar contains a meaningful quantity of anthocyanins. These pigments contribute to the activity of the antioxidant of the watermelon also.[Citation45] For the parameters commented above, the cultivar KK showed the most interesting values.
Watermelon is known for its positive effect on human health, i.e., previous studies have shown that consumption of watermelon increases blood levels of lycopene and β-carotene.[Citation46] These pigment molecules are cited as powerful antioxidants with a protective effect against oxidative stress and are beneficial for human health.[Citation46,Citation47]
Regarding antiradical activity, Citrullus lanatus cultivars showed inhibition percentages (DPPH) ranging from 83.07 ± 0.04% to 91.64 ± 0.02% of 1 mg/mL concentrations. These activities are higher than the DPPH inhibition percentages of 68.24 ± 1.27% at 10 mg/mL concentrations previously reported by authors.[Citation36] According to the ABTS method, the studied cultivars also showed interesting antiradical activities. This antioxidant activity is the source of their biological activities beneficial to health. The antiradical properties of the studied cultivars could be explained by their biochemical composition, in particular their composition in secondary metabolites such as phenolic compounds and carotenoids (lycopene particularly). The contribution of watermelon in antioxidant compounds is an interesting track, because of its thirst-quenching power and it can be consumed during intensive physical activities that accompany muscle stress.
The correlation analysis between the different parameters reveals the existence of positive links between the different secondary metabolites. This would mean that the metabolite contents are positively correlated and can therefore be selected simultaneously. There is also a positive relationship between these secondary metabolites and the ΔE of the pulp. Thus, ΔE could be considered to a lesser extent as a criterion for the selection of cultivars with a high content of phenolic compounds and carotenoids. This correlation is further explained by the contribution of pigmentary secondary metabolites such as carotenoids, especially lycopene has the red color of the fruit.[Citation48]
Conclusion
The examined watermelon cultivars show significant variability in terms of their phytochemical composition. Pulp color parameters vary considerably within cultivars depending on several factors. Nevertheless, it is an essential sensory characteristic to consider. There is a positive relationship between the pulp color parameters (ΔE, L*, b*and C*) and its phenolics and carotenoids content. The phenolic compounds and carotenoids content, especially lycopene, can explain the biological properties of the cultivars. The results obtained, the phytochemical composition, and the antioxidant activity, show that watermelon pulp, in addition to its nutritional role, has an interesting therapeutic potential. Among the studied cultivars, the KK cultivar presented the most interesting nutraceutical potential. Given these results, watermelon pulp could be recommended as a food supplement, especially for the control and prevention of chronic diseases. The results of this study could help vulgarize the positive effect, both nutritional and therapeutic, of the consumption of watermelon pulp on human health.
Acknowledgments
The authors thank the West African Biotechnology Network (RABIOTECH, ISP/IPICS project n°172600000) which financed the mobilities for the collection of samples, purchase of chemical, and the acquisition of lab equipment’s.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Hussain, J., Najeeb, UR., Khan, A.L., ALI, L., Al-Harrassi, A., Shinwari, Z.K., Hussain, H.;, Rizvi, TS. Proximate Based Comparative Assessment of Five Medicinal Plants to Meet the Challenges of Malnutrition. Eur. J. Med. Plants. 2013, 3(3), 444–453. DOI: 10.9734/ejmp/2013/3401.
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus Sabdariffa L. - A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. DOI: 10.1016/j.foodchem.2014.05.002.
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of Watermelon Supplementation on Aortic Hemodynamic Responses to the Cold Pressor Test in Obese Hypertensive Adults. Am. J. Hypertens. 2014, 27(7), 899–906. DOI: 10.1093/ajh/hpt295.
- Aminu, I.; Hadiza, B.; Murtala, Y.; Kamaluddeen, B.; Salisu Maiwada, A.; Jamila Mashi, A.; Abba, B. Nigerian Citrullus Lanatus Fruit and Seed Juice Reduces Cardiovascular Diseases Modifiable Risk Biomarkers in Normal Experimental Rats. Am. J. Hypertens. 2018, 4(2), 1–7. DOI: 10.23937/2474-3690/1510036.
- Paris, H. S. Origin and Emergence of the Sweet Dessert Watermelon, Citrullus Lanatus. Ann. Bot. 2015, 116(2), 133–148. DOI: 10.1093/aob/mcv077.
- Patel, M. B. Water Melon - the Must Melon. Indo Am. J. Pharm. Res. 2019, 9(5), 5–9.
- Dube, J.; Ddamulira, G.; Maphosa, M. Watermelon Production in Africa: Challenges and Opportunities. Int. J. Veg. Sci. 2020, 1–9. DOI: 10.1080/19315260.2020.1716128.
- Oyenihi, O. R.; Afolabi, B. A.; Oyenihi, A. B.; Ogunmokun, O. J.; Oguntibeju, O. O. Hepato- and neuro-protective Effects of Watermelon Juice on Acute ethanol-induced Oxidative Stress in Rats. Toxicol. Rep. 2016, 3, 288–294. DOI: 10.1016/j.toxrep.2016.01.003.
- Olayinka, B. U.; Etejere, E. O. Proximate and Chemical Compositions of Watermelon (Citrullus Lanatus (Thunb.) Matsum and Nakai Cv Red. Int. Food Res. J. 2018, 25(3), 1060–1066.
- Van Rensburg, W. S. J.; Venter, S. L.; Netshiluvhi, T. R.; Van Den Heever, E.; Vorster, H. J.; De Ronde, J. A. Role of Indigenous Leafy Vegetables in Combating Hunger and Malnutrition. S. Afr. J. Bot. 2004, 70(1), 52–59. DOI: 10.1016/S0254-6299(15)30268-4.
- Fraval, S., Yameogo V, Ayantunde A, Hammond J, De Boer IJ, Oosting SJ, Van Wijk MT. Food Security in Rural Burkina Faso : The Importance of Consumption of Own ‑ Farm Sourced Food versus Purchased Food. Agri. Food Sec. 2020, 1–17. DOI: 10.1186/s40066-020-0255-z.
- Hartman, J. L.; Perkins-Veazie, P.; Wehner, T. C. Citrulline and Arginine are Moderately Heritable in Two red-fleshed Watermelon Populations. HortScience. 2019, 54(2), 200–205. DOI: 10.21273/HORTSCI13715-18.
- Sharma, G.; Wu, W.; Dalal, E. N. The CIEDE2000 color-difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30(1), 21–30. DOI: 10.1002/col.20070.
- Dalorima, T.; Khandaker, M. M.; Zakaria, A. J.; Mohd, K. S.; Sajili, M. H.; Badaluddin, N. A.; Hasbullah, M. Organic Matter and Moringa Leaf Extract’s Effects on the Physiology and Fruit Quality of Red Seedless Watermelon (Citrullus Lanatus). Biosci. J. 2019, 35(5), 1560–1574. DOI: 10.14393/BJ-v35n5a2019-49354.
- Ouattara, G. S.; Soro, D.; Chatigre, K. O.; Koffi, E. K. Caractérisation Physicochimique Et Sensorielle de Diverses Formulations de Jus À Base de Pomme de Cajou Et D’ananas. Int. J. Biol. Chem. Sci 2017, 10(6), 2447. DOI:10.4314/ijbcs.v10i6.4.
- AOAC, Official Methods of Analysis of the Association of Official Analytical Chemists Chemists International, p. 250, 1995.
- Dicko, M. H.; Hilhorst, R.; Gruppen, H.; Laane, C.; Van Berkel, W. J. H.; Voragen, A. G. J. Zymography of Monophenolase and o-diphenolase Activities of Polyphenol Oxidase. Anal. Biochem. 2002, 306(2), 336–339. DOI: 10.1006/abio.2002.5707.
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation D’Un Extrait De Propolis Et Identification Des Principaux Constituants. J. Pharm. Belg. 1994, 49(6), 462–468.
- Mole, S., and Waterman, P. G. Tannins as Antifeedants to Mammalian Herbivores—Still an Open Question? In Allelochemicals: Role in Agriculture and Forestry; Washington, DC: Waller, G,ACS Symposium Series,American Chemical Society, 1987, Vol. 330, pp 51–572. DOI:10.1021/bk-1987-0330.ch051.
- Broadhurst, R. B.; Jones, W. T. Analysis of Condensed Tannins Using Acidified Vanillin. J. Sci. Food Agric. 1978, 29, 788–794.
- Lee, J., Durst RW, Wrolstad RE. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. , 2005, 88(5),1269–1278.
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi. 1992, 39(10), 925–928. DOI: 10.3136/nskkk1962.39.925.
- Hinneburg, I.; Damien Dorman, H. J.; Hiltunen, R. Antioxidant Activities of Extracts from Selected Culinary Herbs and Spices. Food Chem. 2006, 97(1), 122–129. DOI: 10.1016/j.foodchem.2005.03.028.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9–10), 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Sánchez-Rangel, J. C.; Benavides, J.; Heredia, J. B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. A. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods. 2013, 5(21), 5990–5999. DOI: 10.1039/c3ay41125g.
- Grassi, S.; Piro, G.; Lee, J. M.; Zheng, Y.; Fei, Z.; Dalessandro, G.; Giovannoni, J. J.; Lenucci, M. S. Comparative Genomics Reveals Candidate Carotenoid Pathway Regulators of Ripening Watermelon Fruit. BMC Genom. 2013, 14(781), 1471–2164.
- Tadmor, Y.; King, S.; Levi, A.; Davis, A.; Meir, A.; Wasserman, B.; Hirschberg, J.; Lewinsohn, E. Comparative Fruit Colouration in Watermelon and Tomato. Food Res. Int. 2005, 38(8–9), 837–841. DOI: 10.1016/j.foodres.2004.07.011.
- Chogou, S. K.; Assogba, R.; Degbey, H.; Abokini, E.; Achigan-Dako, E. G. Market Structure and Performance of Watermelon (Citrullus Lanatus)in Benin. Sci Afr. 2019, 3, e00048. DOI: 10.1016/j.sciaf.2019.e00048.
- Dossou, J.; Soulé, I.; Montgo, M. Evaluation des caractéristiques physico-chimiques et sensorielles de la purée de tomate locale produite à petite échelle au Bénin. Tropicultura. 2007, 25(2), 7.
- Perkins-Veazie, P.; Collins, J. K.; Pair, S. D.; Roberts, W. Lycopene Content Differs among red-fleshed Watermelon Cultivars. J. Sci. Food Agric. 2001, 81(10), 983–987. DOI: 10.1002/jsfa.880.
- Perkins-veazie, P.; Davis, A. R. In Search of High Lycopene Watermelon. Cucurbit Genet. Coop. Rep. 2004, 53, 51–53.
- Amin, M.; Ullah, S.; Rehman, S.; Ullah, Z.; Amir, M. Comparison of Different Types of Water Melon for Their Important Nutrients. J. Biol. Agric. Healthcare. 2014, 4(14), 59–66.
- Soteriou, G. A.; Kyriacou, M. C.; Siomos, A. S.; Gerasopoulos, D. Evolution of Watermelon Fruit Physicochemical and Phytochemical Composition during Ripening as Affected by Grafting. Food Chem. 2014, 165, 282–289. DOI: 10.1016/j.foodchem.2014.04.120.
- Arabshahi-d, S.; Devi, D. V.; Urooj, A. Food Chemistry Evaluation of Antioxidant Activity of Some Plant Extracts and Their Heat, pH and Storage Stability. Food Chem. 2007, 100, 1100–1105. DOI: 10.1016/j.foodchem.2005.11.014.
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of Tea Polyphenols Solution with Different pH at Different Temperatures. Int. J. Food Prop. 2017, 20(1), 1–18. DOI: 10.1080/10942912.2014.983605.
- Ramirez, M. R.; Oyhenart, J. Phenolic Composition, Antioxidant and Enzyme Inhibitory Activities of Cucurbitaceae Fruits. Int. J. Sci. Eng. Appl. Sci. 2018, 4(1), 26–35.
- Bazabang, S.; Monday, N.; Adebisi, S.; Makena, W.; Iliya, I. Hepatoprotective Effects of Aqueous Extract of Watermelon (Citrullus Lanatus) Seeds on Ethanol-Induced Oxidative Damage in Wister Rats. Sub-Saharan Afr. Med. J. 2018, 5(4), 129. DOI:10.4103/ssajm.ssajm_18_18.
- Erhirhie, E.; Ekene, N. Medicinal Values on Citrullus Lanatus (Watermelon): Pharmacological Review. Int. J. Res. Pharm. Biomed. Sci. 2014, 4(4), 1305–1312.
- Seidu, K. T.; Otutu, O. L. Phytochemical Composition and Radical Scavenging Activities of Watermelon (Citrullus Lanatus) Seed Constituents. Croat. J. Food Sci.Technol. 2016, 8(2), 83–89. DOI: 10.17508/cjfst.2016.8.2.07.
- Sharma, S.; Paliwal, S.; Dwivedi, J.; Tilak, A. First Report on Laxative Activity of Citrullus Lanatus. Pharmacol. Online. 2011, 2, 790–797.
- Perkins-Veazie, P.; Collins, J. K.; Davis, A. R.; Roberts, W. Carotenoid Content of 50 Watermelon Cultivars. J. Agric. Food Chem. 2006, 54(7), 2593–2597. DOI: 10.1021/jf052066p.
- Bahmed, I. A.; Meflah, Z. Composition des composés phénoliques et évaluation de leurs potentiels antioxydants : Cas de Citrullus lanatus et Cucumis melo, Université Abdelhamid Ibn Badis Mostaganem, 2018.
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S. H.; Hung, T. Q.; Stejskal, V.; Ringø, E.; Dawood, M. A. O.; Esteban, M. Á. Administration of Watermelon Rind Powder to Nile Tilapia (Oreochromis Niloticus) Culture under Biofloc System: Effect on Growth Performance, Innate Immune Response, and Disease Resistance. Aquaculture 2020, 528, 735574. DOI: 10.1016/j.aquaculture.2020.735574.
- Nwachoko, N.; Oghale, O. Evaluation of Anti-Diarrhoea Potential of Watermelon Seed (Citrullus Lanatus) in Albino Rats. Biochem. Pharmacol. 2017, 06(2), 2–4. DOI: 10.4172/2167-0501.1000228.
- Augustia, V. A. S.; Oktaviani, I. I.; Setyati, W. Anthocyanin and Flavonoid Extracted from Watermelon Rind (Citrullus Lanatus) with Two Different Colors of Watermelon Flesh: Yellow and Red. Mater. Scie. Forum. 2020, 998, 261–265.
- Edwards, A. J.; Vinyard, B. T.; Wiley, E. R.; Brown, E. D.; Collins, J. K.; Perkins-Veazie, P.; Baker, R. A.; Clevidence, B. A. Consumption of Watermelon Juice Increases Plasma Concentrations of Lycopene and β-carotene in Humans. J. Nutr. 2003, 133(4), 1043–1050. DOI: 10.1093/jn/133.4.1043.
- Veljović, M.; Davidović, S.; Pecić, S.; Despotović, S.; Leskošek-Čukalović, I., and Vukosavljević, P. Lycopene Content and Antioxidant Capacity of Tomato Jam, CEFood 2012 - Proceedings of 6th Central European Congress on Food, the University of Belgrade - Faculty of Agriculture, pp. 138–143, 2012.
- Zhao, W.; Lv, P.; Gu, H. Studies on Carotenoids in Watermelon Flesh. Agric. Sci. 2013, 04(7), 13–20. DOI: 10.4236/as.2013.47a003.