 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Agro-industrial waste has proved a huge problem owing to its disposal and impact on the environment. The eco-innovative technologies are applied to utilize waste as a resource. The mandate of the current investigation was to extract and characterize the bioactive molecules with special reference to luteolin from peanut shells through different innovative extraction techniques. This study was divided into two modules, in first the extraction of polyphenols was done through conventional and ultrasonic-assisted extraction (USE) by utilizing different solvents (ethanol 80%, methanol 80%, and water) and time intervals (5, 10 and 15 min for ultrasound and 30,60 & 90 min for conventional extraction technique) alongside HPLC and amino-acid characterization of a peanut shell. The results indicated that ultrasound proved significantly (p < .05) more effective as compared to conventional extraction and among solvent ethanol performed better as compared to methanol and water. Likewise, the time at 10 min also exhibited a significant effect on Peanut shell polyphenyl yield. The resultant highest value for TPC, DPPH, ABTS, FRAP, and β-carotene in ethanol of USE at 10 min was 3.79 ± 0.1 g GAE/100 g PS, 5.05 ± 0.1, 2.13 ± 0.03, 2.97 ± 0.03 and 5.33 ± 0.04 g TE/100 g PS, respectively. The resultant moieties exhibited high Luteolin contents after HPLC characterization and ranged from 1187 mg/g in conventional and 1409 mg/g in Ultrasound extraction. Likewise, the shell exhibited a promising amount of essential and non-essential amino acids. Conclusively, the peanut shell can be valorized due to its rich phytochemicals with special reference to luteolin.
Introduction
Globally, food production and processing generate an enormous amount of waste and by-products that cause serious environmental concerns owing to their permissibility and poor disposal techniques. However, this waste holds a promising phytochemical profile that can play a significant role in ensuring food security besides therapeutic effects. For instance, fruit by-products such as bagasse, peels, trimmings, stems, shells, bran, and seeds account for more than 50% of fresh fruit with higher nutritional or functional properties than the final product.[Citation1] Currently, researchers and scientists are in quest of discovering some innovative processes and technologies not only for waste management but also for the isolation/extraction of ingredients that could prove suitable for human health. Furthermore, changing global environmental patterns and population has caused an inclination in hunger index that may demand those technologies that have the potential for reduction, and recycling of food waste that should ultimately support the sustainability of food systems.[Citation2]
In this context, peanut (Arachis hypogaea) stands out as a potential candidate owing to its global production as an oil and protein-producing crop that yields various by-products rich in protein, fiber, and polyphenols. It belongs to the family Leguminosae and is mostly cultivated in tropical and subtropical regions with an estimated production of 44 million tonnes in 2016. The peanut processing industry generates a huge amount of by-products like peanut meal, peanut skin, peanut shell, peanut vine, etc. The peanut hull has been estimated to be 230–300 g of peanut hull per kg of peanut kernel produced.[Citation3] It is the potential source for various bioactive molecules, polyphenols, amino acids, and secondary metabolites with established safety for human consumption.[Citation4] Besides the rich source of polyphenols and flavonoids, it also contains luteolin, carotene, and isosaponaretin.[Citation5] The polyphenols range from (428.1–739.8 mg gallic acid equivalents/g), flavonoid (142.6–568.0 mg quercetin equivalents/g) and total Luteolin 0.25–1.12 mg/g.[Citation6] Luteolin is a 3′,4′,5,7-tetrahydroxyflavone, which is a naturally occurring flavonoid that provides a multiplicity of health benefits.
The utilization of novel technologies for bioactive extraction can improve extraction yield, and their techno-functional and nutritional properties. In this context, the ultrasonic extraction technique’s advantage is that by increasing mass transfer as well as bursting cellular matrix this technique releases a large amount of the targeted compounds as result enhancing the extraction yield of the desired compound. When the mixture is exposed to ultrasonic extraction; acoustic cavitation occurs and microbubbles develop in the liquid phase, owing to pressure changes it oscillates rapidly before collapsing, resulting in maximum extraction rate. Other innovative extraction techniques include ultrasonic-assisted extraction (UAE), supercritical fluid extraction, and microwave-assisted extraction has gained paramount importance due to higher polyphenol yield, less extraction time, less destructive effect on nutrients, and less complex process conditions.[Citation7,Citation8] The prime objective of the current investigation was to expound on the nutritional and antioxidant profile of peanut shells through innovative technologies.
Material and methods
The current research project was conducted at the Institute of Home and Food Sciences, Government College University Faisalabad. The protocol used for the current project is illustrated below;
Procurement of materials and chemicals
Peanut shells procured from the local processing unit of peanuts and chemicals were purchased from a scientific store in Faisalabad.
Proximate analysis
Assessment of Proximate peanut shells was performed for crude protein, crude fat, crude fiber, carbohydrates, and moisture contents according to the methods of[Citation9] While nitrogen-free extract (NFE) was obtained through the subtraction method.
NFE = (Moisture%+ crude protein%+ crude fiber %+ crude fat%+ carbohydrates %) – 100
Total poly-phenols (TPC) Extraction
Extraction of bioactive moieties (Luteolin) from the extract of peanut shells is carried out through conventional extraction & ultrasonic extraction technique as illustrated by[Citation10] in addition to modifications. For conventional extraction, the time frame was 30, 60 & 90 min, while the ultrasonic extraction was carried out for 5, 10, and 15 min by using Sonic processor of SONIC & MATERIALS. INC (model: VCX750) with frequency of 20 kHz, and a power of 750 W and 230 V with self-cooling system. The samples were prepared with ethanol 80%, methanol 80%, and water, and subjected to constant temperature (60°C). The resultant extracts were subjected to filtration through vacuum filtration, while remnants of solvents were recovered through Rotary Evaporator (EYELA, N-N series, Japan) at a temperature of 40°C. Furthermore, the isolation method was used for the isolation of luteolin from resultant extracts ().
Table 1. Treatments for solvent extraction efficiency by Conventional and Ultrasonic extraction.
Antioxidant profile
DPPH (Radical scavenging assay)
The samples were subjected to a standard method DPPH (1,1 -diphenyl-2- picrylhydrazyl) for the estimation of antioxidant capacity. Finally, the absorbance level for antioxidant capacity was estimated by Spectrophotometer with blank and control samples as explored by[Citation11]
FRAP (Ferric reducing antioxidant power) assay
The antioxidant capacity also assessed by another parameter is ferric reducing antioxidant power (FRAP) where metal ions were chelated. The resultant solution was subjected to absorbance estimation at the wavelength of 700 nm as mentioned by with required modification[Citation12]
ABTS (2, 2 – azino -bis, 3- ethyl-benzo-thiazoline-6 – sulfonic-acid)
The assessment of antioxidant activity is estimated by the most common standard method ABTS. The estimation of absorbance 1 ml solution of ABTS added into the 10 µl extract sample of peanut shell and subjected to spectro-photometer at the wavelength of 734 for the time of 30 min with desired modification, as prescribed by[Citation13]
β –carotene
Estimation of antioxidant capacity is also determined by the method of β-carotene as described by[Citation14] antioxidant activity (AA) is calculated by the following formula:
Free amino acid content estimation
The free amino acid contents were estimated by adapting the guidelines of[Citation15] through the Amino acid analyzer (HI-Tech lab) of the Government college university Faisalabad. In summary, 50 mg of lipolysis sample was hydrolyzed in 6 N HCl for 30 minutes. Fifty milligrams of the sample powder were hydrolyzed for 24 hours at 110 hrs in an ampulla tube with 1 Ml of, and the remaining components were dissolved in 10 mL of 0.01 N HCl solution, thoroughly mixed, and filtered. The free amino acid composition was measured using an automatic amino acid analyzer (L-8900Hitachi, Tokyo, Japan). All of the analyses were carried out in duplicate, and the amount of amino acid was expressed in micrograms per gram sample.
HPLC characterization
HPLC quantification of peanut shell extracts for luteolin has been carried out by adapting the guidelines.[Citation16–18] The Conditions, HPLC[Citation16] (PerkinElmer, Series 200, USA) was comprised of C 18 column. The mobile phase was (A) H2O2 (0.1% acetic acid) and (B) acetonitrile (0.1% acetic acid) at a flow rate of 1.0 ml/min with the following gradient: 0 min–5% B, 5 min–10% B, 10 min–20% B, 15 min–25% B, 20 min–30% B, 30 min–50% and 40 min–65% B and detection ranged is 330 nm.
Statistical analysis
The results were subjected to statistical analysis of each parameter to determine the significance level. Two-way ANOVA was carried out for evaluation of significant trend between means-variance LSD test was implemented. The values were in the mean ± S.D.
Results and discussion
Peanut shell proximate characterization
Proximate composition is a vital role in the compositional assessment of raw materials. Proximate analysis of peanut shell (PS) showed moisture contents, protein, fiber, fats, carbohydrate, ash, and contents were 7.21 ± 0.03%, 4.05 ± 0.05%, 55.95 ± 3.3%, 0.32 ± 0.01%, 23.98 ± 1.1%, 3.29 ± 0.01%, and 5.01 ± 0.02%, respectively (). The result of the current investigation regarding the proximate composition of peanut shells is in line with the earlier findings.[Citation19] They observed a higher amount of fiber and protein in the peanut shell of different peanut cultivars. Moreover,[Citation19] carried out the nutritional exploration of the peanut shell and observed 8.0%, 2.50%, 59.0%, 0.50%, 4.43%, moisture content, ash content, crude fiber, lipid, and crude protein, respectively. Earlier,[Citation20] also elucidate the nutritional and mineral profile of peanut shells and observed that they explicit the promising amount of fiber and protein alongside other constituents. They were of the view that the peanut shell is a good source of protein and fiber and utilization of this in value-added products has proved effective.
Table 2. Peanut shell Compositional analysis.
Extraction and antioxidant profiling of peanut shell
The means showed the highest polyphenol yield in ultrasound followed by conventional extraction.[Citation21] However, the maximum recovery was obtained by aqueous ethanol as compared to aqueous methanol and water. Similarly, the ratio was obtained by ultrasonic (15&90 minutes) and by conventional extraction (10& 60 minutes).
Means exposed highest PS polyphenols extraction rate from ultrasonic whilst lowest for conventional technique as 3.18 ± 0.08 g GAE/100 g of PS and 2.73 ± 0.07 g GAE/100 g of PS, respectively. Among the solvents, ethanol caused a more potent effect as 3.28 ± 0.08 & 2.77 ± 0.12 g GAE/100 g of PS followed by 80% methanol 3.16 ± 0.08 and 2.76 ± 0.09 g GAE/100 g of PS and the least in water as 2.68 ± 0.05 and 3.10 ± 0.06 g GAE/100 g of PS in ultrasound and conventional extraction, respectively. Like[Citation21] The recorded poly-phenol yield at different time intervals in ultrasonic and conventional techniques were 3.79 ± 0.1 g GAE/100 g of PS for 10 min and 3.25 ± 0.1 g GAE/100 g of PS for 60 min, respectively; followed by 3.18 ± 0.09 g GAE/100 g of PS (15 min) and 2.94 ± 0.08 g GAE/100 g of PS (90 min). The lowest amount was 2.56 ± 0.05 g GAE/100 g of PS (5 min) and 2.02 ± 0.08 g GAE/100 g of PS (30 min), respectively ().
Table 3. Impact of different extraction techniques on the Yield of total phenols content (g GAE/100 g of PS).
DPPH
Means regarding DPPH free radical scavenging activity also showed the same trend high 5.04 ± 0.07 g TE/100 g PS in USE and CSE 4.66 ± 0.04 g TE/100 g PS for aqueous ethanol followed by aqueous methanol as 4.81 ± 0.12 g TE/100 g PS and 4.46 ± 0.08 g TE/100 g PS, while lowest for water as 4.41 ± 0.09 and 4.09 ± 0.09 g TE/100 g PS respectively. Influence of time variations on extraction techniques explored the better activity of 5.05 ± 0.1 and 4.68 ± 0.08 g TE/100 g PS in USE at 10 minutes and CSE at 60 minutes than 4.77 ± 0.09 g TE/100 g PS at 15 minutes and 4.42 ± 0.06 g TE/100 g PS 90 minutes and lower 4.44 ± 0.09 g TE/100 g PS g/100 g at 5 minutes and 4.11 ± 0.07 g TE/100 g PS 30 minutes, respectively. Similarly, extraction techniques also impacted a clear difference in scavenging capacity of DPPH explained as 4.75 ± 0.09 g TE/100 g PS high in USE than 4.40 ± 0.07 g TE/100 g PS in CSE extraction technique ().
Table 4. Impact of different extraction techniques on DPPH free radical activity of PS (g TE/100 g PS).
Ferric reducing antioxidant power (FRAP)
Means of FRAP-chelating metal ions activity were affected by the extraction techniques and solvents. The ultrasonic extraction technique exhibited the 1.49 ± 0.02 g TE/100 g PS maximum FRAP activity in contrast to the conventional extraction technique 1.39 ± 0.02 g TE/100 g PS, respectively. The maximal chelating activity during solvents extraction is represented by aqueous ethanol than aqueous methanol and low in the water as 1.86 ± 0.02 g TE/100 g PS, 1.47 ± 0.01 and 1.16 ± 0.03 g TE/100 g PS in ultras-sonic whilst 1.73 ± 0.03, 1.37 ± 0.03 and 1.05 ± 0.02 g TE/100 g PS in conventional, respectively. However, time intervals in ultrasonic showed more strength at 10 min as 2.13 ± 0.03 g TE/100 g PS followed by 15 min 1.47 ± 0.02 g TE/100 g PS and minimum at 5 min 0.88 ± 0.01 g TE/100 g PS as compared to conventional at 60 min, 90 min., nd at 30 min as 0.83 ± 0.01 g TE/100 g PS of peanut shell extract, respectively ().
Table 5. Impact of different extraction techniques on FRAP activity of PS (g TE/100 g PS).
ABTS
Effects of time variations for both extraction methods concluded that the highest antioxidant capacity of PS extract through ABTS was 2.97 ± 0.03 g TE/100 g PS in USE at 10 min, followed by 2.37 ± 0.01 g TE/100 g PS at 15 min and 1.11 ± 0.01 g TE/100 g PS low at 5 min than 0at 60 min 2.70 ± 0.04 g TE/100 g PS, 90 min 1.97 ± 0.03 g TE/100 g PS and 1.01 ± 0.01 g TE/100 g PS for 30 min, respectively. Likewise, among extraction techniques USE technique explored the dominant effect over CSE technique 2.42 ± 0.02 and 2.20 ± 0.02 g TE/100 g PS, respectively. The recorded results for solvents effect on the antioxidant potential of PS extract by leading aqueous ethanol as 2.83 ± 0.02 g TE/100 g PS followed by aqueous methanol 2.59 ± 0.01 g TE/100 g PS and water were observed 1.83 ± 0.03 g TE/100 g PS in USE, while in CSE 2.58 ± 0.04, 2.36 ± 0.02 and 1.66 ± 0.01 g TE/100 g PS, respectively ().
Table 6. Impact of different extraction techniques on ABTS activity of PS (g TE/100 g PS).
β-carotene
Means regarding the β-carotene antioxidant ability of peanut shell extracts indicated that aqueous ethanol impacted a more potent effect than that of methanol and water as 5.53 ± 0.07 5.24 ± 0.04 and 4.64 ± 0.07 g TE/100 g PS in USE technique, whilst CSE technique as 5.13 ± 0.09, 4.90 ± 0.06 and 4.5 ± 0.05 g TE/100 g PS, respectively. However, the recorded effects of time differences and extraction techniques described that 10 min proved more affected as compared to 5.33 ± 0.04, 5.25 ± 0.05, and 4.84 ± 0.09 g TE/100 g PS at 15 min and 5 min in USE followed by CSE at 60 min succeed by 90 min and 30 min as 5.14 ± 0.07, 4.86 ± 0.08 and 4.52 ± 0.04 g TE/100 g PS, respectively. USE extraction explored the same trend with maximum activity as 5.14 ± 0.06 g TE/100 g PS, while CSE extraction showed less 4.84 ± 0.06 g TE/100 g PS, respectively ().
Table 7. Impact of different extraction techniques on β-carotene activity of (g/100 g of PS).
HPLC characterization
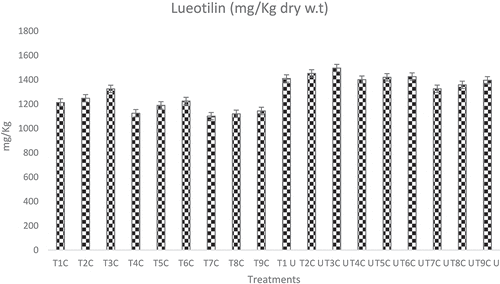
By following[Citation16] HPLC quantification exhibited that the techniques and conditions for extraction elucidated a significant (p ≤ . 005) effect on luteolin contents. Among the extraction techniques, Ultrasound caused a more pronounced effect (1409 mg/Kg) on the Luteolin yield as compared to the conventional extraction (1187 mg/Kg). Likewise, among the solvents, the order of effectiveness in both ultrasound and conventional was Ethanol>Methanol>water ().
Figure 1. Absolute values luteolin carried out through conventional and ultrasound-added extraction by using water, water, methanol and ethanol as solvent. two way ANOVA was applied to check the overall behavior of the study parameter to elaborate the effect of treatments and solvent on luteolin contents. To evaluate the differences among the mean LSD test was (p ≤ . 05).
Amino acid contents
Mean values of free amino acids of peanut shell are given in which showed that the peanut shell powder holds a significant amount of essential amino acids including L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-Phenylalanine, L-Threonine and L-valine as 0.18, 0.39, 0.41, 0.27, 0.04, 0.20, 0.14, and 0.55 mg/g, respectively. Whereas, L-arginine, L-Aspartic acid, L-cysteine, L-glutamic acid, L-glycine, L-tyrosine, proline, and b-alanine were 0.56, 0.68, 0.12, 0.16, 0.04, 0.09, 0.85, and 0.76 mg/g, respectively. However, other free amino acids are not detected except ammonia, and O-Phospho-L-Serine which are 0.34 and 0.05 mg/g, respectively.
Table 8. Amino Acid Profile of peanut shell (mg/g Of Dry Weight).
The hallmark of the investigation is the complete antioxidant profiling alongside Lutenin HPLC characterization and amino acid determination from the peanut shell. The outcomes delineated that the peanut shell showed a promising antioxidant and phenolic profile correlated with the extraction conditions. The ultrasound-aided extraction caused maximum yield as compared to conventional extraction alongside the time and solvent used for the extraction. Polyphenol estimation is the initial step to validate the antioxidant potential of the product and the peanut shell exhibited a strong polyphenol profile. The higher performance of ethanol in the current study is supported by the earlier findings of Shahidi and Peng et al.[Citation22] also observed that high fractions of phenolic moieties recovered by ethanol 80% (v/v) 214 CE mg g − 1. Afterward,[Citation21] probed the TPC contents of peanut shells collected from different cultivars; they observed a significant effect of extraction technique, solvents, and time of extraction. Later, Adhikari et al.[Citation6] conducted the extraction of polyphenols from peanut shells, they applied different solvents (methanol, ethanol, etc) for the extraction of polyphenols from different peanut shells of Korean Cultivars and observed total polyphenol (428.1–739.8 mg gallic acid equivalents/g), flavonoid (142.6–568.0 mg quercetin equivalents/g), and amino acid (5.76–34.56 mg/g) contents with higher, DPPH, FRAP, and SOD activities. They were of the View that the phenolic compounds present in the peanut shells might be due to the self-defense mechanisms of the plants against microorganisms and oxidation in the soil. The current results regarding the antioxidant activity are compared with previous findings,[Citation23–25] they selected three types of electron transfer-based methods (DPPH, ABTS, and FRAP) generally applied to measure the antioxidant activity of bio-active moieties of a peanut shell. They observed that the highest antioxidant activity of DPPH, ABTS, and FRAP was 1.17, 5.30, and 2.70 g TE/100-PS observed at 220 0C, respectively, they conducted that the extraction conditions have influenced the antioxidant profile of peanut shell. Likewise, the peanut shell contains higher flavonoid contents mainly Luteolin which was a principal antioxidative component of peanut shells that increases with the maturity of peanut hulls .[Citation6] In this context, Geethaet al.[Citation16] observed a higher amount of total flavonoids in peanut shells with higher antimicrobial and pharmacological activities. In the Current investigation, the higher amount of lutenin in peanut shell extract are in corroborated with the earlier findings of Qiu et al.[Citation26] using DPPH–HPLC–DAD–TOF/MS methods to identify the flavonoids and antioxidants from the peanut shell and observed lutenin as major flavonoids. The envisaged that the extraction technique, method of analysis, and type of cultivar may play an instrumental role in this context. Adhikari et al.[Citation6] documented that the peanut shell exhibited a good amino acid profile and essential and free amino-acid ranged from 2.16 to 13.26 mg/g dominated by Arginine, aspartic acid, glutamic acid, and leucine whereas, ammonia, cysteine, methionine, and tyrosine were the less abundantly found amino acids
Conclusion
Results concluded that the peanut shell has a notable source of polyphenols, which further can be used for the development of functional foods. It was noticed that the extraction yield of these moieties was significantly influenced by extraction techniques, solvents, and time. The highest extraction for these moieties was observed by ethanol at 10 min of USE as compared to CSE through ethanol at 60 min.
Ethical Approval
This study has nothing to do with human and animal testing.
Interest of conflict
The authors declare no conflict of interest.
Acknowledgments
The authors are thankful for the technical support from Government College University Faisalabad—Pakistan for research support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rodríguez, L. G. R.; Gasga, V. M. Z.; Pescuma, M.; Nieuwenhove, C. V.; Mozzi, F.; Burgos, J. A. S. Fruits and Fruit by-products as Sources of Bioactive Compounds. Benefits and Trends of Lactic Acid Fermentation in the Development of Novel fruit-based Functional Beverages. Int. Food Res. J. 2021, 140, 109854. DOI: 10.1016/j.foodres.2020.109854.
- Bunditsakulchai, P.; Liu, C. Integrated Strategies for Household Food Waste Reduction in Bangkok. Sustainability. 2021, 13(14), 7651. DOI: 10.3390/su13147651.
- Arya, S. S.; Salve, A. R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53(1), 31–41. DOI: 10.1007/s13197-015-2007-9.
- Gam, D. H. H.; Hong, J. W. W.; Kim, J. H. H.; Kim, J. W. W. Skin-Whitening and Anti-Wrinkle Effects of Bioactive Compounds Isolated from Peanut Shell Using Ultrasound-Assisted Extraction. Molecules. 2021, 26(5), 1231. DOI: 10.3390/molecules26051231.
- Hassan, A. B.; Al Maiman, S. A.; Alshammari, G. M.; Mohammed, M. A.; Alhuthayli, H. F.; Ahmed, I. A. M.; Alfawaz, M. A.; Yagoub, A. E. A.; Fickak, A.; Osman, M. A. Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis Hypogaea L.) Pod Shells. Processes. 2021, 9(9), 1542. DOI: 10.3390/pr9091542.
- Adhikari, B.; Dhungana, S. K.; Ali, M. W.; Adhikari, A.; Kim, I. D.; Shin, D. H. Antioxidant Activities, Polyphenol, Flavonoid, and Amino Acid Contents in Peanut Shell. J. Saudi Soc. Agric. Sci. 2019, 18(4), 437–442.
- Jahromi, S. G. Extraction Techniques of Phenolic Compounds from Plants. Plant physiological aspects of phenolic compounds. 2019, 1, 1–18.
- Gupta, D., and Jeyaseelan, C. Ethnic Foods and Concentrates: Its Role in Health Protection. In Herb. Med. J; Canada: Academic Press: 2022; pp 269–289. doi:10.1016/B978-0-323-90572-5.00002-0
- AACC. Approved Methods of the AACC, 10th (ed.). St ed.; AmericanAssociation of Cereal Chemists: Paul; MN, 2000.
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic Content and Antioxidative Capacity of Green and White Tea Extracts Depending on Extraction Conditions and Solvent Used. Food Chem. 2008, 110(4), 852–858. DOI: 10.1016/j.foodchem.2008.02.072.
- Kim, J. S.; Kwon, Y. S.; Chun, W. J.; Kim, T. Y.; Sun, J.; Yu, C. Y.; Kim, M. J. Rhus Verniciflua Stokes Flavonoid Extracts Have anti-oxidant, anti-microbial and α-glucosidase Inhibitory Effect. Food Chem. 2010, 120(2), 539–543. DOI: 10.1016/j.foodchem.2009.10.051.
- Vijayalaxmi, S.; Jayalakshmi, S. K., and Sreeramulu, K. Polyphenols from Different Agricultural Residues: Extraction, Identification and Their Antioxidant Properties. J. Food Sci. Technol. 2015, 52(5), 2761–2769. [Cited 2022 Jan 1]. https://link.springer.com/article/10.1007/s13197-014-1295-9.
- Jitrangsri, K.; Chaidedgumjorn, A., and Satiraphan, M. Effect of Ethanol Percentage upon Various Extraction Methods of Peanut Based on Antioxidant Activity with trans-resveratrol and Total Phenolic Contents. Germany: Springer. DOI: 10.29090/psa.2020.02.018.0056.
- Zhuang, S.; Zheng, W.; Na, Y.; Chen, N.; Gong, F.; Huang, B.; Charles, S. B.; Liu, C.; Cheng, J.; Ma, L., et al. Changes in the Content and Antioxidative Activity of β‐carotene and Its Metabolite Vitamin A during Gastrointestinal Digestion and Absorption and Optimisation of HPLC‐based Detection. Int. J. Food Sci. 2022, 57(2), 1093–1103. DOI: 10.1111/ijfs.15475.
- Mæhre, H. K.; Dalheim, L.; Edvinsen, G. K.; Elvevoll, E. O.; Jensen, I. J. Protein determination—method Matters. Foods. 2018, 7(1), 5. DOI: 10.3390/foods7010005.
- Zhang, B.; Tong, Y.; He, J.; Zhang, F., and Tian, M. Boronate-Modified Polyethyleneimine Dendrimer as Solid-Phase Extraction Adsorbent for the Analysis of Luteolin in Peanut Shell Samples via High Performance Liquid Chromatography. 2021, Available at SSRN 3900946. [cited 2022 Jan 5]. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3900946
- Sharma, A.; Rasane, P.; Dey, A.; Singh, J.; Kaur, S.; Dhawan, K.; Kumar, A.; Joshi, H. S. Optimization of a Process for a Microgreen and Fruit Based Ready to Serve Beverage. Int. J. Food Stud. 2021, 10. DOI: 10.7455/ijfs/10.SI.2021.a4.
- Kale, R. G.; Sawate, A. R.; Kshirsagar, R. B., and Mane, R. P. Studies on Development and Organoleptic Evaluation of beetroot-tamarind RTS Beverage. Int. J. Chem. Studies. 2018, 6(2), 2974–2976. [cited 2022 Jan 1]. https://www.researchgate.net/profile/Rajan-Kale/publication/325057849_Studies_on_development_and_organoleptic_evaluation_of_beetroot-tamarind_RTS_beverage_Kale_RG_Sawate_AR_Kshirsagar_RB_and_Mane_RP/links/5af3ec9e4585157136c929c6/Studies-on-development-and-organoleptic-evaluation-of-beetroot-tamarind-RTS-beverage-Kale-RG-Sawate-AR-Kshirsagar-RB-and-Mane-RP.pdf.
- Varma, A. K.; Singh, S.; Rathore, A. K.; Thakur, L. S.; Shankar, R.; Mondal, P. Investigation of Kinetic and Thermodynamic Parameters for Pyrolysis of Peanut Shell Using Thermogravimetric Analysis. Biomass Convers. Biorefin. 2020, 1–12. DOI: 10.1007/s13399-020-01081-6.
- Shibli, S.; Siddique, F.; Raza, S.; Ahsan, Z.; Raza, I. Chemical Composition and Sensory Analysis of Peanut Butter from Indigenous Peanut Cultivars of Pakistan. Pak. J. Agric. Sci. 2019, 32(1), 159. DOI: 10.17582/journal.pjar/2019/32.1.159.169.
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis Vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20(4), 1076–1080. DOI: 10.1016/j.ultsonch.2012.12.002.
- Shahidi, F.; Peng, H. J. Food Bioact. 2018, 4, 11–68. DOI: 10.31665/JFB.2018.4162.
- Fidrianny, I.; Elviana, D., and Ruslan, K. In Vitro Antioxidant Activities in Various Beans Extracts of Five Legumes from West of Java-Indonesia Using DPPH and ABTS Methods. Int. J. Pharmacogn. Phytochem. Res. 2016, 8(3), 470–476. [cited 2022 Jan 5]. http://impactfactor.org/PDF/IJPPR/8/IJPPR,Vol8,Issue3,Article15.pdf
- Kausar, H.; Saeed, S.; Ahmad, M. M., and Salam, A. Studies on the Development and Storage Stability of cucumber-melon Functional Drink. J. Agric. Res. 2012, 50(2), 239–248. [cited 2022 Jan 5]. https://apply.jar.punjab.gov.pk/upload/1374744164_95_546__165jarpap1(9).pdf.
- Bhalerao, P. P.; Mahale, S. A.; Dhar, R.; Chakraborty, S. Optimizing the Formulation for a pomegranate-amla-muskmelon Based Mixed Fruit Beverage Using Sensory Analysis and Evaluating Its Thermal Stability. LWT. 2020, 133, 109907. DOI: 10.1016/j.lwt.2020.109907.
- Abdulrazak, S.; Otie, D., and Oniwapele, Y. A. Proximate Analysis and anti-nutritional Factors of Groundnut and Melon Husk. Online J. Anim. Feed Res. 2014, 4(2), 25–28. [cited 2022 Feb 2]. http://ojafr.com/main/attachments/article/102/Online%20J.%20Anim.%20Feed%20Res.,%204(2)%2025-28.pdf
