ABSTRACT
Phenolic compounds are important components that affect the taste and health-care function of walnuts. And phenolic compounds in walnut (Juglans sigillata Dode. cv. ‘Qianhe 7’) pellicles at three developmental stages were explored to identify their role. The results showed that the total phenolic content, total flavonoid content, ferric reducing antioxidant power, 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) and 1, 1-diphenyl-2-picrylhydrazyl free radical scavenging activities in pellicles increased gradually during fruits development, and reached the maximum value at maturation period. Syringic acid, gallic acid, catechin, and rutin contents were highly significantly positively correlated with reducing power or highly significantly negatively correlated with IC50 of free radical scavenging activities. Gallic acid and rutin contents were highly significantly positively correlated with phenylalanine ammonia-lyase (PAL) activity. Total phenolic content, syringic acid, catechins, and rutin contents were highly significantly negatively correlated with cinnamate 4-hydroxylase (C4H) and 4-coumarate CoA ligase (4CL) activities. Ferulic acid content was highly significantly positively correlated with polyphenol oxidase (PPO) activity. Overall, PAL, C4H, 4CL and PPO were key enzymes in the metabolism of polyphenols, and they affected the antioxidant capacity of the pellicles by regulating the content of polyphenol during walnut fruit development.
Introduction
As a crucial secondary metabolite, plant phenolic compounds are involved in plant signal transduction, cell division, hormonal regulation, photosynthetic activity regulation, germination and reproduction, as well as in response to a variety of abioticstresses.[Citation1] Plant phenolic compounds can reduce the excess reactive oxygen species (ROS) produced under abiotic stress and balance the oxidation homeostasis in plants by directly scavenging free radicals in the body or regulating the expressions of related genes and enzyme activities.[Citation2] The association between phenolic contents and antioxidant activity has been found in many plants, such as prosomillet[Citation3] and sour orange.[Citation4] In addition, plant phenolic compounds are good for our health in preventing chronic degenerative diseases, such as anti-cancer, anti-type II diabetes, protecting nerves, protecting cardiovascular, regulating blood pressure, fighting atherosclerosis, participating in immune regulation, and improving ophthalmic diseases due to their antioxidant activities, so they are considered as natural compounds with health-care function.[Citation5]
Walnuts (Juglans spp.) are favored by researchers because of their rich phenolic contents and potent antioxidant capacity.[Citation6] Its fruit is composed of epicarp, mesocarp, endocarp and seed. The epicarp and mesocarp are collectively called green husk and the endocarp is also called hard shell. The seeds are the edible part, which is composed of kernel and pellicle.[Citation7] Up to now, the contents and antioxidant activities of polyphenols in different walnut organ, such as flowers, leaves, kernels, pellicles and green husks have been extensively reported.[Citation8] Medic et al.[Citation9] found that total phenolic content in pellicles of six walnut varieties was approximately 1, 000 times than that of kernels. Zhang et al.[Citation10] proved that the polyphenols content and antioxidant activity in the pellicle of walnut ‘Yangbi’ were higher than those in diaphragma juglandis fructus and flowers. Akbari et al.[Citation11] demonstrated that phenolic contents and free radical scavenging ability in pellicles were higher than those of in green husks, hard shells, and kernels. In conclusion, the walnut pellicle is the main organ to accumulate phenolic compounds and antioxidant capacity.[Citation12] Thus, the study on the accumulation law of phenolic compounds in pellicles has an essential guiding role in regulating the quality of kernels.
The accumulation of phenolic substances may be correlated with the activities of related key enzyme inpolyphenolsmetabolism.[Citation13] The interaction of these enzymes affects the accumulation of polyphenols in plants. Total phenolic content (TPC), total flavonoid content (TFC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities were positively correlated with phenylalanine ammonia lyase (PAL), Chalcone synthase (CHS) and polyphenol oxidase (PPO) activities in avocados (p < . 0001 and p < . 05).[Citation14] The content of polyphenols was closely related to the activities of PPO, cinnamate 4-hydroxylase (C4H) and 4-coumarate CoA ligase (4CL) during the fruits development of two olives varieties.[Citation15] In Lonicera japonica, the activities of PAL, C4H and 4CL were positively correlated with chlorogenic acid levels in S1 (young alabastrum)-S5 (silvery flower) stages and luteoloside levels in S1 (young alabastrum)-S4 (whole white alabastrum) stages, respectively.[Citation16] Therefore, the types and contents of polyphenols are controlled by the activities of related enzymes. However, the relationship between polyphenols accumulation and enzyme activity in walnut was only studied in leaves[Citation17] and hard shells.[Citation18] The polyphenol content in pellicles is the highest, and its protection is the key to preventing kernels oxidation.[Citation19] However, the relationship between the content of polyphenols and related metabolic enzymes has not been reported.
J. sigillata is one of the walnut species native to China, mainly distributed in southwest China. ‘Qianhe 7’ has the excellent quality, high yield, robust adaptability and disease resistance. It is very popular with locals and substantial to local economic development. In this study, the dynamic changes of phenolic contents, antioxidant activities, polyphenol metabolism-related enzyme activities in pellicles and their relationships during ‘Qianhe 7’ fruit development were explored. The purpose of our research was to find out the dynamic accumulation pattern of phenolics in pellicles, and laid a theoretical foundation for rationally regulating the quality of walnuts.
Materials and methods
Chemicals
Syringic acid, chlorogenic acid, syringaldehyde, caffeic acid, ferulic acid, catechin, epicatechin, myricetin, ellagic acid, gallic acid, rutin, p-coumaric acid, juglone standard products purchased from Sigma-Aldrich. The purity of syringic acid was more than 99%, and the other was more than 98%.
2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2, 4, 6-tris(2-pyridyl)-1, 3, 5-triazine(TPTZ), 1, 1-diphenyl-2-picrhydrazyl were purchased from Shanghai Yuanye Biotechnology Co., LTD, China. Their purity was 98%. Folin-phenol reagent was purchased from Beijing Laibao Technology Co., LTD, China. Methanol (≥99.9%) and acetic acid (≥99.9%) were chromatographic pure, other reagents without particular description were domestic analytical pure.
Plant material
The experimental materials were taken from Caishen Town (27.13 N, 104.71 E), Hezhang County, Bijie City, Guizhou Province, with an average altitude of 1750 m, an average temperature of 13°C throughout the year, and an average annual rainfall of 835 mm, which belongs to temperate climate. Ten walnut trees ‘Qianhe 7’ inhefull fruit stage with uniform growth were randomly selected. Fruits were collected at six time points in three stages – hard-core stage (1/7, 15/7), kernel-filling stage (1/8, 15/8) and maturation stage (1/9, 10/9). Eight fruits with the same size and no spots were collected on four branches (two per branch), 90 degree apart from each other on each tree. Three biological repetitions were carried out. Samples were brought back to the laboratory with an ice box. After removing green husks and hard shells, pellicles were separated from seeds. Then, a quarter of pellicle of each fruit was quickly removed with a clean surgical blade. The taken pellicles were cut into 2–3 mm diameter pieces, and mixed these pieces from each replicate. Later, samples were divided into two parts; some were used for enzyme activity analysis, the others were used for phenol quantification. All the samples were immediately frozen in liquid nitrogen and stored at −80°C.
Extraction of phenolics from walnut pellicles
Samples (0. 5 g pellicles) were mixed with 5 mL of 50% methanol aqueous solution (v:v), grinded into homogenate and extracted via 40 kHz ultrasound for 40 min at 25°C according to Wang et al .[Citation20] Repeated the extraction twice and combined the extracts. The extracts were centrifuged at 7500 × g for 10 minutes, concentrated under reduced pressure to a paste, and diluted to 5 mL with methanol to obtain the polyphenol extracts that were then stored in the refrigerator for later use.
Total phenolic content (TPC)
TPC was determined via the Folin-phenol method.[Citation21] Gallic acid standard solutions with different concentrations were mixed with 0.4 mL of Folin-phenol reagent and 2.5 mL of 20% Na2CO3 (w:v), and diluted to 10 mL. The absorbance of the mixed solutions at 765 nm was determined after water bath at 25°C for 2 h, and got the standard curve equation of gallic acid: y = 11.925x-0. 0766, R2 = 0. 995. The polyphenol extract was used to replace the gallic acid standard solution in the above system, and the absorbance value was measured under the same conditions. The total phenol content was calculated according to the gallic acid standard curve, and the result was expressed milligrams of gallic acid equivalents per gram of walnut pellicles flesh weight (mg GAE·g−[Citation1] FW).
Total flavonoids content (TFC)
The method of content determination of total flavonoids is referred to Feng et al.[Citation22] Transfer 0.3 mL of rutin standard solution with different concentrations to volumetric flask, then 1 mL of 5% NaNO2 (w: v) was added, 1 mL of 10% Al (NO3) 3 (w: v) was added after 6 min, 3 mL of 1 mol·L−[Citation1] NaOH was added 6 min later, adjusted the volume to 10 mL, and measured the absorbance at 510 nm after water bath at 25°C for 15 min. The standard curve equation of rutin was obtained as follows: Y = 0.0945 x + 0.0276, R2 = 0.9986. Using polyphenol extract solution instead of rutin standard solution in the above system, the absorbance value under the same conditions was determined, and the total flavonoids content was calculated according to rutin standard curve. The results were expressed as milligrams of rutin equivalents per gram of walnut pellicles flesh weight (mg RE·g−[Citation1] FW).
HPLC analysis of phenol compounds
The high-performance liquid chromatography (HPLC) analysis was performed using Shimadzu LC-15C HPLC system equipped with an Agilent C18 column (250 mm × 4.6 mm, 5 µm). Polyphenol extract was filtered with 0.22 µm filter membrane, and the 10 μL filtered liquid was injected by an automatic sampler with 0.9 mL·min−[Citation1] flow rate. The mobile phases consisted of solvent A (0.8% acetic acid (v: v)) and solvent B (methanol). Two elution gradients were used: (1) 0–10 min, 10%–20% B; 10–25 min, 20%–50% B; 25–35 min, 50% B; 35–40 min, 50%–10% B. Chromatograms were recorded at 280 nm for syringic acid, chlorogenic acid, syringaldehyde, caffeic acid, ferulic acid, catechin, epicatechin, gallic acid, p-coumaric acid. (2) 0–10 min, 10%–40% B; 10–25 min, 40%–60% B; 25–30 min, 60%–70% B; 35–40 min, 80%–10% B. Chromatograms were recorded at 251 nm for rutin, ellagic acid, myricetin, quercetin, juglone at 25°C the column temperature.
In vitro antioxidant activity
The ferric reducing antioxidant power was determined according to the method of Benzie et al. .[Citation23] Taking 1.0 mmol·L−[Citation1] FeSO4 as the standard, the FRAP value of samples was expressed in millimoles of FeSO4 needed to achieve the same absorbance. The DPPH and ABTS free radical scavenging assay followed the methods of Jun et al.,[Citation24] and the results were expressed as IC50.
Polyphenol metabolism-related enzymes activities
About 0.2–0.5 g pellicles were rinsed in pre-cooled PBS buffer solution, dried with filter paper, and put into a 5 mL homogenate tube. The homogenizing medium was added to the homogenizing tube in the ratio of 1:4 (m: v). The tissue pieces were cut as soon as possible with small scissors under ice water bath conditions. Held the homogenizer tube in one hand and inserted the lower end into the vessel containing the ice-water mixture, and inserted the tamping rod vertically into the sleeve with the other hand, and fully grinding it to make 20% homogenate. The prepared homogenate was centrifuged at 2000 × g for 15 min, and the supernatant was taken for determination. The activities of 4CL,[Citation25] peroxidase (POD),[Citation26] PPO[Citation27] were determined as previously. The activities of 3-Deoxy-D-arabino-heptulosonate-7-phosphate synthase (DAHPS), chorismate synthase (CS), chorismate mutase (CM), CHS, PAL, C4H, and chalcone isomerase (CHI) were determined by ELISA. The kit was purchased from Shenzhen Zike Biotechnology Co., LTD., China, and all the measurements were carried out according to the reference instructions.
Statistical analysis
Data analyses were performed using the SPSS version 19. Significant differences were determined by Duncan’s multiple-range test. Different lowercase letters above the error line indicated the significant difference at 95% level of each measurement index in different developmental stages (3 replicates).
Results and discussion
Changes of phenolics contents in pellicles during fruits development
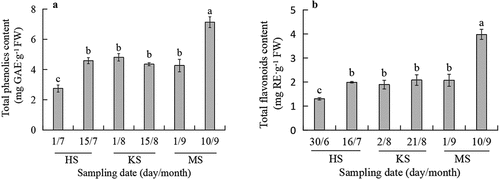
Previous research found that the distribution of polyphenols in walnut trees was uneven, and the change trends among organs were different at different developmental stages. The polyphenol content in walnut leaves[Citation28] and green husks[Citation29] increased first and then decreased in the annual development cycle. In this study, we found the changes of TPC and TFC in pellicles showed the same trend that increased in the hard-core stage (1/7 and 15/7), remained stable from the hard-core stage to the early maturation stage (15/7 ~ 1/9), and increased again during the late stage of mature (1/9 and 10/ 9) (), which inconsistent with the changing trend of leaves and green husks. This may be due to the difference in substance synthesis and the transport relationship between different organs during the development of walnuts. The study on the ultrastructure of pellicles at different development stages revealed that pellicles may be a temporary warehouse for the transport of nutrients from green husks to kernels.[Citation30] In the process of kernel development, the accumulation of organic matter prioritized fat synthesis, and its synthetic raw materials were mainly the sugar produced by photosynthesis in leaves and green husks. The pellicles had significantly developed vascular bundle networks, in which the transportation of sugar ensured the supply of nutrients during the development of kernels.[Citation31] Therefore, the slow increase of the TPC and TFC in pellicles during the hard-core stage in July and the kernel-fulling period in August might be related to the preferential transport of substrates to kernels for the rapid accumulation of fat,[Citation32] while the rapid increase of the TPC and TFC in pellicles in early September might be due to the decrease in the accumulation of fat a few days before kernels mature, which marked the substrates for the synthesis of total phenols and total flavonoids in pellicles sufficient.
Figure 1. Changes of TPC (A) and TFC (B) in pellicles during fruits development. HS means hard-core stage. KS means kernel-filling stage. MS means mature stage. Different letters on top of error bars indicate significant differences of different stages according to Duncan’ s multiple range tests at p < .05 (n = 3).
As shown in , the contents of 10 kinds of monomeric phenols in pellicles showed two change trends during fruit development. The contents of rutin, syringic acid, gallic acid, caffeic acid, p-coumaric acid and syringaldehyde showed a gradual upward trend. In contrast, the contents of catechin, chlorogenic acid, ferulic acid and juglone showed the trend of first increasing, then decreasing and increasing again. The average content of rutin was the highest among 10 kinds of individual phenolic (462.70 mg·100 g−1 FW), and rutin content gradually increased during pellicles development and reached its peak at the maturation stage (870.59 mg·100 g−1 FW). Syringic acid content (266.66 mg·100 g−1 FW) was second only to rutin, and the highest content (444.91 mg·100 g−1 FW) also appeared in the late maturation stage. The other eight monomeric phenols from high to low were chlorogenic acid, catechin, gallic acid, syringaldehyde, caffeic acid, ferulic acid, juglone and pcoumaric acid, and their average contents ranged from 24.36 to 0.14 mg·100 g−1 FW. With the exception of juglone, seven of the eight species showed peaks at late maturation stage (10/9).
Table 1. Changes of individual phenolic content in pellicles during fruits development (mg·100 g−1 FW).
Most of the walnut seed polyphenols concentrated on pellicles are beneficial to the human body.[Citation33] It tastes astringent due to the richness of polyphenols.[Citation34] At present, people usually remove pellicles when eating walnuts and processing walnut by-products, which will significantly reduce the health value of walnuts. Therefore, the study on the synthesis and metabolism of polyphenols in walnut pellicles is helpful to understanding the synthesis and metabolism of polyphenols in walnut seeds and provides theoretical basis for balancing walnut taste and health care.
Changes of antioxidant activity in pellicles during fruits development
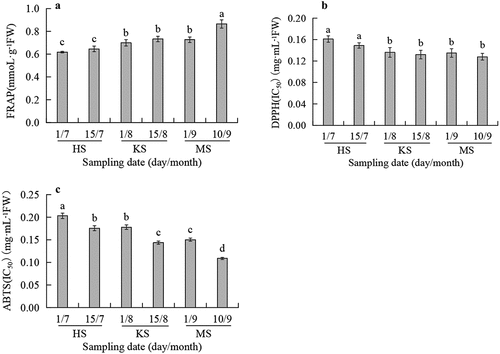
The antioxidant activity of pellicles during walnut fruit development measured by three different methods was shown in . The reducing power of pellicles () increased gradually and reached a maximum with FRAP value of 0.865 mmol·g−[Citation1] FW at the maturation stage. The average value of FRAP was 0.714 mmol·g−[Citation1] FW. DPPH and ABTS free radical scavenging tests were used to evaluate the free radical scavenging ability of pellicles. They were indicated by IC50, and the lower the values, the stronger the antioxidant capacity. The results showed that the DPPH free radical scavenging ability of pellicles was weak in the early stage of fruit development, remained stable from August to maturity (IC50 = 0.136 ~ 0.128 mg·mL−[Citation1] FW) (), and the ABTS free radical scavenging ability increased gradually (), reached the maximum value (IC50 = 0.109 mg·mL−[Citation1] FW) at the maturation stage. The health-care function of the walnut is inseparable from its antioxidant activity. The high antioxidant activity of mature pellicles is related to their protective function. It has been proved that maintaining the integrity of pellicles was critical in reducing the browning and oxidation of kernels.[Citation19]
Figure 2. Changes of FRAP (A), DPPH (B), and ABTS (C) in pellicles during fruit development. HS means hard-core stage. KS means kernel-filling stage. MS means mature stage. Different letters on top of error bars represent significant differences in antioxidant activity during development according to Duncan’s multiple range tests at p < .05 (n = 3).
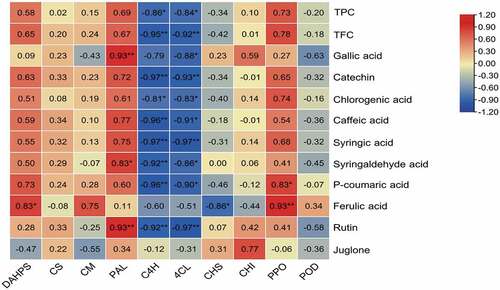
Changes of enzyme activities related to polyphenols metabolism in pellicles during fruits development
Enzymes are catalyzers of biosynthetic metabolism and catabolism, and their activities are significantly correlated with product content.[Citation35] Cumulative effects and delayed expression may lead to an inconsistency between mRNA production and enzyme activity.[Citation36] Therefore, it is more reliable to measure the change of polyphenol content with the change of enzyme activity. Changes in enzyme activities related to polyphenols metabolism were shown in . In the shikimic acid pathway synthesis pathway, the activity of DAHPS in pellicles was relatively high in hard-core stage, decreased in the kernel-filling stage, increased in the late kernel-filling stage to the maturation stage, and reached its peak on September 10. The change of CM activity was similar to DAHPS, which was also high in the hard-core stage, then decreased, remained stable in kernel-filling stage, increased in the maturation stage, and reached its peak on September 10. Zhang et al.[Citation37] proved that the shikimic acid pathway and flavonoid metabolism in grapes had coordinate expression. DAHPS and CM were the entry enzymes of the shikimic acid pathway and phenylpropane metabolism, respectively. Perhaps the shikimic acid pathway and phenylpropane metabolism also have a synergistic expression in pellicles.
In the phenylpropanoid synthesis pathway, PAL activity in pellicles showed a gradual upward trend, with the lowest value at the early hard-core stage and the highest value at the maturation stage. The activities of C4H and 4CL both showed gradual downward trends, which were the highest in the early hard-core stage and the lowest at the maturation stage. The activities of CHS and CHI also had same change trends, both of which increased at first and then decreased, but there is a slight difference in the time when they reached the peak; the activity of CHS reached its peak at the maturation stage, while that of CHI reached its peak at kernel-filling stage (). Persic et al.[Citation38] analyzed the phenylalanine synthesis pathway-related enzyme activities of walnut varieties’ Fernor ‘, ‘Lara,’ and red pellicles walnut during the three developmental stages. In this experiment, the PAL activity of the ‘Qianhe 7’ pellicle was consistent with that of ‘Lara,’ while the CHS and CHI activities were consistent with that of red pellicle walnut. From the above results, it was not possible to draw a good rule. We speculated that this was closely related to walnut varieties, walnut pellicles color and sampling period.
In the polyphenol oxidation pathway, POD activity of pellicles was the highest at the hard-core stage, decreased at the kernel-filling stage, reached the lowest value on September 1, and significantly increased at the maturation stage (), which is consistent with the results in hard shells.[Citation20] The activity of PPO in pellicles remained stable during the early hard-core stage, decreased from the hard-core stage to kernel-filling stage, reached the lowest value on September 1, and significantly increased at the maturation stage, with the highest value on September 10 ().
Correlation analysis
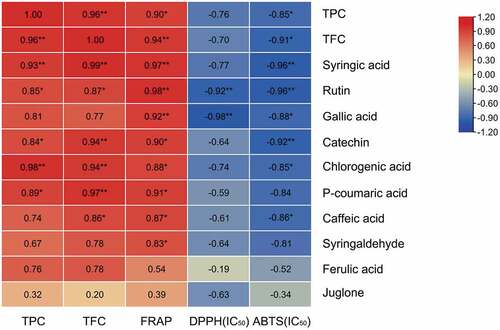
The correlations between the contents of phenolic, monomeric phenols and antioxidant activity were shown in . The scavenging ability of polyphenols to DPPH and ABTS free radicals is expressed by IC50 values. The TPC and TFC in ‘Qianhe 7’ pellicles were positively correlated with reducing ability, but negatively correlated with IC50 values of ABTS and DPPH. It suggested that they were positively correlated with antioxidant activity, which was consistent with previous conclusions.[Citation39] All monomeric phenol contents were positively correlated with TPC and TFC. Except for gallic acid, syringaldehyde, ferulic acid and juglone, the other six monomeric phenols reached significant levels. All monomeric phenol contents were positively correlated with reducing ability but negatively correlated with IC50 values of two kinds of free radical scavenging ability (DPPH, ABTS). Rutin and gallic acid contents were highly significantly positively correlated with reducing ability, but highly significantly negatively correlated with IC50 values of two kinds of free radical scavenging ability (DPPH, ABTS). It indicated that rutin and gallic acid played key roles in the antioxidant activity of pellicles. Syringic acid and catechin contents were highly significantly positively correlated with reducing ability but highly significantly negatively correlated with IC50 value of ABTS free radical scavenging ability. They were also important in the antioxidant activity of pellicles. Chlorogenic acid and caffeic acid contents were significantly positively correlated with reducing ability but significantly negatively correlated with IC50 value of ABTS free radical scavenging ability. The scavenging ability of polyphenols to DPPH and ABTS free radicals was negatively correlated with IC50 value. We found that all monomeric phenols were negatively correlated with IC50 values, so they were all positively correlated with DPPH and ABTS free radical scavenging ability and antioxidant activity. A previous study has found that in green husks, the changes of gallic acid, chlorogenic acid, catechin, rutin, myricetin, and juglone contents were closely related to TPC/TFC,[Citation40] while in pellicles, the changes of p-coumaric acid, rutin, and myricetin contents were closely related with TPC/TFC. In our study, rutin, gallic acid, syringic acid, catechin, chlorogenic acid and caffeic acid contents in pellicles contributed a great deal to the antioxidant activity. These results showed that various monomeric phenols in walnut pellicles cooperated to form a strong antioxidant system and provide a reference for further research and development of functional products related to walnut pellicles.
Figure 3. Correlation among total phenolics, total flavonoids, individual phenolics and antioxidant activity of walnut pellicles. The * and ** Indicate significance at p < .05, p < .01, respectively, n = 6.
The correlation between enzymes activities related to polyphenol metabolism in walnut pellicles was shown in . In the shikimic acid synthesis pathway, the TPC and TFC were positively correlated with the activities of DAHPS, CS and CM, but none reached significant levels, suggesting that these enzymes were not the key rate-limiting enzymes for the polyphenols content in pellicles. In the phenylpropane synthesis pathway, TPC, TFC, and monomeric phenol contents were positively correlated with PAL activity, consistent with the results in pineapples.[Citation41] A coordinated response between PAL, PPO and the concentration of total phenols in the four olive cultivars was also found.[Citation42] The contents of syringaldehyde acid, rutin and gallic acid were significantly positively correlated with PAL activity. It indicated that PAL activity had an important effect on the content of polyphenols in pellicles. The contents of total phenolic and chlorogenic acid were significantly negatively correlated with C4H and 4CL activities, and the contents of total flavonoids, catechins, syringic acid, and rutin were extremely significantly negatively correlated with C4H and 4CL activities. Therefore, these two enzymes may be the key rate-limiting enzymes for polyphenols synthesis. Previous studies had shown a high concentration of polyphenols might inhibit the activities of related enzymes.[Citation43] It was found that catechins in tea could feedback inhibit the expression of PAL, 4CL and C4H genes under certain conditions.[Citation44] We speculated that the C4H and 4CL activities in walnut pellicles also were feedback-regulated by some phenolic substances. In the polyphenol oxidation pathway, there was no significant correlation among POD activity TPC, TFC, and monomeric phenol contents. Most of the correlations were negative. It showed that POD played a small role in the accumulation of polyphenols in walnut pellicles. PPO oxidizes phenols into quinones in damaged plant cells, causing the browning of plant tissues.[Citation45] However, some studies have shown that PPO might perform other functions in intact plant cells. For example, PPO also participated in tyrosine metabolism and the formation of hydroxycoumarin aescinate during the development of walnut leaves.[Citation46] In our study, we found the TPC and TFC were positively correlated with PPO activity, p-coumaric acid content was significantly positively correlated with PPO activity, and ferulic acid content was extremely significantly positively correlated with PPO activity. These may also be due to PPO participating in the biosynthesis of this branch during walnut development.
Conclusion
The pellicle is the main accumulation organ of polyphenols in walnut seeds, which directly affects the nutritional value and taste of walnut kernels. The TPC, TFC, reducing power (FARP) and free radical scavenging ability (DPPH, ABTS) in pellicles of walnut ‘Qianhe 7’ reached the highest level at the maturation stage. The TPC and TFC were positively correlated with antioxidant activity. Rutin was the most abundant monomeric phenol, followed by syringic acid. Rutin, catechin, syringic acid, and caffeic acid, whose contents were highly correlated with antioxidant activity. Moreover, the content of total phenols, total flavonoids and most monomeric phenols were positively correlated with PAL and PPO activities but negatively correlated with that of C4H and 4CL. The synergistic action of PAL, 4CL, C4H and PPO in pellicles affects the antioxidant activity of pellicles by controlling the polyphenol content.
Acknowledgments
This work was supported by National Natural Science Foundation of China (32060673), the Guizhou High Level Innovative Talents Project ([2016]4038), Guizhou Forestry Scientific Research Project (Qian Lin Ke He [2021] No. 07) and Sub-project of Guizhou Walnut Engineering Technology Research Center (Qian Ke He Platform Talent [2019] No. 5202-6).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules. 2019, 24(13), 2452. DOI: 10.3390/molecules24132452.
- Racchi, M. L. Racchi ML. Antixidant Defenses in Plants with Attention to Prunus and Citrus Spp. Antioxidants. 2013, 2(4), 340–369. DOI: 10.3390/antiox2040340.
- Yuan, Y.; Xiang, J. L.; Zheng, B. L.; Sun, J. J.; Luo, D. L.; Li, P. Y.; Fan, J. L. Diversity of Phenolics Including Hydroxycinnamic Acid Amide Derivatives, Phenolic Acids Contribute to Antioxidant Properties of Proso Millet. LWT - Food Sci. Technol. 2021, 1(54), 112611.
- Wen, L. R.; He, M. Y.; Yin, C. X.; Jiang, Y. M.; Luo, D. H.; Yang, B. Phenolics in Citrus Aurantium Fruit Identified by UHPLC-MS/MS and Their Bioactivities. LWT - Food Sci. Technol. 2021, 147, 111671. DOI: 10.1016/j.lwt.2021.111671.
- Leri, M.; Scuto, M.; Ontario, M. L.; Calabrese, V.; Calabrese, E. J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21(4), 1250. DOI: 10.3390/ijms21041250.
- Hayes, D.; Angove, M. J.; Tucci, J.; Dennis, C. Walnuts (Juglans Regia) Chemical Composition and Research in Human Health. Critical Reniews in Food Science and Nutrition. 2016, 56(8), 1231–1241. DOI: 10.1080/10408398.2012.760516.
- Li, Y. Z.; Luo, X.; Wu, C. Y.; Cao, S. Y.; Zhou, Y. F.; Jie, B.; Cao, Y. L.; Meng, H. J.; Wu, G. L. Comparative Transcriptome Analysis of Genes Involved in Anthocyanin Biosynthesis in Red and Green Walnut (Juglans Regia L.). Molecules. 2018, 23(1), 25.
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M., and Amarowicz, R. A. A. Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans Regia L.) Fruit and Tree. Molecules. 2019, 24(11), 2133. DOI: 10.3390/molecules24112133.
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and Quantification of the Major Phenolic Constituents in Juglans Regia L. Peeled Kernels and Pellicless, Using HPLC-MS/MS. Food Chem. 2021, 3(52), 129404. DOI: 10.1016/j.foodchem.2021.129404.
- Zhang, Y. G.; Kan, H.; Chen, S. X.; Thakur, K.; Wang, S. Y.; Zhang, J. G.; Shang, Y. F., and Wei, Z. J. Comparison of Phenolic Compounds Extracted from Diaphragma Juglandis Fructus, Walnut Pellicles, and Flowers of Juglans Regia Using Methanol, Ultrasonic Wave, and Enzyme assisted-extraction. Food Chem. 2020, 3(21), 126672.
- Akbari, V.; Jamei, R.; Esfahlan, A. J.; Esfahlan, A. J. Antiradical Activity of Different Parts of Walnut (Juglans Regia L.) Fruit as a Function of Genotype. Food Chem. 2012, 135(4), 2404–2410. DOI: 10.1016/j.foodchem.2012.07.030.
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and Quantification of Phenolic Compounds in Kernels, Oil and Bagasse Pellets of Common Walnut (Juglans Regia L.). Food Res. Int. 2015, 67, 255–263. DOI: 10.1016/j.foodres.2014.11.016.
- Xiong, L. G.; Li, J.; Li, Y. H.; Yuan, L.; Liu, S. Q.; Huang, J. A.; Liu, Z. H. Dynamic Changes in Catechin Levels and Catechin biosynthesis-related Gene Expression in Albino Tea Plants (Camellia Sinensis L.). Plant Physiol. Biochem. 2013, 71, 132–143. DOI: 10.1016/j.plaphy.2013.06.019.
- Trujillo-Mayol, I.; Badillo-Munoz, G.; Cespedes-Acuna, C.; Alarcon-Enos, J. The Relationship between Fruit Size and Phenolic and Enzymatic Composition of Avocado Byproducts (Persea Americana Mill.): The Importance for Biorefinery Applications. Horticulturae. 2020, 6(4), 91. DOI: 10.3390/horticulturae6040091.
- Xie, Q.; Zhang, S. Y.; Ye, Q. H.; Wang, W.; Chen, Q. X. Dynamic Changes of Polyphenols and Related Enzymes Activity during the Development and Maturation of Chinese Olive (Canarium Album L.). Journal of Fruits Science. 2019, 36, 774–784.
- Kong, D. X.; Li, Y. Q.; Bai, M.; He, H. J.; Liang, G. X.; Wu, H. Correlation between the Dynamic Accumulation of the Main Effective Components and Their Associated Regulatory Enzyme Activities at Different Growth Stages in Lonicera Japonica Thunb. Ind. Crops Prod. 2017, 9(6), 1–22.
- Zhang, R.; Shi, B. B.; Zhang, W. E.; Li, X., and Pan, X. J. Relationship between Polyphenol Content and Enzymes Activities and Antioxidant Capacity in Juglans Sigillata Dode Leaves. Acta Botanica Boreali -Occidentalia Sinica. 2019, 39, 0677–0684.
- Wen, J.; Zhao, S. G.; Wang, H. X.; Zhang, Z. H.; Li, X. B. Changes of Lignin Content and Its Related Enzyme Activities in Endocarp during Walnut Shell Development Period. Acta Horticulturae Sinica 2015, 4(2), 2144–2152.
- Ortiz, C. M.; Vicente, A. R.; Fields, R. P.; Grillo, F.; Labavitch, J. M.; Donis-Gonzalez, I.; Crisosto, C. H. Walnut (Juglans Regia L.) Kernel Postharvest Deterioration as Affected by Pellicles Integrity, Cultivar and Oxygen Concentration. Postharvest. Biol. Technol. 2019, 156, 110948–110978.
- Wang, J.; Liang, S. J.; Ma, H. L.; Zhang, P. P.; Shi, W. N. Effects of Ethephon on Fresh In-Husk Walnut Preservation and Its Possible Relationship with Phenol Metabolism. J. Food Sci. 2016, 81(8), C1921–C1927.
- Conde-Hernandez, L. A.; Guerrero-Beltran, J. A. Total Phenolics and Antioxidant Activity of Piper Auritum and Porophyllum Ruderale. Food Chem. 2014, 142, 455–460. DOI: 10.1016/j.foodchem.2013.07.078.
- Feng, S. M.; Luo, Z. S.; Zhang, Y. B.; Zhong, Z.; Lu, B. Y. Phytochemical Contents and Antioxidant Capacities of Different Parts of Two Sugarcane (Saccharum Officinarum L.) Cultivars. Food Chem. 2014, 151, 452–458. DOI: 10.1016/j.foodchem.2013.11.057.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry. Analytical biochemistry. 1996, 239(1), 70–76. DOI: 10.1006/abio.1996.0292.
- Jun, H. I.; Song, G. S.; Yang, E. I.; Youn, Y.; Kim, Y. S. Antioxidant Activities and Phenolic Compounds of Pigmented Rice Bran Extracts. J. Food Sci. 2012, 7(7), 759–764. DOI: 10.1111/j.1750-3841.2012.02763.x.
- Chen, J. Y.; Wen, P. F.; Kong, W. F.; Pan, Q. H.; Wan, S. B.; Huang, W. D. Changes and Subcellular Localizations of the Enzymes Involved in Phenylpropanoid Metabolism during Grape Berry Development. J. Plant Physiol. 2006, 163(2), 115–127. DOI: 10.1016/j.jplph.2005.07.006.
- Han, Y. W.; Lian, S. Q.; Han, Y. Y.; Li, X. D.; Yan, S. J. Effect of Different Harvest Maturity and Cooling Methods on POD Activity and Browning of Yali Pear. Sci. Technol. Food Ind. 2016, 37(14), 320–323 + 338.
- Winters, A. L.; Minchin, F. R.; Michaelson-Yeates, T. P. T.; Lee, M. R. F.; Morris, P. Latent and Active Polyphenol Oxidase (PPO) in Red Clover (Trifolium Pratense) and Use of a Low PPO Mutant to Study the Role of PPO in Proteolysis Reduction. J. Agric. Food Chem. 2008, 56(8), 2817–2824. DOI: 10.1021/jf0726177.
- Shi, B. B.; Zhang, W. E.; Li, X.; Pan, X. J. Phenolics Content and Antioxidant Activity of Walnut (Juglans Sigillata) Leaves. Acta Horticulturae Sinica. 2017(44), 23–32.
- Shi, B. B.; Zhang, W. E.; Li, X.; Pan, X. J. Seasonal Variations of Phenolic Profiles and Antioxidant Activity of Walnut (Juglans Sigillata Dode) Green Husks. Int. J. Food Prop. 2017, 20(sup3), S2635–S2646. DOI: 10.1080/10942912.2017.1381706.
- Wu, G. L.; Zhang, L. Y.; Pan, Q. H.; Shen, Y. Y., and Zhang, D. P. Phloem Unloading in Developing Walnut Fruit Is Symplasmic in the Seed Pericarp and Apoplasmic in the Fleshy Pericarp. Plant Cell Physiol. 2004, 45(10), 1461–1470. DOI: 10.1093/pcp/pch169.
- Wu, G. L.; Liu, Q. L.; Teixeira da Silva,; da Silva Jat, J. A. Ultrastructure of Pericarp and Seed Capsule Cells in the Developing Walnut (Juglans Regia L.) Fruits. S. A. J. Botany. 2009, 75(1), 128–136. DOI: 10.1016/j.sajb.2008.09.001.
- Hu, Z. W. Study on the Changes of Main Mineral Element Contents in Leaves and Fruitss during the kernel-filling Period of Precocious Walnut; Hebei Agricultural University: Hebei, 2011.
- Trandafir, I.; Cosmulescu, S.; Botu, M.; Nour, V. Antioxidant Activity, and Phenolic and Mineral Contents of the Walnut Kernel (Juglans Regia L.) as a Function of the Pellicle Color. Fruits. 2016, 71(3), 177–184. DOI: 10.1051/fruits/2016006.
- Figueroa, F.; Marhuenda, J.; Zafrilla, P.; Villano, D.; Martinez-Cacha, A.; Tejada, L.; Cerda, B.; Mulero, J. High-performance Liquid chromatography-diode Array Detector Determination and Availability of Phenolic Compounds in 10 Genotypes of Walnuts. Int. J. Food Prop. 2016, 20, 1–33.
- Robinson, P. K. (2015). Enzymes: Principles and Biotechnological Applications. Essays in Biochemistry. 2015, 59: 1–41. DOI: 10.1042/bse0590001.
- Ren, T. Y.; Zheng, P. C.; Zhang, K. X.; Liao, J. R.; Xiong, F.; Shen, Q.; Ma, Y. C.; Fang, W. P.; Zhu, X. J. Effects of GABA on the Polyphenol Accumulation and Antioxidant Activities in Tea Plants (Camellia Sinensis L.) under heat-stress Conditions. Plant Physiol. Biochem. 2021, 159, 363–371. DOI: 10.1016/j.plaphy.2021.01.003.
- Zhang, Z. Z.; Li, C. Y. N., XX; Zhang, M. X.; Wen, Y. Q.; Duan, C. Q.; Pan, Q. H. Three Types of Ultraviolet Irradiation Differentially Promote Expression of Shikimate Pathway Genes and Production of Anthocyanins in Grape Berries. Plant Physiol. Biochem. 2012, 57, 74–83. DOI: 10.1016/j.plaphy.2012.05.005.
- Persic, M.; Mikulic-Petkovsek, M.; Halbwirth, H.; Solar, A.; Veberic, R.; Slatnar, A. Red Walnut: Characterization of the Phenolic Profiles, Activities and Gene Expression of Selected Enzymes Related to the Phenylpropanoid Pathway in Pellicle during Walnut Development. J. Agric. Food Chem. 2018, 66(11), 2742–2748. DOI: 10.1021/acs.jafc.7b05603.
- Sabraoui, T.; Taleb, N.; Boubker, E.; Rabiaa, M. A., and Benbachir, M. Determination of Punicalagins Content, Metal Chelating, and Antioxidant Properties of Edible Pomegranate (Punica Granatum L) Peels and Seeds Grown in Morocco. Int. J. Food Sci. Technol. 2020, 8885889.
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules. 2021, 26(10), 3013. DOI: 10.3390/molecules26103013.
- Yeoh, W. K.; Ali, A. Ultrasound Treatment on Phenolic Metabolism and Antioxidant Capacity of fresh-cut Pineapple during Cold Storage. Food Chem. 2017, 216, 247–253. DOI: 10.1016/j.foodchem.2016.07.074.
- Ortega-Garcia, F.; Peragon, J. Phenol Metabolism in the Leaves of the Olive Tree (Olea Europaea L.) Cv. Picual, Verdial, Arbequina, and Frantoio during Ripening. J. Agric. Food Chem. 2010, 58(23), 12440–12448. DOI: 10.1021/jf102827m.
- Alberstein, M.; Eisenstein, M.; Abeliovich, H. Removing Allosteric Feedback Inhibition of Tomato 4-coumarate:CoA Ligase by Directed Evolution. Plant J. 2012, 69(1), 57–69. DOI: 10.1111/j.1365-313X.2011.04770.x.
- Rani, A.; Singh, K.; Sood, P.; Kumar, S.; Ahuja, P. S. p-Coumarate: CoA Ligase as a Key Gene in the Yield of Catechins in Tea [Camellia Sinensis (L.) O. Kuntze]. Functional & Integrative Genomics. 2006, 9, 271–275.
- Lin, Y. F.; Lin, H. T.; Lin, Y. X.; Zhang, S.; Chen, Y. H.; Jiang, X. J. The Roles of Metabolism of Membrane Lipids and Phenolics in Hydrogen peroxide-induced Pericarp Browning of Harvested Longan Fruit. Postharvest. Biol. Technol. 2016, 111, 53–61. DOI: 10.1016/j.postharvbio.2015.07.030.
- Araji, S.; Grammer, T. A.; Gertzen, R.; Anderson, S. D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M. L.; Solar, A.; Leslie, C. A., and Dandekar, A. M. Novel Roles for the Polyphenol Oxidase Enzyme in Secondary Metabolism and the Regulation of Cell Death in Walnut. Plant Physiol. 2014, 164(3), 1191–1203. DOI: 10.1104/pp.113.228593.