ABSTRACT
Bactris gasipaes Kunth belongs to the Arecaceae family and is known in various parts of the world under various names. This fruit presents several hybridizations and wild species containing diverse percentages of nutritional and functional constituents. These constituent properties link its parts, such as pulps, peels, and seeds rich in oils, with a predominance of polyunsaturated fatty acids, carotenoids, and polyphenols. Consequentially, these fruits possess pharmacological, anti-inflammatory, antimicrobial, and antioxidant properties, among other characteristics. Additionally, B. gasipaes byproducts, including starches, fibers, and oils, have a high potential for technological applications. The diversity of the varieties has led to a notable increase in research on a multidisciplinary scale, adding value to these varieties with a focus on their industrial applications and by consolidating acquired patents over the years. However, its industrial potential is still poorly explored, a fact that has presented itself as an opportunity for new research and the design of novel products. This review provides an updated view of the different strands and varieties of peach palm, their identification, technological potential in the food industry, and their bioactive functional properties. Finally, ongoing research on a new variety of this species in the Amazon is also discussed.
Introduction
Advancements in the fields of environmental, agronomical, pharmaceutical, chemical, and food sciences have provided significant insights on numerous plant species, allowing for the recognition of fruitful variants and their associated scientific, technological, and industrial applications. Moreover, advances in the precision and accuracy of various analytical platforms have increased the number of new knowledge branches.
The study of food matrix constituents in plant species has expanded beyond the edible parts and has been extensively explored.[Citation1–5] These studies are comprehensive scientific inducers that expand production chains, add value to byproducts, and minimize the disposal of inputs characterized as waste. The practicality of the latter lies in their nutritional and functional constituents, which exhibit good technological properties for the supplementation of raw materials in various industrial sectors.[Citation2,Citation4,Citation6]
Advances in research on oilseed-producing Amazonian palms have prompted the emergence of various ethnographic and pharmaceutical studies aimed at environmental conservation. The associated research particularly focuses on the identification and application of the bioactive constituents in traditional Amazonian medicine and food, including the oleaginous peach palm fruits.[Citation7–9]
Peach palm (Bactris gasipaes) belongs to the Arecaceae family, of which approximately 400 species have been reported. Palm trees and their fruits are referred to by numerous vernacular names, including chontaduro (Colombia), chonta (Ecuador), pejibaye (Costa Rica), and peach palm (North America and other South American countries, including Brazil).[Citation2,Citation10,Citation11]
According to the Brazilian Agricultural Research Corporation,[Citation12] Brazil is considered among the largest global producers, consumers, and exporters of peach palm, with more than 30,000 hectares of planted land. The main Brazilian cultivators of palm predominantly used for palm heart extraction are São Paulo, Bahia, Santa Catarina, and Paraná. Moreover, it is estimated that more than two-thirds of the entire planted area in Brazil is cultivated with B. gasipaes. Although rational peach palm planting is limited in the northern region of the country, this area is home to one of the largest fruit productions using traditional extractive culture, which maintains the practice of environmental conservation [Embrapa 2019b).
According to,[Citation13] the maintenance of traditional Amazonian peach palm culture and the palm heart industry have been growing since the 1990s, with a sharp increase in import rates in recent decades. Moreover, the presence of peach palms in different territories has expanded their commercial area and associated research based on product and byproduct constituents in several countries. Nonetheless, peach palm species and varieties found in the Amazon region have been the main focus of these intensive studies.[Citation2,Citation3,Citation10,Citation14]
Of particular interest are the peach palm fruits, which manifest in a variety of conformations and colors as a result of successive hybridization stages in relation to provenance-specific evolutionary and edaphoclimatic conditions. Moreover, environmental factors such as rainfall, the effective time of sunlight, relative humidity, and soil nutrition, among others, affect the species’ productivities. In general, flowering and fruiting occurs from August to October and December to March, respectively, depending on the area of cultivation.[Citation15] Each peach palm produces 5–10 bunches yearly, containing approximately 100 fruits each; however, up to 25 bunches have been produced in some circumstances.[Citation15]
The different peach palm varieties elicit distinct nutritional benefits in terms of the bioactive components of their different fruit parts, as demonstrated by research on the direct consumption of the fruit. The peach palm fruit can be considered to have a high content of insoluble fiber[Citation16] and, even with a low protein content, the peach palm has all the essential amino acids.[Citation17] In addition, the starch extracted from peach palm pulp can be considered as an alternative for the production of biodegradable thermoplastic.[Citation18] The lipid content is quite expressive, with a main composition of palmitic acid[Citation19,Citation20] and oleic acid.[Citation21,Citation22] The fruit also has a varied carotenoid profile, provided by its yellow-red pigmentation.[Citation3,Citation10] Therefore, this study revisited and presents an updated view on the different aspects of the peach palm, namely, its identification, technological potential as food, and bioactive functional properties of different fruit varieties. Moreover, a novel Amazonian peach palm variety is discussed, for which limited literature exists. The studies cited in this review were obtained using descriptors such as: “peach palm,” “Bactris gasipaes,” “chontaduro,” “chonta,” and “pejibaye.” These terms with “Amazon oilseeds,” “nutraceuticals,” “properties,” “fatty acids,” “bioactive compounds,” and “functional foods” were combined using the Boolean descriptor “AND.” Content has been published in online databases, including ScienceDirect, Web of Science, Scopus, SciELO, and Google Scholar. Sources in any publication year or study field were considered.
General aspects of peach palms
From cultivation to profitability
Among the palm trees of the Arecaceae family, the peach palm (B. gasipaes) has been cultivated on a domestic and commercial scale and has high economic potential.[Citation15] According to georeferenced records from the Global Biodiversity Information Facility,[Citation23] the peach palm is widely distributed from Honduras to Bolivia and is mainly concentrated in the Brazilian state of Pará. The most accepted origin of this palm is attributed to the occurrence of several hybridizations following its domestication, regardless of its wild-type species.[Citation15] Furthermore,[Citation24] demonstrated a significantly high genetic diversity among the Bactris species in South America, particularly in the western Amazon.
The peach palm tree is a multi-stemmed tree, which can have green, brown or yellow thorns; of up to 10 cm in length that can reach 20 m in height and 15‒25 cm in diameter at maturity.[Citation15] The leaves are pinnate, irregularly arranged in groups and arranged in several planes (); with intrafoliar inflorescence.[Citation25] These palms are cultivated in hot (24‒28°C), humid climates at an altitude of up to 900 m, with annual precipitation of 2,500 mm. Moreover, male flowers are characteristically larger and greater in quantity than their female counterparts
Figure 1. Photographs of Bactris gasipaes showing a whole tree (a), manual harvesting (b), and fruits after harvesting (c).

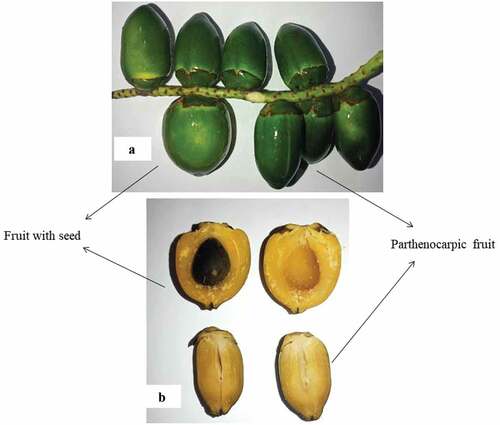
In central Amazonia, peach palm flowering occurs from August to October, while fruiting mainly occurs between December and March, depending on the climate and soil conditions .[Citation15] An average of 10 bunches are produced annually; however, up to 25 bunches of varying sizes and weights can develop during the rainy seasons with good soil quality. The first peach palm bunches are harvested 3‒4 years after planting, when the trunk of the palm reaches 10 cm in diameter. Furthermore, harvesting is done manually using knives equipped with a trimmer or sickle () and is recommended to be performed when at least half of the fruits in a bunch are ripe, thereby enhancing their resistance to deterioration ( Factors such as pollination, poor soil nutrition, dry season, competition, pest insects, and the occurrence of certain diseases can lead to plant termination, thereby reducing the overall cluster and fruit production efficiency .[Citation15] Moreover,[Citation26] demonstrated that climatic factors, such as wind and rain, can result in ineffective pollination, thereby contributing to the development of parthenocarpic fruits (absence of seeds), a common characteristic of the species.
According to the agricultural census of the Brazilian Institute of Geography and Statistics (IBGE), approximately 5 million palm trees and a total of 8,873 tons of peach palm bunches were produced in Brazil in 2017, with predominant production in Bahia, Amazonas, and Pará.[Citation27] These data substantiate the importance of peach palm commercialization in Brazil and the consequential economic and cultural value of the fruit for the population.[Citation15]
Fruit for human consumption
The peach palm fruits are arranged in bunches and are characteristically fleshy and drupe-like (a single seed); however, parthenocarpic fruits can also occur because of the high genetic variability of the species. Moreover, this high genetic variability also leads to diversification in terms of size, skin and pulp color, and nutritional composition.[Citation15] The color of the fruits manifests as shades of green, yellow, orange, and red; however, some fruits are without any color (albino). In addition, a variety of fruit shapes exist, including ovoid, globose, or conical-globose (). This variability is attributed to flowering, and occurs following pollination between different plants.[Citation26] show fruits with and without seeds, respectively, present in the same bunch.
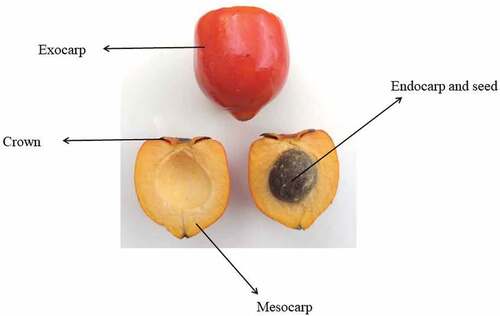
Considering that the peach palm fruits are of the drupe type, they consist of a fibrous shell (exocarp); fleshy pulp (mesocarp), which varies from starchy to oily; and an endocarp that encompasses a fibrous and oily white almond. These fruits are 1‒1.5 cm in diameter, and may grow as large as 7 cm[Citation15] (). Although the varieties and types of peach palms are generally distinguished based on fruit color and whether or not they contain seeds, they can also be classified according to the thickness and weight of their pulp. Accordingly, the fruits can be differentiated as microcarps (<20 g), mesocarps (21‒70 g), and macrocarps (>70 g).[Citation15]
The peach palm pulp is mainly consumed after boiling in salted water. The skin and seeds are subsequently removed, and the pulp may either be consumed directly or used in culinary preparations, such as purees, jellies, risottos, and flour. Moreover, the fruits are usually peeled with the teeth or hands and are consumed as an accompaniment with coffee.[Citation28] The fruit cooking process (105°C for 20 min) is necessary to inhibit anti-nutritional factors (trypsin inhibitors), eliminate oxalate crystals in the peel,[Citation29] and inactivate pulp peroxidase enzymes, as these components can irritate the throat mucosa.[Citation28]
Considering that peel color is not directly related to fruit quality,[Citation28] fruit preference is rather based on the sensory perception of oiliness. Thus, less oily fruits have higher moisture, starch, and fiber contents, resulting in a drier pulp that is considered distasteful by consumers.[Citation14,Citation28]
Nutritional value of peach palm pulp
Nutritional composition
Varying peach palm characteristics (peel and pulp color, presence or absence of seeds, etc.) and maturation stages significantly affects the fruit pulp composition, as shown in . The lipid content presented the greatest variation (1.9‒13.7%) among the other peach palm constituents. Moreover, variations were also significant, but less representative, for moisture (51.7‒64.0%), ash (0.7‒2.4%), protein (1.8‒4.6%), carbohydrate (26.4‒38.8%), and fiber (3‒6%) contents. Moreover, it is important to consider that the fruits are usually cooked, thereby also altering the product composition.
Table 1. Nutritional composition of peach palm pulp.
Despite not having a high protein content, the peach palm mesocarp contains all the essential amino acids,[Citation17] found in the yellow fruit particularly leucine (3.9 g.kg−1), lysine (3.7 g.kg−1), tyrosine (3.4 g.kg−1), arginine (3.1 g.kg−1), threonine (2.9 g.kg−1), valine (2.7 g.kg−1), and histidine (1.4 g.kg−1). Furthermore, these authors found lower values of the same amino acids in the red fruit: 3.0 g.kg−1 of leucine, 2.6 g.kg−1 of lysine, 2.7 g.kg−1 of tyrosine, 2.8 g.kg−1 of arginine, 2.1 g.kg−1 of threonine, 2.1 g.kg−1 of valine, and 1.0 g.kg−1 of histidine. In addition, significant levels of glutamic acid (4.98 g.kg−1) and aspartic acid (4.33 g.kg−1) are also present.[Citation16]
The main constituents of peach palm pulp are carbohydrates, particularly starch (35–54 g.100 g−1), which can potentially be used for various purposes[Citation36,Citation2,Citation37] The white, odorless peach palm starch consists of 19.3% amylose and contains low levels of ash, proteins, lipids, and fibers[Citation2,Citation14,Citation38] could not demonstrate that fruit ripening promoted morphological changes in the peach palm starch granules. Following nutrient content characterization in peach palm fruit varieties,[Citation35] observed the highest levels of lipids in microcarp and carbohydrates in macrocarp fruits, corroborating that macrocarp fruits have the driest pulp. Furthermore, cooked peach palm pulp has a low glycemic index (35.0), attributed to the slow absorption of associated glucose into the bloodstream.[Citation39]
[Citation16] observed a higher content of insoluble dietary fiber (5.5%) than soluble dietary fiber [1, 1%) in three Amazonian peach palm populations. Moreover, similar total dietary fiber levels were observed in peach palm fruits by,[Citation36] (6.7%) and[Citation35] (6.0%). As such, peach palm pulp was classified as fiber-rich food.[Citation40] Insoluble dietary fibers remain intact during digestion and are involved in reducing the risk of coronary heart disease and type 2 diabetes.[Citation41,Citation42] Additionally,[Citation43] investigated the chemical structure of peach palm fruit pectins and found acetate (16.2%) and propionate (6.2%) to be the major constituents.
Lipid profile
Considering that peach palm fruits generally have a high lipid content, it is important to consider its lipid profile, particularly the quantity and quality of the fatty acids present in the pulp. presents the saturated and unsaturated fatty acid contents of peach palm oil, indicating that palmitic and oleic acids are the main contributing fatty acids. According to the compiled data, the oil extracted from the red-colored peach palm pulp predominantly contains unsaturated fatty acids,[Citation21,Citation22] whereas that from the orange-colored fruits primarily contains saturated fatty acids.[Citation19]
Table 2. Lipid profile in peach palm oil (% of fatty acid).
[Citation22] compared lipid extraction methods for the chichagui and gasipaes peach palm fruit varieties and found a higher yield using supercritical extraction than by using the other methods. Moreover, the chosen extraction method did not affect the fatty acid profile of the fruits; however, the oil of the yellow gasipaes variety extracted using supercritical extraction had a higher palmitic acid content than that extracted using the other methods. Among the most functionally relevant unsaturated fatty acids found in peach palm pulp, omega-9 (oleic acid) predominates, followed by omega-6 (linoleic acid) and omega-3 [linolenic acid), regardless of the fruit color. According to the categorizations of,[Citation5] the oil extracted from peach palm pulp can be characterized as an intermediate source of omega-6 (4.9‒8.6%).
Moreover,[Citation45] analyzed the relationship between the unsaturated fatty acids content and antioxidant capacity using a kinetic oxidation model by DPPH assay. The authors observed that peach palm oil was highly susceptible to oxidation, attributed to its high omega-9 content. Fatty acids are susceptible to oxidation due to the presence of double bonds in their structure. This oxidation process occurs by free radical chain reactions and can cause a loss of nutritional and sensory qualities in oils.[Citation46]
The omega-6 content of red peach palm pulp (4.9%) was higher than that found in buriti (2.9%), bacaba (4.3%), and tucumanzinho (2.6%) oils, and the omega-3 content was higher than that found in the oils of all fruits analyzed by.[Citation44] Moreover, peach palm oil is liquid at room temperature as a result of its high oleic acid content,[Citation21, Citation22] Contrarily, due to the high content of saturated fatty acids as found by,[Citation19] ,and[Citation20] in orange and red peach palm pulp oils, respectively, these oils can be solid at room temperature.[Citation19]
The Food and Agriculture Organization of the United Nations (FAO 2010) recommends the consumption of 2.5‒9% omega-6 and 0.5‒2% omega-3. This consumption beneficially aids in the regulation of inflammatory processes, oxidative stress, and endothelial function.[Citation47] The health effects of fatty acids have been evaluated extensively in clinical human trials. In one such pilot study, an increase in daily omega-3 (3.4 g) intake for 6 months resulted in reduced benign epithelial proliferation (Ki-67) in premenopausal women, thereby contributing to the prevention of breast cancer.[Citation48]
Fatty acids are bioactive compounds present in fruits that can act as cardioprotective components ,[Citation20,Citation49] This was supported by[Citation50] who demonstrated a significant reduction in cardiovascular injuries, particularly strokes, in an at-risk population following supplementation with extra virgin olive oil (≈1 L per week) and nuts (15 g). Moreover, a randomized clinical trial involving 235 participants supplemented with the same foods as in the previous study observed a reduction in blood pressure, total cholesterol, and fasting glucose levels.[Citation51]
Fatty acids directly affect the body’s defense mechanisms via the synthesis of specific antibodies, which participate in the elimination of microorganisms through the release of cytokines, complement system activation, and reactive oxygen species generation. Polyunsaturated fatty acids and bioactive lipids act upon viruses by breaking their protein envelopes, inducing intracellular fluid loss and oxidative phosphorylation, and inhibiting cellular respiration,[Citation52–54] [Citation55] proposed that patients severely affected by COVID-19 should prioritize the consumption of long-chain fatty acids, especially omega-3 and omega-9 fatty acids. These fatty acids can react upon different stages of viral infection, mainly at viral entry and during replication.
Oleic acid specifically has been reported as a precursor to beneficial effects. Rats supplemented with a complex of rice starch with oleic acid showed decreased body weight, improved serum lipid levels, oxidative stress, and liver function, as well as improved butyrate production.[Citation56] The same findings were reported by,[Citation57] when supplementing rats with peanut oil and extra virgin olive oil, both rich in oleic acid. In this study, in addition to the benefits for lipid profiles and body weight, the use of oleic acid supplements promoted a reduction in insulin resistance and hepatic steatosis. Moreover, a meta-analysis showed that consumption of oleic acid had a significant effect on metabolic syndrome, probably by balancing the oxidation and storage of lipid stearoyl-CoA desaturase 1.[Citation58]
Carotenoids
Among the fat-soluble constituents of peach palms, carotenoids are also considered important bioactive nutrients and are present in high concentrations in the fruits (). Carotenoids are natural pigments related to the yellow, orange, and red colors of fruits and can be present in the diet (β-carotene, lutein, and lycopene) or involved in photosynthetic processes (β-carotene, spheroidene, lutein, violaxanthin, and zeaxanthin).[Citation61] shows the carotenoid profiles of the peach palm pulp and peel. Carotenoids can be divided into two main groups according to their chemical composition, namely carotenes, which are hydrocarbons (e.g., α-carotene, β-carotene, γ-carotene, and lycopene), and xanthophylls, which contain oxygen atoms (e.g., β-cryptoxanthin, lutein, zeaxanthin, astaxanthin, fucoxanthin, and peridinin).[Citation62]
Table 3. Carotenoid screening results (qualitative) in raw and cooked pulp and peel of different colored peach palm fruit.
Carotenoids are stored in the cellular chromoplast, which can be globular (containing lipid globules of various sizes wherein a high content of carotenoids are dissolved), tubular (have thin internal tubules), crystalloid (involving carotenoids dissolved in liquid and liquid-crystalline), and membranous chloroplasts (concentrated in membrane layers). Considering this, carotenoid absorption in the human body is dependent on its release from the food matrix and subsequent dissolvement in dietary lipids; however, many fruits and vegetables do not contain sufficient lipids to favor such dissolution.[Citation63] These same authors evaluated the carotenoid deposition and chromoplastic anatomy of yellow and orange peach palm fruits and found mainly β-carotene. In addition, these authors noted that the associated chromoplasts were of the globular type and that peach palm carotenoids were likely to be highly bioavailable.
Previous studies have found that yellow, orange, and red peach palms are rich in carotenoids and the stage of fruit maturation strongly influences the total carotenoid content thereof.[Citation3,Citation5,Citation33] Moreover, fresh fruits of the orange peach palm variety had the highest total carotenoid content,[Citation3] followed by the red[Citation33,Citation59,Citation60] and yellow[Citation33] fruits with a much lower total carotenoid content. As such, a relationship exists between the color intensity and carotenoid content of the peach palm. Furthermore,[Citation64] extracted carotenoids from peach palm pulp by ultrasound and added it to a mayonnaise emulsion. After performing the in vitro digestion, the authors found that the carotenoids in this emulsion were 11 times more bioaccessible than those in the freeze-dried fruit.
Carotenoids are subject to instability, which can be influenced by their chemical composition (carotene or xanthophyll), molecular structural configuration (cis or trans), esterification, the type of cellular matrix (fruit, root, leaf), as well as processing and storage conditions, depending on the permeability of the packaging material to oxygen and exposure to light.[Citation61] In this way,[Citation31] reported higher content of total carotenoids after cooking the peach palm fruit, compared to the fresh fruit, which may be related to the release of carotenoids from the cell walls. According to,[Citation65] carotenoids are prone to the formation of isomers after cooking and drying processes, and a consequent decrease in their bioactivity. The formation of the carotenoid isomers Z-β-carotene, Z-γ-carotene, and Z-lycopene have already been detected in peach palm fruits by some authors.[Citation3,Citation10,Citation33,Citation60]
[Citation59] analyzed six varieties of fresh and cooked peach palms (boiled for 30 min) from different countries and observed total carotenoid contents of 1.1‒22.3 mg.100 g in raw fruits and 1.3‒21.1 mg.100 g in cooked fruits. Although the range of variation was practically the same, the authors observed that cooked fruits generally presented higher contents of total carotenoids, which was attributed to the formation of isomers (cis-trans). This may further be ascribed to the combination of β-carotene and lycopene, which may be responsible for the higher associated antioxidant activity.[Citation60]
The health benefits of carotenoids are attributed to their bioaccessibility and bioavailability.[Citation66] Among the antioxidants, carotenoids are the most efficient natural singlet oxygen inactivators. This action occurs by transferring energy from the singlet oxygen molecules to carotenoids or by scavenging peroxyl radicals via chemical interactions. Moreover, owing to their lipophilic character, carotenoids inhibit the oxidation of low-density lipoproteins (LDL).[Citation67,Citation68]
The main functionality of pro-vitamin A carotenoids has been elucidated by previous studies. A derivative of this vitamin, Retinol, has been directly related to visual health, as it acts on the retina and visual chromophore of mammals. The main carotenoids involved in this action are β-carotene and β-cryptoxanthin, which possess unsubstituted β-ionone rings.[Citation67,Citation69] The total carotenoid content quantified in fruits must be converted to vitamin A, which considers that 1 μg of β-carotene equals 0.167 μg of retinol equivalent (RE) [IOM 2005).[Citation20] found 139.01 μg of RE in oil extracted from red peach palm pulp.
Peach palm products and byproducts
Research indicates that products and byproducts generated during the harvesting, processing, and consumption of peach palms may contain compounds with nutritional and functional properties, which, when used as a base ingredient or supplement in food products, can increase the functional composition of the product.[Citation70, Citation71]
Flour of peach palm pulp and peel
visualizes the flour obtained from different colored peach palms. The peach palm flour was obtained from the mesocarp (pulp) or exocarp (peel) of the fruits following removal of the liquid fraction (mostly water) via thermal steaming at a temperature lower than the boiling temperature of water. Peach palm flour has a low hygroscopicity up to 50% relative humidity, but requires greater care when handled or stored in environments with a relative humidity above 50%.[Citation30]
Figure 5. Flour obtained from different colored peach palms. a: white peach palm flour; b: green peach palm flour; c: yellow peach palm flour; and d: red peach palm flour.

Numerous previous studies have been aimed at the characterization of peach palm flour[Citation3,Citation35,Citation72,Citation73] could differentiate between microcarp, mesocarp, and macrocarp peach palm flours based on their physicochemical properties. They observed that microcarp flour had the highest lipid content and proved to be a source of dietary fiber, whereas macrocarp and mesocarp flours were fiber-rich. Moreover, macrocarp flour had the highest carbohydrate content and was characterized as being denser and drier than the others. Additionally, scanning electron microscopy confirmed that drying at 55°C did not affect the structure of the starch granules in the flour.
Recently,[Citation73] analyzed flour obtained from Colombian peach palm pulp, comparing unripe and ripe and raw and cooked fruits. Cooking increased the protein content of unripe and ripe peach palm flour, whereas a considerable reduction in the carbohydrate and mineral (sulfur, calcium, phosphorus, magnesium, and sodium) content was observed in both types of flour. These authors suggested that peach palm pulp flour may be an important food supplement for children, the elderly, and breastfeeding mothers. Accordingly, a pie enriched with peach palm flour was found to contribute approximately 30% of the daily β-carotene needs recommended for the elderly.[Citation74]
Regarding the stability to heat treatment (baking) and the effects of the drying process,[Citation31] evaluated the changes in carotenoid profiles during the production of peach palm flour. It was observed that the carotenoid content decreased by 28% in the flour compared to that of the cooked pulp, which may be attributed to enzymatic and oxidative reactions, and the molecules degraded due to prolonged drying at 72°C. These results corroborate those obtained by,[Citation33] who compared the carotenoid content in the pulp and flour of yellow and red peach palms, observing a reduction in the total carotenoid content in the flours, particularly in red peach palm flour.
The thermoplastic extrusion process was also used to evaluate changes in the functionality of peach palm flour. Despite the significant decrease in the carotenoid content of the extruded peach palm flour, there was an increase in the carotenoid content of the product when corn was added to the peach palm flour. This behavior can be attributed to the protection of carotenoids by the protein-carbohydrate matrix and/or the formation of carotenoid isomers Z-β-carotene, Z-γ-carotene, and Z-lycopene[Citation33] The incorporation of extruded peach palm flour into corn breakfast cereal yielded a texture with lower compression force and an increase in fiber, carotenoid, and iron content, resulting in a low sodium content, which is an important feature for industrially manufactured products.[Citation72] Nevertheless, in the field of industrial applications, sausages produced with purple tilapia showed higher yields, better consistency, and texture properties when combined with peach palm flour.[Citation75]
Peach palm peel flour is another important product obtained from peach palm fruits. Both peach palm pulp and peel flours have a low water activity (Wa), ensuring a longer shelf life and greater stability of the product. In addition, the levels of macronutrients can increase considerably subsequent to the elimination of water. The peach palm peel flour has been reported to contain 13.47% lipids, 6.18% proteins, and a high carbohydrate content (62.81%), in addition to being considered a source of carotenoids with high antioxidant activity.[Citation14]
[Citation3] were the first to quantify carotenoids in peach palm peel flour, observing higher levels of these constituents than in pulp flour. Moreover, the authors observed activity for vitamin A due to the presence of β-carotene, γ-carotene, and their respective isomers and found that peach palm peel flour is an important source of carotenoids [1500 μg.100−1 g of flour).
Peach palm peel flour can be used as nutritional supplements in the preparation of food products. Through sensory analysis[Citation76] found that the addition of up to 7.5% peach palm peel flour in cake formulations improved the texture, color, odor, flavor, and overall acceptance thereof. Similar results were obtained by,[Citation77] for a wheat bread formulation, in which the addition of 5% or more peach palm husk flour improved the sensory acceptability of the product.
In another study, increasing the peach palm peel flour oil in sausage formulations improved the associated color parameters due to the presence of carotenoids and, consequently, reduced the amount of nitrite in the product.[Citation78] These results indicated that peach palm peel flour oil might serve as a potential ingredient in the formulation of pigmented foods, thereby replacing synthetic dyes and making products healthier.
Peach palm starch
Starch, which is another byproduct obtained from peach palm pulp, is white, odorless, and contains residual levels of ash, proteins, lipids, and fibers.[Citation2,Citation38] Peach palm starch granules are heterogeneous in shape and size and are related to fruit maturation stage.[Citation2] As such, starches from unripe fruits do not show distinct morphologies.[Citation73]
According to[Citation2] starch extracted from peach palm is characterized as being bimodal, with a greater number of medium chains and a perfect crystalline structure.[Citation37] characterized and compared starches of microcarp, mesocarp, and macrocarp peach palm varieties and found a higher starch yield in macrocarp fruits. Moreover,[Citation18] produced and characterized a biodegradable thermoplastic based on peach palm starch, which demonstrated high tensile strength and thermal degradation. These results indicate that peach palm starch may serve as an important environmentally friendly alternative in the production of this type of material, which is of great interest to the packaging industry.
Peach palm heart
Peach palm hearts are divided into three parts: basal, central, and apical, possessing different physical and chemical characteristics.[Citation79] According to,[Citation80] the central part, which is sold with cylindrical cuts, is of particular interest, as it has the highest total carotenoid content. The basal region is cubic, whereas the palm heart of the apical region has irregular shapes, containing the highest content of total phenolic compounds. The peach palm heart has low acidity (pH>4.5) and does not withstand heat treatment at sterilization temperatures (>100°C). As such, their pH must be decreased to approximately 4.3 to avoid contamination with Clostridium botulinum spores. The palm hearts are subsequently packed in acidified brine in cans or glass, and, after exhaustion to remove the air, the product is subjected to pasteurization (<100°C).
[Citation80] evaluated the changes in quality and nutritional content of different peach palm parts during 120 days of storage. It was observed that storage time influenced the carotenoid content present in the three regions of the palm heart; however, the most significant reductions were observed for phenolic compounds and fibers in the apical portion. A recent study evaluated the use of different packages to increase the shelf life of peach palm hearts.[Citation81] A reduction was observed in the product firmness with all the tested packages; however, the perforated, microperforated, and non-perforated polyethylene packages were more efficient in preserving the product than the other packages tested.
Peach palm production generates additional residues that require research to evaluate their composition and possible uses.[Citation79] evaluated the flours obtained from the middle sheath and unused stem part of the peach palm and observed that the flour from the latter had a higher lipid, protein, dietary fiber, and, in general, ash and mineral content than the flour from the former. In contrast, the flour from the middle sheath had a higher carotenoid content, whereas the flour from the stem had higher levels of phenolic compounds and antioxidant activity. These results demonstrate the industrial potential of these byproducts.
Functional activities of peach palm
Despite being scarce, some studies have reported that extracts from different fractions of peach palms have important functional activities. Similar results were obtained in in vivo and in vitro experiments using peach palm products and byproducts, a summary of which is provided in .[Citation83] found that combining peach palm flour with a caffeine-based diet promoted an increase in plasma and liver vitamin A concentration in Wistar rats. These results suggested that the peach palm is an important source of vitamin A.
Table 4. Experimental applications of functional activities of peach palm.
In experimental clinical trials, the aqueous extract of peach palm pulp promoted a decrease in serum triglyceride levels in rats, probably due to the presence of lectin in the extract.[Citation82] Currently, the effect of lectin on metabolism has been elucidated, especially regarding its antihyperglycemic activity and lipid levels. In the study by[Citation90] rats subjected to diabetes and supplemented with lectin isolated from red seaweed had a decrease in insulin resistance. This effect was obtained by calculating the homeostasis model for evaluating the resistance of β cells [HOMA-β) and was attributed to the lectin’s ability to reduce free radicals. In the research by[Citation91, Citation92] native γ-conglutin with lectin activity showed an antihyperglycemic effect due to its ability to bind to glucose receptors on HepG2 cells, including insulin receptors.
These results corroborate those obtained in tests carried out with broiler chickens, in which peach palm oil promoted a decrease in the serum concentration of LDL-c and a more favorable LDLc: HDLc ratio.[Citation84] Moreover,[Citation86] observed a decrease in total cholesterol and an increase in high-density lipoprotein in Wistar rats (lactating and infant) supplemented with red peach palm pulp. This capacity may be directly related to the composition of unsaturated fatty acids in the pulp and oil of peach palm.[Citation20–22]
An important use in clinical-nutritional management was observed when feed supplemented with peach palm pulp flour was administered to Wistar rats[Citation86] and lambs,[Citation87] resulting in the prevention of weight gain. Recently,[Citation88] observed a change in the feeding behavior of goats fed diets in which corn was replaced by peach palm pulp flour. Here, the use of up to 10% peach palm flour resulted in reduced feed consumption and, consequently, increased chewing time, which was attributed to the fat and fiber composition of the flour. These effects have been reported for the presence of fiber in foods, which can alter the gut microbiota, produce short-chain fatty acids, reduce macronutrient absorption, and promote satiety.[92] These results indicated that peach palm flour has the potential to fight against obesity in humans.[Citation89] Observed that a hydroethanolic extract of peach palm pulp has a non-cytotoxic effect in vitro on lung fibroblasts [MRC-5). Moreover,[Citation85] observed that oil extracted with hexane from peach palm bark possessed antimicrobial characteristics for strains of Staphylococcus aureus 24 h after the addition of 10 µL oil. This potential was attributed to the sensitivity of this microorganism to compounds with antibiotic potential, showing that further studies on the antimicrobial effect of peach palm can be better explored.
Future perspectives
Considering the phases of public policies for Amazonian environmental protection and the associated design of food security strategies, the expansion of knowledge regarding traditional Amazonian food species, such as the peach palm, is essential to expand the applications and uses of this fruit. The associated studies are presented as perspectives for the dissemination of knowledge regarding plant matrices with high nutritional, functional, and technological potential, thus adding value to the fruit species of the Amazonian biodiversity.
In future perspectives for peach palm fruits, ongoing research on a novel peach palm variety deserves to be highlighted, namely, the characterization and applications of an albino fruit. This indicates an increase in other peach palm varieties that can be added to the already well-established varieties, such as red and yellow peach palms. In this sense, research aimed at listing the properties and potential of this white variety of peach palm using the same strategies already carried out for the other varieties is emerging.
Along with the sociocultural aspects of the traditional Amazonian diet, research, such as those mentioned in the course of this work, aims to promote, in parallel, the survival of the food culture, reduction of food shortages, and the implementation of management and consumption policies, aiming to assist in sustainable food security. Considering the recent technological and knowledge-based advancements, the potential of new peach palm species is yet to be explored.
Thus, advances in research on peach palm products, byproducts, and their applications in the design of new industrial segments have been reported based predominantly on red and yellow peach palm varieties. However, this integrative review addresses, in addition to the listed objectives, the occurrence of mutations and albinism in peach palm fruits (). Herein, the need for research on this new white or albino variety is highlighted, considering that it has already been incorporated in traditional Amazonian diets. Moreover, the sensory aspects, physical, nutritional, and functional composition of this new variety of peach palm remains unknown in the scientific literature.
In this review, we present preliminary data from our research group, which includes a novel variety of peach palm and the analyses of its macro- and micronutrient content; bioactive constituents; yield rates, starch constituents and properties; lipid constituents, extracted by different methods from different parts of the peach palm (peel, pulp, and seeds); carotenoid and potential antioxidant profile of extracts; applications of products and byproducts as raw materials in the replacement and supplementation of formulations; thermogravimetric-differential and spectroscopic stabilities; and optical and morphological patterns, among other research fields.
Conclusion
This review presents an updated view of the current literature addressing the botanical aspects, chemical composition, nutritional-functional properties, applications, and potentialities of different peach palm varieties, in addition to reporting, for the first time, a new variety of white peach palm. Several research groups worldwide have demonstrated the properties and potential of the products and byproducts of peach palm varieties, especially red and yellow varieties. This review aimed to highlight the constituents and application potential of red, green, yellow, and orange peach palms, with emphasis on their products and byproducts. The most recent studies have demonstrated the high functional potential of the bioactive compounds present in both the pulp and peel of the peach palm, such as lipids, carotenoids, starches, and fibers, among others. A considerable number of studies have examined the fatty acid profiles of the fruits. Furthermore, the isolation of peach palm starch and its application to the formulations of products has also been a strong indicator of its supplemental potential. The carotenoid profile of peach palms has been implicated in the metabolic homeostasis maintenance of fat-soluble vitamins, particularly pro-vitamin A. Nevertheless, previous studies mainly focused on the evaluation of biochemical parameters, and limited literature exists on the in vivo characterization of peach palms and its byproducts.
Analytical results from numerous studies are presented in the current review, highlighting the utilization of different technological applications in the elucidation of the fundamental chemical and functional constituents of peach palms. Furthermore, the applicability of these constituents in the design of new products for the nutraceutical, pharmaceutical, and dermocosmetic sectors, among others, is also reviewed. Highlights include the isolation and bioactive applications (blends) of peach palm constituents, nanotechnology in active packaging, starch and lipid extraction, peach palm-based enrichment and combinations in products, technological productions involving the peach palm production chain, and the aggregation of value of the red and yellow varieties of the peach palm species.
The results presented by publications with a high impact factor demonstrate that, on a scientific basis, the knowledge and applications of traditional Amazonian cuisine have already been empirically reported. This scientific evidence has increased the knowledge pool and diversified the applications of raw materials from the Amazonian biodiversity, such as that of the peach palm. Rapid technological advances promote the emergence of new insights regarding Amazonian fruits. These studies are essential and must consider different approaches to effectively add to the already disseminated literature and to those aspects discussed in this review. Finally, this work presents the first approaches, in various aspects, toward the research development of the white peach palm (albino) at the Federal University of Pará, in the center of the Brazilian Amazon.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), for the financial support through the Master scholarship of Stephanie Dias Soares (Process 132850/2020-6) and also thank to PROAP/PROPESP for the financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Costa, R. D. S.; Rodrigues, A. M. C.; Laurindo, J. B.; Silva, L. H. M. Development of Dehydrated Products from Peach palm–tucupi Blends with Edible Film Characteristics Using Refractive Window. J. Food Sci. Technol. 2019, 56(2), 560–570. DOI: 10.1007/s13197-018-3454-x.
- Felisberto, M. H. F.; Costa, M. S.; Boas, F. V.; Leivas, C. L.; Franco, C. M. L.; De Souza, S. M.; Clerici, M. T. P. S.; Cordeiro, L. M. C. Characterization and Technological Properties of Peach Palm (Bactris Gasipaes Var. Gasipaes) Fruit Starch. Food Res. Int. 2020, 136:1‒8. DOI: 10.1016/j.foodres.2020.109569.
- Matos, K. A. N.; Lima, D. P.; Barbosa, A. P. P.; Mercadante, A. Z.; Chisté, R. C. Peels of Tucumã (Astrocaryum Vulgare) and Peach Palm (Bactris Gasipaes) are Byproducts Classified as Very High Carotenoid Sources. Food Chem. 2019, 272(1), 216. DOI: 10.1016/j.foodchem.2018.08.053.
- Queiroz, L. S.; de Souza, L. K. C.; Thomaz, K. T. C.; Leite Lima, E. T.; da Rocha Filho, G. N. Activated Carbon Obtained from Amazonian Biomass Tailings (Acai Seed): Modification, Characterization, and Use for Removal of Metal Ions from Water. J. Environ. Manage. 2020, 270, 1‒8. doi: 10.1016/j.jenvman.2020.110868.
- Santos, O. V.; Gonçalves, B. S.; Macêdo, C. S.; Conceição, L. R. V.; Costa, C. E. F.; Monteiro Júnior, O. V.; Souza, A. L. G.; Lannes, S. C. S. Evaluation of Quality Parameters and Chromatographic, Spectroscopic, and Thermogravimetric Profile of Patauá Oil (Oenocarpus Bataua). Food Sci. Technol. 2020a, 40(1), 76‒82. DOI: 10.1590/fst.01619.
- Oliveira, D. A.; Mezzomo, N.; Gomes, C.; Ferreira, S. R. S. Encapsulation of Passion Fruit Seed Oil by Means of Supercritical Antisolvent Process. J. Supercrit. Fluids. 2017, 129, 96‒105. doi: 10.1016/j.supflu.2017.02.011.
- Peixoto Araujo, N. M.; Arruda, H. S.; Marques, D. R. P.; de Oliveira, W. Q.; Pereira, G. A.; Pastore, G. M. Functional and Nutritional Properties of Selected Amazon Fruits: A Review. Food Res. Int. 2021, 147(110520:1‒19), 110520. DOI: 10.1016/j.foodres.2021.110520.
- Sato, M. K.; de Lima, H. V.; Noronha Costa, A.; Rodrigues, S.; Mooney, S. J.; Clarke, M.; Silva Pedroso, A. J.; de Freitas Maia, C. M. B. Biochar as a Sustainable Alternative to Açaí Waste Disposal in Amazon, Brazil. Process Saf. Environ. Prot. 2020, 39:36‒46. DOI: 10.1016/j.psep.2020.04.001.
- Silva, J. S.; Ortiz, D. W.; Garcia, L. G. C.; Asquieri, E. R.; Becker, F. S.; Damiani, C. Effect of Drying on Nutritional Composition, Antioxidant Capacity and Bioactive Compounds of Fruits Coproducts. Food Sci. Technol. 2020, 40(4), 810. DOI: 10.1590/fst.21419.
- Chisté, R. C.; Costa, E. L. N.; Monteiro, S. F.; Mercadante, A. Z. Carotenoid and Phenolic Compound Profiles of Cooked Pulps of Orange and Yellow Peach Palm Fruits (Bactris Gasipaes) from the Brazilian Amazonia. J. Food Compost. Anal. 2021, 99, 103873. DOI: 10.1016/j.jfca.2021.103873.
- Rojas-Garbanzo, C.; Pérez, A. M.; Vaillant, F.; Pineda-Castro, M. L. Pineda-Castro. Physicochemical and Antioxidant Composition of Fresh Peach Palm (Bactris Gasipaes Kunth) Fruits in Costa Rica. Brazilian journal food technology. 2016, 191, 1‒9. DOI:10.1590/1981-6723.9715.
- Embrapa – Empresa Brasileira de Pesquisa Agropecuária. 2019a. Transferência de tecnologia florestal - Pupunha. Banco de dados on-line da Embrapa. [cited 2022 Feb 25]. https://www.embrapa.br/florestas/transferencia-detecnologia/pupunha/tema
- Franchetti, M., and Rozane, D. E. Palmito pupunha: Do plantio a colheita (UNESP, São Paulo), 2017; pp 175.
- Martinez, J. M.; Moreno-Caicedo, L. P. Loaiza-Loaiza, O. A. Sensory Dimensions of peach-palm Fruit (Bactris Gasipaes) and Implications for Future Genetics. Agronomía Mesoamericana. 2021, 321, 77‒92. DOI:10.15517/am.v32i1.41348.
- Aguiar, J. P. L.; Yuyama, K.; Souza, F. D. C. D. A. Caracterização dos frutos de Pupunheira (Bactris gasipaes Kunth) cultivada na vila do Equador, RR: O que há de novo? Scientia Amazonia 2019, 8(1), 1‒5.
- Yuyama, L. K. O.; Aguiar, J. P. L.; Yuyama, K.; Clement, C. R.; Macedo, S. H. M.; Fávaro, D. I. T.; Afonso, C.; Vasconcellos, M. B. A.; Pimentel, S. A.; Badolato, E. S. G., et al. Chemical Composition of the Fruit Mesocarp of Three Peach Palm (Bactris Gasipaes) Populations Grown in Central Amazonia, Brazil. Int. J. Food Sci. Nutr. 2003, 54(1), 49‒56. DOI: 10.1080/096374803/000061994.
- Leterme, P.; García, M. F.; Londono, A. M.; Rojas, M. G.; Buldgen, A.; Souffrant, W. B. Chemical Composition and Nutritive Value of Peach Palm (Bactris Gasipaes Kunth) in Rats. J. Sci. Food Agric. 2005, 85:1505‒12. DOI: 10.1002/jsfa.2146.
- Melo Neto, B. A.; Fornari Junior, C. C. M.; Silva, E. G. P.; Franco, M.; Reis, N. S.; Bonomo, R. C. F.; Almeida, P. F.; Pontes, K. V. Biodegradable Thermoplastic Starch of Peach Palm (Bactris Gasipaes Kunth) Fruit: Production and Characterization. Int. J. Food Prop. 2017b, 20(S3), 2430. DOI: 10.1080/10942912.2017.1372472.
- Santos, M. F. G.; Alves, R. E.; Brito, E. S.; Silva, S. M.; Silveira, M. R. S. Quality Characteristis of Fruits and Oils of Palms Native to the Brazilian Amazon. Rev. Bras. Frutic. 2017b, 39:1‒6. DOI: 10.1590/0100-29452017.
- Santos, O. V.; Soares, S. D.; Dias, P. C. S.; Duarte, S. D. P. A.; Santos, M. P. L.; Nascimento, F. D. C. A. Chromatographic Profile and Bioactive Compounds Found in the Composition of Pupunha Oil (Bactris Gasipaes Kunth): Implications for Human Health. Revista de Nutrição. 2020b, 33, e190146. DOI: 10.1590/1678-9805202033e190146.
- Mujica, F.; Viky, C.; Del Carmen Rodriguez, S. M. Evaluation of Oil Properties of Fruit Pulp Pijiguao (Bactris Gasipaes HBK) for Use in Cosmetics Industry. Revista Ingenieria UC 2017, 24(3), 314.
- Restrepo, J.; Estupinán, J. A.; Colmenares, A. J. Estudio comparativo de las fracciones lipídicas de Bactris gasipaes Kunth (chontaduro) obtenidas por extracción Soxhlet y por extracción con CO2 supercrítico. Química aplicada y analítica. 2016, 451, 5‒9. DOI:10.15446/rev.colomb.quim.v45n1.57199.
- GBIF – Global Biodiversity Information Facility. 2019. Bactris Gasipaes Var. Gasipaes Kunth. GBIF Online Database. [cited 2022 Mar 15]. https://www.gbif.org/pt/species/2733062
- Galluzzi, G.; Dufour, D.; Thomas, E.; Van Zonneveld, M.; Salamanca, A. F. E.; Toro, A.; Rivera, A.; Duque, H. S.; Baron, H. S.; Gallego, G., et al. An Integrated Hypothesis on the Domestication of Bactris Gasipaes. PLoS ONE.2015, 10(12), 1‒25. DOI: 10.1371/journal.pone.0144644.
- Lorenzi, H. Bactris in Flora E Funga Do Brasil. 2022. Jardim Botânico do Rio de Janeiro.: <https://floradobrasil.jbrj.gov.br/FB22106>. August 27, 2022.
- Silva, M. G. C. P. C.; Vieira, E. S. Descrição morfológica dos frutos de pupunheira no sul da bahia-acesso yurimáguas, Peru. Agrotrópica. 2012, 24(3), 133. DOI: 10.21757/0103-3816.2012v24n3p133-136.
- IBGE – Instituto Brasileiro de Geografia e Estatística. 2017. Censo Agropecuário, resultados definitivos. Pupunha – Cachos de frutas | Brasil. Banco de dados on-line do IBGE. [cited 2022 Mar 18]. https://censoagro2017.ibge.gov.br/templates/censo_agro/resultadosagro/agricultura.html? Locality = 0 & theme = 78309.
- Santos, M. A. S.; Protázio, D. C.; Costa, G. P.; Rebello, F. K.; Martins, C. M.; Bezerra, A. S.; Nogueira, A. S. Profile of Peach Palm Fruit Consumers in the Metropolitan Region of Belém, Pará, Brazilian Amazon. International Journal for Innovation Education and Research. 2021, 91, 550. DOI:10.31686/ijier.vol9.iss1.2929.
- Melo, C. M. T.; Costa, L. L.; Pereira, F. C.; De Castro, L. M.; Nepumoceno, S. Análises físico-químicas do fruto “in natura” da pupunha. Revista Inova Ciência & Tecnologia 2017, 3(1), 13.
- Ferreira, C. D.; Pena, R. S. Hygroscopic Behavior of Pupunha Flour (Bactris Gasipaes). Food Sci. Technol. 2003, 23(2), 5–251. DOI: 10.1590/S0101-20612003000200025.
- Rojas-Garbanzo, C.; Pérez, A. M.; Pineda-Castro, M. L.; Vaillant, F. Major Physicochemical and Antioxidant Changes during peach-palm (Bactris Gasipaes H.B.K.) Flour Processing. Fruits. 2012, 67(6), 415. DOI: 10.1051/fruits/2012035.
- Carvalho, A. V.; Beckman, J. C.; de Almeida Maciel, R.; Farias Neto, J. T. Características físicas e químicas de frutos de pupunheira no estado do Pará. Rev. Bras. Frutic. 2013a, 35(3), 763. DOI: 10.1590/S0100-29452013000300013.
- Basto, G. J.; Carvalho, C. W. P.; Soares, A. G.; Costa, H. T. G. B.; Chávez, D. W. H.; Godoy, R. L. O.; Pacheco, S. Physicochemical Properties and Carotenoid Content of Extruded and Nonextruded Corn and Peach Palm (Bactris Gasipaes, Kunth). LWT - Food Sci. Technol. 2016, 69:312‒18. DOI: 10.1016/j.lwt.2015.12.065.
- Santos, B. W.; Ferreira, F. M.; Souza, V. F. D.; Clement, C. R.; Rocha, R. B. Análise discriminante das características físicas e químicas de frutos de pupunha (Bactris gasipaes Kunth) do alto Rio Madeira, Rondônia, Brasil. Científica. 2017a, 45(2), 154. DOI: 10.15361/1984-5529.2017v45n2p154-161.
- Pires, M. B.; Amante, E. R.; Lopes, A. S.; Rodrigues, A. M. D. C.; Silva, L. H. M. D. Peach Palm Flour (Bactris Gasipaes Kunth): Potential Application in the Food Industry. Food Sci. Technol. 2019, 39(3), 613. DOI: 10.1590/fst.34617.
- Melo Neto, B. A.; Fernandes, B. S.; Fornari Junior, C. C. M.; Franco, M.; Bonomo, R. C. F.; Almeida, P. F.; Pontes, K. V. Thermal-morphological Characterization of Starch from peach-palm (Bactris Gasipaes Kunth) Fruit (Pejibaye). Int. J. Food Prop. 2017a, 20(5), 1007–1015. DOI: 10.1080/10942912.2016.1192645.
- Pires, M. B.; Amante, E. R.; Petkowicz, C. L. O.; Esmerino, E. A.; Rodrigues, A. M. D. C.; Silva, L. H. M. D. Impact of Extraction Methods and Genotypes on the Properties of Starch from Peach Palm (Bactris Gasipaes Kunth) Fruits. Food Sci. Technol. 2021, 150, 111983. DOI: 10.1016/j.lwt.2021.111983.
- Valencia, G. A.; Moraes, I. C. F.; Lourenço, R. V.; Sobral, P. J. D. A.; Sobral, P. J. D. A. Physicochemical, Morphological, and Functional Properties of Flour and Starch from Peach Palm (Bactris Gasipaes K.) Fruit. Starch - Stärke. 2015, 67(1–2), 163. DOI: 10.1002/star.201400097.
- Jiménez, G.; Gómez, G.; Pérez, A. M.; Blanco-Metzler, A. Estimation of Glycemic Index of Peach Palm (Bactris Gasipaes) Cooked Fruits and Chips, and Pitahaya (Hylocereus Spp.) Pulp. Archivos latinoamericanos de nutrición 2012, 62(3), 242.
- IOM. Institute of Medicine. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press; 2005: Washington, D.C.
- Jefferson, A.; Adolphus, K.; Castanas, E.; Kampa, M. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutrit. 2019, 6, 6. DOI: 10.3389/fnut.2019.00006.
- Tosh, S. M.; Bordenave, N. Emerging Science on Benefits of Whole Grain Oat and Barley and Their Soluble Dietary Fibers for Heart Health, Glycemic Response, and Gut Microbiota. Nutr. Rev. 2020, 78:13‒20. DOI: 10.1093/NUTRIT/NUZ085.
- Cantu-Jungles, T. M.; Cipriani, T. R.; Iacomini, M.; Hamaker, B. R.; Cordeiro, L. M. C. A Pectic Polysaccharide from Peach Palm Fruits (Bactris Gasipaes) and Its Fermentation Profile by the Human Gut Microbiota in Vitro. Bioactive Carbohydrates and Dietary Fiber. 9:1‒6, 2016. DOI:10.1016/j.bcdf.2016.11.005
- Santos, R. C.; Chagas, E. A.; Melo Filho, A. A.; Takahashi, J. A.; Montero, I. F.; Santos, G. F.; Chagas, P. C.; Melo, A. C. G. R. Chemical Characterization of Oils and Fats from Amazonian Fruits by 1H NMR. Chem. Eng. Trans. 2018, 64:235‒40. DOI: 10.3303/CET1864040.
- Rufino, M. D. S. M.; Nazareno, L. S. Q.; Alves, R. E.; Fernandes, F. A. N. Kinetic Modeling and Evaluation of Free radical-scavenging Behavior in Oils: Application to Four Tropical and Subtropical Fruits in a DPPH System. Food Sci. Technol. 2020, 40(2), 440. DOI: 10.1590/fst.03819.
- Kozlowska, M.; Gruczynska, E. Comparison of the Oxidative Stability of Soybean and Sunflower Oils Enriched with Herbal Plant Extracts. Chem. Pap. 2018, 72(10), 2607–2615. DOI: 10.1007/s11696-018-0516-5.
- Calder, P. C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45(5), 1105. DOI: 10.1042/BST20160474.
- Fabian, C. J.; Kimler, B. F.; Philips, T. A.; Box, J. A.; Kreutzjan, A. L.; Carlson, S. E.; Hidaka, B. H.; Metheny, T.; Zalles, C. M.; Mills, G. B., et al. Modulation of Breast Cancer Risk Biomarkers by high-dose Omega-3 Fatty Acids: Phase II Pilot Study in Premenopausal Women. Cancer Prev. Res. 2015, 8(10), 912. DOI: 10.1158/1940-6207.CAPR-14-0335.
- Elagizi, A.; Lavie, C. J.; Keefe, E. O.; Marshall, K.; Keefe, J. H. O.; Milani, R. V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients. 2021, 204(13), 1–12. DOI: 10.3390/nu13010204.
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M. I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J., et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with extra-virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378(25), 1‒15. DOI: 10.1056/nejmoa1800389.
- Doménech, M.; Roman, P.; Lapetra, J.; Garcia, F. J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutierrez, V.; Lamuela-Raventós, R. M., et al. Mediterranean Diet Reduces 24-hour Ambulatory Blood Pressure, Blood Glucose, and Lipids: One-year Randomized, Clinical Trial. Hypertension.2014, 64(1), 69‒76. DOI: 10.1161/HYPERTENSIONAHA.113.03353.
- Darwesh, A. M.; Bassiouni, W.; Sosnowski, D. K.; Seubert, J. M. Can N-3 Polyunsaturated Fatty Acids Be Considered a Potential Adjuvante Therapy for COVID-19-associated Cardiovascular Complications? Pharmacol. Ther. 2021, 219:1‒27. DOI: 10.1016/j.pharmthera.2020.107703.
- Rogero, M. M.; Leão, M. C.; Santana, T. M.; Pimentel, M. V. M. B.; Carlini, G. C. G.; Silveira, T. F. F.; Gonçalves, R. C.; Castro, I. A. Potential Benefits and Risks of Omega-3 Fatty Acids Supplementation to Patients with COVID-19. Free Radical Biol. Med. 2020, 156:190‒9. DOI: 10.1016/j.freeradbiomed.2020.07.005.
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May Omega-3 Fatty Acid Dietary Supplementation Help Reduce Severe Complications in Covid-19 Patients? Biochimie. 2020, 179, 275–280. DOI: 10.1016/j.biochi.2020.09.003.
- Romano, L.; Bilotta, F.; Dauri, M.; Macheda, S.; Pujia, A.; de Santis, G. L.; Tarsitano, M. G.; Merra, G.; Renzo, L. D. I.; Esposito, E., et al. Short Report – Medical Nutrition Therapy for Critically Ill Patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24(7), 39–4035. DOI: 10.26355/EURREV_202004_20874.
- Zheng, B.; Wang, T.; Wang, H.; Chen, L., and Zhou, Z. 2022. Studies on Nutritional Intervention of Rice starch-oleic Acid Complex (Resistant Starch Type V) in Rats Fed by high-fat Diet. Carbohydr. Polym. 246: 1–10. doi: 10.1016/j.carbpol.2020.116637.
- Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients. 2019, 11(12), 1–13. DOI: 10.3390/nu11123005.
- Pastor, R.; Bouzas, C.; Tur, J. A. Beneficial Effects of Dietary Supplementation with Olive Oil, Oleic Acid, or Hydroxytyrosol in Metabolic Syndrome: Systematic Review and meta-analysis. Free Radical Biol. Med. 2021, 172, 372–385. DOI: 10.1016/j.freeradbiomed.2021.06.017.
- Jatunov, S.; Quesada, S.; Díaz, C.; Murillo, E. Carotenoid Composition and Antioxidant Activity of the Raw and Boiled Fruit Mesocarp of Six Varieties of. Bactris gasipaes. Archivos latinoamericanos de nutricion 2010, 60(1), 99‒104.
- Quesada, S.; Azofeifa, G.; Jatunov, S.; Jiménez, G.; Navarro, L.; Gómez, G. Carotenoids Composition, Antioxidant Activity and Glycemic Index of Two Varieties of. Bactris gasipaes. Emirates Journal of Food and Agriculture 2011, 23(6), 482.
- Rodriguez-Amaya, D. B. Update on Natural Food Pigments – A mini-review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2018, 124:200‒5. DOI: 10.1016/j.foodres.2018.05.028.
- Boon, C. S.; Mcclements, D. J.; Weiss, J.; Decker, A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50(6), 515. DOI: 10.1080/10408390802565889.
- Hempel, J.; Amrehn, E.; Quesada, S.; Esquivel, P.; Jiménez, V. M.; Heller, A.; Carle, R.; Schweiggert, R. M. Lipid-dissolved γ-carotene, β-carotene, and Lycopene in Globular Chromoplasts of Peach Palm (Bactris Gasipaes Kunth) Fruits. Plants. 2014, 240(5), 1037. DOI: 10.1007/s00425-014-2121-3.
- Mesquita, L. M. S.; Neves, B. V.; Pisani, L. P.; De Rosso, V. V. Mayonnaise as a Model Food for Improving the Bioaccessibility of Carotenoids from Bactris Gasipaes Fruits. LWT- Food Sci. Technol. 2020, 122(109022), 1‒7. DOI: 10.1016/j.lwt.2020.109022.
- Cortés, C.; Esteve, M. J.; Frígola, A.; Torregrosa, F. Identification and Quantification of Carotenoids Including Geometrical Isomers in Fruit and Vegetables Juices by Liquid Chromatography with Ultraviolet-Diode Array Detection. J. Agric. Food Chem. 2004, 52(8), 2203–2212. DOI: 10.1021/jf035505y.
- Xavier, A. A. O.; Mercadante, A. Z. The Bioaccessibility of Carotenoids Impacts the Design of Functional Foods. Curr. Opin. Food Sci. 2019, 26(26), 1–8. DOI: 10.1016/j.cofs.2019.02.015.
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients. 2019, 11(10), 1‒6. DOI: 10.3390/nu11102388.
- Blaak, E. E.; Canfora, E. E.; Theis, S.; Frost, G.; Groen, A. K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Harsselaar, J. V., et al. Short-chain Fatty Acids in Human Gut and Metabolic Health. Beneficial Microbes.2020, 11(5), 411. DOI: 10.3920/BM2020.0057.
- Lawler, T.; Liu, Z.; Tinker, L.; Johnson, E.; Hammond, B. R.; Gangnon, R.; Engelman, C.; Wallace, R.; Liu, Y.; Bailey, S. T., et al. Relationship between Mediterranean Diet Pattern and Macular Pigment Optical Density in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an Ancillary Study of the Women’s Health Initiative (WHI). Investigative Ophthalmology & Visual Science. 2020, 61(7), 4977.
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M. A. C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I. M.; Montanari, A.; López, M. J., et al. 2017. Processing, Valorization and Application of Biowaste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability (Switzerland). 9(8), 1–46, DOI: 10.3390/su9081492.
- Danesi, E. D. G.; Granato, D.; Iwassa, I. J.; Pinzon, C.; Bolanho, B. C. Effects of Industrial Byproducts from Orange, Peach Palm and Soybean on the Quality Traits and Antioxidant Activity of Flours: A Response Surface Approach. Int. Food Res. J. 2018, 25(3), 1219.
- Santos, I. L.; Schmiele, M.; Aguiar, J. P. L.; Steel, C. J.; Paiva, E. P.; Souza, F. C. A. Evaluation of Extruded Corn Breakfast Cereal Enriched with Whole Peach Palm (Bactris Gasipaes, Kunth) Flour. Food Sci. Technol. 2020c, 40(2), 458. DOI: 10.1590/fst.04019.
- Torres‐Vargas, O. L.; Luzardo‐Ocampo, I.; Hernandez‐Becerra, E.; Rodríguez‐García, M. E. Physicochemical Characterization of Unripe and Ripe Chontaduro (Bactris Gasipaes Kunth) Fruit Flours and Starches. Starch - Stärke. 2021, 73(7–8), 2000242. DOI: 10.1002/star.202000242.
- Fernández-Cordero, P.; Mora-Molina, J.; Obando-Ulloa, J. M.; Arguedas-Gamboa, P. Desarrollo de una torta precocida nutracéutica a partir de materiales vegetales biofortificados para adultos mayores. Revista Tecnología En Marcha. 2018, 311, 110. DOI:10.18845/tm.v31i1.3501.
- Zapata, J. I. H.; La Pava, G. C. R. Propiedades texturales y sensoriales de salchichas de tilapia roja (Oreochromis sp.) con adición de harina de chontaduro (Bactris gasipaes). Ingeniería y Desarrollo. 2015, 332, 198‒215. DOI:10.14482/inde.33.2.6332.
- Martínez-Giron, J.; Figueroa-Molano, A. M.; Ordónez-Santos, L. E. Effect of the Addition of Peach Palm (Bactris Gasipaes) Peel Flour on the Color and Sensory Properties of Cakes. Food Sci. Technol. 2017, 37(3), 418. DOI: 10.1590/1678-457X.14916.
- Ordónez-Santos, L. E.; Martínez-Giron, J.; Figueroa-Molano, A. M. Effect of the Addition of Peach Palm (Bactris Gasipaes) Peel Flour on the Color and Sensory Properties of Wheat Bread. Revista de Ciências Agrárias. 2016, 39(3), 456. DOI: 10.19084/RCA16008.
- Pinzón-Zárate, L. X.; Hleap-Zapata, J. I.; Ordonez-Santos, L. E. Análisis de los parámetros de color en salchichas frankfurt adicionadas con extracto oleoso de residuos de chontaduro (Bactris gasipaes). Informacion Tecnologica. 2015, 265, 45‒54. DOI:10.4067/S0718-07642015000500007.
- Bolanho, B. C.; Danesi, E. D. G.; Beléia, A. D. P. Carbohydrate Composition of Peach Palm (Bactris Gasipaes Kunth) Byproducts Flours. Carbohydr. Polym. 2015, 124:196‒200. DOI: 10.1016/j.carbpol.2015.02.021.
- Stevanato, N.; Ribeiro, T. H.; Giombelli, C.; Cardoso, T.; Wojeicchowski, J. P.; Dalva, E.; Danesi, G.; Barros, B. C. B. Effect of Canning on the Antioxidant Activity, Fiber Content, and Mechanical Properties of Different Parts of Peach Palm Heart. Journal of food processing preservation. e14554:1‒8, 2020. DOI:10.1111/jfpp.14554
- Ribeiro, J. C.; Pereira, M. G.; Gadioli, J. L.; Almeida, J. C. R. Litterfall Dynamics and Nutrient Cycling in an Experimental Plantation of Peach Palm (Bactris Gasipaes Kunth). Floresta e Ambiente. 2021, 272, 1‒9. DOI:10.1590/2179-8087.021018.
- Gómez, G.; Quesada, S.; Nanne, C. I. Efecto de factores antinutricionales en el pejibaye (Bactris gasipaes) sobre el metabolismo de ratas jovenes. Agronomfa Costarricense 1998, 22(2), 191‒198.
- Yuyama, L. K. O.; Yonekura, L.; Aguiar, J. O. L.; Sousa, R. F. S. Biodisponibilidade de vitamina a da pupunha (Bactris gasipaes kunth) em ratos. Acta Amazonica. 1999, 29(3), 497‒500. DOI: 10.1590/1809-43921999293500.
- Baldizán, G.; Oviedo, M.; Michelangeli, C.; Vargas, R. E. Effects of Peach Palm Oil on Performance, Serum Lipoproteins and Hemostasis in Broilers. British Poul. Sci. 2010, 51(6), 784. DOI: 10.1080/00071668.2010.526925.
- Araújo, M. L.; Silva, C. F. C.; Souza, R. M.; Melhorança Filho, A. L. Atividade antimicrobiana de óleos extraídos de açaí e de pupunha sobre o desenvolvimento de Pseudomonas aeruginosa e. Staphylococcus aureus. Bioscience Journal 2013, 29(4), 90–985.
- Carvalho, R. P.; Lemos, J. R. G.; Sales, R. S. A.; Martins, M. G.; Nascimento, C. H.; Bayona, M.; Marcon, J. L.; Monteiro, J. B. The Consumption of Red Pupunha (Bactris Gasipaes Kunth) Increases HDL Cholesterol and Reduces Weight Gain of Lactating and Postlactating Wistar Rats. Journal of aging research and clinical practice 2013b, 2(3), 257.
- Dos Santos, A. B.; Pereira, M. L. A.; De Oliveira Silva, H. G.; De Carvalho, G. G. P.; De Jesus Pereira, T. C.; Ribeiro, L. S. O.; Azevedo, J. A. G.; Silva, M. G. C. P. C.; Sousa, L. B. Intake, Digestibility and Performance of Lambs Fed Diets Containing Peach Palm Meal. Trop. Anim. Health Prod. 2016, 48(3), 15–509. DOI: 10.1007/s11250-015-0982-5.
- Pereira, T. C. J.; Ribeiro, L. S. O.; Pereira, M. L. A.; Pires, A. J. V.; De Carvalho, G. G. P.; Pereira, C. A. R. Feeding Behavior of Goat Kids Fed Diets Containing Peach Palm Meal. Acta Scientiarum. 2020, 42(e47088), 1‒8. DOI: 10.4025/actascianimsci.v42i1.47088.
- Faria, J. V.; Valido, I. H.; Paz, W. H.; Da Silva, F. M.; De Souza, A. D.; Acho, L. R.; Lima, E. S.; Boleti, A. P. A.; Marinho, J. V. N.; Salvador, M. J., et al. Comparative Evaluation of Chemical Composition and Biological Activities of Tropical Fruits Consumed in Manaus, Central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836. DOI: 10.1016/j.foodres.2020.109836.
- Alves, M. F. A.; Barreto, F. K. A.; Vasconcelos, M. A.; Nascimento Neto, L. G.; Carneiro, R. F.; Silva, L. T.; Nagano, C. S.; Sampaio, A. H.; Teixeira, E. H. Antihyperglycemic and Antioxidante Activities of a Lectin from the Marine Red Algae, Bryothamnion Seaforthii, in Rats with streptozotocin-induced Diabetes. Int. J. Biol. Macromol. 2020, 158, 773–780. DOI: 10.1016/j.ijbiomac.2020.04.238.
- Grácio, M.; Rocha, J.; Pinto, R.; Ferreira, R. B.; Solas, J.; Eduardo-Figueira, M.; Sepodes, B.; Ribeiro, A. C. A Proposed lectin-mediated Mechanism to Explain the in Vivo Antihyperglycemic Activity of γ-conglutin from Lupinus Albus Seeds. Food Sci. Nutr. 2021, 9(11), 5980–5996. DOI: 10.1002/fsn3.2520.
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of Insoluble and Soluble Fibers Isolated from Barley on Blood Glucose, Serum Lipids, Liver Function and Caecal short-chain Fatty Acids in Type 2 Diabetic and Normal Rats. Food Chem. Toxicol. 2020, 135, 1–8. DOI: 10.1016/j.fct.2019.110937.