ABSTRACT
A new method based on solid-phase extraction (SPE) combined with headspace solid-phase microextraction (HS-SPME) was established for the determination of terpenes in Baijiu. Terpenes were concentrated on the C18 solid-phase extraction column, and an additional enrichment of the terpenes was performed using HS-SPME for fillers of the C18 solid-phase extraction column. The conditions of the SPE and SPME were optimized, and the optimal conditions were using the Thermo C18 extraction column, elution water of 1 mL, an extraction temperature of 70°C, and an extraction time of 50 min. Under these optimal conditions, four terpenes were randomly selected to verify the analytical method in terms of linearity, the limit of detection, the limit of quantification, precision, and accuracy. The results showed that the SPE-HS-SPME method has high sensitivity and a high recovery rate, which was also accurate and reliable in the quantitative analysis. It is a feasible new method for the detection of terpene substances in Baijiu.
Introduction
Baijiu is a special distilled liquor in China, and ranks as one of the top six distilled liquor in the world, along with brandy, whiskey, vodka, gin, and rum .[Citation1] The traditional process of brewing Baijiu comprises Jiuqu preparation, substrate hydrolysis, fermentation, solid-state distillation, aging, and blending .[Citation2] Baijiu is usually brewed from sorghum alone, but can also be brewed from a combination of corn, rice, wheat, peas, millet, and sorghum. The grains are mixed with Jiuqu to saccharify and ferment under solid-state conditions, and the fermented mixture is distilled to produce the base liquor of Baijiu .[Citation3] Baijiu is mainly composed of water and alcohol solution, accounting for 98% (v/v), and the rest of the ingredients are trace flavor compounds that can determine the flavor type of Baijiu .[Citation4] Baijiu can be divided into three major categories, namely, Jiangxiangxing Baijiu, Nongxiangxing Baijiu, Qingxiangxing Baijiu, and nine minor categories of Jianxiangxing Baijiu, namely Fengxiangxing Baijiu, Mixiangxing Baijiu, Dongxiangxing Baijiu, Zhimaxiangxing Baijiu, Texiangxing Baijiu, Chixiangxing Baijiu, Laobaiganxiangxing Baijiu, and Fuyuxiangxing Baijiu. To date, 510 esters, 249 alcohols, 140 acids, 18 lactones, 102 aldehydes, 160 ketones, 48 acetals, 82 sulfur compounds, 155 nitrogen compounds, 138 heterocycles, 170 aromatic compounds, 84 hydrocarbons, 104 terpenes, and 60 others have been reported in Baijiu .[Citation5]
Terpene compounds are a class of natural unsaturated hydrocarbons derived from the polymerization of two or more isoprene molecules; there are many types of terpenoids, including chain, ring, olefinic bonds with different degrees of saturation, and oxygenated compounds. These oxygenated derivatives include alcohols, aldehydes, ketones, carboxylic acids, and esters. Terpene compounds can be structurally decomposed into isoprene (C5H8) residues and classified as monoterpenes (10 carbons), sesquiterpenes (15 carbons), diterpenes (20 carbons), triterpenes (30 carbons), and tetraterpenes or carotenes (40 carbons) .[Citation6] Monoterpenes are derived from geranyl pyrophosphate[Citation7] and contain two isoprene units. A representative example of monoterpenes is limonene. Sesquiterpenes are derived from musk farnesyl diphosphate[Citation7,Citation8] and contain three isoprene units. The representative examples of sesquiterpenes are caryophyllene[Citation9] and farnesol .[Citation10] Monoterpenes and sesquiterpenes are common constituents of plant scents and essential oils .[Citation11] Diterpenes are derived from geranylgeranyl pyrophosphate,[Citation7] and contain four isoprene units. Representative examples of diterpenes are paclitaxel[Citation12] and rographolide .[Citation13] Triterpenes contain six isoprene units that can be condensed from two molecules of farnesyl pyrophosphate,[Citation7] and squalene is the most common triterpenoid .[Citation14] Tetraterpenes contain eight isoprene units that can be condensed from two molecules of geranylgeranyl pyrophosphate[Citation7]; carotenoids are an important representative example of tetraterpenoid .[Citation15]
Terpene compounds are widely found in nature, especially in many plants[Citation16–18] and edible plant fruits or their peels[Citation19]; they usually exist in the form of free glycosides and glycosides. Terpene compounds play a variety of roles in nature. Terpene compounds can be used not only as protective compounds against various organisms, but also as a medium of communication between species .[Citation8] They have antiviral, antiproliferative, antioxidant, anxiolytic, antihypertensive, and anti-inflammatory properties .[Citation20–22] In Baijiu, terpene compounds can improve the flavor of wine and relieve alcohol damage .[Citation23,Citation24] Therefore, the detection of terpene compounds in Baijiu is particularly important. It will help the further exploration of the terpene compounds in Baijiu, and the discovery of the several physiological activities that terpene compounds show as health factors in Baijiu. In addition, the detection of terpene compounds will lay a foundation for studying the synthesis pathways of terpene compounds.
Currently, research on terpene compounds mainly focuses on wine and other fermented fruit wines. Barbera et al.[Citation25] studied 4 basic terpene alcohols of 15 different Sicilian musk wines, which were produced in different years, and found that the number of terpene compounds decreased with aging, thus reducing the quality characteristics of these wines. Muñoz Redondo et al.[Citation26] monitored 35 commercial sparkling wines from different grape varieties, different geographic regions, and different ages, and analyzed the effects of aging factors, grape varieties, and origin on terpene content. Qi et al.[Citation27] found that in the production of kiwi wine, skin immersion could produce a good characteristic aroma, resulting in a significant increase in terpene compounds. Hodel et al.[Citation28] explored the behavior of common volatile terpene compounds in the distillation of gin. The concentration values of 10 typical terpenes in the distillate of gin were measured during distillation, and the effects of different distillation equipment configurations and distillation operations on the concentration of terpenes in the distillate were observed. In a study analyzing the ability of Hanseniaspora osmophila to produce characteristic cider in pure fermentation and mixed fermentation with Torulaspora quercuum, Wei et al.[Citation29] found that terpene compounds contributed significantly to the sensory description of the mild and fruity aroma of cider. Although now wine, sparkling wine, kiwi fruit wine, gin, and terpenoids in cider, as well as the influencing factors of terpenoids in alcohol (e.g., brewing raw materials, brewing method, distillation equipment and operation, and the brewing time) are studied, reports of analysis of terpene compounds in liquor are relatively few.
In the current research, gas chromatography (GC) as a powerful instrumental analysis method with high resolution and sensitivity can identify a large number of analytes. It is the most commonly used method to analyze the matrix of alcoholic beverages. In addition, GC coupled with different detectors has been applied to a wide range of alcohol products. The qualitative and quantitative methods for the determination of terpene compounds in alcoholic beverages are solid-phase extraction (SPE),[Citation30] headspace solid-phase microextraction (HS-SPME),[Citation31] and liquid-liquid extraction (LLE),[Citation32] which are combined with gas chromatography-mass spectrometry (GC-MS) or gas chromatography-tandem mass spectrometry (GC-MS-MS). Mariusz and Henryk[Citation33] established a new method named SPE-SPME to determine the content of terpene compounds in wine; the SPE column was used for separating polar (bound) and nonpolar (free) terpenes in wine, and then SPME was used for SPE. The results showed that the SPE-SPME method performed more effectively than the SPE method and had great potential in the analysis of terpene compounds.
Owing to the low concentration of terpene compounds in Baijiu, it is difficult to use traditional methods to extract terpene compounds completely. The SPE method combined with the HS-SPME method was developed and used to extract the terpene compounds in Baijiu in our study. The influencing factors of SPE (type of extraction column and volume of elution water) and HS-SPME (extraction temperature and extraction time) were investigated and optimized. The proposed method was used to identify and quantify the terpene compounds in Laobaiganxiangxing Baijiu samples. Based on this, qualitative analysis of Laobaiganxiangxing Baijiu samples was conducted using this method, and the possibility of quantifying terpene compounds in Baijiu was evaluated using this method.
Materials and methods
Chemicals
The standards of squalene (≥98%), caryophyllene (≥80.0%), limonene (>95%), and p-Cymene (99%) were purchased from Sigma-Aldrich. Methanol (HPLC grade) and ethanol (HPLC grade) were purchased from Sigma-Aldrich (Pozna´n, Poland) and the SPME fibers (50/30 μm CAR/DVB/PDMS) were purchased from Supelco (Pozna´n, Poland). Ultrapure water was obtained from the system of Thermo Scientific Barnstead (Thermo Fisher Scientific, USA).
Samples
Laobaiganxiangxing Baijiu was purchased from Hengshui Laobaigan Ltd., Inc. (Hebei). The Baijiu sample SZ was purchased from Shanzhuanglaojiu Ltd., Inc. (Hebei). LZ was purchased from Luzhou Laojiao Ltd., Inc. (Sichuan), FT was purchased from Maotai Ltd., Inc. (Guizhou), GT was purchased from Guotai Ltd., Inc. (Guizhou), YX was purchased from Yulinquan Ltd., Inc. (Yunnan), and CX Baijiu was purchased from Jiangjin Ltd. Inc. (Chongqing).
SPE method
The SPE column was first activated with 25 mL of methanol, washed with 12 mL of water to remove the methanol, and then 50 mL of Baijiu was added to the pretreated C18 SPE column. After the sample has passed through the column, the column was cleaned with ultrapure water. The residual Baijiu and water in the SPE column fillers were discharged through pressurization. The residue in the gaskets and fillers were removed into a 20 mL headspace bottle, which was sealed with the headspace bottle cap, waiting for the enrichment of HS-SPME. In this part, taking the number of terpene compounds as the index, the SPE column with the highest extraction capacity for terpene compounds was determined, and the volume of elution water was optimized.
HS-SPME method
The headspace bottle with gaskets and fillers was equilibrated at 70°C for 10 min, and the SPME fiber was exposed in the headspace bottle at 70°C for 50 min. The extraction conditions of the HS-SPME method were investigated in this experiment. Taking the total peak area of terpene compounds as the index, the extraction time and extraction temperature were optimized. The schematic of the extraction process is shown in the graphical abstract.
GC-MS conditions
A Thermo Trace 1300 (Thermo Fisher Scientific, USA) equipped with the ISQ MS (Thermo Fisher Scientific, USA) was used to analyze the target compound. The samples were injected with a TriPlus RSH automatic injector (Thermo Fisher Scientific, USA). The SPME fiber was placed at the GC injection port and desorbed at 250°C for 4 min in a splitless mode. Helium (purity > 99.999%) was used as the carrier gas; the flow rate was 1 mL/min. The compounds were separated on an HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA). The temperature program is shown in , and described as follows: 40°C for 1 min, increased to 200°C at 4°C/min, followed by an increase of 20°C/min to 250°C, and kept for 10 min. The temperature of the transfer line and ion source was 250°C, and the temperature of the quadrupole was 150°C. The MS operated in electron ionization mode at 70 eV. The mass scan range was 50–400 atomic mass units.
Table 1. Temperature program.
Method validation
The analytical method was verified from the aspects of linearity, the limit of detection, the limit of quantification, precision, and accuracy. Five standard solutions of different analytes with increasing concentrations were prepared. Under optimized conditions, a standard curve was established using selective ion scanning (SIM) with the peak area (y) of the substance to be quantified as the ordinate and the mass concentration (x) of the substance to be quantified as the abscissa, and the slope of the correction curve was determined to estimate the sensitivity.
The mass concentration with a signal-to-noise ratio of 3:1 was selected as the limit of detection, and the mass concentration with a signal-to-noise ratio of 10:1 was selected as the limit of quantification. A known volume of the standard and internal standard solutions were added to the pretreated sample to prepare the spiked sample. The recovery rate of each analyte in Baijiu was estimated.
Detection of samples
To investigate the generalizability of the method, the terpenes of three typical flavors of Baijiu, namely Nongxiangxing Baijiu (LZ and SZ), Jiangxiangxing Baijiu (FT and GT), and Qingxiangxing Baijiu (CX and YX), were determined based on the optimized conditions.
Results and discussion
Optimization of SPE
Solid-phase microextraction has a good detection effect on most trace substances, and considering the competitive adsorption effect of a large amount of ethanol in Baijiu on the SPME fibers, this method was applied to the enrichment of terpene compounds in Baijiu and the separation of target compounds from ethanol. The SPE column and the volume of the elution water were optimized to obtain the best extraction conditions.
Selection of SPE column: Four types of C18 SPE columns with a solid phase content of 500 mg/6 mL produced by different manufacturers were tested and compared in this study, and the Laobaiganxiangxing Baijiu was selected as the sample for condition optimization. Under the same extraction conditions, taking the number of terpene compounds as an index, the packing of four SPE columns was investigated. The result is shown in .
Figure 1. Comparison of terpene extraction capacity of SPE extraction columns from different manufacturers.
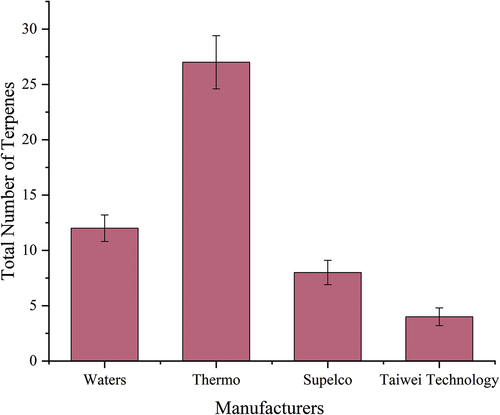
Owing to the differences in fillers and production processes of SPE columns produced by different manufacturers, the extraction effects of different SPE columns on terpene compounds are very different. More than 25 terpenes were detected from the Laobaiganxiangxing Baijiu using the Thermo C18 SPE column. The extraction efficiency of terpene compounds was much higher than that of the C18 SPE columns of the other three manufacturers. Thus, the Thermo C18 SPE column was chosen for further optimization and validation in our experiment. In the study of the precursors of fragrances extracted from grapes, Ibarz et al.[Citation34] also found that C18 resin was more suitable for the determination of terpenes and norisoprenes in grape musts and skins. Therefore, in the detection, the SPE column needs to be selected according to the characteristics of the sample and the detected compound, so that the detection can achieve better results.
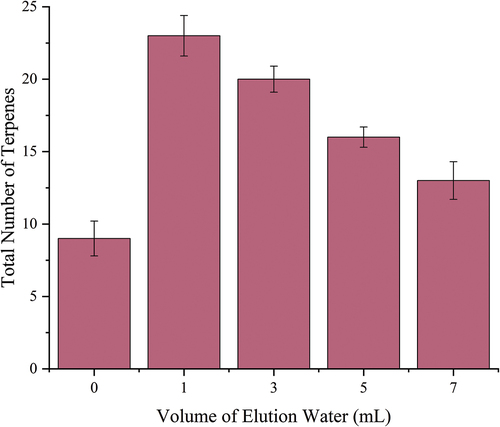
Investigation of elution water volume: After Baijiu was passed through the column, a certain volume of solvent was used to elute the impurities. This process can effectively remove residual ethanol and further exclude the influence of ethanol on later detection. The volume of elution water (1 mL, 3 mL, 5 mL, and 7 mL) was investigated. The result is shown in . Since the adsorption capacity of different compounds to the solid phase is different, the elution volume will affect the types of terpene compounds in the solid phase. In our experiment, as the volume of the ultrapure water increased, the solid phase retained the types of terpene compounds and produced a corresponding loss, and the amount decreased accordingly. The solid phase was eluted without ultrapure water, considering that its residual impurities may have an effect on the extraction and adsorption of SPME, but the result is not satisfactory. Therefore, 1 mL is the best elution volume to elute the terpene compounds in the SPE column.
Optimization of HS-SPME
Based on the enrichment of target compounds using SPE, the packing material of the SPE column was extracted using HS-SPME. After determining the optimal conditions for SPE, the extraction temperature and extraction time were selected to optimize the extraction conditions.
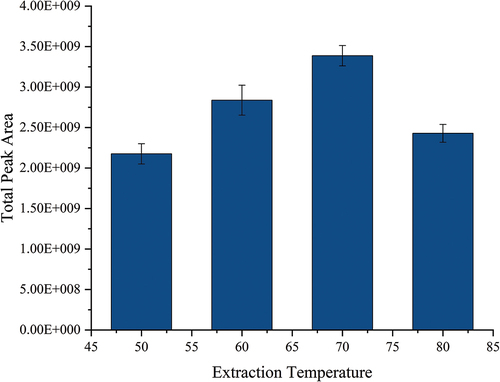
Investigation of the extraction temperature: Extraction temperatures of 50°C, 60°C, 70°C, and 80°C were tested. The peak area of the extracted terpene compounds is compared in . As the results show, when the temperature was between 50 and 70°C, with the increase in extraction temperature, the release rate of the terpene compounds in the solid-phase fillers increased, and more target compounds were released into the headspace, which promoted the adsorption of the SPME fibers, and the peak area of terpene compounds gradually increased. When the extraction temperature reached 70°C, the peak area of the terpene compounds reached the maximum. We consider this temperature as a critical point because once it is passed, the extraction rate does not increase but starts to decrease when the temperature is increased again. For this phenomenon, Câmara et al.[Citation29] suggested that temperature has distinctive effects on terpene compounds. More analytes will be released into the headspace when the temperature is increased, and the extraction efficiency will be improved because of the enhanced mass transfer. However, due to thermodynamic reasons, they have a negative effect on analyte adsorption by the coating; the extraction of the fiber coating does not increase but decreases when the temperature continues to increase. Thus, 70°C was selected as the optimal extraction temperature in our experiment.
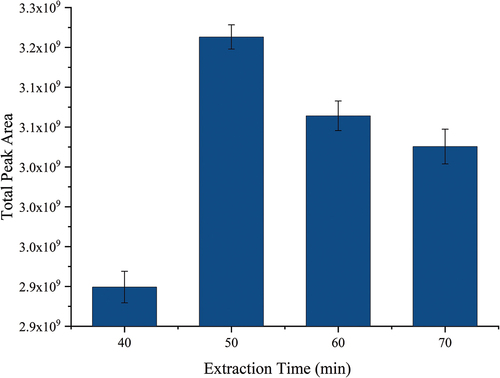
Investigation of the extraction time: Different extraction times from 30–60 min were examined and compared. shows the relationship between different extraction times and the total peak area of terpene compounds. As shown in , with the extension of extraction time, the concentration of analytes in the fiber increased, and the extraction number of terpenes increased gradually until equilibrium was reached. When the extraction time was 50 min, the concentration of analytes in the extracted fiber reached equilibrium, and the peak area of terpenes reached the maximum. When the extraction time was prolonged, the concentration of analytes in the fiber decreased, and the peak area of terpenes also decreased. Therefore, the extraction time of 50 min is more suitable. In summary, the optimal combination was determined to be Thermo C18 SPE column, 1 mL elution solvent volume, extraction temperature of 70°C, and adsorption time of 50 min. Under the optimized extraction conditions, the quantity and response intensity of terpenoids obtained are higher.
Based on the optimization conditions, a total of 23 terpene compounds were detected in Laobaiganxiangxing Baijiu; the results are shown in . Among them, α-Gurjunene had the highest content, accounting for 24.35% of the total content of terpenes, followed by caryophyllene, accounting for 18.41% of the total content. Squalene and α-Bulnesene accounted for 12.05% and 9.09% of the total content of terpenes, respectively.
Table 2. Terpenes of Laobaiganxiangxing baijiu.
Method validation
We randomly selected 4 substances from the 23 terpene compounds, and adopted the selective ion scanning mode. A standard curve was established, and the method was comprehensively evaluated through the linear range and correlation coefficient. Finally, the detection limit and quantification limit, the relative standard deviation, and the recovery rate were determined. The results are shown in .
Table 3. Regression equation, correlation coefficient and recovery of 4 components.
Five gradients of functional components were mixed with standard solutions for GC-MS analysis, and linear regression was performed with mass concentration-peak area to draw a standard curve. The R2 of the four functional components in their respective linear ranges were from 0.990 (p-Cymene) to 0.994 (d-Limonene); if all are greater than 0.9900, the linear relationship is good. The detection limit and quantification limit were calculated at 3 times and 10 times of signal-to-noise ratio respectively. Estimated from the lowest detectable peak, when the signal-to-noise ratio is 3, the limit of detection is from 0.24 μg/L for caryophyllene to 6.69 μg/L for squalene; when the signal-to-noise ratio is 10, the limit of quantification is 0.75 μg/L for caryophyllene to 21.84 μg/L for squalene. The relative standard deviations of the samples were calculated using this method, and the relative standard deviations of the four functional components are all not higher than 6.00%. By adding nearly the same number of standard products, the recovery rate of addition is from 71.69% for caryophyllene to 120.23% for squalene, which proves that the method has good precision.
Detection of samples
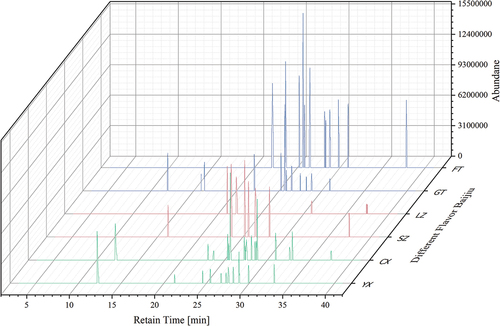
The optimized SPE-HS-SPME-GC-MS method was used to determine the terpene compounds in the Baijiu samples. The chromatograms of the terpenoids of the six Baijiu samples are shown in . A total of 11 terpenoids were detected in the two Jiangxiangxing Baijiu samples; geranylacetone and phytone were detected in both SZ and LZ. A total of 21 terpenoids were detected in two types of Jiangxiangxing Baijiu and six substances; among them, trans, trans-2, 4-decadienal, elemene, copaene, geranyl isovalerate, cedrol, and geranylacetone were detected in both FT and GT. A total of 19 terpenoids were detected in YX and CX; D-limonene, α-acorenol, geranyl isovalerate, (+)-gamma-gurjunene, α-curcumene, α-muurolene, and (-)-isolongifolol were detected in the two Qingxiangxing Baijiu samples. A total of 37 terpenoids were detected in the three typical flavors of Baijiu, while alpha-cedrene, phytanone, geranyl isovalerate, and elemene were detected in all three typical flavors of Baijiu.
The sources of terpenoids in Baijiu may be released from the brewing materials under specific brewing conditions, or the migration of the carbon atoms and rearrangement of the skeleton will form terpenes under the action of suitable microorganisms. This is also the main reason for the existence of terpenes in all types of liquors, such as Jiangxiangxing Baijiu and Nongxiangxing Baijiu .[Citation35] In the study on the mechanism of formation of terpenoids in Qingxiangxing Baijiu, Wu et al.[Citation36] found that brewer’s yeast contributes significantly to the terpenoids in Qingxiangxing Baijiu, and suggested that differences in the structure and chemical composition of the raw material also have a significant effect on the type of terpenoids. This explains the significant differences in the type and content of terpenoids in different flavors of Baijiu.
At present, there are few reports on the detection methods of terpenes in Baijiu. Hu et al.[Citation37] identified 52 terpenes from Dong Flavor Baijiu using HS-SPME-GC-MS. Fan et al.[Citation38] used the LLE-GC-MS method to detect a large number of terpenes in Yanghe Blue Classics Flavor Baijiu, and a total of 30 species were detected. However, report on the detection of terpenoids in Laobaiganxiangxing Baijiu is relatively rare. The SPE-HS-SPME method developed by us detected 23 terpenoids in Laobaiganxiangxing Baijiu, and was also applicable to the detection of terpenoids in other flavors of Baijiu through validation. SPE was used for fractionation; the procedure involves the concentration of extracts to a relatively small volume and subsequent analysis using GC/MS systems. This method combines the advantages of SPME, its selectivity and sensitivity with the standard approach to bound volatiles based on SPE.
Conclusion
A new method for the detection of terpenes in Baijiu was adopted. The SPME of the SPE column packing can be used to qualitatively and quantitatively determine the selected terpenes at a lower concentration. The effects of the type of SPE column, the volume of the elution solvent, the extraction temperature of HS-SPME, and the adsorption time on the quantity and peak area of terpenoids were compared. The optimal combination was determined to be Thermo C18 SPE column, 1 mL elution solvent volume, extraction temperature of 70°C, and adsorption time of 50 min. This optimized method was used to detect 23 terpenes in the Laobaiganxiangxing Baijiu. Four substances were randomly selected to verify the analytical method in terms of linearity, the limit of detection, the limit of quantification, precision, and accuracy, and the results were satisfactory. The method was applied to determine the terpenes of the three typical flavors of Baijiu, and 37 terpenoids were detected, which shows that this is a new and feasible method for detecting terpenoids in Baijiu.
Credit author statement
Minxue Feng: Methodology, Validation, Formal analysis, Investigation, Writing-original draft, Writing-review & editing. Chenyao Li: Investigation. Chao Wang: Supervision, Resources, Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing-original draft, Writing-review & editing. Guojun Zhu: Supervision. Jun Lu: Supervision. Yefu Chen: Supervision. Dongguang Xiao: Supervision. Xuewu Guo: Supervision, Resources, Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing-original draft, Writing-review & editing.
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (No. 2017M611169), the Hebei Province Postdoctoral Research Projects (No. B2018003031) and the Open Foundation of Key Laboratory of Wuliangye-flavor Liquor Solid-state Fermentation, China National Light Industry, 2019JJ013.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data not available due to commercial restrictions.
Additional information
Funding
References
- Zheng, X. W.; Han, B. Z. B. Chinese Liquor: History, Classification and Manufacture. J. Ethnic Foods. 2016, 3(1), 19–25. DOI: 10.1016/j.jef.2016.03.001.
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese Liquor Fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. DOI: 10.1016/j.tifs.2017.02.016.
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66(22), 5425–5432. DOI: 10.1021/acs.jafc.8b00692.
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent Advances in Baijiu Analysis by Chromatography Based technology–A Review. Food Chem. 2020, 324, 126899. DOI: 10.1016/j.foodchem.2020.126899.
- Hong, J.; Tian, W.; Zhao, D. Research Progress of Trace Components in sesame-aroma Type of Baijiu. Food Res. Int. 2020, 137(2), 109695. DOI: 10.1016/j.foodres.2020.109695.
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in Grapes and Wines: Occurrence, Biosynthesis, Functionality, and Influence of Winemaking Processes. Compr. Rev. Food Sci. Food Saf. 2020, 19(1), 247–281. DOI: 10.1111/1541-4337.12516.
- McGarvey, D. J.; Croteau, R. Terpenoid Metabolism. The Plant Cell. 1995, 7(7), 1015. DOI: 10.2307/3870054.
- Yu, F.; Utsumi, R. Diversity, Regulation, and Genetic Manipulation of Plant Mono- and Sesquiterpenoid Biosynthesis. Cell. Mol. Life Sci. 2009, 66(18), 3043–3052. DOI: 10.1007/s00018-009-0066-7.
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical Characterization and Pharmacokinetics Evaluation of β-caryophyllene/β-cyclodextrin Inclusion Complex. Int. J. Pharmaceutics. 2013, 450(1–2), 304–310. DOI: 10.1016/j.ijpharm.2013.04.013.
- Ku, C. M.; Lin, J. Y. Farnesol, a Sesquiterpene Alcohol in Essential Oils, Ameliorates Serum Allergic Antibody Titres and Lipid Profiles in ovalbumin-challenged Mice. Allergologia et Immunopathologia. 2016, 44(2), 149–159. DOI: 10.1016/j.aller.2015.05.009.
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N. S.; Valsamma, R. K.; George, V. Caryophyllene-rich Rhizome Oil of Zingiber Nimmonii from South India: Chemical Characterization and Antimicrobial Activity. Phytochemistry. 2006, 67(22), 2469–2473. DOI: 10.1016/j.phytochem.2006.08.003.
- Malik, S.; Cusidó, R. M.; Mirjalili, M. H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the Anticancer Drug Taxol in Taxus Baccata Suspension Cultures: A Review. Process Biochem. 2011, 46(1), 23–34. DOI: 10.1016/j.procbio.2010.09.004.
- Chao, W. W.; Lin, B. F. Isolation and Identification of Bioactive Compounds in Andrographis Paniculata (Chuanxinlian). Chin.Med. 2010, 5(1), 17-17. DOI: 10.1186/1749-8546-5-17.
- Reddy, L. H.; Couvreur, P. Squalene: A Natural Triterpene for Use in Disease Management and Therapy. Adv. Drug Delivery Rev. 2009, 61(15), 1412–1426. DOI: 10.1016/j.addr.2009.09.005.
- Soukoulis, C.; Bohn, T. A Comprehensive Overview on the micro-and nano-technological Encapsulation Advances for Enhancing the Chemical Stability and Bioavailability of Carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58(1), 1–36. DOI: 10.1080/10408398.2014.971353.
- Mendoza-Poudereux, I.; Muñoz-Bertomeu, J.; Navarro, A.; Arrillaga, I.; Segura, J. Enhanced Levels of s-linalool by Metabolic Engineering of the Terpenoid Pathway in Spike Lavender Leaves. Metab. Eng. 2014, 23, 136–144. DOI: 10.1016/j.ymben.2014.03.003.
- Ahmed, A. A.; Mohamed, A. E. H. H.; Karchesy, J.; Asakawa, Y. Salvidorol, a nor-abietane Diterpene with a Rare Carbon Skeleton and Two Abietane Diterpene Derivatives from Salvia Dorrii. Phytochemistry. 2006, 37(5), 424–428. DOI: 10.1016/j.phytochem.2005.12.009.
- Mahmoud, A. A.; Al-Shihry, S. S.; Son, B. W. Diterpenoid Quinones from Rosemary (Rosmarinus Officinalis L.). Phytochemistry. 2005, 66(14), 1685–1690. DOI: 10.1016/j.phytochem.2005.04.041.
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic Engineering of Yeast, Filamentous Fungi and Bacteria for Terpene Production and Applications in Food Industry. Food Res. Int. 2021, 147(4), 110487. DOI: 10.1016/j.foodres.2021.110487.
- Pardo, E.; Rico, J.; Gil, J. V.; Orejas, M. De Novo Production of Six Key Grape Aroma Monoterpenes by a Geraniol synthase-engineered S.cerevisiae Wine Strain. Microb. Cell Fact. 2015, 14(1), 1–8. DOI: 10.1186/s12934-015-0306-5.
- Chen, Y.; Zhu, Z.; Chen, J.; Zheng, Y.; Limsila, B.; Lu, M.; Gao, T.; Yang, Q.; Chao, F.; Liao, W. Terpenoids from Curcumae Rhizoma: Their Anticancer Effects and Clinical Uses on Combination and versus Drug Therapies. Biomed. Pharmacother. 2021, 138(6), 111350. DOI: 10.1016/j.biopha.2021.111350.
- Fan, W. L.; Xu, Y. Review of Important Functional Compounds Terpenes in Baijiu (Chinese Liquor) (In Chinese). Liquor Making. 2013, 40(6), 11–16. DOI: 10.3969/j.1002-8110.2013.06.008.
- Guo, X. W.; Fan, E. D.; Ma, B. T.; Li, Z. X.; Zhang, Y. H.; Hang, Z. M.; Chen, Y. F.; Xiao, D. G. Recent Progress in Micro Components of Chinese Baijiu (In Chinese). Food Sci. 2020, 41(11), 267–276. DOI: 10.7506/spkx1002-6630-20190704-065.
- Hortelano, S.; González-Cofrade, L.; Cuadrado, I.; de Las Heras, B. Current Status of Terpenoids as Inflammasome Inhibitors. Biochem. Pharmacol. 2019, 172, 113739. DOI: 10.1016/j.bcp.2019.113739.
- Barbera, D.; Avellone, G.; Filizzola, F.; Monte, L. G.; Catanzaro, P.; Agozzino, P. Determination of Terpene Alcohols in Sicilian Muscat Wines by hs-spme-gc-ms. Nat. Prod. Res. 2013, 27(6), 541–547. DOI: 10.1080/14786419.2012.676553.
- Muñoz-Redondo, J. M.; Ruiz-Moreno, M. J.; Puertas, B.; Cantos-Villar, E.; Moreno-Rojas, J. M. Multivariate Optimization of Headspace solid-phase Microextraction Coupled to Gas chromatography-mass Spectrometry for the Analysis of Terpenoids in Sparkling Wines. Talanta. 2020, 208, 120483. DOI: 10.1016/j.talanta.2019.120483.
- Qi, Y.; Liu, M.; Yang, K.; Fan, M. Effect of Skin Maceration Treatment on Aroma Profiles of Kiwi Wines Elaborated with Actinidia Deliciosa “Xuxiang” and A. Chinensis “Hort16A.” J. AOAC Int. 2019, 102(2), 683–685. DOI: 10.5740/jaoacint.18-0290.
- Hodel, J.; O’Donovan, T.; Hill, A. E. Influence of Still Design and Modelling of the Behaviour of Volatile Terpenes in an Artificial Model Gin. Food Bioprod. Process. 2021, 129, 46–64. DOI: 10.1016/j.fbp.2021.07.002.
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical Composition, Sensorial Properties, and aroma-active Compounds of Ciders Fermented with Hanseniaspora Osmophila and Torulaspora Quercuum in Co- and Sequential Fermentations. Food Chem. 2020, 306, 125623-125623. DOI: 10.1016/j.foodchem.2019.125623.
- Carballeira Lois, L.; Cortés Diéguez, S.; Gil de la Peña, M. L.; Fernández Gómez, E. SPE-GC Determination of Aromatic Compounds in Two Varieties of White Grape during Ripening. Chromatographia. 2001, 53(S1), S350–S355. DOI: 10.1007/BF02490355.
- Câmara, J. S.; Alves, M. A.; Marques, J. C. Development of Headspace solid-phase microextraction-gas chromatography–mass Spectrometry Methodology for Analysis of Terpenoids in Madeira Wines - Sciencedirect. Anal. Chim. Acta. 2006, 555(2), 191–200. DOI: 10.1016/j.aca.2005.09.001.
- Hernanz, D.; Gallo, V.; Recamales, A. F.; Meléndez-Martínez, A. J.; Heredia, F. J. Comparison of the Effectiveness of solid-phase and ultrasound-mediated liquid-liquid Extractions to Determine the Volatile Compounds of Wine. Talanta. 2008, 76(4), 929–935. DOI: 10.1016/j.talanta.2008.04.049.
- Dziadas, M.; Jeleń, H. H. Analysis of Terpenes in White Wines Using SPE–SPME–GC/MS Approach. Anal. Chim. Acta. 2010, 677(1), 43–49. DOI: 10.1016/j.aca.2010.06.035.
- Ibarz, M. J.; Ferreira, V.; Hernández-Orte, P.; Loscos, N.; Cacho, J. Optimization and Evaluation of a Procedure for the Gas chromatographic-mass Spectrometric Analysis of the Aromas Generated by Fast Acid Hydrolysis of Flavor Precursors Extracted from Grapes. J. Chromatogr. A. 2006, 1116(1–2), 217–229. DOI: 10.1016/j.chroma.2006.03.020.
- Zhang, C. S.; Yang, R. G.; Zhu, L. Y.; Wu, S. M. Strategies to Improve the Yield of Terpenoids Synthesized by Microorganisms (In Chinese). Chin. J. Biol. Eng. 2017, 37(1), 97–103.
- Wu, Q.; Zhu, W.; Wang, W.; Xu, Y. Effect of Yeast Species on the Terpenoids Profile of Chinese light-style Liquor. Food Chem. 2015, 168, 390–395. DOI: 10.1016/j.foodchem.2014.07.069.
- Hu, G. Y.; Fan, W. L.; Xu, Y.; Jia, Q. Y.; Ran, X. H. Research on Terpenoids in Dongjiu (In Chinese). Liquor-Making Sci. Technol. 2011, 7, 29–33. doi: 10.13746/j.njkj.2011.07.024.
- Fan, W. L.; Xu, Y.; Yang, Y. D.; Zhang, Y. B.; Zhu, G. S.; Zhou, X. H.; Chen, X. Volatile Compounds of Supple and Mellow Flavor Type in Yanghe’s Lansejidian Liquors Detected by liquid-liquid Extraction Coupled with Fractionation (In Chinese). Liqour Making. 2012, 39(1), 21–29. DOI: 10.3969/j.1002-8110.2012.01.012.