ABSTRACT
The current research was conducted to investigate and compare the role of functional foods such as probiotics and prebiotics alone or in combinations on the markers of liver damage, oxidative stress markers and inflammatory markers in the nonalcoholic fatty liver disease (NAFLD) induced rats. According to the results, G3, G4 and G6 gained significant weight at the end of the study. Moreover, the weights of different organs (liver, Spleen and kidney) revealed that G1 (control negative) fed on a standard diet gained the lowest liver weight, G2 (control positive) fed on high fat diet (HFD) gained maximum weight (9.63 ± 0.46 g). In addition, the symbiotic group (G8) lowered the liver organ weight (6.77 ± 0.20 g), indicating that HFD induced fat deposition in body and other body organs. Furthermore, results of serum liver function markers were concerned, Maximum reduction of Alanine aminotransferase (ALT) was found among the G8 (Probiotics, GOS and Inulin) and G4 (GOS). Aspartate aminotransferase (AST) was significantly reduced among all the treatments compared to the control positive. Alkaline phosphate (ALP) was reduced among G5, G6 and G7. Gamma-glutamyl transferase (GGT) remained unaffected during the trial. As far as the results of tumor necrotic factor (TNF) were concerned, probiotics and GOS groups had a significant reduction in the levels of TNF after 19 weeks of study. Upon the prebiotics and probiotics therapy, a strong association was found with the hepatic inflammation at various degrees and hepatic and portal inflammation was reduced among the symbiotic groups significantly (Chi-square value = 49.04**; P-value = 0.000; ** = Highly significant (P < .01). Overall histopathological analysis revealed reduction in the fatty vacuoles and inflammation in the treatment groups compared to control in hepatocytes. The progression of fibrosis was also reduced among the treatment groups. Conclusively, the biomarkers of NAFLD were reduced among the treatment groups compared to the control positive.
Introduction
Liver, one of the most vital organs, functions as a center of metabolism of nutrients such as carbohydrates, proteins and lipids and excretion of waste metabolites. Liver disease is a leading cause of mortality worldwide and constitutes a wide range of diseases with varied or unknown etiologies.[Citation1] There are many factors responsible for the toxicity of the liver, such as, viral infections, certain drugs, alcohol, toxic industrial chemicals and autoimmune disease.[Citation2] Today’s growing problem of the liver is a fatty liver disease that has turned into a universal disorder. It is a progressive disease that leads to more severe forms of liver injuries. Normally liver contains some fat but if it exceeds 5% – 10% of the total weight then it is named as fatty liver (steatosis).[Citation3] If the fat deposition is followed by necro-inflammatory changes in the liver, then it is named steatohepatitis that leads to fibrosis, cirrhosis and in some cases, liver carcinoma.[Citation4]
NAFLD is a very common liver problem affecting 20–30% of the general population of the Western world whereas in Pakistan the prevalence has reached 14%.[Citation5] The key factors responsible for this chronic liver disease that are most commonly becoming a serious public health concern are obesity, diabetes mellitus (DM type 2), cardiovascular disease and environmental factors such sedentary lifestyle with unhealthy hypercaloric dietary patterns.[Citation6] NAFLD is often associated with dyslipidemia, central obesity and hyperglycemia commonly categorized as metabolic syndrome or insulin resistance syndrome.[Citation7] The characteristic features of this disease include an elevation in liver enzyme levels and fat accumulation in hepatocytes followed by necrosis of liver that is similar to alcoholic hepatitis but not due to heavy alcohol consumption.[Citation8]
Currently, limited treatment options are available to cure this disease including an active lifestyle to lose weight as the first line strategy and there is no authentic pharmacological drug that persists for NAFLD. In current era, dietary restrictions and lifestyle changes are approved medications available for NAFLD therapy.[Citation9] Furthermore, functional foods and certain therapeutic agents are being evaluated for the management of this entity. The human gut microflora plays a significant role in health management and normal hepatocyte function by modulating the alterations to the type and number of gut microbiota. Now food substances that bring about a healthy effect on the composition of gut flora including additives, prebiotics and probiotics have achieved more interest.[Citation10] Probiotics are bacteria that maintain the balance of the gut microbiota. The most widely used probiotics include the species of lactobacilli, streptococci commonly categorized as LAB and bifidobacteria which are consumed by adding to fermented milk products or ingested in lyophilized forms.[Citation11] The other known probiotic strains include Bifidobacterium, Lactobacillus, Bacillus, Enterococcus, Escherichia, Streptococcus, and some fungal Saccharomyces.[Citation12] Probiotic intervention studies are claimed to produce calamity in the risk factors associated with liver infection. Being a noninvasive, inexpensive and safe alternate strategy that can improve different types of liver disorders, while reducing the pathophysiological symptoms. Moreover, probiotics intervention has no side effects as compared to other treatment options such as the use of certain drugs.[Citation13]
Prebiotics is any food ingredient that cannot be digested and affects the host in a favorable way by selectively motivating the activity or growth of one or a fixed number of microbes in the large intestine.[Citation14] Most dietary fibers particularly soluble fibers have prebiotic activity; however, only two groups of nutritional compounds i-e inulin-type and galacto-oligosaccharides (GOS) are identified as prebiotics.[Citation15] Prebiotics reduce the risk factors for colorectal diseases by restraining the growth of adenomas and carcinomas in the colon and by positively affecting the immune system.[Citation16,Citation17] There are several other claims regarding the health benefits of prebiotics including diarrhea suppression, constipation relief, reduction of risk of osteoporosis and cardiovascular disease due to insulin resistance and dyslipidemia, improvement in glucose tolerance, reduced absorption of cholesterol and fat by binding the bile acids, delayed gastric emptying and reduction of obesity and risk of type II diabetes. The term symbiotic denotes the combination product of probiotics with specific prebiotics that result in a healthy microflora population in the gut. Examples of symbiotic combinations include the Bifidobacterium-inulin and Bifidobacterium fructooligosaccharides etc. Symbiotics promote the viability of probiotics and impart specific health benefits.[Citation18]
The present study was carried out to evaluate the preventive role of prebiotics and probiotics is a safe, inexpensive and noninvasive strategy in the optimal management of nonalcoholic fatty liver disease by exploring the effect of acute supplementation with multi-strain probiotics alone or in combination with prebiotics on the markers of liver injury, plasma levels of cytokines, parameters of exudative stress and the markers of lipid peroxidation in the model study.
Material and method
Study design
This study was carried out at Department of Food Science & Human Nutrition, University of Veterinary and Animal Sciences, Lahore. The study protocol was approved by the UVAS animal ethics committee. The rats were kept in collective cages under controlled conditions of temperature (24 ± 2°C), light (12 h light/dark cycle) and relative humidity (50%). The animals were fed basal diet and water. We included 96 male Wistar rats (Weight: 125–150 gm, age: six weeks) into eight groups (G1-G8) with each group containing 12 rats (n = 12). In this study, a probiotic preparation, Bio-Kult, contains 14 different strains (Bifidobacterium bifidum, B. longum, B. breve, B. infantis, Lactobacillus acidophilus, L. casei, L. delbrueckii ssp. bulgaricus, L. plantarum, L helveticus, L. rhamnosus, L. salivarius, Lactococcus lactis ssp. lactis, Streptococcus thermophilus, Bacillus subtilis) of live bacteria was used. Minimum 2 billion live microorganisms were present per capsule (2 billion CFU/capsule).
The study duration of 21 weeks was divided into two periods: 1) induction of NAFLD (weeks 1–7), and 2) treatment phase (weeks 8–21). During the NAFLD induction phase (week 1–7), G1 (control negative) fed the standard rat chow while all the other groups (G2-G8) fed high fat-high sugar diet. In the treatment phase (weeks 8–21), all groups were given different treatments. G1 (control negative) fed standard rat chow, G2 (control positive) fed high fat diet (cholesterol raising agents cholic acid 0.5%) and high sugar diet (high sucrose diet 40% sucrose) but no treatment was given. Groups G3 to G8 fed high fat-high sugar with different treatment regimens. Probiotics treatment was given to G3, prebiotics GOS was given to G4 and G5 was supplemented with prebiotics inulin. Combinations of probiotics with prebiotics inulin and prebiotics GOS fed to G6 and G7 respectively while G8 was given all the treatment combinations of probiotics + prebiotics inulin + prebiotics GOS.
The dose of prebiotics was calculated according to the human equivalent dose (HED) equation: HED = animal dose in mg/kg × (animal weight in Kg/ human weight in kg)0.33(Reagan-Shaw et al. 2008). The tube feeding method was used to orally feed prebiotics by adding in saline water for 8 weeks based on each group’s mean body weight at the start of the intervention and was updated on weekly basis according to changes in the mean body weight of the groups. The lyophilized probiotics was given by adding in the drinking water. The concentration of probiotics blend added to the saline water was 1 × 108−9 CFU/ml, representing a dose per kg of body mass equivalent for a human. In order to homogenize the condition of the experiment, groups G1 (Control negative) and G2 (Control positive) were orally given a similar amount of water.
Blood and tissue samples collection
At the end of the intervention, animals of all groups were sacrificed in the overnight fasting state by exsanguination under urethane anesthesia (45 mg/kg body weight). Blood samples were collected from the heart through cardiac puncture of anesthetized animals and were poured into heparinized tubes (2 U/µL). After collection samples were stored at a very low temperature (−80°C) for further analysis. Liver tissue samples from both hepatic lobes were taken for histopathological analysis.
Organs to body weight ratio
After sacrificing the rats, different organs including heart, kidney, liver, spleen and small intestine were collected and weighed. The small intestine length was also measured after collection. These parameters were used to assess the effect of the HFHS diet on different anatomical parameters of the rats fed on different treatments compared to the control.
Markers of liver damage
The serum samples were analyzed for aspartate aminotransferase (AST), alanine aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) using kits prepared by Analyticon, Lichtenfels, Germany.[Citation19]
Inflammatory markers
TNF-α levels were measured by homogenizing the portions of liver tissues using the colorimetric commercial rat kit (Cat. No. RT00, Quantikine Rat TNF-α/immunoassay, R&D System) following the manufacturer’s instructions.
Histopathological analysis
Neutral buffered formalin
After dissection of the rats, liver was separated and stored in neutral buffered formalin (10%) and formalin was prepared using the below-mentioned method and chemicals shown in . According to the method of Bancroft and Gamble,[Citation20] all the chemicals were taken in above-mentioned proportions and mixed to make neutral buffered formalin.
Table 1. Method for formation of neutral buffered formalin.
Dehydration
Afterward the fixed tissues were placed in different concentrations of alcohol in a below-mentioned manner . For the current purpose, the dehydration of tissues was done by following the method of Bancroft and Gamble .[Citation20]
Table 2. Method for tissue dehydration.
Clearing
Afterward pure xylene was used to clear the alcohol and form the tissues using the method of Bancroft and Gamble .[Citation20] For this purpose, pure xylene was applied for three hours on tissues to clear the samples.
Impregnation with paraffin wax
Tissue samples were placed in paraffin wax after clearing with pure xylene using below-mentioned method. The temperature of paraffin wax was maintained between the range of 50–58°C for six hrs.[Citation20]
Sectioning
Afterward paraffin wax fixed tissues were cut using a microtome with 5 µm thick blade. After slight warming with in water bath (58°C) tissues were placed on thin transparent clean glass slides covered with thin layer of egg albumen.[Citation20]
Hematoxylin and eosin staining
Staining of prepared slides was done using the below-mentioned methods and chemicals ().[Citation20] .
Table 3. Method for Hematoxylin and Eosin staining.
Mounting
The drop of mounting material (DPX) was placed on every stained tissue section and a cover slip was applied. Afterward, microscopy was performed to observe changes between different groups of trial at 100x.[Citation20]
Markers of lipid peroxidation
Liver malondialdehyde assay
The quantitative measurement of lipid peroxidation was assessed following the method of Wills[Citation21] .
Estimation of superoxide dismutase (SOD) activity
The estimation of SOD activity was performed according to the method of Dieterich et al.[Citation22] In brief, after removing, fresh liver samples were immediately homogenized with 50 mM of sodium phosphate buffer at 37°C (1 ml, pH 7.8) and 1 mM of diethylenetriamine Penta acetic acid (DTPA). By adding pyrogallol acid (0.2 mM/L, 37°C for 3 min) the reaction was initiated and the absorbance was measured at 420 nm. SOD activity was expressed as U/mg of protein, where one unit (1 U) of the enzyme was defined as the amount of SOD required to inhibit the rate of oxidation of pyrogallol by 50%.
Evaluation of reduced glutathione (GSH) levels
The estimation of GSH in the liver samples was carried out following the method described by Jollow et al. .[Citation23]
Ethical consideration
All the methods and procedures were approved by the animal ethical committee before conducting the trial and an ethical certificate was issued.
Statistical analysis
Data obtained from biochemical tests were analyzed using statistical package for social sciences (SPSS) version 25.0 and one-way analysis of variance (ANOVA) was used under Complete Randomized Design (CRD).[Citation24] while data obtained from the body weight, feed intake and water intake was assessed through repeated measure ANOVA. The significantly different parameters and means difference was tested through LSD and Tukey test and level of significance was defined as P ≤ .05.
Results and discussion
Effect on body weight
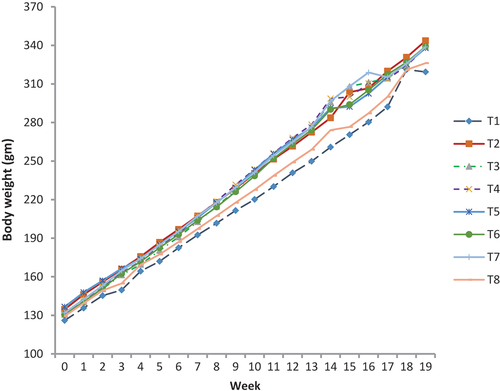
The high-fat diet caused a sudden and significant increase in weight from the baseline for seven weeks. At baseline, the differences in body weight were not noticeable but became significant after eight weeks. The initial weight and final weight in all groups are presented in . Group G2 (Control positive) Group 3, 4 and 6 gained the significant weight as compared to other groups at the end of study. There is a strong relationship between the consumption of processed foods (sugar, salt etc.) and the occurrence of no communicable diseases (NDCs). Unhealthy dietary habits and a sedentary lifestyle can contribute to NDCs and many kinds of liver abnormalities including nonalcoholic fatty liver diseases (NAFLD). Current results confirm that the body weight was significantly reduced among the symbiotic groups containing combination of treatments (Probiotics, GOS and Inulin) as compared to control negative and control positive. Many strategies have been used to halt the progression or management of NAFLD and one of them includes a reduction in the weight. Prebiotics, probiotics and symbiotics are also known to help in weight reduction. In the present study, the maximum reduction in weight was found among the group having probiotics and two types of prebiotics (GOS and Inulin) also shown in .
Table 4. Effects of different treatments on body weights in rats.
Probiotics can be helpful in the manner that they play a major role in the gut through the gut “microbiota-brain axis (MBA)” as they can regulate specific hormones that are responsible for maintaining appetite, storage and energy expenditure.[Citation25] Prebiotics can also help in weight reduction by the number of mechanisms including satiation and satiety, inhibiting the absorption of free fatty acids and bile salts and controlling the glycemic response.[Citation26] In combination with probiotics, it can reduce weight following the aforementioned mechanisms. Several studies have been documented to support the fact in both human and animal trail that weight can be reduced by supplementing the diet with probiotics and prebiotics.[Citation27] Asgharian et al.[Citation28] tested symbiotics on the lipid profile of patients with NAFLD condition conclude that these symbiotics can reduce body weight as well as improves the parameters of the lipid profile. The weight reduction can be explained through the regulation of glucagon-like peptide and Peptide YY and down-regulation of the ghrelin (hunger hormone).
Weights of body and organs (Liver, Spleen and Kidney)
Means for liver organ indicated that G1 (control negative) fed on standard diet gained the lowest weight while G2 (control positive) fed on HFD and without any treatment gained maximum weight 9.63 ± 0.46 g. A significant difference was noted among other treatment groups except control. G8 with a Combination of probiotics and prebiotic inulin and prebiotic GOS lowered the liver organ weight to 6.77 ± 0.20 g. Similarly, weight of other body organs of spleen, kidney, liver was found higher in G2 (Control positive) fed on HFD diet as compared to G1 (control negative) fed on standard diet. This is a clear indication that HFD induced fat deposition in body and other body organs. Among different treatment groups G8 with probiotic+ prebiotic inulin+ prebiotics GOS showed lower organ weight than other treatments ().
Table 5. Weights of body and organs (Liver, Spleen and Kidney).
Effect of prebiotics and probiotics treatment on various anatomical parameters
Means for the effect of prebiotics and probiotics on various anatomical parameters of the heart, lungs and intestine indicated that G1 (control negative) fed on a standard diet gained the lowest weight in the case of the heart (0.82 ± 0.033 g), lungs (1.77 ± 0.065 g) and intestine (5.98 ± g) and highest length in case of the intestine (110.75 ± 1.48 cm). Whilst, G2 (control positive) fed on a high fat-high sugar diet and without any treatment gained maximum weight in case of the heart (0.84 ± 0.045 g), lungs (1.87 ± 0.101 g) and intestine (8.42 ± 0.36 g) and lowest length in case of the intestine (101.67 ± 1.83 cm). A significant difference was noted among other treatment groups and control groups. Among different treatments groups, in heart, G6 with probiotic+ prebiotic inulin showed lower weight than other treatments, while in the lungs, the lowest weight was shown by G7 (probiotic+ prebiotic GOS) and in the intestine, lowest weight was shown by G8 (probiotic+ prebiotic inulin+ prebiotic GOS). As far as the highest weights were concerned, G3, G6 and G7 showed the highest results in the case of the heart, lungs and intestine respectively ().
Table 6. Effect of prebiotics and probiotics treatment on various anatomical parameters.
Liver function tests
Alanine aminotransferase (ALT)
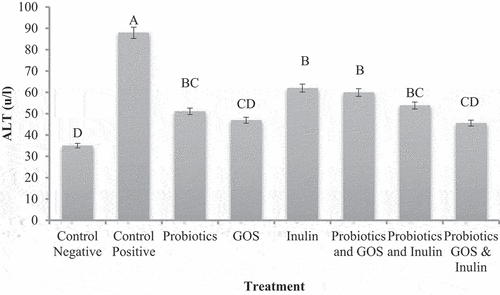
Means values are depicted in for ALT in G1 (control negative) and G2 (control positive and different treatment groups from G3 to G8 significant differences. The results indicated that the ALT activity significantly increased in groups fed on HFD than normal group fed on a standard diet. This was a clear sign that HFD had a deleterious effect on hepatocytes. The ALT levels were higher at 87.92 ± 2.26µ/l in G2 (control positive) than 35.00 ± 1.02µ/l in G1 (control negative. Treatment with probiotics and prebiotics alone or in combination improved the ALT levels in HFD-fed groups. Among all the treatments, a prominent decrease in ALT activity 45.58 ± 3.7µ/l was observed in G8 and given a combination of probiotics+Inulin+GOS; However, these are statistically similar to G4 given GOS 46.92 ± 3.37µ/l. Due to reduction in weight, all the biomarkers of NAFLD including liver enzymes, scores of steatosis and lobular inflammation can be reduced. But the main problem with weight reduction is adherence to typical hypocaloric diet and physical activity (Trappoliere et al. 2005). Alleviation in the ALT was found after the administration of probiotics combination on NAFLD obese children and adolescents.[Citation27] Symbiotics can reduce the hepatic aminotransferases and IL-6 after 2 months of symbiotic supplementation in rats fed with a hypercaloric diet.[Citation29] These beneficial effects also improve the overall inflammation including hepatic enzyme concentration specifically ALT concentration,[Citation30] which can also be related to a detrimental effect in oxidative stress and satiety stimulation in the liver cells. A multi-strain probiotic reduces the liver fat, aminotransferases and other inflammatory mediators after 8 weeks.[Citation31] Overall the biomarkers of NAFLD were reduced among the treatment groups compared to the control positive.
Aspartate aminotransferase (AST)
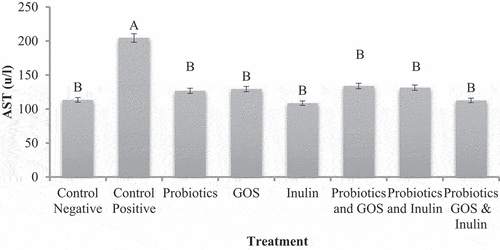
The mean values presented in revealed that AST values were higher 204.50 ± 5.67µ/l in HFD fed group than normal group fed on a standard diet 113.42 ± 2.28µ/l. Probiotics and prebiotics treatments significantly improved AST levels in groups G3 to G8. Prominent effect 108.67 ± 5.02µ/l was observed in G5 group that was given inulin followed by G8 112.67 ± 3.76µ/l, however, both groups are statistically similar. It was proved that the consumption of functional ingredients such as probiotics and prebiotics improves the AST activity in HFD-induced fatty liver disease. The same findings were reported by Monem,[Citation32] who probed the effect of supplementation of L Acidophilus on liver enzymes in nonalcoholic fatty liver disease and found a significant reduction in AST after one month of treatment. Similarly, Famouri et al.[Citation27] explored the effect of probiotics (L acidophilus, L Lactis and B Bifidum) on obese children and adolescents suffering from nonalcoholic fatty liver disease and found significant alleviation in AST.
Alkaline phosphate (ALP)
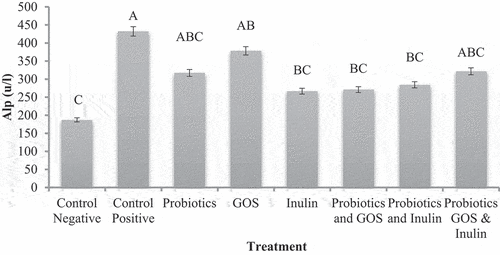
A significant effect was observed in ALP activity in different treatment groups fed on a standard diet or HFD. depicted ALP hepatic enzyme was significantly higher 432.17 ± 19.02µ/l in G2 (Control positive) fed on HFD than G1 (control negative) fed on a standard diet. Treatment with probiotics and prebiotics significantly improved the ALP hepatic enzyme in G3 to G8 however, in G5 (Inulin) major improvement was observed 266.67 ± 24.97µ/l followed by G6 (probiotics+GOS) 271 ± 27.12µ/l that is statistically similar to G5. Manzhalii et al.[Citation33] analyzed the effect of multiple probiotics and their combination on NASH human model for 12 weeks and found that there was a significant reduction in the stiffness of liver.
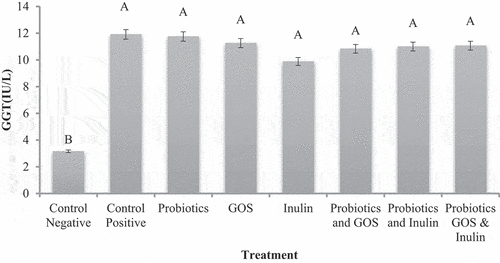
Gamma-glutamyl transferase (GGT)
Gamma-glutamyl transferase (GGT) was significantly higher in HFD group G2 11.91 ± 0.95IU/L than normal group G1 fed on standard diet. A significant improvement was observed in all treatments groups except for control but in G5 (inulin) major improvement was noted at 9.88 ± 0.60IU/L. Other treatments also improved the GGT hepatic enzymes in HFD-induced fatty liver disease (). Gut dysbiosis is the structural or functional disturbance of gut microbiota resulting in the progression of NAFLD and disturbances in lipid profile, inflammation an increase in reactive oxygen species and steatosis of the liver which can be reduced or managed by the proper probiotic therapy. As the probiotics can be manipulated by different prebiotics, so the supplementation of different symbiotics can produce favorable results or even more effective results compared to the individual effects of probiotics and prebiotics individually. Regarding the GGT, it was found to remain unaffected during the trial.[Citation34]
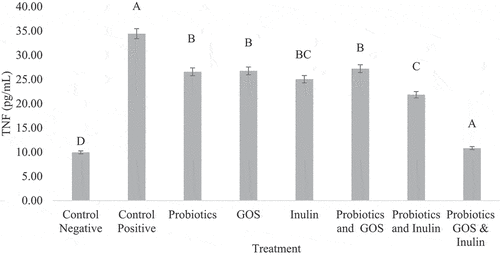
Tumor necrotic factor (TNF)
Overall the combination of prebiotics and probiotics was more effective against lowering the cholesterol level than the alone supplementation of probiotics and prebiotics. Overall G8 was the most effective treatment for lowering the cholesterol level of among treatments compared to the control positive group in the present study (). Probiotics and GOS groups had a significant reduction in the levels of TNF after 19 weeks of study.
A clinical trial conducted to measure the efficacy of symbiotic supplementation in NAFLD concluded that there was a significant decrease in liver enzymes, inflammation and TNF was found at the end of the trial.[Citation35] These findings were in-lined with the present study as the symbiotic group (G8) with 2 prebiotics and probiotics significantly reduced the liver enzymes as well as TNF after 19 weeks of trail. The lipid profile parameters were also reduced among all the prebiotic and probiotic groups but the effect was synergistic in the symbiotic group having two prebiotics and probiotics (G8). The same finding were observed in a study in which the author concluded that probiotics, prebiotics or in combination symbiotics can maintain the intestinal barriers as the damaged intestinal barrier has been associated with the development of NAFLD .[Citation36]
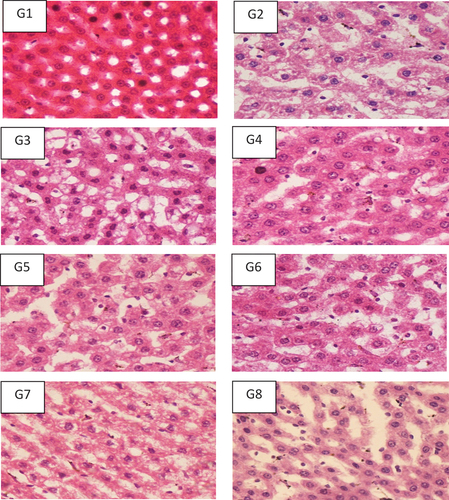
Figure 7. Hepatic histopathological analysis of the rats fed with probiotics, prebiotics and combination of pro/prebiotics100x after staining with Hematoxylin and Eosin stain.
Effect on liver malondialdehyde, SOD, GPx and Catalase
Means indicated that the liver malondialdehyde significantly increased in groups fed on HFD than normal group fed on a standard diet (). The liver malondialdehyde levels were higher 97.30 ± 0.94 nmol/g wet issue in G2 (control positive) than 26.05 ± 0.78 nmol/g wet issue in G1 (control negative). Treatment with probiotics and prebiotics alone or in combination improved the liver malondialdehyde in HFD-fed groups. Moreover, superoxide dismutase values were higher (12.89 ± 0.44 IU/mg) in normal group fed on standard diet than in HFD fed group (11.60 ± 0.54 IU/mg). Probiotics and prebiotics treatments significantly improved superoxide dismutase levels in groups G3 to G8. A prominent effect (16.92 ± 0.26a) was observed in G8 group that was given inulin followed by G3 (15.33 ± 0.52 IU/mg); however, both groups are statistically similar. GPx was significantly higher (32.06 ± 0.50 IU/mg) in G1 (control negative) fed on standard diet than in G2 (Control positive) fed on HFD. Treatment with probiotics and prebiotics significantly improved the GPx in G3 to G8. Moreover, catalase was significantly lower in HFD group G2 (7.29 ± 0.20 IU/mg) than in normal group G1 fed on the standard diet. Significant improvement was observed in different treatment groups but in G8 (probiotics + both prebiotics) major improvement was noted (15.31 ± 0.40 IU/mg). Other treatments also improved the catalase in HFD-induced fatty liver disease .
Table 7. Liver Malondialdehyde, SOD, GPx and Catalase.
An experimental study was conducted to test the impact of symbiotic and vitamin supplementation particularly that of antioxidant Vitamin E on hypertension and inflammatory markers in individuals with NAFLD for a period of 56 days. According to the study design, there were total of four groups who were given alpha-tocopherol in a dose 400 International Units and symbiotic supplement in amount 2 × 108 Colony Forming Units per gram. The composition of symbiotic was based on 7 probiotic species and a prebiotic FOS. The parameters assessed twice in the study were anthropometrics, SBP & DBP, serum malondialdehyde (MDA), nitric oxide (NO) and tumor TNFα. The synergistic effect of Vitamin E and Symbiotic decreased SBP, serum MDA, TNFα levels and enzymes live in NAFLD participants. However, no effect was observed on DBP and concentration of nitric oxide in serum. The supposed mechanism behind the addition of Vitamin E to treatment therapy is that it can control blood pressure and oxidative damage along with symbiotics.[Citation37]
Effect on Hepatic inflammation
Upon the prebiotics and probiotics therapy strong association was found with the hepatic inflammation at various degrees and hepatic and portal inflammation was reduced among the symbiotic groups (group 8) significantly (Chi-square value = 49.04**; P-value = 0.000; ** = Highly significant (P < .01). presents the effect of prebiotics and probiotics on various parameters of hepatic inflammation. Our results showed that lobular and portal inflammation were lower in the rats given pro and prebiotics versus the control positive groups. These results affirm the protective role of functional diets. Overall, Group 8 rats were more protected than others. Upon the prebiotics and probiotics therapy, a strong association was found with the hepatic inflammation at various degrees and hepatic and portal inflammation was reduced among the symbiotic groups significantly as evidenced by the statistical model mentioned above.
Table 8. Association of prebiotics and probiotics treatment on hepatic inflammation.
Conclusion
Nonalcoholic fatty liver disease is the most prevalent disorder in Asia and South Asia. The key factors responsible for this chronic liver disease that are most commonly becoming a serious public health concern are obesity, diabetes mellitus (DM type 2), cardiovascular disease and environmental factors such sedentary lifestyle with unhealthy hypercaloric dietary patterns. An increase in the trend of using functional foods and nutraceuticals has been observed rather than the use of drugs and allopathic treatments in the management of NCDs. The present study was conducted to investigate and compared the role of functional foods or ingredients such as probiotics and prebiotics alone or in combinations on the markers of liver damage, oxidative stress markers, inflammatory markers, lipid profile and bacterial counts in the high fat diet-induced experimental model of NAFLD. After the completion of the trial, the weight was significantly reduced among the Symbiotic group containing a combination of treatments (Probiotics, GOS and Inulin). Maximum reduction of ALT was found among the G8 (Probiotics, GOS and Inulin) and G4 (GOS). AST was significantly reduced among all the treatments compared to the control positive. ALP was reduced among G5, G6 and G7 only. GGT remained unaffected during the trial. Creatinine was significantly reduced among G6 and G8 only. Total bilirubin was reduced only in G5. Lipid profile was also reduced among the treatment groups compared to the control positive group. Overall histopathological analysis revealed reduction in the fatty vacuoles and inflammation in hepatocytes in the treatment groups compared to control. The progression of fibrosis was also reduced among the treatment groups. Overall the biomarkers of NAFLD were reduced among the treatment groups compared to the control positive.
Declaration
The work described has not been published before (except in the form of an abstract, a published lecture or an academic thesis). It is not under consideration for publication elsewhere.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Asrani, S. K.; Devarbhavi, H.; Eaton, J.; Kamath, P. S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70(1), 151–171. DOI: 10.1016/j.jhep.2018.09.014.
- Ando, Y.; Jou, J. H. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin. Liver Dis. 2021, 17(1), 23. DOI: 10.1002/cld.1045.
- Wong, W. K.; Chan, W. K. Nonalcoholic Fatty Liver Disease: A Global Perspective. Clin. Ther. 2021, 43(3), 473–499. DOI: 10.1016/j.clinthera.2021.01.007.
- Powell, E. E.; Wong, V. W. S.; Rinella, M. Non-alcoholic Fatty Liver Disease. Lancet. 2021, 397(10290), 2212–2224. DOI: 10.1016/S0140-6736(20)32511-3.
- Loomba, R.; Friedman, S. L.; Shulman, G. I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell. 2021, 184(10), 2537–2564. DOI: 10.1016/j.cell.2021.04.015.
- Muthiah, M. D.; Cheng Han, N.; Sanyal, A. J. A Clinical Overview of Non‐alcoholic Fatty Liver Disease: A Guide to Diagnosis, the Clinical Features, and complications—what the Non‐specialist Needs to Know. Diabetes Obes. Metab. 2022, 24(S2), 3–14. DOI: 10.1111/dom.14521.
- Chang, E.; Park, C. Y.; Park, S. W. Role of Thiazolidinediones, Insulin Sensitizers, in Non‐alcoholic Fatty Liver Disease. J. Diabetes Investig. 2013, 4(6), 517–524. DOI: 10.1111/jdi.12107.
- Marceau, P.; Biron, S.; Hould, F. S.; Marceau, S.; Simard, S.; Thung, S. N.; Kral, J. G. Liver Pathology and the Metabolic Syndrome X in Severe Obesity. J. Clin. Endocrinol. Metab. 1999, 84(5), 1513–1517. DOI: 10.1210/jcem.84.5.5661.
- Negi, C. K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the Molecular Targets and Emerging Pharmacotherapeutic Interventions for Nonalcoholic Fatty Liver Disease. Metabolism. 2022, 126, 154925. DOI: 10.1016/j.metabol.2021.154925.
- Cunningham, M.; Azcarate-Peril, M. A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H. D.; Hunter, K.; Manurung, S.; Obis, D., et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29(8), 667–685. DOI: 10.1016/j.tim.2021.01.003.
- Montrose, D. C.; Floch, M. H. Probiotics Used in Human Studies. J. Clin. Gastroenterol. 2005, 39(6), 469–484. DOI: 10.1097/01.mcg.0000165649.32371.71.
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27(3), 202–209. DOI: 10.4103/0255-0857.53201.
- Wu, H.; Chiou, J. Potential Benefits of Probiotics and Prebiotics for Coronary Heart Disease and Stroke. Nutrients. 2021, 13(8), 2878. DOI: 10.3390/nu13082878.
- Lim, C. C.; Ferguson, L. R.; Tannock, G. W. Dietary Fibres as “Prebiotics”: Implications for Colorectal Cancer. Mol. Nutr. Food Res. 2005, 49(6), 609–619. DOI: 10.1002/mnfr.200500015.
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137(3), 830S–837S. DOI: 10.1093/jn/137.3.830S.
- Ashwell, M. Concepts of Functional Foods. ILSI Europe Concise Monograph Series. Nutr. Food Sci. 2004, 34(1), 47–47. https://doi.org/10.1108/nfs.2004.34.1.47.3
- Saavedra, J. M.; Tschernia, A. Human Studies with Probiotics and Prebiotics: Clinical Implications. Br. J. Nutr. 2002, 87(S2), S241–S246. DOI: 10.1079/BJN/2002543.
- Sekhon, B. S.; Jairath, S. Prebiotics, Probiotics and Synbiotics: An Overview. J. Pharm. Sci. Res. 2010, 1(2), 13–36.
- Lasker, S.; Rahman, M. M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S. B.; Subhan, N.; Ahsan, G. U., et al. High-fat diet-induced Metabolic Syndrome and Oxidative Stress in Obese Rats are Ameliorated by Yogurt Supplementation. Sci. Rep. 2019, 9(1), 1–15. DOI: 10.1038/s41598-019-56538-0.
- Bancroft, J. D., and Gamble, M., Eds. Theory and Practice of Histological Techniques; China: Elsevier health sciences, 2008.
- Wills, E. Mechanisms of Lipid Peroxide Formation in Animal Tissues. Biochem. J. 1966, 99(3), 667–676. DOI: 10.1042/bj0990667.
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene Expression of Antioxidative Enzymes in the Human Heart: Increased Expression of Catalase in the end-stage Failing Heart. Circulation. 2000, 101(1), 33–39. DOI: 10.1161/01.CIR.101.1.33.
- Jollow, D. J.; Mitchell, J. R.; Zampaglione, N.; Gillette, J. R. Bromobenzene-induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3, 4-bromobenzene Oxide as the Hepatotoxic Metabolite. Pharmacol. 1974, 11(3), 151–169. DOI: 10.1159/000136485.
- Steel, R. G.; Dickey, J. H. Pinciples and Procedures of Statistics a Biometrical Approach, 1997, (No. 519.5 S8).
- de Clercq, N. C.; Groen, A. K.; Romijn, J. A.; Nieuwdorp, M. Gut Microbiota in Obesity and Undernutrition. Adv. Nutr. 2016, 7(6), 1080–1089. DOI: 10.3945/an.116.012914.
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients. 2013, 5(4), 1417–1435. DOI: 10.3390/nu5041417.
- Famouri, F.; Shariat, Z.; Hashemipour, M.; Keikha, M.; Kelishadi, R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 64(3), 413–417. DOI: 10.1097/MPG.0000000000001422.
- Asgharian, A.; Mohammadi, V.; Gholi, Z.; Esmaillzadeh, A.; Feizi, A.; Askari, G. 2017. The Effect of Synbiotic Supplementation on Body Composition and Lipid Profile in Patients with NAFLD: A Randomized, Double Blind, placebo-controlled Clinical Trial Study. Iran. Red. Crescent. Med. J 19(4) DOI: 10.5812/ircmj.42902.
- Tagliari, E.; Campos, A. C.; Costa-Casagrande, T. A.; Salvalaggio, P. R. The Impact of the Use of Symbiotics in the Progression of Nonalcoholic Fatty Liver Disease in a Rat Model. ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo). 2017, 30(3), 211–215. DOI: 10.1590/0102-6720201700030011.
- Çakır, M.; İşbilen, A. A.; Eyüpoğlu, İ.; Sağ, E.; Örem, A.; Şen, T. M.; Kaklıkkaya, N.; Gjtjg, K. Effects of long-term Synbiotic Supplementation in Addition to Lifestyle Changes in Children with obesity-related non-alcoholic Fatty Liver Disease. Turkish j. gastroenterol: official j. Turkish Soci Gastroenterol. 2017, 28(5), 377–383. DOI: 10.5152/tjg.2017.17084.
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; TJAoh, B. Efficacy of Probiotics and Smectite in Rats with non-alcoholic Fatty Liver Disease. J. Hepatol. 2018, 17(1), 153–161.
- Monem, S. M. A. Probiotic Therapy in Patients with Nonalcoholic Steatohepatitis in Zagazig University Hospitals. Euroasian j. hepato-gastroenterol. 2017, 7(1), 101. DOI: 10.5005/jp-journals-10018-1226.
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; WJJodd, S. Treatment Efficacy of A Probiotic Preparation for Non‐alcoholic Steatohepatitis: A Pilot Trial. J.digestive dis. 2017, 18(12), 698–703. DOI: 10.1111/1751-2980.12561.
- Perumpail, B. J.; Li, A. A.; John, N.; Sallam, S.; Shah, N. D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. J. D. The Therapeutic Implications of the Gut Microbiome and Probiotics in Patients with NAFLD. Diseases. 2019, 7(1), 27.
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Hekmatdoost, A.; Hekmatdoost, A. Synbiotic Supplementation in Lean Patients with non-alcoholic Fatty Liver Disease: A Pilot, Randomised, double-blind, placebo-controlled, Clinical Trial. Br. J. Nutr. 2017, 117(5), 662–668. DOI: 10.1017/S0007114517000204.
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; CJJoa, X.; chemistry, F. Intestinal Barrier Function–Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. agric. food chem. 2019, 67(10), 2754–2762. DOI: 10.1021/acs.jafc.9b00080.
- Ekhlasi, G.; Zarrati, M.; Agah, S.; Hosseini, A. F.; Hosseini, S.; Shidfar, S.; Aarbshahi, S. S. S.; Razmpoosh, E.; FJEj, S. Effects of Symbiotic and Vitamin E Supplementation on Blood Pressure, Nitric Oxide and Inflammatory Factors in non-alcoholic Fatty Liver Disease. EXCLI j. 2017, 16, 278. DOI: 10.17179/excli2016-846.