?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The structural modification of starch improves its physicochemical and functional properties and develops new material. Our study aimed to modify rice starch obtained from three varieties using a solvent-free system involving gulupa seed oil and lipase B from Candida antarctica. We characterized six starches (native and modified), performed a digestibility test, and determined the particle sizes, zeta potentials, and calorimetric differences between the materials. All the starches presented a charged surface between −41.6 and −58.9 mV. The modified starches’ fatty acid profiles changed according to the rice variety used, namely, the proportions of C14, C16, and C18 acids. The modified Clearfield, F60, and Oryzica 1 starches showed fatty acid content increased 85%, 23%, and 73%, respectively. The features presented by the three varieties of starch open up the possibility of their industrial application; furthermore, the starch modification process presents economic and environmental advantages.
Introduction
By-products tend to be wasted in most Colombian production chains, e.g., broken rice, which is priced 50% lower than whole rice. Starch is the majority component of the rice grain and has variable functional properties due to its botanical origin, and this has attracted the attention of relevant industries, such as food; however, its retrograde tendency, low thermal stability, and low affinity with organic solvents have limited its applicability in pharmacy and cosmetic fields.[Citation1] Alternatively, chain residues, such as gulupa seed, can be useful for obtain active principles. Oil is extracted from its seeds, providing a source of fatty acids (FAs) with industrial applicability; however, there have been few studies regarding the applicability of this oil.
The structural modification of starches has improved their physicochemical and functional properties. In recent years, modification alternatives that avoid using aggressive solvents have been sought to obtain materials recognized by the FDA (Food and Drug Administration) as GRAS (generally recognized as safe); additionally, these alternatives may provide economic advantages in their production, e.g., using different production-chain by-products, removing environmental and health implications, and utilizing green chemistry principles.[Citation2,Citation3]
Reaction conditions directly using FAs or oils (particularly the latter) obtained from agricultural by-products have provided an alternative to polymers modification, and are recognized as safe for different applications .[Citation4,Citation5] Oils are a reaction medium that allow for greater contact between polymer and an acyl donor without the hydrolysis present in aqueous media; however, there are few investigations regarding the oil’s lipid composition that evaluate its functionality in modified starches (MS). Rajan et al. used lauric acid recovered from coconut oil as an acyl donor in a microwave system to achieve a 1.1 degree of substitution (DS).[Citation6] Gulupa seed oil has different FAs that could modify starch properties; furthermore, its low-cost extraction, which produces a juice by-product, mean that it is a low-cost reaction medium framed in circular economy processes, which have shown trends of using the by-products that increase in correlation to gulupa seed production.[Citation7]
Starch acylation and transesterification highlights the changes they generate. The insertion of FAs into starch increases hydrophobicity, improving its ethyl acetate, acetone, and other-solvent solubility. These modifications generate changes in its swelling power (SP), water solubility (WS), and water absorption capacity (WAC) properties; furthermore, its ability to self-associate and form hydrophobic nuclei improve its applicability in pharmacy and cosmetic fields as encapsulating agents for active compounds.[Citation8–10]
Our study aimed to modify starch obtained from three rice varieties grown in Tolima Department, Colombia, using a solvent-free system, the gulupa (Passiflora edulis Sims. f. edulis) seed oil by-product of this production chain, and lipase as a catalyst. By doing so, we establish an alternative framed in green chemistry that can modify polymer’s physicochemical properties, expanding the applicability of this material in focus industries, such as pharmaceuticals and cosmetics.
Materials and methods
Plant material
We used rice varieties (F60, Oryzica 1, and Clearfield) supplied by the Colombian Federation of Rice Growers. We carried out starch extraction by an alkaline method.[Citation11] We obtained gulupa seed oil as follows: We separated the seeds from the pulp, washed with distilled water, and dried at 45 ± 2°C in an air-circulation oven. Later, we ground the seeds in a blade mill to obtain a homogeneous flour. Then, we obtained the oil by using soxhlet with n-hexane until exhaustion. We separated the oil from the solvent in a rotary evaporator and stored at −20°C until use.
Starch modification
We suspended different varieties of rice starches in gulupa oil in a 1.5: 10 (w/v) ratio. We used Candida antarctica lipase-B (40 U/mL) as a catalyst. We carried out the reaction for 3 hours at 50°C with constant stirring at 100 rpm. We stopped the reaction by adding ethanol (96%) and centrifuged the materials at 6000 rpm for 10 min with hexane to eliminate oil excess. We dried the polymers at 40°C for 24 hours in an airflow oven and stored under a nitrogen atmosphere in amber flasks.[Citation4]
Material characterization
Infrared spectroscopy (IR): We performed infrared spectroscopy (Thermo Scientific NICOLTET 380) of native starch (NS) and MS to monitor the impact of starch modification. We tracked spectra bands between 1640 and 1760 cm−1 related to the presence of group carbonyl formed by material acylation, as well as the OH-group band formed in the range from 3500–3200 cm−1.[Citation12] We removed the starches (native and modified) for one day at 40°C; then, we left them to cool in a desiccator before passing them through the instrument.
Fatty acids profile variation analysis: We performed fatty acid profile variation analysis of the materials followed by gas chromatography after material derivatization, which determined the FA content of native and modified rice starches. We carried out direct transesterification by acid catalysis ethanol/H2SO4 at 2% (v/v). We carried out the quantification in a GC-FID equipped with a column: DB 23 (Agilent Tech.) 30 m x 0.25 mm x 0.25 µm, with a flow of 1 mL/min of H2 and a heating ramp: initial temperature 150°C for 1 min, ramp from 25°C/min to 180°C, and ramp from 10°C/min to 230°C 3–5 min. We used the FAME supelco standard (ref: crm47885), which comprised 37 compounds in 1 mL of CH2Cl2, for the determination. We determined the percentage variation of FA for MS concerning their native polymers by following equation:
where ΔAG is the percentage of FA variation, [AGM] is the total concentration of FA in MS, and [AGTN] is the total concentration of FA in NS
X-ray diffraction (XRD): We determined modification impact on the starches’ crystalline conformation by monitoring the diffraction patterns of NS and MS using a Malvern-PANalytical model Empyean 2012 X-ray diffractometer.
DSC: We determined thermal transition temperatures using a differential scanning calorimeter (DSC Q100 de TA Instrument). We heated the sample from 20°C to 200°C with a heating rate of 10°C/min.
Functional properties: We determined the swelling power (SP), water solubility index (WSI), and water absorption index (WAI) following the methodology proposed by Mendoza et al.[Citation13] We deposited 1 g (bs) of starch in a centrifuge tube and added 25 mL of distilled water. We heated a suspension in a water bath at 60°C for 30 minutes, with mechanical stirring every 10 minutes. Subsequently, we centrifuged at 4500 rpm for 15 min. We immediately discarded the supernatant in previously dry beakers at 60°C and weighed. Then, we dried the supernatant at 70°C for 12 hours after weighing the time beaker to obtain the weight of soluble starch portion. Next, we weighed the centrifuge tubes to obtain the weight of the gel according to EquationEquations 2(2)
(2) –Equation4
(4)
(4) .
Scanning electron microscopy: We verified the surface and shape of the NS and MS granules using field emission SEM at an acceleration voltage of 10 kV. We sprayed the dehydrated samples on a double-adhesive tape fixed on circular copper pieces and then covered with a thin film (~15 nm) of gold to avoid the accumulation of electrical charges under an electron beam.[Citation14]
Starch digestibility: We dissolved the materials (native and modified starches) in water (2500 mg/L), pregelatinized by treatment at 60°C for 1 hour, and added α-amylase enzymes (5000 U/mL) and amyloglucosidase (5000 U/mL) for 3 h. We measured the degradation kinetics as a function of the glucose released using the 3,5-dinitrosalicylic acid (DNS) method.[Citation15]
Particle size and zeta potential: We analyzed the particle sizes and zeta potentials of native and modified materials in a dynamic light scattering system (DLS). We prepared samples at 1 mg/mL in water, and the type of material we selected in the equipment was polystyrene latex. We used Cell DTS0012 for particle size and DTS1070 for zeta potential.
Statistical analysis
We analyzed our results using the Statgraphic Centurión XVI software. We implemented three replications for each test, and the results we obtained are expressed as the mean ± standard error of mean. We determined significant differences between means for 95% confidence (P < .05) using an analysis of variance after verifying data normality and performing a multiple range test.
Results and discussion
Chemical modifications of rice starches
shows the lipid profile of gulupa oil, which presents a majority composition of FA C18. Montoya et al.[Citation4] obtained starches with variable physico–chemical properties using acylation with long-chain FA (C:18). MS properties, which determine their future applications, depend on the FA used. The insertion of myristic, palmitic, and oleic acids give the polymer a greater resistance to enzymatic hydrolysis, can improve its thermal resistance, and modify the starch’s solubility. Using gulupa seed oil is an alternative for obtaining polymers with better physicochemical properties because of its FA diversity, allowing its application in industries such as pharmaceuticals, cosmetics, and food.
Table 1. Lipid profile of Gulupa oil.
shows starch extraction yield according to our studied varieties. Rice grain has a majority composition of starch (±90%), and obtaining this polymer is linked to rice variety and extraction method. Arns et al.[Citation16] used NaOH (0.1%) for 18 hours and heat treatment at different times (10, 30, and 60 min), and observed that after a long time less polymer was extracted. The control treatment (not subjected to heat) displayed the highest extraction yield (66.5%). Furthermore, Pinkaew et al.[Citation17] demonstrated that extraction with NaOH (0.2%) for 3 hours using pre-germinated rice achieves a yield of 30%. In our study, we obtained starch extraction yields greater than 70% on a dry basis for all used varieties, with a purity percentage of 99%.
Table 2. Extraction yield of native rice starches and yield of modified product according to rice variety used.
Once we modified the three materials, we discovered differences on composition of FAs in starches. shows the polymer’s modification yield. The modification system we used allowed us to recover the total weight of starch and established an average insertion of 0.036 grams of oil per each gram of starch used.
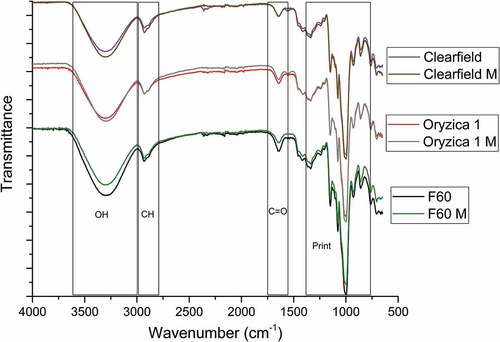
shows the IR spectra of native and modified polymers. The starches show characteristic bands at 1077, 1148, and 1005 cm−1 corresponding to the stretching of CO and CC bonds.[Citation18] Additionally, the stretches at 929, 858, and 760 cm−1 are products of a hydroglucose ring in the molecule.[Citation19] The 2930 and 3300 cm−1 bands are given by C-H and OH bonds, respectively.[Citation20]
Decreased bands at 3300 cm−1 are related to the acylation of polymer; however, Xiao et al. reported that the 1700 cm−1 band is directly related to an extension of carbonyl-group vibration. We found no differences in these bands compared with NS in the spectra we obtained, which could be explained by the low DS achieved under enzyme-catalyzed systems, as reported by Najafi et al.,[Citation21] who observed that starch with less DS (0.04) showed no changes in this band. Considering the catalytic nature of lipases, a transesterification process may be taking place in which the FAs present in NS are replaced, thus producing starches with different compositions.[Citation22]
The modified Clearfield, F60, and Oryzica 1 starches increased the FA content by 85%, 23%, and 73%, respectively. We determined the variations of each FA in the lipid compositions of modified polymers for the native materials (). Negative and positive values represent increases and decreases, respectively, in the composition percentage that each FA represents in MS to NS. The values that the FAs represent for each modified material vary, becoming most noticeable in myristic, palmitic, oleic, and linoleic acids. Lim et al.[Citation23] established that the presence of myristic and palmitic acids increases the amount of resistant starch. Montoya et al.[Citation4] showed that starch modification with linoleic acid increases values of WSI, WAI, and SP.
Table 3. Lipid profile variation of MS.
The lipid profile variations of modified polymers concerning their native materials are notorious, with changing proportions of C14, C16, and C18 acids. Alternatively, the FA proportions that we removed and added to the starches allowed us to establish that two modification processes were being carried out corresponding to acylation attributed to most of the added FAs and a transesterification, which could be responsible for the decrease in the amounts of some of these acids in the modified materials.
Morphological and functional characterization of NS and MS
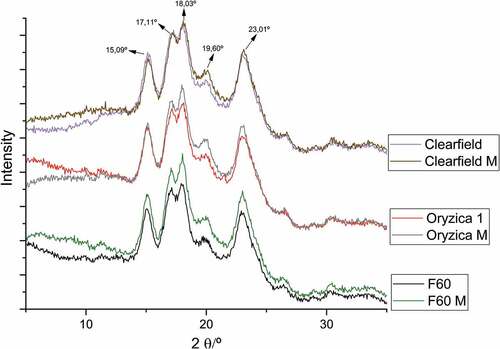
Starches can be classified into types A, B, or C, based on X-ray diffraction patterns that reflect the crystallinity properties of their underlying structures. shows their diffractograms. Native and modified polymers presented the same patterns, with characteristic peaks at 2° 9°, 15°, 17°, 18°, 19°, and 23° indicating a type A crystallinity[Citation24,Citation25] however, modified materials showed peak intensities differences related to increases in polymer crystallinity[Citation26] more notable in the F60 variety.
Results from studies on starch modification showed that the crystalline structure of the native polymer was destroyed with esterification, and a characteristic peak is formed at 2° 9°. Modified polymers did not present these features because the changes take place in an amorphous region of the polymer where enzymes can easily access; however, crystalline regions remain unchanged due to their complexity and tendency to form helices.[Citation13,Citation27] Issola et al.[Citation28] showed that the crystalline structure of the polymer can remain intact through transesterification processes; additionally, Najafi et al. showed that MS with low DS do not present considerable changes in the crystalline structure. Bajaj et al.[Citation29] showed that MS with low DS have little impact on granule size, avoiding its increase and improving properties such as retrogradation, which is highly relevant when considering their use in industries such as food and pharmaceuticals for coating food matrices and as active ingredients, respectively, where polymers with reduced molecular sizes are required to protect these molecules.
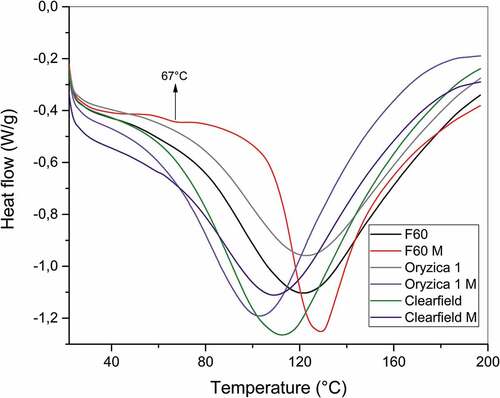
We performed DSC analysis to obtain the thermal transitions of materials (), which indicate that no glass transition (Tg) occurred for NS of different varieties. Additionally, the MS of the Oryzica 1 and Clearfield varieties did not show clear Tg. The inter- and intramolecular hydrogen bonding and degree of crystallinity we observed in the XRD spectra probably prevented starch from demonstrating any thermal transition properties.[Citation30] However, the modified materials showed changes in their melting temperatures (Tm) with respect to the native materials: F60: 121°C; F60M: 129°C; Orizyca1: 122°C; Oryzica1M: 103°C; Clearfield: 112°C; and ClearfieldM: 109°C. In addition, the modified F60 variety showed Tg at 67°C, and was the only starch modified enough to clearly achieve the transition; nevertheless, the graph shows that this transition probably also occurs for the other starches in previous values, which agrees with the results obtained in other investigations related to starch acetylation.[Citation31] On the other hand, it allows us to observe the differences of a amylose–lipid complexation process where these values generally decrease compared with the NS.[Citation32] The differences in the transition parameters between NS and MS are given by the degree of modification obtained by each material, and F60 M presents a greater degree of change.[Citation33]
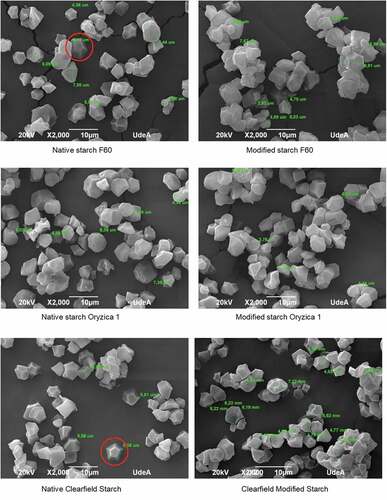
shows the morphologies of NS and MS. NS have a polyhedral shape, with diameters between 2 and 8 µm and a smooth granule surface. We observed a more defined shape for some granules of native polymers, e.g., native starches such as F60 and Clearfield have a dodecahedral structure; however, most of the granules of different materials have an irregular shape. Therefore, the modification of the starches did not generate changes in granules size. The granule shapes did not show great variations either; nevertheless, there were decreases in the number of dodecahedral structures in the F60 and Clearfield varieties.
One of the main differences between NS and MS was the high tendency of the latter to form agglomerates. Increases in the hydrophobicity of the materials caused by acylation and transesterification processes promoted the starches’ ability to spontaneously self-associate, forming hydrophobic nuclei.[Citation9,Citation10] This characteristic is required in pharmaceutical fields when obtaining polymers for active compounds encapsulation – this agglomerates formation is an advantage because it generates a denser barrier against enzymatic attacks and pH changes when administrated orally.[Citation34]
shows the functional properties of native and modified materials, as well as changes in the composition of the FAs of treated polymers with alterations generated on their functional features. SP and WAI decreased in MS due to an increase in long-chain FAs that confer a greater hydrophobic character to the polymers, leading to a decrease in absorption and water retention capacities. Otherwise, the WSI increased in the modified F60 and Oryzica 1 varieties. Additionally, given structural reorganization as a consequence of steric hindrance, starch granules were weakened and the leaching of amylose chains was improved, which triggers an increase in this index.[Citation24] One of the polymers that presented the greatest differences concerning its native material was the Clearfield variety, evidencing a direct relationship with the level of variation in the lipid profile of this material ().
Table 4. Functional properties of NS and MS.
shows the size variations and particle distributions of the materials. The modified polymers present differences concerning native materials, including the polydispersity index (PI) and zeta potential of each material. The modified starch F60 was a polydisperse sample, although sizes of 3.38 and 155 (d.nm) were not very abundant. In general, an increase in particle size for both modified F60 and Clearfield polymers was associated with an increase in long-chain FAs. Xiao et al.[Citation21] elucidated that the greater degree of substitution in esterified starches, the greater the increase in particle size. As for PI, it represents the degree of variation in the molecular weights of a polymer, and when the value of this index is less than or equal to 1.0, the polymer is mostly monodisperse, as is the case for the starches in our study ().
Table 5. Particle size, polydispersity index and zeta potential of native and modified polymers.
Regarding the zeta potential observed for work materials, Najafi et al. reported that polymers with values lower than −18 mV are not very stable and form aggregations. In our study, all polymers presented a charged surface between −41.6 and – 58.9 mV, which indicates that in colloidal solutions they tend to be stable and do not form aggregations, which is relevant for their possible industrial application.
Enzymatic hydrolysis resistance
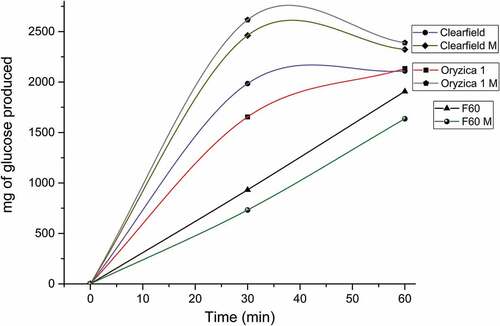
We evaluated enzymatic hydrolysis resistance, which is an aspect to consider when implementing polymers in industries such as pharmaceuticals, cosmetics, and food. shows the results of α-amylase- and amyloglucosidase-mediated digestibility tests.
The modified Clearfield and Oryzica 1 varieties increased their hydrolysis rates compared with native materials; however, the modified F60 variety presented a decrease in digestibility rate, which is shown on DSC thermograms () that show a higher degree of modification in this starch. Based on diffractograms (), increases in peak intensity have been related to a greater tolerance to enzymatic degradation; furthermore, the percentage that myristic, palmitic and oleic acids represent in the composition () has been reported to decrease the hydrolysis rate. On the other hand, a decrease in the levels of these acids for other varieties leads to an increase in polymer degradability.[Citation23,Citation35] Additionally, we observed that the modified F60 starch’s morphology () presents a greater amount of agglomerates, which increase resistance to enzymatic attacks. Moreover, structural reorganization in the F60 variety, a consequence of steric hindrance, leads to a favorable conformation for resistance to enzymatic degradation, hindering the enzyme–substrate union.[Citation36]
The features exhibited by modified polymers can present inconveniences for their application in the confectionery industry due to their low SP level; however, this decrease in SP is favorable for its use in cosmetics, allowing greater stability in formulations because of the low absorption and water retention capacities of polymers, which allows the possibility of generating emulsions because of the zeta potential exhibited by these materials.[Citation37] Additionally, easy degradation and improvements in thermal properties due to changes in the FA compositions of the Clearfield and Oryzica 1 MS varieties may present them as options for the production of bioplastics.[Citation38]
Additionally, increases in the solubility of these starches makes new applications in the food industry possible, such as the generation of sweeteners in the case of the Clearfield and Oryzica 1 varieties. On the contrary, the F60 variety can be used as an additive in addition to a nutritional load that contributes to the maintenance of a low glycemic index due to its resistance to enzymatic degradation.[Citation39] Moreover, this material, due to its resistance to enzymatic attacks, is stable in colloidal solutions because of its zeta potential and hydrophobic nature, and its increased WSI allows us to suppose its possible application in the pharmaceutical industry as a wall material, especially for the encapsulation of active principles that require this type of process to keep up their activities.[Citation4,Citation10]
Conclusion
Our starch modification process, which used mild reaction conditions, demonstrated the possibility of using agricultural by-products from two agricultural chains to produce materials with potential industrial applications, especially in food, cosmetics, and pharmaceuticals. The three varieties of starch presented various features that opened the possibility for synthesizing new materials with potential industrial uses; furthermore, our starch modification process could also generate economic and environmental advantages. The characteristics of the F60 variety are promising, and could have potential future application in the pharmaceutical industry, as well as being used as a wall material. The properties exhibited by Clearfield and Oryzica 1 MS enable their use in producing bioplastics; moreover, they could be used by the cosmetics industry to improve the stability of the formulations they create. Finally, using gulupa oil and rice starch to obtain polymers with high industrial applicability strengthens the rice and gulupa production chains through an alternative circular economy that favors the principles of green chemistry.
CRediT authorship contribution statement
Alvaro Esteban Aldana Porras: investigation, writing—original draft, and methodology. Diego Fernando Montoya Yepes: conceptualization, methodology, and investigation. Walter Murillo Arango: supervision and validation. Ángel Arturo Jiménez Rodríguez: methodology and investigation. Jonh Jairo Méndez Arteaga: data curation and writing—review and editing.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rhowell, N. T., Jr; Bonto, A. P.; Sreenivasulu, N. Enhancing the Functional Properties of Rice Starch through Biopolymer Blending for Industrial Applications: A Review. Int. J. Biol. Macromol. 2021, 192, 100–117. DOI: 10.1016/j.ijbiomac.2021.09.194.
- Zarski, A.; Bajer, K.; Zarska, S.; Kapusniak, J. From High Oleic Vegetable Oils to Hydrophobic Starch Derivatives: I. Development and Structural Studies. Carbohydr. Polym. 2019 Jun 15;[Epub 2019 Mar 14];214:124–130. PMID: 30925980. 10.1016/j.carbpol.2019.03.034
- Melis, S.; Morales, W. R. M.; Delcour, J. A. Lipases in Wheat Flour Bread Making: Importance of an Appropriate Balance between Wheat Endogenous Lipids and Their Enzymatically Released Hydrolysis Products. Food Chem. 2019, 298, 125002. DOI: 10.1016/j.foodchem.2019.125002.
- Montoya, D.; Barbosa, L. O.; Méndez, J.; Murillo, W. Morphological, Structural, and Functional Evaluation of Rice Starch Acylated in a System Catalyzed by the B‐Lipase of Candida Antarctica. Starch‐Stärke. 2020, 72(11–12), 2000010. DOI: 10.1002/star.202000010.
- Aldana Porras, A. E.; Montoya Yepes, D. F.; Murillo Arango, W.; Méndez Arteaga, J. J. Aspergillus Niger y Rhizopus oryzae inmovilizados para la producción de almidón modificado. Biotecnología. En. El. Sector Agropecuario Y. Agroindustrial. 2021, 19(2):69–81. DOI:10.18684/bsaa.v19.n2.2021.1544.
- Rajan, A.; Sudha, J. D.; Abraham, T. E. Enzymatic Modification of Cassava Starch by Fungal Lipase. Ind. Crops Prod. 2008, 27(1), 50–59. DOI: 10.1016/j.indcrop.2007.07.003.
- Mayilvahanan, A.; Ramchary, A.; Niraikulam, A.; Marichetti Kuppuswami, G.; Numbi Ramudu, K. A Green Process for Starch Oleate Synthesis by Cryptococcus Sp. MTCC 5455 Lipase and Its Potential as an Emulsifying Agent. Starch‐Stärke. 2019, 71(1–2), 1700325. DOI: 10.1002/star.201700325.
- Montoya, D.; Murillo, W.; Jímenez, A.; Mendez, J.; Aldana, A. E. Encapsulation of Phenols of Gulupa Seed Extract Using Acylated Rice Starch: Effect on the Release and Antioxidant Activity. J. Funct. Foods. 2021, 87, 104788. DOI: 10.1016/j.jff.2021.104788.
- Breternitz, N. R.; de Vasconcelos Fidelis, C. H.; Silva, V. M.; Eberlin, M. N.; Hubinger, M. D. Volatile Composition and Physicochemical Characteristics of Mussel (Perna Perna) Protein Hydrolysate Microencapsulated with Maltodextrin and n-OSA Modified Starch. Food Bioprod. Process. 2017, 105, 12–25. DOI: 10.1016/j.fbp.2017.05.008.
- Shao, P.; Zhang, H.; Niu, B.; Jin, W. Physical Stabilities of Taro Starch Nanoparticles Stabilized Pickering Emulsions and the Potential Application of Encapsulated Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 2032–2039. DOI: 10.1016/j.ijbiomac.2018.07.076.
- Wang, L.; Wang, Y. J. Rice Starch Isolation by Neutral Protease and high-intensity Ultrasound. J. Cereal Sci. 2004, 39(2), 291–296. DOI: 10.1016/j.jcs.2003.11.002.
- Horchani, H.; Chaâbouni, M.; Gargouri, Y.; Sayari, A. Solvent-free lipase-catalyzed Synthesis of long-chain Starch Esters Using Microwave Heating: Optimization by Response Surface Methodology. Carbohydr. Polym. 2010, 79(2), 466–474. DOI: 10.1016/j.carbpol.2009.09.003.
- Mendoza, J. S.; Díaz, R.; Hernández, J.; Quintero, A. F. Effect of the Acetylation Process on Native Starches of Yam (Dioscorea Spp.). Revista. Facultad. Nacional de. Agronomía. Medellín. 2016, 69(2), 7997–8006. DOI: 10.15446/rfna.v69n2.59144.
- Sutaphanit, P.; Chitprasert, P. Optimisation of Microencapsulation of Holy Basil Essential Oil in Gelatin by Response Surface Methodology. Food Chem. 2014, 150, 313–320. DOI: 10.1016/j.foodchem.2013.10.159.
- Wang, D.; Ma, X.; Yan, L.; Chantapakul, T.; Wang, W.; Ding, T.; Liu, D.; Liu, D. Ultrasound Assisted Enzymatic Hydrolysis of Starch Catalyzed by Glucoamylase: Investigation on Starch Properties and Degradation Kinetics. Carbohydr. Polym. 2017, 175, 47–54. DOI: 10.1016/j.carbpol.2017.06.093.
- Arns, B.; Bartz, J.; Radunz, M.; Do Evangelho, J. A.; Pinto, V. Z.; da Rosa Zavareze, E.; Dias, A. R. G. Impact of heat-moisture Treatment on Rice Starch, Applied Directly in Grain Paddy Rice or in Isolated Starch. LWT Food Sci. Technol. 2015, 60(2), 708–713. DOI: 10.1016/j.lwt.2014.10.059.
- Pinkaew, H.; Thongngam, M.; Wang, Y. J.; Naivikul, O. Isolated Rice Starch Fine Structures and Pasting Properties Changes during pre-germination of Three Thai Paddy (Oryza Sativa L.) Cultivars. J. Cereal Sci. 2016, 70, 116–122. DOI: 10.1016/j.jcs.2016.05.009.
- Granza, A. G.; Travalini, A. P.; Farias, F. O.; Colman, T. A. D.; Schnitzler, E.; Demiate, I. M. Effects of Acetylation and acetylation–hydroxypropylation (dual-modification) on the Properties of Starch from Carioca Bean (Phaseolus Vulgaris L.). J. Therm. Anal. Calorim. 2015, 119(1), 769–777. DOI: 10.1007/s10973-014-4092-9.
- Najafi, S. H. M.; Baghaie, M.; Ashori, A. Preparation and Characterization of Acetylated Starch Nanoparticles as Drug Carrier: Ciprofloxacin as a Model. Int. J. Biol. Macromol. 2016, 87, 48–54. DOI: 10.1016/j.ijbiomac.2016.02.030.
- Acevedo, L.; Nieto, L.; Sanchez, L. T.; Pinzon, M. I.; Villa, C. C. Development of Native and Modified Banana Starch Nanoparticles as Vehicles for Curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. DOI: 10.1016/j.ijbiomac.2018.01.063.
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G. Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated Starch Nanocrystals: Preparation and Antitumor Drug Delivery Study. Int. J. Biol. Macromol. 2016, 89, 456–464. DOI: 10.1016/j.ijbiomac.2016.04.037.
- Lukasiewicz, M.; Kowalski, S. Low Power Microwave‐assisted Enzymatic Esterification of Starch. Starch‐Stärke. 2012, 64(3), 188–197. DOI: 10.1002/star.201100095.
- Lim, J. H.; Kim, H. R.; Choi, S. J.; Park, C. S.; Moon, T. W. Complexation of Amylosucrase‐Modified Waxy Corn Starch with Fatty Acids: Determination of Their Physicochemical Properties and Digestibilities. J. Food Sci. 2019, 84(6), 1362–1370. DOI: 10.1111/1750-3841.14647.
- El Halal, S. L. M.; Colussi, R.; Pinto, V. Z.; Bartz, J.; Radunz, M.; Carreño, N. L. V.; da Rosa Zavareze, E.; Zavareze, E. D. R. Structure, Morphology and Functionality of Acetylated and Oxidised Barley Starches. Food Chem. 2015, 168, 247–256. DOI: 10.1016/j.foodchem.2014.07.046.
- Adak, S.; Banerjee, R. A Green Approach for Starch Modification: Esterification by Lipase and Novel Imidazolium Surfactant. Carbohydr. Polym. 2016, 150, 359–368. DOI: 10.1016/j.carbpol.2016.05.038.
- Sukhija, S.; Singh, S.; Riar, C. S. Physicochemical, Crystalline, Morphological, Pasting and Thermal Properties of Modified Lotus Rhizome (Nelumbo Nucifera) Starch. Food Hydrocolloids. 2016, 60, 50–58. DOI: 10.1016/j.foodhyd.2016.03.013.
- Shah, A.; Masoodi, F. A.; Gani, A.; Ashwar, B. A. Physicochemical, Rheological and Structural Characterization of Acetylated Oat Starches. LWT. 2017, 80, 19–26. DOI: 10.1016/j.lwt.2017.01.072.
- Issola, A. G.; Kamlo, A. N.; Yona, A. M.; Ndikontar, M. Chemical Modification of Cassava Starch by Transesterification Using VegeTable Oil/Aluminum Chloride. J. Renewable Mater. 2018, 6(6), 642–650.
- Bajaj, R.; Singh, N.; Kaur, A. Properties of Octenyl Succinic Anhydride (OSA) Modified Starches and Their Application in Low Fat Mayonnaise. Int. J. Biol. Macromol. 2019, 131, 147–157. DOI: 10.1016/j.ijbiomac.2019.03.054.
- Nasseri, R.; Moresoli, C.; Yu, A.; Yuan, Z.; Xu, C. C. Structural Dependence of the Molecular Mobility in Acetylated Starch. Polymer. 2021, 215, 123371. DOI: 10.1016/j.polymer.2020.123371.
- Wang, R.; Wang, J.; Liu, M.; Strappe, P.; Li, M.; Wang, A.; Zhou, Z.; Liu, J.; Blanchard, C.; Zhou, Z. Association of Starch Crystalline Pattern with Acetylation Property and Its Influence on Gut Microbota Fermentation Characteristics. Food Hydrocolloids. 2022, 128, 107556. DOI: 10.1016/j.foodhyd.2022.107556.
- Nasseri, R.; Moresoli, C.; Yu, A.; Yuan, Z.; Xu, C. C. Structural Dependence of the Molecular Mobility in Acetylated Starch. Polymer. 2021, 215, 123371. DOI: 10.1016/j.polymer.2020.123371.
- Ren, F.; Wang, S. Effect of Modified Tapioca Starches on the Gelling Properties of Whey Protein Isolate. Food Hydrocolloids. 2019, 93, 87–91. DOI: 10.1016/j.foodhyd.2019.02.025.
- Bhaskar Gangurde, A.; Ali, M.; Pawar, J.; Dhanraj Amin, P. Encapsulation of Vitamin E Acetate to Convert Oil to Powder Microcapsule Using Different Starch Derivatives. J. Pharm. Invest. 2016, 47(6), 559–574. DOI: 10.1007/s40005-016-0287-3.
- Sun, S.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. An Investigation into the Structure and Digestibility of starch-oleic Acid Complexes Prepared under Various Complexing Temperatures. Int. J. Biol. Macromol. 2019, 138, 966–974.
- Simsek, S.; El, S. N. In Vitro Starch Digestibility Estimated Glycemic Index and Antioxidant Potential of Taro (Colocasia Esculenta L. Schott) Corm. Food Chem. 2015, 168, 257–261. DOI: 10.1016/j.foodchem.2014.07.052.
- Marto, J.; Pinto, P.; Fitas, M.; Gonçalves, L. M.; Almeida, A. J.; Ribeiro, H. M. Safety Assessment of starch-based Personal Care Products: Nanocapsules and Pickering Emulsions. Toxicol. Appl. Pharmacol. 2018, 342, 14–21. DOI: 10.1016/j.taap.2018.01.018.
- Mose, B. R.; Maranga, S. M. A Review on starch-based Nanocomposites for Bioplastic Materials. J. Mater. Sci. Eng. B. 2011, 1(2B), 239.
- Koh, G. Y.; Rowling, M. J. Resistant Starch as a Novel Dietary Strategy to Maintain Kidney Health in Diabetes Mellitus. Nutr. Rev. 2017, 75(5), 350–360. DOI: 10.1093/nutrit/nux006.