?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study examined the effects of two fat-to-lean ratios (7:3 and 6:4) and five cooking times (30 min, 60 min, 90 min, 120 min and 150 min) on water migration in and the nutritional quality and fatty acid composition of traditional Chinese pork meatballs. Accordingly, this work has established a quality control reference for the industrial-scale research and development of pork meatball production. Low-field nuclear magnetic resonance was used to determine the distribution and migration of water in pork meatballs during the cooking process, and gas chromatography–mass spectrometry was used to determine the fatty acid composition of pork meatballs. The results show that the water in pork meatballs migrated and thus became more strongly bound as the cooking time increased. The relaxation time of water in pork meatballs with a fat-to-lean ratio of 7:3 was significantly higher than that of water in pork meatballs with a fat-to-lean ratio of 6:4 (P < .05). The length of cooking time did not have a significant effect on the protein or fat content of pork meatballs (P > .05). When the pork meatballs were cooked for 150 min, the calorie value of pork meatballs with a fat-to-lean ratio of 7:3 was significantly greater than that of pork meatballs with a fat-to-lean ratio of 6:4. The hardness and tenderness of pork meatballs with a fat-to-lean ratio of 7:3 were significantly lower than those of pork meatball with a fat-to-lean ratio of 6:4 (P < .05), but the elasticity and chewiness of the former were greater than that of the latter. During cooking, the improvement in the thiobarbituric acid reactive substances (fat oxidation) value of pork meatballs with a fat-to-lean ratio of 6:4 was greater than that of pork meatballs with a fat-to-lean ratio of 7:3. The main fatty acids in both types of pork meatballs were oleic acid (C18:1 n9c), linoleic acid (C18: 2), palmitic acid (C16:0) and stearic acid (C18:0), with oleic acid (C18:1n9c) being predominant. These results show that in terms of taste and textural requirements, and unsaturated fatty acid content, pork meatballs with a fat-to-lean ratio of 7:3 and a cooking time of 150 min can best meet the texture preferences and nutritional needs of consumers.
Introduction
Consumer demand for convenience meat products, such as ready-to-eat and semi-prepared pork products, has increased in recent years. Large pork meatballs, also called as Shi Zi Tou, big meatballs, sunflower big meatballs, Shandong cuisine, Si Xi Wan Xi, are among the most famous traditional foods in China. This traditional dish is made from pork belly and is characteristically tender. Qing Barnyard Banknotes states that ‘Pork meatballs are named after pork rounds, a similar dish. The pork is half fat and half lean, thinly cut, coarsely chopped and combined with egg whites to enable it to solidify or mixed with shrimp and crab powder.’[Citation1] The high concentrations of fat and fatty acids give pork meatballs an appealing flavor and a smooth and tender mouthfeel. They are fatty but not greasy and melt in the mouth. Pork belly ribs, which contain fine muscle fibers and a high water content, should be used to prepare this dish.[Citation2]
Research on pork meatballs has focused mainly on process optimization, process standards and routine nutritional analyses. To date, no reports have described the changes in the fatty acid composition of the pork meatballs during processing. Wang et al.,[Citation3] Shen et al.[Citation4] and Li et al.[Citation5] studied the changes in fats and fatty acids in braised pork during processing under different conditions. However, the processes used to produce braised pork and pork meatballs are considerably different. Kun et al.[Citation6] investigated the combined effects of a low-frequency magnetic field (LF-MF, 3.8 mT) and pH (5–7) on the quality of a pork myofibrillar protein gel. The application of the LF-MF led to various changes in the water ratio and migration rate. In addition, it has been shown that treatment with an ice-structuring protein effectively restrained water migration and mi-crostructural destruction in pork patties subjected to freeze-thaw cycles.[Citation3] However, studies on the effects of different fat-to-lean ratios and cooking process conditions on water migration and nutritional quality in pork patties have not been reported.The fat-to-lean ratio is another important factor that affects emulsion formation and gel stabilization in pork meatballs.[Citation7] Traditionally, pork meatballs have a high fat content, which stabilizes the meat and improves their firmness, juiciness and flavor. These improvements are attributed to the ability of the added fat to emulsify with protein and water in the meat. Compared with pork meatballs prepared using only lean meat, those containing a certain proportion of fat have a softer taste. However, pork meatballs containing a large proportion of fat are greasy, which adversely affects their quality. The addition of excess fat to pork meatballs will also increase their content of cholesterol, which is harmful to human health.[Citation8] In particular, the pork meatballs made in Yangzhou city of China with a fat-to-lean ratio of 7:3 or 6:4 are perceived as fresh and tender.[Citation9] Therefore, this article describes the effects of different fat-to-lean ratios and cooking times on the water distribution, nutritional content, texture (including tenderness), thiobarbituric acid reactive substances (TBARS) value and fatty acid composition ratio of pork meatballs. The correlations between these factors are also explored to determine a scientific and healthy cooking mode and provide theoretical support for quality control and research and development in industrial production settings.
Materials and methods
Sample collection
Fat and lean pork belly tissues with uniform layering were selected from the left abdominal ribs of ternary cross-bred pigs that had been slaughtered 18 hours earlier.
Preparation of pork meatballs
Pork meatballs were prepared according to the traditional Chinese process,[Citation10] as follows. Pork belly tissue was washed and dried, the skin was removed, and the meat was frozen at −20°C for 2 h. Subsequently, the meat was finely diced, and the fat and lean meat were separated and retained for further use. Pork meatballs with fat-to-lean ratios of 6:4 and 7:3 were prepared using the following process: one egg, 10 g of chopped green onion, 10 g of chopped ginger, 15 g of starch, 2 g of salt and 90 g of cooking wine were added to 500 g of meat mixture. The mixture was stirred evenly in a clockwise direction until it formed a paste, and was then chilled in a refrigerator (4°C) for 1 h. Next, 90-g pieces of paste were flattened into circles between the palms of two hands. The resulting pork circles were added to a pot of boiling water on an induction cooker, and the mixture was boiled at 500 W until the pork circles had become meatballs. The pork meatballs were then transferred to a saucepan containing boiling water. The resulting mixture was boiled at 1000 W, and was then simmered at 200 W, for a total time of 150 min. The soup was then drained off and the pork meatballs were left to equilibrate at 25°C in an air-conditioned room, for later analysis. Samples were collected every 30 min during the cooking process, and the preparation was repeated five times.
Water distribution and migration
Low-field nuclear magnetic resonance (LF-NMR) was used to determine the water distribution of the pork meatballs at each sampling time point. Thirty grams of each sample were pressed into a cylinder with a diameter of 3.5 cm and height of 3 cm, which was then wrapped carefully with polytetrafluoroethylene tape and placed in a special NMR tube. The relaxation time of each sample was measured in a constant-temperature nuclear magnetic field generated by a radio-frequency coil. The Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence was used to determine the T2 spin-spin relaxation time in the sample. The following sample collection parameter settings were applied: receiving gain = 50 dB, echo interval = 400 μs, number of sampling points = 4,096, scan number = 32 and recycle delay interval = 2000 ms. Three measurements were obtained per sample, and the average value was used for inversion and analysis.
Macronutrient and calorie value analysis
The protein and moisture contents of pork meatballs were determined by the methods of AOAC International.[Citation11] Fat was extracted by the method of Folch et al.[Citation12] Calorie values were determined using the following method. Each sample was cut into cubes measuring 0.2 cm3, which were then distributed evenly in the test tray of a Calorie Answer analyzer (CA-HM, JWP, Japan). The time required to determine the calorie value was 5 min.
Texture profile analysis
Samples were collected at each time point and cut into 30 mm (length) × 10 mm (width) × 5 mm (height) pieces. These pieces were subjected to texture profile analysis with the P36R probe of a TMS-Pro Texture Analyzer, which has a maximum detection force of 2500 N, using the following test conditions: pretest speed = 3 mm/s; test speed = 1 mm/s; posttest speed = 5 mm/s; compression distance = 10 mm and trigger force = 5.0 N. Each sample was subjected to two compressions separated by an interval of 3.0 s.
Shear force measurement
Samples were collected at each time point and cut into pieces measuring 30 mm × 10 mm × 5 mm, as above. These pieces were analyzed with a digital muscle tenderness tester (C-LM3B), using a 1000-N sensor equipped with a cutting fixture, at a sampling frequency of 10 Hz and a starting force of 2 N.
Fat oxidation (TBARS) analysis
Fat oxidation was evaluated using the method of Díaz et al.,[Citation13] with some modifications. Ten grams of each test sample were ground, mixed with 50 mL of 7.5% trichloroacetic acid (containing 0.1% EDTA), shaken for 30 min and filtered twice. Next, 5 mL of the supernatant were combined with 5 mL of 0.02 mol/L thiobarbituric acid (TBA) and incubated in a water bath at 90°C for 40 min. A blank sample containing only reagent was also incubated as the control condition. Subsequently, the samples were cooled for 1 h and centrifuged at 16 000 rpm for 5 min. The supernatant from each sample was mixed with 5 mL of chloroform and shaken thoroughly. The samples were then allowed to separate into layers. The supernatant was collected, and the absorbance values at 532 and 600 nm (A532 and A600) were measured. The TBARS value of each sample was then calculated using EquationEquation (1)(1)
(1) and is expressed as the mass (mg) of malondialdehyde (MDA) per kilogram of meat:
Notes:”155” indicates the extinction coefficient.”10” indicates the mass of the sample”72.6” indicates the relative molecular mass of malondialdehyde
Fatty acid content and analysis
The method of Campo et al.[Citation14] was used for fatty acid methyl esterification, with slight modifications. First, the fat was extracted from the sample according to the method of Folch et al.[Citation12] Then, 40–50 mg of extracted fat was placed in a test tube and treated with 3 mL of 0.5 N NaOH-CH3OH solution, and the resulting mixture was heated in a water bath at 55°C for 30 min to dissolve the oil droplets. The solution was then cooled to room temperature, treated with 3 mL of 14% boron trifluoride–methanol and 1–2 drops of 2,2-dimethoxypropane (a water scavenger) and heated in a water bath at 55°C for 30 min to methylate the fatty acids. After this time, the mixture was cooled to room temperature, treated with 2 mL of n-hexane, 2 mL of saturated NaCl solution and 100 μL of methyl octanoate (an internal standard) and then stood at 4°C to separate. The supernatant was quantitatively removed from the separated mixture and passed through a 0.22-μm pore size organic-phase filter membrane. Finally, the filtrate was analyzed by gas chromatography-mass spectrometry (GC-MS).
GC conditions: For GC, a DB-Wax chromatographic column (30 m × 0.25 mm × 0.25 µm) was used with high-purity nitrogen (99%) as the carrier gas, splitless injection and a flow rate of 1.0 mL/min. The injection port temperature was 250°C, and the heating programme was 50°C for 1 min, then heated at 4°C/min to 200°C, held for 5 min, then heated at 4°C/min to 220°C and held for 20 min.
MS conditions: For MS, an electron ionization (EI) ion source was used with an emission current of 200 µA, electron energy of 70 eV, interface temperature of 250°C, ion source temperature of 200°C, delay time of 5 min, mass scan range m/z of 35–450 and acquisition scan mode.
Composition analysis: The mass spectrum data obtained by GC-MS were subjected to a computerized search, and the standard mass spectra provided by the mass spectrum library and the retention time of the standard product were analyzed to detect the components.
Content analysis: The content of each component in the sample was calculated according to the concentration of the added internal standard substance and the ratio of the peak areas of different components in the sample to the peak area of the internal standard.
Statistical analyses
All experimental sample preparations were repeated five times. The data were processed using Origin 8.5 software, and differences between the data were determined by single-factor analysis of variance. All data of each sample group were analyzed using a general linear model. The linear and quadratic associations of the dependent variables with the independent variables were determined via regression analysis with SPSS 19.0 software. The analysis results are expressed as means ± standard deviations (mean ± SD), and Duncan’s new multiple-range method was used to make multiple comparisons between means. Significance was determined at the 0.05 level (P < .05).
Results and discussion
Effects of fat-to-lean ratio and cooking time on water distribution in pork meatballs
LF-NMR is a rapid, effective method of determining the moisture content and state changes in a material. Typical moisture relaxation distribution maps were obtained by fitting the CPMG relaxation curves of pork meatballs cooked for different times. depicts the water migration relaxation diagram of pork meatballs with a fat-to-lean ratio of 7:3, while depicts that of pork meatballs with a fat-to-lean ratio of 6:4. For these samples, respectively depict the corresponding areas occupied by the T21 and T22 peaks in ratios expressed as A21 and A22. As shown in the figures, two relaxation peaks were observed in the pork ball samples with different fat-to-lean ratios and cooking times. The T21 peak corresponded to strongly bound water (relaxation time in the range of 0–10 ms) and the T22 peak to weakly bound water (10–100 ms).[Citation15] Cheng et al.[Citation16] attributed the T21 signal to water strongly bound to protein macromolecules and the T22 signal (weakly bound water) to water immobilized in the myofibril network; the latter has the largest peak, with A22 values as high as 90% in this study.
Figure 1. Inversion diagram of the lateral relaxation time of water (T2) in pork meatballs with a fat-to-lean ratio of 7:3 and cooked for various times. Lowercase letters (a, b and c) represent significant differences (P < .05).
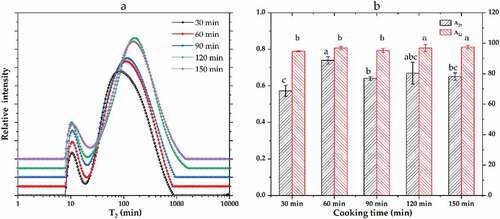
Figure 2. Inversion diagram of the lateral relaxation time of water (T2) in pork meatballs with a fat-to-lean ratio of 6:4 and cooked for various times. Lowercase letters (a, b and c) represent significant differences (P < .05).
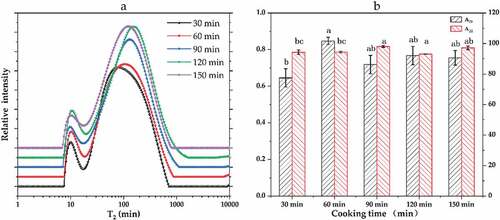
As shown in and , the T21 and T22 relaxation times increased significantly as the cooking time increased because the long-term thermal denaturation of muscle protein changed the internal water structure in the samples.[Citation17] shows a significant difference in the A21 peak area of the pork meatballs with a fat-to-lean ratio of 7:3 as the cooking time varies (P < .05), whereas the A22 peak area does not vary significantly with the cooking time (P > .05). Specifically, A21 gradually increased with increasing cooking time, peaked after 90 min of cooking, and then gradually decreased. The results indicate that the extended cooking time helped the water to migrate to a strongly bound form. shows that the T21 and T22 relaxation times also increased as the cooking time was extended in the pork meatballs with a fat-to-lean ratio of 6:4; however, the relaxation time decreased significantly in samples cooked for 150 min. A possible explanation is that the longest cooking time reduced the content of myofibril. The concentration of sodium chloride in meat products can increase the water-holding capacity of myofibrillar protein, thereby affecting these products’ water balance.[Citation18] shows a significant difference in the proportions of the A21 peak areas in the pork meatballs with a fat-to-lean ratio of 6:4 under different cooking times (P < .05), whereas no significant difference was observed between the A22 values at different cooking times (P > .05). As the cooking time increased, A21 gradually increased and then decreased. This phenomenon is consistent with the data from the samples with a fat-to-lean ratio of 7:3, although in the samples with a ratio of 6:4, A21 reached a peak value after cooking for 60 min rather than 90 min.
Table 1. Effects of fat-to-lean ratio and cooking time on low-field nuclear magnetic resonance parameters (T21 and T22 relaxation times) of pork meatballs.
Mean values ± SD in the same row with different superscript letters (a, b and c) are significantly different (P < .05).
The T21 and T22 relaxation times differed significantly between the two types of samples. The significantly longer relaxation time in the 7:3 group compared with the 6:4 group (P < .05) may be because the excessive fat was able to tightly bind the water. Fat macromolecules increased the mobility of protons, decreased the mobility of water, and increased the relative contribution of the T21 peak, indicating that fat has a significant effect on the ability of proteins to bind protons. This is in contrast to the findings of LF-NMR studies reported by Shao[Citation19] on the effect of the protein hydration state on the fluidity, moisture content and fat content of meat paste.
Effect of fat-to-lean ratio and cooking time on the nutritional composition of pork meatballs
Protein and fat are important nutrients that determine the quality of meat. The effects of the fat-to-lean ratio and cooking time on the nutrient content of pork meatballs are shown in . During the cooking process, the protein and fat content of the pork meatballs did not change significantly compared with that of the raw pork (P > .05). However, different fat-to-lean ratios and cooking times increased the protein and fat contents of pork meatballs (P < .05) compared with raw pork. The protein and fat contents of pork meatballs also fluctuated with increased cooking time. This is mainly attributable to the loss of moisture during the cooking process, which results in the denaturation of intramuscular protein[Citation20] and the contraction of muscle fibers, and thus reduces the water-retention capacity of muscle tissue.[Citation21] The moisture content of the pork meatballs decreased with increasing cooking time, which is consistent with the results of Vasanthi et al.[Citation22] in a study on the stewing of buffalo meat at 100°C.
Table 2. Changes in basic nutritional indices during the processing of pork meatballs (g/100 g).
The fluctuation of the fat content with increased cooking time is also due to the tendency of fat to be released but then to degrade into volatile flavor substances during prolonged cooking.[Citation23] Specifically, heating shrinks the connective tissue in the adipose tissue of meat, and the resulting pressure increase crushes the adipose cells and thus releases fat into the cooking liquid.[Citation24] However, this fat is then melted and decomposed to fatty acids and flavor substances, which reduces the fat content.[Citation25] Our finding that the fat content in pork meatballs initially increased and then decreased with increased cooking time is consistent with the findings described in related literature.[Citation4,Citation11] In terms of compositional changes with increasing cooking time, the fat content of the pork meatballs with a fat-to-lean ratio of 7:3 became significantly greater than that in the pork meatballs with a fat-to-lean ratio of 6:4 (P < .05), but the differences between the protein content of these two types of pork meatballs was not significant (P > .05). Increased cooking time also increased the calorie value of pork meatballs, and the calorie value of pork meatballs with a fat-to-lean ratio of 7:3 was significantly greater than that of those with a fat-to-lean ratio of 6:4.
Mean values ± SD in the same column with different superscript letters (a, b, c) are significantly different (P < .05).
Changes in the texture of pork meatballs during cooking
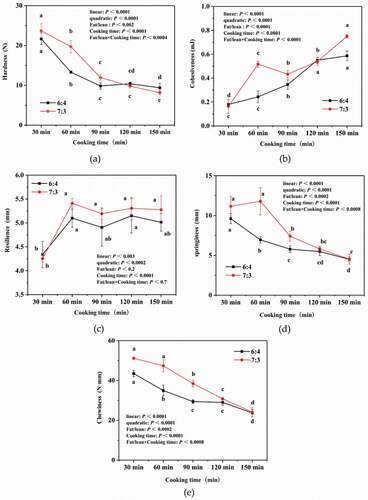
The hardness, resilience and chewiness of pork meatballs were significantly affected by the fat-to-lean ratio and the cooking time (P < .05). Overall, the hardness, resilience and chewiness of the pork meatballs significantly decreased (P < .05) with increased cooking time, while their cohesion increased. However, pork meatballs with a fat-to-lean ratio of 7:3 had significantly lower hardness and chewiness but higher elasticity than those with a fat-to-lean ratio of 6:4. This is attributable to the fact that as cooking time increases, the protein denaturation of myofibrils approaches completion. The resulting gelatinization of collagen and the destruction of connective tissue weakens adhesive forces in muscle tissue, thereby decreasing its hardness and chewiness.[Citation26,Citation27]
Meat tenderness is one of the most important eating qualities that influences consumer acceptance.[Citation28] During processing, meat proteins are denatured, and subcutaneous and intermuscular fat are dissolved.[Citation28] These dissolved fats may be absorbed into the lean tissues, thereby making the meat smoother and more tender. In addition, prolonged heating destroys the endomysium and perineurium, which may also increase meat tenderness.[Citation22] Tenderness and chewiness are important parameters that are used to assess the edible quality of stewed pork meatballs. In Chinese cooking, stewed pork meatballs that ‘melt in the mouth’ are desirable. According to , after 150 min of cooking, the hardness and chewiness of both types of pork meatballs decreased, but their Cohesion increases. The pork meatballs with a fat-to-lean ratio of 7:3 have a lower hardness than those with a fat-to-lean ratio of 6:4, but the cohesion of the former is higher than that of the latter. The elasticity and cohesion of pork meatballs are determined by the properties and interactions of water, elastin, collagen and muscle fibers. We found that cooking time had a significant effect on the texture of pork meatballs (P < .05), which is consistent with the results of Vasanthi et al.[Citation22] The texture index shows that the best cooking time for both types of pork meatballs is 150 min, and that pork meatballs with a fat-to-lean ratio of 7:3 better meet consumers’ tenderness preferences than pork meatballs with a fat-to-lean ratio of 6:4.
Figure 3. Effects of two fat-to-lean ratios (6:4 and 7:3) and various cooking times on the textural aspects of pork meatballs. Different lowercase letters (a, b, c) represent significant differences (P < .05).
Shear force measurement
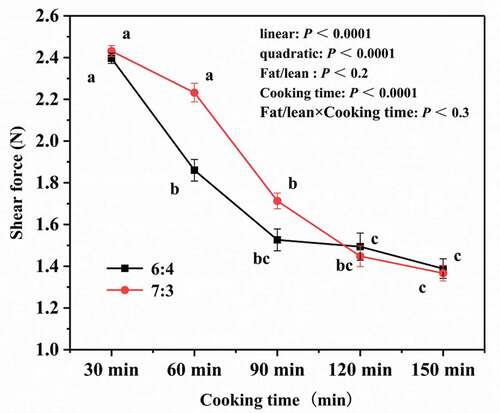
Tenderness is an important indicator of meat digestibility and can be measured by the magnitude of the force required to shear a sample. The cooking time had a significant effect on the shear force of both types of pork meatballs (P < .05). As shown in , their shear force was significantly reduced from 30 min to 150 min of cooking (P < .05). In addition, from 30 min to 120 min, the shear force of pork meatballs with a fat-to-lean ratio of 7:3 was greater than that of those with a fat-to-lean ratio of 6:4, but after 120 min the converse was true. Prolonged heating of meat dissolves collagen and/or decreases cross-linking between collagen molecules and denatures myofibril protein, leading to a decrease in shear force.[Citation29,Citation30] Thus, cooking strongly affects the tenderness of meat, especially when conducted at a low temperature over a long period of time.[Citation31]
Fat oxidation (TBARS) determination
The TBARS value is a measure of the quantity of secondary products, such as malondialdehyde, which are produced by the oxidative decomposition of unsaturated fatty acids in oils. These secondary products react with TBA, and the proportion of these reactions is calculated to give a TBARS value that indicates the extent of secondary oxidation of the fat in a meat sample.[Citation32] TBARS values are widely used to evaluate the extent of fat oxidation of meat products. The changes in the TBARS values of both types of pork meatballs with various cooking times are shown in . After cooking for 30 min, the TBARS values of pork meatballs with fat-to-lean ratios of 6:4 and 7:3 were 0.23 mg MDA/kg and 0.145 mg MDA/kg, respectively. As cooking time increased, the TBARS value of pork meatballs with a fat-to-lean ratio of 6:4 gradually decreased, and then remained constant after 60 min. The results of Shi Xiaona et al.[Citation33] who studied the degradation and oxidation of fat during the processing of red meat are consistent with this paper.In pork meatballs with a fat-to-lean ratio of 7:3, the TBARS value slowly decreased until a cooking time of 90 min, and slowly increased thereafter.
Figure 5. Changes in the thiobarbituric acid reactive substances (TBARS) values in pork meatballs with different fat-to-lean ratios and cooking times. Lowercase letters (a, b and c) represent significant differences (P < .05).
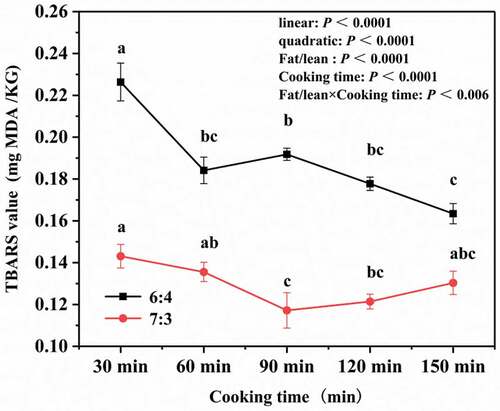
Hernández et al.[Citation34] processed pork by boiling and found that its TBARS value was initially 0.40 mg MDA/kg and increased significantly with increased cooking time, indicating that fat oxidation continued with prolonged heating. Other studies have also shown that the TBARS value may increase after boiling or high-temperature roasting, and that this change is positively correlated with the cooking temperature.[Citation35] However, the TBARS values of the two types of pork meatballs studied in this paper show a decreasing trend over cooking time. There are two possible reasons for this. First, the pork meatballs were stewed by continual heating at a low power (200 W) in a closed (lidded) container. This isolation of the pork meatballs from oxygen in the air would have prevented continued oxidation of fats, thereby leading to the reduced TBARS value. Second, the pork meatballs contained onions, ginger and other components with antioxidant properties, which may have inhibited the oxidation of fats. Fat oxidation is crucial to the production of pork flavors, but is dependent on temperature: moderate oxidation under heating will produce palatable flavor substances, but oxidation at room temperature will produce a rancid taste.[Citation36]
Fatty acid content
The content of most fatty acids in pork meatballs was significantly affected by the fat-to-lean ratio, cooking time and the interaction between these factors (P < .05, ). The main fatty acids in pork meatballs were oleic acid (C18:1 n9c), linoleic acid (C18: 2), palmitic acid (C16:0), stearic acid (C18:0) and palmitoleic acid (C16:1), with oleic acid (C18:1 n9c) present in the highest proportion. It can be seen from that compared with raw pork, the SFA content in pork meatballs with a fat-to-lean ratio of 6:4 was significantly reduced during the cooking process (P < .05), while the SFA content in pork meatballs with a fat-to-lean ratio of 7:3 was increased. Changes in fatty acid content tended to be minor. The SFA content of pork meatballs with a fat-to-lean ratio of 7:3 ratio was slightly lower than that of those with a fat-to-lean ratio of 6:4. It appears that, in terms of SFA content, 150 min was the optimal cooking time for these pork meatballs. The decrease in the palmitic acid (C16:0) and stearic acid (C18:0) content of pork meatballs may account for the decrease in the SFA content. In addition, the C10:0, C12:0 and arachidic acid (C20:0) content of pork meatballs was low. SFAs are considered to be a possible cause of cancer and cardiovascular disease, and a diet high in C16:0 leads to high plasma concentrations of low-density lipoprotein cholesterol (LDL-C).[Citation37] An increase in the concentration of SFAs in the human body is considered a precursor to hypertension and hyperlipidemia.[Citation38] Therefore, a reduction in the SFA content of pork meatballs during cooking may be beneficial to the health of consumers. The monounsaturated fatty acid (MUFA) content of both types of pork meatballs increased significantly compared to that of raw pork (P < .05). However, the cooking time had little effect on the MUFA content of pork meatballs (P > .05). The MUFA content of the pork meatballs with a fat-to-lean ratio of 7:3 was higher than that of those with a fat-to-lean ratio of 6:4 (). Our results for other fatty acids are inconsistent with the results of Li et al.[Citation5] This can be attributed to differences between our study and that of Li et al., in terms of the parts of the pork belly that were used (as there are variations in the fat content and fatty acid composition of the fat and lean layers of pork belly[Citation39,Citation40]) and in the cutting and cooking methods that were utilized.
Table 3. Changes in fatty acid content during the processing of pork meatballs (mg/100 g muscle).
The polyunsaturated (PUFA) content of both types of pork meatballs was significantly affected by the cooking time and the fat-to-lean ratio (P < .05). In particular, although the PUFA content in pork meatballs with a fat-to-lean ratio of 7:3 did not change significantly from 60 to 150 min of cooking, it significantly increased compared with that in raw pork (P < .05). In contrast, the PUFA content in pork meatballs with a fat-to-lean ratio of 6:4 significantly decreased over cooking time compared with that in raw pork (P < .05), which is opposite to the results reported by Li et al. .[Citation5] This increase in the PUFA content of braised pork with prolonged stewing may have been due to the generation of highly active antioxidants during the braising process, such as Maillard reaction products (MRPs). MRPs specifically scavenge hydroxyl radicals and superoxides and retard the formation of peroxides,[Citation28,Citation41] and fat-to-lean-meat ratios affect the generation of MRPs from meat products.[Citation42] The unsaturated fatty acid (UFA) content of both types of pork meatballs increased significantly (P < .05) as cooking time increased. The UFA/SFA content of the pork meatballs was less than that of raw pork but increased during the cooking process, especially in pork meatballs with a fat-to-lean ratio of 6:4. This is aligned with the results of Ruihua et al.,[Citation43] who showed that the ratio of UFA to (UFA/SFA) increased after the pork was cooked. Thus, cooking improves the nutritional value of pork, in addition to enhancing its flavor. UFAs lower plasma concentrations of LDL-C, which can help to prevent atherosclerosis and cardiovascular diseases. Some long-chain saturated FAs are key components of the human nervous system, where they strengthen nerve conduction and brain tissue. They are also important skeletal components of enzyme tissues and impart flexibility to blood vessels.[Citation44] It is known that the fat-to-lean ratio of a meat product has a more significant effect on its textural properties than does the starch content.[Citation7] In addition, the tenderness and flavor of meat are positively correlated with its SFA and MUFA content, and negatively correlated with its PUFA content. Braising meat also considerably alters its flavor and texture.[Citation45]
Mean values ± SD with different superscript letters (a, b and c) in the same row are significantly different (P < .05). SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; UFA, unsaturated fatty acid.
Conclusion
We studied the effects of two fat-to-lean ratios and various cooking times on the water migration in and nutritional quality of Chinese traditional pork meatballs. The changes in water distribution, basic nutrients, texture, shear force, TBA value and fatty acid content of the pork meatballs during the cooking process were observed. We found that the fat-to-lean ratio and cooking time had a significant effect on the water distribution within pork meatballs (P < .05), as the water migrated and became more strongly bound as cooking time increased. Pork meatballs with a fat-to-lean ratio of 6:4 exhibited more water migration than those with a fat-to-lean ratio of 7:3. A two-factor interactive analysis of cooking time and fat-to-lean ratio reveals that cooking time did not have a significant effect on the protein and fat content of pork meatballs (P > .05). However, increased cooking time led to significant increases in the calorie value of both types of pork meatballs (P < .05). In addition, when cooked for 150 min, the calorie value of pork meatballs with a fat-to-lean ratio of 7:3 was significantly greater than that those with a fat-to-lean ratio of 6:4. The cooking time and fat-to-lean ratio also significantly affected the hardness, elasticity, chewiness and shear value of the pork meatballs (P < .04). A cooking time of 150 min led to decreases in the hardness and chewiness of both types of pork meatballs. Finally, the hardness and shear force values of the pork meatballs with a fat-to-lean ratio of 7:3 were lower than those of the pork meatballs with a fat-to-lean ratio of 6:4, which shows that the former are more suited to the demands of consumers for tender meat products.
Fatty acids are important nutrients for the human body, and their composition and content are related to the nutritional composition of the meat consumed. We found that the raw pork composition and cooking time had a profound influence on the ultimate fatty acid composition and content of pork meatballs. The main fatty acids in raw pork were determined to be UFAs, followed by SFAs and MUFAs. In contrast, oleic acid (C18:1 n9c), linoleic acid (C18:2), palmitic acid (C16:0) and stearic acid (C18:0) were the main fatty acids in pork meatballs, which shows that cooking significantly decreased the SFA content of pork meatballs (P < .05). UFAs have important physiological functions, and it is therefore notable that the UFA content of both types of pork meatballs increased significantly during cooking (P < .05). Due to improvements in living standards and changes in lifestyles, consumers pay increasing attention to food ingredients and cooking modes. This study shows that in terms of texture, shear force and UFA content, pork meatballs with a fat-to-lean ratio of 7:3 and a cooking time of 150 min can better meet the texture and nutritional demands of consumers than those with a fat-to-lean ratio of 6:4. We note that our use of onion, ginger, cooking wine and other condiments during the preparation of the pork meatballs may have affected the extent of fat oxidation and fatty acid composition. These aspects require exploration in future work.
Author contributions
Methodology, H.Z.; data curation, Q.W.; writing—original draft, W.Z.; writing—review and editing, Z.Y., Z.C. and J L.; supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Qing, K. X.;. Barnyard Banknotes[M]; Zhong Hua Book Company: Beijing, 1986; Vol. 3, pp 89.
- Zhou, X. Y.; Tang, J. H.; Chen, J.; Wang, X. Optimization of Key Process Conditions for the Making of Yangzhou-style Large meatballs[J]. Food Sci. 2010, 31(16), 145–150.
- Wang, Y. F.; Zhang, Q.; Bian, W. Y.; Ye, L.; Yang, X. B.; Song, X. Q. Preservation of Traditional Chinese Pork Balls Supplemented with Essential Oil Microemulsion in a phase-change Material Package. Journal of the Science of Food and Agriculture. 2020, 100(5), 2288–2295. DOI: 10.1002/jsfa.10262.
- Shen, Q.; Wang, M. T.; Tian, J. H.; Hu, L. L.; Ren, S. J.; Chen, J. C.; Ye, X.; Liu, D. Effects of Chinese Pickled and Dried Mustard on Nutritional Quality, Sensory Quality, and Shelf Life of Steamed Pork belly[J]. Food Sci. Nutr. 2018, 6(4), 747–756.
- Li, Y. Q.; Li, C. B.; Zhao, F.; Lin, X. S.; Bai, Y.; Zhou, G. H. The Effects of long-duration Stewing Combined with Different Cooking and Heating Methods on the Quality of Pork belly[J]. J. Food Process. Pres. 2016, 40(1), 94–102. DOI: 10.1111/jfpp.12587.
- Yang, K.; Zhou, Y. H.; Guo, J. J.; Feng, X. L.; Wang, X.; Wang, L. M.; Ma, J.; Sun, W. Low Frequency Magnetic Field Plus High pH Promote the Quality of Pork Myofibrillar Protein Gel: A Novel Study Combined with Low Field NMR and Raman spectroscopy[J]. Food Chemistry. 2020, 326, 9. DOI: 10.1016/j.foodchem.2020.126896.
- Banon, S.; Diaz, P.; Nieto, G.; Castillo, M.; Alvarez, D. Modelling the Yield and Texture of Comminuted Pork Products Using Color and Temperature. Effect of fat/lean Ratio and starch[J]. Meat Science. 2008, 80(3), 649–655. DOI: 10.1016/j.meatsci.2008.03.001.
- Zhang, J.; Zhang, Y. Q.; Wang, Y.; Xing, L. J.; Zhang, W. G. Influences of ultrasonic-assisted Frying on the Flavor Characteristics of Fried meatballs[J]. Innov. Food Sci. Emerg. Technol. 2020, 62, 102365. DOI: 10.1016/j.ifset.2020.102365.
- Mao, Y. Y.; Zhu, X. Analysis on the Flavor Formation of Yangzhou meatballs[J]. China Condiment 2003, (11), 33–36.
- Zhu, W. Z.; Xu, Y.; Liu, W.; Wang, Q. Y.; Sha, W. X.; Zhou, X. Y.; Yang, Z. P. Cooking Time on the Nutritional Quality and Volatile Flavor Compounds of Pork Meat ball[J]. Food and Fermentation Industry 2021, 47(4), 208–214.
- Li, Y. Q.; Li, C. B.; Li, H.; Lin, X. S.; Deng, S. L.; Zhou, G. H. Physicochemical and Fatty Acid Characteristics of Stewed Pork as Affected by Cooking Method and time[J]. Int. J. Food Sci. Tech. 2016, 51(2), 359–369. DOI: 10.1111/ijfs.12968.
- Folch, J. L. M.; Sloane-Stanley, G.; Stanley, G. H. S. A Simple Method for the Isolation and Purification of Total Lipids from Animal tissues[J]. J. Biol. Chem. 1957, 226(226), 497–509. DOI: 10.1016/S0021-9258(18)64849-5.
- Diaz, P.; Linares, M. B.; Egea, M.; Auqui, S. M.; Garrido, M. D. TBARs Distillation Method: Revision to Minimize the Interference from Yellow Pigments in Meat products[J]. Meat Sci. 2014, 98(4), 569–573. DOI: 10.1016/j.meatsci.2014.06.012.
- Campo, M. M.; Muela, E.; Olleta, J. L.; Moreno, L. A.; Santaliestra-Pasias, A. M.; Mesana, M. I.; Sañudo, C. Influence of Cooking Method on the Nutrient Composition of Spanish Light lamb[J]. Journal of Food Composition and Analysis. 2013, 31(2), 185–190.
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of Multiple freeze-thaw Cycles on the Quality of Instant Sea Cucumber: Emphatically on Water Status of by LF-NMR and MRI[J]. Food Res. Int. 2018, 109, 65–71. DOI: 10.1016/j.foodres.2018.04.029.
- Cheng, S.; Wang, X.; Yang, H.; Lin, R.; Wang, H.; Tan, M. Characterization of Moisture Migration of Beef during Refrigeration Storage by low-field NMR and Its Relationship to Beef quality[J]. Journal of the Science of Food and Agriculture. 2020, 100(5), 1940–1948. DOI: 10.1002/jsfa.10206.
- Bouhrara, M.; Clerjon, S.; Damez, J. L.; Chevarin, C.; Portanguen, S.; Kondjoyan, A.; Bonny, J.-M. Dynamic MRI and Thermal Simulation to Interpret Deformation and Water Transfer in Meat during heating[J]. J. Agric. Food Chem. 2011, 59(4), 1229–1235.
- Tsuruhashi, K.; Ooizumi, T.; Akahane, Y.; Sakai, A. T. Internal Migration of Sodium Chloride, Sorbitol and Moisture in Fish Meat during Soaking and Cold preservation[J]. Fish. Sci. 2003, 69(4), 836–841. DOI: 10.1046/j.1444-2906.2003.00695.x.
- Shao, J.-H.; Deng, Y.-M.; Song, L.; Batur, A.; Jia, N.; Liu, D.-Y. Investigation the Effects of Protein Hydration States on the Mobility Water and Fat in Meat Batters by LF-NMR technique[J]. LWT - Food Science and Technology. 2016, 66, 1–6. DOI: 10.1016/j.lwt.2015.10.008.
- Palka, K.; Daun, H. Changes in Texture, Cooking Losses, and Myofibrillar Structure of Bovine M-semitendinosus during heating[J]. Meat Sci. 1999, 51(3), 237–243. DOI: 10.1016/S0309-1740(98)00119-3.
- Chian, F. M.; Kaur, L.; Astruc, T.; Venien, A.; Stubler, A. S.; Aganovic, K. Shockwave Processing of Beef Brisket in Conjunction with Sous Vide Cooking: Effects on Protein Structural Characteristics and Muscle microstructure[J]. Food Chem. 2021, 343,128500.
- Vasanthi, C.; Venkataramanujam, V.; Dushyanthan, K. Effect of Cooking Temperature and Time on the physico-chemical, Histological and Sensory Properties of Female Carabeef (Buffalo) meat[J]. Meat Science. 2007, 76(2), 274–280. DOI: 10.1016/j.meatsci.2006.11.018.
- Larsen, D.; Quek, S. Y.; Eyres, L. Effect of Cooking Method on the Fatty Acid Profile of New Zealand King Salmon (Oncorhynchus tshawytscha)[J]. Food Chem. 2010, 119(2), 785–790. DOI: 10.1016/j.foodchem.2009.07.037.
- Chen, L. Effects of ultrasound-assisted Cooked Pork Belly on Nutritional and Texture characteristics[D].Hunan Agricultural University. 2016,32–33.
- He, J.; Liu, H. Z.; Balamurugan, S.; Shao, S. Q. Fatty Acids and Volatile Flavor Compounds in Commercial plant-based burgers[J]. Journal of Food Science. 2021, 86(2), 293–305. DOI: 10.1111/1750-3841.15594.
- Gopinath, S. P.; Anthony, M. X. K.; Nagarajarao, R. C.; Jaganath, B.; Krishnaswamy, S. G. T. Standardisation of Process Parametres for ready-to-eat Squid Masala in Indigenous polymer-coated tin-free Steel cans[J]. Int. J. Food Sci. Tech. 2007, 42(10), 1148–1155. DOI: 10.1111/j.1365-2621.2006.01274.x.
- Kong, F. B.; Tang, J.; Rasco, B.; Crapo, C. Kinetics of Salmon Quality Changes during Thermal processing[J]. Journal of Food Engineering. 2007, 83(4), 510–520. DOI: 10.1016/j.jfoodeng.2007.04.002.
- Alfaia, C. M.; Lopes, A. F.; Prates, J. Cooking and Diet Quality: A Focus on meat[M]. Diet Quality. 2013,102–103.
- Zhu, X. S.; Ruusunen, M.; Gusella, M.; Zhou, G. H.; Puolanne, E. High post-mortem Temperature Combined with Rapid Glycolysis Induces Phosphorylase Denaturation and Produces Pale and Exudative Characteristics in Broiler Pectoralis Major muscles[J]. Meat Sci. 2011, 89(2), 181–188. DOI: 10.1016/j.meatsci.2011.04.015.
- Ishiwatari, N.; Fukuoka, M.; Sakai, N. Effect of Protein Denaturation Degree on Texture and Water State of Cooked meat[J]. Journal of Food Engineering. 2013, 117(3), 361–369. DOI: 10.1016/j.jfoodeng.2013.03.013.
- Baldassini, W. A.; Neto, O. R. M.; Fernandes, T. T.; Ament, H. D.; Luz, M. G.; Santiago, B. M. Testing Different Devices to Assess the Meat Tenderness: Preliminary results[J]. J. Food Sci. Technol. 2021,58(6) , 2441–2446.
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of Chitosan, Aqueous Extract of Ginger, Onion and Garlic on Quality and Shelf Life of stewed-pork during Refrigerated storage[J]. Food Chem. 2013, 141(3), 1655–1660.
- Shi, X. N.; Huang, F.; Zhang, L. Study on the Changes of Fat Degradation, Oxidation and Volatile Flavor Substances during the Processing of Red meat[J]. Modern Food Sci and Technol. 2017, 33(3), 257–265.
- Hernandez, P.; Navarro, J. L.; Toldra, F. Lipids of Pork Meat as Affected by Various Cooking techniques[J]. Food Science and Technology International. 1999, 5(6), 501–508. DOI: 10.1177/108201329900500608.
- Peiretti, P. G.; Medana, C.; Visentin, S.; Dal Bello, F.; Meineri, G. Effect of Cooking Method on Carnosine and Its Homologues, Pentosidine and Thiobarbituric acid-reactive Substance Contents in Beef and Turkey meat[J]. Food Chem. 2012, 132(1), 80–85. DOI: 10.1016/j.foodchem.2011.10.035.
- Mottram, D. S. The Effect of Cooking Conditions on the Formation of Volatile Heterocyclic Compounds in pork[J]. Journal of the Science of Food and Agriculture. 1985, 36(5), 377–382. DOI: 10.1002/jsfa.2740360510.
- Webb, E. C.; O’Neill, H. A. The Animal Fat Paradox and Meat quality[J]. Meat Sci. 2008, 80(1), 28–36. DOI: 10.1016/j.meatsci.2008.05.029.
- Russell, F. D.; Burgin-Maunder, C. S. Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic acids[J]. Marine Drugs. 2012, 10(12), 2535–2559. DOI: 10.3390/md10112535.
- Soladoye, P. O.; Shand, P. J.; Aalhus, J. L.; Gariepy, C.; Juarez, M. Review: Pork Belly Quality, Bacon Properties and Recent Consumer trends[J]. Can. J. Anim. Sci. 2015, 95(3), 325–340. DOI: 10.4141/cjas-2014-121.
- Trusell, K. A.; Apple, J. K.; Yancey, J. W. S.; Johnson, T. M.; Galloway, D. L.; Stackhouse, R. J. Compositional and Instrumental Firmness Variations within Fresh Pork bellies[J]. Meat Sci. 2011, 88(3), 472–480. DOI: 10.1016/j.meatsci.2011.01.029.
- Hwang, I. G.; Kim, H. Y.; Woo, K. S.; Lee, J.; Jeong, H. S. Biological Activities of Maillard Reaction Products (Mrps) in a sugar–amino Acid Model system[J]. Catedra. 2011, 126(1), 221–227.
- Wang, Z. L.; Cai, R.; Yang, X. D.; Gao, Z. P.; Yuan, Y. H.; Yue, T. L. Changes in Aroma Components and Potential Maillard Reaction Products during the stir-frying of Pork slices[J]. Food Control. 2021, 123, 107855. DOI: 10.1016/j.foodcont.2020.107855.
- Ruihua, W.; Qian, W.; Wanzhou, J.; Jianchu, C.; Xingqian, Y.; Donghong, L. Effects of Cooking Methods on Lipid Oxidation and Fatty Acid Profiles in Intramuscular Tissues of pork[J]. Chinese J Food Sci 2017, 17(7), 61–68.
- Neff, M. R.; Bhavsar, S. P.; Braekevelt, E.; Arts, ,. M. T. Effects of Different Cooking Methods on Fatty Acid Profiles in Four Freshwater Fishes from the Laurentian Great Lakes region[J]. Food Chemistry. 2014, 164(12), 544–550. DOI: 10.1016/j.foodchem.2014.04.104.
- Meng, Q. W.; Sun, S. S.; Sun, Y. C.; Li, J. A.; Wu, D.; Shan, A. S.; Shi, B.; Cheng, B. Effects of Dietary Lecithin and L-camitine on Fatty Acid Composition and Lipid Metabolic Genes Expression in Subcutaneous Fat and Longissimus Thoracis of growing-finishing pigs[J]. Meat Sci. 2018, 136, 68–78. DOI: 10.1016/j.meatsci.2017.10.012.