 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Chocolate biscuits are one type of biscuit that contains antioxidants, albeit in small amounts. Incorporating functional compounds into the product is an option for enhancing its quality. This study investigated chocolate biscuits’ physicochemical, antioxidant, and sensory properties incorporated with encapsulated mangosteen peel extract in percentages of 1%, 3%, 5%, and 7%. The increase in mangosteen peel extract microcapsules significantly affected the total value of polyphenols, flavonoids, peroxide number, color, and texture profile. But, there were no significant differences in the organoleptic characteristics of color, aroma, taste, texture, and overall acceptance. Total polyphenols, flavonoids, texture profile, a* and b* values, and browning index were all positively correlated with increasing the concentration of encapsulated in the formula. However, there was a negative correlation between antioxidant activity, peroxide number, and L*. It obtained the best recipe for its physicochemical, organoleptic, and antioxidant properties at 5%. Overall, the panelists approved the chocolate biscuits with mangosteen peel extract microcapsules. This product’s development is an alternative to antioxidant-rich functional foods.
Introduction
Biscuits are one of the most popular snacks because they have a pleasant taste and aroma, are ready to eat, come in various shapes, have a long shelf life, and are reasonably priced.[Citation1] The chocolate biscuit is one of the most popular and widely produced varieties.[Citation2] Commercial chocolate biscuits are primarily composed of carbohydrates and fat, with low levels of functional compounds that act as antioxidants.[Citation3] Antioxidant compounds, particularly those in the flavonoid family, are required to combat free radicals and prevent oxidative stress, leading to degenerative diseases.[Citation4] However, the consumption of functional food products is currently on the rise. It demonstrates that food product orientation not only serves to meet daily nutritional needs but also plays a role in providing a more positive impact on health.[Citation5]
One effort can be made to obtain biscuit products with high functional value by incorporating antioxidant-rich bioactive compounds into their formulations. Mangosteen peel extract is known to contain compounds that have strong antioxidant properties. For example, polyphenol compounds found in mangosteen peel extracts, such as xanthones, flavonoids, anthocyanins, tannins, saponins, and other phenolic compounds, are high in antioxidants.[Citation6]
However, mangosteen peel extract cannot be added directly when used in product formulations because polyphenolic compounds are unstable, easily oxidized, reactive, and sensitive to environmental and processing factors such as heat, light, and oxygen.[Citation7–9] Furthermore, it can impart a bitter and astringent flavor that consumers dislike. As a result, an encapsulation method is required to preserve the antioxidant compounds found in mangosteen peel. Encapsulation can prevent or reduce compound degradation after roasting, bitter and astringent taste in food, and fat oxidation.[Citation10]
The concentration of mangosteen peel extract microcapsules added to biscuit products must also be considered. It will affect the physicochemical and functional properties of the chocolate biscuits produced. The addition of high-concentration mangosteen peel extract microcapsules boosts the content of polyphenols, flavonoids, and antioxidant activity. It will, however, negatively impact the resulting chocolate biscuits’ physical and organoleptic properties. Extraction and encapsulation methods for mangosteen peel have been utilized extensively. However, adding these encapsulants to chocolate biscuit products has not been thoroughly investigated. Therefore, this study will examine chocolate biscuits’ physicochemical, antioxidant, and sensory properties incorporated with encapsulated mangosteen peel extract at different concentrations.
Materials and methods
Materials
Mangosteen peel was obtained from mangosteen fruit with the Puspahiang variety from Tasikmalaya, West Java, Indonesia, with the fruit sugar content of 15.0 °brix, skin color dark purple, oval fruit shape, harvest time September-April. Alkalized cocoa powder (Van Houten, PT. Perusahaan Industri Ceres, Bandung-Indonesia) was obtained from a supermarket with a pH of 7.21. Maltodextrin with a DE value of 10‒12 and Arabic gum is used as coating material. Biscuit ingredients include low-protein flour, refined sugar, margarine, milk powder, eggs, baking powder, and vanilla, purchased from the supermarket. Chemical analysis materials (analytical grade) include aluminum chloride, gallic acid, ethanol, hexane, glacial acetic acid, chloroform, 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium iodide, potassium carbonate, quercetin standard, methanol, sodium carbonate, Folin Ciocalteu reagent, and sodium thiosulfate.
Mangosteen peel extraction
The mangosteen peel was extracted using ultrasonic-assisted extraction.[Citation11] Mangosteen peel was blanched in hot water at 95°C for 5 minutes, immersed in cold water at 4°C, drained, and freeze-dried for 48 hours at −50°C. It reduced the dried mangosteen peel in size to obtain mangosteen peel powder. The mangosteen peel powder was extracted using ethanol (1:5 mass/volume) and an ultrasonication probe (Sonicator Q125, QSonica, USA) with a 65% amplitude for 45 minutes. The extracted supernatant was evaporated with a rotary evaporator (R-300, BUCHI, Switzerland) and then lyophilized with a freeze dryer (ALPHA 1–4 LSC, CHRIST, Germany). For future analysis, freeze-dried extracts stored in the freezer can be used.
Mangosteen peel extract encapsulation
The encapsulation of the mangosteen peel extracts was modified by Sarabandi et al.[Citation12] Maltodextrin: Arabic gum (80: 20 w/w) with 20% total solids (w/v) dissolved in distilled water containing 10% w/v extract of mangosteen peel, agitated using a magnetic stirrer at 3000 rpm for 15 minutes. Then the solution was hydrated for 18 hours at ±8°C. In each process, 2 liters of the feed solution is dried using a spray dryer (Mini Spray Dryer B-290, BUCHI, Switzerland) at a temperature of inlet 170°C, flow rate 15 mL/min, and atomizer nozzle pressure of 1 atm.
Chocolate biscuit preparation
The biscuits were prepared using the creaming technique by Dordoni et al.[Citation13] lists the formulations of each sample. The egg yolk was added, combined with margarine and powdered sugar using a mixer. Furthermore, low-protein flour was added, along with whole-milk powder, cocoa powder, baking powder, and vanilla. Then, mangosteen peel extract was encapsulated at 1%, 3%, 5%, and 7% of the total weight of the dough, respectively. The process includes kneading the dough until it is smooth, flattening it to a thickness of 0.3 cm, and molding. Twenty minutes are required to bake biscuits at 150°C in a digital electric oven (KBO-300DRA, Kirin, Indonesia).
Table 1. Chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
Biscuit storage
Biscuits were stored in an Alufoil standing pouch with a resealable zipper (120 mm×200 mm; Thickness: 95 microns) at room temperature 26 ± 2°C. The storability was studied for (0, 7, and 35) days, and biscuits were analyzed for peroxide value.
Extraction of dough and biscuit samples
Extraction of each sample of dough and biscuits was carried out in 2 steps: fat extraction and polyphenol extraction, referring to the research of Ioannone et al.[Citation14] with slight modification. In the first step, 4 g of the sample was defatted three times by extracting the fat using 25 mL of hexane. Each time, the supernatant was removed, and the solid was dried with nitrogen flux to obtain a fat-free solid sample. Then in the second step, 1 g of the defatted sample was added with 7 mL of 96% ethanol, followed by ultrasonication-assisted extraction for 45 minutes and an amplitude of 65%. A vacuum filter separated the mixture between the solid and the supernatant. The extract and solvent supernatant was then evaporated using a rotary evaporator. The solvent-free extract was freeze-dried and ready for chemical analysis (total polyphenol content, flavonoid content, and antioxidant activity).
Total polyphenol content determination
The determination of total polyphenol content (TPC) is referred to by Muzykiewicz et al.[Citation15] with a slight modification. A test tube was filled with 0.5 mL of the sample. Then 2.5 mL of 10% Folin Ciocalteu was added. The sample sat for 5 minutes and then was added 2 mL of Na₂CO₃ and incubation for 15 minutes at 45°C in a water bath (DK-98-(II)A series, Huanghua Faithful Instrument Co., Ltd., China). At a wavelength of 765 nm, a UV−Vis spectrophotometer (Lambda 35, PerkinElmer, USA) was used to measure absorbance. The standard used was gallic acid. TPC is expressed as milligram Gallic Acid Equivalent/gram (mg GAE/g).
Total flavonoid content determination
Total flavonoid content (TFC) was analyzed according to Mahloko et al.[Citation16] with slight modifications. 0.5 mL of sample solution was added with 3 mL of methanol, 0.2 mL of 10% aluminum chloride solution, and 0.2 mL of 1 M potassium acetate solution. It was then incubated for 15 minutes at 45°C in a water bath (DK-98-(II)A series, Huanghua Faithful Instrument Co., Ltd., China). The absorbance was measured at a wavelength of 450 nm with a spectrophotometer UV-Vis (Lambda 35, PerkinElmer, USA). Quercetin was used as a standard. TFC was expressed as a milligram Quercetin Equivalent/gram sample (mg QE/g).
Antioxidant activity determination
Antioxidant activity was used to calculate DPPH’s free radical scavenging activity, expressed in IC50 values.[Citation17] 2 mL of DPPH solution was added to the sample solution. After 30 minutes of incubation in a dark room, the absorbance at 517 nm was measured with a spectrophotometer UV-Vis (Lambda 35, PerkinElmer, USA). The percentage of inhibition was calculated based on the following equation 1:
Peroxide value determination
Peroxide value was determined according to Duta et al.[Citation18] with slight modifications. In a closed Erlenmeyer flask, a 5 gram sample was added to 30 mL of an acetic acid-chloroform (3:2) solution. If it is homogeneous, 0.5 mL of a saturated potassium iodide solution was added and let stand for 1 minute. It was then titrated with 0.01 sodium thiosulfate until the yellow color almost disappeared, and then 0.5 mL of 1% starch solution was added. Continue titration until the blue color disappears. The peroxide number is expressed in milli-equivalents of peroxide per kilogram of the sample (meq/kg). The peroxide value was calculated based on the following equation 2:
Texture profile analysis
Texture profile analysis (TPA) was carried out by referring to a research by Lao et al.[Citation19] with some modifications. A texture profile analyzer (TA.XTplusC, Stable Micro System Ltd., UK) was used to conduct the compression test. The biscuit sample was placed on the instrument and pressed with a probe model TA44. The pretest speed is 2.00 mm/s, the test speed is 0.50 mm/s, the posttest rate is 10 mm/s, the distance is 0.5 mm, and the trigger force is 5.00 g. The texture profile results were analyzed using Exponent Lite Express 6.1.16.0 software. Texture profile analysis includes hardness and fracturability, measured until the sample breaks due to pressure.[Citation20] The texture profile can be depicted using a force versus time graph (), where the first peak (1st peak) before the peak force indicates the fracturability (p) value, while the peak force (Z1) indicates the hardness value.
Figure 1. Typical instrument texture profile analysis (TPA) graph (Modified image from Liu et al.[Citation21]
![Figure 1. Typical instrument texture profile analysis (TPA) graph (Modified image from Liu et al.[Citation21]](/cms/asset/7d7dd921-438f-437b-a18d-8364c455559c/ljfp_a_2159429_f0001_oc.jpg)
Color analysis
A slight modification to the color testing protocol was established by Arifin et al.[Citation22] The biscuit sample was placed on a cup and then onto a Spectrophotometer (CM-5, Konica Minolta, Japan). Using the software Spectra Magic NX, color attributes were analyzed. The samples L* (lightness), a* (redness), and b* (yellowness) values were used to describe their color. The calculation of the browning index was determined based on the research of Sung and Chen[Citation23] by following equation 3 below:
Sensory evaluation
Sensory evaluation was carried out using a hedonic scoring test following the steps that had been carried out by Mareta,[Citation24] with some modifications. This evaluation was attended by twenty semi-trained panelists consisting of ten male panelists and ten female panelists, with ages ranging from 20–22 years. The criteria for the panelists included: 1) Those who can distinguish and communicate reactions to the organoleptic assessment being evaluated, 2) Those who are healthy and not colorblind, 3) Those who were neither full nor hungry before the organoleptic test, 4) Those who do not smoke, and 5) Those who were not wearing perfume.
Panelists were asked to rate the product’s color, aroma, flavor, texture (toughness and breaking strength), and overall acceptability. The values employed are 1 (dislike very much), 2 (dislike), 3 (neither like nor dislike), 4 (like), and 5 (like very much).
Statistical analysis
This study has five treatment variations incorporating encapsulated mangosteen peel extract on chocolate biscuits. Each treatment had three batches, and each batch was measured in duplicate. The IBM SPSS Statistics software for Windows, Version 26.0 (IBM Corp., Armonk, NY), was used for statistical analysis. The significant effect of treatment on the analysis parameters was determined using one-way ANOVA. The Duncan Multiple Range Test (DMRT) is used to continue the comparison test if there is a significant difference at the 95% confidence level. The data are presented as mean ± standard deviation (SD).
Results and discussion
Total polyphenol content
The TPC of mangosteen peel extract and encapsulated peel extract were 869.32 ± 0.22 mg GAE/g extract and 79.83 ± 0.01 mg GAE/g sample, respectively, as shown in . The TPC of mangosteen peel extract is affected by mangosteen variety and maturity level, postharvest technique, storage conditions, drying method, extraction method, solvent type, and other factors.[Citation25] Varieties can affect a substance’s structure, rheology, hardness, and chemical composition. It has different sample surface susceptibility to ultrasonic shock waves and cavitation bubbles.[Citation26] Ultrasonic-assisted extraction (UAE) is better than conventional methods such as soxhlet extraction, maceration, or Clevenger distillation.[Citation27] The UAE method can extract phenolic compounds with less solvent, short duration extraction, good polyphenol recovery, low energy cost, safe and environmentally friendly, and high productivity and efficiency.[Citation27–29] Therefore, it will affect the sample’s TPC. Meanwhile, the TPC of the encapsulated mangosteen peel extract was affected by the encapsulation method as well as the ratio of coating material to core material.
Table 2. The total polyphenol content of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
The concentration variation of the encapsulated mangosteen peel extract had a significant effect (p < .05) on the TPC in the dough and chocolate biscuits produced, as shown in . TPC levels in the dough and chocolate biscuit samples ranged from 2.17 to 8.16 mg GAE/g and 1.38 to 7.05 mg GAE/g, respectively (). The TPC in the dough and chocolate biscuit samples increased significantly (p < .05) as the concentration of the encapsulated mangosteen peel extract increased. The dough and chocolate biscuit samples in F4 had the highest TPCs at 8.16 ± 0.03 mg GAE/g and 7.05 ± 0.02 mg GAE/g, respectively. When compared to the total polyphenols in the F0 (2.17 ± 0.01 mg GAE/g and 1.38 ± 0.01 mg GAE/g), the total polyphenols of dough and chocolate biscuits in F4 were 276.04% and 410.87% higher than in F0. The TPC of samples F1, F2, F3, and F4 was derived from dough raw materials (cocoa powder, margarine, and wheat flour) and encapsulated mangosteen peel extract, resulting in a more excellent total polyphenol value than F0. In contrast, cocoa powder, margarine, and wheat flour were the only sources of the total polyphenols in the F0. In addition, the total polyphenols of alkalized cocoa powder contain 10.67‒24.92 mg GAE/g,[Citation30] margarine 0.27 mg GAE/mL,[Citation31] and whole wheat flour 241.32‒283.72 GAE mg/100 g.[Citation32] There are differences in TPC and TFC results of chemical analysis and calculations in dough and biscuits for all sample treatments (shown in ). The TPC and TFC in the dough analytically were higher than theoretically. TPC and TFC analytically include all raw materials used for making dough. So, there are sources of polyphenols and flavonoids derived from encapsulated extracts and all dough materials which contribute to polyphenols. While theoretically, TPC and TFC are only from the encapsulated extract. Then the difference analytically and theoretically in biscuits is influenced by the baking process. According to Abdel-Aal and Rabalski,[Citation33] baking can increase the amount of free phenolic in bread, cakes, and muffins but decrease the amount of bound phenolic. It is because baking can convert bound phenolic to free phenolic.
Table 3. Total flavonoid content of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
shows a decrease in TPC in all samples after the biscuit-baking process. Without the encapsulated mangosteen peel extract, the highest total polyphenol concentration in F0 decreased by 36.35% compared to F1, F2, F3, and F4, with concentrations ranging between 13.12% and 13.63%. In the F0, no compounds can protect the heat-sensitive antioxidant sources of cocoa powder, margarine, and wheat flour, which will degrade them during the baking process. However, with thermal treatment, phenolic acids like ferulic acid can be degraded into aromatic compounds like guaiacol, 4-methyl guaiacol, 4-ethyl guaiacol, 4-vinyl guaiacol, and vanillin.[Citation34,Citation35]
Encapsulation with maltodextrin and gum arabic coating reduces polyphenolic compound degradation during biscuit manufacturing. Maltodextrin can prevent the oxidation of the encapsulated material by forming an amorphous glass matrix during the encapsulation process.[Citation36] Gum arabic is a type of coating that can provide effective oxidation protection and is resistant to high temperatures,[Citation37] thereby minimizing the degradation of bioactive compounds during the biscuit baking process. According to Dordoni et al.,[Citation13] the total polyphenols of encapsulated grape skin extract biscuits decreased by 16.49 ± 4.39% after roasting at 180°C. In contrast, biscuits containing unencapsulated grape skin extract decreased by 40%.
Total flavonoid content
TFC of mangosteen peel extract was 91.54 ± 0.45 mg QE/g extract, as shown in . The encapsulated mangosteen peel extract contained 11.75 ± 0.02 mg QE per gram of sample. The TFC of mangosteen peel extract is affected by the plant’s geographic location, climate, environmental conditions, cultivation methods, harvest time, and postharvest processing.[Citation38] Meanwhile, the total flavonoids of the encapsulated mangosteen peel extract can be influenced by the ratio of maltodextrin and gum arabic used as a coating. The high concentration of gum arabic used will form a layer of shell resistant to destructive changes due to its high emulsification ability.[Citation39]
Variations in the concentration of the encapsulated mangosteen peel extract had a significant effect (p < .05) on the TFC of the dough and chocolate biscuits produced, according to . The TFC in all dough and chocolate biscuit samples was significantly different. The TFC in the dough and chocolate biscuit samples increased as the encapsulated mangosteen peel extract concentration increased. The total flavonoids in the dough and chocolate biscuit F4 were the highest, with 5.17 ± 0.03 mg QE/g and 4.73 ± 0.03 mg QE/g, respectively. Compared to the TFC sample in F0, the TFC of F4 dough and chocolate biscuits increased by 200.58% and 284.55%, respectively.
After baking the dough into biscuits, the TFC in all samples decreases, as shown in . F0 experienced the highest decrease in TFC by 28.39%, while F1, F2, F3, and F4 dropped lower than F0 ranging from 8.35‒8.89%. It is due to the encapsulation of antioxidant-rich mangosteen peel extract. During heat exposure, flavonoids with one or more hydroxyl groups readily bind to one or more sugars found in maltodextrin and gum arabic.[Citation40] Therefore, minimizing the loss of TFC compounds due to oxidation and thermal treatment is possible. Due to breaking molecular chains and oxidation, flavonoid degradation can occur, rapidly forming other compounds with volatile properties.[Citation41] Thermal treatment converts anthocyanins to chalcone glycosides, which are then deglycosylated to liberate chalcones.[Citation42] As depicted in , chalcones then form various products, including volatile aldehydes and phenolic acids. Catechin compounds are also sensitive to heat. Finally, it will degrade and epimerize to epicatechins during thermal processing. During thermal treatment, quercetin, also present in mangosteen peel extract, is typically relatively stable.
Figure 2. Thermal degradation mechanism of anthocyanins.[Citation43]
![Figure 2. Thermal degradation mechanism of anthocyanins.[Citation43]](/cms/asset/a0dcbd23-cd5b-412d-88e6-e05310149faa/ljfp_a_2159429_f0002_oc.jpg)
Antioxidant activity
Antioxidant activity was measured by DPPH radical scavenging activity expressed as an IC50 value (ppm). The IC50 represents the sample concentration necessary to inhibit 50% DPPH free radicals. The lower the IC50, the higher the antioxidant activity (Chen et al., 2022). Antioxidant activity is classified as very strong (IC50 <50 ppm), strong (IC50 50‒100 ppm), moderate (IC50 100‒150 ppm), weak (IC50 150‒200 ppm), and very weak (IC50 >200 ppm).[Citation44] shows that the IC50 value for mangosteen peel extract was 3.84 ± 0.01 ppm. After the extract was encapsulated, the IC50 value was 39.34 ± 0.25 ppm. The antioxidant activity of the two samples was classified as very strong. Compared with ascorbic acid with IC50 of 2.86 ± 0.02 ppm, mangosteen peel extract showed a higher IC50 value, but it still has a very strong antioxidant activity.
Table 4. Antioxidant activity of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
shows that the concentration variation of the encapsulated mangosteen peel extracts significantly impacted the IC50 value of the dough and chocolate biscuits produced (p < .05). The IC50 values of all dough and chocolate biscuit samples differ significantly. The IC50 value of dough and chocolate biscuit samples tends to decrease as more encapsulated mangosteen peel extract is added to the formulation. These results demonstrate an increase in the antioxidant activity of the sample. The IC50 values for the dough and biscuit in F4 were the lowest, at 101.66 ± 0.14 and 106.80 ± 0.06 ppm, respectively. Comparing the F0 with IC50 values of 302.66 ± 0.12 and 360.12 ± 0.08 ppm, respectively, reveals that the IC50 of dough and chocolate biscuits in F4 is 197.72% and 237.19% lower, respectively. According to these results, the F4 has more excellent antioxidant activity than the F0. According to , dough and biscuit samples F0 and F1 have very weak antioxidant activity, dough and biscuits F2 are weak, and dough and biscuits F3 and F4 are of moderate strength. The strength of antioxidant activity will increase along with the increasing content of total polyphenols and flavonoids in foodstuffs. According to Silviani et al.,[Citation45] there is a positive correlation between total polyphenols and the IC50 of cherry fruit flour biscuits.
The IC50 value increased in all samples during baking. The increase ranged between 5.06 and 5.82% for F1, F2, F3, F4, and 18.98% for F0. The increase in the IC50 value after baking indicates a reduction in the antioxidant activity’s potency. Despite this, several studies have found no significant difference in antioxidant strength after baking bread products. It is due to the Maillard reaction’s formation of reductant intermediates and melanoidin compounds during the roasting process.[Citation46] Reductones may act as chain-breakers for radical reactions by donating a hydrogen atom, which gives them potential as antioxidants.[Citation17,Citation47] In the meantime, melanoidin compounds can serve as metal chelates and prooxidant compounds. It should be noted, however, that the Maillard reaction products are undesirable compounds because their formation involves the destruction of essential amino acids and the production of anti-nutritional compounds.[Citation48]
Peroxide value
shows that on the 0th and 7th days of storage, no peroxide compounds were produced by any samples. Nonetheless, after 35 days of storage, peroxide compounds were detected. The concentration variation of the encapsulated mangosteen peel extracts significantly affected (p < .05) the peroxide number of chocolate biscuits. In addition, the peroxide levels of each sample varied significantly. The peroxide value of the chocolate biscuit () tends to decrease as the concentration of the encapsulated mangosteen peel extract increases. The lowest peroxide concentration in F4 is 0.21 ± 0.02 meq/kg. Compared to the peroxide concentration at F0 of 0.36 ± 0.03 meq/kg, the peroxide concentration at F4 is 41.66% lower than at F0. These findings suggest that the addition of encapsulated mangosteen peel extract can reduce lipid oxidation by inhibiting the initiation or propagation of oxidation chain reactions.[Citation49]
Figure 3. Peroxide value of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations during storage.
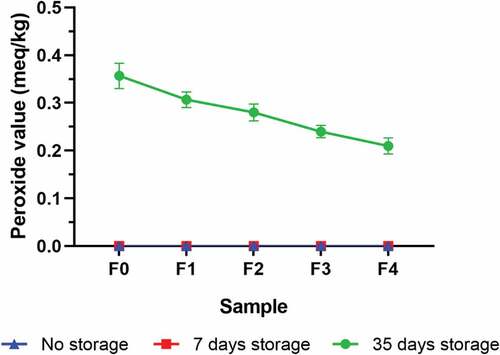
Peroxide compounds formed after 35 days of storage can be caused by fluctuating temperatures. The higher storage temperature can increase the oxidation reaction rate by forming alkyl free radicals.[Citation50] Astrari et al.[Citation51] state that spirulina biscuits also increased peroxide after being stored at a higher temperature. In addition, an Alufoil standing pouch with a zipper also affects product fat oxidation. Due to its low permeability to oxygen, it is difficult for oxygen trapped in the packaging to be removed.[Citation52,Citation53] Exposure to oxygen can also accelerate oxidation reactions because oxygen will react with alkyl free radicals and produce peroxide radicals, known as the propagation stage.[Citation54,Citation55]
In general, using ingredients like margarine influences the formation of peroxide compounds in chocolate biscuits. These ingredients contain unsaturated fatty acids, including oleic, linoleic, linolenic, eicosanoic, and palmitoleic acids, susceptible to oxidation during baking and storage.[Citation56] Jacobsen and Lyngby[Citation57] state that unsaturated fatty acids can bind oxygen to the double bond to produce hydroperoxide compounds, which can then be converted into aldehydes, lactones, and acroleins. These compounds produce a rancid flavor and odor.[Citation58]
Instrumental texture profile analysis
The variation in concentration of encapsulated mangosteen peel extract had a significant effect (p < .05) on the hardness and fracturability of the chocolate biscuits, as shown in . All chocolate biscuit samples’ hardness and fracturability varied significantly. In addition, there is a tendency for the hardness and fracturability of chocolate biscuit samples to increase with increasing concentrations of encapsulated mangosteen peel extract. F4 had the highest hardness and fracturability values, 22.62 ± 0.19 N and 9.75 ± 0.25 mm, respectively. In F0, the peroxide control values were 15.54 ± 0.27 N and 4.39 ± 0.09 mm. Finally, it indicates that the hardness and fracture ability of the F4 is 45.56% and 122.09% greater than those of the F0 sample, respectively.
Table 5. Texture profile characteristic of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
The TPC is thought to affect the texture of the resulting chocolate biscuits. Polyphenols can act as reducing agents for disulfide bonds and increase thiol groups and other covalent bonds that can affect dough rheology, including viscosity, strength, and elasticity.[Citation59] Polyphenol compounds can also inhibit starch granules from absorbing water during gelatinization because hydrophilic polyphenols can compete with starch for water.[Citation60] Therefore, the hardness and fracturability of the product will be higher as the TPC in the product increases. Mosafa et al.[Citation61] state that adding 1.4%–1.9% of maltodextrin can increase biscuits’ hardness and fracturability. It is because maltodextrin can form a gel in water. Some of the water will immobilize and significantly reduce the water available to the gluten for hydration, increasing the hardness and breaking strength of the resulting biscuit. Meanwhile, adding gum arabic by 0.3‒0.5% can also increase the hardness and fracturability of biscuits because of its ability to bind water and other components producing a stronger and harder biscuit.[Citation62]
Color analysis
shows that the variation of the encapsulated mangosteen peel extract incorporated in the biscuit formula has a significant effect (p < .05) on the color parameters (L*, a*, b*). The L* values of F1 and F2 samples significantly differ from those of F3 and F4. However, there is a tendency that the higher the concentration of the encapsulated mangosteen peel extract used, the lower the L* value of the chocolate biscuits, which indicates the darker color of the chocolate biscuits produced. The encapsulated mangosteen peel extract is still slightly purplish due to anthocyanins.
Table 6. Color analysis of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
The color tends to be dark in chocolate biscuits due to the use of alkaline cocoa powder. The alkalization process can cause an increase in pH in cocoa which causes the polyphenol oxidase enzyme. Its enzyme activity is optimal in alkaline conditions (pH±8) and can oxidize polyphenol compounds from cocoa and produce brown pigments.[Citation63] In addition, the Maillard reaction during the biscuit baking process also gives the biscuit brown color because it creates a brown melanoidin pigment.[Citation64] The Maillard reaction is a non-enzymatic browning reaction that occurs between carbohydrates as reducing sugars and protein amino groups at high temperatures.[Citation65]
The value of a* (redness) in F1 does not differ significantly from F2, but it does differ significantly (p < .05) from samples F3 and F4. The * values of samples with darker colors (lower L* values) are greater. According to Murata,[Citation66] it is due to the red pigmentation produced by the browning and Maillard reactions. Moreover, b* (yellowness) and browning index varied significantly across all samples. The sample’s b* value increased as the amount of encapsulated mangosteen peel extract increased. It is due to the presence of α-mangosteen and β-mangosteen in the mangosteen peel, which can contribute to the yellow color.[Citation67] In addition, the mangosteen peel contains flavan-3,4-diols and tannin compounds, which contribute to yellow-to-brown pigmentation. Finally, the product will become browner as the browning index in the sample increases. shows the chocolate biscuit’s color and appearance.
Sensory evaluation
shows that based on statistical data analysis, there are no significant differences (p > .05) between the attributes of each treatment. Consequently, no further Mann-Whitney tests are required. The organoleptic test revealed that the panelists enjoyed all the chocolate biscuit samples. The F3 is typically chosen over the other samples. The lack of a significant difference in the panelists’ preferences for each sample’s color attribute is also supported by the instrumental L* (lightness) value (), which compares samples F1 and F2 with F3 and F4. Different results were obtained for the hardness and fracture ability properties based on instrumental tests, indicating significant differences between each sample. Due to the slight variance between samples, panelists’ preference levels tend to be comparable. In addition, panelists are suspected of experiencing the placebo effect, which is the capacity to combine past experiences and preconceived notions with the sample’s sensory perceptions.[Citation68] Lastly, it leads panelists to believe that all samples share the same characteristics.
Figure 5. Spider web graph of sensory attributes of chocolate biscuits incorporated with encapsulated mangosteen peel extract in different formulations.
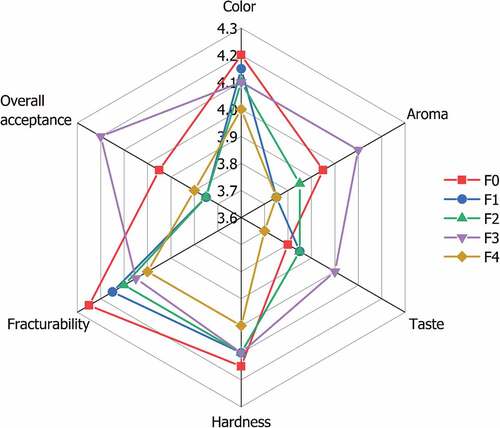
The aroma and flavor attributes () demonstrated that adding 1% to 7% encapsulated mangosteen peel extract did not affect these two characteristics. The Maillard reaction can produce pyrrolidine and piperidine compounds (proline derivatives) during baking. It is responsible for the chocolate biscuits’ aroma.[Citation69] Due to powdered sugar, milk powder, and cocoa powder, chocolate biscuits have a dominantly sweet flavor with a slight bitterness characteristic of chocolate. However, due to the presence of pyrazine and ester compounds in cocoa powder, chocolate has a distinctive flavor.[Citation70]
Based on the data of the physicochemical and organoleptic parameters, F3 exhibited the most desirable characteristics. After sample F4, F3 had the highest TPC () and TFC (). The IC50 value () and peroxide number () of F3 are comparable to F4. The F3 has a better color and texture profile than the F4. In addition, the results of the organoleptic test revealed that F3 was the sample the panelists accepted and preferred the most when compared to the other four samples (F0, F1, F2, and F3).
Pearson correlation coefficient (r) between variables
According to the Pearson Correlation analysis (), exhibited a correlation (p < .05) between parameters. Correlation coefficients (r) in indicate a very close relationship between variables. Positive correlation coefficients show a positive relationship between variables and vice versa. There was a positive correlation between TPC, TFC, hardness, fracturability, a*, b*, and browning index. Nonetheless, it correlates negatively with IC50, peroxide number, and L*. Meanwhile, if the r value is close to 1 or −1, it indicates a stronger relationship; while r is closer to 0, it indicates a weaker relationship. The r values ranging from 0.00–0.10 indicate a very weak or negligible correlation; 0.10–0.39 weak correlation; 0.40–0.69 moderate correlation; 0.70–0.89 strong correlation; and 0.90–1.00 very strong correlation.[Citation71]
Figure 6. The Pearson correlation coefficient (r) between variables. TPC (total polyphenol content); TFC (total flavonoid content); IC50 (antioxidant activity); POV (peroxide value); H (hardness); F (fracturability); L* (lightness); a* (redness); b* (yellowness); BI (browning index). The correlation is statistically significant at p < .05.
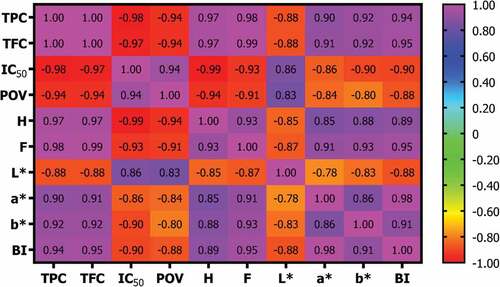
TPC very strongly positively correlates with TFC, as flavonoids are one of the largest polyphenol groups with a diphenylpropane (C6-C3-C6) skeleton consisting of two aromatic rings connected by three chains of carbon atoms.[Citation72] TPC and TFC are very strongly positively correlated with hardness and fracturability due to the presence of polyphenolic compounds that interact with protein and starch in wheat flour, thereby increasing the hardness and fracturability of chocolate biscuits. The TPC and TFC in the sample also very strongly positively correlated with a* (redness), b* (yellowness), and browning index values but strongly negatively correlated with L* (lightness) values. Mangosteen peel contains anthocyanins with contributing red pigments and α-mangosteen and β-mangosteen, which can give a yellow color.[Citation73,Citation74] Therefore, the greater TPC and TFC, the higher the intensity of the red and yellow colors in the sample. But on the contrary, the lightness value decreases, and the sample tends to be darker.
The very strong negative correlation between TPC‒TFC and IC50 is shown in . The higher TPC and TFC, the lower the IC50 value, indicating that the sample has stronger antioxidant activity. The strength of antioxidant activity is influenced by polyphenol and flavonoid compounds, which act as antioxidants by breaking free radical chain bonds and scavenging various reactive species.[Citation8,Citation17,Citation63] The TPC and TFC have a negative correlation with peroxide value. It indicates that the value of the peroxide number decreases as the sample’s TPC and TFC increase. The polyphenol and flavonoid compounds act as antioxidants and can inhibit fat oxidation, resulting in a lower peroxide value. These compounds inhibit the formation of free radicals at the initiation stage and prevent chain reactions at the propagation stage.
Conclusion
Different encapsulated mangosteen peel extracts significantly affected the chocolate biscuits’ physicochemical properties but not the sensory properties. The encapsulated mangosteen peel extract can increase the total values of TPC, TFC, hardness, fracturability, a*, b*, and browning index of chocolate biscuits while decreasing the IC50, peroxide number, and L* values. The hardness, fracturability, a*, b*, and browning index were positively correlated with TPC and TFC. In contrast, it negatively correlates with IC50 value, peroxide number, and L*. The F3 containing 5% encapsulated mangosteen peel extract was the optimal formulation. The chocolate biscuits containing the encapsulated mangosteen peel extract can be accepted by the panelists and have the potential to be developed as an alternative to antioxidant-rich functional foods.
Acknowledgments
The authors gratefully acknowledge Universitas Padjadjaran and the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for their support and facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alves, C.; José, C.; Ramírez, L.; Maria, A.; Peixoto, A.; Couto, G. Evaluation of Nutritional Characteristics and Consumers Acceptance of Gluten‑free Sweet Biscuits Made from Rice Based Pregelatinized Composite Flours Containing Orange Pomace and Soy Protein Isolate. SN Appl. Sci. 2021, 3(2), 1–13. DOI: 10.1007/s42452-021-04209-z.
- Wihenti, A. I.; Setiani, B. E.; Hintono, A.; Analisis Kadar, A.; Tebal, B. Dan Tekstur Biskuit Cokelat Akibat Perbedaan Transfer Panas. J. Apl. Teknol. Pangan. 2017, 6(2), 69–73. DOI: 10.17728/jatp.186.
- Irmayanti, F.; Nurman, L.; S. Formulasi Biskuit Kaya Serat Dan Antioksidan Dari Tepung Ubi Jalar Kuning Varietas Lokal Aceh Dengan Fortifikasi Pasta Buah Jamblang (Syzgium Cumini). Agriovet. 2019, 1(2), 136–152.
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5. DOI: 10.1017/jns.2016.41.
- Petrescu, D. C.; Vermeir, I.; Petrescu-Mag, R. M. Consumer Understanding of Food Quality, Healthiness, and Environmental Impact: A Cross-National Perspective. Int. J. Environ. Res. Public Health. 2020, 17, 1. DOI: 10.3390/ijerph17010169.
- Widyarman, A. S.; Lay, S. H.; Wendhita, I. P.; Tjakra, E. E.; Murdono, F. I.; Binartha, C. T. O. Indonesian Mangosteen Fruit (Garcinia Mangostana L.) Peel Extract Inhibits Streptococcus Mutans and Porphyromonas Gingivalis in Biofilms in Vitro. Contemporary Clinical Dentistry. 2019, 10(1), 123–128. DOI: 10.4103/ccd.ccd_758_18.
- Indiarto, R.; Indriana, L. P. A.; Andoyo, R.; Subroto, E.; Nurhadi, B. Bottom–up Nanoparticle Synthesis: A Review of Techniques, Polyphenol-Based Core Materials, and Their Properties. Eur. Food Res. Technol. 2022, 248(1), 1–24. DOI: 10.1007/s00217-021-03867-y.
- Indiarto, R.; Subroto, E.; Sukri, N.; Djali, M. Cocoa (Theobroma Cacao L.) Beans Processing Technology: A Review of Flavonoid Changes. Asian Journal of Plant Sciences. 2021, 20(4), 684–693. DOI: 10.3923/ajps.2021.684.693.
- Indiarto, R.; Pratama, A. W.; Sari, T. I.; Theodora, H. C. Food Irradiation Technology: A Review of the Uses and Their Capabilities. SSRG Int. J. Eng. Trends Technol. 2020, 68, 12. DOI: 10.14445/22315381/IJETT-V68I12P216.
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants. 2020, 9(10), 1–36. DOI: 10.3390/antiox9100923.
- Plaza, M.; Domínguez-Rodríguez, G.; Sahelices, C.; Marina, M. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2021, 11(12), 5625. DOI: 10.3390/app11125625.
- Sarabandi, K.; Jafari, S. M.; Mahoonak, A. S.; Mohammadi, A. Application of Gum Arabic and Maltodextrin for Encapsulation of Eggplant Peel Extract as a Natural Antioxidant and Color Source. Int. J. Biol. Macromol. 2019, 140, 59–68. DOI: 10.1016/j.ijbiomac.2019.08.133.
- Dordoni, R.; Garrido, G. D.; Marinoni, L.; Torri, L.; Piochi, M.; Spigno, G. 2019 . Enrichment of Whole Wheat Cocoa Biscuits with Encapsulated Grape Skin Extract. International Journal of Food Science. 2019, 1–11. DOI: 10.1155/2019/9161840.
- Ioannone, F.; Di Mattia, C. D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. F. Proanthocyanidins and Antioxidant Activity Changes during Cocoa (Theobroma Cacao L.) Roasting as Affected by Temperature and Time of Processing. Food Chem. 2015, 174, 256–262. DOI: 10.1016/j.foodchem.2014.11.019.
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Siemak, J.; Klimowicz, A. Antioxidant Activity and Polyphenol Content in Extracts from Various Parts of Fresh and Frozen Mangosteen. Acta Scientiarum Polonorum Technologia Alimentaria. 2020, 19(3), 261–270. DOI: 10.17306/J.AFS.0788.
- Mahloko, L. M.; Silungwe, H.; Mashau, M. E.; Kgatla, T. E. Bioactive Compounds, Antioxidant Activity and Physical Characteristics of Wheat-Prickly Pear and Banana Biscuits. Heliyon. 2019, 5(10), e02479. DOI: 10.1016/j.heliyon.2019.e02479.
- Indiarto, R.; Pranoto, Y.; Santoso, U.; Supriyanto. In Vitro Antioxidant Activity and Profile of Polyphenol Compounds Extracts and Their Fractions on Cacao Beans. Pakistan J. Biol. Sci. 2019, 22(1), 34–44. DOI: 10.3923/pjbs.2019.34.44.
- Duta, D. E.; Culetu, A.; Mohan, G. Sensory and Physicochemical Changes in Gluten-Free Oat Biscuits Stored under Different Packaging and Light Conditions. J. Food Sci. Technol. 2019, 56(8), 3823–3835. DOI: 10.1007/s13197-019-03853-z.
- Lao, Y. X.; Yu, Y. Y.; Li, G. K.; Chen, S. Y.; Li, W.; Xing, X. P.; Wang, X. M.; Hu, J. G.; Guo, X. B. Effect of Sweet Corn Residue on Micronutrient Fortification in Baked Cakes. Foods. 2019, 8(7), 1–13. DOI: 10.3390/foods8070260.
- Indiarto, R.; Nurhadi, B.; Tensiska, S.; Istiqamah, E.; J, Y. Effect of Liquid Smoke on Microbiological and Physico-Chemical Properties of Beef Meatballs during Storage. Food Res. 2020, 4(2), 522–531. DOI: 10.26656/fr.2017.4(2).341.
- Liu, Y. X.; Cao, M. J.; Liu, G. M. 2019. Texture Analyzers for Food Quality Evaluation. In Evaluation Technologies for Food Quality, Zhong, J., Wang, X. Woodhead Publishing Series in Food Science, Technology and Nutrition, Eds., 441–463. https://www.sciencedirect.com/science/article/pii/B9780128142172000172. Sawston, Cambridge, United Kingdom: Woodhead Publishing: DOI: 10.1016/B978-0-12-814217-2.00017-2.
- Arifin, H. R.; Djali, M.; Nurhadi, B.; Azlin-Hasim, S.; Masruchin, N.; Vania, P. A.; Hilmi, A. Corn Starch-Based Bionanocomposite Film Reinforced with ZnO Nanoparticles and Different Types of Plasticizers. Front. Sustain. Food Syst. 2022, 6. DOI: 10.3389/fsufs.2022.886219.
- Sung, W. C.; Chen, C. Y. Influence of Cookies Formulation on the Formation of Acrylamide. J. Food Nutr. Res. 2017, 5(6), 370–378. DOI: 10.12691/jfnr-5-6-3.
- Mareta, D. T. Hedonic Test Method for Measuring Instant Pindang Seasoning Powder Preferences. J. Sci. Appl. Technol. 2019, 3(1), 34. DOI: 10.35472/jsat.v3i1.195.
- Ghasemzadeh, A.; Jaafar, H. Z. E.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-Mangostin-Rich Extracts from Mangosteen Pericarp: Optimization of Green Extraction Protocol and Evaluation of Biological Activity. Molecules. 2018, 23(8), 1–16. DOI: 10.3390/molecules23081852.
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. DOI: 10.1016/j.ultsonch.2021.105506.
- Chemat, F.; Rombaut, N.; Sicaire, A. G.; Meullemiestre, A.; Fabiano-Tixier, A. S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. DOI: 10.1016/j.ultsonch.2016.06.035.
- Chemat, S.; Aissa, A.; Boumechhour, A.; Arous, O.; Ait-Amar, H. Extraction Mechanism of Ultrasound Assisted Extraction and Its Effect on Higher Yielding and Purity of Artemisinin Crystals from Artemisia Annua L. Leaves. Ultrason. Sonochem. 2017, 34, 310–316. DOI: 10.1016/j.ultsonch.2016.05.046.
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A. G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M. A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health. 2021, 18(17), 9153. DOI: 10.3390/ijerph18179153.
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between Antimicrobial, Antioxidant Activity, and Polyphenols of Alkalized/Nonalkalized Cocoa Powders. J. Food Sci. 2017, 82(4), 1020–1027. DOI: 10.1111/1750-3841.13672.
- Nadeem, M.; Imran, M.; Taj, I.; Ajmal, M.; Junaid, M. Omega-3 Fatty Acids, Phenolic Compounds and Antioxidant Characteristics of Chia Oil Supplemented Margarine. Lipids Health Dis. 2017, 16(1), 1–12. DOI: 10.1186/s12944-017-0490-x.
- Zhang, Y.; Truzzi, F.; D’amen, E.; Dinelli, G. Effect of Storage Conditions and Time on the Polyphenol Content of Wheat Flours. Processes. 2021, 9(2), 1–11. DOI: 10.3390/pr9020248.
- Abdel-Aal, E. S. M.; Rabalski, I. Effect of Baking on Free and Bound Phenolic Acids in Wholegrain Bakery Products. J. Cereal Sci. 2013, 57(3), 312–318. DOI: 10.1016/j.jcs.2012.12.001.
- Schmid, V.; Steck, J.; Mayer-Miebach, E.; Behsnilian, D.; Briviba, K.; Bunzel, M.; Karbstein, H. P.; Emin, M. A. Impact of Defined Thermomechanical Treatment on the Structure and Content of Dietary Fiber and the Stability and Bioaccessibility of Polyphenols of Chokeberry (Aronia Melanocarpa) Pomace. Food Res. Int. 2020, 134, 109232. DOI: 10.1016/j.foodres.2020.109232.
- Zhao, Q.; Yao, S.; Ou, S. Y. Maillard Volatiles in Baked Products as Affected by Feruloylated Oligosaccharides from Maize Bran. Int. J. Food Prop. 2017, 20(12), 3266–3273. DOI: 10.1080/10942912.2017.1285788.
- González-Ortega, R.; Faieta, M.; Di Mattia, C. D.; Valbonetti, L.; Pittia, P. Microencapsulation of Olive Leaf Extract by Freeze-Drying: Effect of Carrier Composition on Process Efficiency and Technological Properties of the Powders. J. Food Eng. 2020, 285, 110089. DOI: 10.1016/j.jfoodeng.2020.110089.
- Ramos, P.; Broncel, M. Influence of Storage Conditions on the Stability of Gum Arabic and Tragacanth. Molecules. 2022, 27, 5. DOI: 10.3390/molecules27051510.
- Medisa, D.; Anita Nugraheni, D. Effect of Regional Variation on the Total Flavonoid Level of Ethanol Extract of Mangosteen (Garcinia Mangostana) Peels. Indones. J. Med. Heal. 2017, 2(3), 146–153.
- Ebrahimi, B.; Homayouni Rad, A.; Ghanbarzadeh, B.; Torbati, M.; Falcone, P. M. The Emulsifying and Foaming Properties of Amuniacum Gum (Dorema Ammoniacum) in Comparison with Gum Arabic. Food Sci. Nutr. 2020, 8(7), 3716–3730. DOI: 10.1002/fsn3.1658.
- Naufalin, R.; Erminawati, W.; Febryani, R.; T, A.; Latifasari, N. Antioxidant Activity of Kecombrang Preserving Powder Using Foam Mat Drying Method. IOP Conf. Ser. Earth Environ. Sci. 2021, 746, 1. DOI: 10.1088/1755-1315/746/1/012017.
- Speisky, H.; Shahidi, F.; Camargo, A. C. Revisiting the Oxidation of Flavonoids : Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants. 2022, 11(133), 1–28. DOI: 10.3390/antiox11010133.
- Nistor, M.; Pop, R.; Daescu, A.; Pintea, A.; Socaciu, C.; Rugina, D. Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules. 2022, 27(13), 1–37. DOI: 10.3390/molecules27134254.
- Juanying, O.;. Application of Polyphenols in Foods and Food Models; 1st. Cambridge, Massachusetts, United States: Academic Press. https://www.sciencedirect.com/science/article/pii/S1043452621000140. 2021 Incorporation of polyphenols in baked products ; Vol. 98 Advances in Food and Nutrition Research. 10.1016/bs.afnr.2021.02.009.
- Savitri, E. S.; Holil, K.; Resmisari, R. S. Phytochemistry Screening and Antioxidant Activities of Extract Pomegranate, Grape, Fig, and Olive in the Various Solvent. J. Biodjati. 2022, 7(1), 132–139. DOI: 10.15575/biodjati.v7i1.13424.
- Silviani, D.; Marliyati, S. A.; Kustiyah, L. Pengaruh Pemanfaatan Tepung Buah Kersen (Muntingia Calabura L.) Dan Substitusi Gula Terhadap Kandungan Gizi. Antioksidan Dan Organoleptik Biskuit. Media Gizi Indones. 2022, 17(1), 33–42. DOI: 10.20473/mgi.v17i1.33-42.
- Blanch, G. P.; Castillo, M. L. R. D. Effect of Baking Temperature on the Phenolic Content and Antioxidant Activity of Black Corn (Zea Mays L.) Bread. Foods. 2021, 10, 1–8.
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard Reaction Products as Food-Born Antioxidant and Antibrowning Agents in Model and Real Food Systems. Food Chem. 2019, 275, 644–660. DOI: 10.1016/j.foodchem.2018.09.083.
- ALjahdali, N.; Carbonero, F. Impact of Maillard Reaction Products on Nutrition and Health: Current Knowledge and Need to Understand Their Fate in the Human Digestive System. Crit. Rev. Food Sci. Nutr. 2019, 59(3), 474–487. DOI: 10.1080/10408398.2017.1378865.
- Sinaga, R. N.; Zulaini, Z. The Effect of Mangosteen Rind Extract (Garcinia Mangostana L.) toward Stress Oxidative Parameter, Leukocytes, Leukocytes Type Counts on Male Rats (Rattus Norvegicus) with Excessive Physical Activity. Open Access Maced J. Med. Sci. 2020, 8(A), 904–909. DOI: 10.3889/oamjms.2020.5448.
- Liu, K.; Liu, Y.; Chen, F. Effect of Storage Temperature on Lipid Oxidation and Changes in Nutrient Contents in Peanuts. Food Sci. Nutr. 2019, 7, 2280–2290. DOI: 10.1002/fsn3.1069.
- Astrari, M. D.; Dewita, Suparmi. Shelf life of Spirulina biscuit with different packaging. Jurnal Online Mahasiswa. 2015, 2(2) 1–7. https://jom.unri.ac.id/index.php/JOMFAPERIKA/article/view/6227/5927
- Zabihzadeh Khajavi, M.; Ebrahimi, A.; Yousefi, M.; Ahmadi, S.; Farhoodi, M.; Mirza Alizadeh, A.; Taslikh, M. Strategies for Producing Improved Oxygen Barrier Materials Appropriate for the Food Packaging Sector. Food Eng. Rev. 2020, 12. DOI: 10.1007/s12393-020-09235-y.
- Rosida, D. F. Estimation of Shelf Life of Bangkalan Zalacca (Salacca Zalacca (Gaertner) Voss) Chips Using Vacuum Frying Technology and Aluminum Foil and PVC Plastic Packaging. Adv. Eng. Res. 2020, 194, 181–188.
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F. J.; Zhang, W.; Lorenzo, J. M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants. 2019, 8(10), 1–31. DOI: 10.3390/antiox8100429.
- Indiarto, R.; Rezaharsamto, B. The Physical, Chemical, and Microbiological Properties of Peanuts during Storage: A Review. Int. J. Sci. Technol. Res. 2020, 9(3), 1909–1913.
- Culetu, A.; Ionescu, V.; Todasca, M. C.; Duta, D. E. Evaluation of the Storage-Associated Changes in the Fatty Acid Profile of Oat-Based Gluten-Free Cookies Prepared with Different Fats. Food Sci. Biotechnol. 2020, 29(6), 759–767. DOI: 10.1007/s10068-019-00720-7.
- Jacobsen, C.; Lyngby, K. Oxidative Rancidity; Elsevier: Kongens Lyngby. Denamrk. 2018. DOI: 10.1016/B978-0-12-814026-0.21672-7.
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules. 2022. DOI: 10.3390/molecules27155014.
- Girard, A. L.; Awika, J. M. Effects of Edible Plant Polyphenols on Gluten Protein Functionality and Potential Applications of Polyphenol–Gluten Interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19(4), 2164–2199. DOI: 10.1111/1541-4337.12572.
- Xu, J.; Wang, W.; Li, Y. D. P. Bread Quality, and Associated Interactions with Added Phenolic Compounds : A Review. J. Funct. Foods. 2019, 52, 629–639. DOI: 10.1016/j.jff.2018.11.052.
- Mosafa, L.; Nadian, N.; Hojatoleslami, M. Investigating the Effect of Whole Oat Flour, Maltodextrin and Isomalt on Textural and Sensory Characteristics of Biscuits Using Response Surface Methodology. J. Food Meas. Charact. 2017. DOI: 10.1007/s11694-017-9559-5.
- China, M. A. H.; Oguzor, U. C.; Ujong, A. E. Effect of Gum Arabic Incorporation on the Proximate Composition and Sensory Properties of Biscuits Produced from Flour Blends of Wheat and Water Yam Effect of Gum Arabic Incorporation on the Proximate Composition and Sensory Properties of Biscuits Produce. Asian Food Sci. J. 2020, 18(1), 1–11. DOI: 10.9734/AFSJ/2020/v18i130201.
- Indiarto, R.; Pranoto, Y.; Santoso, U. S. Evaluation of Physicochemical Properties and Antioxidant Activity of Polyphenol-Rich Cacao Bean Extract through Water Blanching. Pakistan J. Nutr. 2019, 18(3), 278–287. DOI: 10.3923/pjn.2019.278.287.
- Dong, L.; Qiu, C.; Wei, F.; Yu, Z.; Zhang, Y.; Wang, S. The Effect of Microwave Baking Conditions on the Quality of Biscuits and the Control of Thermal Processing Hazards in the Maillard Reaction. Front. Nutr. 2022, 9, 825365. DOI: 10.3389/fnut.2022.825365.
- Bertrand, E.; El Boustany, P.; Faulds, C. B.; Berdagué, J.-L. The Maillard Reaction in Food: An Introduction. Ref. Module Food Sci. 2018. DOI: 10.1016/b978-0-08-100596-5.21459-5.
- Murata, M. Browning and Pigmentation in Food through the Maillard Reaction. Glycoconj. J. 2021, 38(3), 283–292. DOI: 10.1007/s10719-020-09943-x.
- John, O. D.; Mouatt, P.; Majzoub, M. E.; Thomas, T.; Panchal, S. K.; Brown, L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia Dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2019, 21, 1. DOI: 10.3390/ijms21010272.
- Colloca, L. The Fascinating Mechanisms and Implications of the Placebo Effect. Int. Rev. Neurobiol. 2018, 138. DOI: 10.1016/S0074-7742(18)30027-8.
- Alasti, F. M.; Asefi, N.; Maleki, R.; SeiiedlouHeris, S. S. Investigating the Flavor Compounds in the Cocoa Powder Production Process. Food Sci. Nutr. 2019, 7(12), 3892–3901. DOI: 10.1002/fsn3.1244.
- Mohamadi Alasti, F.; Asefi, N.; Maleki, R.; SeiiedlouHeris, S. S. Investigating the Flavor Compounds in the Cocoa Powder Production Process. Food Sci. Nutr. 2019, 7(12), 3892–3901. DOI: 10.1002/fsn3.1244.
- Schober, P.; Schwarte, L. A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126(5), 1763–1768. DOI: 10.1213/ANE.0000000000002864.
- Mutha, R. E.; Tatiya, A. U.; Surana, S. J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics : An Overview. Futur. J. Pharm. Sci. 2021, 7(25), 1–13.
- Ibrahim, M. Y.; Hashim, N. M.; Mariod, A. A.; Mohan, S.; Abdulla, M. A.; Abdelwahab, S. I.; Arbab, I. A. α-Mangostin from Garcinia Mangostana Linn: An Updated Review of Its Pharmacological Properties. Arab. J. Chem. 2016, 9(3), 317–329. DOI: 10.1016/j.arabjc.2014.02.011.
- Chaovanalikit, A.; Mingmuang, A.; Kitbunluewit, T.; Choldumrongkool, N.; Sondee, J.; Chupratum, S. Anthocyanin and Total Phenolics Content of Mangosteen and Effect of Processing on the Quality of Mangosteen Products. Int. Food Res. J. 2012, 19(3), 1047–1053.

