?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cashew tree has two parts of food industry and nutrition interest, i.e the nut and the juicy apple. This study aimed to carry out a phytochemical characterization, determine the antioxidant properties and evaluate the influence of temperature on the phytonutrient composition of cashew fruits. The contents of ascorbic acids, phytates, and tannins, of various pigments were determined. Antioxidant activities were determined by four methods using standard methods. The apples presented the best antioxidant activities for FRAP, SOD, and LPO 98.54 ± 5.48, 834.12 ± 13.47, and 84.9 ± 4.15, respectively; while walnuts showed the best antioxidant activities for free radical scavenging which was 26.14 ± 3.21. Tannin, phytate, and anthocyanin contents decreased by more than 50% when increasing temperatures between 50 and 100°C. Ascorbic acid tends to disappear completely in fruit at temperatures of 100°C. Extractable lycopene increased by about 25% with increasing temperature. β-carotene was found to be insensitive to temperature increase. These results provide valuable information that can help in the control of cashew fruit processing in the food industry. They also provide important information on the nutraceutical potential of cashew fruit.
Introduction
Cashew tree (Anacardium occidentale L.) is a cash crop that contributes to the socio-economic development of several countries in the world[Citation1] The plant is grown in several countries in Africa (West and East), South America, and Asia (India, Vietnam, etc.). Burkina Faso is one of the largest producers of cashew nuts in the world. It ranks 6th and 12th among cashew nut-producing countries in Africa and the world, respectively[Citation2] It should be noted that the cashew tree is a plant that can reach more than 10 m in height depending on the climate and the nature of the soil. Producers are interested in its cultivation for its fruits, but its bark, leaves, and roots are traditionally used by traditional healers. It is adapted to the humid tropical climate. The organic fruit of the cashew tree is a hard-shelled nut with a swollen stalk forming an apple called a “false fruit.” Both parts are edible and also traditionally used for therapeutic purposes, making them both a food and medicinal plant[Citation3] The nut includes the shell, the kernel, and the balsam; however, it is the kernel that is especially valued because it contains a lot of fat. The almond is used to make peanuts, and sometimes as a substitute for milk powder in chocolate making,[Citation4] while the shell is often used to produce energy through pyrolysis[Citation5] Nuts contain many bioactive compounds that give them antioxidant and t, anti-inflammatory properties[Citation6,Citation7] The apple is juicy and consumed by local populations; however, for conservation reasons and its astringent taste, it is not highly appreciated. This apple is rich in pigments, which explains its color which is between yellow and red. Its moderate consumption is also explained by certain prejudices saying that it is incompatible with certain foods such as milk[Citation8] For some authors, these findings are explained by the presence of anti-nutritional factors that inhibit the incorporation of these nutrients such as minerals[Citation9–11] Studies have shown that cashew apple extracts have prebiotic effects on different strains of potentially probiotic Lactobacillus, making cashew fruits a possible value-added ingredient for the food industry[Citation12] Other studies have shown that cashew apples and nuts contain many nutrients such as proteins, minerals, vitamin C, lycopene, carbohydrates, essential amino acids,[Citation13,Citation14] etc. It has also been proven that cashew fruits contain several other phytochemicals such as tannins, phytates, β-carotene, chlorophyll, etc.[Citation1,Citation15,Citation16] Therefore, cashew products are very important from a nutritional and medicinal point of view. However, some compounds such as vitamin C, anthocyanins, and lycopene contained in cashew are sensitive to temperature and UV light[Citation15,Citation16] This requires a control of the factors acting on the composition of these micronutrients. Approaches to know the factors at the origin of these variations in microelements in cashew fruits showed that ascorbic acid content was very sensitive to the increase of temperature[Citation16,Citation17] and the tannin level decreased following a long soaking in water[Citation1] Pigments and anti-nutritional factors depend on the physical and chemical transformations that the fruits undergo[Citation18]The content of anti-nutritional factors in plant products can be considerably reduced by applying certain treatments[Citation19] such as soaking, heating, etc. This can greatly reduce their complexation reaction with certain minerals. Several studies did not address the majority of microelements and the variation of fruit content with increasing temperature. Therefore, it was necessary to extend the determination of the microelements content to several other parameters and specially to follow the evolution of the content of these elements with increasing temperature variations. The present work aimed to quantify phytonutrients in cashew fruits, determine the antioxidant activity, and assess the stability of cashew phytonutrients depending on temperatures.
Materials and methods
Plant material
The cashew fruit consists of two parts: the nut covered with shell and the apple whose color can turn from yellow to red (). The study involved eighteen apple and cashew samples collected from thirty orchards in three regions of Burkina Faso.
Sample collection
Samples were collected from the three largest cashew-producing regions of Burkina Faso, namely the Southwest (Gaoua, 10° 17’ 57” N, 3° 15’ 3” W), the “Hauts-Bassins” (Bobo-Dioulasso, 11° 10’ 37.7” N, 4° 17’ 52.4” W) and the Cascades (Banfora, 10° 37’ 60” N, 4° 46’ 0” W). A total of 60 kg of fresh apples and 60 kg of cashew nuts were collected. Samples from the “Cascades” are coded CK1, CK2, CK3 for nuts and CA1, CA2, CA3 for apples; those from the “Sud-Ouest” SK1, SK2, SK3 for nuts and SA1, SA2, SA3 for apples; and those from the “Hauts Bassins” HK1, HK2, HK3 for nuts and HA1, HA2, HA3 for apples ().
Sample preparation
The nuts are crushed with manual pruning shears and then shelled manually. The almonds (kernel) were obtained after a manual sorting operation. The almonds separated from the shells were dried in a ventilated dryer (Prolab dryer) at 50°C for 24 h and then shelled to obtain dry almonds (2% – 4% water). The almonds were de-oiled in a Soxhlet with n-hexane. The de-oiled almonds were oven dried for at 50°C for 2 hours to evaporate the hexane and the resulting products were used for some trials and tests[Citation20] The apples were cut and dried in a ventilated dryer at 40°C for 48 h to obtain dry almonds (5% −7%.) The dried apples were ground with a blender (MICROTRON®MB800) and the powder was collected for analysis. The different steps are summarized on the diagram in . The moisture content of the obtained powder was 7 ± 2% for both apples and almonds.
Figure 3. Different steps in the production of kernel and apple powders prior to analysis[Citation21]
![Figure 3. Different steps in the production of kernel and apple powders prior to analysis[Citation21]](/cms/asset/4bb9031d-46a7-410a-a503-46033dc1d489/ljfp_a_2163661_f0003_b.gif)
Extraction method
The powders (0.5 g) of apples and cashews from each region were extracted with 10 ml of acetone:water (80:20, v/v). The mixture was stirred for 24 h, then placed under ultrasound for 2 h and centrifuged at 4500 rpm for 15 min[Citation22] The supernatant was used for quantification of total phenols, total flavonoids, tannins, phytates and antioxidant activities.
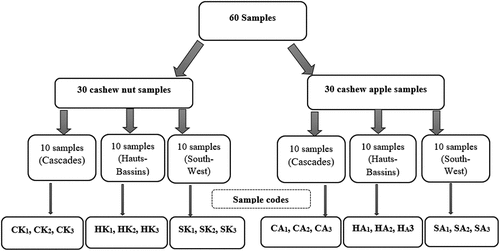
To evaluate the effect of temperature on the content of anti-nutritional factors and pigments, fresh apples and shelled nuts were exposed to different temperatures. Four batches of 500 mg of each fresh apple and almond sample were ground in 500 mL of distilled water and placed at 50°C, 75°C or 100°C in a study to monitor the effect of temperature. The levels of ascorbic acid, various pigments, and anti-nutritional factors were quantified at 15, 30, 60, and 120 min.
Ascorbic acid and piments content
Ascorbic acid was estimated on the basis of the decolorization of 2,6-dichlorophenolindophenol (DCPIP) by ascorbic acid[Citation23] with slight modifications. To an aliquot of the extract of almonds and apples (50 μL) was added 150 µL of DCPIP (0.2 mM). Ascorbic acid contents are expressed as mg EAA/100 g DM of sample. For β-Carotene, total chlorophyll, and lycopene analysis 300 mg of fresh apples and almonds were mixed with 3 ml of 95% ethanol. The mixture was kept for 10 min in ice and centrifuged at 4500 rpm for 1 min. β-Carotene, total chlorophyll, and lycopene contents were determined by adapting previously describedmethods,[Citation24,Citation25] respectively. For the β-carotene and lycopene, absorbances were read at different wavelengths and the contents were calculated according to the following equations:
The absorbance of the supernatant was measured at 665 nm and 649 nm for photosynthetic pigments using the equation below:
Total anthocyanin content (TAC) was determined by the differential pH method (AOAC 2005.02)[Citation26] with some modifications[Citation27] This method uses two buffer systems: potassium chloride buffer (25 mM, pH 1.0) and sodium acetate buffer (400 mM, pH 4.5). The absorbances were read with a spectrophotometer at two wavelengths (520 and 700 nm).
TAC was calculated as cyanidin-3-glucoside equivalents
Mw: molecular weight of cyanidin-3-glucoside (449.2 Da). DF: dilution factor. ελ: Molar extinction coefficient (26 900 M−1. cm−1)
Anti-nutritional factors
The contents of hydrolysable tannins were quantified[Citation28] using tannin acid as standard. The results were expressed as mg tannic acid equivalent (GAE) per g dry extract (mgGAE/100 g). The hydrolysable tannins were determined by the following formula:
where A: absorbance; Mw: molecular weight of tannic acid (1701.19 g/mol); V: volume of extract used; DF: dilution factor; ε mole: 2169 mol; W: sample weight (g). The determination of phytate was based on a spectrophotometric assay using phytic acid as standard[Citation29] The assay was performed with 2.0 ml of Wade reagent (0.03% (w/v) FeCl3 and 0.3% sulfosalicylic acid) and 3.0 ml of the eluted sample
Phenolic compounds
Total phenolics compounds were determined according to the slightly modified method of Singleton[Citation30] It is based on the transfer of electrons in alkaline medium from phenolate ions to phosphomolybdic/phosphotungstic acid complexes leading to formation of blue-colored reaction products that are determined spectrophotometrically at approximately 760 nm. Gallic acid is used as reference compound. Total flavonoid contents were determined by the colorimetric method of Dowd[Citation31] Total flavonoid contents were expressed in mg of quercetin equivalent (QE) per gram of fresh matter (mg QE/g).
Antioxidant activity of apple and cashew kernel extracts
Antioxidant activity as measured by the FRAP method, a modified version of Benzie & Strain[Citation32] was applied for the determination of ferric reducing antioxidant power (FRAP). Results were calculated as µg ascorbic acid equivalent (EAA) DM of the samples. The ability of cashew apple and nut extracts to scavenge the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was evaluated at 517 nm[Citation33] The IC50 (50% inhibitory concentration) are calculated graphically by linear or logarithmic regressions of the percent inhibition against different concentrations of each of the extracts tested[Citation34]
Superoxide dismutase (SOD) activity
Cashew powder (10 mg) powder was homogenized with 2 mL of 50 mM sodium phosphate buffer, pH 7.8. SOD activity was determined as previously described[Citation34] with slight modifications[Citation35] Absorbances were read at 420 nm. A modified 2-thiobarbituric acid method[Citation36] was used to determine the lipid peroxidation (LOP) inhibitory activity of the extracts[Citation37] The ability of the extracts to inhibit lipid peroxidation of lecithin is expressed as percentage inhibition according to the following formula:
Ac = Absorbance of the control, and Ae = Absorbance with sample
Statistical analyses
Figures and calculations were done using GraphPad Prism version 8.4.3, Excel 2016, respectively. XLSTAT 2016 was used for analyses of variance (p < .05). Principal component analysis was performed using R software version 4.0.2 (2020).
Results and discussion
Vitamin C, pigments, phytates, and tannins content
Fruits are generally abundant in pigments that give them different colors. For the cashew fruit, the apples are very rich in various pigments (). Except for chlorophyll, almonds contain very few pigments. Lycopene is the most abundant pigment in apples (294.5 ± 24 mg/100g), followed by anthocyanins (88.64 ± 11.5 g/100g), β-carotene (54.2 ± 8.94 mg/100g), and chlorophyll (27.48 ± 6.45 mg/100g). These pigments give the yellow-red or dark orange color to apples and also have important antioxidant properties. A study conducted in Venezuela showed similar data on raw cashew apple juice, where lycopene and β-carotene contents of 580 ± 50.0 mg/100g and 40 ± 6.2 mg/100g, respectively, were found[Citation38] Lycopene is of interest because it may help prevent diabetes mellitus, cancer, liver disorders, and some cardiac complications, and reduce the risk of oxidative stress-related diseases[Citation39] Our results are similar to those of[Citation15] who found levels in the order of 60 mg/100g of cashew apples in a study conducted in Brazil. β-Carotene is a nutritionally essential provitamin A because it is metabolized to retinol, the oxidation of which produces retinal essential for good vision[Citation25] The anthocyanin levels in our cashew apples were much higher than those found in a study conducted in Brazil which was 21.16 mg/100 g[Citation40] This difference could be explained by the different types of cashew varieties that produce fruits with different phenotypes[Citation41] Rainfall, biotic stress, edaphic, meteorological, and photoperiod conditions could also influence the concentration of anthocyanins that give color to apples. Anthocyanins which are antioxidant pigments, have attracted much interest for their potential preventive and/or therapeutic health effects including obesity prevention, cardiovascular disease, antibacterial, anti-inflammatory, and anticancer effects[Citation25] In addition, a study showed a strong correlation between anthocyanin content and antioxidant activity of fruits[Citation42]
Table 1. Pigment and antinutritional factors content of cashew nuts and apple.
Tannins and phytates are sometimes referred to as antinutritional compounds because of their complexation with certain molecules and minerals in the body, reducing their bioavailability. Antinutritional factors are defined as compounds that decrease the bioavailability of nutrients by interfering with their absorption. Antinutritional factors present in fruits and oilseeds form complexes with proteins, iron, and enzymes in the gastrointestinal tract and reduce their bioavailability[Citation10] Our results show that they are present in both parts of the western Anacardium fruit. Tannins were more abundant in apples (204.8 ± 26.5 mg/100g) than in almonds (64.32 ± 12.4 mg/100g), whereas phytates are present at higher levels in almonds (89.34 ± 18.45 mg/100g) compared with apples (41.72 ± 9.2 mg/100g) (). The abundance of tannins in apples would partly explain their astringent taste. Condensed tannins (proanthocyanidins) can form complexes with proteins and reduce their digestibility. They hurt the absorption of iron, copper, and zinc, as well as the reserves of these micronutrients[Citation9] Therefore, pregnant women and children are not advised to consume large amounts of foods rich in tannins[Citation11] Fortunately, hydrolysable tannins can be degraded in apples by applying enzymatic or thermal treatments[Citation43] On the other hand, at low doses tannins can be useful for human health due to their antioxidant activity and inhibition of the growth of various groups of microorganisms such as fungi, yeasts, viruses, and bacteria[Citation44] In addition, it has been reported that the consumption of foods containing low levels of tannins contributes to the reduction of high blood pressure and serum lipid constituents[Citation45] Phytic acid is also known to reduce the absorption of certain minerals such as divalent cations (copper, zinc, iron, etc.)[Citation46]
Unlike nuts which contain only traces (18.13 ± 6.2 g/100g DM), cashew apples are very rich in ascorbic acid (387.45 ± 17.4 mg/100g) (). The results differ significantly according to the collection area, this is explained by the fact that the three sample collection sites are in the same climatic zone with very little variation in soil type. The differences revealed by the statistical analysis (p < .05) can be explained by the cashew varieties grown in the area. These values are similar to those found by[Citation40] who found ascorbic acid levels of 279.37 mg/100 g in apples produced in Brazil. Another study in Ghana on cashew apple juice found average ascorbic acid levels of 231.4 mg/10 mL[Citation47] This difference is attributable to the extraction method because we performed a continuous extraction cycle which may explain the high levels of ascorbic acid in our cashew apple extracts. Thus, cashew apples are an important source of vitamin C. Ascorbic acid is particularly important for strengthening the immune system involved in the turnover and function of certain white blood cells[Citation48] Cashew apple consumption would be an alternative to the growing demand for vitamin C, especially for low-income populations. Ascorbic acid is known to be a potent antioxidant that helps boost the immune system. In the early days of the Covid-19 pandemic, foods rich in ascorbic acid were highly recommended to address the SARS-CoV-2[Citation49] coronavirus. In synergy with vitamin E, beta-carotene, selenium, zinc, and other minerals, ascorbic acid can scavenge excess free radicals in the body, which accelerate cellular aging[Citation50] As such, it helps prevent cardiovascular disease, certain cancers, cataracts, and neurodegenerative diseases.
The analyses reveal significant differences for the studied parameters according to the area of collection, this could be explained by the nature of the cultivation soil, and the climate and also could be related to a response to the aggressors. affirmed that the fertilization with micronutrients in the soil affects the nutritional status and the photosynthetic activity of plants[Citation51]
Effect of temperature on the content of pigments and anti-nutritional factors
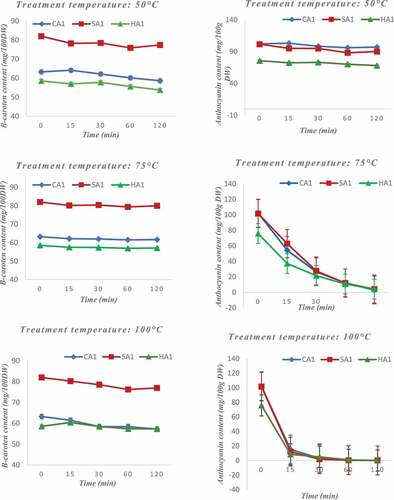
Pigments and anti-nutritional factors were subjected to different temperatures and their contents were quantified as a function of time (0 to 2 h). The concentration of tannins decreased significantly with temperature (). At 50°C, a progressive discoloration of about 20% is observed after 2 h of heating of fresh almond and apple crushes. This decrease becomes more pronounced as the temperature is increased. Thus, during 2 h, the tannin content decreases by 50% at 75°C and by 75% at 100°C. The decrease is more important for apples than for almonds. Nuts contain high level of fat, so the tannins are complex in the fatty parts which are more resistant to heat. Tannins are therefore thermolabile and their degradation increases with temperature and depends on the nature of the sample that contains them. shows that phytates are also sensitive to temperature increases. Their content decreases with time when they are subjected to higher temperatures. Thus, at 50°C, 75°C, and 100°C for 2 h, a decrease in the concentration of about 40%, 50%, and 64% respectively is observable (). There is no significant difference in the rate of degradation with increasing temperature (50°C, 75°C and 100°C) exposure of the samples as a function of time, so another factor would be associated with the temperature effect. A previous study showed that soaking decreases the phytate content of foods[Citation38] Therefore, to significantly reduce the phytate content in plant-based products, they must be soaked in hot water for a long time[Citation17] Knowledge of techniques to reduce the concentration of anti-nutrients is very important from a nutritional point of view because these factors decrease the absorption of some micronutrients such as minerals,[Citation9] whose are important for the body.
Figure 4. Influence of temperature on the content of anti-nutritional factors in apples and cashew nuts (tannins and phytates).
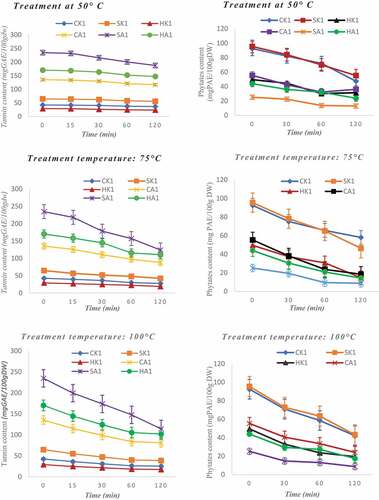
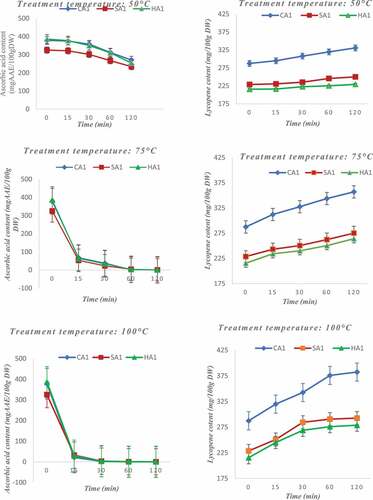
Pigments are temperature-sensitive compounds that react differently to temperature variations. The study was concerned only with apples because cashew nuts do not contain many pigments. Thus, the ascorbic acid and anthocyanin contents of cashew apples decrease strongly with increasing temperature. Lycopene contents, on the contrary, increase with the rise in temperature. As for β-carotene contents, no significant variation was observed with increasing temperature. Ascorbic acid content decreased by about 25% at 50°C for 2 h (). At 75°C, the ascorbic acid content decreases by about 70% after only 15 min of heating, after 2 h there are only traces of ascorbic acid in the apples. The same observation is made at 100°C cooking time. Ascorbic acid is very sensitive to temperature increases as it starts to degrade from 50°C during a long heating period. It has been shown that exposure of fruits to increasingly higher temperatures would very significantly reduce the ascorbic acid content in these[Citation51,Citation52] Knowing the behavior of ascorbic acid as a function of temperature is important when one knows the nutritional contribution of ascorbic acid in the body. Lycopene content increased by about 15%, 22%, and 35% during heating to 50°C, 75°C, and 100°C, respectively (). Lycopene was the only pigment whose concentration increased. This increase could be explained by the fact that when apples are heated to a high temperature, the bioavailability of lycopene increases[Citation15] Heating releases lycopene molecules complex to macromolecules and thus increases their concentration. Heating has no significant effect on β-carotene content because its content does not change significantly during heating (). The β-carotene is thermostable and does not react to temperature increase. Carotenoids are the primary pigments of plants that give them distinctive hues such as yellow and orange. In the case of cashew apples, it has been shown that the major carotenoid is β-carotene[Citation53] This means that heating does not greatly influence the color of the apples which are mostly yellow and red. As for the anthocyanins, they were resistant to heating at 50°C. However, from 75°C of heating for 2 h, they undergo a great degradation leading to a decrease of more than 80% of their content (). At 100°C for 2 h, anthocyanins disappear since only traces remain. When using apples to preserve the anthocyanins they contain, heat treatment at prolonged temperatures should be avoided as this may cause deterioration. An earlier study also showed that temperatures of about 70°C destroyed the pigments governing cashew colors, particularly anthocyanins[Citation16,Citation54] High levels of anthocyanins greatly influence the antioxidant activity of fruit extracts[Citation42] Knowledge of these properties allows us to predict the appropriate treatment depending on the purpose of the formulations we want to achieve by including cashew products. Variation in pigment and anti-nutrient levels have a direct effect on the antioxidant and organoleptic properties of apples and almonds. It was found that temperature has a significant impact on the composition of pigments and anti-nutritional factors in apples and cashews. Indeed, the temperature deteriorates some components such as ascorbic acid, phytates, and hydrolysable tannins[Citation55]
Phenolic compounds and antioxidant activities of apples and almonds
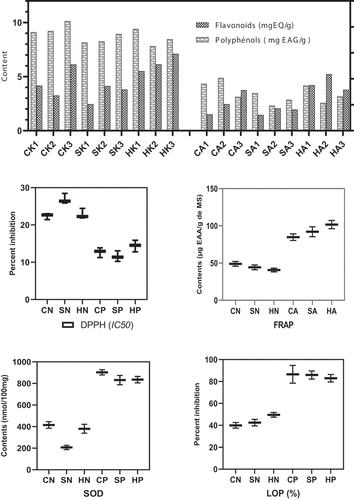
Phenolic compounds found in fruits and vegetables have attracted much interest because of their antioxidant potential[Citation56] The use of synthetic phenolic antioxidants is of increasing concern because of their negative effects on human health. Therefore, it has been proposed to replace these synthetic substances with antioxidant extracts from various foods. More than 8000 different phenolic compounds have been characterized, notably from fruits and vegetables[Citation57] In this study, total phenolic compound and flavonoid contents were quantified, and the results show that walnuts contain high levels of polyphenols and flavonoids compared with apples, which contain moderate amounts. Polyphenol contents ranged from 7.8 to 10.5 mg GAE/g DM for walnuts and from 2.7 to 5.01 mg GAE/g DM for apples (). For flavonoids, concentrations ranged from 3.6 to 7.01 mg EQ/g for walnuts and 1.7 to 4.98 mgEQ/g for apples (). Levels of phenolics in cashew apples from Burkina Faso are comparable to those of Cruz Reina et al., who found levels of 190 ± 9.02 mg GAE/100 mL of cashew apple from Colombia[Citation58] Indeed, the presence of phenolic compounds in the different parts of the plants is a natural defense against pests[Citation28] In addition, they confer antioxidant and anti-inflammatory properties to the different plant parts. Moreover, these compounds may contain active principles of interest to treat certain diseases, especially to prevent metabolic diseases[Citation59] Thus, they reduce oxidative stress, which contributes to decreasing the risk of metabolic diseases that cause many deaths in recent years.
For the evaluation of antioxidant activity, different methods including DPPH, FRAP, SOD, and LPO have been used (). For FRAP, SOD, and LPO assays, apple extracts showed the best antioxidant activities (109.4 ± 2.47 µgEAA/g DM and 927.121 ± 25.54 mmol/100 g DM, respectively and 83% inhibition) while for DPPH assay walnut extracts showed the highest activities (121.25 ± 6.2 µg/mL DM). This means that the antioxidants contained in apples have very high iron reducing power, high potential for superoxidase activity, and high capacity to inhibit lipid peroxidation compared to walnuts. In contrast, the antioxidants in walnuts are better at reducing the DPPH (2,2’-diphenyl-1-picryl hydroxyl) radical. Cordaro et al., also showed that cashew nuts had a strong potential to trap the DPPH free radical[Citation60] These significant differences in the antioxidant power of the extracts may result from the nature of the antioxidants in apples and almonds. Indeed, it appears that apples contain high level of ascorbic acid, tannins, and pigments (lycopene, β-carotene, anthocyanin chlorophyll), whereas walnuts do not contain them. On the contrary, walnuts are rich in total polyphenols and flavonoids. This could explain the differences of the antioxidant activities. In general, both parts of the cashew fruit have interesting antioxidant power, which is very important from a nutritional point of view, as nowadays we are witnessing an increase in the number of deaths related to diseases caused by oxidative stress. Reactive oxygen species (ROS) are involved in different mechanisms that participate in normal signaling pathways and processes such as cancer development, neurodegenerative diseases, and cardiovascular diseases[Citation38] Andrade et al, stated that cashew fruits are very important to fight against oxidative stress[Citation7] Consumption of products from cashew fruits would be beneficial in the fight against metabolic diseases.
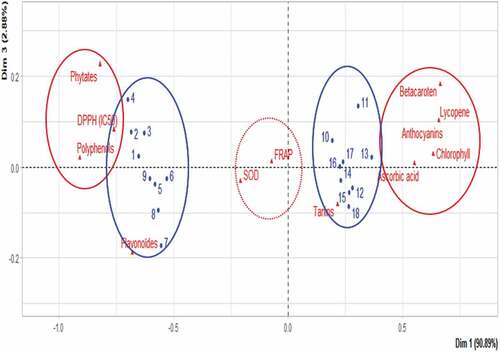
Correlation and principal component analysis (ACP)
The principal component analysis reveals several groups of different typologies. Four groups are distinguished. Naturally, the samples of the two parts of the cashew fruit form very distinct groups (). The first group, 1 to 9 gathers the almond samples and the second, 10 to 18 the apple samples. This shows that the two parts of the cashew fruit have very different nutritional characteristics. It was also revealed that apples and almonds have dissimilar agro-morphological characteristics[Citation61] The remaining two groups are {phytates, polyphenols, DPPH} and {β-carotene, chlorophyll, ascorbic acid, anthocyanin, lycopene}. This shows on the one hand a close correlation between the phytate and polyphenol content and the antioxidant activity of the extracts according to the DPPH test. On the other hand, the contents of β-carotene, lycopene, ascorbic acid, tannins, anthocyanins, and chlorophyll are very strongly correlated. Also, the group constituted by almond samples showed a positive correlation between phytate contents, polyphenols, and antioxidant activity according to the DPPH test. On the other hand, the contents of β-carotene, ascorbic acid, anthocyanins, lycopene, and chlorophylls strongly correlated to the group of samples consisting of cashew apples. There was also a positive correlation between apples and iron reduction capacity and super oxidase activity. Of all the tests for antioxidant activity, apples had the highest activity. This can be explained by their high content of vitamin C and lycopene which are powerful antioxidants[Citation36,Citation38] All this shows that the two parts of the cashew fruit are very important in terms of nutrition.
Conclusion
The phytochemical composition, antioxidant activities, temperature sensitivity of microelements, and anti-nutritional factors of apples and cashew nuts from three localities in Burkina Faso were evaluated. The origin of the samples did not influence the parameters analyzed, but apples and cashew nuts have very different phytochemical composition and antioxidant properties. Regarding the sensitivity of pigments and anti-nutritional factors to temperature variation, the concentrations of phytates, tannins, ascorbic acid, and anthocyanins decreased at different frequencies with increasing temperatures. Extractable lycopene level increased with increasing temperature. However, extractable β-carotene level was insensitive to the increase in temperature. Apples showed the best antioxidant activities. These findings revealed the nutritional potential of cashew fruits and provide a database for possible uses of cashew products in the food industry. These results also prompt further pharmacological research to identify bioactive compounds.
Acknowledgments
The African Biotechnology Network (RABIOTECH-ISP/IPICS project) is appreciated for supporting publication fees and academic mobilities.
Disclosure statement
The authors have not declared any conflict of interest.
Additional information
Funding
References
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M. F. B.; Carneiro, J. N. P.; Bezerra, C. F.; Silva, T. G. D.; Coutinho, H. D. M.; Amina, B., et al. Anacardium Plants: Chemical,nutritional Composition and Biotechnological Applications. Biomolecules. 2019, 9(9), 1–34. DOI: 10.3390/biom9090465.
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on August 25, 2022).
- Oliveira, N. N.; Mothé, C. G.; Mothé, M. G.; Oliveira, L. G. D. Cashew Nut and Cashew Apple: A Scientific and Technological Monitoring Worldwide Review. J. Food Sci. Technol. 2020, 57(1), 12–21. DOI: 10.1007/s13197-019-04051-7.
- Ogunwolu, S. O.; Henshaw, F. O.; Mock, H. P.; Santros, A.; Awonorin, S. O. Functional Properties of Protein Concentrates and Isolates Produced from Cashew (Anacardium Occidentale L.) Nut. Food Chem. 2009, 115(3), 852–858. DOI: 10.1016/j.foodchem.2009.01.011.
- Godjo, T.; Tagutchou, J.-P.; Naquin, P.; Gourdon, R. Valorisation des coques d’anacarde par pyrolyse au Bénin. Environnement, Ingénierie & Développement. 2015, N°70-nov(N°70), 11–18.
- Trevisan, M. T. S.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R. W. Characterization of Alkyl Phenols in Cashew (Anacardium Occidentale) Products and Assay of Their Antioxidant Capacity. Food Chem. Toxicol. 2006, 44(2), 188–197. DOI: 10.1016/j.fct.2005.06.012.
- Andrade, T. D. J. A. D. S.; Araújo, B. Q.; Citó, A. M. D. G. L.; Silva, J. D.; Saffi, J.; Richter, M. F.; Ferraz, A. D. B. F. Antioxidant Properties and Chemical Composition of Technical Cashew Nut Shell Liquid (Tcnsl). Food Chem. 2011, 126(3), 1044–1048. DOI: 10.1016/j.foodchem.2010.11.122.
- Emmanuelle, D.; Joseph, D.; Victor, A.; Mohamed, M. S. A Review of Cashew (Anacardiumoccidentale L.) Apple: Effects of Processing Techniques, Properties and Quality of Juice. Afr. J. Biotechnol. 2016, 15(47), 2637–2648. DOI: 10.5897/AJB2015.14974.
- Petroski, W.; Minich, D. M. Is There Such A Thing as “anti-nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020, 12(10), 1–32. DOI: 10.3390/nu12102929.
- Amon, A.; Olga, A.; Souleymane, T.; Fatoumata, C.; Gbogouri, G. A.; Kouakou, B. Evaluation of Technological Treatments Impact on Nutritional Value and anti-nutritional Factors of Cashew kernel-based Flour (Anacardium Occidentale) Grown in Côte D. Ivoire. Int. J. Food Sci. Nutr. 2018, 3(1), 20–28.
- Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by Tannin Constituents of Selected Edible Nuts. Int. J. Mol. Sci. 2009, 10(12), 5485–5497. DOI: 10.3390/ijms10125485.
- Duarte, F. N. D.; Rodrigues, J. B.; Costa Lima, M. D.; Lima, M. D. S.; Pacheco, M. T. B.; Pintado, M. M. E.; Souza Aquino, J. D.; Souza, E. L. D. Potential Prebiotic Properties of Cashew Apple (Anacardium Occidentale L.) agro-industrial Byproduct on Lactobacillus Species. J. Sci. Food Agric. 2017, 97(11), 3712–3719. DOI: 10.1002/jsfa.8232.
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional Composition of Raw Fresh Cashew (Anacardium Occidentale L.) Kernels from Different Origin. Food Sci. Nutr. 2016, 4(2), 329–338. DOI: 10.1002/fsn3.294.
- Sharma, P.; Gaur, V. K.; Sirohi, R.; Larroche, C.; Kim, S. H.; Pandey, A. Valorization of Cashew Nut Processing Residues for Industrial Applications. Ind. Crop Prod. 2020, 152(January), 112550. DOI: 10.1016/j.indcrop.2020.112550.
- Assunção, R. B.; Mercadante, A. Z. Carotenoids and Ascorbic Acid Composition from Commercial Products of Cashew Apple (Anacardium Occidentale L.). J. Food Compos. Anal. 2003, 16(6), 647–657. DOI: 10.1016/S0889-1575(03)00098-X.
- Dao, T. P.; Nguyen, D. V.; Tran, T. Y. N.; Pham, T. N.; Nguyen, P. T. N.; Bach, L. G.; Nguyen, V. H.; Do, V. Q.; Nguyen, V. M.; Tran, T. T. Effects of Tannin, Ascorbic Acid, and Total Phenolic Contents of Cashew (Anacardium Occidentale L.) Apples Blanched with Saline Solution. Food Res. 2021, 5(1), 409–416. DOI: 10.26656/fr.2017.5(1).454.
- Dao, T. P.; Vu, D. N.; Nguyen, D. V.; Pham, V. T.; Tran, T. Y. N. Study of Jelly Drying Cashew Apples (Anacardium Occidentale L.) Processing. Food Sci. Nutr. 2022, 10(2), 363–373. DOI: 10.1002/fsn3.2565.
- Idris, W. H.; AbdelRahaman, S. M.; ElMaki, H. B.; Babiker, E. E.; Tinay, A. H. E. Effect of Malt Pretreatment on Phytate and Tannin Level of Two Sorghum (Sorghum Bicolor) Cultivars. Int. J. Food Sci. Technol. 2006, 41(10), 1229–1233. DOI: 10.1111/j.1365-2621.2006.01190.x.
- Carlson, D.; Poulsen, H. D. Phytate Degradation in Soaked and Fermented Liquid Feed - Effect of Diet, Time of Soaking, Heat Treatment, Phytase Activity, pH and Temperature. Anim. Feed Sci. Technol. 2003, 103(1–4), 141–154. DOI: 10.1016/S0377-8401(02)00288-2.
- Bai, S. H.; Brooks, P.; Gama, R.; Nevenimo, T.; Hannet, G.; Hannet, D.; Randall, B.; Walton, D.; Grant, E.; Wallace, H. M. Nutritional Quality of Almond, Canarium, Cashew and Pistachio and Their Oil Photooxidative Stability. J. Food Sci. Technol. 2019, 56(2), 792–798. DOI: 10.1007/s13197-018-3539-6.
- Dakuyo, R.; Konaté, K.; Sanou, A.; Kaboré, K.; Sama, H.; Bazié, D.; Diao, M.; Dicko, M. H.; Morales-Quintana, L. Comparison of Proximate and Phytonutrient Compositions of Cashew Nuts and Apples from Different Geographical Areas of Burkina Faso. BioMed. Res. Int. 2022, 2022, 1800091. DOI: 10.1155/2022/1800091.
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine Max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods. 2022, 11(12), 1775. DOI: 10.3390/foods11121775.
- Mehta, N.; Patani, P.; Singhvi, I. Colorimetric Estimation of Ascorbic Acid from Different Varities of Tomatoes Cultivated in Gujarat. World J. Pharm. Res. 2018, 7(4), 1376–1384.
- Kovalevskaya, R. Z.; Zhukava, H. A.; Adamovich, B. V. Modification of the Method of Spectrophotometric Determination of Chlorophyll A in the Suspended Matter of Water Bodies. J. Appl. Spectrosc. 2020, 87(1), 72–78. DOI: 10.1007/s10812-020-00965-9.
- Wu, X.; Sun, C.; Yang, L.; Zeng, G.; Liu, Z.; Li, Y. β-carotene Content in Sweet Potato Varieties from China and the Effect of Preparation on β-carotene Retention in the Yanshu No. 5. Innov. Food Sci. Emerg. Technol. 2008, 9(4), 581–586. DOI: 10.1016/j.ifset.2008.06.002.
- Lee, J. AOAC Official Method 2005.02 Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines pH Differential Method First Action 2005. Official Methods of Analysis of AOAC International. 2006, 37.1.68.
- Hasperué, J. H.; Rodoni, L. M.; Guardianelli, L. M.; Chaves, A. R.; Martínez, G. A. Use of LED Light for Brussels Sprouts Postharvest Conservation. Sci. Hortic. (Amsterdam). 2016, 213(25), 281–286. DOI: 10.1016/j.scienta.2016.11.004.
- Price, M. L.; Butler, L. G. Rapid Visual Estimation and Spectrophotometric Determination of Tannin Content of Sorghum Grain. J. Agric. Food Chem. 1977, 25(6), 1268–1273. DOI: 10.1021/jf60214a034.
- Latta, M.; Eskin, M. A Simple and Rapid Colorimetric Method for Phytate Determination. J. Agric. Food Chem. 1980, 28(6), 1313–1315. DOI: 10.1021/jf60232a049.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of folin-ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178.
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation D’Un Extrait De Propolis Et Identification Des Principaux Constituants. J. Pharm. Belg. 1994, 49(6), 462–468.
- Ahmed, Z. B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Heyden, Y. V. Determination of Optimal Extraction Conditions for Phenolic Compounds From: Pistacia Atlantica Leaves Using the Response Surface Methodology. Anal. Methods. 2016, 8(31), 6107–6114.
- Velázquez, E.; Tournier, H. A.; Mordujovich De Buschiazzo, P.; Saavedra, G.; Schinella, G. R. Antioxidant Activity of Paraguayan Plant Extracts. Fitoterapia. 2003, 74(1–2), 91–97. DOI: 10.1016/S0367-326X(02)00293-9.
- Scherer, R.; Godoy, H. T. Antioxidant Activity Index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl Method. Food Chem. 2009, 112(3), 654–658. DOI: 10.1016/j.foodchem.2008.06.026.
- Mesa-Herrera, F.; Quinto-Alemany, D.; Díaz, M. A Sensitive, Accurate, and Versatile Method for the Quantification of Superoxide Dismutase Activities in Biological Preparations. React. Oxyg. Species. 2019, 7(19), 10–20.
- Wills, E. D. Mechanisms of Lipid Peroxide Formation in Animal Tissues. Biochem. J. 1966, 99(3), 667–676. DOI: 10.1042/bj0990667.
- Ruberto, G.; Baratta, M. T.; Deans, S. G.; Dorman, H. J. D. Antioxidant and Antimicrobial Activity of Foeniculum Vulgare and Crithmum Maritimum Essential Oils. Planta Med. 2000, 66(8), 687–693. DOI: 10.1055/s-2000-9773.
- Chaparro, L.; Dhuique-Mayer, C.; Castillo, S.; Vaillant, F.; Servent, A.; Dornier, M. Concentration and Purification of Lycopene from Watermelon Juice by Integrated microfiltration-based Processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 153–160. DOI: 10.1016/j.ifset.2016.08.001.
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M. A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M., et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants. 2020, 9(8), 1–27. DOI: 10.3390/antiox9080706.
- Lopes, M. M. A. D.; Miranda, M. R. A. D.; Moura, C. F. H.; Filho, J. E. Compostos bioativos e atividade antioxidante total de pedúnculos de caju (Anacardium occidentale L.) durante o amadurecimento de clones de cajueiro anão-precoce. Cienc. E Agrotecnologia. 2012, 36(3), 325–332.
- Semporé, J. N.; Songré-Ouattara, L. T.; Tarpaga, W. V.; Bationo, F.; Dicko, M. H. Morphological Characterization and Quality Assessment of Cashew (Anacardium Occidentale L.) Nuts from 53 Accessions of Burkina Faso. J. Agric. Food Res. 2021, 6(September), 100219. DOI: 10.1016/j.jafr.2021.100219.
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive Compounds, Antioxidant Activity and Fruit Quality Evaluation of Eleven Blood Orange Cultivars. J. Sci. Food Agric. 2022, 102(7), 2960–2971. DOI: 10.1002/jsfa.11636.
- Abdullah, S.; Pradhan, R. C.; Aflah, M.; Mishra, S. Efficiency of Tannase Enzyme for Degradation of Tannin from Cashew Apple Juice: Modeling and Optimization of Process Using Artificial Neural Network and Response Surface Methodology. J. Food Process. Eng. 2020, 43(10). DOI: 10.1111/jfpe.13499.
- Adegunwa, M. O.; Kayode, B. I.; Kayode, R. M. O.; Akeem, S. A.; Adebowale, A. A.; Bakare, H. A. Characterization of Wheat Flour Enriched with Cashew Apple (Anacardium Occidentale L.) Fiber for Cake Production. J. Food Meas. Charact. 2020, 14(4), 1998–2009. DOI: 10.1007/s11694-020-00446-9.
- Chung, K. T.; Wong, T. Y.; Wei, C. I.; Huang, Y. W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38(6), 421–464. DOI: 10.1080/10408699891274273.
- Gibson, R. S.; Raboy, V.; King, J. C. Implications of Phytate in plant-based Foods for Iron and Zinc Bioavailability, Setting Dietary Requirements, and Formulating Programs and Policies. Nutr. Rev. 2018, 76(11), 793–804. DOI: 10.1093/nutrit/nuy028.
- Lowor, S. T.; Agyeute-Badu, C. K. Mineral and Proximate Composition of Cashew Apple (Anarcadium Occidentale L.) Juice from Northern Savannah, Forest and Coastal Savannah Regions in Ghana. Am. J. Food Technol. 2009, 4(4), 154–161. DOI: 10.3923/ajft.2009.154.161.
- Moyses-Neto, M.; Brito, B. R. S.; Araújo Brito, D. J. D.; Barros, N. D. C.; Dantas, M.; Salgado-Filho, N.; Costa, R. S.; Silva, G. E. B. Vitamin C-induced Oxalate Nephropathy in A Renal Transplant Patient Related to Excessive Ingestion of Cashew Pseudofruit (Anacardium Occidentale L.): A Case Report 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Nephrol. 2018, 19(1), 1–4. DOI: 10.1186/s12882-018-1060-9.
- Xie, C.; Jiang, L.; Huang, G.; Pu, H.; Gong, B.; Lin, H.; Ma, S.; Chen, X.; Long, B.; Si, G., et al. Comparison of Different Samples for 2019 Novel Coronavirus Detection by Nucleic Acid Amplification Tests. Int. J. Infect. Dis. 2020, 93, 264–267. DOI: 10.1016/j.ijid.2020.02.050.
- Sirmali, R.; Giniş, Z.; Sirmali, M.; Solak, O.; Şeliman, B.; Ağaçkiran, Y.; Delibaş, N. Vitamin C as an Antioxidant: Evaluation of Its Role on Pulmonary Contusion Experimental Model. Turkish J. Med. Sci. 2014, 44(6), 905–913.
- Silva, R. R. D.; Rodrigues, L. U.; Fidélis, R. R.; Faria, Á. J. G. D.; Nascimento, V. L. Nutritional and Morphophysiological Responses of Soybean to Micronutrient Fertilization in Soil. Commun. Plant Sci. 2019, 9(1), 93–99. DOI: 10.26814/cps2019016.
- Dioha, I.; Olugbemi, O.; Onuegbu, T.; Shahru, Z. Determination of Ascorbic Acid Content of Some Tropical Fruits by Iodometric Titration. Int. J. Biol. Chem. Sci. 2012, 5(5), 2180. DOI: 10.4314/ijbcs.v5i5.37.
- Kafache, D.; Deli, M.; Galani, B. R. T.; Agume, A. N.; Bouba, A. A.; Njintang, N. Y. Physicochemical and in Vitro Antioxidant Properties of Juice and Cake Filters from Carissa Edulis Vahl Fruits. J. Explor. Res. Pharmacol. 2022, 7(3), 000–000.
- Nhi, T. T. Y.; Quy, N. N.; Truong, L. D.; Phat, D. T.; Phong, H. X. Comparison of Pretreatment Methods on Total Ascorbic Acid, Total Phenolic Content, and Color of Soursop (Annona Muricata L.) Pulp. Steam Blanching, Hot Water Blanching, and microwave-assisted Blanching. J. Food Process. Preserv. 2022, 46(11). DOI: 10.1111/jfpp.17017.
- Oboulbiga, B. E.; Parkouda, C.; Dabiré, C.; Guissou, A. W. D. B.; Traore, K.; Semde, Z.; Douamba, Z.; Sawadogo-Lingani, H.; Dicko, M. H. Storage Stability of Dried Tomato Slices during Storage as Affected by Salt and Lemon Pretreatments. Int. J. Food Prop. 2022, 25(1), 450–462.
- Alda, L. M.; Gogoa, I.; Bordean, D.; Gergen, I.; Alda, S.; Moldovan, C.; Ni, L. Lycopene Content of Tomatoes and Tomato Products. J. Agroaliment. Process Technol. 2009, 15(4), 540–542.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D. G.; Lightfoot, D. A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017, 6(4), 42. DOI: 10.3390/plants6040042.
- Cruz Reina, L. J.; Durán-Aranguren, D. D.; Forero-Rojas, L. F.; Tarapuez-Viveros, L. F.; Durán-Sequeda, D.; Carazzone, C.; Sierra, R. Chemical Composition and Bioactive Compounds of Cashew (Anacardium Occidentale) Apple Juice and Bagasse from Colombian Varieties. Heliyon. 2022, 8(5), e09528. DOI: 10.1016/j.heliyon.2022.e09528.
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M. F. B.; Carneiro, J. N. P.; Bezerra, C. F.; Silva, T. G. D.; Coutinho, H. D. M.; Amina, B., et al. Antioxidant, Antimicrobial, and Anticancer Effects of Anacardium Plants: An Ethnopharmacological Perspective. Front. Endocrinol. (Lausanne). 2020, 11(June), 1–16. DOI: 10.3389/fendo.2020.00295.
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’amico, R.; Peritore, A. F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G., et al. Cashew (Anacardium Occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of carrageenan-induced Paw Edema. Antioxidants. 2020, 9(8), 1–19. DOI: 10.3390/antiox9080660.
- Dakuyo, R.; Konaté, K.; Bazié, D.; Sanou, A.; Kabakde, K.; Sama, H.; Balmoussa, S.; Konkobo, F. A.; Dicko, M. H. Correlating the Morphology of Anacardium Occidentale L . Fruits from 30 Orchards with Their Physicochemical and Nutritional Properties. Front. Plant Sci. 2022, 13(December), 1033577. DOI: 10.3389/fpls.2022.1033577.