 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the present study, the application of high-intensity ultrasound (USon) on the rheological properties of guamuchil seed protein isolate (GPI) was evaluated. GPI dispersions (30% w/v) were subjected to USon treatments (20 kHz; 200, 400, and 600 W) for 15 and 30 min. Strain, frequency, and temperature sweep tests showed that the storage (G) and loss (G”) moduli increased, while loss tangent (Tan δ) decreased by USon. The results showed a dominance of G’ over G”, indicating the formation of a stronger and more elastic dispersion due to USon, especially at high power (600 W for 15 and 30 min). Compared to native GPI, the USon treatments that caused the largest decreases in apparent viscosity (ηapp) and the most notable shear thinning behavior were at low (200 W) and high (600 W) power. The modification of the rheological characteristics of GPI by USon could be associated with structural changes, which modifies the inter and intramolecular interactions. The rheologically improved GPI by USon could represent an alternative to diversify its possible use as a new food ingredient.
INTRODUCTION
The global demand for high-quality protein is increasing due to population growth and people’s interest in protein-rich foods.[Citation1] At present, food proteins in the human diet are mainly derived from plants and animals. In the case of plant proteins, many sources as by-products of the processing of oilseeds, legumes, cereals, and fruits have been recovered as protein isolates to be used in food products depending of its functional quality.[Citation2] Nevertheless, the production processes of protein isolates can affect the functional properties and thus limit their use in the food industry.[Citation3]
The extraction process to produce protein isolates can have negative effects on the functional and nutritional properties. Exposure to solvents, heating, dehydration, homogenization, drying, and other treatments can change the structure, composition, conformation, and/or surface charge of the proteins, which together modify the functionality of these biopolymers.[Citation4] Among the most widely used extraction methods are alkaline extraction-isoelectric precipitation, salt extraction-dialysis, ultrafiltration, and micellar precipitation.[Citation5] Alkaline extraction followed by isoelectric precipitation is the most common method used in the food industry to obtain proteins due to its simplicity, rapidity, and low cost.[Citation6] However, to improve yield, harsh conditions applied in this process can cause undesirable changes in the structure of the recovered protein. Denaturation, aggregation, racemization, and the formation of cross-linked amino acids are related to alkaline extraction at high pH, temperature, and exposure time, resulting in lower nutritional quality, and changes in functional properties such as poor solubility, foaming, and emulsification.[Citation4,Citation7] Thus, the use of new technologies can modulate changes in the structure of proteins that could reduce the disadvantages associated with the extraction process and improve the functional properties of the protein obtained.
The functional properties of protein isolates can be modified by physical, chemical, and biological methods to improve its application as food ingredients.[Citation8] To date, physical methods such as high hydrostatic pressure,[Citation9] microwaves,[Citation10] pulsed electric field,[Citation11] gamma radiation[Citation12] and ultrasound,[Citation13] which are considered emerging green technologies, have been used to change the functional properties of proteins. Among the physical treatments, USon is an environmentally friendly, efficient, non-thermal, safe and nontoxic technology, that has demonstrated improving of the protein functionality.[Citation14]
USon are sound waves with frequencies higher than the upper audible limit of human hearing (>16 kHz). The effect of USon (frequency in the range of 16–100 kHz, power in the range of 10–100 W/cm2) on proteins is mainly due to a phenomenon known as cavitation. Cavitation is a phenomenon that includes the formation, growth, and rapid collapse of bubbles. The collapse of bubbles generates some cavitational effects, such as extreme temperatures and pressures, high shear forces, shock waves, turbulence, and reactive radicals, which can induce or accelerate chemical and physical changes in proteins.[Citation15] Chemical and physical changes induce conformational modifications in protein molecules and alter their physicochemical and functional properties, such as solubility, emulsion, foaming, viscosity, and gelation.[Citation16]
Among the functional properties, the rheological properties play an important role in predicting the behavior of foods during consumption, as well as during processing and handling, which can be deduced from studies of deformation and flow of matter.[Citation17] Rheological measurements allow evaluating the viscoelastic properties that can be defined from the storage (or elastic) modulus G’ and the loss (or viscous) modulus G”.[Citation18]
The effect of USon on the rheological properties of plant protein isolates obtained from soy, lupine, chickpea, and pumpkin has been recently reported. USon caused disruption in covalent as well as non-covalent bonds, leading to protein unfolding and formation of new inter and intramolecular interactions, which improved the elasticity (G’) of soy[Citation19] and lupine[Citation20] protein isolate gels.[Citation21,Citation22] reported that USon improved the fluidity and reduced the ηapp of chickpea and pumpkin protein isolates, respectively, due to the cavitation and mechanical effect of USon, which accelerate the disruption of aggregates into smaller protein particles. The results of these studies showed that USon effect depends both on the native characteristics of these polymers, as well as on the sonication conditions, such as frequency, intensity, time, etc.
It should be noted that the time and power of ultrasound affect the protein functional properties, including the rheological ones. In some cases, increasing exposure to ultrasound waves had detrimental effects on these properties.[Citation23] Conversely, other studies have seen improvements in functional properties with increased sonication time and power.[Citation19] Therefore, to improve the functional and rheological properties of proteins by USon are necessary studies for the determination of the best sonication time and power.
On the other hand, guamuchil (Pithecellobium dulce) from the family of Leguminosae is a tree which is native to Mexico and is widely distributed in America, the Philippines, Southern Florida, Cuba, the Caribbean, Hawaii, India, Bangladesh, and East Africa.[Citation24] It is primarily used for its fruit eaten either fresh or processed or in the preparation of sour-sweet beverages similar to lemonade.[Citation25] According to,[Citation2] defatted flour proceeding from the guamuchil seeds is an important resource to produce protein isolates because contains 33% of proteins. In this study also the authors demonstrated that USon improved the functional properties of GPI obtained from guamuchil seed defatted flour, which was derived from structural and physicochemical changes of the proteins caused by such treatment. However, since the GPI is a new protein source, and other studies are necessary to complete its characterization and find their potential use as food ingredient, as is the case of rheological behavior. Therefore, the objective of this work was to evaluate the effect of USon on the flow behavior and viscoelastic properties of GPI.
MATERIALS AND METHODS
Materials
GPI dispersions (10% w/v) were prepared at pH 7, adding GPI in distilled water and 0.1 N NaOH, by magnetic stirring for 30 min. An ultrasound system model CPX750 (Vernon Hills, Illinois, U.S.A.) provided with a titanium probe (2.54 diameter) was employed to sonicate the GPI dispersions in a 1 L glass beaker, which was placed in an ice-water bath to maintain the temperature below 15°C. The USon treatments were generated from at frequency of 20 kHz and power levels of 200, 400 and 600 W for 15 and 30 min (pulse time: on time 5 s, off time 1 s), in addition to a control treatment without USon. The exposed treatments at 200 W, 400 W and 600 W power received an ultrasonic intensity of 36–38 W/cm−2, 54–57 W/cm−2 and 107–109 W/cm−2, respectively, which was measured by calorimetry.[Citation2] Finally, all above protein dispersions were freeze-dried and stored at 4°C in airtight containers. The GPI was characterized according to, [Citation26] methods, which presented the next proximal composition: 75.17–76.40% protein, 0.97–1.13% fat, 1.19–3.84% moisture, 2.28–2.72% ash, and 17.55–20.12% total carbohydrates.
Rheological characterization
Rheological measurements were carried out using a controlled stress rheometer, according to the method of, [Citation27] with some modifications as are described below. GPI dispersions (30%, w/v) were prepared in distilled water, stirred for 3 h at 25°C and then allowed to stand for 24 h at 4°C. All rheological measurements were performed at 25°C using an equipment HR-1 (TA Instruments, Delaware, USA) provided with plate-cone geometry (60 mm diameter and 64 μm gap), and a Peltier temperature control system. The storage (G’) and loss (G”) moduli values were collected and analyzed with a TRIOS software, version 4.0 (TA Instruments, USA). The following sequence of measurements was done.
Strain sweep: The GPI dispersions were subjected to an amplitude strain in a range of 0.5 to 100% under a constant frequency of 1.0 Hz. Strain sweep analyses were performed in order to determine the linear viscoelastic region (LVR), where G’ and G” are independent of amplitude strain.[Citation28]
Frequency sweep: Frequency sweep analyses of the GPI dispersions were performed at a range of 0.1 to 10 Hz. The used value for the frequency sweep depended on the strain sweep (Section 2.2.1).
Temperature sweep: Temperature sweep analyses of the GPI suspensions were performed in the temperature range of 25 to 95°C with a scan rate of 5°C/min. The loss tangent or phase angle (Tan δ) was recorded as a temperature function.
Apparent viscosity (ηapp): ηapp of GPI dispersions was determined under a shear rate range (γ) of 0.1–100 s−1 for 4 min. Then, the experimental data of ηapp were fitted to Cross model to describe the flow behavior, as expressed in EquationEquation 1(1)
(1) .[Citation29]
where η is the shear viscosity (Pa·s), η∞ is the infinity shear viscosity (Pa·s), η0 is the zero shear viscosity (Pa·s), C is the Cross time constant (s), m* is the Cross rate constant (dimensionless), and γ represent the shear rate (s−1).
Statistical analysis
All rheological measurements were performed in triplicate and the resulting values expressed as means ± standard deviations. The experiment results were subjected to one-way analysis of variance using the StatSoft Statistica version 7.1 (TIBCO Software, Inc. California, USA). Significant differences (p < .05) between treatments were determined using Fisher’s test.
RESULTS AND DISCUSSION
Strain sweep
Strain sweep provides information on linear viscoelastic region (LVR) and protein structure stability.[Citation30] The G’ indicates the amount of energy that is stored in the material in each deformation cycle, while G” is an indication of the amount of energy dissipated as heat.[Citation31] shows the effect of USon on the G’ and G” moduli of GPI dispersions in function of strain sweep.
Figure 1. Impact of high-intensity ultrasound on the moduli of storage G’ (A) and loss G” (B) of guamuchil protein isolate in function of strain sweep. For each treatment the numerator and denominator represent the power and time of exposure to ultrasound, respectively.
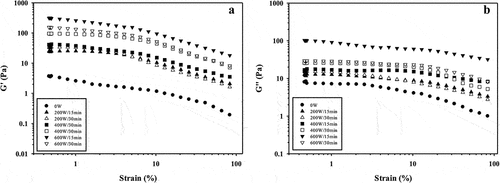
For all GPI dispersions, G’ () was higher than G” () over the strain range tested, except for 0W, indicating that USon formed a more elastic dispersion (solid nature), while the control treatment (0W) formed more viscous dispersions (fluid character). The obtained results reflect that GPI treated by USon was stronger and more structured compared to non-treated GPI.[Citation32]
Also, for all USon treatments, G’ and G” remained almost constant as strain increased and then suddenly decreased (). With basis in this trend the LVR was defined in the range of 0.5–2.5 strain (%) for sonicated GPI dispersions, while for the control treatment (0 W) was of 0.5–0.8 strain (%). Therefore, USon increased the LVR range of the GPI, probably due to the enhancement of particle interactions, which produce better stabilization of the guamuchil proteins.[Citation33] On the other hand, different trends on the G” was observed by effect of USon on the diverse treatments (). The limit of the LVR of 0 W, 200 W/15 min and 200 W/30 min treatments was 2%, while that for 400 W/15 min, 400 W/30 min, 600 W/15, and 600 W/30 min treatments the limit of LVR was 15%.
A previous study by,[Citation34] in USon-treated chicken breast myofibrillar proteins had strain sweep changes similar to those observed for GPI dispersions from this study, where both LVR region range and G’ values increased with ultrasonic treatment. On the contrary, a reduction on G’ was observed on soy and porcine myofibrillar proteins by USon effect according with studies reported by [Citation30,Citation23] respectively. This could be a consequence of the partial denaturation of proteins, which decreases intermolecular interactions between protein molecules because of ultrasound.
Frequency sweep
shows the USon effect depending of the frequency on the G’ and G” moduli of GPI dispersions. As can be observed, the increase of the frequency and the USon treatment provoked a higher augment of the G’ and G” values in comparison than control treatment, same behavior that have been observed in protein dispersions of album[Citation35] and quinoa.[Citation36]
Figure 2. Impact of high-intensity ultrasound on the moduli of storage G’ (A) and loss G” (B) of guamuchil protein isolate as function of frequency sweep. For each treatment the numerator and denominator represent the power and time of exposure to ultrasound, respectively.
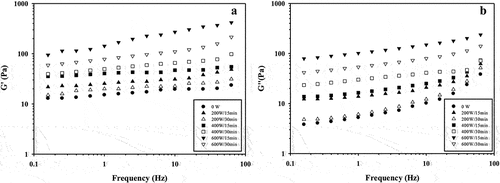
Furthermore, in this study, G’ showed higher values in comparison with G” on studied frequency range, suggesting the dominance of elastic components over viscous components of GPI dispersions in the oscillation process (). In particular, 0 W, 200 W/15 min, and 400 W/30 min treatments had a cross-over point between G’ and G” at frequencies of 20, 63, and 25 Hz, respectively. However, as the frequency increased, the values of G” also arose and exceeded the G’ values, indicating that USon treatment modified the viscoelastic properties of the proteins, due probably to conformation changes of the molecules.[Citation37]
In this study, the values of G’ and G” of the GPI dispersions treated with USon were higher than the control treatment (0 W), being the maximum and minimum values for both rheological parameters those corresponding to 600 W/15 min and 200 W/30 min treatments, respectively. The G’ values at 0.1–63 Hz for 0 W ranged from 35.5 to 55.0 Pa, while for 200 W/30 min from 59.2 to 214.7 Pa, and for 600 W/15 min from 94.7 to 417.3 Pa (). In the case of G”, its values ranged from 3.8 to 38.4 Pa for 0 W, 41.7 to 140.3 Pa for 200 W/30 min, and 78.9 to 236.6 Pa for 600 W/15 min (), which indicate that the USon treatment enhanced the elastic and viscous properties of the GPI dispersions.
In contrast [Citation23] confirmed that the G’ and G” values became smaller as USon [200 W; 15, 30, 60 and 120 min) treatment time increased, which may be because USon treatment induces protein unfolding and weakens non-covalent bonds, such as intermolecular hydrogen bonds.
In addition, [Citation38] observed that the USon treatments (200, 400, 600, 800, and 1000 W for 15 min] induced a reduction in the G’ and G” values of chicken myofibrillar proteins with the increase USon power, which suggests that the diminishing in both moduli depend on the protein type and sonication conditions.
The same behavior of the moduli where G’ > G” after of the application of USon was observed in sunflower,[Citation39] chickpea,[Citation21] and date palm pollen[Citation18] proteins. However, these authors not observed cross-over points between G’ and G” in the frequency sweep. These studies determined that ultrasonic cavitation produces molecular unfolding of the proteins, resulting a smaller particle size and greater surface hydrophobicity, higher protein solubility, as well as secondary structure modification, leading to increase intermolecular interactions between functional groups of protein molecules.[Citation27,Citation40]
In contrast, [Citation41,Citation38] observed that the USon treatment induced a reduction in the G’ and G” values of the whey protein isolate and chicken myofibrillar proteins, respectively, which suggests that the diminishing in both moduli may depend on the protein type and sonication conditions.
Temperature sweep
Knowledge of rheological behavior of protein isolates with increase in temperature can be used to control the mouth feel and texture of various food products such as yogurts, pudding and sausages.[Citation39] shows the effect of USon on G’, G” and Tan δ of the GPI as a function of temperature (from 25 to 95°C). In general, G’ and G” of GPI augmented with the increase of the temperature (). First, G’ and G” of GPI slightly increased up to approximately 65°C, and then both moduli increased sharply up to the maximum temperature of 95°C. In addition, G’ was predominantly larger than G” throughout the whole measurement, exhibiting both moduli a similar pattern, which implies a system mainly elastic gel-like structure. These results are in agreement with those previously discussed in the frequency sweep section (3.2).
Figure 3. Impact of high-intensity ultrasound on the moduli of storage G’ (A) and loss G” (B), and loss tangent of guamuchil protein isolate as function of temperature sweep. For each treatment the numerator and denominator represent the power and time of exposure to ultrasound, respectively.
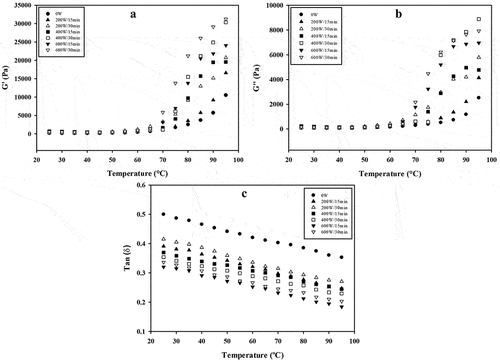
As is shown in , all USon conditions significantly increased (p < .05) the G’ and G” values of GPI with respect to 0 W. The highest and lowest values of G’ and G” of sonicated GPI were for the 600 W/15 min and 200 W/30 min treatments, respectively (). In addition, the G’ values in the temperature range of 25–95°C for 0 W varied from 195.5 to 10,537.9 Pa, while for 200 W/30 min from 249.4 to 20,731.6 Pa, and for 600 W/15 min from 588.1 to 31,235.6 Pa (), which means augments of 27.6–96.7% and 200.8–196.4%, respectively.
In addition, the G’ values in the temperature range of 25–95°C for 200 W/30 min and 600 W/15 min had increases of 27.6–96.7% and 200.8–196.4%, respectively, in comparison with 0 W, while in the case of G”, its values augmented from 27.7% to 129.9% for 200 W/30 min, and 143.1–215.6% for 600 W/15 min, in comparison with 0 W (), which demonstrated that USon had a high impact in such rheological parameters.
The augment in G’ during heating could be explained by the effect of mechanical waves of USon, with the consequent unfolding of the proteins where more functional groups (hydrophilic and hydrophobic residues) were exposed, creating new bonds (hydrogen, hydrophobic, disulfide, and electrostatic) which led to protein aggregates and a network formation.[Citation42,Citation43]
The results of this study with GPI about the gelation patterns and the impact of USon on the increase of G’ and G” of GPI were similar to those obtained by [Citation35,Citation44,Citation40] for albumin, beef myofibrillar and tuna myofibrillar proteins, respectively.
On the contrary, [Citation45,Citation18] observed different gelation patterns between G’ and G” in field pea protein isolate and palm pollen protein concentrate, respectively. According to these studies were observed crossover points between G’ and G” for field pea protein isolate at 84.0°C, while that for palm pollen protein concentrate at 53.1°C, indicating the specific temperatures of gel formation for each protein source.
According to, [Citation27] proteins that form strong gels are due to the high percentage of β-sheet. β-sheets oriented in parallel or antiparallel direction have a larger contact surface compared to α-helix and random coil secondary structures and thus can enhance intermolecular hydrogen bonds, which could stabilize the gel network. However, no gel formation was observed in the GPI. In fact, according to, [Citation45] a high β-sheet content induces greater thermal stability, and a higher temperature is then required to ensure the gelation of the protein. According to, [Citation2] the β-sheet content of guamuchil proteins is the predominant secondary structure, which could favor its thermal stability as was observed in the temperature scanning test (25 to 95°C), resulting in a delay of the clear appreciation of the formation of a gel, probably because the protein denaturation temperature is above the temperature range studied ().
On the other hand, Tan δ is a parameter that is used to obtain information about the structure of gels, emulsions and dispersions. Tan δ indicates the ratio of G’ and G”, which determine the contribution of the viscous and elastic components to the final structure.[Citation42] High values of Tan δ (>1) indicate the formation of weaker structures (more viscous) and low values of Tan δ (<1) indicate the formation of stronger structures (more elastic).[Citation17]
The impact of USon on Tan δ of GPI is shown in . All sonicated treatments showed a significant reduction (p < .05) in Tan δ with respect to 0 W, indicating the formation of strong and elastic dispersions. However, the largest reduction of Tan δ, in the temperature range of 25–95°C was 36.0–48.6%, respectively, for 600 W/15 min, while the smallest decrease of 16.0–28.6% was for 200 W/30 min, compared to 0 W (). According to, [Citation35] the reduction in Tan δ values is due to the fact that USon generates a greater interaction between protein molecules, resulting in a larger capacity to form hydrogen bonds, disulfide bridges, hydrophobic and electrostatic interactions, which was observed in this study.
In a study with black bean protein isolate,[Citation46] it has been reported that low (150 W) and high (450 W) power USon treatment augmented the strength of the formed gels (Tan δ < 1), because of cavitation produces unfolding of the proteins and the subsequent formation of new inter and intramolecular interactions as occurred in this study.
Apparent viscosity (ηapp)
ηapp of the non-Newtonian fluids is very important to understand their behavior in any process involving fluid flow such as extraction, purification, filtration, and extrusion. This property is defined as the ratio of shear stress to shear rate at a specified shear rate.[Citation47,Citation48] The viscosity of protein suspensions is related to the functional groups, molecular weight, shape, size, charge, molecular flexibility of said polymers, and their interaction with solvents.[Citation49]
As can be seen in , the ηapp of GPI of all treatments decreased significantly (p < .05) with the increase of the shear rate, as well as with USon application, which is one of the typical shear thinning behavior of the non-Newtonian fluids.[Citation50] The higher and lower differences between ultrasonicated treatments and control in ηapp at 0.1 s−1 were observed for 600 W/30 min (92.5%) and 200 W/15 min (6.7%), respectively, while that for these same treatments at shear rate of 100 s−1 the ηapp was reduced 95.5% and 18.3% ().
Figure 4. Effect of ultrasound on the apparent viscosity (ηapp) of guamuchil protein isolate as function of shear rate (γ).
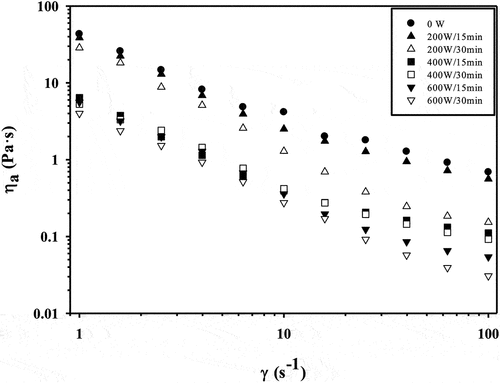
Shear thinning behavior can be explained by a breakdown of intra and intermolecular interactions during shear rate, since as shear rate increases, chains of protein molecules become oriented and aligned in the direction of flow, resulting in a decrease in internal friction and resistance, such as was observed in this study.[Citation51] According to, [Citation52] USon increase the random coil content that led to an important shear thinning behavior. The augment in this structure type by the effect of USon was shown in our previous study.[Citation2,Citation15] reported that USon cavitation produces a certain degree of molecular unfolding of the proteins, which causes a better ordering of the proteins along the flow field, exhibiting less resistance to flow, which results in a lower viscosity as observed in .
The behavior of the ηapp reduction by effect of USon on GPI is in agreement with results obtained by [Citation20,Citation53] for soybean protein isolates and [Citation54] for a whey protein isolate [Citation55] studied the rheology of soybean protein isolate solutions, finding that as the USon treatment [20 kHz for 30, 60, and 120 s) time increased, the ηapp significantly decreased with respect to the control sample, which could be due to the increase in the solubility of the protein.
[Citation56] also observed that the longer sonication time [450 W for 5, 10 and 15 min] reduced the ηapp of chicken breast meat protein isolate, which could be due to the diminishing in particle size and surface charge by effect of sound waves on proteins; this behavior is typical of shear thinning materials where the ηapp decreases as the shear rate increases.
On the contrary [Citation57,Citation58] confirmed that USon treatment increased the ηapp of whey protein isolates, which can be attributed to the cavitation effect that causes the unfolding of the proteins, making them less compact and more resistant to flow. On the other hand [Citation49,Citation59] did not found changes in ηapp by effect of USon on whey and lactoalbumin protein isolates, respectively, which may be due to the particular characteristics of each protein sources and the specific conditions of ultrasound treatment.[Citation60]
shows the effect of USon on the rheological parameters of GPI according to the Cross model. Each of these rheological parameters have a special means. η0 provides information on the stability of suspensions and emulsions, η∞ is related with aspects of high shear processing, m* measure the degree of dependency of viscosity on shear rate in the region of shear thinning, and 1/C determine the onset of shear thinning region.[Citation17]
Table 1. Impact of high-intensity ultrasound on η0 (zero viscosity), η∞ (infinity viscosity), m* (rate constant), and 1/C (critical shear rate) parameters of guamuchil protein isolate generated by Cross model.
A significant reduction (p < .05) in η0,η∞ and 1/C parameters at higher values of γ was observed in the sonicated treatments with respect to 0 W. 600 W/30 min exhibited the greater decrease in η0 (0.001 x106 Pa·s), η∞ (0.011 Pa·s), and 1/C [0.010 s−1) with respect to 0 W. The reduction in the I/C parameter with respect γ is an indicator of onset of shear thinning behavior, which were observed earlier for the GPI treated with USon than control treatment. According to a report of [Citation21] from chickpea protein isolate, USon cavitation (high power] accelerated the collision of aggregates and depolymerized the larger protein aggregates into smaller protein particles, exhibiting less resistance to flow, which resulted in lower values of η0,η∞ and 1/C as observed in this study (). On the contrary, the m* parameter augmented in the GPI by the application of USon in comparison with 0 W (0.900), being 600 W/30 min the treatment that exhibited the higher increment (1.335), which confirmed the shear thinning behavior (). Knowledge of these rheological properties in proteins systems could be of utility for improving or modifying the texture or during high shear processing operations such as pumping and filling.[Citation61]
Conclusion
USon treatment changed the rheological properties of GPI, which altered its viscoelasticity properties according to G’, G”, and Tan δ parameters, as was revelated by the strain, frequency and temperature sweeps, as well as the flow behavior denoted by ηapp, especially at high sonication power (600 W). The USon provoked stronger and more elastic GPI dispersions. At both low (200 W) and high (600 W) sonication powers, primarily, the onset of the shear thinning behavior of GPI dispersions according to the Cross model. Modification on rheological properties of GPI could be the consequence of ultrasonic cavitation, possibly due to the alteration on the structure, conformation and particle size of guamuchil proteins derived from changes on the intra and intermolecular interactions and the subsequent unfolding of proteins by effect of ultrasonic waves. USon is a useful technology for modifying the rheological properties of GPI for the production of guamuchil proteins with improved rheology functionality for specific applications in foods. Studies on the sensory characteristics related to rheological properties of the GPI are need to diversify its potential applications in the food industry.
CRediT authorship contribution statement
Nitzia Thalía Flores-Jiménez: Investigation, Methodology, Writing-original draft preparation & editing. José Armando Ulloa: Funding acquisition, Investigation, Writing-original draft, Writing-review & editing, Conceptualization, Supervision, Data curation. Rosa Isela Ortiz-Basurto: Equipment, Software, Conceptualization, Supervision, Visualization. Judith Esmeralda Urías-Silvas: Software, Resources, Supervision, Validation.
Acknowledgments
The authors acknowledge the help received from the National Council for Science and Technology of Mexico for the scholarship (601948) to M.Sc. Nitzia Thalía Flores Jiménez and the Patronage to Administer the Special Tax Destined to the Autonomous University of Nayarit for providing research funds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yu, X.-Y.; Zou, Y.; Zheng, Q.-W.; Lu, F.-X.; Li, D.-H.; Guo, L.-Q.; Lin, J.-F. Physicochemical, Functional and Structural Properties of the Major Protein Fractions Extracted from Cordyceps Militaris Fruit Body. Food Res. Int. 2021, 142, 110211. DOI: 10.1016/j.foodres.2021.110.
- Flores-Jiménez, N. T.; Ulloa, J. A.; Urías-Silvas, J. E.; Ramírez-Ramírez, J. C.; Bautista-Rosales, P. U.; Gutiérrez-Leyva, R. Influence of high-intensity Ultrasound on Physicochemical and Functional Properties of a Guamuchil Pithecellobium Dulce (Roxb.). Ultrason. Sonochem. 2022, 84, 105976. DOI: 10.1016/j.ultsonch.2022.105976.
- Nasrabadi, M. N.; Doost, A. S.; Mezzenga, R. Modification Approaches of plant-based Proteins to Improve Their techno-functionality and Use in Food Products. Food Hydrocolloids. 2021, 118, 106789. DOI: 10.1016/j.foodhyd.2021.106789.
- Miranda, C. G.; Speranza, P.; Kurozawa, L. E.; Kawazoe Sato, A. C. Lentil Protein: Impact of Different Extraction Methods on Structural and Functional Properties. Heliyon. 2022, 8(11), e11775. DOI: 10.1016/j.heliyon.2022.e11775.
- Cui, L.; Bandillo, N.; Wang, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Functionality and Structure of Yellow Pea Protein Isolate as Affected by Cultivars and Extraction pH. Food Hydrocolloids. 2020, 108, 106008. DOI: 10.1016/j.foodhyd.2020.106008.
- López, D.; Ingrassia, R.; Busti, P.; Wagner, J.; Boeris, V.; Spelzini, D. Effects of Extraction pH of Chia Protein Isolates on Functional Properties. LWT-Food Sci. Technol. 2018, 97, 523–529. DOI: 10.1016/j.lwt.2018.07.036.
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An Efficient ultrasound-assisted Extraction Method of Pea Protein and Its Effect on Protein Functional Properties and Biological Activities. LWT-Food Sci. Technol. 2020a, 127, 109348. DOI: 10.1016/j.lwt.2020.109348.
- Biswas, B.; Sit, N. Effect of Ultrasonication on Functional Properties of Tamarind Seed Protein Isolates. J. Food Sci. Technol. 2020, 57(6), 2070–2078. DOI: 10.1007/s13197-020-04241-8.
- Ahmed, J.; Al-Ruwaih, N.; Mulla, M.; Rahman, M. H. Effect of High Pressure Treatment on Functional, Rheological and Structural Properties of Kidney Bean Protein Isolate. LWT-Food Sci. Technol. 2018, 91, 191–197. DOI: 10.1016/j.lwt.2018.01.054.
- Zheng, Y.; Li, Z.; Zhang, C.; Zheng, B.; Tian, Y. Effects of microwave-vacuum pre-treatment with Different Power Levels on the Structural and Emulsifying Properties of Lotus Seed Protein Isolates. Food Chem. 2020, 311, 125932. DOI: 10.1016/j.foodchem.2019.12.
- Wang, H.; Wang, N.; Chen, X.; Wu, Z.; Zhong, W.; Yu, D.; Zhang, H. Effects of Moderate Electric Field on the Structural Properties and Aggregation Characteristics of Soybean Protein Isolate. Food Hydrocolloids. 2022a, 133, 107911. DOI: 10.1016/j.foodhyd.2022.107911.
- Wang, X. B.; Wang, C. N.; Zhang, Y. C.; Liu, T. T.; Lv, J. P.; Shen, X.; Guo, M. R. Effects of Gamma Radiation on Microbial, Physicochemical, and Structural Properties of Whey Protein Model System. J. Dairy Sci. 2018, 101(6), 4879–4890. DOI: 10.3168/jds.2017-14085.
- Flores-Jiménez, N. T.; Ulloa, J. A.; Urías-Silvas, J. E.; Ramírez-Ramírez, J. C.; Rosas-Ulloa, P.; Bautista-Rosales, P. U.; Silva-Carrillo, Y.; Gutiérrez-Leyva, R. Effect of high-intensity Ultrasound on the Compositional, Physicochemical, Biochemical, Functional and Structural Properties of Canola (Brassica Napus L.) Protein Isolate. Food Res. Int. 2019, 121, 947–956. DOI: 10.1016/j.foodres.2019.01.025.
- Su, J.; Cavaco-Paulo, A. Effect of Ultrasound on Protein Functionality. Ultrason. Sonochem. 2021, 76, 105653. DOI: 10.1016/j.ultsonch.2021.105653.
- Ren, X.; Li, C.; Yang, F.; Huang, Y.; Huang, C.; Zhang, K.; Yan, L. Comparison of Hydrodynamic and Ultrasonic Cavitation Effects on Soy Protein Isolate Functionality. J. Food Eng. 2020, 265, 109697. DOI: 10.1016/j.jfoodeng.2019.10969.
- Chen, W.; Ma, H.; Wang, Y. Y. Recent Advances in Modified Food Proteins by High Intensity Ultrasound for Enhancing Functionality: Potential Mechanisms, Combination with Other Methods, Equipment Innovations and Future Directions. Ultrason. Sonochem. 2022, 85, 105993. DOI: 10.1016/j.ultsonch.2022.105993.
- Pérez-Orozco, J. P.; Sánchez-Herrera, L. M.; Ortiz-Basurto, R. I. Effect of Concentration, Temperature, pH, co-solutes on the Rheological Properties of Hyptis Suaveolens L. Food Hydrocolloids. 2019, 87, 297–306. DOI: 10.1016/j.foodhyd.2018.08.0.
- Sebii, H.; Karra, S.; Blecker, C.; Karoui, R.; Attia, H.; Besbes, S. Effect of Sonication and Succinylation on Rheological Properties and Secondary Structures of Date Palm Pollen Protein Concentrate. Rheol. Acta. 2021a, 60(9), 543–551. DOI: 10.1007/s00397-021-01291-3.
- Liang, Y.; Teng, F.; He, M.; Jiang, L.; Yu, J.; Wang, X.; Li, Y.; Wang, Z. Effects of Ultrasonic Treatment on the Structure and Rehydration Peculiarity of freeze-dried Soy Protein Isolate Gel. Food Struct. 2021, 28, 100169. DOI: 10.1016/j.foostr.2020.100169.
- Lo, B.; Kasapis, S.; Farahnaky, A. Effect of Low Frequency Ultrasound on the Functional Characteristics of Isolated Lupin Protein. Food Hydrocolloids. 2022, 124, 107345. DOI: 10.1016/j.foodhyd.2021.107345.
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of High Intensity Ultrasound on Physicochemical, Interfacial and Gel Properties of Chickpea Protein Isolate. LWT-Food Sci. Technol. 2020b, 129, 109563. DOI: 10.1016/j.lwt.2020.109563.
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity Ultrasonic Treatment on the Physicochemical, Structural, Rheological, Behavioral, and Foaming Properties of Pumpkin (Cucurbita Moschata Duch.)-seed. LWT-Food Sci. Technol. 2022, 155, 112952. DOI: 10.1016/j.lwt.2021.112952.
- Kim, Y. J.; Lee, M.; Kim, H.; Kim, S.-M.; Yong, B.-K.; Choi, H. I. Improvement of Structural, Physicochemical, and Rheological Properties of Porcine Myofibrillar Proteins by high-intensity Ultrasound Treatment for Application as Pickering Stabilizers. Ultrason. Sonochem. 2023, 92, 106263. DOI: 10.1016/j.ultsonch.2022.106263.
- Chandra Sekhar, S.; Karuppasamya, K.; Vedaraman, N.; Kabeel, A. E.; Sathyamurthy, R.; Elkelawy, M.; Alm Eldin Bastawissi, H. Biodiesel Production Process Optimization from Pithecellobium Dulce Seed Oil: Performance, Combustion, and Emission Analysis on Compression Ignition Engine Fuelled with diesel/biodiesel Blends. Energy Convers. Manag. 2018, 161, 141–154. DOI: 10.1016/j.enconman.2018.01.074.
- Chaudhari, B. B.; Annapure, U. S. Physiochemical and Rheological Characterization of Pithecellobium Dulce (Roxb.) Benth Gum Exudate as a Potential Wall Material for the Encapsulation of Rosemary Oil. Carbohydr. Polym. Technol. Appl. 2020, 1, 100005. DOI: 10.1016/j.carpta.2020.100005.
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VI, 2000.
- Li, Y.; Zeng, Q.-H.; Liu, G.; Peng, Z.; Wang, Y.; Zhu, Y.; Liu, H.; Zhao, Y.; Wang, J. J. Effects of Ultrasoundassisted Basic Electrolyzed Water (BEW) Extraction on Structural and Functional Properties of Antarctic Krill (Euphausia Superba) Proteins. Ultrason. Sonochem. 2021, 71, 105364. DOI: 10.1016/j.ultsonch.2020.105364.
- Liu, P.; Xu, H.; Zhao, Y.; Yang, Y. Rheological Properties of Soy Protein Isolate Solution for Fibers and Films. Food Hydrocolloids. 2017, 64, 149–156. DOI: 10.1016/j.foodhyd.2016.11.001.
- Hauswirth, S. C.; Bowers, C. A.; Fowler, C. P.; Schultz, P. B.; Hauswirth, A. D.; Weigand, T.; Miller, C. T. Modeling Cross Model non-Newtonian Fluid Flow in Porous Media. J. Contam. Hydrol. 2020, 235, 103708. DOI: 10.1016/j.jconhyd.2020.103708.
- Hu, H.; Wu, J.; Li-Chan, E. C. Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of Ultrasound on Structural and Physical Properties of Soy Protein Isolate (SPI) Dispersions. Food Hydrocolloids. 2013, 30(2), 647–655. DOI: 10.1016/j.foodhyd.2012.08.001.
- Aliyari, M. A.; Salami, M.; Hosseini, E.; Emam-Djomeh, Z.; Karboune, S.; Waglay, A. Biophysical, Rheological, and Functional Properties of Complex of Sodium Caseinate and Olive Leaf Aqueous Polyphenolic Extract Obtained Using ultrasound-assisted Extraction. Food Biophys. 2021, 16(3), 325–336. DOI: 10.1007/s11483-021-09671-1.
- Wu, C.; Navicha, W. B.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effects of Removal of non-network Protein on the Rheological Properties of heat-induced Soy Protein Gels. LWT-Food Sci. Technol. 2018, 95, 193–199. DOI: 10.1016/j.lwt.2018.04.077.
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T. A. Physics of Agarose Fluid Gels: Rheological Properties and Microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. DOI: 10.1016/j.crfs.2021.06.003.
- Chen, J.; Zhang, X.; Chen, Y.; Zhao, X.; Anthony, B.; Xu, X. Effects of Different Ultrasound Frequencies on the Structure, Rheological and Functional Properties of Myosin: Significance of Quorum Sensing. Ultrason. Sonochem. 2020, 69, 105268. DOI: 10.1016/j.ultsonch.2020.105268.
- Mir, N. A.; Riar, C. S.; Singh, S. Structural Modification in Album (Chenopodium Album) Protein Isolates Due to Controlled Thermal Modification and Its Relationship with Protein Digestibility and Functionality. Food Hydrocolloids. 2020, 103, 105708. DOI: 10.1016/j.foodhyd.2020.105708.
- Mir, N. A.; Riar, C. S.; Singh, S. Structural Modification of Quinoa Seed Protein Isolates (Qpis) by Variable Time Sonification for Improving Its Physicochemical and Functional Characteristics. Ultrason. Sonochem. 2019, 58, 104700. DOI: 10.1016/j.ultsonch.2019.10.
- Espert, M.; Hernández, M. J.; Sanz, T.; Salvador, A. Rheological Properties of Emulsion Templated Oleogels Based on Xanthan Gum and Different Structuring Agents. Curr. Res. Food Sci. 2022, 5, 564–570. DOI: 10.1016/j.crfs.2022.03.001.
- Zhang, Z.; Regenstein, J.; Zhou, P.; Yang, Y. Effects of High Intensity Ultrasound Modification on Physicochemical Property and Water in Myofibrillar Protein Gel. Ultrason. Sonochem. 2017, 34, 960–967. DOI: 10.1016/j.ultsonch.2016.08.008.
- Malik, M. A.; Saini, C. S. Rheological and Structural Properties of Protein Isolates Extracted from Dephenolized Sunflower Meal: Effect of High Intensity Ultrasound. Food Hydrocolloids. 2018, 81, 229–241. DOI: 10.1016/j.foodhyd.2018.02.052.
- Liu, X.; Sun, X.; Wei, Y.; Ma, Y.; Sun, P.; Li, X. Effects of Ultrasonic Treatment on physico-chemical Properties and Structure of Tuna (Thunnus Tonggol) Myofibrillar Proteins. J. Food Compost. Anal. 2022, 108, 104438. DOI: 10.1016/j.jfca.2022.104438.
- Frydenberg, R. P.; Hammershøj, M.; Andersen, U.; Greve, M. T.; Wiking, L. Protein Denaturation of Whey Protein Isolates (Wpis) Induced by High Intensity Ultrasound during Heat Gelation. Food Chem. 2016, 192, 415–423. DOI: 10.1016/j.foodchem.2015.07.037.
- Huang, Y.; Zhang, Y.; Zhang, D.; Chen, L.; Bao, P.; Fang, H.; Zhou, C. Combination Effects of Ultrasonic and Basic Amino Acid Treatments on Physicochemical Properties of Emulsion Sausage. J. Food Meas. Charact. 2021, 15(2), 2088–2097. DOI: 10.1007/s11694-020-00800-x.
- Sebii, H.; Karra, S.; Bchir, B.; Nhouchi, Z.; Ghribi, A. M.; Karoui, R.; Blecker, C.; Besbes, S. Effect of Succinylation on the Secondary Structures, Surface, and Thermal Properties of Date Palm Pollen Protein Concentrate. J. Food Sci. Technol. 2021b, 58(2), 632–640. DOI: 10.1007/s13197-020-04577-1.
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of Ultrasound Treatment for Improving the Physicochemical, Functional and Rheological Properties of Myofibrillar Proteins. Int. J. Biol. Macromol. 2018, 111, 139–147. DOI: 10.1016/j.ijbiomac.2017.12.167.
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J. Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocolloids. 2015, 43, 679–689. DOI: 10.1016/j.foodhyd.2014.07.024.
- Li, L.; Zhou, Y.; Teng, F.; Zhang, S.; Qi, B.; Wu, C.; Tian, T.; Wang, Z.; Li, Y. Application of Ultrasound Treatment for Modulating the Structural, Functional, and Rheological Properties of Black Bean Protein Isolates. Int. J. Food Sci. Technol. 2020, 55, 1637–1647. DOI: 10.1111/ijfs.
- Mitra, P.; Nepal, K.; Tavade, P. Effect of Whey and Soy Proteins Fortification on the Textural and Rheological Properties of value-added Yogurts. Appl. Food Biotechnol. 2022, 2, 100195. DOI: 10.1016/j.afres.2022.100195.
- Tian, Z.; Duan, L.; Wu, L.; Shen, L.; Li, G. Rheological Properties of glutaraldehyde-crosslinked Collagen Solutions Analyzed Quantitatively Using Mechanical Models. Mater. Sci. Eng. C. 2016, 63, 10–17. DOI: 10.1016/j.msec.2016.02.047.
- Vargas, S. A.; Delgado-Macuil, R. J.; Ruiz-Espinosa, H.; Rojas-López, M.; Amador-Espejo, G. G. High-intensity Ultrasound Pretreatment Influence on Whey Protein Isolate and Its Use on Complex Coacervation with Kappa Carrageenan: Evaluation of Selected Functional Properties. Ultrason. Sonochem. 2021, 70, 105340. DOI: 10.1016/j.ultsonch.2020.105340.
- Piñeiro-Lago, L.; Franco, I.; Tovar, C. A. Changes in Thermoviscoelastic and Biochemical Properties of Atroncau Blancu and Roxu Afuega’l Pitu Cheese (PDO) during Ripening. Food Res. Int. 2020, 137, 109693. DOI: 10.1016/j.foodres.2020.109.
- Agi, A.; Junin, R.; Gbadamosi, A.; Abbas, A.; Azli, N. B.; Oseh, J. Influence of Nanoprecipitation on Crystalline Starch Nanoparticle Formed by Ultrasonic Assisted weak-acid Hydrolysis of Cassava Starch and the Rheology of Their Solutions. Chem. Eng. Process. 2019, 142, 107556. DOI: 10.1016/j.cep.2019.107556.
- Wei, Y.; Lin, Y.; Xie, R.; Xu, Y.; Yao, J.; Zhang, J. The Flow Behavior, Thixotropy and Dynamical Viscoelasticity of Fenugreek Gum. J. Food Eng. 2015, 166, 21–28. DOI: 10.1016/j.jfoodeng.2015.05.015.
- Wang, T.; Wang, N.; Li, N.; Ji, X.; Zhang, H.; Yu, D.; Wang, L. Effect of high-intensity Ultrasound on the Physicochemical Properties, Microstructure, and Stability of Soy Protein isolate-pectin Emulsion. Ultrason. Sonochem. 2022b, 82, 105871. DOI: 10.1016/j.ultsonch.2021.105871.
- Albano, K. M.; Nicoletti, V. R. Ultrasound Impact on Whey Protein concentrate-pectin Complexes and in the O/W Emulsions with Low Oil Soybean Content Stabilization. Ultrason. Sonochem. 2018, 41, 562–571. DOI: 10.1016/j.ultsonch.2017.10.01.
- Karki, B.; Lamsal, B. P.; Grewell, D.; Pometto, A. L.; van Leeuwen, J.; Khanal, S. K.; Jung, S. Functional Properties of Soy Protein Isolates Produced from Ultrasonicated Defatted Soy Flakes. J Am. Oil Chem. Soc. 2009, 86(10), 1021–1028. DOI: 10.1007/s11746-009-1433-0.
- Li, K.; Li, S.-Y.; He, -Y.-Y.; Wang, Y.-Q.; Zhang, Y.-X.; Zhao, -Y.-Y.; Du, M.-T.; Wang, Y.; Wang, Y.-T.; Bai, Y.-H. Application of ultrasound-assisted Alkaline Extraction for Improving the Solubility and Emulsifying Properties of Pale, Soft, and Exudative (Pse)-like Chicken Breast Meat Protein Isolate. LWT-Food Sci. Technol. 2022, 172, 114234. DOI: 10.1016/j.lwt.2022.114234.
- Krešić, G.; Lelas, V.; Jambrak, A. R.; Herceg, Z.; Brnčić, S. R. Influence of Novel Food Processing Technologies on the Rheological and Thermophysical Properties of Whey Proteins. J. Food Eng. 2008, 87(1), 64–73. DOI: 10.1016/j.jfoodeng.2007.10.02.
- Zhang, T.; Zhao, Y.; Tian, X.; Liu, J.; Ye, H.; Shen, X. Effect of Ultrasound Pretreatment on Structural, Physicochemical, Rheological and Gelation Properties of Transglutaminase cross-linked Whey Protein Soluble Aggregates. Ultrason. Sonochem. 2021, 74, 105553. DOI: 10.1016/j.ultsonch.2021.105553.
- Jambrak, A. R.; Mason, T. J.; Lelas, V.; Krešić, G. Ultrasonic Effect on Physicochemical and Functional Properties of α-lactalbumin. LWT-Food Sci. Technol. 2010, 43(2), 254–262. DOI: 10.1016/j.lwt.2009.09.001.
- Arredondo-Parada, I.; Torres-Arreola, W.; Suárez-Jiménez, G. M.; Ramírez-Suárez, J. C.; Juárez-Onofre, J. E.; Rodríguez-Félix, F.; Marquez-Rios, E. Effect of Ultrasound on Physicochemical and Foaming Properties of a Protein Concentrate from Giant Squid (Dosidicus Gigas) Mantle. LWT-Food Sci. Technol. 2020, 121, 108954. DOI: 10.1016/j.lwt.2019.108954.
- Koocheki, A.; Taherian, A. R.; Bostan, A. Studies on the Steady Shear Flow Behavior and Functional Properties of Lepidium Perfoliatum Seed Gum. Food Res. Int. 2013, 50(1), 446–456. DOI: 10.1016/j.foodres.2011.05.002.
