ABSTRACT
Berberine is a metabolite of many medicinal plants. Chemically, berberine belongs to the isoquinoline alkaloids family. The roots of plants grown at lower elevations have a higher concentration of berberine as compared to plants grown at higher elevations. Berberine is a natural substance, which is now among the patent pharmaceuticals for reducing chronic disorders and malignancies. Berberine is also classified as a nutraceutical. Nutraceuticals have been associated with various health benefits via modifying microRNA (miR) expression systems, apoptosis, gene activity, chemical signals/pathways and activation of transcription factors thus aiding in the prevention from various ailments. The pharmacokinetic studies revealed that berberine is poorly absorbed via intestinal walls therefore, its concentration level is extremely low in body fluid. However, the quantity of berberine and its active derivatives in organs is higher as compared to blood as it stably present in tissues. Berberine has an effective role in the body as an anticancer, antidiabetic, antiobesity, antioxidant, antiinflammatory, neuroprotective, hepatoprotective, antiaging and cardioprotective agent. In this review, the therapeutic potentials of berberine are discussed to understand its nutraceutical importance.
Introduction
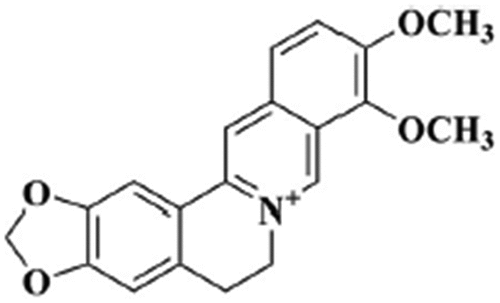
Berberine (BBR) (5, 6dihydro9, 10dimethoxybenzo[g]1, 3benzodioxolo [5, 6a] quinolizinium) () is a medicinal plant metabolite that belongs to the isoquinoline alkaloids family.[Citation1–3] It is found in plants such as Barberry (Berberis species including B. Vulgaris, B. Asiatica, B. Lycium, B. aristata and B. pseudumbellata), Yellowroot (Xanthorhiza simplicissima), Amur corktree (Phellodendron amurense), Chinese goldthread (Coptis chinensis), Prickly poppy (Tinospora cordifolia, Argemone mexicana), Goldenseal (Hydrastis Canadensis)and Californian poppy (Eschscholzia californica).[Citation4,Citation5] BBR, a benzylisoquinoline alkaloid, is important in the production of lead compounds in pharmacology.[Citation2] BBR is a tasteless, deep yellow powder that has a high solubility in water and ethanol. The abundant quantity of BBR is present in B. vulgaris’ roots, rhizomes, stem, and bark.[Citation6] According to some studies, the root contains the highest concentration of BBR (1.6–4.3%), and plants grown at low elevations have more BBR than plants that grow at higher altitudes in most Berberis species.[Citation7–9] BBR concentrations varies in different species of the same genus as B. Asiatica have a higher BBR content (4.3%) than B. Lycium (4.0%) and B. aristata (4.0%). Srivastava et al.[Citation10] stated that B. aristata roots had more BBR (2.8%) than B. Asiatica roots (2.4%).[Citation7] In contrast to B. aristata roots, in which the BBR quantity (1.9%) is better for the winter crops, the highest output of BBR for B. pseudumbellata was attained in the summer harvest, with 2.8% in the roots and 1.8% in stem bark.[Citation11]
BBR is now a patented pharmaceutical for the treatment of chronic disorders and malignancies.[Citation12,Citation13] Understanding of pharmacokinetic factors should be utilized to incorporate measures to boost absorption sites to improve or enhance BBR’s poor bioavailability.[Citation14] Ginseng, curcumin, and BBR are natural chemicals originating from traditional folk medicine that influence the gut microbiota by lowering the components of intestinal microflora and their metabolites.[Citation15] Nutraceuticals have been associated with health benefits via modulating microRNA (miR) expression, this causes apoptosis and aids in the reduction of aging, diabetes, and cardiovascular diseases (CVDs). BBR as antiinflammatory and antioxidant molecule decreases arterial inflammation and improves vascular health, insulin resistance, and overall cholesterollowering benefits.[Citation5]
Review methodology
The data of the current review article was carried out using different sites including Science Direct, Google scholar, PubMed, scientific databases comprising Scopus, Google Scholar, Science Hub, Library genesis and Cochrane Library. For the purpose, the collection of data was done by using the subject heading Berberine, Medicinal Plant, Nutraceuticals, Sources of Berberine, Pharmaceuticals Properties of Berberine, and Health benefits of Berberine. Furthermore, data regarding role of berberine as antiinflammatory, antioxidant, anticancer, antidiabetic, antiobesity, neuroprotective, hepatoprotective, antiaging and cardioprotective agent was collected. In addition, the authors collected the latest available literature from primary and secondary sources.
Antioxidant potential
The antioxidant effects of BBR have been well established as it plays a pivotal role in the inhibition and treatment of Alzheimer’s disease.[Citation16,Citation17] Jiang and colleagues[Citation18] discovered that BBR can protect immortal functioning human melanocyte cell line PIG1 from oxidative damages. BBR reduces reactive oxygen species (ROS) generation, increases total nuclear factor erythroid 2 related factor 2 (Nrf2) levels and antioxidant response elements (ARE) activity, blocks nuclear factorB (NFB) activation, and regulates microphthalmiaassociated transcription factor (Mitf) levels.[Citation18] Pirmoradi and colleagues[Citation19] tested various dosages of BBR (50 and 100 mg/kg/daily) in chronic cerebral hypoperfusion (CCH) model rats and found prevention from hippocampus neurodegeneration They also observed reduction in malondialdehyde (MDA) level, relapse in caspase 3 neuronal activity, and improvement in antioxidative enzymes activity including superoxide dismutases (SOD) and catalase (CAT) following TwoVessel Occlusion (2VO) surgery.[Citation19] Several research groups investigated that diets supplemented (100 mg/kg) in dibutyltin dichloride (DBTC) caused acute pancreatitis in Wistar albino rats by lowering fasting blood glucose, increasing level of antioxidative enzymes like SOD, CAT, and Glutathione (GSH), as well as lowering peroxidation parameters.[Citation20–22]
BBR inhibits doxorubicin (DOX)induced cardiac apoptosis in diabetic animal models and human kidney cells through inhibition of PI3K/Akt transduction pathways’ AMP/ATP ratio and AMPK.[Citation23] BBR affects the neurotransmitters and receptor systems of the central nervous system.[Citation6] BBR can also help to heal the damaged intestinal mucosa and immunological barrier, which helps to prevent intestinal endotoxins from entering the bloodstream and maintain metabolic equilibrium in the host.[Citation23] In vivo studies with liquid crystal nanoparticles (LCNPs) reservoirs containing a dissolution rate of BBR oleate significantly reduced psoriasis symptoms and inflammatory cytokines in psoriatic individuals.[Citation24,Citation25] BBR has antiAlzheimer’s and antiParkinson’s disease qualities.[Citation26]
Pharmacokinetic study
Although BBR is poorly absorbed by the intestinal walls and thus is available at extremely low blood levels in animal pharmacokinetic investigations. The quantity of BBR and consequently its dynamic byproducts are more in organs than in circulation after oral treatment.[Citation26,Citation27] Tan and colleagues[Citation28] investigated the bioavailability of drugs of BBR as well as its bioactive derivatives in rats after an oral dose of 200 mg/kg and discovered that BBR was rapidly spread in the liver, kidneys, muscle, lungs, brain, heart, and pancreas in increasing order. BBR levels in the liver appear to be almost 10 times greater than the blood, with a spike (68.19 ng/g) discovered 8 hours after oral administration. The AUC (0t) (area under the concentrationtime curve) for metabolites in the liver is 30fold higher than in plasma.[Citation28] The mismatch between BBR’s limited bioavailability and therapeutic qualities is explained in part by the regional distributions of BBR and also its constituents.
Health endorsing perspectives
Cancer insurgence
The rising prevalence of cancer is due to an increase in life expectancy, urban lifestyles, and changing environmental conditions. A variety of chemical medications have been employed to treat cancer, but their usage is limited due to the induction of genotoxic, carcinogenic, and teratogenic consequences. Phytochemicals from plants are effective chemopreventive agents. The oxidative and inflammatory role of BBR is important to reduce oncogenesis. [18,] For different types of cancers, BBR shows protective effects.[Citation29] The impact of BBR on the induction of cell cycle arrest in distinct cancer cell type is given in .
Table 1. The impact of BBR on the induction of cell cycle arrest in distinct cancer cell types.
Song and colleagues[Citation30] discovered that BBR has anticancer properties in the A549 xenografted tumor model through a variety of mechanisms, including inducing apoptosis by obstructing mitochondria membrane potential, increasing caspase 3–9 actions, increasing cytochrome C release, activating proapoptotic Bax, and hindering antiapoptotic Bcl2 levels.[Citation30] BBR inhibited methotrexateinduced renal toxicity in rats by lowering the expression of the Keap1, P38 mitogenactivated protein kinase (MAPK), and NFB genes, as well as a significant upregulation of the Nrf2 gene. BBR also reserved the transcription of the Bax and caspase 3 proteins despite growing Bcl2 synthesis. BBR also boosted GSH and SOD activity while lowering thiobarbituric acid reactive substances (TBARS) and NO2.[Citation31] BBR has been proven to prevent human murine melanoma (B16F10), human MDAMB231, and MCF7 breast cancer cells by inhibiting PI3K/AKT pathway and reducing EGFR and AKT phosphorylation.[Citation32,Citation33] BBR has an anticancer role and suppresses the development of human malignant pleural mesothelioma (MPM) cell line NCI H2452. It suppressed NCI H2452 cell proliferation in dosage and timedependent way or may trigger apoptosis, probably by an inherent mitochondrial process involving caspase 9. BBR also triggered apoptosis, as supported by the accumulation of LC3 II and a decrease in p62 expression.[Citation34] Multiple investigations by many scientists and analysts identified anticancer mechanisms like genetic alterations, suppression of progression and invasive phases, initiation of autophagy, and interactions with DNA that might cause DNA damage.[Citation35,Citation36]
In three human embryonal rhabdomyosarcoma (RMS) cell lines, ERMS1, KYM1, and RD, BBR reduced cell growth and slowed the cell cycle during the G1 phase.[Citation1,Citation37] BBR MDAMB231 cells caused DNA breaks and paused cells in S stage of the cell cycle, suppressed XRCC1 activity, and restored cancer cell resistance.[Citation13] BBR decreased ZEB1 and Snail transcription and dysregulated the expression of TRI, TRII, Smad2/pSmad2, and Smad3/pSmad3 in the TAFlike myofibroblast cell line CCD18Co. It also induced apoptosis, increased Bax expression, negatively regulated Bcl2, phosphorylated p38 MAPK, and raised p38 MAPK expression.[Citation38]
In ovarian cancer cell lines, OVCAR3 and POCCLs, BBR substantially reduced cell growth, and invasion, including induction of G0/G1 downregulation in a dose and timedependent manner. It also raised the likelihood of normal apoptosis, necrotic neurodegeneration morphology, suppressed PCNA and Ki67 production, and elevated the expression and activity of Caspase-3, Caspase-8, RIPK3, and MLKL.[Citation39]
One of the most prevalent cancer and the second largest cause of cancer-related death worldwide is gastric cancer. Gastric cancer is typically treated with cisplatin (DDP). It is vital to find how BBR affects DDP susceptibility in gastric cancer and what pathways are involved. IC50 values of DDP in the BGC-823/DDP and SGC-7901/DDP cells were substantially more than in the original cells, showing that they were DDP-resistant gastric cancer cells. BBR inhibited cell survival in BGC-823 and SGC-7901 cells in a quantity-dependent mode, although its inhibitory effects were considerably reduced in DDP-resistant cells. After being pre-treated with BBR, BGC-823/DDP and SGC-7901/DDP cells were particularly sensitive to DDP. BBR injection decreased the amounts of multidrug inhibition protein 1 and multidrug resistance-1 protein in the BGC-823/DDP and SGC7901/DDP cells in a concentration-dependent manner. Furthermore, in BGC-823/DDP and SGC-7901/DDP cells, cross with BBR and DDP resulted in a considerable increase in cell mortality. According to animal research, BBR treatment of SGC-7901/DDP cells exposed them to DDP in vivo. In BGC-823/DDP and SGC-7901/DDP cells treated with DDP, BBR reduced the PI3K/AKT/mTOR signaling. Finally, it was observed that BBR increases DDP sensitivity in stomach cancerous cells. Further research found that BBR-mediated DDP sensitivity is related to reduced drug transporter transcription (multi-drug resistance-1 and multi-drug resistance-associated protein 1), increased apoptosis, and decreased PI3K/AKT/mTOR activation.[Citation40]
BBR has been demonstrated to promote cellular damage in glioblastoma cells when coupled with solid lipid curcumin particles by inhibiting the PI3K/Atk/mTOR system.[Citation41] A series of experiments revealed that extracts of Coptidis Rhizoma, a dried rhizome of Coptis chinensis, and its active metabolite BBR had anticancer activity. BBR inhibits IL-8 synthesis via the EGFR/MEK/ERK pathway, which may lower cell invasiveness and proliferation in triple-negative breast cancer cells.[Citation42] BBR inhibits the VEGFR2/ERK signaling, which may block angiogenesis in glioblastoma xenografts. BBR has also been demonstrated to improve colorectal cancer apoptosis by influencing the cancer vulnerability candidate 2 (CASC2)/AU-Binding Factor 1 (AUF1)/B-Cell CLL/Lymphoma 2 (Bcl-2) Axis.[Citation43] This study showed that BBR triggered mitochondrial apoptosis and inhibited the development of human gastric cancer cells. The BBR-induced apoptotic consequences is mainly due to impact of BBR on cellular lipid homeostasis.[Citation44]
The p53 protein, also known as tumor protein p53, is often mutated and is essential for tumorigenesis. Because of its extremely short half-life, P53 protein levels in cells are low.[Citation45] Phosphorylation, acetylation, and methylation activate the p53 protein in response to stress conditions like hypoxia as well as DNA damage. Its concentration in the cell rapidly rises, and it then reaches the nucleus, where it regulates the subsequent expression of genes and has a role in the cell cycle, apoptosis, antibiotic resistance, phagocytosis, growth, and some other activities.[Citation46] In human malignancies, TP53 is the most often altered gene. TP53 mutations are seen in more than half of all cancers.[Citation47,Citation48] BBR induced G1 stage arrest in U87 cells by phosphorylating wild-type p53 (wtp53), increasing p21 protein production, and decreasing cyclin D1 levels, all of which resulted in wtp53 phosphorylation. BBR reduced mutant p53 (mutp53) levels in U251 cells and caused G2 phase arrest, as well as p21, cyclin D1, and cyclin B1 levels. The cell cycle arrest effects of wtp53 transduction were increased. BBR also inhibited the growth of glioma in a mouse tumor model in vivo.[Citation49] BBR promoted apoptosis and mortality in temozolomide-resistant cells after temozolomide administration in a way that was connected to ERK1/2 signaling in some experiments. BBR improved glioblastoma sensitivity to temozolomide when given in vivo via ERK1/2 signaling pathways.[Citation50] illustrates BBR’s antitumor activity through diverse molecular mechanisms and pathways i.e. inducing autophagy and apoptosis, preventing the advancement of the cell cycle, and suppressing invasion and metastasis of various types of cancers.[Citation51]
Diabetes prevention
Millions of people worldwide are afflicted by the potentially fatal metabolic disease known as diabetes mellitus (DM). Diabetes patients are at an elevated risk of death and morbidity due to an increase in atherothrombotic events brought on by platelet activation and apoptosis, which cause macro and microvascular occlusions. The buildup of ROS brought on by enhanced aldose reductase (AR) and NADPH oxidase (NOX) activities during DM causes platelet hyper-reactivity and death. Through altering the actions of AR, NOX, and glutathione reductase, BBR was found to decrease platelet activation and superoxide generation in high glucose (HG)treated platelets. It is involved in blocking the release of calcium, ERK activation, release of dense granule, and platelet stickiness. BBR also inhibited HG-induced platelet mortality, mitochondrial dysfunction, and Bax activation via the p38-p53 pathway.[Citation23,Citation52] Hypoxia-inducible factor-3 (HIF3A) methylation has previously been associated with insulin resistance (IR) in people with gestational diabetes (GDM). In IR 3T3-L1 adipocytes, BBR greatly increased glucose uptake, adiponectin generation, and cell differentiation. In IR adipocytes, there was also a rise in HIF3A production and a reduction in HIF3A methylation. Furthermore, blocking HIF3A largely reversed the effects of BBR on increasing insulin levels, as well as the insulin-related gene expression alterations in IR fat tissue generated by BBR treatment.[Citation53]
Insulin resistance, problems with glucose and lipid metabolism, and hypothalamus-pituitary-adrenal (HPA) axis activity are all associated with type 2 diabetes. BBR reduced fasting blood sugar, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels in experimental rats. Moreover, the levels of glucagon, insulin, insulin resistance, insulin sensitivity, and high-density lipoprotein cholesterol variations were decreased. The study found decreased levels of orexin-A, the OX2R receptor, corticotropin-releasing hormone, pituitary, and plasma adrenocorticotropic hormone, as well as serum and urine corticosterone. Model rat skeletal muscles showed increased expression of GLUT4 mRNA and protein after BBR treatment.[Citation54] BBR in vivo experiment dramatically enhanced erectile function, lowered the expression levels ratio of phosphorylated Janus kinase 2, and improved oxidative stress in DM rat models produced by streptozotocin (STZ).[Citation30]
In the hippocampus, BBR restored several abnormal alterations in signal molecules linked to inflammation, as well as cholinergic and insulin signal transduction. BBR also decreased cerebrospinal fluid (CSF)/blood glucose levels, inflammation, and acetyl cholinesterase enzyme activity (AChE). After BBR therapy, diabetic rats’ acetylcholine levels were raised. Besides, in the diabetic hippocampus, BBR improved spatial learning memory.[Citation39,Citation53] In an experimental DM model created by intraperitoneal injection of STZ, BBR suppressed microglia and astrocyte activation in the spinal cords of diabetic mice. Inflammatory proteins like inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) as well as pro-inflammatory cytokines like tumor necrosis factor, interleukin-6, and interleukin-1 were reduced by BBR. BBR decreased STZ-induced nerve pain in diabetic rats, and this effect was correlated with a decline in the activation and irritation of the neuroglia, both of which are associated with DM.[Citation55] The diabetic nephropathy (DN) rat model was created using STZ (35 mg/kg) and a high-lipid diet. In a rat kidney model, BBR lowers elevated biochemical markers and enhances aberrant expression of phosphatidylinositol 3-kinase, protein kinase B, and phosphorylated Akt. In vitro, a co-stimulating factor reduced podocyte adhesion activity, resulting in podocyte dysfunction as seen by lower production of nephrin, podocin, and adhesive molecule 31, all of which were dramatically restored by BBR and LY294002 therapy. Furthermore, in the BBR (30 and 60 mol/L) and LY294002 (40 mol/L) treated groups, PI3K and phosphorylated Akt levels were reduced, whereas Akt expression was unaffected.[Citation56]
Diabetic kidney disease is the most prevalent cause of final renal failure among diabetic patients, and is associated with a high incidence of death and disability. Treatment with BBR (30 M) increased Bax, cytochrome C, caspase 9, and caspase 3 are upregulated under HG and hypoxia, while Bcl-xL, an anti-apoptotic factor, is downregulated. BBR protects against palmitateinduced apoptosis in human kidney 2 (HK-2) proximal tubular cells and normal rat renal tubular epithelial (NRK-52E) cells. It also avoided hypoxia and HG. BBR modulates HIF-1 and PI3K/Akt signaling pathway in renal tubular cells, which results in protection from hypoxia/HG -induced apoptosis.[Citation23] Certain aspects that contribute to the progression of diabetic kidney disease in diabetic mouse models include increased plasma-free fatty acid levels and disrupted mitochondrial dynamics. BBR reduced podocyte mortality, increased ROS production, and mitochondria fission and dysfunction in vivo and in vitro. It may keep podocyte mitochondria in shape by blocking dynamin-related protein 1 (Drp1) activation caused by palmitic acid (PA).[Citation57,Citation58]
BBR can also help with insulin secretion, gluconeogenesis in the liver, glycolysis in peripheral tissue cells, gut microbiota, glucose absorption in the intestine, and lipid metabolism regulation.[Citation59] The second most frequent kind of dementia is vascular dementia, which is closely connected to diabetes. Endothelial dysfunction in diabetes is connected to endothelial cell ectopic production of miR-133a. STZ (50 mg/kg/day) was administered to rats for five days to cause hyperglycemia and vascular dementia. Pregnancy-related diabetes decreased GTPCH1 gene expression and BH4 levels and increased ectopic translations of the miR-133a molecule in the vascular endothelium of STZ-injected rats. These effects were restored by BBR treatment (1.0 g/kg/day, 8 weeks). BBR prevented hyperglycemia from lowering blood flow by preventing acetylcholine-induced vasorelaxation in the middle cerebral artery. BBR’s beneficial effects on acetylcholine-induced vasorelaxation in the middle cerebral arteries of rats were diminished by miR-133a agomirs, although endothelial dysfunction was prevented by supplementing with L-sepiapterin. BBR was found to increase BH4 levels and NO production while decrease miR-133a expression in cultivated endothelium cells that had been treated for extreme hyperglycemia. BBR lowers the incidence of vascular dementia in diabetics because it inhibits the creation of ectopic miR-133a in endothelial cells.[Citation60]
Oxidative stress
Due to increased oxygen demand and low antioxidant levels, neurons are known to be vulnerable to oxidative stress. As a result, when the number of damaged components grows sufficiently and the balance between pro-oxidants and antioxidants is upset, oxidative stress occurs. The body has both enzymatic and non-enzymatic antioxidant systems to combat oxidative stress. These antioxidants include non-enzymatic antioxidants such as thiols (T-SHs) and reduced glutathione as well as enzymes like Glutathione-S-transferase. Oxidative stress arises when ROS outnumber biological antioxidant activity, resulting in the buildup of hazardous chemicals that damage proteins and enzymes while also destroying lipids.[Citation16,Citation61] Gender variations in oxidative stress production have been observed, and oxidative stress and associated signaling system alterations might have varied clinical ramifications at various phases of life. Because of its increased respiration activity, elevated concentration of oxidizing substrates, and absence of antioxidant defense, the brain is particularly vulnerable to oxidative stress. Mitochondria are vulnerable to oxidative damage and are a key component of the aging theory. Oxidative stress causes significant damage to mitochondrial membranes, resulting in reduction of overall mitochondrial activity. Aging is marked by a continuous reduction in biological function and physical fitness, as well as an increased risk of age-related disorders including skeletal muscular sarcopenia.[Citation61] BBR was given intraperitoneally 30 minutes before detorsion at a dosage of 200 mg/kg, and it lowered tissue malondialdehyde, total oxidant status, and total antioxidant status.[Citation62] BBR preserved renal tissues by regulating oxidative stress and altering the transcription factors Nrf2 and NF-B.[Citation31] By suppressing Bax and caspase-3 and increasing Bcl2 expression, BBR prevents apoptosis.[Citation63]
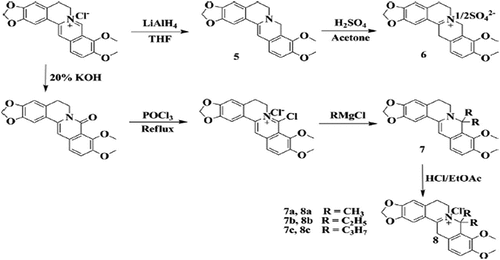
BBR derivatives () were synthesized which are dhBBR, 5 (dihydro-BBR) and Di-Me, 7 and 8 (8, 8-dimethyl-dihydro BBR) to solve the poor absorption and bioavailability problems of pure BBR. Both dhBBR and Di-Me exhibited analogous properties in in vitro results concerning BBR relating to the AMPK activation process, inhibition of the mitochondrial respiration process, and stimulation of glucose uptake. Both these compounds showed the ability to counter the increased adiposity, accumulation of tissue triglyceride, and insulin resistance in diet-induced obese mice.[Citation64]
Anti-obesity
The uncoupling protein 1 gene is associated with mitochondrial energy use in brown adipose tissue. A high-fat diet mouse model received 100 mg/kg/d of BBR in 0.9% normal saline. Four-week-old C57BL/6 J male mice show significant differences in energy expenditure, transcription of thermogenic genes (including UCP1), the cell stress protein inositol-requiring enzyme 1, and macrophage phenotype (M1 and M2) in white and brown adipose tissue. In rats given a high-fat diet, BBR therapy increased blood glucose levels, metabolic activity, and UCP1 expression in white adipose tissue while pro-inflammatory cytokines, macrophage recruitment, and M2 macrophage polarization was decreased. In polarized M2 macrophages, the expression of IRE1 and apoptosis genes was reduced. When given a high-fat diet, BBR improved metabolic activity in mice.[Citation65]
BBR assists in treating problems with lipid metabolism, improving insulin sensitivity, and lowering blood glucose levels. It works therapeutically in a similar way as frequently given drugs. It simultaneously addresses hyperlipidemia, hyperglycemia, and insulin resistance without causing side effects, making it a potential treatment option for breast cancer patients with metabolic issues or those at high risk for the disease.[Citation66] BBR helps to cure a variety of metabolic issues by lowering blood sugar, boosting insulin levels, and improving insulin sensitivity. It operates in a similar manner to commonly prescribed medications. It simultaneously treats hyperlipidemia, hyperglycemia, and insulin resistance without having any negative side effects, and it might be a viable option for breast cancer patients who are otherwise healthy but are rising or affected by the disease and have metabolic problems.[Citation66] The mTOR/P70S6K/4EBP1 pathway is used by the cell to regulate autophagy in this scenario. After being exposed to BBR, there is a protracted weight-loss impact. The ambient fat oxidation condition of triglycerides in adipocytes is enhanced by increased ATGL expression (AMPK-mediated).[Citation67]
In adipogenesis, peroxisome proliferator-activated receptors (PPARs) play a crucial role in transcription. BBR inhibits the PPAR and C/EBP pathways, which reduce adipocyte development. BBR also inhibits the proliferation of preadipocytes.[Citation68] BBR induced G1 phase arrest in U87 cells by phosphorylating wtp53, raising p21 protein expression, lowering cyclin D1 levels, and phosphorylating wtp53. BBR reduced mutp53 levels in U251 cells and caused G2 phase arrest, as well as p21, cyclin D1, and cyclin B1 levels. The cell cycle arrest effects of wtp53 transduction were increased. BBR also inhibited the growth of gliomas in a mouse tumor model in vivo.[Citation49]
Cardio protective
By increasing hepatic LDL reproduction (LDLR) and reducing LDLR modulator proportion convertase subtilisin/Kexin type 9 production and secretion, BBR decreases the growth of smooth muscle cells and reduces endothelial dysfunction. By increasing glucose consumption in muscle cells and adipose tissues while blocking glucose uptake in the intestinal epithelium, BBR has a net hypoglycemic effect. In hyperlipidemic rats, it reduces aortic lesions and LDL-C and TC levels in a manner similar to statins. BBR lowers blood glucose levels, improves glucose tolerance, and slows the gain of body fat and body weight in diabetic mice. The usefulness of BBR in people has been investigated in several clinical investigations. With no major side effects, BBR significantly lowers TC, triglycerides, and LDL-C levels in hypercholesterolemic patients while significantly raises HDL-C levels. BBR improves lipid and glucose profiles in people with metabolic syndrome, lower BMI and waist circumference, and lessen glycemia and plasma cholesterol in diabetic persons.[Citation69] BBR has been shown an anti-cardiovascular effect on a variety of molecular targets, including AMPK, SIRT1, LDLR, PCSK9, and PTP1B.[Citation70–72]
In STZ-induced diabetic rats, ischemia/reperfusion (I/R)-induced arrhythmias were treated for 6 weeks with resveratrol (5 mg/kg, intralperitonially (i.p.)), BBR (10 mg/kg, i.p.), and glibenclamide (5 mg/kg, i.p.). Resveratrol alone and in conjunction with glibenclamide reduced the incidence of numerous types of arrhythmias, the arrhythmia score, and the length of the arrhythmic episode during the reperfusion phase. By addressing an underlying issue that is not only connected to the restoration of Kir6.2 subunit protein production but also to the other subunits or ion channels that sustain cardiac action potential, the combination of resveratrol and glibenclamide may prevent reperfusion-induced arrhythmias.[Citation37,Citation73] In a mouse model of cardiac cell injury, BBR therapy reduced the serum production of inflammatory molecules such as interleukin (IL) 6, tumor necrosis factor, IL 10, and IL 17A in mice with anoxia reoxygenation damage. Following anoxia reoxygenation injury, BBR therapy reduced apoptosis in cardiac cells by controlling the expression of apoptosis-related genes. It decreased body weight, blood cholesterol levels, blood pressure, and heart rate by reducing circumferential fragmentation and segmentation of cardiac cells. In comparison to untreated mice, BBR administration reduced the expression of nuclear factor (NF) B and mitogen-activated protein kinase (MAPK) p38 in cardiac cells from mice with anoxia reoxygenation damage. However, induction of BBR decreases NF-B activity and expression as well as BBRprovide prevention from myocardial apoptosis in myocardial cells that were isolated from experimental mice when p38 MAPK was overexpressed.[Citation55]
According to Zhang and colleagues[Citation74] findings, trimetazidine coupled with BBR reduced blood pressure via increasing NO levels in the blood and improved endothelium-dependent brachial artery dilation function.[Citation74] BBR and silymarin had a synergistic impact on serum lipids and fasting plasma glucose, lowering total cholesterol, and triglycerides, increasing high-density lipoprotein cholesterol and lowering low-density lipoprotein cholesterol.[Citation75] BBR relaxes the endothelium by increasing arginine-induced NO synthesis via endothelial nitric oxide synthase (eNOS) (), a key component in the vasodilation process. It increases NO levels and upregulates eNOS mRNA. BBR also increases NO production by promoting eNOS phosphorylation and coupling with HSP 90 (heat shock proteins). BBR also blocks COX-2 production, which decreases endothelial contraction. Changes in the ratio of prothrombotic/antithrombotic and vasodilator/vasoconstrictor effects can be caused by COX 1 or 2 activity imbalances.[Citation76] BBR was found to have a beneficial effect on TNF-induced endothelium contraction as well as an increase in PI3K/AKT/eNOS mRNA levels.[Citation77]
To protect the heart during conditions of ischemia, BBR modifies the activity of AMP-activated kinase (AMPK), protein kinase B (PKB) phosphorylation, the JAK/STAT pathway, and glycogen synthase kinase 3 (GSK3).[Citation78] AMPK is a crucial enzyme that regulates lipid and glucose metabolism, mitochondrial and endoplasmic reticulum function, and apoptosis during ischemia.[Citation79] When damaging stimuli are present, BBR activates the PI3K/AKT pathway, which is thought to be a compensatory mechanism for preventing pro-inflammatory and apoptotic responses. Through modification of TLR4, activation of this system is linked to a decrease in ischemia damage (toll-like receptor 4) signal transduction via a mediator.[Citation2] Among other crucial physiological processes, the serine/threonine protein kinase GSK3 controls metabolism, differentiation, proliferation, and death. Because BBR prevents this enzyme from functioning, it can exert its cardioprotective benefits.[Citation80] Improving blood lipid profiles directly reduces the risks of cardiovascular diseases. Extensive studies provide evidence that demonstrates the beneficial effects of BBR on the blood lipid profile and in turn has a cardio-protective effect against CVDs. Several human clinical trials have been done in order to validate the positive effects of BBR (). Several human trials have been completed with diverse health/disease (or disorders) conditions including those with hyper-cholesterolemia, hyper-lipidemic, and cardiovascular diseases. The human trials exhibited reductions of about 11–29% in TC and 8–25% in LDL-C i.e. bad cholesterol).[Citation81]
Table 2. Effect of BBR on blood lipid profiles.
Anti-inflammatory role
BBR is also acts as an anti-inflammatory agent (). [Citation82] Systemic lipopolysaccharide (LPS) causes neuroinflammation, which causes behavioral and cognitive problems. LPS was delivered intraperitoneally to adult male rats at a rate of 1 mg/kg to promote neuroinflammation, and BBR was given intravenously at doses of 10 or 50 mg/kg one hour later for seven days. In the LPS group, BBR at 50 mg/kg (but not 10 mg/kg) enhanced performance in the novel object recognition test (NORT), enhanced spatial recognition memory in the Y maze, and avoided learning and memory deficits in passive avoidance tasks. By raising antioxidant enzyme levels and decreasing nuclear factor-kappa B (NF-B), toll-like receptor 4 (TLR4), tumor necrosis factor (TNF), and interleukin 6 (IL-6) levels as well as lowering hippocampal acetylcholinesterase (AChE), malondialdehyde (MDA), protein carbonyl, caspase 3 activity, and DNA fragmentation, BBR improved antioxidant capacity. Additionally, BBR raised glial fibrillary acidic protein (GFAP), sirtuin 1, 3-nitrotyrosine (3-NT), cyclooxygenase 2 (COX 2), and mitogen-activated protein kinase (MAPK p38) levels in the hippocampus while maintaining brain-derived neurotrophic factor (BDNF) levels.[Citation83] BBR has recently been discovered to boost Bifidobacterium and Lactobacillus development, inhibit Escherichia coli growth, and reduce LPS levels in the gut.[Citation23] In lipopolysaccharide (LPS)-induced RAW264.7 macrophages cells, BBR (1.25 M) decreased pro-inflammatory cytokines TNF-, IL-6, IL-1, PGE2, and NO, and suppressed the mRNA expressions of COX-2 and iNOS, respectively, in vitro and in vivo investigations.[Citation36,Citation84]
Table 3. The use of BBR to treat systemic inflammation.
Although CDC6 is a crucial regulator of pre-RC assembly and DNA replication in eukaryotic cells, it is unclear how it affects keratinocyte growth and psoriasis. Researchers looked at how CDC6 is expressed in psoriatic skin and how it affects human keratinocyte proliferation. In psoriatic lesions, epidermal cells produce more CDC6, which may be induced in keratinocytes by the important signaling pathway IL-22/STAT3 that has been linked to the pathophysiology of psoriasis. When CDC6 is lacking, keratinocyte proliferation is decreased. BBR reduces keratinocyte development by inhibiting CDK4/6-RB-CDC6 signaling in keratinocytes. The control of JAK1, JAK2, and TYK2 by BBR prevents STAT3 activation and is the mechanism via which it exerts its anti-proliferative effects. Finally, we demonstrated that BBR could stop imiquimod-induced skin lesions that resembled psoriasis and the activation of CDC6 and p-STAT3 in mice.[Citation85]
The anti-inflammatory potential of BBR was studied in ovalbumin (OVA) sensitized inside Guinea pigs. Twenty- four healthy Guinea pigs were selected for the study. Airway inflammation was induced at days 0 and 14 utilizing OVA injections through the peritoneal routes as well as by inhalation route on days 25, 26, and 27, in each experimental group except the normal control group. BBR (1.8 mg/ per kg) was injected through peritoneal routes. TLC (Total leukocyte count) in blood samples of BBR-treated groups was considerably in low than OVA-sensitized groups. TLC of the broncho-alveolar lavage (BAL) () fluid in BBR-treated groups was considerably lower than in the OVA-sensitized groups. The Eosinophil % in blood samples of BBR-treated groups was considerably lower than OVA-sensitized groups. BBR treatment had reduced Total leukocyte count and eosinophil % in both blood samples and BAL fluids when compared to OVA-sensitized groups.[Citation86]
Anti-aging
BBR increases AMPK signaling and the activity of its downstream targets, including mTOR/rpS6, Sirtuin1/FOXO3, Nrf2, nicotinamide adenine dinucleotide (NAD+), NF-B. Most of these functions increase AMPK control when it comes to mitochondrial oxidative stress.[Citation87–89] In rats of normal aging, BBR therapy administered over six months greatly reduced insulin resistance and cognitive impairments. Rats that were 24 months old had better alignment and fewer muscle fibers after receiving BBR treatment. Serum and skeletal muscle ROS levels in 24-month-old rats were reduced by BBR. BBR increased the expression of p-AMPK, SIRT1, and PGC-1 proteins, as well as ATP production, in the skeletal muscle of elderly rats. BBR preserves muscular function in skeletal muscle by stimulating the AMPK/SIRT1/PGC-1 pathway.[Citation90,Citation91]
The effects of 50 and 100 mg/kg BBR were effective in preventing cognitive issues, increasing ROS, and ensuing increase in protein and lipid oxidation in the cerebral cortex and hippocampus, as well as aminolevulinate dehydratase inhibition in the cerebral cortex, in a model of sporadic dementia of the Alzheimer’s type induced by intracerebroventricular (ICV) injection of STZ. In the cerebral cortex and hippocampus of ICV-STZ rats, BBR therapy also protected total thiol loss and resulted in decrease of glutathione and glutathione S-transferase activity.[Citation16,Citation92]
BBR prevents D-galactose (D-gal)-induced renal aging in experimental rats by decreasing urea and creatinine concentrations, malondialdehyde, 8-hydroxy-2′-deoxyguanosine, retrieving changes in kidney histopathology, and activating the heme oxygenase-1 enzyme,[Citation93] Fv-0klo9 suppresses pro-inflammatory mediators, lowers tensin homolog lost on chromosome ten (PTEN) expression, enhances Akt activity, and inhibits the anti-apoptotic marker (Bcl-2).[Citation93] BBR also protects against neuroinflammation induced by amyloid-beta (A) accumulation in the brain, a common feature of neurodegenerative disease etiology. Additionally, it guards against heightened cytokine production, ROS, NF-B, and microglial activation, all of which are involved in the inflammatory phase of Alzheimer’s disease.[Citation94] In human neuroglioma H4 cells that persistently express Swedish-type APP, BBR therapy considerably decreased A levels, according to Asai and colleagues,[Citation95] with an IC50 of about 5 M. They continued by demonstrating how BBR controlled this decrease by up- and down-regulating -secretase activity, which led to a change in the processing of APP from the amyloidogenic to the non-amyloidogenic route.[Citation95]
Zhu et al.[Citation96] demonstrated that BBR reduces A synthesis by reducing BACE1 expression through activation of the ERK1/2 pathway using Swedish APP-expressing HEK293 cells. The effects of BBR on A and BACE1 might be reversed if ERK1/2 was inhibited using the MEK1/2 antagonist U0126.[Citation96] According to Zhang et al.,[Citation97] BBR has an impact on A metabolism via the AMPK pathway. Because it inhibited BACE1 expression and stimulated AMPK, BBR decreased the synthesis of A in neuroblastoma cells and primary cultured neurons.[Citation97] BBR’s ability to inhibit amyloid-beta precursor protein (APP) processing and A-induced neurotoxicity has recently been demonstrated in several in vivo experiments. Panahi et al.[Citation98] discovered that BBR administration lowered BACE1 activity protected the hippocampus against degeneration and treated chemically-induced AD-like behavioral derangements using an Al-maltol-induced AD rabbit model.[Citation98] However, Haghani et al.[Citation99] used bilateral A injection in the prefrontal cortex to produce a rat model of Alzheimer’s disease and examined BBR’s effects on A-induced cognitive impairment and neurotoxicity. Their results revealed that BBR administration could lessen the damaging effects of A on memory, learning, and the electrophysiological characteristics of hippocampal pyramidal neurons.[Citation99]
Additionally, a study by Durairajan et al.[Citation100] showed that sustained BBR therapy reduced tau hyperphosphorylation, gliosis, and cognitive deficits in a well-established transgenic mouse model of Alzheimer’s disease (TgCRND8 animals).[Citation100] It is possible to evaluate the potential and restrictions of BBR as a Parkinson’s disease treatment agent as a result of several in vitro and in vivo research.[Citation101] Bae et al.[Citation102] found that BBR protects dopaminergic neurons against cell death brought on by the neurotoxin 6-hydroxydopamine, which causes parkinsonism, using an in vitro model of Parkinson’s disease (6-OHDA).[Citation102] Kim and colleagues[Citation101] also looked at the in vivo neuroprotective benefits of BBR using the 1-methyl-4-phenyl-1,2,3,6-trerahydropyridine (MPTP) animal model of Parkinson’s disease, which shares many traits with the illness. Their results suggested that BBR may enhance motor balance and coordination by decreasing MPTP-induced substantia nigra dopaminergic neuronal death and striatal fiber loss.[Citation101]
Apoptosis in the hippocampus, which has been connected to MPTP-induced short-term memory loss, has also been shown to be inhibited by BBR. The results of these studies taken together offer some insight into the potential use of BBR as a Parkinson’s disease treatment. Shin et al.[Citation103] from South Korea discovered that long-term L-DOPA-BBR combination therapy in a rat model of Parkinson’s disease had adverse effects, despite the fact that BBR is typically acknowledged as safe for human use.[Citation103] In earlier research, BBR exacerbated 6-OHDA-induced cytotoxicity in PC12 cells and accelerated the degeneration of dopaminergic neuronal cells in rats with 6-OHDA-lesioned substantia nigra.[Citation104] Although their findings contradict those of other studies that suggest BBR protects against dopaminergic neuronal loss caused by 6-OHDA or MPTP and suppresses hippocampal apoptosis. More study is required to determine the potential side effects and limitations of using BBR alone or in combination to treat Parkinson’s disease. Due to its ability to increase autophagy levels and reduce inflammation in macrophages brought on by oxidized LDL, BBR has the potential to be a useful therapeutic agent in the treatment of atherosclerosis.[Citation78,Citation105–107]
In animal models, BBR has also been shown to promote autophagy and decrease lung fibrosis by blocking the PI3K/Akt-mTOR signaling cascade.[Citation108] Additionally, BBR has anti-tumor effects and can increase the effectiveness of some chemotherapeutic drugs and radiation on cancer cells by stimulating autophagy and death in cancer cells, according to a growing body of research.[Citation109–111] Jiang and coworkers[Citation18] recently examined the impact of BBR on the buildup of harmful poly Q-HTT in cellular and animal models of Huntington’s disease (HD). In HEK293 cells transfected with a mutant HTT that has 120 CAG repeats in exon 1 and in a transgenic HD animal model that produces mutant HTT that has 82 glutamine repeats in the poly Q tract, their research revealed that BBR may drastically upregulate autophagy to eliminate poly Q-HTT. HD mice’s neurological deficits are improved by autophagy clearance of poly Q-HTT aggregates, which also lengthens rotarod performance, muscular strength, motor coordination, and lifespan.[Citation18]
Hypertension
Hypoxia-induced hyperproliferation of pulmonary artery smooth muscle cells (PASMCs) is a crucial element in the development of pulmonary arterial hypertension (PAH). The right ventricular systolic pressure (RVSP) and the right ventricle/left ventricle plus septum were both reduced by BBR. BBR blocked hypoxia-induced elevations in proliferating cell nuclear antigen (PCNA) and smooth muscle actin expression. BBR therapy also increased the expression of the bone morphogenetic protein type II receptor (BMPRII) and its downstream components Psmad1/5 while lowering the expression of transforming growth factor (TGF) and its downstream molecules Psmad2/3. Hypoxia also caused a significant decrease in peroxisome proliferator-activated receptor expression, which was recovered by BBR treatment.[Citation112] Excessive proliferation, migration, and antiapoptosis of pulmonary artery smooth muscle cells characterize the progression of pulmonary vascular remodeling (PASMCs).
Patients with pulmonary arterial hypertension (PAH) have higher levels of circulating catecholamines, which suggests that neurotransmitters produced by sympathetic over-activity may play a role in PAH. The PA is mostly sympathetically innervated. The essential mechanism, nevertheless, is still unknown. In a study, boyden chamber migration and wound-healing assays were used to evaluate migration, the proliferating cell nuclear antigen and the cell counting kit8 assay to assess PASMC proliferation, the proliferating cell nuclear antigen and the cell counting kit8 assay to assess PASMC migration, and western blot analysis to investigate protein expression. In both in vivo and in vitro experiments, we found that the protein phosphatase 2A (PP2A) catalytic subunit (Y307) was phosphorylated at a higher level in PAH patients and PAH models than in controls. Furthermore, BBR, and/or PP2A overexpression prevented the migration and multiplication of PASMCs that were induced by neutrophil elastase (NE). The effects of PP2A inhibition on NE-induced PAH were not reversed by BBR. As a result, PP2A contributes to the development of PAH, and BBR could treat PAH by reducing it through PP2A signaling pathways.[Citation113]
BBR (2 g/h) infusion into the hypothalamic paraventricular nucleus (PVN) via the ROS/Erk1/2/iNOS pathway lowers blood pressure and decreases sympathoexcitation. It reduced plasma levels of norepinephrine, MAP, PVN Fra-like activity, and norepinephrine, as well as NOX2, NOX4, Erk1/2, iNOS, and activated Cu/Zn-SOD in the PVN. BBR lowers hypertension and sympathoexcitation in 2K1C renovascular hypertensive rats via the ROS/Erk1/2/iNOS pathway.[Citation114] Liu and colleagues[Citation39] evaluated in vitro effectiveness of the Src-selective inhibitor 1-(1,1-dimethyl ethyl)-1-(4-methyl phenyl)-1 H-pyrazolo[3,4-d] pyrimidine-4-amine (PP1) through inhibition of Src (Tyr416) phosphorylation, and found that BBR inhibited pulmonary arterial hypertension-pulmonary artery smooth muscle cells (PAH-PASMC) proliferation and migration by inhibiting hypoxia-inducible factor-1 (HIF-1) expression via the Akt/mTOR signal pathway. In Sugen (SU) 5416/hypoxia (SU-PAH) mice, the Src-selective inhibitor PP1 and BBR significantly reduced distal pulmonary vascular remodeling, right ventricular systolic pressure (RVSP), and right ventricular hypertrophy.[Citation39]
Anti-allergic
Treatment with BBR decreased the number of cytokines that IL-33 generated in mast cells, thwarting the combined effects of IL-33 and IgE-mediated mast cell activation. By deactivating NF-B and p38 signaling, it reduces IL-33-mediated inflammation in mast cells. Rat peritoneal mast cells (RPMCs) produced cytokines in response to IL-33 in vivo, including IL-6, TNF-, IL-13, and MCP-1, although ST2 expression was unaffected. BBR changed IL-33 signaling by blocking nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) transcription and p38 phosphorylation brought on by IL-33, but not ERK or JNK. In vitro and in vivo experiments revealed that BBR treatment in rats reduced cytokine production by suppressing IL-33-induced plasma cytokine levels.[Citation36]
Hepatoprotective
BBR can help to prevent metabolic dysregulation and nonalcoholic fatty liver disease (NAFLD). By preventing methylation of the microsomal triglyceride transfer protein (MTTP) gene promoter, which typically increases gene expression in NAFLD, BBR therapy reduced fatty liver alterations in an animal model of obesity with a high-fat diet.[Citation65] The effects of BBR at 100 mg/kg on sodium nitrate-induced toxicity included significant decreases in alkaline phosphatase, alanine aminotransferase, MDA level, TNF expression, caspase-3 activity, and TGF-1 concentration, as well as increases in reduced glutathione, glutathione reductase, glutathione S-transferase, and glutathione peroxidase.[Citation115] Zhu and colleagues[Citation116] reported that BBR (12 M) alleviated oxidative stress caused by hydrogen peroxide (H2O2) in the liver cell line L02 by upregulating sirtuin 1 expression levels in a time-dependent manner.[Citation116]
It also suppressed the respiratory electron chain and activated AMPK, reducing endogenous oxidants and the constitutive DNA damage response while decreasing mTOR/S6 signaling. BBR suppresses mTOR/S6 and so has anti-aging properties, as well as reduces the level of constitutive DNA damage response.[Citation117]
Nephroprotective role
The common condition of chronic kidney injury in individuals with hypertension and diabetes mellitus is atherosclerosis of the renal artery, which is brought on by oxidative stress and inflammation. In a study, 69 people with HT and DM were given standard medication to control their blood pressure and blood sugar levels and investigated the preventive effects of BBR on the kidneys. The patients received 300 mg of BBR every day for 24 months, spaced out by 2-week intervals every 5 months. After treatment, there was decrease in c-reactive protein (CRP), MDA, and SOD levels, but not in creatinine, arterial pressure, or glycemia. These results confirm BBR’s anti-inflammatory and antioxidant properties, which support kidney function.[Citation118]
In another study, HgCl2 was used to induce hepato-renal damages and examined BBR’s reno-protective characteristics. By increasing oxidative stress, this substance caused hepato-renal damages including increase in lipid peroxidation and NO levels, lower the glutathione and SOD levels and the activity of other protective enzymes). In compared to the control group, HgCl2 injection raised the levels of AST (aspartate aminotransferase), ALT (alanine aminotransferase), and ALP (alkaline phosphatase). These enzymes were dramatically reduced by BBR pretreatment. Additionally, urea and creatinine levels in the HgCl2 group were significantly greater when compared to the control group, but these changes were stopped by pretreatment with BBR. According to the researchers, the intervention group exhibited higher levels of pro-oxidants and lower levels of antioxidants. These findings back up BBR’s hepatoprotective and renal-protective effects. In other studies, CCl4-induced hepatotoxicity was demonstrated to have a comparable effect in animal models.[Citation119]
The potential decrease of nephrotoxicity brought on by cisplatin may be made possible by BBR. In an animal model experiment, BBR was given orally for two days, beginning two days after cisplatin, in doses of 1, 2, and 3 mg/kg. The animals were killed after receiving their final dosages of BBR, and the pathologist examined the kidneys. The reduction in NF-kB, TNF, COX2, and iNOS levels, along with the considerable improvement in histology, all testify to the anti-inflammatory characteristics of BBR.[Citation120]
Reproductive role
BBR, in combination with coenzyme Q10 (Co Q10) and/or alone, increase sperm parameters and decrease sperm DNA damage in rats with varicocele (VCL).[Citation121] The researchers wanted to discover if BBR might protect the testes of experimental varicocele-induced rats against the effects of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), inhibin B (INHB), testosterone (T), and alkaline phosphatase (Alk-p). 30 adult male Wistar rats were randomly assigned to the control (n = 6) control-sham (n = 6) and experimental (n = 18) groups for this investigation. The experimental groups had experimental varicocele, whereas the control-sham group had a standard laparotomy. The experimental group was separated into three groups following varicocele (VCL) induction: untreated VCL-induced, 50 mg/kg, and 100 mg/kg BBR-treatment (intra-peritoneally). The mice were killed after 60 days, and blood testosterone levels and alkaline phosphatase activity in the testicles were assessed. When compared to the control and control-sham groups, non-treated VCL-induced rats showed a substantial decrease in blood T and INHB levels and a noticeable rise in GnRH, FSH, LH, and Alk-p activity. The control and control-sham groups showed no significant changes. Meanwhile, serum T and INHB levels increased significantly in each BBR-treated group, but GnRH, FSH, LH, and alkaline phosphatase activity in testis tissue decreased significantly. According to these findings, elevating blood testosterone and INHB levels enhances testicular endocrine competence and protects Leydig cells from inflammatory and oxidative damage induced by varicocele. Furthermore, BBR modifies serum sex hormone levels in experimental varicocele and lowers varicocele-induced inflammatory responses by reducing GnRH, FSH, LH, and alkaline phosphatase activity.[Citation53,Citation121]
Polycystic ovarian syndrome (PCOS) is a prevalent endocrine and metabolic disorder in women of reproductive age. Recent research has found a link between gut microbiota and metabolic diseases such as type 2 diabetes, obesity, and PCOS. This study used dihydrotestosterone (DHT)-induced PCOS rats to assess the composition, diversity, and abundance of the gut microbiota using Illumina MiSeq sequencing (PE300). Probiotics, BBR, and Diane-35 (a combination of estrogen and progesterone) were investigated as PCOS therapies. Constant estrous cycles, the disappearance of mature ovarian follicles, insulin resistance, and obesity were all observed in DHT-induced rats. Treatment with probiotics and dianne-35 enhanced the metabolic and reproductive processes in PCOS rats. In PCOS-like mice, diane-35 and probiotics restored gut microbiota diversity, and enhancedthe reproductive function. Contrarily, PCOS was unaffected by BBR, which reduced the quantity and variety of the gut flora. These results highlighted the role of gut flora in PCOS’s metabolic and reproductive issues and open up possibilities for dietary counseling specific to PCOS.[Citation23,Citation39]
Conclusion
BBR is important in the production of principal compounds in pharmacology. BBR has great potential to be used as antioxidant, anti-cancer, anti-diabetic, anti-obesity, cardio-protective, anti-inflammatory, anti-aging agent, neuroprotective, antihypertensive, anti-allergic, hepatoprotective, and nephroprotective agent. It plays a pivotal role in many biochemical pathways and acts on diverse group of cellular targets like growth factors, transcription factors, inflammatory factors and their receptors, cell signaling molecules, cytokines, enzymes and genes. As a natural compound, BBR has great potency to be used against large number of metabolic ailments and related complexities with little or no side effects to healthy cells. Due to limitation of its absorption and bioavailability, it is necessary to develop more novel derivatives or formulations to establish its therapeutic effectiveness in large-scale clinical trials. C-1: very short??/
C-2: English and clarity should be checked by an expert
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium Majus L. and Berberis Thunbergii DC. toward Hematopoietic Cancer Cell Lines. Toxins. 2019, 11(9), 485. DOI: 10.3390/toxins11090485.
- Neag, M. A.; Mocan, A.; Echeverría, J.; Pop, R. M.; Bocsan, C. I.; Crişan, G.; Buzoianu, A. D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9(9), 557. DOI: 10.3389/fphar.2018.00557.
- Baldwin-Lien, B.; Wei, W.; Zhao, H.; Wang, A. Berberine Compared to Metformin Study. Sat. 2019, 1, 16.
- Lu, Q.; Fu, Y.; Li, H.; Ebrahimnejad, M.; Kholghi, G.; Zarrindast, M.-R. Berberine and Its Derivatives Represent as the Promising Therapeutic Agents for Inflammatory Disorders. Pharmacol Rep. 2022, 74(1), 1–13. DOI: 10.1007/s43440-021-00339-8.
- Cicero, A. F.; Baggioni, A. Berberine and Its Role in Chronic Disease Anti-inflammatory Nutraceuticals and Chronic Diseases; Springer International Publishing Switzerland, 2016; pp 27–45.
- Fan, J.; Zhang, K.; Jin, Y.; Li, B.; Gao, S.; Zhu, J.; Cui, R. Pharmacological Effects of Berberine on Mood Disorders. J. Cellular Mol. Med. 2019, 23(1), 21–28. DOI: 10.1111/jcmm.13930.
- Andola, H. C.; Gaira, K. S.; Rawal, R. S.; Rawat, M. S. M.; Bhatt, I. D. Habitat-dependent Variations in Berberine Content of Berberis Asiatica Roxb. Ex. DC. in Kumaon, Western Himalaya. Chem. Biodiversity. 2010, 7(2), 415–420. DOI: 10.1002/cbdv.200900041.
- Srivastava, S. K.; Rai, V.; Srivastava, M.; Rawat, A. K. S.; Mehrotra, S. Estimation of Heavy Metals in Different Berberis Species and Its Market Samples. Environ. Monit. Assess. 2006, 116(1), 315–320. DOI: 10.1007/s10661-006-7395-x.
- Singh, S. Quantitative Analysis of Berberine in Argemone Mexicana Linn(Papaveraceae) Using HPLC and HPTLC. Adv. Plant Sci. 2014, 27, 209–211.
- Srivastava, S. K.; Singh Rawat, A. K.; Mehrotra, S. Pharmacognostic Evaluation of the Root of Berberis Asiatica. Pharm. Biol. 2004, 42(6), 467–473. DOI: 10.1080/13880200490886256.
- Rajasekaran, A.; Pokhriyal, R.; Singh, Y. (2009). Quantitative Estimation of Berberine in Roots of Different Provenances of Berberis Aristata DC by HPLC and Study of Their Antifungal Properties. Pharmacognosy Magazine. 5(20), 355.
- Verma, S.; Sharma, D. Berberine: A Pioneer Remedy for Various Ailments. Pharm. Innovation J. 2018, 7(10), 194–200.
- Brown, C. O.; Tseng, P. Y.; Lin, I. Y.; Tsai, C. E.; Chen, C. K. Therapeutic Uses of Berberine Formulations, U.S Patent US20150320738 A1, 2016.
- Liu, C. S.; Zheng, Y. R.; Zhang, Y. F.; Long, X. Y. Research Progress on Berberine with a Special Focus on Its Oral Bioavailability. Fitoterapia. 2016, 109, 274–282. DOI: 10.1016/j.fitote.2016.02.001.
- Pan, C.; Guo, Q.; Lu, N. Role of Gut Microbiota in the Pharmacological Effects of Natural Products. Evid. Based Complement. Altern. Med. 2019, 2019, 2682748. DOI: 10.1155/2019/2682748.
- de Oliveira, J. S.; Abdalla, F. H.; Dornelles, G. L.; Palma, T. V.; Signor, C.; da Silva Bernardi, J.; de Andrade, C. M.; Lenz, L. S.; de Oliveira, V. A.; Chitolina Schetinger, M. R. Neuroprotective Effects of Berberine on Recognition Memory Impairment, Oxidative Stress, and Damage to the Purinergic System in Rats Submitted to Intracerebroventricular Injection of Streptozotocin. Psychopharmacology. 2019, 236(2), 641–655. DOI: 10.1007/s00213-018-5090-6.
- Pervez, S.; Saeed, M.; Ali, M. S.; Fatima, I.; Khan, H.; Ullah, I. Antimicrobial and Antioxidant Potential of Berberisinol, a New Flavone from Berberis Baluchistanica. Chem. Nat. Compd. 2019, 55(2), 247–251. DOI: 10.1007/s10600-019-02660-4.
- Jiang, L.; Iwahashi, H. The Roles of radio-functional Natural Chemicals for the Development of Cancer Radiation Therapy. Reviews on Environmental Health. 2019, 34(1), 5–12. DOI: 10.1515/reveh-2018-0057.
- Pirmoradi, Z.; Yadegari, M.; Moradi, A.; Khojasteh, F.; Mehrjerdi, F. Z. Effect of Berberine Chloride on Caspase-3 Dependent Apoptosis and Antioxidant Capacity in the Hippocampus of the Chronic Cerebral Hypoperfusion Rat Model. Iran. J. Basic Med. Sci. 2019, 22(2), 154. DOI: 10.22038/ijbms.2018.31225.7534.
- Putta, S.; Qureshi, A. A.; Kilari, E. K. Berberine Attenuates the Acute Pancreatitis Induced by Dibutyltin Dichloride (DBTC) in Albino Wistar Rats. FASEB. J. 2019, 33(S1), 820–824. DOI: 10.1096/fasebj.2019.33.1_supplement.820.4.
- Jia, Q.; Zhang, L.; Zhang, J.; Pei, F.; Zhu, S.; Sun, Q.; Duan, L. Fecal Microbiota of Diarrhea-Predominant Irritable Bowel Syndrome Patients Causes Hepatic Inflammation of Germ-Free Rats and Berberine Reverses It Partially. Biomed Res. Int. 2019, 2019, 4530203. DOI: 10.1155/2019/4530203.
- Kumar, V.; Gupta, P.; Hassan, M. I. Mechanism and Implications of Traditional Chinese Medicine in Amyotrophic Lateral Sclerosis Therapy. J. Pro.and Pro. 2019, 10, 131–147.
- Zhang, B.; Yue, R.; Chen, Y.; Yang, M.; Huang, X.; Shui, J.; Chin, J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2019, 2019, 2634898. DOI: 10.1155/2019/2634898.
- Freag, M. S.; Torky, A. S.; Nasra, M. M.; Abdelmonsif, D. A.; Abdallah, O. Y. (2019). Liquid Crystalline Nanoreservoir Releasing a Highly skin-penetrating Berberine Oleate Complex for Psoriasis Management. Nanomedicine. 2019, 14(8), 931–954. DOI: 10.2217/nnm-2018-0345.
- Ghareeb, D. A. Berberine Is multi-targets Therapeutic Weapon; Medical Biotechnology Resolves the Mystery. J. Alexandria Sci. 2019, 1, 2.
- Kumar, A.; Dhull, D. K.; Dhull, D. K.; Dhull, D. K.; Dhull, D. K.; Dhull, D. K. Current Knowledge and Pharmacological Profile of Berberine: An Update. Eur. J. Pharmacol. 2015, 761(761), 288–297. DOI: 10.1016/j.ejphar.2015.05.068.
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and Pharmacokinetic Properties of Berberine: A Review of Recent Research. J. Pharm. Pharmacol. 2009, 61(7), 831–837. DOI: 10.1211/jpp.61.07.0001.
- Tan, X. S.; Ma, J. Y.; Feng, R.; Ma, C.; Chen, W. J.; Sun, Y. P.; Jiang, J. D.; Huang, M.; He, C.-Y.; Shou, J.-W. (2013). Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PloS one. 2013, 8(10), e77969. DOI: 10.1371/journal.pone.0077969.
- Xiong, R. G.; Huang, S. Y.; Wu, S. X.; Zhou, D. D.; Yang, Z. J.; Saimaiti, A.; Zhao, C. N.; Shang, A.; Zhang, Y. J.; Gan, R. Y., et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules. 2022, 27(14), 4523.
- Song, J.; Lin, C.; Yang, X.; Xie, Y.; Hu, P.; Li, H.; Hu, W.; Hu, H. Mitochondrial Targeting Nanodrugs self-assembled from 9-O-octadecyl Substituted Berberine Derivative for Cancer Treatment by Inducing Mitochondrial Apoptosis Pathways. J. Controlled Release. 2019, 294, 27–42. DOI: 10.1016/j.jconrel.2018.11.014.
- Hassanein, E. H.; Shalkami, A. G. S.; Khalaf, M. M.; Mohamed, W. R.; Hemeida, R. A. The Impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 Signaling Pathways in the Protective Effects of Berberine against methotrexate-induced Nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. DOI: 10.1016/j.biopha.2018.10.088.
- Ri, M. H.; Ma, J.; Jin, X. Development of Natural Products for anti-PD-1/PD-L1 Immunotherapy against Cancer. J. Ethnopharmacol. 2021, 281, 114370. DOI: 10.1016/j.jep.2021.114370.
- Jabbarzadeh Kaboli, P.; Leong, M. P. Y.; Ismail, P.; Ling, K. H. Antitumor Effects of Berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 Breast Cancer Cells Using Molecular Modelling and in Vitro Study. Pharmacol Rep. 2019, 71(1), 13–23. DOI: 10.1016/j.pharep.2018.07.005.
- Yao, Z.; Wan, Y.; Li, B.; Zhai, C.; Yao, F.; Kang, Y.; Lin, D. Berberine Induces Mitochondrial‑mediated Apoptosis and Protective Autophagy in Human Malignant Pleural Mesothelioma NCI‑H2452 Cells. Oncol. Rep. 2018, 40(6), 3603–3610. DOI: 10.3892/or.2018.6757.
- Abrams, S. L.; Follo, M. Y.; Steelman, L. S.; Lertpiriyapong, K.; Cocco, L.; Ratti, S.; McCubrey, J. A.; Candido, S.; Libra, M.; Murata, R. M. Abilities of Berberine and Chemically Modified Berberines to Inhibit Proliferation of Pancreatic Cancer Cells. Adv. Biol. Reg. 2019, 71, 172–182. DOI: 10.1016/j.jbior.2018.10.003.
- Li, X. D.; Wang, Z.; Wang, X. R.; Shao, D.; Zhang, X.; Li, L.; Dong, W. F.; Chang, Z.-M.; Dong, W.-F. Berberine-loaded Janus Gold Mesoporous Silica Nanocarriers for chemo/radio/photothermal Therapy of Liver Cancer and radiation-induced Injury Inhibition. Int. J. Nanomed. 2019, 14, 3967. DOI: 10.2147/IJN.S206044.
- Shinji, S.; Nakamura, S.; Nihashi, Y.; Umezawa, K.; Takaya, T. Berberine and Palmatine Inhibit the Growth of Human Rhabdomyosarcoma Cells. Biosci., Biotechnol., Biochem. 2020, 84(1), 63–75. DOI: 10.1080/09168451.2019.1659714.
- Huang, C.; Wang, X. L.; Qi, F. F.; Pang, Z. L. Berberine Inhibits epithelial-mesenchymal Transition and Promotes Apoptosis of tumour-associated fibroblast-induced Colonic Epithelial Cells through Regulation of TGF-β Signalling. J. Cell Commun. Signaling. 2020, 14(1), 53–66. DOI: 10.1007/s12079-019-00525-7.
- Liu, M.; Gao, L.; Zhang, N. Berberine Reduces Neuroglia Activation and Inflammation in streptozotocin-induced Diabetic Mice. Inter. J. Immuno. Pharmacol. 2019, 33, 2058738419866379. DOI: 10.1177/2058738419866379.
- Kou, J. Y.; Li, Y.; Zhong, Z. Y.; Jiang, Y. Q.; Li, X. S.; Han, X. B.; Yang, L. M.; Tian, Y.; Yang, L. M. Berberine-sonodynamic Therapy Induces Autophagy and Lipid Unloading in Macrophage. Cell Death & Disease. 2018, 8(1), e2558–e2558. DOI: 10.1038/cddis.2016.354.
- Maiti, P.; Plemmons, A.; Dunbar, G. L. Combination Treatment of Berberine and Solid Lipid Curcumin Particles Increased Cell Death and Inhibited PI3K/Akt/mTOR Pathway of Human Cultured Glioblastoma Cells More Effectively than Did Individual Treatments. PloS one. 2019, 14(12), e0225660. DOI: 10.1371/journal.pone.0225660.
- Kim, S.; You, D.; Jeong, Y.; Yu, J.; Kim, S. W.; Nam, S. J.; Lee, J. E. Berberine down-regulates IL-8 Expression through Inhibition of the EGFR/MEK/ERK Pathway in triple-negative Breast Cancer Cells. Phytomedicine. 2018, 50, 43–49. DOI: 10.1016/j.phymed.2018.08.004.
- Dai, W.; Mu, L.; Cui, Y.; Li, Y.; Chen, P.; Xie, H.; Wang, X. Berberine Promotes Apoptosis of Colorectal Cancer via Regulation of the Long non-coding RNA (Lncrna) Cancer Susceptibility Candidate 2 (CASC2)/AU-binding Factor 1 (AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) Axis. Med. Sci. Monitor: Inter. Med. J. Experi. and Clinical Res. 2019, 25, 730. DOI: 10.12659/MSM.912082.
- Nishi, K.; Suzuki, K.; Sawamoto, J.; Tokizawa, Y.; Iwase, Y.; Yumita, N.; Ikeda, T. Inhibition of Fatty Acid Synthesis Induces Apoptosis of Human Pancreatic Cancer Cells. Anticancer Res. 2016, 36(9), 4655–4660. DOI: 10.21873/anticanres.11016.
- Yue, X.; Zhao, Y.; Xu, Y.; Zheng, M.; Feng, Z.; Hu, W. Mutant p53 in Cancer: Accumulation, gain-of-function, and Therapy. J. Mol. Biol. 2017, 429(11), 1595–1606. DOI: 10.1016/j.jmb.2017.03.030.
- Levine, A. J.; Hu, W.; Feng, Z. The P53 Pathway: What Questions Remain to Be Explored? Cell Death & Different. 2006, 13(6), 1027–1036. DOI: 10.1038/sj.cdd.4401910.
- Muller, P. A.; Vousden, K. H. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell. 2014, 25(3), 304–317. DOI: 10.1016/j.ccr.2014.01.021.
- Freed-Pastor, W. A.; Prives, C. Mutant p53: One Name, Many Proteins. Genes Dev. 2012, 26(12), 1268–1286. DOI: 10.1101/gad.190678.112.
- Liu, Z.; Chen, Y.; Gao, H.; Xu, W.; Zhang, C.; Lai, J.; … Huang, H. Berberine Inhibits Cell Proliferation by Interfering with wild-type and Mutant P53 in Human Glioma Cells. OncoTargets Ther. 2020, 13, 12151. DOI: 10.2147/OTT.S279002.
- Qu, H.; Song, X.; Song, Z.; Jiang, X.; Gao, X.; Bai, L.; Yao, Z.; Na, L.; Yao, Z. Berberine Reduces Temozolomide Resistance by Inducing Autophagy via the ERK1/2 Signaling Pathway in Glioblastoma. Can. Cell Inter. 2020, 20(1), 1–13. DOI: 10.1186/s12935-020-01693-y.
- Samadi, P.; Sarvarian, P.; Gholipour, E.; Asenjan, K. S.; Aghebati-Maleki, L.; Motavalli, R.; Yousefi, M.; Yousefi, M. Berberine: A Novel Therapeutic Strategy for Cancer. IUBMB Life. 2020, 72(10), 2065–2079. DOI: 10.1002/iub.2350.
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K. S. Berberine Mitigates High glucose-potentiated Platelet Aggregation and Apoptosis by Modulating Aldose Reductase and NADPH Oxidase Activity. Free Radical Biol. Med. 2019, 130, 196–205. DOI: 10.1016/j.freeradbiomed.2018.10.453.
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine Improves Insulin Resistance in Adipocyte Models by Regulating the Methylation of hypoxia-inducible factor-3α. Biosci. Rep. 2019, 39(10), 10. DOI: 10.1042/BSR20192059.
- Mi, J.; He, W.; Lv, J.; Zhuang, K.; Huang, H.; Quan, S. Effect of Berberine on the HPA-axis Pathway and Skeletal Muscle GLUT4 in Type 2 Diabetes Mellitus Rats. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019, 12, 1717. DOI: 10.2147/DMSO.S211188.
- Zhao, L.; Liu, S.; Wang, M.; Zhi, M.; Geng, X.; Hou, C.; … Zhao, D. Berberine Restored Nitrergic and Adrenergic Function in Mesenteric and Iliac Arteries from streptozotocin-induced Diabetic Rats. J. Ethnopharmacol. 2019, 244, 112140. DOI: 10.1016/j.jep.2019.112140.
- Ni, W. J.; Zhou, H.; Ding, H. H.; Tang, L. Q. Berberine Ameliorates Renal Impairment and Inhibits Podocyte Dysfunction by Targeting the Phosphatidylinositol 3-kinase–protein Kinase B Pathway in Diabetic Rats. J Diabetes Invest. 2020, 11(2), 297–306. DOI: 10.1111/jdi.13119.
- Qin, X.; Zhao, Y.; Gong, J.; Huang, W.; Su, H.; Yuan, F.; … Lu, F. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-mediated Mitochondrial Fission and Dysfunction. Theranostics. 2019, 9(6), 1698. DOI: 10.7150/thno.30640.
- Rezaeiamiri, E.; Bahramsoltani, R.; Rahimi, R. Plant-derived Natural Agents as Dietary Supplements for the Regulation of Glycosylated Hemoglobin: A Review of Clinical Trials. Clin. Nutr. 2020, 39(2), 331–342. DOI: 10.1016/j.clnu.2019.02.006.
- Pang, B.; Zhao, L. H.; Zhou, Q.; Zhao, T. Y.; Wang, H.; Gu, C. J.; Tong, X. L. Application of Berberine on Treating Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015(2015), 1–12. DOI: 10.1155/2015/905749.
- Yin, S.; Bai, W.; Li, P.; Jian, X.; Shan, T.; Tang, Z.; Guo, T.; Ping, S.; Li, Q.; Miao, Z. Berberine Suppresses the Ectopic Expression of miR-133a in Endothelial Cells to Improve Vascular Dementia in Diabetic Rats. Clini. Experi. Hyper. 2019, 41(8), 708–716. DOI: 10.1080/10641963.2018.1545846.
- Simioni, C.; Zauli, G.; Martelli, A. M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L. M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget. 2018, 9(24), 17181. DOI: 10.18632/oncotarget.24729.
- Kazaz, I. O.; Mentese, A.; Demir, S.; Kerimoglu, G.; Colak, F.; Bodur, A.; Alver, A.; Kutlu, O.; Turedi, S. Berberine Inhibits the ischemia-reperfusion Induced Testicular Injury through Decreasing Oxidative Stress. Am. J. Emergency Med. 2020, 38(1), 33–37. DOI: 10.1016/j.ajem.2019.04.001.
- Ahmed, T.; Nabavi, S. F.; Nabavi, S. F.; Nabavi, S. F.; Nabavi, S. M.; Nabavi, S. M. Berberine and Neurodegeneration: A Review of Literature. Pharmacol Rep. 2015, 67(5), 970–979. DOI: 10.1016/j.pharep.2015.03.002.
- Verma, S. K.; Thareja, S. An Overview on Chemistry of Natural Aldose Reductase Inhibitors for the Management of Diabetic Complications. Studi. Nat. Prod. Chem. 2020, 65, 381–429.
- Lin, J.; Cai, Q.; Liang, B.; Wu, L.; Zhuang, Y.; He, Y.; Lin, W. Berberine, a Traditional Chinese Medicine, Reduces Inflammation in Adipose Tissue, Polarizes M2 Macrophages, and Increases Energy Expenditure in Mice Fed a High-Fat Diet. Med. Sci. Monitor: Inter. Med. J. Experi. and Clinical Res. 2019, 25, 87. DOI: 10.12659/MSM.911849.
- Cazzaniga, M.; Bonanni, B. Relationship between Metabolic Disorders and Breast Cancer Incidence and Outcomes. Is There a Preventive and Therapeutic Role for Berberine? Anticancer Res. 2018, 38(8), 4393–4402. DOI: 10.21873/anticanres.12741.
- Li, C.; Guan, X. M.; Wang, R. Y.; Xie, Y. S.; Zhou, H.; Ni, W. J.; Tang, L. Q. Berberine Mitigates High glucose-induced Podocyte Apoptosis by Modulating Autophagy via the mTOR/P70S6K/4EBP1 Pathway. Life Sci. 2020, 243, 117277. DOI: 10.1016/j.lfs.2020.117277.
- Wang, H.; Zhu, C.; Ying, Y.; Luo, L.; Huang, D.; Luo, Z. Metformin and Berberine, Two Versatile Drugs in Treatment of Common Metabolic Diseases. Oncotarget. 2018, 9(11), 10135. DOI: 10.18632/oncotarget.20807.
- Pirillo, A.; Catapano, A. L. Berberine, a Plant Alkaloid with lipid-and glucose-lowering Properties: From in Vitro Evidence to Clinical Studies. Atherosclerosis. 2015, 243(2), 449–461. DOI: 10.1016/j.atherosclerosis.2015.09.032.
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Shen, A. Z.; Hassan, S. T. S.; Šmejkal, K.; Malaník, M. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics. 2019, 9(7), 1923. DOI: 10.7150/thno.30787.
- Rivellese, A. A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The Possible Role of Nutraceuticals in the Prevention of Cardiovascular Disease. High Blood Pressure & Cardiovascular Prevention. 2019, 26(2), 101–111. DOI: 10.1007/s40292-019-00309-5.
- Hadi, A.; Arab, A.; Ghaedi, E.; Rafie, N.; Miraghajani, M.; Kafeshani, M. Barberry (Berberis Vulgaris L.) Is A Safe Approach for Management of Lipid Parameters: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Complementary Ther. Med. 2019, 43, 117–124. DOI: 10.1016/j.ctim.2019.01.017.
- Kaya, S. T.; Bozdogan, O.; Ozarslan, T. O.; Taskin, E.; Eksioglu, D.; Erim, F.; Yasar, S.; Yasar, S. The Protection of Resveratrol and Its Combination with Glibenclamide, but Not Berberine on the Diabetic Hearts against reperfusion-induced Arrhythmias: The Role of Myocardial K ATP Channel. Arch. Physiol. Biochem. 2019, 125(2), 114–121. DOI: 10.1080/13813455.2018.1440409.
- Zhang, X.; Guan, T.; Yang, B.; Chi, Z.; Wan, Q.; Gu, H. F. Protective Effect of Berberine on High Glucose and hypoxia-induced Apoptosis via the Modulation of HIF-1α in Renal Tubular Epithelial Cells. Am. J. Transl. Res. 2019, 11(2), 669.
- Fogacci, F.; Grassi, D.; Rizzo, M.; Cicero, A. F. Metabolic Effect of berberine–silymarin Association: A Meta-analysis of Randomized, Double-blind, Placebo-controlled Clinical Trials. Phytotherapy Res. 2019, 33(4), 862–870. DOI: 10.1002/ptr.6282.
- Liu, L.; Liu, J.; Huang, Z.; Yu, X.; Zhang, X.; Dou, D.; Huang, Y. Berberine Improves Endothelial Function by Inhibiting Endoplasmic Reticulum Stress in the Carotid Arteries of Spontaneously Hypertensive Rats. Biochem. Biophys. Res. Commun. 2015, 458(4), 796–801.
- Xiao, M.; Men, L. N.; Xu, M. G.; Wang, G. B.; Lv, H. T.; Liu, C. Berberine Protects Endothelial Progenitor Cell from Damage of TNF-α via the PI3K/AKT/eNOS Signaling Pathway. Eur. J. Pharmacol. 2014, 743, 11–16. DOI: 10.1016/j.ejphar.2014.09.024.
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; … Chen, L. Berberine Pretreatment Confers Cardioprotection against ischemia–reperfusion Injury in a Rat Model of Type 2 Diabetes. Journal of Cardiovascular Pharmacology and Therapeutics. 2016, 21(5), 486–494. DOI: 10.1177/1074248415627873.
- Zaha, V. G.; Qi, D.; Su, K. N.; Palmeri, M.; Lee, H. Y.; Hu, X.; Young, L. H.; Shulman, G. I.; Rabinovitch, P. S.; Russell, R. R. AMPK Is Critical for Mitochondrial Function during Reperfusion after Myocardial Ischemia. J. Mol. Cell. Cardiol. 2016, 91, 104–113. DOI: 10.1016/j.yjmcc.2015.12.032.
- Park, D. W.; Jiang, S.; Liu, Y.; Siegal, G. P.; Inoki, K.; Abraham, E.; Zmijewski, J. W. GSK3β-Dependent Inhibition of AMPK Potentiates Activation of Neutrophils and Macrophages and Enhances Severity of Acute Lung Injury. Am. J. Physiol. 2014, 307(10), L735–L745.
- Wang, Y.; Zidichouski, J. A. Update on the Benefits and Mechanisms of Action of the Bioactive Vegetal Alkaloid Berberine on Lipid Metabolism and Homeostasis. Cholesterol. 2018, 2018, 1–17. DOI: 10.1155/2018/7173920.
- Mohammadian Haftcheshmeh, S.; Momtazi-Borojeni, A. A. Berberine as A Promising Natural Compound for the Treatment of Periodontal Disease: A Focus on Anti-inflammatory Properties. J. Cellular Mol. Med. 2021, 25(24), 11333–11337. DOI: 10.1111/jcmm.17019.
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Roghani, M.; Roghani, M. Berberine Ameliorates lipopolysaccharide-induced Learning and Memory Deficit in the Rat: Insights into Underlying Molecular Mechanisms. Metabolic Brain Disease. 2019, 34(1), 245–255. DOI: 10.1007/s11011-018-0349-5.
- Vita, A. A.; Pullen, N. A. The Influence of Berberine on co-stimulatory Molecule Expression and T Cell Activation. Am. Assoc. Immnol. 2018, 171–11. DOI: 10.4049/jimmunol.200.Supp.175.11.
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H. Berberine Downregulates CDC6 and Inhibits Proliferation via Targeting JAK-STAT3 Signaling in Keratinocytes. Cell Death & Disease. 2019, 10(4), 274. DOI: 10.1038/s41419-019-1510-8.
- Zaidi, T. S.; Kausar, R.; Malik, M.; Sarfraz, J.; Shafiq, A.; Chiragh, S. Comparison of Berberine and Dexamethasone on Blood and Bronchial Inflammatory Cells of Ovalbumin Sensitized Guinea Pigs. Esculapio202. 2021, 17(1), 34–38. DOI: 10.51273/esc21.251717.
- Xu, Z.; Feng, W.; Shen, Q.; Yu, N.; Yu, K.; Wang, S.; Guo, Y.; Shioda, S.; Guo, Y. Rhizoma Coptidis and Berberine as a Natural Drug to Combat Aging and aging-related Diseases via anti-oxidation and AMPK Activation. Aging and Disease. 2017, 8(6), 760. DOI: 10.14336/AD.2016.0620.
- McCubrey, J. A.; Lertpiriyapong, K.; Steelman, L. S.; Abrams, S. L.; Cocco, L.; Ratti, S.; Martelli, A. M.; Candido, S.; Libra, M.; Montalto, G. Regulation of GSK-3 Activity by Curcumin, Berberine and Resveratrol: Potential Effects on Multiple Diseases. Adv. Biol. Reg. 2017, 65, 77–88. DOI: 10.1016/j.jbior.2017.05.005.
- Martel, J.; Ojcius, D. M.; Ko, Y. F.; Chang, C. J.; Young, J. D. Antiaging Effects of Bioactive Molecules Isolated from Plants and Fungi. Med. Res. Rev. 2019, 39(5), 1515–1552. DOI: 10.1002/med.21559.
- Yu, Y.; Zhao, Y.; Teng, F.; Li, J.; Guan, Y.; Xu, J.; Chen, L.; Guan, F.; Zhang, M.; Chen, L. Berberine Improves Cognitive Deficiency and Muscular Dysfunction via Activation of the AMPK/SIRT1/PGC-1a Pathway in Skeletal Muscle from Naturally Aging Rats. j. nutr. health aging. 2018, 22(6), 710–717. DOI: 10.1007/s12603-018-1015-7.
- Sharma, R.; Padwad, Y. In Search of Nutritional Anti-Aging Targets: TOR Inhibitors, SASP Modulators, and BCL-2 Family Suppressors. Nutrition. 2019, 65, 33–38. DOI: 10.1016/j.nut.2019.01.020.
- Cai, Z.; Wang, C.; Yang, W. Role of Berberine in Alzheimer’s Disease. Neuropsychiatr Dis Treat. 2016, 12, 2509. DOI: 10.2147/NDT.S114846.
- El-Horany, H. E.-S.; Gaballah, H. H.; Helal, D. S. Berberine Ameliorates Renal Injury in a Rat Model of D-galactose-induced Aging through a PTEN/Akt-dependent Mechanism. Arch. Physiol. Biochem. 2018, 126(2), 1–9.
- Shal, B.; Ding, W.; Ali, H.; Kim, Y. S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. DOI: 10.3389/fphar.2018.00548.
- Asai, M.; Iwata, N.; Yoshikawa, A.; Aizaki, Y.; Ishiura, S.; Saido, T. C.; Maruyama, K. Berberine Alters the Processing of Alzheimer’s Amyloid Precursor Protein to Decrease Abeta Secretion. Biochem. Biophys. Res. Commun. 2007, 352(2), 498–502. DOI: 10.1016/j.bbrc.2006.11.043.
- Zhu, F.; Wu, F.; Ma, Y.; Liu, G.; Li, Z.; Sun, Y. A.; Pei, Z. Decrease in the Production of beta-amyloid by Berberine Inhibition of the Expression of beta-secretase in HEK293 Cells. BMC neuro. 2011, 12(1), 1–8. DOI: 10.1186/1471-2202-12-125.
- Zhang, H.; Zhao, C.; Cao, G.; Guo, L.; Zhang, S.; Liang, Y.; Qin, C.; Su, P.; Li, H.; Zhang, W. Berberine Modulates amyloid-β Peptide Generation by Activating AMP-activated Protein Kinase. Neuropharmacology. 2017, 125, 408–417. DOI: 10.1016/j.neuropharm.2017.08.013.
- Panahi, N.; Mahmoudian, M.; Mortazavi, P.; Hashjin, G. S. Effects of Berberine on β-secretase Activity in a Rabbit Model of Alzheimer’s Disease. Arch. Med. Sci. 2013, 9(1), 146–150. DOI: 10.5114/aoms.2013.33354.
- Haghani, M.; Shabani, M.; Tondar, M. The Therapeutic Potential of Berberine against the Altered Intrinsic Properties of the CA1 Neurons Induced by Aβ Neurotoxicity. Eur. J. Pharmacol. 2015, 758, 82–88. DOI: 10.1016/j.ejphar.2015.03.016.
- Durairajan, S. S.; Liu, L. F.; Lu, J. H.; Chen, L. L.; Yuan, Q.; Chung, S. K.; Huang, L.; Li, X. S.; Huang, J. D.; Li, M. Berberine Ameliorates β-amyloid Pathology, Gliosis, and Cognitive Impairment in an Alzheimer’s Disease Transgenic Mouse Model. Neurobiol. Aging. 2012, 33(12), 2903–2919. DOI: 10.1016/j.neurobiolaging.2012.02.016.
- Kim, M.; Cho, K. H.; Shin, M. S.; Lee, J. M.; Cho, H. S.; Kim, C. J.; Shin, D. H.; Yang, H. J. Berberine Prevents Nigrostriatal Dopaminergic Neuronal Loss and Suppresses Hippocampal Apoptosis in Mice with Parkinson’s Disease. Int. J. Mol. Med. 2014, 33(4), 870–878. DOI: 10.3892/ijmm.2014.1656.
- Bae, J.; Lee, D.; Kim, Y. K.; Gil, M.; Lee, J. Y.; Lee, K. J. Berberine Protects 6-hydroxydopamine-induced Human Dopaminergic Neuronal Cell Death through the Induction of Heme Oxygenase-1. Mol. Cells. 2013, 35(2), 151–157. DOI: 10.1007/s10059-013-2298-5.
- Shin, K. S.; Choi, H. S.; Zhao, T. T.; Suh, K. H.; Kwon, I. H.; Choi, S. O.; Lee, M. K. Neurotoxic Effects of Berberine on long-term L-DOPA Administration in 6-hydroxydopamine-lesioned Rat Model of Parkinson’s Disease. Arch. Pharmacal Res. 2013, 36(6), 759–767. DOI: 10.1007/s12272-013-0051-4.
- Kwon, I. H.; Choi, H. S.; Shin, K. S.; Lee, B. K.; Lee, C. K.; Hwang, B. Y.; Lim, S. C.; Lee, M. K. Effects of Berberine on 6-hydroxydopamine-induced Neurotoxicity in PC12 Cells and a Rat Model of Parkinson’s Disease. Neurosci. Lett. 2010, 486(1), 29–33. DOI: 10.1016/j.neulet.2010.09.038.
- Fan, X.; Wang, J.; Hou, J.; Lin, C.; Bensoussan, A.; Chang, D.; Liu, J.; Wang, B. Bensoussan A., Chang D., Liu J., Wang B. Berberine Alleviates ox-LDL Induced Inflammatory Factors by up-regulation of Autophagy via AMPK/mTOR Signaling Pathway. J. Transl. Med. 2015, 13(1), 92. DOI: 10.1186/s12967-015-0450-z.
- Kou, J. Y.; Li, Y.; Zhong, Z. Y.; Jiang, Y. Q.; Li, X. S.; Han, X. B.; Liu, Z. N.; Tian, Y.; Yang, L. M. Berberine-sonodynamic Therapy Induces Autophagy and Lipid Unloading in Macrophage. Cell Death Dis. 2017, e8(1), 2558.
- Zhou, H.; Feng, L.; Xu, F.; Sun, Y.; Ma, Y.; Zhang, X.; Liu, H.; Xu, G.; Wu, X.; Shen, Y., et al. Berberine Inhibits palmitate-induced NLRP3 Inflammasome Activation by Triggering Autophagy in Macrophages: A New Mechanism Linking Berberine to Insulin Resistance Improvement. Biomed. Pharmacother. 2017, 89, 864–874. DOI: 10.1016/j.biopha.2017.03.003.
- Chitra, P.; Saiprasad, G.; Manikandan, R.; Sudhandiran, G. Berberine Inhibits Smad and non-Smad Signaling Cascades and Enhances Autophagy against Pulmonary Fibrosis. J. Mol. Med. 2015, 93(9), 1015–1031. DOI: 10.1007/s00109-015-1283-1.
- Peng, P. L.; Kuo, W. H.; Tseng, H. C.; Chou, F. P. Synergistic tumor-killing Effect of Radiation and Berberine Combined Treatment in Lung Cancer: The Contribution of Autophagic Cell Death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70(2), 529–542.
- Lee, K. H.; Lo, H. L.; Tang, W. C.; Hsiao, H. H.; Yang, P. M. A Gene Expression signature-based Approach Reveals the Mechanisms of Action of the Chinese Herbal Medicine Berberine. Sci. Rep. 2014, 4(1), 6394. DOI: 10.1038/srep06394.
- Halicka, H. D.; Garcia, J.; Li, J.; Zhao, H.; Darzynkiewicz, Z. Synergy of 2-deoxy-D-glucose Combined with Berberine in Inducing the lysosome/autophagy and Transglutaminase activation-facilitated Apoptosis. Apoptosis. 2017, 22(2), 229–238. DOI: 10.1007/s10495-016-1315-5.
- Chen, M.; Shen, H.; Zhu, L.; Yang, H.; Ye, P.; Liu, P.; , and Chen, S. Berberine Attenuates Hypoxia-induced Pulmonary Arterial Hypertension via Bone Morphogenetic Protein and Transforming Growth factor-β Signaling. J. Cell. Physiol. 2019, 234(10), 17482–17493.
- Luo, J.; Gu, Y.; Liu, P.; Jiang, X.; Yu, W.; Ye, P.; Chao, Y.; Yang, H.; Zhu, L.; Zhou, L. Berberine Attenuates Pulmonary Arterial Hypertension via Protein Phosphatase 2A Signaling Pathway Both in Vivo and in Vitro. J. Cell. Physiol. 2018, 233(12), 9750–9762. DOI: 10.1002/jcp.26940.
- Tian, H.; Kang, Y.-M.; Gao, H.-L.; Shi, X.-L.; Fu, L.-Y.; Li, Y.; Jia, X.-Y.; Liu, K.-L.; Qi, J.; Li, H.-B. Chronic Infusion of Berberine into the Hypothalamic Paraventricular Nucleus Attenuates Hypertension and Sympathoexcitation via the ROS/Erk1/2/iNOS Pathway. Phytomedicine. 2019, 52, 216–224. DOI: 10.1016/j.phymed.2018.09.206.
- Akhzari, M.; Shafiee, S. M.; Rashno, S.; Akmali, M. Berberine Attenuated Oxidative Stress Induced by Sodium Nitrite in Rat Liver. Jundishapur Journal of Natural Pharmaceutical Products. 2019, 14(1), e68532. DOI: 10.5812/jjnpp.68532.
- Zhu, X.; Guo, X.; Mao, G.; Gao, Z.; Wang, H.; He, Q.; Li, D. Hepatoprotection of Berberine against Hydrogen Peroxide-induced Apoptosis by Upregulation of Sirtuin 1. Phytotherapy Res. 2013, 27(3), 417–421. DOI: 10.1002/ptr.4728.
- Zhao, H.; Halicka, H. D.; Li, J.; Darzynkiewicz, Z. Berberine Suppresses gero-conversion from Cell Cycle Arrest to Senescence. Aging (Albany NY). 2013, 5(8), 623. DOI: 10.18632/aging.100593.
- Dai, P.; Wang, J.; Lin, L.; Zhang, Y.; Wang, Z. Renoprotective Effects of Berberine as Adjuvant Therapy for Hypertensive Patients with Type 2 Diabetes Mellitus: Evaluation via Biochemical Markers and Color Doppler Ultrasonography. Exp. Ther. Med. 2015, 10(3), 869–876. DOI: 10.3892/etm.2015.2585.
- Othman, M. S.; Safwat, G.; Aboulkhair, M.; Abdel Moneim, A. E. The Potential Effect of Berberine in mercury-induced Hepatorenal Toxicity in Albino Rats. Food Chem. Toxicol. 2014, 69, 175–181. DOI: 10.1016/j.fct.2014.04.012.
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Škoda, M.; Mikelić, L.; Crnčević-Orlić, Ž. Berberine Exerts Nephroprotective Effect against cisplatin-induced Kidney Damage through Inhibition of oxidative/nitrosative Stress, Inflammation, Autophagy and Apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. DOI: 10.1016/j.fct.2013.09.003.
- Najaran, H.; Bafrani, H. H.; Rashtbari, H.; Izadpanah, F.; Rajabi, M. R.; Kashani, H. H.; Mohammadi, A. Evaluation of the Serum Sex Hormones Levels and Alkaline Phosphatase Activity in Rats’ Testis after Administering of Berberine in Experimental Varicocele. Oriental Pharmacy and Experi. Med. 2019, 19, 157–165. DOI: 10.1007/s13596-019-00369-x.