ABSTRACT
Gynostemma pentaphyllum belongs to the family Cucurbitaceae which is native to China where it is also termed an immortal herb. It is an emerging herb gaining fame for its rich phytochemistry. It is loaded with superior phytochemicals with immense therapeutic potential. The key phytochemicals include saponins and sterols. Saponins are a wide class of bioactive found in Gynostemma pentaphyllum and 100 different saponins have been reported to date by different researchers in this herb. Several sterols have been demonstrated in this plant including ergostanol, sitosterol and stigmasterol. All these bioactive substances possess superior therapeutic and pharmacological properties that have been investigated in different studies as presented in this comprehensive review. Taxonomic classification, botanical description, and geographical distribution are briefly covered in the first section of this review. In contrast, the phytochemistry, therapeutic properties, and pharmacological features of Gynostemma pentaphyllum are thoroughly explained in the second section.
Introduction
Gynostemma pentaphyllum is indeed a perennial creeping in the Gynostemma genus. Cucurbitaceae is a plant family that includes cucumbers, gourds, and melons.[Citation1] Jiaogulan is a popular name for G. pentaphyllum.[Citation2] Gynostemma has 21 species, the majority of which are found in southwestern China. One of the more common species is pentaphyllum, which may be found in India, Nepal, Bangladesh, Sri Lanka, Myanmar, Korea, and Japan.[Citation1] At a height of 300–3200 m, G. pentaphyllum grows natively in mountain forests, hillside meadows, timber, scrub, stream sides, roadsides, and shrubs. It also grows in shady and moist areas.[Citation3]
G. pentaphyllum has been used for more than 500 years, and the majority of the research on it is Chinese. Throughout the Ming Dynasty (1368–1644 AD.), the textbook “Herbs for Famine” recounts the plant’s use as a vegetable, appropriate for eating, or a nutritional complement through starvation instead of as a therapeutic herb.[Citation4] G. pentaphyllum was used by the famous herbalist Li Shi-Zhen (1578 A.D.) for the treatment of hematuria, pharyngeal edema, cancers, and trauma.[Citation5,Citation6] According to Traditional Chinese Medicine (TCM) theories, the flavor and texture of G. pentaphyllum, are somewhat sour, balanced, warming, and strengthening, and the herb can proactively decrease inflammation.[Citation1] Hyperlipidemia, muscle twitches, chest tightness, tingling sensations in extremities, disorientation, migraine, amnesia, tinnitus, involuntary sweating, overall fatigue, enlargement of the belly, and mucous and blood obstruction are included in its treatment.[Citation3] As a result, G. pentaphyllum must include some of the most current Chinese Materia Medica vocabulary, whereby it is used in TCM for warmth clearance, cleansing, anti-inflammatory agent, cardiac palpitation, tiredness syndrome, respiratory problems, and purgative for cough relief.[Citation7] It’s used as a therapeutic agent, antipyretic, anti-inflammatory, and stimulant in Japan.[Citation8] Extracts from the Southeast Asian plant G. pentaphyllum (Cucurbitaceae) have been shown to have anticancer, cholesterol-lowering, immuno-potentiating, antioxidants, and hypoglycemia properties.[Citation9–11]
G. pentaphyllum is a perennial creeping plant belonging to the Gynostemma genus that can be found in India, Nepal, Bangladesh, Sri Lanka, Myanmar, Korea, and Japan. Taxonomic Hierarchy of Gynostemma pentaphyllum was shown in . According to traditional Chinese medicine (TCM) ideas, G. pentaphyllum has a flavor and texture that is a little bit sour, balanced, warm, and strengthening, and the herb “would be utilized to boost barriers against illness and toward anti-inflammation.” G. pentaphyllum must be included in the most recent Chinese medical lexicon since it is utilized in TCM for respiratory issues, heart palpitations, exhaustion syndrome, cleaning, anti-inflammatory, and purgative alleviation of coughing. In Japan, it’s utilized as a stimulant, antipyretic, and medicinal agent.
Table 1. Taxonomic Hierarchy of Gynostemma pentaphyllum.
Morphology and flowering season
The plant is usually referred to as Jiaogulan in European countries including English and German.[Citation12] Alternative titles encompass Chinese; xiancao (roughly “immortal grass,” but more precisely “herb of immortality”), English; five-leaf ginseng, sweet tea vine, Japanese; amachazuru, Korean; dungkulcha, Latin; Gynostemma pentaphyllum, Thai; jiaogulan, and Vietnamese; Giảo cổ lam.
depicted the leaves, flowers, and fruits of G. pentaphyllum. The morphology is characterized by thin, branching branches that are arris, smooth, or hairy. The leaves are pinnately constructed, membranous, and have 4 to 7 foliates. Leaves are ovate-elliptic to oblong, ovate or sharp, and infrequently upturned. These are malodorous over surfaces, dark green on peak & pale green on the below side. Two complicated tendrils are thin G. pentaphyllum is a dioecious flowering plant. Male blooms are conical and inflorescent, measuring 15–20 cm in diameter. The tiny, highly branched tube has branches that are 4–5 (up to 15) cm long. The stalk is 2–3 mm long and thread-like. The small calyx has 5 triangular, apiculate lobes, each 0.7 mm in length. The light green or white corolla has five clefts. The acuminate, single-veined flowers are ovate-lanceolate and somewhat notched (2.5–3.0 mm length, 1 mm broad). The stamens are made up of five connate threads that create a column with anthers at the top.
Female flowers resemble male blooms but are considerably shorter. The globose 2–3 loculated ovaries. The 3 styles are all short and lobed, with a bifid stigma. There might be a few small stamens left over. A smooth, globose tiny berry around 4–6 mm in diameter while mature makes up the fruit. The seeds are dark brown in hue and crushed by uneven hairy or follicular nodules. The seed has an oblique tip and a heart-shaped bottom. March to November is the blooming period in the northern hemisphere (September to May in the southern side); April to December is the fruiting period. September to October is harvest time in China.[Citation3]
G. pentaphyllum has slim stems with thin, delicate leaves that are organized and have 3 to 9 (typically 5 to 7 leaves). Long and flexible, the leaflets are widest underneath the center and descend to a lance-shaped end. Both surfaces are scratchy to feel, and the top part is dark green with a bright green beneath. Male and female flowers grow on distinct plants.[Citation13] Male blooms are numerous, measuring 10–15 cm (max. 30 cm) in diameter. The petals of the 5-cleft corolla are light green or white. Female flowers are identical to male blooms; however, they are considerably smaller. The globe-shaped ovary has 2–3 cavities. The stigma and the three styles are both small and split into two pieces. The fruit is a glossy, spherical, tiny berry with a dimension of 5–6 mm that becomes black once it matures. The seeds are dark brown and has an oblique apex and a heart-shaped foot. In the northern latitude, the inflorescence is from March to November, while the fruiting season is from April to December.[Citation7]
Cultivation and harvesting
G. pentaphyllum grows best in soil that is more than 30 cm in depth, loaded with humic substances, nitrogen, phosphorus, and moisture (pH range 6.0 to 8.0, including an optimal pH of 6.5 to 7.0). A rich sandy soil with good aeration and water retention is ideal.[Citation13] The amount of light required to develop G. pentaphyllum is critical.[Citation14,Citation15] Producing Gynostemma Pentaphyllum from seed requires dedication and can take a long period. A flexible and easy-to-grow medium is required for effective proliferation. A blend of minerals (vermiculite, perlite) and organic substances is ideal (topsoil, potting soil). During April and May, G. Pentaphyllum seeds must be sown on a window ledge or in an inside greenhouse. If you wish to test it outside, you’ll only be able to do so if there are no further frosts. A bright and warm location is required for growing. G. Pentaphyllum is a dark germinator, which means the seeds must be pounded into the soil for 3 to 4 cm (1.5 inches). It is necessary to soak the seed in hot water for 24 hours before planting it. The medium must be kept moist but not too wet at all times. In essence, cultivation on the rooftop and patio is conceivable. G. Pentaphyllum plants, on the other hand, must be planted in large plant pots.[Citation16]
Cutting propagation of G. Pentaphyllum is achievable and encouraged. Make a diagonal cut underneath the axils of the leaves of each leaf piece for optimal efficiency. For 3 to 4 weeks, the sliced portion is placed in a cup of water. The cuttings could be planted in a container loaded with soil once the roots reach a sufficient height of approximately 2 to 4 cm (1 inch). Consider that the soil never entirely dried up and is constantly somewhat damp while pouring. It’s preferable to spray early in the morning or late in the sunset. A plant can be harmed by too much wetness. Because G. Pentaphyllum is a perennial herb, it must be fertilized regularly. The majority of available on the market flower or herb soil is pre-fertilized and contains suitable nutrient storage.[Citation15]
G. pentaphyllum cultivation is inexpensive, simple, and requires no special care or supervision. So, when the vine reaches a length of 2–3 meters, G. pentaphyllum can be harvested. The plant is harvested 4–5 times a year in the subtropics and tropics. Collection may occur every 20–30 days depending on growth conditions and luxuriance. A high-yield patch might generate 4000–5000 kg of dry herb per hectare.[Citation2,Citation15,Citation17–19] depicted Gynostemma pentaphyllum (var. Ginpent) crops that were grown in Leno, Brescia, Italy.
Geographical distribution
For several underdeveloped nations, medicinal plants including their inherent chemicals serve an imperative function in the therapy of a variety of ailments. The value of therapeutic herbs is increasing rapidly as a result of their numerous advantages.[Citation20,Citation21] Several medical plant varieties are critically endangered by overharvesting and ecosystem loss as a result of increased interest in medicinal plants in the local and global pharma industry. This is in addition to the impact of human activities.[Citation22] Synthetic farming is the most practical technique to safeguard wild assets while satisfying consumer needs. However, the effective artificial culture of medicinal plants of high quality is dependent not only on rich hereditary sources but also on favorable environmental circumstances.[Citation23,Citation24] As a result, assessing the ecological requisites for elevated therapeutic flora by associating the material of the target species’ efficient elements with external surroundings will assist additional efficient plant farming while also contributing to species sustainability.[Citation25] Furthermore, cultivation is premised on particular knowledge of medicinal plants’ habitat prerequisites.[Citation23]
Gynostemma pentaphyllum is a popular Chinese medicinal plant that is an herbaceous climber plant of the gourd family. Its native distribution is restricted to wet places beneath forests at altitudes ranging from 200 to 3200 meters.[Citation26] Due to its powerful capacity to acclimate to the environment, it is broadly dispersed in Assam, Bangladesh, Borneo, China North-Central, China South-Central, China Southeast, East Himalaya, India, Hainan, Japan, Jawa, Korea, Kuril Is., Laos, Lesser Sunda Is., Malaysia, Maluku, Myanmar, Nansei-Shoto, Nepal, New Guinea, Philippines, Sri Lanka and Sumatera.[Citation27] The time of sprouting leaves in the growing phase of G. pentaphyllum in the south of China seems to be from late March to the earliest April. The leaf-expanding phase lasts until the end of April, preceded by a time of maximum growth. Between July and September, the blossoming season begins. Plant growth slows in mid-November when the above-ground sections of the plant wither.[Citation19]
G. pentaphyllum was added to the List of National Protected Plants because of its broad array of growth, high yield, and market perspective and it became popular in Europe at the turn of the century.[Citation28] G. pentaphyllum studies now concentrate mostly on pharmaceutical studies, reproductive genetic sources, and functional genomics.[Citation29,Citation30] Only a handful of these, notably for high-quality G. pentaphyllum, mention the species’ likely dispersal region.
Usage in past
G. pentaphyllum is a plant that is commonly used in traditional medicine as an herbal tea. However, it might also exist and be found as an alcohol extract or as a nutritional supplement. Since it originated in a part of central China, a region where traditional Chinese medicine (TCM) emerged, it has not extensively engaged in TCM. As a result, it was not included in the TCM system’s standard pharmaceuticals. Before that, it was mostly used in the hilly provinces of southern China and northern Vietnam as a local plant. Locals refer to it as the “immortality herb” since a significant proportion of old individuals in Guizhou Province have documented frequently ingesting the plant.[Citation31] G. pentaphyllum is widely utilized in traditional medicine to heal bronchitis, asthma, liver problems, and adipose tissue abnormalities.[Citation32–34] This herb has relaxing and memory-enhancing properties and is very beneficial in the treatment of insomnia.[Citation35]
Phytochemistry
Saponins
As indicated in , the most recent literature analysis on the SciFinder database discovered above 100 saponins extracted and characterized from G. pentaphyllum by researchers in China in 1976. From these, 8 saponins are identical to protopanaxadiol-type ginsenosides Rb1 (Gypenoside III), Rc, Rb3 (Gypenoside IV), Rd (Gypenoside VIII), F2, Rg3, malonyl-Rb1, and malonyl-Rd originated in P. ginseng. Rf, a protopanaxatriol was also disclosed (Ma et al., 1995). Such these ginsenosides account for approximately a quarter of the plant’s entire gynosaponin and are the first ginseng saponins discovered beyond the Araliaceae family.
Table 2. Dammarane-type saponin structures in G. pentaphyllum.
The remaining saponoid component is largely made up of gypenosides, which are distinctively attributed to G. pentaphyllum. Gymnema sylvestra also contains the gypenosides XXVIII, XXXVII, LV, LXII, and LXIII. P. notoginseng contains Gypenoside XVII, IX (notoginsenoside Fd), and XV, as well as Gypenoside XVII and IX in P. quinquefolium. The dried herb’s total saponin concentration is estimated to be around 2.4%.[Citation9] The overall saponin concentration is greatest before blooming, according to studies. The total saponin content varies depending on the species, emergent location, and instance of collection.[Citation68]
The principal sugar types occurring in positions C-3 (b) & C-20 include b-D-glucose, b-D-xylose, a-L-arabinose, and a-L-rhamnose. Hydroxyl, methyl, aldehyde, alcohol, and the least frequent, ketone, are all functional groups that can be encountered at position C-19. At C-2 (a) and C-12 (b), a hydroxyl group can also be detected ( and ). The first ocotillone-type saponins ( and ) containing an epoxy ring at C-17 have also been identified, with structures 3b; 12b; 23S; 24 R-tetrahydroxy-20S, 25-epoxydammarane and (20S, 24S)-20,24-epoxydammarane 3b; 12b; 25-triol.[Citation71] Gynoside A, Gynogenin II, and Gynosaponin TN1 are all crystal structures of gypenosides that have been identified by Razmovski-Naumovski et al.[Citation72]
Figure 3. G. pentaphyllum dammarane skeleton with characteristic linkages.
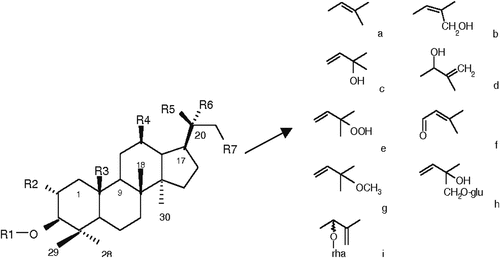
Figure 4. G. pentaphyllum cyclopentenone (j) and epoxy dammarane-type glycosides.
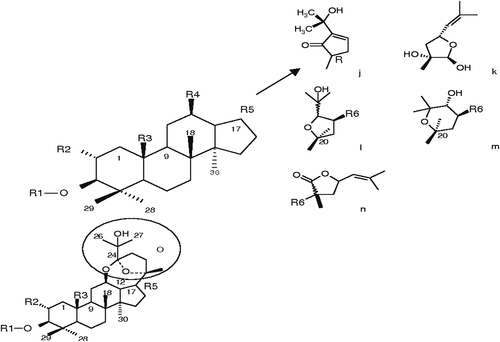
Table 3. G. pentaphyllum cyclopentenone and epoxy dammarane-type glycoside compounds.
Other components
Ergostanol, sitosterol, and stigmasterol are among the frequent sterols found in G. pentaphyllum in minute quantities (0.0001%). In the Cucurbitaceae family, cholestanols with a 24,24-dimethyl substitution are abundant. One of the most common sterol components is chondrillasterol and its (24S)-epimer, spinasterol. 24-alkyl-∆[Citation5]-, ∆[Citation7]-, ∆[Citation8]-sterols and 24,24-dimethyl-∆[Citation7] -sterols are the most common configurations at C-24. The composition of the 24, and 24-dimethyl-∆[Citation7]-sterols is remarkable since the side chain (C-24) contains acyclic, quaternary carbon groups, which are abundant in marine sponges. 4a-methyl sterols, 14a-methyl sterols, 24,24- dimethylsterols, (24 R/α)- and (24S/β) epimers of 14α also occur. G. pentaphyllum was the first non-marine organism to produce acetylenic sterols. Flavonoids, omuibn, ombuoside, rutin, carbohydrates, vitamins, minerals, carotenoids, and amino acids are among the other ingredients documented. Allantoin and vitexin were found by Yin et al.,[Citation62] and it has been stated that there are no alkaloids present.[Citation74]
Toxicological studies
In rats, a concentrated extract of Gynostemma pentaphyllum did not result in mortality or toxic symptoms. The extract was taken daily for 90 days without producing any fatal or negative consequences. Neutrophil, monocyte, sugar, and serum alkaline phosphatase levels were among the hematological and blood biochemistry values that were shown to be statistically different from the control group. However, these values were still within the limits of normal rats.[Citation75]
Pharmacological properties
The saponins, which have been the subject of pharmacological investigations in China, are primarily responsible for G. pentaphyllum’s therapeutic benefits.[Citation3] The plant’s adaptability has given it the moniker “immortality herb.”[Citation1]
Anti-cancer effect
In 1993, 59 individuals who had established malignant tumors participated in experimental testing to see how G. pentaphyllum affected them. The findings revealed that patients administered with a G. pentaphyllum formula had 11.9 and 8.5% cancer recurrence and spread, respectively. This was compared to 72.4 and 55.2% in the control group. The frequency of T lymphocyte activation and acid-naphthyl acetate esterase (ANAE+) capacity rose by 8.1% after G. pentaphyllum therapy, according to the findings of Wang et al. .[Citation76] A second 5-year observational trial found that treating cancer patients with the G. pentaphyllum formula resulted in substantial decreases in cancer recurrence and spread frequencies, as well as lower mortality and enhanced immunity performance within those individuals. G. pentaphyllum was also exposed to boost the immune system of cancer sufferers following chemotherapy. This was seen in an improved T lymphocyte activation rate and lower IgG and IgM levels in women with breast cancer. Moreover, G. pentaphyllum improved lung cancer patients’ immune function following treatment. G. pentaphyllum prescription can function in harmony with chemotherapeutic agents, according to findings of recent research.[Citation77]
Mechanisms of action
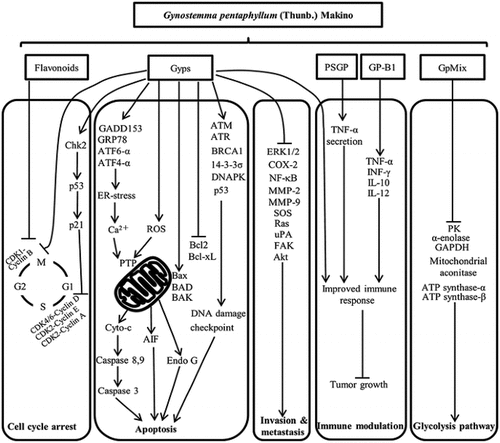
Cell cycle blockage, apoptosis initiation, prevention of colonization and metastasis, glycolysis restriction, and immune regulation have all been postulated as mechanisms of action for G. pentaphyllum’s anti-cancer properties ().
Cell cycle blockage
SAS human oral cancer cells,[Citation78] WEHI-3 leukemia cells,[Citation79] A549 human lung adenocarcinoma cells, and HL-60 human myeloid leukemia cells were all detained in the G0/G1 phase by gypenosides (Gyps).[Citation80] Gyps also caused cell cycle delay by altering the expression of cyclin-dependent kinase 2 (CDK2), (CDK4), and (CDK6), which are all cell cycle regulators.[Citation81] Gyps treatment of SCC-4 human tongue cancer cells resulted in the activation of checkpoint kinase 2 (Chk2). As a result of such action, p53 and its targets p21 and p16 were upregulated. This resulted in lower concentrations of cyclin D and cyclin E and G0/G1 cell cycle arrests.[Citation80] Regulating the activity of cyclins, flavonoids, and saponins derived from G. pentaphyllum caused cell cycle delay in the S and G2/M phases in PC-3 human prostate cancer cells.[Citation82]
Apoptosis initiation
G. pentaphyllum pushes its anti-cancer properties by causing cellular death via multiple signaling channels, according to several researchers. Gyps activates the creation of Bax/Bak holes on the exterior mitochondrial membrane by downregulating the anti-apoptotic proteins Bcl-2 and Bcl-xL and upregulating the pro-apoptotic proteins Bax, Bad, and Bak.[Citation83] The activation of starter caspases-8 and −9, triggering by the dissociation of effector caspase-3, results in the escape of cytochrome c and other pro-apoptotic proteins into the cytoplasm, triggering apoptosis.[Citation80] Subsequent DNA breakage and chromatin condensation, the development of Bax/Bak holes in response to Gyps treatment resulted in the liberation of apoptosis-inducing factor (AIF) & endonuclease G (EndoG) from the mitochondria.
Apoptosis has also been observed to be induced by different components and fractions of G. pentaphyllum. Flavonoids and water extracts from G. pentaphyllum, for example, trigger apoptosis in tumor cells by regulating the Bcl-2 protein family.[Citation84] Moreover, since tumor cells had higher superoxide dismutase activities than healthy cells, an ethanolic extract from G. pentaphyllum preferentially altered the intracellular H2O2 content to excessive levels.[Citation85]
Prevention of colonization and metastasis
By suppressing nuclear factor kappa B (NF-B) & matrix metalloproteinase-9 (MMP-9) in SCC4 human tongue cancer cells, Gyps inhibited their penetration and motility.[Citation86] Gyps also prevented SAS cells from invading and migrating, as evidenced by in vitro lesions and Boyden Chamber experiments.[Citation87] At a dosage of 100 g/mL, Gyps also reduced the proliferation of SW-480 human colon cancer cells in vitro. Medical trials have shown this impact. Persons with incurable malignant tumors administered with the G. pentaphyllum formula, for instance, exhibited an 8.5% cancer metastasis probability compared to 54.9% in the control group.[Citation88]
Glycolysis restriction
Completely unregulated energy metabolism that could evolve to a condition characterized as “aerobic glycolysis” is one of the characteristics of cancer cells.[Citation89] Therefore, targeting glucose metabolism for development of new cancer medicines has shown promising results. In the context of normal cells, GpMix, a combination of triterpenoid saponins from G. pentaphyllum, substantially suppressed the proliferation of cancer cells.[Citation90]
Immune regulation
The anti-cancer properties of G. Pentaphyllum are mediated by its immune-regulation properties. For instance, Yang et al.[Citation91] discovered that a water-soluble polysaccharide from G. pentaphyllum herb tea (PSGP) has indirect anticancer action toward SW-1116 human colorectal adenocarcinoma cells and HT-29 via increasing macrophage immune response and TNF production in a dose-dependent manner. Furthermore, GP-B1, an acidic polysaccharide derived from G. pentaphyllum, not only suppressed cancer cell proliferation but in addition boosted cellular immune response by mounting TNFα-, IFN-γ, IL-10, and IL-12 levels in the serum of melanoma-B16-bearing animals.[Citation92] Gyps’ anti-cancer effect was linked to the xenografted mice’ enhanced immunological systems.
Antidiabetic effects
For two weeks, saponin components from G. pentaphyllum effectively reduced plasma glucose levels in diabetic rats caused by streptozotocin (STZ).[Citation93] It was recently demonstrated that G. pentaphyllum phanoside enhanced insulin secretion from isolated rat pancreatic islets. Phenosides boost glucose tolerance & amplify plasma insulin concentrations in hyperglycemic rats when administered orally.[Citation70] Gypenosides improved insulin receptor sensitivity and lowered exogenous glucose-induced hyperglycemia in Zucker diabetic fatty rats.[Citation94]
Ability to inhibit α-glucosidase
To digest carbohydrates, the small intestine’s brush border contains an enzyme called α-glucosidase that is membrane-bound. Acarbose, miglitol, and voglibose are the ingredients that make up the inhibitors of this enzyme, which can delay and lower postprandial blood glucose levels.[Citation95] G. pentaphyllum inhibited α-glucosidase in a dose-dependent manner. G. pentaphyllum showed a higher inhibition of α-glucosidase in the in vitro assay of inhibition activity. G. pentaphyllum may be able to encapsulate the substrate, enzyme, or both, improving its capacity to block α-glucosidase.[Citation96]
Influence of gypenosides upon pro-inflammatory mediator mRNA expression in LPS-stimulated RAW264.7 macrophage cells
Inhibiting the mRNA transcripts of major proinflammatory mediators such as IL-6, IL-1, COX-2, and TNF-α may assist in lessening inflammatory responses. These mediators are involved in several inflammatory responses. These mediators are involved in several inflammatory pathways. The gypenosides inhibit the transcription of COX-2, IL-6, and IL-1 mRNA. shows that a larger gypenoside therapy dosage was linked with a positive suppressive impact on cytokine mRNA expression.[Citation98]
Figure 6. The mechanism through which gypenosides reduce inflammatory reactions.
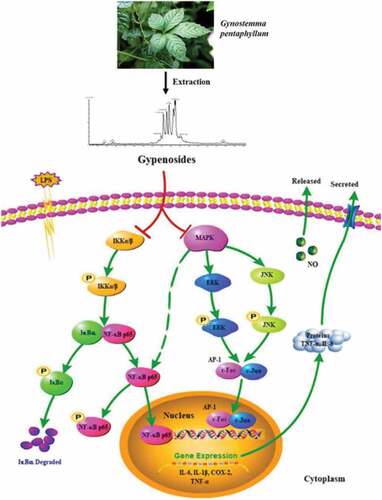
In LPS-stimulated RAW264.7 macrophage cells, the influence of gypenosides on the release of pro-inflammatory mediators
The released protein levels of IL-6 and TNF-α were evaluated in the media of LPS-stimulated RAW264.7 macrophage cells to explore the anti-inflammatory action of gypenosides. After LPS stimulation, both IL-6 and TNF-α protein levels were considerably elevated (p.01), and gypenoside administration reduced IL-6 and TNF-α release in the growth media. A substantial inhibiting effect on IL-6 was observed at gypenoside concentrations of 150 μg/ml (p <.05) and 200 μg/ml (p <.01), whereas a substantial suppression for TNF- was reported in the 100–200 μg/ml (p <.01) concentration levels.[Citation97] Modifications in IL-6 and TNF-protein levels were associated with variations in their mRNA expression levels. In LPS-stimulated RAW264.7 macrophage cells, gypenosides were shown to inhibit IL-6 and TNF-α release as well as NO generation.[Citation99]
In RAW264.7 macrophage cells, gypenosides inhibited LPS-stimulated NFκB induction
NF-κB is a critical transcription component engaged in the control of inflammatory mediators, according to prior research.[Citation100,Citation101] NF-κB ordinarily occurs as a dormant compound in the cytoplasm of unstimulated cells, consisting of the p65 subunits linked to suppressive proteins of the IκBα family. The IκBα kinase complex (IKK), a key upstream kinase for phosphorylation of IκBα and subsequent IκBα degradation in macrophages, is triggered whenever cells are stimulated by LPS. This route permits unattached NF-κB p65 to translocate into the nucleus, where it triggers the production of subsequent proinflammatory cytokines.[Citation102] Soy saponins were shown to alleviate inflammation by decreasing NF-κB activation in macrophages in previous research by Zha et al.[Citation103] As a result, gypenosides affect the NF-κB cascade in LPS-stimulated RAW264.7 macrophage cells to have anti-inflammatory action.[Citation97]
Antioxidant activity
Antioxidant activity may be found in a broad spectrum of bioactive elements of plants, fungi, & mammals, particularly polysaccharides.[Citation104] Based on multiple test methodologies and activity indicators, antioxidant properties have been the subject of considerable study. Because traditional Chinese medicines are nutraceutical and therapeutic in nature.[Citation91]
Several studies have demonstrated that G. pentaphyllum polysaccharides possess antioxidant potential in vitro and in vivo. GPA1, GPA2, and GPA3 extracted from G. pentaphyllum utilizing a mixture of water extraction & gel permeation chromatography were recently shown to have antioxidant activity.[Citation105] In vitro, GPA3 displayed a higher scavenging performance of 2,2- diphenyl-1-picrylhydrazyl (DPPH) and hydroxyl radicals, a higher ferrous ion chelating activity, and a higher reducing power than GPA1 and GPA2. G. pentaphyllum three components of polysaccharides, GMA, GMB, and GMC, were purified and tested for antioxidant activity.[Citation106] The findings showed that GMC had a substantial superoxide radical scavenging effect and prevented 1,2,3-phentriol self-oxidation, which might be linked to the physiochemical and monosaccharide makeup of these polysaccharides. depicts the mode of action of polysaccharide antioxidant properties.
Figure 7. Mode of action of polysaccharides from Gynostemma pentaphyllum’s antioxidant and immunomodulatory capabilities.
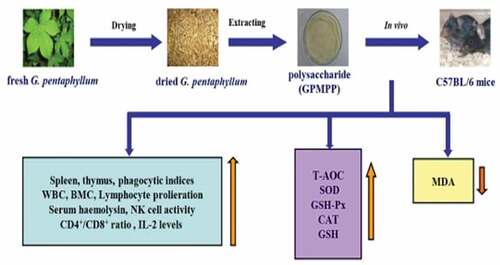
Immunomodulatory activity
Organic polysaccharides that operate as immunotherapies and/or biologically responsive modifiers are thought to have a significant functional role in immunomodulation.[Citation108] G. pentaphyllum polysaccharides are disclosed to increase cell-mediated immunity, humoral immunity, and nonspecific immunity in investigations. As illustrated in , the immunostimulatory actions of G. pentaphyllum polysaccharide adjuvant (GPMPP) were previously examined in rats by Shang et al.[Citation107] GPMPP significantly enhanced splenic and thymic indices, activated macrophages and NK cells, and had a dose-dependent effect on normal and Con A/LPS-stimulated splenocytes in C57BL/6 mice, according to their findings. GPMPP enhanced CD4 + T lymphocyte counts and the CD4+/CD8+ ratio in Cy-immunosuppressed mice’ serum and spleen in a dose-dependent manner, as well as IL-2 concentrations. Additionally, GPMPP increased SOD, GSH-Px, T-AOC, GSH, and CAT levels while decreasing MDA levels. The findings suggested that GPMPP may play a significant role in immune system oxidative injury avoidance and that GPMPP had immunomodulatory action in vivo.[Citation109]
Hepatoprotective activity
Oxidative stress and fatty degeneration are linked to NAFLD. Hepatic oxidative injury can lead to lipid peroxidation, which can lead to liver cell death. As a result of oxidative stress, the liver’s internal antioxidant system, such as antioxidant enzymes, protect it. Exogenous antioxidants, like certain vitamins and nutritional supplements, also can help to clear up reactive oxygen and nitrogen species (ROS/RNS) and prevent oxidative damage.[Citation110–113] In rat studies, prior investigations have established the preventive benefits of GP for NAFLD.[Citation114] Mice models, on the other hand, may more precisely mimic the histology and pathophysiological characteristics of human NAFLD, as per current research by Ganz et al. .[Citation115]
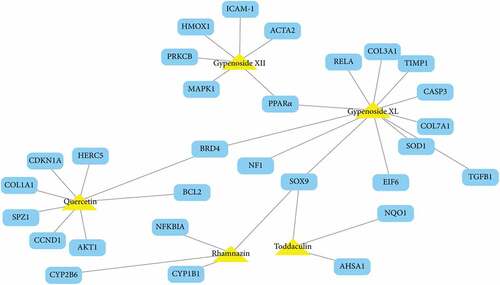
An in-silico technique was used to estimate the probable active components of G. pentaphyllum as well as their active processes. The link between GP-derived compounds and NAFLD-associated targeted genes or proteins was shown in the drug-target network (). The findings showed that six possible active components in G. pentaphyllum had favorable pharmacokinetic characteristics and may interact with several intracellular targets, which could be linked to NAFLD. Gypenoside XL, a water-soluble fraction of G. pentaphyllum, had the most NAFLD-related targets (10), followed by quercetin (8 connections) and Gypenoside XII (6 connections). For the 29 NAFLD-related targets, the network findings revealed that peroxisome proliferator-activated receptor alpha (PPARα) had the most (3) compound linkages (campesterol, Gypenoside XII, and Gypenoside XL), followed by BRD4 (Gypenoside XL and quercetin) and SOX9 (Gypenoside XL and Rhamnazin). The remaining 26 targets had only one chemical interaction. Gypenoside XL and PPARα, BRD4, and SOX9, for example, are high-degree nodes with many linkages in the network that may create various biological impacts and perform a potentially essential role in treating NAFLD. The STITCH, TTD, PharmGKB, and HIT databases were used to compile all of the data.
Figure 8. (a). NALFD is part of a compound-target network. The yellow triangles indicate active G. pentaphyllum compounds, whereas the blue rectangles reflect probable NAFLD target genes, with the gray line representing the compound-target relationship. Source: Shang et al.[Citation107] (b). The six possible anti-NAFLD compounds from G. pentaphyllum and their matching chemical compositions.
![Figure 8. (a). NALFD is part of a compound-target network. The yellow triangles indicate active G. pentaphyllum compounds, whereas the blue rectangles reflect probable NAFLD target genes, with the gray line representing the compound-target relationship. Source: Shang et al.[Citation107] (b). The six possible anti-NAFLD compounds from G. pentaphyllum and their matching chemical compositions.](/cms/asset/e19ca7f2-11fe-4596-aa90-481cdbe81c4e/ljfp_a_2185566_f0008b_oc.jpg)
Neuroprotective activity
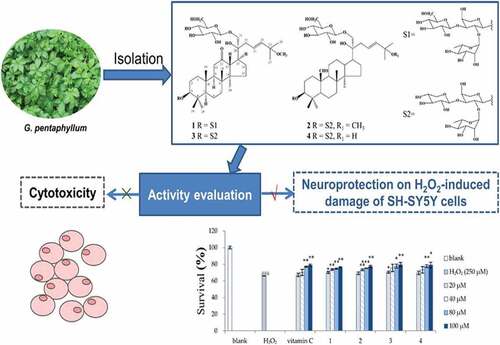
In a study, active G. pentaphyllum substances that prevent SH-SY5Y cells from hydrogen peroxide-induced cell death were extracted and discovered. Employing different chromatographic techniques, four novel dammarane-type saponins were recovered from G. pentaphyllum. HRESIMS and NMR spectra identified them as gypenoside S1 (1), gypenoside S3 (2), gypenoside S2 (3), and gypenoside S4 (4), respectively. The MTT technique was used to test their cytotoxic activity against three human cancer cell lines: A549 (lung), HepG2 (liver), and SH-SY5Y (nerve). On three cancer cell lines, they demonstrated modest cytotoxicity with IC50 values of greater than 100 μM.
Moreover, they seemed to have dose-dependent protective effects against hydrogen peroxide-induced SH-SY5Y cell death. They increased cell survival from 66% to more than 69% at a concentration of 20 μM, whereas vitamin C increased it to 67%. shows that compounds 3 and 4 restored more than 79% at 100 μM. According to this investigation, G. pentaphyllum possesses antioxidative potential, and G. pentaphyllum saponins are regarded as active chemicals with a nontoxic and neuroprotective impact.[Citation116]
Figure 9. The neuro-protective influence of Gynostemma pentaphyllum is depicted graphically.
Anti-inflammatory actions
Inflammation in the body has been linked to the chronic and metabolic syndrome of diabetes .[Citation117,Citation118] To determine whether or not G. pentaphyllum had possible anti-inflammatory properties in hypoglycemia, the levels of four key cytokines (IL-4, IL-6, IL-10, and TNF-α) were examined. These four inflammation markers virtually reached average levels after receiving 0.5 mL of 800 g/mL polysaccharides of G. pentaphyllum once daily for 4 weeks.[Citation119] According to this finding, G. pentaphyllum may have anti-inflammatory benefits for diabetic mice. When several studies looked at the connection between anti-inflammatory drugs and diabetes, they found identical outcomes. According to Liu et al.,[Citation120] Pleurotus citrinopileatus polysaccharide can reduce hepatotoxicity by raising IL-10 levels while lowering CYP2E1, TNF-, α, and IL-6 levels. Future research will focus on G. pentaphyllum underlying anti-inflammatory function to enhance its antidiabetic properties.
Conclusion
Gynostemma pentaphyllum contains immense therapeutic potential. It has attracted the attention of numerous researchers linked to herbal medicine, so its biology, chemistry and toxicology have been updated in the available literature. In many scientific studies conducted on animals as well as on humans, G. pentaphyllum extracts were shown to manage lipid profiles. In addition, this herb has demonstrated pharmacological potential against cardiovascular system and immune systems. However, the exact mechanism of action is still unknown and can only be elucidated by isolating the gypenosides of this herb and studying them at the molecular level. Its potential for activation or antagonism of specific nuclear receptors regulating the homeostasis of different metabolic pathways can only be determined by understanding the aglycone and sugar sequence of the gypenosides found in this herb. Moreover, the potential applications of this herb in clinical settings against hyperlipidemia, cancer, diabetes and other lifestyle-related maladies can be best understood by thoroughly investigating its pharmacological effects along with its safety aspects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Blumert, M.; Liu, J. L. Jiaogulan China’s “Immortality” Herb. In Torchlight Publishing Inc. Badger: USA, April 1999.
- Guo, W. Y.; Wang, W. X. Cultivation and utilisation of Gynostemma pentaphyllum. Publishing House of Electronics Science and Technology University: China, 1993; 1–261.
- China Pharmaceutical University. Yan, Y.-Q., ed. Encyclopedia of Chinese Herbs Vol. 2 1st 1878–1882. China Medicine, Science and Technology Publisher: China. 1996.
- Cheng, J. G. Investigation of the Plant Jiaogulan and Its Analogous Herb, Wulianmei. Zhong Cao Yao. 1990, 21(9), 424.
- Razmovski-Naumovski, V.; Huang, T. H. W.; Tran, V. H.; Li, G. Q.; Duke, C. C.; Roufogalis, B. D. Chemistry and Pharmacology of. Gynostemma Pentaphyllum. Phytochem. Rev. 2005, 4(2), 197–219. DOI: 10.1007/s11101-005-3754-4.
- SZ, L. Compendium of materia medica [M]. Shanxi, China: Shanxi Science and Technology Publishers. 2014, 327, 1–327.
- Wu, Y. G., ed. Dictionary of Chinese Materia Medica. In Compendium of materia medica. Vol. 2. 1st. Shanghai Science and Technological Publishing House: Shanghai, China. 1998; 1088.
- Tanner, M. A.; Bu, X.; Steimle, J. A.; Myers, P. R. The Direct Release of Nitric Oxide by Gypenosides Derived from the Herb. Gynostemma Pentaphyllum. Nitric Oxide. 1999, 3(5), 359–365. DOI: 10.1006/niox.1999.0245.
- Bai, M. S.; Gao, J. M.; Fan, C.; Yang, S. X.; Zhang, G.; Zheng, C. D. Bioactive dammarane-type Triterpenoids Derived from the Acid Hydrolysate of Gynostemma Pentaphyllum Saponins. Food Chem. 2010, 119(1), 306–310. DOI: 10.1016/j.foodchem.2009.06.033.
- Zhou, H. P. The Saponin Constituents and Pharmacology of Gynostemma Pentaphyllum. Chin. Pharm. Bull. 1988, 23, 720–724.
- Le, D. D.; Kim, W.; Lim, S.; Kim, S. C.; Choi, G. Identification of Three Groups of Ginsenoside Biosynthetic UDP-glycosyltransferases from Gynostemma Pentaphyllum. Plant Sci. 2021, 313, 111069. DOI: 10.1016/j.plantsci.2021.111069.
- Michael, B.; Jialiu, L. Jiaogulan: China’s “Immortality” Herb; Torchlight Publishing: Badger CA, 2003; pp 66–70.
- Wang, Q. Y.; Guo, Q. S.; Sun, J. Y.; Zhang, S. D.; Li, Y. H. Studies on Sex Identification and Variation of Endogenous Hormones in Female and Male Plants of Gynostemma Pentaphyllum. China J. Chinese Materia Medica. 2004, 29(9), 837–840.
- Huang, C.; Wu, Z.; Yao, Y.; Xu, X. Photosynthetic Characteristics of Gynostemma Pentaphyllum under Shade. Ying Yong Sheng Tai Xue Bao= J. Appl. Ecol. 2004, 15(11), 2099–2103.
- Razmovski-Naumovski, V.; Li, G. Q.; Duke, C. C. Gynostemma Pentaphyllum Cultivation in Sydney, Australia and Its Comparison with Products from China. Journal of Applied Horticulture. 2005, 7(2), 99–104. DOI: 10.37855/jah.2005.v07i02.25.
- Chen, Z.; Zhao, Y.; Ma, X.; Lu, R.; Song, J. Introduction and Cultivation of Gynostemma Pentaphyllum Mark. in Beijing. Zhongguo Zhong Yao Za Zhi. 1991, 16(4), 208–254.
- Zhao, Z.; Guo, Y.; Wei, H.; Ran, Q.; Gu, W. Predictions of the Potential Geographical Distribution and Quality of a Gynostemma Pentaphyllum Base on the Fuzzy Matter Element Model in China. Sustainability. 2017, 9(7), 1114. DOI: 10.3390/su9071114.
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer Effects of Gynostemma Pentaphyllum (Thunb.) Makino (Jiaogulan). Chin.Med. 2016, 11(1), 1–16. DOI: 10.1186/s13020-016-0114-9.
- Chang, C. K.; Chang, K. S.; Lin, Y. C.; Liu, S. Y.; Chen, C. Y. Hairy Root Cultures of Gynostemma Pentaphyllum (Thunb.) Makino: A Promising Approach for the Production of Gypenosides as an Alternative of Ginseng Saponins. Biotechnol. Lett. 2005, 27(16), 1165–1169. DOI: 10.1007/s10529-005-8653-7.
- Beeran, A. A.; Rao, C. M.; Rao, C. M.; Udupa, N. The Enriched Fraction of Elephantopus Scaber Triggers Apoptosis and Inhibits multi-drug Resistance Transporters in Human Epithelial Cancer Cells. Pharmacogn. Mag. 2015, 11(42), 257–268. DOI: 10.4103/0973-1296.153077.
- Williamson, E. M.; Lorenc, A.; Booker, A.; Robinso, N. The Rise of Traditional Chinese Medicine and Its Materia Medica: A Comparison of the Frequency and Safety of Materials and Species Used in Europe and China. J. Ethnopharmacol. 2013, 149(2), 453–462. DOI: 10.1016/j.jep.2013.06.050.
- Lu, C. Y.; Gu, W.; Dai, A. H.; Wei, H. Y. Assessing Habitat Suitability Based on Geographic Information System (GIS) and Fuzzy: A Case Study of Schisandra Sphenanthera Rehd. Et Wils. in Qinling Mountains, China. Ecol. Model. 2012, 242, 105–115. DOI: 10.1016/j.ecolmodel.2012.06.002.
- Guo, Y. L.; Wei, H. Y.; Lu, C. Y.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ. 2016, 4, e2554. DOI: 10.7717/peerj.2554.
- Chávez-Dulanto, P. N.; Thiry, A. A.; Glorio-Paulet, P.; Vögler, O.; Carvalho, F. P. Increasing the Impact of Science and Technology to Provide More People with Healthier and Safer Food. Food Energy Secur. 2021, 10(1), e259. DOI: 10.1002/fes3.259.
- Lentini, P. E.; Wintle, B. A. Spatial Conservation Priorities are Highly Sensitive to Choice of Biodiversity Surrogates and Species Distribution Model Type. Ecography. 2015, 38(11), 1101–1111. DOI: 10.1111/ecog.01252.
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals. 2020, 13(10), 313. DOI: 10.3390/ph13100313.
- Committee of Flora of China. Angiospermae. Flora of China, 1986, 73, 265–277.
- Cui, J. F.; Eneroth, P.; Bruhn, J. G. Gynostemma Pentaphyllum: Identification of Major Sapogenins and Differentiation from Panax Species. Eur. J. Pharm. Sci. 1999, 8(3), 187–191. DOI: 10.1016/S0928-0987(99)00013-5.
- Le, Y. Study on Saponin Content in Gynostemma Pentaphyllum. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2010. (In Chinese)
- Mishra, R. N.; Joshi, D. Jiao Gu Lan (Gynostemma Pentaphyllum): The Chinese rasayan-current Research Scenario. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1483–1502.
- Bensky, D.; Gamble, A.; Clavey, S.; Stöger, E. September Chinese Herbal Medicine: Materia Medica. 3rd. Eastland Press, 2004.
- Wong, W. Y.; Lee, M. M.; Chan, B. D.; Ma, V. W.; Zhang, W.; Yip, T. T.; Wong, W. T.; Tai, W. C. Gynostemma Pentaphyllum Saponins Attenuate Inflammation in Vitro and in Vivo by Inhibition of NF-κB and STAT3 Signaling. Oncotarget. 2017, 8(50), 87401–87414. DOI: 10.18632/oncotarget.20997.
- Li, K.; Ma, C.; Li, H.; Dev, S.; He, J.; Medicinal Value, Q. X. Medicinal Value and Potential Therapeutic Mechanisms of Gynostemma Pentaphyllum (Thunb.) Makino and Its Derivatives: An Overview. Curr. Top. Med. Chem. 2019, 19(31), 2855–2867. DOI: 10.2174/1568026619666191114104718.
- Hong, M.; Cai, Z.; Song, L.; Liu, Y.; Wang, Q.; Feng, X. Gynostemma Pentaphyllum Attenuates the Progression of Nonalcoholic Fatty Liver Disease in Mice: A Biomedical Investigation Integrated with in Silico Assay. Evid. Based Complement. Altern. Med. 2018, 2018, 8384631. DOI: 10.1155/2018/8384631.
- Liu, H.; Li, X.; Duan, Y.; Xie, J. B.; Piao, X. L. Mechanism of Gypenosides of Gynostemma Pentaphyllum Inducing Apoptosis of Renal Cell Carcinoma by PI3K/AKT/mTOR Pathway. JEthnopharmacol. 2021, 271, 113907. DOI: 10.1016/j.jep.2021.113907.
- Takemoto, T. Gynosaponins Extraction from Gynostemma Pentaphyllum. Nippon Shoji Co., Ltd. Assignee. Jpn Kokai Tokkyo Koho JP. 1984, 59(80,696), 84.
- Takemoto, T. Nippon Shoji Co. Ltd. Gynosaponins G, K, and progynosaponin A2 extraction from Gynostemma pentaphyllum. Japan Kokai Tokkyo Koho, JKXXAF JP, Japan, 59080697, A2. 1984.
- Kuwabara, M.; Kawanishi, F.; Komiya, T.; Oshio, H. Dammarane Saponins of Gynostemma Pentaphyllum Makino and Isolation of malonylginsenosides-Rb1, -rd, and Malonylgypenoside V. Chem. Pharm. Bull. 1989, 37(1), 135–139. DOI: 10.1248/cpb.37.135.
- Takemoto, T.; Odashima, T. Antitumor Saponins from Gynostemma Pentaphyllum. Rhoto Pharmaceuticals Ltd. Japan Kokai Tokkyo Koho. JKXXAF JP. 1983, 58059921, A2.
- Takemoto, T.; Arichi, S.; Arihara, S.; Nakajima, T.; Okuhira, M.; Uchida, Y. Gynosaponins and Drug Preparations Containing These Compounds. Ger. Offen. 1981, DE 80-3042117 19801107. CAN 96: 24789. AN 1982: 24789.
- Takemoto, T.; Arichi, S.; Arihara, S.; Nakajima, T.; Okuhira, M.; Yoshihiro, U. Gynosaponins, Their Use and a Process for Preparing the Same. 1982, Patent 4339422, SN: 205377, INTL: A01N 031/00, US: 424/182.
- Takemoto, T.; Arihara, S.; Yoshikawa, K. Studies on the Constituents of Cucurbitaceae Plants. XIV. On the Saponin Constituents of Gynostemma Pentaphyllum Makino. Yakugaku Zasshi. 1986, 106(8), 664–670. DOI: 10.1248/yakushi1947.106.8_664.
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Gynostemma Pentaphyllum MAKINO. I. Structures of Gypenoside I-XIV. Yakugaku Zasshi. 1983, 103(2), 173–185. DOI: 10.1248/yakushi1947.103.2_173.
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Gynostemma Pentaphyllum MAKINO. II. Structures of Gypenoside XV–XXI. Yakugaku Zasshi. 1983, 103(10), 1015–1023. DOI: 10.1248/yakushi1947.103.10_1015.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Hino, K.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. XII. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (8). Yakugaku Zasshi. 1984f, 104, 1115–1162.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Kawasaki, J.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. XI. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (7). Yakugaku Zasshi. 1984e, 104(10), 1043–1049. DOI: 10.1248/yakushi1947.104.10_1043.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. VIII. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (4). Yakugaku Zasshi. 1984a, 104(4), 332–339. DOI: 10.1248/yakushi1947.104.4_332.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. IX. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (5). Yakugaku Zasshi. 1984b, 104(7), 724–730. DOI: 10.1248/yakushi1947.104.7_724.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. VII. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (3). Yakugaku Zasshi. 1984c, 104(4), 325–331. DOI: 10.1248/yakushi1947.104.4_325.
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Nakajima, T.; Okuhira, M. Studies on the Constituents of Cucurbitaceae Plants. X. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (6). Yakugaku Zasshi. 1984d, 104(9), 939–945. DOI: 10.1248/yakushi1947.104.9_939.
- Guo, S.; Wang, Q.; Wang, G.; Ma, Y. Advances in the Researchs on Fiveleaf Gynostemma (Gynostemma Pentaphyllum). Zhongcaoyao. 1987, 18(7), 325–328.
- Yoshikawa, K.; Takemoto, T.; Arihara, S. Studies on the Constituents of Cucurbitaceae Plants. XV. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (10). Yakugaku Zasshi. 1986, 106(9), 758–763. DOI: 10.1248/yakushi1947.106.9_758.
- Yoshikawa, K.; Mitake, M.; Takemoto, T.; Arihara, S. Studies on the Constituents of Cucurbitaceae Plants. XVII. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (12). Yakugaku Zasshi. 1987a, 107(5), 355–360. DOI: 10.1248/yakushi1947.107.5_355.
- Yoshikawa, K.; Takemoto, T.; Arihara, S. Studies on the Constituents of Cucurbitaceae Plants. XVI. On the Saponin Constituents of Gynostemma Pentaphyllum Makino. (II). Yakugaku Zasshi. 1987, 107(4), 262–267. DOI: 10.1248/yakushi1947.107.4_262.
- Yoshikawa, K.; Arimitsu, M.; Kuki, K.; Takemoto, T.; Arihara, S. Studies on the Constituents of Cucurbitaceae Plants. XVIII. On the Saponin Constituents of Gynostemma Pentaphyllum MAKINO. (13). Yakugaku Zasshi. 1987c, 107(5), 361–366. DOI: 10.1248/yakushi1947.107.5_361.
- Nagai, M.; Izawa, K.; Nagumo, S.; Sakurai, N.; Inoue, T. Two Glycosides of a Novel Dammarane Alcohol from Gynostemma Pentaphyllum. Chemical and Pharmaceutical Bulletin. 1981, 29(3), 779–783. DOI: 10.1248/cpb.29.779.
- Huang, T. H.-W.; Razmovski-Naumovski, V.; Salam, N. K.; Duke, R. K.; Tran, V. H.; Duke, C. C.; Roufogalis, B. D. A Novel LXR-A Activator Identified from the Natural Product Gynostemma Pentaphyllum. Biochem. Pharmacol. 2005, 70(9), 1298–1308.
- Fang, Z. P.; Zeng, X. Y. Structure of Gypentonoside A from Gynostemma Pentaphyllum Makino. Acta Pharmaceut. Sin. 1996, 31, 680–683.
- Hu, L. H.; Chen, Z. L.; Xie, Y. Y. New Triterpenoid Saponins from Gynostemma Pentaphyllum. J. Nat. Prod. 1996, 59(12), 1143–1145. DOI: 10.1021/np960445u.
- Hu, L. H.; Chen, Z. L.; Xie, Y. Y. Dammarane-type Glycosides from Gynostemma Pentaphyllum. Phytochemistry. 1997, 44(4), 667–670. DOI: 10.1016/S0031-9422(96)00577-8.
- Liu, X.; Ye, W. C.; Hsaio, H. W. W.; Che, C. T.; Zhao, S. X. Studies on Chemical Constituents of Gynostemma Pentaphyllum. Zhongguo Yaoke Daxue Xuebao. 2003, 34, 21–24.
- Yin, F.; Hu, L.; Lou, F.; Pan, R. Dammarane-type Glycosides from Gynostemma Pentaphyllum. J. Nat. Prod. 2004b, 67(6), 942–952. DOI: 10.1021/np0499012.
- Yin, F.; Hu, L.; Pan, R. Novel dammarane-type Glycosides from Gynostemma Pentaphyllum. Chem. Pharmaceut. Bull. 2004a, 52(12), 1440–1444. DOI: 10.1248/cpb.52.1440.
- Piacente, S.; Pizza, C.; De Tommasi, N.; De Simone, F. New dammarane-type Glycosides from Gynostemma Pentaphyllum. J. Nat. Prod. 1995, 58(4), 512–519. DOI: 10.1021/np50118a005.
- Qin, Z.; Zhao, L.; Bi, S.; You, L. Saponin Constituents and Resource of Gynostemma Pentaphyllum. Tianran Chanwu Yanjiu Yu Kaifa. 1992, 4(1), 83–98.
- Ma, Y. C.; Zhu, J.; Benkrima, L.; Luo, M.; Sun, L. H.; Sain, S.; Kont, K.; Plaut-Carcasson, Y. J. A Comparative Evaluation of Ginsenosides in Commercial Ginseng Products and Tissue Culture Samples Using HPLC. J. Herbs, Spices, Med. Plants. 1995, 3(4), 41–50. DOI: 10.1300/J044v03n04_06.
- Chen, S.; Jeffrey, C. Gynostemma pentaphyllum (Thunberg) Makino, Bot. Mag. (Tokyo). 16: 179. 1902. In Flora of China. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria: Cambridge, United States. Retrieved 2 May 2018.
- Ding, S.; Zhu, Z. Resources of Genus Gynostemma and Determination of Their Total Saponins Contents. Zhongcaoyao. 1992, 23(12), 627–629.
- Mackay, M.; Wei, J. X.; Chen, Y. G. Structure of a New dammarane-type Triterpene from Gynostemma Pentaphyllum (Thurb.) Makino. Acta Crystallogr. Sec. C: Crystal Struct. Commun. 1991, C47(4), 790–793. DOI: 10.1107/S0108270190008290.
- Norberg, A.; Hoa, N. K.; Liepinsh, E.; Van Phan, D.; Thuan, N. D.; Jornvall, H.; Sillard, R.; Ostenson, C. G. A Novel Insulin-releasing Substance, Phanoside, from the Plant Gynostemma Pentaphyllum. Journal of Biological Chemistry. 2004, 279(40), 41361–41367. DOI: 10.1074/jbc.M403435200.
- Liu, X.; Ye, W.; Mo, Z.; Yu, B.; Zhao, S.; Wu, H.; Che, C.; Jiang, R.; Mak, T. C.; Hsiao, W. W. Five New ocotillone-type Saponins from Gynostemma P Entaphyllum. J. Nat. Prod. 2004, 67(7), 1147–1151.
- Razmovski-Naumovski, V.; Duke, R. K.; Turner, P.; Duke, C. C. (20S)-2α, 3β, 12β-Trihydroxydammar-24-ene 20-O-β-d-glucopyranoside (Gynosaponin TN1) as the 2.5-methanol Solvate. Acta Crystallographica Section E: Structure Reports Online. 2005, 61(5), o1239–o1241. DOI: 10.1107/S1600536805009773.
- Yan, H.; Wang, X.; Niu, J.; Wang, Y.; Wang, P.; Liu, Q.; Aykin-Burns, N. Anti-cancer Effect and the Underlying Mechanisms of Gypenosides on Human Colorectal Cancer SW-480 Cells. PloS one. 2014, 9(4), e95609. DOI: 10.1371/journal.pone.0095609.
- Arbain, D.; Cannon, J. R.; Afriastini Kartawinata, K.; Djamal, R.; Bustari, A.; Dharma, A.; Rosmawaty Rivai, H.; Zaherman Basir, D.; Sjafar, M.; Sjaiful Nawfa, R., et al. Survey of Some West Sumatran Plants for Alkaloids. Econ. Bot. 1989, 43(1), 73–78. DOI: 10.1007/BF02859327.
- Chiranthanut, N.; Teekachunhatean, S.; Panthong, A.; Khonsung, P.; Kanjanapothi, D.; Lertprasertsuk, N. Toxicity Evaluation of Standardized Extract of Gynostemma Pentaphyllum Makino. J. Ethnopharmacol. 2013, 149(1), 228–234. DOI: 10.1016/j.jep.2013.06.027.
- Wang, J.-R.; Zhao, J.-B. The Effect of Preventing Recurrence of Cancer Metastasis on Jiaogulan Soup in Clinical Study. Zhejiang Zhong Yi Za Zhii. 1993, 28, 529–530.
- Zhou, K.; Bai, S. The Quality of Randomized Parallel Controlled Study of Chemotherapy in Advanced Gastric Cancer and Improve Survival of Yiqi Jianpi Qingre Huoxue Method. Shiyong Zhongyi Neike Zazhi. 2015, 29, 42–44.
- Lu, K. W.; Chen, J. C.; Lai, T. Y.; Yang, J. S.; Weng, S. W.; Ma, Y. S.; Lin, H. Y.; Wu, R. S.; Wu, K. C.; Wood, W. G., et al. Gypenosides Suppress Growth of Human Oral Cancer SAS Cells in Vitro and in a Murine Xenograft Model: The Role of Apoptosis Mediated by caspase-dependent and caspase-independent Pathways. Integr. Cancer Ther. 2012, 11(2), 129–140. DOI: 10.1177/1534735411403306.
- Hsu, H. Y.; Yang, J. S.; Lu, K. W.; Yu, C. S.; Chou, S. T.; Lin, J. J.; Chen, Y. Y.; Lin, M. L.; Chueh, F. S.; Chen, S. S., et al. An Experimental Study on the Antileukemia Effects of Gypenosides in Vitro and in Vivo. Integr. Cancer Ther. 2011, 10(1), 101–112. DOI: 10.1177/1534735410377198.
- Chen, J.-C.; Lu, K.-W.; Lee, J.-H.; Yeh, -C.-C.; Chung, J.-G. Gypenosides Induced Apoptosis in Human Colon Cancer Cells through the Mitochondriadependent Pathways and Activation of Caspase-3. Anticancer Res. 2006, 26(6B), 4313–26.106.
- Chen, J.-C.; Lu, K.-W.; Tsai, M.-L.; Hsu, S.-C.; Kuo, C.-L.; Yang, J.-S.; Hsia, T.-C.; Yu, C.-S.; Chou, S.-T.; Kao, M.-C. Gypenosides Induced G0/G1 Arrest via CHk2 and Apoptosis through Endoplasmic Reticulum Stress and Mitochondriadependent Pathways in Human Tongue Cancer SCC-4 Cells. Oral Oncol. 2009, 45(3), 273–283. DOI: 10.1016/j.oraloncology.2008.05.012.
- Cheng, T. C.; Lu, J. F.; Wang, J. S.; Lin, L. J.; Kuo, H. I.; Chen, B. H. Antiproliferation Effect and Apoptosis Mechanism of Prostate Cancer Cell PC-3 by Flavonoids and Saponins Prepared from Gynostemma Pentaphyllum. J. Agric. Food Chem. 2011, 59(20), 11319–11329. DOI: 10.1021/jf2018758.
- Wang, Q. F.; Chiang, C. W.; Wu, C. C.; Cheng, C. C.; Hsieh, S. J.; Chen, J. C.; Hsieh, Y. C.; Hsu, S. L. Gypenosides Induce Apoptosis in Human Hepatoma Huh-7 Cells through a calcium/reactive Oxygen species-dependent Mitochondrial Pathway. Planta Med. 2007, 73(6), 535–544. DOI: 10.1055/s-2007-967200.
- Yuan, G.; Wei, J.; Zhou, J.; Guo, X.; Yang, M. Apoptosis of Human Hepatoma Cells Induced by Gynostemma Pentaphyllum Makino. Chin. German J. Clin. Oncol. 2006, 5(3), 173–7.113. DOI: 10.1007/s10330-005-0436-z.
- Schild, L.; Chen, B. H.; Makarov, P.; Kattengell, K.; Heinitz, K.; Keilhoff, G. Selective Induction of Apoptosis in Glioma Tumour Cells by a Gynostemma Pentaphyllum Extract. Phytomedicine. 2010, 17(8–9), 589–597. DOI: 10.1016/j.phymed.2009.12.002.
- Lu, H. F.; Chen, Y. S.; Yang, J. S.; Chen, J. C.; Lu, K. W.; Chiu, T. H.; Liu, K. C.; Yeh, C. C.; Chen, G. W.; Lin, H. J., et al. Gypenosides Induced G0/G1 Arrest via Inhibition of Cyclin E and Induction of Apoptosis via Activation of Caspases-3 and −9 in Human Lung Cancer A-549 Cells. Vivo. 2008, 22, 215–221.
- Lu, K. W.; Chen, J. C.; Lai, T. Y.; Yang, J. S.; Weng, S. W.; Ma, Y. S.; Lu, P. J.; Weng, J. R.; Chueh, F. S.; Wood, W. G., et al. Gypenosides Inhibits Migration and Invasion of Human Oral Cancer SAS Cells through the Inhibition of Matrix Metalloproteinase-2-9 and urokinase-plasminogen by ERK1/2 and NFκB Signaling Pathways. Human & Experimental Toxicology. 2011, 30(5), 406–415. DOI: 10.1177/0960327110372405.
- Yan, H.; Wang, X.; Wang, Y.; Wang, P.; Xiao, Y. Antiproliferation and Antimigration Induced by Gypenosides in Human Colon Cancer SW620 and Esophageal Cancer Eca-109 Cells. Hum. Exp. Toxicol. 2014, 33(5), 522–533. DOI: 10.1177/0960327113497771.
- Hanahan, D.; Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell. 2011, 144(5), 646–674. DOI: 10.1016/j.cell.2011.02.013.
- Hsiao, W.; Tai, W. C. S.; Mo, Z.; Wu, P. K. The Assessment of anti-cancer Activities and Saponin Profiles of Gynostemma Pentaphyllum Saponins Obtained from Different Regions of China. J. Biotechnol. 2008, 136, S22–3. DOI: 10.1016/j.jbiotec.2008.07.038.
- Yang, L.; Zhang, L. M. Chemical Structural and Chain Conformational Characterization of Some Bioactive Polysaccharides Isolated from Natural Sources. Carbohydr. Polym. 2009, 76(3), 349–361. DOI: 10.1016/j.carbpol.2008.12.015.
- Li, X. L.; Wang, Z. H.; Zhao, Y. X.; Luo, S. J.; Zhang, D. W.; Xiao, S. X.; Peng, Z. H. Isolation and Antitumor Activities of Acidic Polysaccharide from Gynostemma Pentaphyllum Makino. Carbohydrate Polymers. 2012, 89(3), 942–947. DOI: 10.1016/j.carbpol.2012.04.040.
- Jang, Y. J.; Kim, J. K.; Lee, M. S.; Ham, I. H.; Whang, W. K.; Kim, K. H.; Kim, H. J. Hypoglycemic and Hypolipidemic Effects of Crude Saponin Fractions from Panax Ginseng and Gynostemma Pentaphyllum. Yakhak Hoechi. 2001, 45(5), 545–556.
- Megalli, S.; Roufogalis, B. D. Cholesterol Lowering for Healthy Hearts: A Herbal Approach. Aust. J. Pharm. 2005, 86, 326–330.
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, Hypolipidemic and Antioxidant Effects of Sarcandra Glabra Polysaccharide in Type 2 Diabetic Mice. Food Funct. 2014, 5(11), 2850–2860. DOI: 10.1039/C4FO00430B.
- Wang, Z.; Yang, L.; Wu, J.; Zhang, H.; Zhu, L.; Zhan, X. Potential Application of a low-viscosity and high-transparency Xanthan Gum Produced from Xanthomonas Campestris CCTCC M2015714 in Foods. Preparative Biochemistry & Biotechnology. 2018, 48(5), 402–407. DOI: 10.1080/10826068.2018.1451884.
- Wang, B.; Li, M.; Gao, H.; Sun, X.; Gao, B.; Zhang, Y.; Yu, L. Chemical Composition of Tetraploid Gynostemma Pentaphyllum Gypenosides and Their Suppression on Inflammatory Response by NF-κB/MAPKs/AP-1 Signaling Pathways. Food Science & Nutrition. 2020, 8(2), 1197–1207. DOI: 10.1002/fsn3.1407.
- Ogawa, M.; Osada, H.; Hasegawa, A.; Ohno, H.; Yanuma, N.; Sasaki, K.; Ohmori, K.; Shirai, J.; Kondo, H.; Ohmori, K. Effect of interleukin-1β on Occludin mRNA Expression in the Duodenal and Colonic Mucosa of Dogs with Inflammatory Bowel Disease. J. Veterinary Internal Med. 2018, 32(3), 1019–1025. DOI: 10.1111/jvim.15117.
- Baek, K. S.; Yi, Y. S.; Son, Y. J.; Yoo, S.; Sung, N. Y.; Kim, Y.; Cho, J. Y.; Aravinthan, A.; Kim, J.-H.; Cho, J. Y. In Vitro and in Vivo anti-inflammatory Activities of Korean Red Ginsengderived Components. J. Ginseng Res. 2016, 40(4), 4–437. DOI: 10.1016/j.jgr.2016.08.003.
- Jeon, Y. J.; Kim, B. H.; Kim, S.; Oh, I.; Lee, S.; Shin, J.; Kim, T. Y. Rhododendrin Ameliorates Skin Inflammation through Inhibition of NFκB, MAPK, and PI3K/Akt Signaling. Eur. J. Pharmacol. 2013, 714(1–3), 7–14. DOI: 10.1016/j.ejphar.2013.05.041.
- Yang, Y. J.; Yi, L.; Wang, Q.; Xie, B. B.; Dong, Y.; Sha, C. W. Anti-inflammatory Effects of Physalin E from Physalis angulata on lipopolysaccharide-stimulated RAW264.7 Cells through Inhibition of NF-κB Pathway. Immuno. and Immunotoxicology. 2017, 39(2), 74–79. DOI: 10.1080/08923973.2017.1282514.
- Noort, A. R.; van Zoest, K. P. M.; Weijers, E. M.; Koolwijk, P.; Maracle, C. X.; Novack, D. V.; Tas, S. W.; Schlingemann, R. O.; Tak, P. P.; Tas, S. W. NF-κB -inducing Kinase Is a Key Regulator of inflammation-induced and tumour-associated Angiogenesis. The Journal of Pathology. 2014, 234(3), 375–385. DOI: 10.1002/path.4403.
- Zha, L. Y.; Chen, J. D.; Sun, S. X.; Mao, L. M.; Chu, X. W.; Deng, H.; Cao, W.; Li, X.; Liu, Z.; Cao, W. Soyasaponins Can Blunt Inflammation by Inhibiting the Reactive Oxygen species-mediated Activation of PI3K/AKT/NF-κB Pathway. PLoS ONE. 2014, 9(9), e107655. DOI: 10.1371/journal.pone.0107655.
- Zhang, J.; Xiao, X.; Dong, Y.; Shi, L.; Xu, T.; Wu, F. The anti-obesity Effect of Fermented Barley Extracts with Lactobacillus Plantarum Dy-1 and Saccharomyces Cerevisiae in diet-induced Obese Rats. Food Funct. 2017, 8(3), 1132–1143. DOI: 10.1039/C6FO01350C.
- Li, B.; Zhang, X.; Wang, M.; Jiao, L. Characterization and Antioxidant Activities of Acidic Polysaccharides from Gynostemma Pentaphyllum (Thunb.) Markino. Carbohydr. Polym. 2015, 127, 209–214. DOI: 10.1016/j.carbpol.2015.03.069.
- Wang, Z.; Luo, D. Antioxidant Activities of Different Fractions of Polysaccharide Purified from Gynostemma Pentaphyllum Makino. Carbohydr. Polym. 2007, 68(1), 54–58. DOI: 10.1016/j.carbpol.2006.07.022.
- Shang, X.; Chao, Y.; Zhang, Y.; Lu, C.; Xu, C.; Niu, W. Immunomodulatory and Antioxidant Effects of Polysaccharides from Gynostemma Pentaphyllum Makino in Immunosuppressed Mice. Molecules. 2016, 21(8), 1085. DOI: 10.3390/molecules21081085.
- Meng, X.; Liang, H.; Luo, L. Antitumor Polysaccharides from Mushrooms: A Review on the Structural Characteristics, Antitumor Mechanisms and Immunomodulating Activities. Carbohydr. Res. 2016, 424, 30–41. DOI: 10.1016/j.carres.2016.02.008.
- Yang, X. B.; Zhao, Y.; Yang, Y.; Ruan, Y. Isolation and Characterization of Immunostimulatory Polysaccharide from an Herb Tea, Gynostemma Pentaphyllum Makino. J. Agric. Food Chem. 2008, 56(16), 6905–6909. DOI: 10.1021/jf801101u.
- Zhai, X.; Chen, X.; Lu, J.; Zhang, Y.; Sun, X.; Huang, Q.; Wang, Q. Hydrogen-rich Saline Improves non-alcoholic Fatty Liver Disease by Alleviating Oxidative Stress and Activating Hepatic PPARα and PPARγ. Mol. Med. Rep. 2017, 15(3), 1305–1312. DOI: 10.3892/mmr.2017.6120.
- Huang, F.; Zhao, S.; Yu, F.; Yang, Z.; Ding, G. Protective Effects and Mechanism of Meretrix Meretrix Oligopeptides against Nonalcoholic Fatty Liver Disease. Mar. Drugs. 2017, 15(2), 31. DOI: 10.3390/md15020031.
- Leong, P. K.; Ko, K. M. A double-edged Sword in Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. DOI: 10.1155/2016/6171658.
- Hong, M.; Li, S.; Wang, N.; Tan, H. Y.; Cheung, F.; Feng, Y. A Biomedical Investigation of the Hepatoprotective Effect of Radix Salviae Miltiorrhizae and Network pharmacology-based Prediction of the Active Compounds and Molecular Targets. Int. J. Mol. Sci. 2017, 18(3), 620. DOI: 10.3390/ijms18030620.
- Wang, H. H.; Portincasa, P.; de Bari, O.; Liu, K. J.; Garruti, G.; Neuschwander-Tetri, B. A.; Wang, D. Q. H. Prevention of Cholesterol Gallstones by Inhibiting Hepatic Biosynthesis and Intestinal Absorption of Cholesterol. European Journal of Clinical Investigation. 2013, 43(4), 413–426. DOI: 10.1111/eci.12058.
- Ganz, M.; Bukong, T. N.; Csak, T.; Saha, B.; Park, J. K.; Ambade, A.; Szabo, G.; Szabo, G. Progression of non-alcoholic Steatosis to Steatohepatitis and Fibrosis Parallels Cumulative Accumulation of Danger Signals that Promote Inflammation and Liver Tumors in a High fat–cholesterol–sugar Diet Model in Mice. J. Transl. Med. 2015, 13(1), 1–14. DOI: 10.1186/s12967-015-0552-7.
- Zhai, X. F.; Zu, M. L.; Wang, Y. R.; Cui, W. Y.; Duan, Y.; Yang, C.; Piao, X. L. Protective Effects of Four New Saponins from Gynostemma Pentaphyllum against Hydrogen peroxide-induced Neurotoxicity in SH-SY5Y Cells. Bioorg. Chem. 2021, 106(January), 104470. DOI: 10.1016/j.bioorg.2020.104470.
- Merone, L.; McDermott, R. Nutritional Anti -inflammatories in the Treatment and Prevention of Type 2 Diabetes Mellitus and the Metabolic Syndrome. Diabetes Res. Clin. Pr. 2017, 127, 238–253. DOI: 10.1016/j.diabres.2017.02.019.
- Das, U. N. Vitamin C for Type 2 Diabetes Mellitus and Hypertension. Archives of Medical Research. 2019, 50(2), 11–14. DOI: 10.1016/j.arcmed.2019.05.004.
- Zichao, W.; Zhe, W.; Wenhui, H.; Jiming, S.; Xiaotian, C.; Kaiqiang, D.; Qi, S.; Huiru, Z. Antioxidant and anti-inflammatory Activities of an anti-diabetic Polysaccharide Extracted from Gynostemma Pentaphyllum Herb. Int. J. Biol. Macromol. 2020, 145, 484–491. 141-8130. DOI: 10.1016/j.ijbiomac.2019.12.213.
- Liu, X.; Pang, H.; Gao, Z.; Zhao, H.; Zhang, J.; Jia, L. Antioxidant and Hepatoprotective Activities of Residue Polysaccharides by Pleurotus Citrinipileatus. Int. J. Biol. Macromol. 2019, 131, 315–322. DOI: 10.1016/j.ijbiomac.2019.03.074.