ABSTRACT
Diabetic peripheral neuropathy is one of the most prevalent complications of diabetes mellitus. It is the most common type of neuropathy characterized by decreased sensory functions in the lower extremities and substantial neuropathic pain. Based on clinical characterization, it is classified into symmetric and asymmetric neuropathy. The pathological changes and neuronal function impairment during diabetes are associated with various pathways, including polyol pathway activation, advanced glycation end-products formation, oxidative stress, protein kinase C activation, poly ADP-ribose polymerase, and hexosamine pathways. Demyelination, axonal atrophy, nerve fibre loss, reduced regeneration, and loss of neurovascular interactions are hallmarks. Although some symptomatic and supportive therapies, such as tricyclic agents, antiarrhythmics, opioid analgesics, incretin, aldose reductase, and protein kinase C inhibitors, are in practice, the outcome is not promising. To fill this gap, natural product-based therapy can prove prodigious as an effective alternative. This review aims to comprehend the available literature on the role of various biological molecules in ameliorating diabetic peripheral neuropathy. These molecules play a key role in reducing oxidative-nitrosative stress, aldose reductase activity, and neuronal apoptosis. They control glucose and HbA1c% levels and improve nerve conduction velocity, axonal regeneration, and antioxidant species (catalase, superoxide dismutase, malondialdehyde). They are known for their attenuating thermal and mechanical hyperalgesia and tactile allodynia. Therefore, there is a need to evaluate these molecules at the pre-clinical and clinical levels for efficacy. Hence, natural molecules may act as promising players against diabetic peripheral neuropathy and are a ray of hope for suffering individuals.
Introduction
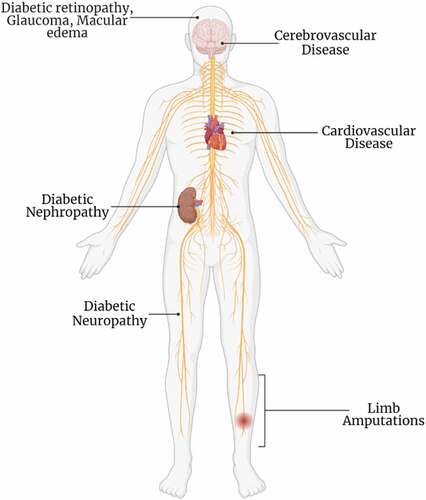
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia due to a lack of insulin secretion (type 1) or insulin receptors insensitivity (type 2). Both type 1 and type 2 diabetic patients present symptoms of polyuria, polydipsia, polyphagia, weight loss, slow-healing wounds, lack of energy, extreme tiredness, and blurred vision[Citation1].[Citation2] Prolonged hyperglycemia results in damage to the eyes, kidneys, and nerves, that increases the risk for the development of cardiovascular diseases (CVD). DM also causes blindness, cerebrovascular diseases, renal failure, and limb amputations.[Citation3,Citation4] A brief description of micro-and macro vascular complications is mentioned in .
According to International Diabetes Federation (IDF), 463 million adults (20–79 years) worldwide have diabetes, making it the largest global epidemic of the 21st century. Based on the 2019 estimation, this number will be moved to 578.4 million (10.2%) by 2030 and 700.2 million (10.9%) by 2045. 77 million people in India, 31 million in the United States, 19.4 million in Pakistan, and 116 million in China have been diagnosed with diabetes in 2019. These figures will be moved to 101 million in India, 34.4 million in the United States, 26.2 million in Pakistan, and 140 million in China by 2030.[Citation5]
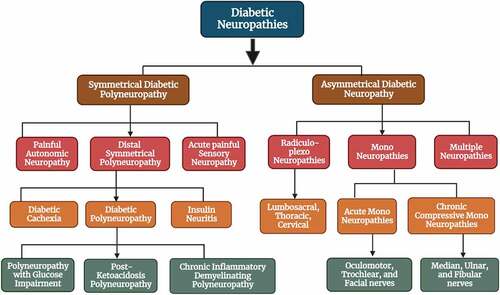
Diabetic peripheral neuropathy (DPN) is an extremely complex pathophysiological and heterogeneous group of disorders that damages both somatic and autonomic divisions of the nervous system. Despite the type of DM, DPN is the most common complication affecting the lifestyle of millions of people. DPN pain is defined as burning, jabbing, aching, sharp tingling, cramping, cold, or allodynia; usually worse at night. Acute DPN pain is sometimes linked with weight loss and depression and it is now termed “diabetic neuropathic cachexia.”[Citation6] Commonest type; distal symmetrical neuropathy accounts for 75% of DPN while asymmetrical neuropathies are of acute onset which arises from ischemic infarction of Vasa Nervosa and affects cranial nerves, or thoracic nerves.[Citation7] A detail of DPN-associated neuropathies is given in . The next section of this study also includes a thorough description of the pathways connected to the functional impairment caused by DPN. The main aim of this study is to comprehend the literature on the role of various natural substances in treating DPN. Although DPN is being treated with a variety of symptomatic and supportive medicines, the results are not encouraging. Natural product-based therapy can be a highly effective substitute to close this gap.
Pathophysiology of diabetic peripheral neuropathy
DPN has been extensively studied for the past 20 years and its pathology has been explained very well, but the pathogenesis is still unclear. Reported known pathologies in DPN include axonal atrophy, demyelination, nerve fiber loss, and reduced regeneration of nerves. Two well-known pathogenesis for DPN includes metabolic versus vascular which are believed to be involved at all stages of DPN. Impaired glycemic control (both direct glucose measures and levels of glycated hemoglobin) is linked with the occurrence of neuropathy.
Glia cells are fundamental to the pathogenic mechanisms of DPN, which is obvious from the integral role played during the maintenance of peripheral nerve structure and function.[Citation8] Schwann cells (SCs) are the passive insulator and most abundant cells, ensheathing the axons of peripheral nerves either in the form of non-myelinating or myelinating cells.[Citation9] SCs sustain peripheral nerve structure and function by the establishment of unmyelinated axons, myelination of myelinated axons, and secretion of neurotrophic factors. During DPN, a decrease in small myelinated and unmyelinated axons emerges earlier than large myelinated fibers. Aggregation of glycogen particles, hyperplasia of SCs (basal lamina), and edematous cell cytoplasm were seen in electron microscopic studies of humans.[Citation10]
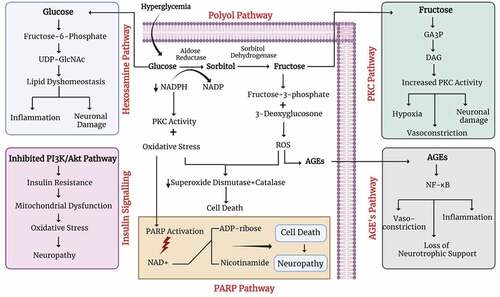
Metabolic abnormality in SCs functioning contributes to the pathogenesis of DPN. Previous researches emphasize that oxidative stress plays a chief role in the pathogenesis of diabetes-induced nerve injury.[Citation8] Aldose reductase (AR) that is a key enzyme of the polyol pathway is present in SCs, therefore any abnormality in SCs leads to enhance cascade of reactions due to excessive production of AR. This leads to the accumulation of sorbitol as well as fructose and was seen in cultured SCs. It was evaluated in a randomized (12-month), double-blind, and placebo-controlled trial that AR inhibition increases the regeneration of myelinated nerve fibers as well as improves segmental demyelination, myelin wrinkling, and paranodal demyelination.[Citation9] During diabetes, SCs functioning gets disturbed and causes fiber loss, compromised nerve homeostasis, and communication between axon and glial cells,[Citation8,Citation11] as suggested by animal models and human-based studies.[Citation12] Hyperglycemia, on a molecular level, is related to five different pathways: the polyol pathway, the PARP pathway, the advanced glycation end-product (AGE) pathway, the protein kinase C (PKC) pathway, and the hexosamine pathway (). Studies recommend that these five pathways and oxidative stress; essential to the pathogenesis of neurovascular dysfunction are interdependent. Sensory, motor, and autonomic neurons get affected by hyperglycemia after the activation of above mentioned five pathways.[Citation13]
Metabolic pathways
PARP Pathway: Poly ADP-ribose polymerase (PARP) pathway get activated due to higher level of oxidative stress and eventually result in neuronal dysfunction. Oxidative-nitrosative stress contributes to nerve conduction loss, metabolic alterations, neurovascular dysfunction, compromised neurotrophic support, abnormal sensation, as well as pain. Peripheral nerve, dorsal root, sympathetic ganglia, and vasculature of the peripheral nervous system (PNS) show enhanced oxidative-nitrosative stress in animals with both type 1 and type 2 diabetes. Activation of PARP, a nuclear enzyme, is one of the important downstream effectors of oxidative-nitrosative injury and is associated with the breakdown of nicotinamide adenine dinucleotide (NAD+) into nicotinamide and ADP-ribose residues. The process consequences can be (1) NAD+ reduction and energy failure, (2) alterations in transcriptional regulation and gene expression, and (3) poly (ADP-ribosyl) activation and inhibition of glyceraldehyde 3-phosphate dehydrogenase that subsequently leads to diversion of the glycolytic flux to many pathways involved in diabetes-associated complications. Recent studies support the fact that activation of PARP is an essential step in the pathogenesis of diabetes-related problems.[Citation14,Citation15]
AGE Pathway: AGEs are a major player in both diabetic pathophysiology and diabetic complications as well. Irreversible glycation products known as AGEs are formed in the Maillard reaction, in which glucose reacts with amino groups on proteins to make Amadori products that ultimately produce AGEs. These products can bind with cell surface receptors, known as receptor advanced glycation end products (RAGE), which initiates an injurious nuclear factor kappa B (NF-κB) mediated downstream signaling cascade. Activation of RAGE results in vasoconstriction, inflammation, and loss of neurotrophic support in rodent models,[Citation16] while the presence of higher levels of AGEs in the peripheral nerves of patients with DPN and T2D has also been reported.[Citation17]
Polyol Pathway: The polyol pathway is based upon two enzyme systems; first AR which converts glucose into sorbitol that is further converted into fructose by sorbitol dehydrogenase (SDH). All these reactions use NADPH and NAD as their co-factor. Therefore, the intracellular NADPH concentration reduces which in turn activates the PKC pathway.[Citation18] NADPH also acts as a co-factor of glutathione reductase which implicates the reestablishment of cellular redox status through the transformation of oxidized glutathione (GSSG) into reduced glutathione (GSH), therefore, the decrease in NADPH concentration directly cause cellular oxidative stress.[Citation19] Briefly, the polyol pathway increases the higher uptake of glucose and enhances the AGEs formation which upon binding of AGEs with its receptor yields reactive oxygen species.[Citation20] Excessive reactive oxygen products lessen the capability of the antioxidant enzymes.g., superoxide dismutase (SOD), catalase, etc., resulting in oxidative stress, DNA destruction, and cell death. Pre-clinical studies confirmed the polyol pathway activation in the streptozotocin (STZ) rat model of type 1 diabetes and the consequent harmful downstream effects. AR inhibitors in the STZ rat model or AR-deficient mice show improved diabetes-mediated discrepancies in behavior, nerve conduction velocity (NCV), and nerve structure as well.[Citation21,Citation22] As a result, AR inhibitors have become the main target to counteract diabetes-associated complications.
PKC Pathway: PKC is a family (consisting of at least 12 isoforms) of lipid-activated serine-threonine kinase enzymes.[Citation23] PKCs have multidisciplinary roles in the commencement and advancement of DPN. Hyperglycemia not only increases the PKC and diacylglycerol (DAG) levels but also increases AR activity and the de novo DAG synthesis.[Citation24] Constantly activated PKC causes many metabolic damages by mediating insulin resistance, upsetting the function of Na+/K+ ATPase, fluctuating the expression of vascular endothelial growth factor (VEGF), and transforming growth factor β (TGF-β), which leads to vasoconstriction, hypoxia, and neuronal damage.[Citation25]
Hexosamine Pathway: In response to hyperglycemia-induced DPN, the rate of glycolysis was seen to be increased which leads to stimulating metabolic pathways and enhancing neuronal injury. Fructose-6-phosphate (intermediate of glycolysis) moves toward the hexosamine pathway and initiates a cascade of events to form uridine 5-diphosphate-N-acetylglucosamine (GlcNac). This GlcNac is a sugar moiety and allows serine-threonine residues to bind with common transcription factors such as Sp-1 (specificity protein 1) which further promotes inflammation, lipid dyshomeostasis, and injury of peripheral nerves.[Citation26]
Insulin Signaling: Insulin is normally known to maintain the glucose level, but in the case of neurons, it is only essential for regulating neuronal functions. Insulin receptors are commonly found over peripheral axons enduring the epidermis as well as neuronal cell bodies (dorsal root ganglion). However, it was reported that the level of insulin receptors increases upon diabetes and physical injury. It was seen that exogenous insulin can improve mechanical sensation and nerve fiber density in the hind paw of mice.[Citation27] Additionally, another study showed that insulin administration through the intranasal route increases sensory nerve fibers in plantar footpads and reduces the pathogenesis of diabetes.[Citation28] Insulin also possesses neurotrophic effects, hence promoting the survival and growth of neurons. Insulin resistance or deficiency leads to reduce neurotrophic signaling and contributes to the pathophysiology of DPN. Inhibition of Insulin mediated phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway was reported to cause oxidative stress and mitochondrial dysfunction and promote neuropathy.[Citation29] Moreover, it leads to cause cellular disturbances such as apoptosis, DNA damage, and endoplasmic reticulum stress as well as the recruitment of macrophages.[Citation30] Although the underlying mechanism may be different in both type 1 and type 2 diabetes, thus it is clear that tight glucose control is not only the way to treat diabetes-associated neuropathy.[Citation31]
Vascular pathways
Neurovascular interaction is the key feature that provides nutrient supply to each part that is why reduced nerve blood flow was considered one of the main factors in the development of DPN. Numerous in vivo and in vitro experiments indicate that nerve blood flow (NBF) improvements in the diabetes model could lead to reducing the risk factor for the development of diabetes-associated complications.[Citation32] Neurotrophic factors such as nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), neurotrophin 3 (NT3), neurotrophin 4 (NT4), and brain-derived neurotrophic factor (BDNF) play important roles in the survival of neuronal populations. For instance, it was seen that the release of NGF from the target site secures the survival of sensory and sympathetic neurons. Hence, deficiency of any of the neurotrophic factors leads to cause neuro-developmental disorders.[Citation33] In diabetic conditions, expression of NGF, NT3, and BDNF reduces in peripheral nerves via reducing retrograde and anterograde axonal transport. It was explored that intrathecal delivery of NT3 and NGF improves the innervation of myelinated fiber in the dermal footpad of mice. Further studies have also shown that BDNF, and NGF also regulate angiogenesis and revascularization. NGF either directly promotes vascular cell growth or indirectly enhances the level of VEGF, whereas ciliary neurotrophic factor (CNTF) and GDNF were found non-angiogenic molecules.[Citation34]
Treatment strategies
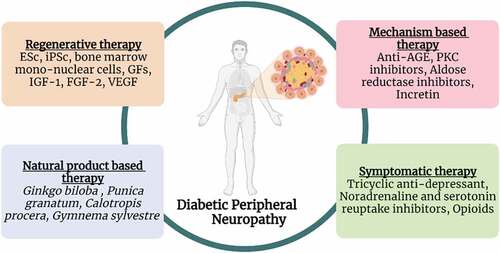
Several treatment strategies including symptomatic, mechanism-based, natural product-based and regenerative therapies have been used to treat DPN (, ). Detail of each treatment is given below.
Table 1. Treatment strategies against diabetic peripheral neuropathy.
Symptomatic treatment
One of the treatment strategies to ameliorate DPN is the symptomatic treatment of pain. Various agents have been investigated in clinical trials to alleviate painful symptoms for the management of DPN. Here some therapies are given below in detail.[Citation53] Tricyclic Agents (First-Line Therapy): Tricyclic agents (TCAs) are the first line of choice for the treatment of neuropathic pain associated with diabetes due to low cost. However, in clinical care, it is not used in excess due to the severity and increased frequency of its side effects.[Citation53] These are also known as antidepressants and exert their effects by acting as antagonists of histamine, 5-hydroxytryptamine (5-HT), alpha-adrenergic, N-methyl-D-aspartate (NMDA), and muscarinic receptors as well as by inhibiting noradrenaline and serotonin reuptake from the synaptic cleft.[Citation54] In addition, some TCAs may possess sodium channel blockade (amitriptyline) to alleviate diabetes-associated pain.[Citation55]
In a double-blind placebo study, the dose-dependent effect of mirogabalin (15, 20, 30 mg/day) depicts a significant pain-relieving effect in DPN patients.[Citation56] The use of mirogabalin is common in Japan due to its binding affinity and long duration of action. The effect of pregabalin and mirogabalin were assessed in a retrospective study and the results depict that pregabalin exhibits adverse effects while mirogabalin is effective and safe to reduce neuropathic pain associated with diabetes. Additionally, a randomized, placebo-controlled trial demonstrates that duloxetine (the first FDA-approved drug) can significantly improve pain scores and be used for the treatment of DPN.[Citation57,Citation58]
Antiarrhythmics: Antiarrhythmics such as mexiletine are reported to treat DPN and act as an alternative agent for those patients without cardiac disease. Similar to mexiletine, lidocaine was introduced to treat DPN via administering it transcutaneously in the form of patches in clinical practice. It was seen in an open-label study that four lidocaine patches (5%) for 18 h on daily basis can improve neuropathic pain and quality of life.[Citation59] However, intravenous administration of lidocaine at a dose of 5–7.5 mg/kg over 4 h was found to relieve pain for up to 28 days from intractable DPN.[Citation60]
Opioid Analgesics (Second-Line Therapy): Opioid analgesics have been used as first-line therapy in some selected clinical situations.[Citation61] However, due to their potential for abuse, it is considered either second or third-line therapy.[Citation62] Numerous studies have suggested the positive impacts of potent opioids in the management of peripheral neuropathy.[Citation63] Opioids including morphine, methadone, and oxycodone are used in treating neuropathic pain.[Citation64] Another study evaluated the impact of strong opioids in reducing neuropathic pain.[Citation65] However, further studies are required to find out the long-term risk factors of opioid use in the management of neuropathic pain.[Citation35]
Aldose Reductase Inhibitor: Aldose reductase inhibitors (ARIs) are used against AR that is a key enzyme of the polyol pathway to specifically reduce the conversion rate of fructose into sorbitol and act as a particular therapeutic strategy for DPN. The detail of ARIs has been discussed in previous studies.[Citation66–68] In a small clinical study, epalrestat was introduced as ARIs in patients suffering from DPN. It was found that epalrestat markedly improves NCV in the median nerve and also ameliorates autonomic nerve function. However, this study highlighted that it is only favorable for those patients with controlled blood sugar levels and there is a further need to explore its efficacy in a large-scale study.[Citation69,Citation70] The only ARI, epalrestat, is being used in India, China, and Japan to treat patients suffering from DPN. AT-001, a new ARI, is currently in clinical progress (NCT04083339) and can reduce N-terminal pro-β-type natriuretic peptide and sorbitol levels in the blood as mentioned in pre-clinical studies.[Citation71]
PKC Inhibitors: PKC is a regulatory protein involved in the maintenance of nerve function and plays a key role in the development of DPN. PKC inhibitors are most commonly used to inhibit neuropathic pain associated with diabetes. Ruboxistaurin possesses antioxidant properties and is used as a PKC inhibitor. Ruboxistaurin has been reported in improving endoneurial blood flow and NCV in a diabetic rat model, as well as in clinical trials.[Citation72] In randomized, double-blind studies the effect of robuxistaurin was evaluated with different doses (32 mg/day or 64 mg/day) for 1 year. The findings of this study reported that only those patients having less severe DPN indicate an improvement in primary as well as secondary measures; however, due to greater side impacts the use of ruboxistaurin is withdrawn.[Citation73]
Anti-AGE Agents: Anti-AGE agents inhibit the accumulation of AGEs and also prevent the interaction of AGEs with their receptors (RAGE) to prevent aggravation of oxidative stress during DPN. Aspirin, aminoguanidine, and benfotiamine act as anti-AGE agents due to their antioxidant properties and prevent the formation of AGEs.[Citation41,Citation74] In vitro study indicates that aspirin suppresses the formation of pentosidine (cross-linking AGEs) by trapping free radicals. Aminoguanidine reacts with AGEs precursor; 3-deoxyglucosone and traps reactive carbonyl, thereby reducing the formation of AGEs. However, aminoguanidine causes toxicity and has been withdrawn from clinical trials. It has been reported that benfotiamine enhances the activity of the transketolase enzyme to direct AGEs substrates in an alternative pathway known as the pentose phosphate pathway, thus resulting in the suppression of hyperglycemia-induced damage. Benfotiamine also inhibits UDP-N-acetylglucosamine (inducer of hexosamine pathway) to prevent the formation of tissue AGEs. Combinatorial treatment of cyanocobalamin, and pyridoxamine with benfotiamine was found to improve motor functions, symptom score, and vibration perception threshold in a more efficient manner than alone.[Citation72]
Incretin: An incretin hormone called glucagon-like peptide-1 (GLP-1) stimulates glucose-dependent insulin secretion and also has neurotrophic effects. Pro-glucagon is broken down in intestinal L cells to produce GLP-1, an insulinotropic hormone, that is glucose-dependent. They have unique G-coupled protein receptors, the GLP-1 receptor (GLP-1 R) and the GIP receptor (GIPR), via which they have biological effects. GLP-1 R, present in the peripheral nerve, has been seen to mediate ERK-signaling in the sciatic nerve of diabetic rodents to guard against large motor fiber function and small C fiber structure by a mechanism unrelated to glycemic control.[Citation75]
Moreover, GLP-1 may function as a neuroprotective agent, promote neurite outgrowth of adult sensory neurons in vitro, and have direct nutritional effects on the nervous system. It also protects sensory neuropathy brought on by pyridoxine, suggesting that it can be used to treat diabetic neuropathies. In addition, it has been demonstrated that GLP-1 has advantages in microvascular complications of diabetes, one of the mechanisms that result in DPN, by reducing endothelium dysfunction, an early sign of atherosclerosis, arterial endothelial wall injury, and decrease peripheral nerve nutrition.[Citation76]
Glucagon-like peptide-1 receptor agonists (GLP-1RA) and dipeptidyl peptidase (DPP)-4 inhibitors are two incretin-based treatments that are now often used to treat people with type 2 diabetes. In STZ-induced diabetic rats, GLP-1RA has been demonstrated to have positive effects on DPN despite the glucose-lowering impact. GLP-1RA treatment for four weeks improved hypoalgesia and motor and sensory NCV. Additionally, GLP-1RA stimulates the ERK signaling pathway in peripheral neurons and/or Schwann cells produced from diabetic rats and mice, preventing the slowing of NCV.[Citation77]
Regeneration Therapy: Regeneration therapy improves the functionality of nerve cells and vessels along with the regeneration of damaged tissue associated with DPN. Regeneration therapies include stem cells isolated from fat tissue and bone marrow, bone marrow mono-nuclear cells comprising of precursor and stem cells, and stem cells obtained from the induced pluripotent stem (iPS) and embryonic stem (ES) cells.[Citation78]
Growth factors (GFs) also gain attention due to their important role in promoting neuronal survival and repair as well as the functional integrity of damaged nerves. Some GFs are neurotrophic such as NT3 and are found to induce neural regeneration while other GFs are angiogenic and involved to mediate the growth of blood vessels to provide oxygen and nutrients to nerves for their functional recovery. Notably, FGF-2, VEGF, IGF-1, BDNF, and NGF possess both neurotrophic and angiogenic properties and thus can be used to treat DPN.[Citation79] It has been found in rabbit and rat models of diabetes that VEGF-encoding plasmids can normalize NCV, and enhance angiogenesis of vasa nervosa, and nerve fiber density. Interestingly, plasmid VEGF has also shown promising therapeutic effects in a randomized double-blind trial of humans.[Citation80]
Although there is a further, need to explore the beneficial impact of VEGF in a large sample size to introduce this product in the treatment of DPN. Similarly, IGF-1, IGF-2, SHH, VEGF-A, VEGF-C, and statin have been presented to restore micro-circulation in damaged nerves along with functional recovery, hence suggesting protective effects of growth factors against the development of neuropathy associated with diabetes.[Citation81] On the other hand, administration of IGF-1, IGF-2, GDNF, CTNF, and NGF has also shown neuroprotective effects in an animal model with DPN.[Citation13] These findings strongly suggested that cell therapy which can target both neurotrophic and angiogenic processes has more advantages than single protein or gene therapy to ameliorate DPN.
Vitamin D: A recent study has evaluated the link between pain and vitamin D and shown that neurons are related to nociceptive calcitonin gene-associated peptides, which have particular vitamin D phenotypes.[Citation82] As we come to know from a previous study that NGF was found to be depleted in an animal model with diabetes as well as in patients suffering from diabetic neuropathy.[Citation83] Recently, it has been shown that the expression level of NGF was maintained in diabetic animals (sciatic nerve) via using CB1093 (vitamin D analog). Additionally, tacalcitol (active vitamin D3) was found to induce NGF formation in human epidermal keratinocytes. In another study, vitamin D3 has been shown to promote axonal regeneration in the spinal cord compression model and also reduce demyelination in the cuprizone experimental model of demyelination.[Citation84]
Natural product-based therapy
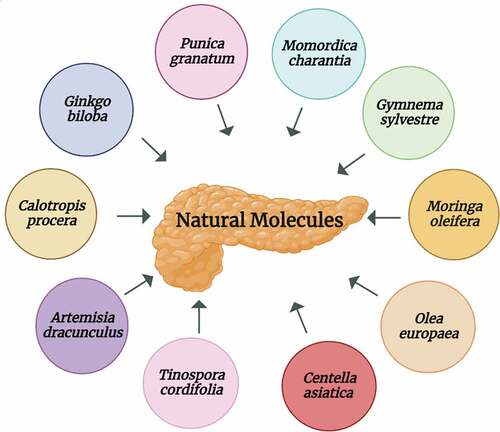
Despite all symptomatic and mechanistic approaches, patients suffering from DPN have inadequate therapies available yet. Synergistic and multi-target characteristics of herbals and nutraceuticals are the common features to treat diabetes and its complications such as DPN. Many prescription medicines possess unwanted impacts and have a narrow therapeutic window, whereas natural molecules provide an effective, safe, and valuable therapeutic approach.[Citation85–87] A schematic presentation of natural molecules is given in .
Ginkgo biloba: Ginkgo biloba L. (Gb) belongs to the Ginkgoaceae family and possesses a broad range of properties due to the presence of potent components such as terpene trilactones, and flavonols including quercetin, myricetin, and kaempferol.[Citation88] Extract of Ginkgo biloba (EGb) leaves is also reported to be cardioprotective and neuroprotective. Quercetin, a free radicals scavenger, is responsible for the antioxidant activity of EGb. EGb can ameliorate neurological disorders such as depression, short-term memory loss, Alzheimer’s disease as well as DM. EGb is also known as a potent vasodilator having antiplatelet properties and is used to improve microcirculation. It is reported that EGb treatment can improve NCV in patients suffering from DPN.[Citation89] A randomized, placebo, double-blind study on 156 patients indicates significant improvements in the functionality of peripheral sensorimotor nerves within 6 months after the administration of EGb 761 (Ginkgo biloba leaves extract). Therefore, this study strongly recommended that EGb 761 can be developed as a safe drug for managing clinical diabetic sensorimotor polyneuropathy.[Citation90]
In addition, a study indicated that EGb 761 can be used to treat diabetes-associated peripheral neuropathy at a dose of 50 mg/kg by eliminating superoxide anion and hydroxyl radicals.[Citation91–93] EGb treatment can also prevent pathological alterations in peripheral nerves associated with DM and enhances axonal regeneration.[Citation94]
Punica granatum: Punica granatum L., a fruiting plant, is commonly known as pomegranate and is found in warm-temperature areas and subtropics. Various parts of this plant including peel, juice, leaves, bark, and flower are in Ayurvedic and traditional medicines due to their health-promoting effects. As Pomegranate (PG) possesses anti-inflammatory, immunomodulatory, and antioxidant properties, therefore this plant has been used as antidepressant, antidiabetic, anthelminthic, antifungal, antiulcer, antibacterial, and antiviral agent. Leaves of PG are rich sources of flavones (apigenin and luteolin), tannins (punicafolin and punicalin), and glycosides.[Citation95,Citation96] Extract of PG flowers exhibits blood glucose-lowering effects. Further, it was investigated in Zucker diabetic fatty (ZDF) rat model that methanolic extract of PG flowers inhibits the enzymatic activity of glucosidase and improves hyperglycemic levels in type 2 diabetes.[Citation97] Recent research suggested that ellagic acid, a potent constituent, is responsible for the antihyperglycemic activity of PG. In a mouse model, the dose-dependent study, PG extract has shown promising antidiabetic effects thus it is speculated that it can be used in the symptomatic treatment of DPN. In addition, these PG extracts are beneficial in the treatment of macro-and microvascular complications of DM.[Citation98]
Calotropis procera: Calotropis procera Linn. belongs to the Asclepiadaceae family and possesses multifactorial medicinal properties. Calotropis procera (C. procera) has antioxidant, antibacterial, anti-inflammatory, antifertility, analgesic, antidiabetic, proteolytic, and hepatoprotective properties. Therefore, different parts of C. procera have been used in the treatment of liver, and abdomen diseases as well as in piles, ulcers, diabetes, and leprosy.[Citation99–102] C. procera has potent constituents such as triterpenoids, resins, alkaloids, glycosides, saponins, tannins, and flavonoids. Due to the presence of these constituents, C. procera leaves also possess anti-hyperglycemic activity.[Citation100,Citation103]
Different parts of C. procera such as roots, leaves, and stems ameliorate peripheral neuropathy following STZ-induced diabetes. Among them, methanolic extract of C. procera root (100 mg/kg) was found to attenuate thermal and mechanical hyperalgesia, HbA1c% level, and tactile allodynia due to its multiple and potent antioxidant and hypoglycemic activity.[Citation104] C. procera (root extract), in a recent study, exhibits significant improvements in hyperalgesic activity with enhanced HbA1c levels in treated groups as compared to normal rats.[Citation105]
Artemisia dracunculus: Artemisia is a genus of the family Asteraceae and has economic and therapeutic importance with greater than 500 species.[Citation106] Artemisia dracunculus L. (A. dracunculus), also known as tarragon, is a widely used spice in French and Russia. It has potent components including coumarins, flavonoids, phenols (carbonic acids), and essential oils. Methyleugenol (essential oil), a robust pain reliever, is responsible for the antinociceptive property of A. dracunculus. In vitro studies highlighted the hepatoprotective, antihyperglycemic, and anti-inflammatory effects of A. dracunculus.[Citation107]
Ethanolic extract of A. dracunculus (PMI-5011) alleviates the progression of DPN by inhibiting AR activity, oxidative stress, NFκB inflammatory mechanism while improving insulin receptor signaling and sorbitol pathway. PMI-5011 has the potential to alleviate NCV, small-fiber dysfunctioning, and promotes regeneration of sensory nerve fibers via reducing oxidative-nitrosative stress.[Citation108] Similarly, another study evaluated the potent role of PMI-5011 in alleviating peripheral nerve dysfunction and considered it as a nontoxic as well as an effective treatment strategy for DPN.[Citation109]
Gymnema sylvestre: Gymnema sylvestre, also known as “gurmar,” belongs to the Asclepiadaceae family and grows in tropical areas. Gymnema sylvestre (G. sylvestre) has been used for medicinal purposes in the traditional Ayurvedic system mostly in India to treat diabetes.[Citation110,Citation111] Leaves of G. sylvestre contain gymnemic acid that is a combination of acidic glycosides, anthroquinones, alkaloids, and triterpenoid saponins. Anti-inflammatory and antioxidative properties of G. sylvestre leaves extract was found to be responsible for reducing nerve tissue damage in an STZ-induced DPN model of wistar rats.[Citation112] Moreover, it was seen that the aqueous extract of G. sylvestre leaves reduces hyperalgesia due to its AR inhibitory activity in the experimental DPN model.[Citation113]
Momordica charantia: Momordica charantia L. is commonly known as bitter gourd belongs to the Cucurbitaceae family. Earlier, Momordica charantia (M. charantia) seed oil was used to relieve pain in patients with diabetic neuropathy, rheumatoid arthritis, and spondylitis when administered through a topical route.[Citation114] The potent neuroprotective activity of M. charantia is observed against the cerebral ischemia-reperfusion-induced neuronal injury model. It was found that M. charantia acts by modulating hyperglycemia and decreasing ROS production.[Citation115] Moreover, administration of M. charantia at a dosage of 200–800 mg/kg could reduce withdrawal latency without influencing motor and sensory functions.[Citation116]
Moringa oleifera: Moringa oleifera Lam. known as Moringa in literature belongs to the family Moringaceae. Moringa oleifera (M. oleifera) is a rich source of minerals (magnesium, iron, copper, zinc, potassium, manganese, calcium) and vitamins (B, C, A, D, E, K) and exhibits more than 40 antioxidant species. Previous studies have depicted the use of leaves, gums, flowers, seeds, and bark of M.oleifera in relieving vitamin deficiency, cardiovascular protection, normalizing blood glucose levels, and also exhibiting anti-inflammatory as well as free-radicals scavenging properties of these parts of the plant.[Citation117,Citation118]
It was evaluated in the diabetic model with neuropathic pain that M. oleifera treatment (at a dose of 100 and 200 mg/kg) reverses the decreased withdrawal latency and withdrawal threshold intensity in hot plate and Von Frey filament tests, respectively. Besides this, an elevation in the level of MDA and decrease in GSH-Px and SOD was reversed in an injured nerve of rats after M. oleifera extract administration. Conclusively, the antioxidative capacity of M. oleifera possesses a strong neuroprotective activity to reduce diabetes-associated neuropathy.[Citation119] A recent pre-clinical study has also confirmed the potent hypoglycemic, as well as antioxidant activity of M. oleifera due to its insulin secretagogue and alpha-glucosidase inhibitory activity.[Citation120]
Centella asiatica: Centella asiatica, an important traditional plant, belongs to Apiaceae family and is used against diarrhea, measles, leukorrhea, syphilis, and toothache due to its potent therapeutic potential. Centella asiatica (C. asiatica) has anti-oxidant, anti-viral, anti-ulcer, anti-microbial, and anti-filarial activities due to the presence of potent bioactive compounds such as Asiatic acid, madecassic acid, α-copaene, α-terpinene, and β-caryophyllene.[Citation121] The potent bioactive compound of C. asiatica such as triterpenes has been found as a potential candidate in the treatment of DPN, as it was seen in the pilot, placebo-controlled clinical study. The patients were assessed based on the alleviation of burning sensation, the elevation of numbness, and the reduction in pain. Thus C. asiatica represents a well-tolerated and effective drug regimen in the treatment of DPN.[Citation122]
Olea europaea: Olea europaea is also known as olive (zaytoun) and belongs to Oleaceae family. Olea europaea (O. europaea) has been used traditionally as a hypotensive, laxative, diuretic, febrifuge, and emollient. It is a rich source of phytoconstituents such as flavonoids, glycosides, polyunsaturated fatty acids, and secoiridoid. It also possesses anti-microbial, anti-oxidant, anti-diabetic, and anti-viral properties. It also causes health-promoting impacts on cardiovascular disorders.[Citation123]
Ethanolic extract of O. europaea leaves has potent hypoglycemic activity and is used to attenuate inflammatory markers to prevent the progression of diabetes-induced complications.[Citation124] Furthermore, O. europaea leaves suppress thermal hyperalgesia via reducing neuronal apoptosis and diabetes-induced neuronal damage to prevent or attenuate diabetic neuropathic pain.[Citation125]
Tinospora cordifolia: Tinospora cordifolia is a deciduous shrub and is found in tropical areas of India, Srilanka, Bangladesh, and China. Tinospora cordifolia (T. cordifolia) belongs to the Menispermaceae family and contains phytoconstituents such as steroids, alkaloids, glycosides, diterpenoid lactones, polysaccharides, and aliphatic compounds in large amounts. Moreover, it possesses anti-hyperglycemic, anti-neoplastic, anti-oxidant, and hepatoprotective activities.[Citation126] In the experimental diabetic neuropathy model, it was seen that T. cordifolia attenuates hyperalgesia by inhibiting AR activity.[Citation127]
Phoenix dactylifera: Phoenix dactylifera, belongs to Arecaceae family and is a rich source of dietary fibers, carbohydrates, proteins, fatty acids, minerals, amino acids, and vitamins. Phoenix dactylifera (P. dactylifera) exhibits antioxidant potential due to the presence of a wide range of phenolic components such as flavonoids, p-coumarins, ferulic, procyanidins, and sinapic acids. It has antifungal, antibacterial, antitumoral, antimutagenic, and gastroprotective as well as neuroprotective properties.[Citation128,Citation129] P. dactylifera (date fruit) extract prevents the deterioration of peripheral nerves against STZ-induced diabetes, thus protecting neurons, and can be used as a potential preventive approach for DPN.[Citation128]
Citrullus colocynthis: Citrullus colocynthis L., bitter apple (Cucurbitaceae), is commonly found in Aisa and Africa. Due to antinociceptive activity, Citrullus colocynthis has been used by ancient Persian physicians to treat diabetes-associated neuropathy. It has anesthetic, anti-ulcerogenic, and antioxidant activities.[Citation130,Citation131] It decreases the number of degenerated and demyelinated nerve fibers. It also lowers blood glucose levels and enhances antioxidant production (superoxide dismutase, catalase, malondialdehyde) in adose – response relationship (100 mg/kg) in an animal model of DPN.[Citation132]
Coriandrum sativum: Coriandrum sativum L., a member of Apiaceae family is valued for its medicinal and nutritional properties.[Citation133] Coriandrum sativum (C. sativum) is a source of multiple potent constituents such as polyphenols (gallic acid, ferrulic acid, rutin, anethole, caffeic acid, caroteinoids, borneol), flavonoids (quercetin, rutin, and isoquercetin), terpenes, coumarins, tannins, tocopherols, fatty acids, and vitamin C. It possesses several pharmacological activities including anti-inflammatory, antidyslipidemic, neuroprotective, and cardioprotective. It is found that CPE (C. sativum petroleum ether extract) can reduce the production of AGEs and increases the level of glutathione and superoxide dismutase in STZ-nicotinamide (NAD) induced diabetic (type 2) model.[Citation134] In addition, CHA (C. sativum-hydroalcohol) extract attenuates nitrosative-oxidative stress, hyperglycemia, TNF-α, and formation of AGEs, thus potentially managing DPN.[Citation135]
Phytoconstituents and Diabetic Peripheral Neuropathy: Natural compounds that act as ARI are always a better choice to target the polyol pathway to ameliorate DPN. Quercetin, a potent flavonoid, possesses anti-nociceptive activity and has been shown to enhance the nociceptive threshold in diabetic rats in a previous study.[Citation136] Similarly, rutin was also found to exhibit a protective impact due to its metal chelating property. It was seen that rutin chelates the transition metals, thus suppressing the Fenton reaction that further participates to prevent the development of DPN.[Citation137]
Another flavonoid naringin was found to act as ARI and used due to its free radical scavenging property. It was also seen in diabetic rats that naringin exhibits a neuroprotective effect by downregulating cytokine-associated TNF-α formation in the diabetic model.[Citation44] Puerarin (isoflavonoid) has been found to cause dilation of blood vessels that leads to improving micro-circulation, thus enhancing NCV by reducing blood viscosity. Co-treatment of curcumin and anti-oxidant has been found to inhibit the production of TNF-α and nitric oxide (NO) in diabetic rats due to its antihyperalgesic property.[Citation138] Also, baicalin was found to inhibit PKC, LOX pathway, oxidative stress, and AR against DPN in diabetic rats.[Citation43,Citation138]
There is a long history of using natural substances as medications to cure various illnesses.[Citation139,Citation140] Chlorogenic acid possesses anti-inflammatory and anti-oxidant properties and is used to ameliorate DPN via targeting AR in the polyol pathway. It has also anti-hyperalgesic activity and is used as a potent ARI.[Citation141] Moreover, epigallocatechin-gallate was also reported due to its ability to inhibit oxidative stress in diabetic rats.[Citation142] Similarly, ellagic acid has antioxidant activity and is found to exhibit a protective effect. It was also seen that ellagic acid reduces nitrate and malondialdehyde (MDA) levels in rat models having diabetes.[Citation143]
Conclusion
Diabetes mellitus, also known as a disorder of carbohydrate metabolism, is characterized by insufficient production of insulin or the inability of the body to respond to this hormone. Diabetic peripheral neuropathy is one of the common and severe complications of diabetes mellitus and adversely affects the lifestyle of millions of people throughout the world. Several supportive therapies including tricyclic agents, opioid analgesics, antiarrhythmics, anti-AGE agents, protein kinase C, and aldose reductase inhibitors have been used to treat this condition. In addition to these therapies, neurotrophic, and angiogenic factors such as FGF-2, VEGF, IGF-1, BDNF, and NGF possess beneficial effects in ameliorating DPN. Despite the availability of various treatment options, we are still lacking the optimal treatments for DPN. It is the need for the present era to design or discover the potent compounds that would execute the complete sensorimotor functional regain. In this regard, natural molecules are getting more attention in treating several diseases in general and DPN in particular. Natural molecules provide more reliable and effective therapy with fewer side impacts. A plethora of natural molecules has been suggested with antidiabetic, antioxidant, anti-inflammatory, anti-bacterial, anti-viral, anti-nociceptive, and neuroprotective effects against DPN. This study discussed the neuroprotective potential of numerous plants and their potential to attenuate thermal and mechanical hyperalgesia and tactile allodynia associated with DPN. Natural molecules have been found to improve NCV, manage glucose levels, reduce neuronal apoptosis, and the production of reactive oxygen species. They also increase the level of antioxidants and enhance axonal regeneration. However, validation of therapeutic activities of natural molecules needs to be tested in clinical trials to confirm the efficacy and safety of the natural product formulations.
Author contributors
R.A., H.A., M.S.J., A.R., S.A.M., M.M., F.I., I.U.K., F.S., T.I., M.A.S. and T.S. have equally contributed to conceptualization, literature analysis, and review drafting; Supervision: M.A.S. and G.H.; Project administration: G.H., and M.A.S. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The listed author(s) express gratitude to their representative institutes and universities for providing access to the literature.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kharroubi, A. T. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes. 2015, 6(6), 850. DOI: 10.4239/wjd.v6.i6.850.
- Galtier, F. Definition, Epidemiology, Risk Factors. Diabetes. Metab. 2010, 36(6), 628–651. DOI: 10.1016/j.diabet.2010.11.014.
- Chobanian, A. V.; Bakris, G. L.; Black, H. R.; Cushman, W. C.; Green, L. A.; Izzo, J. L.; Jones, D. W.; Materson, B. J.; Oparil, S.; Wright, J. T., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. J. Am. Med. Assoc. 2003, 289(19), 2560.
- Aynalem, S. B.; Zeleke, A. J. Prevalence of Diabetes Mellitus and Its Risk Factors Among Individuals Aged 15 Years and Above in Mizan-Aman Town, Southwest Ethiopia, 2016: A Cross Sectional Study. Int. J. Endocrinol. 2018, 2018, 1–7. DOI: 10.1155/2018/9317987.
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A. A.; Ogurtsova, K., et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas 9th. Diabetes res. clin. pract. 2019, 157, 107843. DOI: 10.1016/j.diabres.2019.107843.
- Bellelli, A.; Santi, D.; Simoni, M.; Greco, C. Diabetic Neuropathic Cachexia: A Clinical Case and Review of Literature. Life (Basel, Switzerland). 2022, 12(5). DOI: 10.3390/LIFE12050680.
- Kasznicki, J. Advances in the Diagnosis and Management of Diabetic Distal Symmetric Polyneuropathy. Arch. Med. Sci. 2014, 2, 345. DOI: 10.5114/AOMS.2014.42588.
- Mizisin, A. P. Mechanisms of Diabetic Neuropathy: Schwann Cells. Handb. Clin. Neurol.2014, 126, 401–428. DOI: 10.1016/B978-0-444-53480-4.00029-1.
- Brown, A. M.; Evans, R. D.; Black, J.; Ransom, B. R. Schwann Cell Glycogen Selectively Supports Myelinated Axon Function. Ann. Neurol. 2012, 72(3), 406–418. DOI: 10.1002/ana.23607.
- Naruse, K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv. Exp. Med. Biol. 2019, 1190, 345–356. DOI: 10.1007/978-981-32-9636-7_22.
- Eckersley, L. Role of the Schwann Cell in Diabetic Neuropathy. Int. Rev. Neurobiol. 2002, 50, 293–321.
- Gonçalves, N. P.; Vægter, C. B.; Pallesen, L. T. Peripheral Glial Cells in the Development of Diabetic Neuropathy. Front. Neurol. 2018, 9. DOI: 10.3389/fneur.2018.00268.
- Leinninger, G. M.; Vincent, A. M.; Feldman, E. L. The Role of Growth Factors in Diabetic Peripheral Neuropathy. J. Peripher. Nerv. Syst. 2004, 9(1), 26–53. DOI: 10.1111/j.1085-9489.2004.09105.x.
- Obrosova, I. G.; Drel, V. R.; Pacher, P.; Ilnytska, O.; Wang, Z. Q.; Stevens, M. J.; Yorek, M. A. Oxidative-Nitrosative Stress and Poly(adp-Ribose) Polymerase (PARP) Activation in Experimental Diabetic Neuropathy: The Relation is Revisited. Diabetes. 2005, 54(12), 3435–3441. DOI: 10.2337/diabetes.54.12.3435.
- Komirishetty, P.; Areti, A.; Gogoi, R.; Sistla, R.; Kumar, A. Poly(adp-Ribose) Polymerase Inhibition Reveals a Potential Mechanism to Promote Neuroprotection and Treat Neuropathic Pain. Neural. Regen. Res. 2016, 11(10), 1545–1548. DOI: 10.4103/1673-5374.193222.
- Lukic, I. K.; Humpert, P. M.; Nawroth, P. P.; Bierhaus, A. The RAGE Pathway: Activation and Perpetuation in the Pathogenesis of Diabetic Neuropathy. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc, 2008; Vol. 1126, pp. 76–80.
- Mišur, I.; Žarković, K.; Barada, A.; Batelja, L.; Miličević, Z.; Turk, Z. Advanced Glycation End Products in Peripheral Nerve in Type 2 Diabetes with Neuropathy. Acta Diabetol. 2004, 41(4), 158–166. DOI: 10.1007/s00592-004-0160-0.
- Ramana, K. V. Aldose Reductase: New Insights for an Old Enzyme. Biomol. Concepts. 2011, 2(1–2), 103–114. DOI: 10.1515/bmc.2011.002.
- Alter, M. L.; Ott, I. M.; Von Websky, K.; Tsuprykov, O.; Sharkovska, Y.; Krause-Relle, K.; Raila, J.; Henze, A.; Klein, T.; Hocher, B. DPP-4 Inhibition on Top of Angiotensin Receptor Blockade Offers a New Therapeutic Approach for Diabetic Nephropathy. Kidney Blood Press. Res. 2012, 36(1), 119–130. DOI: 10.1159/000341487.
- Yamagishi, S. I.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of Advanced Glycation End Products (AGEs) and Oxidative Stress in Vascular Complications in Diabetes. Biochim. Biophys. Acta - Gen. Subj. 2012, 1820(5), 663–671. DOI: 10.1016/j.bbagen.2011.03.014.
- Schemmel, K. E.; Padiyara, R. S.; D’souza, J. J. Aldose Reductase Inhibitors in the Treatment of Diabetic Peripheral Neuropathy: A Review. J. Diabetes Complications. 2010, 24(5), 354–360. DOI: 10.1016/j.jdiacomp.2009.07.005.
- Ho, E. C. M.; Lam, K. S. L.; Yuk, S. C.; Yip, J. C. W.; Arvindakshan, M.; Yamagishi, S. I.; Yagihashi, S.; Oates, P. J.; Ellery, C. A.; Chung, S. S. M., et al. Aldose Reductase–Deficient Mice are Protected from Delayed Motor Nerve Conduction Velocity, Increased C-Jun NH2-Terminal Kinase Activation, Depletion of Reduced Glutathione, Increased Superoxide Accumulation, and DNA Damage. Diabetes. 2006, 55(7), 1946–1953.
- Steinberg, S. F. Structural Basis of Protein Kinase C Isoform Function. Physiol. Rev. 2008, 88(4), 1341–1378. DOI: 10.1152/physrev.00034.2007.
- Chandrasekaran, K.; Zilliox, L. A.; Russell, J. W. Diabetic Neuropathy – Research Basic and Translational Research in Diabetic Neuropathy. Microvasc. Dis. Diabetes Hum. 2020, 129–155.
- Geraldes, P.; King, G. L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. DOI: 10.1161/CIRCRESAHA.110.217117.
- Du, X. L.; Edelstein, D.; Rossetti, L.; Fantus, I. G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-Induced Mitochondrial Superoxide Overproduction Activates the Hexosamine Pathway and Induces Plasminogen Activator Inhibitor-1 Expression by Increasing Sp1 Glycosylation. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 12222–12226. DOI: 10.1073/pnas.97.22.12222.
- Guo, J.; Whittemore, R.; He, G. P. The Relationship Between Diabetes Self-Management and Metabolic Control in Youth with Type 1 Diabetes: An Integrative Review. J. Adv. Nurs. 2011, 67, 2294–2310. DOI: 10.1111/j.1365-2648.2011.05697.x.
- Francis, G.; Martinez, J.; Liu, W.; Nguyen, T.; Ayer, A.; Fine, J.; Zochodne, D.; Hanson, L. R.; Frey, W. H.; Toth, C. Intranasal Insulin Ameliorates Experimental Diabetic Neuropathy. Diabetes. 2009, 58, 934–945. DOI: 10.2337/db08-1287.
- Kim, B.; Feldman, E. L. Insulin Resistance in the Nervous System. Trends Endocrinol. Metab. 2012, 23, 133–141. DOI: 10.1016/j.tem.2011.12.004.
- Eguchi, K.; Manabe, I. Macrophages and Islet Inflammation in Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 152–158. DOI: 10.1111/dom.12168.
- Pop-Busui, R.; Herman, W. H.; Feldman, E. L.; Low, P. A.; Martin, C. L.; Cleary, P. A.; Waberski, B. H.; Lachin, J. M.; Albers, J. W. DCCT and EDIC Studies in Type 1 Diabetes: Lessons for Diabetic Neuropathy Regarding Metabolic Memory and Natural History. Curr. Diab. Rep. 2010, 10, 276–282. DOI: 10.1007/s11892-010-0120-8.
- Han, J. W.; Sin, M. Y.; Yoon, Y. S. Cell Therapy for Diabetic Neuropathy Using Adult Stem or Progenitor Cells. Diabetes Metab. J. 2013, 37, 91–105. DOI: 10.4093/dmj.2013.37.2.91.
- Huang, E. J.; Reichardt, L. F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. DOI: 10.1146/annurev.neuro.24.1.677.
- Kermani, P.; Rafii, D.; Jin, D. K.; Whitlock, P.; Schaffer, W.; Chiang, A.; Vincent, L.; Friedrich, M.; Shido, K.; Hackett, N. R., et al. Neurotrophins Promote Revascularization by Local Recruitment of TrkB+ Endothelial Cells and Systemic Mobilization of Hematopoietic Progenitors. J. Clin. Invest. 2005, 115, 653–663. DOI: 10.1172/jci22655.
- Khdour, M. R. Treatment of Diabetic Peripheral Neuropathy: A Review. J. Pharm. Pharmacol. 2020, 72, 863–872. DOI: 10.1111/jphp.13241.
- Snyder, M. J.; Gibbs, L. M.; Lindsay, T. J. Treating Painful Diabetic Peripheral Neuropathy: An Update. Am. Fam. Physician. 2016, 94(3), 227–234.
- Atef, M.; El-Sayed, N.; Mostafa, Y.; Ahmed, A. Recent Updates in Treatment of Diabetic Neuropathy. Rec. Pharm. Biomed. Sci. 2019, 0, 15–27. DOI: 10.21608/rpbs.2019.12385.1033.
- Javed, S.; Petropoulos, I. N.; Alam, U.; Malik, R. A. Treatment of Painful Diabetic Neuropathy. Ther. Adv. Chronic Dis. 2015, 6, 15–28. DOI: 10.1177/2040622314552071.
- Hosseini, A.; Abdollahi, M. Diabetic Neuropathy and Oxidative Stress: Therapeutic Perspectives. OXID. MED. CELL LONGEV. 2013, 2013, 1–15. DOI: 10.1155/2013/168039.
- Stevens, M.; Shakher, J. Update on the Management of Diabetic Polyneuropathies. Diabetes, Metab. Syndr. Obes. Targets Ther. 2011, 289. DOI: 10.2147/dmso.s11324.
- Edwards, J. L.; Vincent, A. M.; Cheng, H. T.; Feldman, E. L. Diabetic Neuropathy: Mechanisms to Management. Pharmacol. Ther. 2008, 120, 1–34. DOI: 10.1016/j.pharmthera.2008.05.005.
- Li, R.; Ma, J.; Wu, Y.; Nangle, M.; Zou, S.; Li, Y.; Yin, J.; Zhao, Y.; Xu, H.; Zhang, H., et al. Dual Delivery of NGF and bFgf Coacervater Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Schwann Cells Apoptosis. Int. J. Biol. Sci. 2017, 13, 640–651. DOI: 10.7150/ijbs.18636.
- Stavniichuk, R.; Drel, V. R.; Shevalye, H.; Maksimchyk, Y.; Kuchmerovska, T. M.; Nadler, J. L.; Obrosova, I. G. Baicalein Alleviates Diabetic Peripheral Neuropathy Through Inhibition of Oxidative-Nitrosative Stress and P38 MAPK Activation. Exp. Neurol. 2011, 230, 106–113. DOI: 10.1016/j.expneurol.2011.04.002.
- Kandhare, A. D.; Raygude, K. S.; Ghosh, P.; Ghule, A. E.; Bodhankar, S. L. Neuroprotective Effect of Naringin by Modulation of Endogenous Biomarkers in Streptozotocin Induced Painful Diabetic Neuropathy. Fitoterapia. 2012, 83, 650–659. DOI: 10.1016/j.fitote.2012.01.010.
- Bachewal, P.; Gundu, C.; Yerra, V. G.; Kalvala, A. K.; Areti, A.; Kumar, A. Morin Exerts Neuroprotection via Attenuation of ROS Induced Oxidative Damage and Neuroinflammation in Experimental Diabetic Neuropathy. BioFactors. 2018, 44, 109–122. DOI: 10.1002/biof.1397.
- Yang, R.; Li, L.; Yuan, H.; Liu, H.; Gong, Y.; Zou, L.; Li, S.; Wang, Z.; Shi, L.; Jia, T., et al. Quercetin Relieved Diabetic Neuropathic Pain by Inhibiting Upregulated P2X4 Receptor in Dorsal Root Ganglia. J. Cell. Physiol. 2019, 234, 2756–2764. DOI: 10.1002/jcp.27091.
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin Ameliorates Diabetic Neuropathy by Lowering Plasma Glucose and Decreasing Oxidative Stress via Nrf2 Signaling Pathway in Rats. Eur. J. Pharmacol. 2016, 771, 84–92. DOI: 10.1016/j.ejphar.2015.12.021.
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic Acid Prevents Diabetic Nephropathy by Inhibiting Oxidative Stress and Inflammation Through Modulation of the Nrf2/HO-1 and NF-ĸb Pathways. Int. Immunopharmacol. 2018, 54, 245–253. DOI: 10.1016/j.intimp.2017.11.021.
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T., et al. Epigallocatechin Gallate Upregulates NRF2 to Prevent Diabetic Nephropathy via Disabling KEAP1. Free Radic Biol. Med. 2017, 108, 840–857. DOI: 10.1016/j.freeradbiomed.2017.04.365.
- Ahad, A.; Ganai, A. A.; Mujeeb, M.; Siddiqui, W. A. Ellagic Acid, an NF-Κb Inhibitor, Ameliorates Renal Function in Experimental Diabetic Nephropathy. Chem. Biol. Interact. 2014, 219, 64–75. DOI: 10.1016/j.cbi.2014.05.011.
- Sharaf El Din, U. A. A.; Mansour Salem, M.; Abdulazim, D. O. Recent Advances in Management of Diabetic Nephropathy. J. Clin. Exp. Nephrol. 2017, 02, 1–22. DOI: 10.21767/2472-5056.100035.
- Vincent, A. M.; Callaghan, B. C.; Smith, A. L.; Feldman, E. L. Diabetic Neuropathy: Cellular Mechanisms as Therapeutic Targets. Nat. Rev. Neurol. 2011, 7, 573–583. DOI: 10.1038/nrneurol.2011.137.
- Spallone, V. Management of Painful Diabetic Neuropathy: Guideline Guidance or Jungle? Curr. Diab. Rep. 2012.
- Chong, M. S.; Hester, J. Diabetic Painful Neuropathy: Current and Future Treatment Options. Drugs. 2007, 67, 569–585. DOI: 10.2165/00003495-200767040-00006.
- Rudroju, N.; Bansal, D.; Teja Talakokkula, S.; Gudala, K.; Hota, D.; Bhansali, A.; Ghai, B. Comparative Efficacy and Safety of Six Antidepressants and Anticonvulsants in Painful Diabetic Neuropathy: A Network Meta-Analysis. Pain Physician. 2013, 16, E705–14.
- Baba, M.; Matsui, N.; Kuroha, M.; Wasaki, Y.; Ohwada, S. Mirogabalin for the Treatment of Diabetic Peripheral Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled Phase III Study in Asian Patients. J. Diabetes Investig. 2019, 10(5), 1299–1306. DOI: 10.1111/jdi.13013.
- Tetsunaga, T.; Tetsunaga, T.; Nishida, K.; Misawa, H.; Takigawa, T.; Yamane, K.; Tsuji, H.; Takei, Y.; Ozaki, T. Short-Term Outcomes of Mirogabalin in Patients with Peripheral Neuropathic Pain: A Retrospective Study. J. Orthopaedic Surg Res. 2020, 15, 1–8. DOI: 10.1186/s13018-020-01709-3.
- Javed, S.; Alam, U.; Malik, R. A. Mirogabalin and Emerging Therapies for Diabetic Neuropathy. J. Pain Res. 2018, Volume 11, 1559–1566. DOI: 10.2147/JPR.S145999.
- Barbano, R. L.; Herrmann, D. N.; Hart-Gouleau, S.; Pennella-Vaughan, J.; Lodewick, P. A.; Dworkin, R. H. Effectiveness, Tolerability, and Impact on Quality of Life of the 5% Lidocaine Patch in Diabetic Polyneuropathy. Arch. Neurol. 2004, 61, 914. DOI: 10.1001/archneur.61.6.914.
- Viola, V.; Newnham, H. H.; Simpson, R. W. Treatment of Intractable Painful Diabetic Neuropathy with Intravenous Lignocaine. J. Diabetes Complications. 2006, 20, 34–39. DOI: 10.1016/j.jdiacomp.2005.05.007.
- Dworkin, R. H.; O’connor, A. B.; Backonja, M.; Farrar, J. T.; Finnerup, N. B.; Jensen, T. S.; Kalso, E. A.; Loeser, J. D.; Miaskowski, C.; Nurmikko, T. J., et al. Pharmacologic Management of Neuropathic Pain: Evidence-Based Recommendations. Pain. 2007, 132, 237–251. DOI: 10.1016/j.pain.2007.08.033.
- Finnerup, N. B.; Sindrup, S. H.; Jensen; Jensen, T. S. T.S. The Evidence for Pharmacological Treatment of Neuropathic Pain. Pain. 2010, 150, 573–581. DOI: 10.1016/j.pain.2010.06.019.
- Gimbel, J. S.; Richards, P.; Portenoy, R. K. Controlled-Release Oxycodone for Pain in Diabetic Neuropathy: A Randomized Controlled Trial. Neurology. 2003, 60, 927–934. DOI: 10.1212/01.WNL.0000057720.36503.2C.
- Chou, R.; Fanciullo, G. J.; Fine, P. G.; Adler, J. A.; Ballantyne, J. C.; Davies, P.; Donovan, M. I.; Fishbain, D. A.; Foley, K. M.; Fudin, J., et al. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. J. Pain. 2009, 10, 113–130.e22. DOI: 10.1016/j.jpain.2008.10.008.
- Mcnicol, E. D.; Midbari, A.; Eisenberg, E. Opioids for Neuropathic Pain. Cochrane Database Syst. Rev. 2013, 2019. DOI: 10.1002/14651858.CD006146.pub2.
- Oates, P. J. Polyol Pathway and Diabetic Peripheral Neuropathy. Int. Rev. Neurobiol. 2002, 50, 325–392. DOI: 10.1016/s0074-7742(02)50082-9.
- R, M.; R, O. Targeting Aldose Reductase for the Treatment of Diabetes Complications and Inflammatory Diseases: New Insights and Future Directions. J. Med. Chem. 2015, 58, 2047–2067. DOI: 10.1021/JM500907A.
- Chalk, C.; Benstead, T. J.; Moore, F. Aldose Reductase Inhibitors for the Treatment of Diabetic Polyneuropathy. Cochrane Database Syst. Rev. 2007, 2010. DOI: 10.1002/14651858.CD004572.PUB2.
- Ramirez, M. A.; Borja, N. L. Epalrestat: An Aldose Reductase Inhibitor for the Treatment of Diabetic Neuropathy. Pharmacotherapy. 2008, 28, 646–655. DOI: 10.1592/phco.28.5.646.
- Hotta, N.; Akanuma, Y.; Kawamori, R.; Matsuoka, K.; Oka, Y.; Shichiri, M.; Toyota, T.; Nakashima, M.; Yoshimura, I.; Sakamoto, N., et al. Long-Term Clinical Effects of Epalrestat, an Aldose Reductase Inhibitor, on Diabetic Peripheral Neuropathy: The 3-Year, Multicenter, Comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006, 29, 1538–1544. DOI: 10.2337/dc05-2370.
- Jannapureddy, S.; Sharma, M.; Yepuri, G.; Schmidt, A. M.; Ramasamy, R. Aldose Reductase: An Emerging Target for Development of Interventions for Diabetic Cardiovascular Complications. Front. Endocrinol. (Lausanne). 2021, 12, 78. DOI: 10.3389/FENDO.2021.636267.
- Oyenihi, A. B.; Ayeleso, A. O.; Mukwevho, E.; Masola, B. Antioxidant Strategies in the Management of Diabetic Neuropathy. Biomed Res. Int. 2015, 2015, 1–15. DOI: 10.1155/2015/515042.
- Vinik, A. I.; Bril, V.; Kempler, P.; Litchy, W. J.; Tesfaye, S.; Price, K. L.; Bastyr, E. J. Treatment of Symptomatic Diabetic Peripheral Neuropathy with the Protein Kinase C β-Inhibitor Ruboxistaurin Mesylate During a 1-Year, Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Clin. Ther. 2005, 27, 1164–1180. DOI: 10.1016/j.clinthera.2005.08.001.
- Haupt, E.; Ledermann, H.; Köpcke, W. Benfotiamine in the Treatment of Diabetic Polyneuropathy - a Three-Week Randomized, Controlled Pilot Study (BEDIP Study). Int. J. Clin. Pharmacol. Ther. 2005, 43, 71–77. DOI: 10.5414/CPP43071.
- Jolivalt, C. G.; Fineman, M.; Deacon, C. F.; Carr, R. D.; Calcutt, N. A. GLP-1 Signals via ERK in Peripheral Nerve and Prevents Nerve Dysfunction in Diabetic Mice. Diabetes Obes. Metab. 2011, 13, 990–1000. DOI: 10.1111/j.1463-1326.2011.01431.x.
- Le, T. D.; Nguyen, N. P. T.; Tran, H. T. T.; Cong, T. L.; Nguyen, L. H. T.; Nhu, B. D.; Nguyen, S. T.; Ngo, M. V.; Dinh, H. T.; Nguyen, H. T., et al. Diabetic Peripheral Neuropathy Associated with Cardiovascular Risk Factors and Glucagon-Like Peptide-1 Concentrations Among Newly Diagnosed Patients with Type 2 Diabetes Mellitus. Diabetes, Metab. Syndr. Obes. Targets Ther. 2022, 15, 35–44. DOI: 10.2147/DMSO.S344532.
- Kawanami, D.; Matoba, K.; Sango, K.; Utsunomiya, K. Incretin-Based Therapies for Diabetic Complications: Basic Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2016, 17, 1223. DOI: 10.3390/IJMS17081223.
- Kawano, T. A Current Overview of Diabetic Neuropathy – Mechanisms, Symptoms, Diagnosis, and Treatment. Peripheral Neuropathy; InTech. 2014. DOI: 10.5772/58308.
- Schratzberger, P.; Walter, D. H.; Rittig, K.; Bahlmann, F. H.; Pola, R.; Curry, C.; Silver, M.; Krainin, J. G.; Weinberg, D. H.; Ropper, A. H., et al. Reversal of Experimental Diabetic Neuropathy by VEGF Gene Transfer. J. Clin. Invest. 2001, 107, 1083–1092. DOI: 10.1172/JCI12188.
- Ropper, A. H.; Gorson, K. C.; Gooch, C. L.; Weinberg, D. H.; Pieczek, A.; Ware, J. H.; Kershen, J.; Rogers, A.; Simovic, D.; Schratzberger, P., et al. Vascular Endothelial Growth Factor Gene Transfer for Diabetic Polyneuropathy: A Randomized, Double-Blinded Trial. Ann. Neurol. 2009, 65, 386–393. DOI: 10.1002/ana.21675.
- Ii, M.; Nishimura, H.; Kusano, K. F.; Qin, G.; Yoon, Y. S.; Wecker, A.; Asahara, T.; Losordo, D. W. Neuronal Nitric Oxide Synthase Mediates Statin-Induced Restoration of Vasa Nervorum and Reversal of Diabetic Neuropathy. Circulation. 2005, 112, 93–102. DOI: 10.1161/CIRCULATIONAHA.104.511964.
- Tague, S. E.; Smith, P. G. Vitamin D Receptor and Enzyme Expression in Dorsal Root Ganglia of Adult Female Rats: Modulation by Ovarian Hormones. J. Chem. Neuroanat. 2011, 41, 1–12. DOI: 10.1016/j.jchemneu.2010.10.001.
- Tague, S. E.; Clarke, G. L.; Winter, M. K.; McCarson, K. E.; Wright, D. E.; Smith, P. G. Vitamin D Deficiency Promotes Skeletal Muscle Hypersensitivity and Sensory Hyperinnervation. J. Neurosci. 2011, 31, 13728–13738. DOI: 10.1523/JNEUROSCI.3637-11.2011.
- Basit, A.; Basit, K. A.; Fawwad, A.; Shaheen, F.; Fatima, N.; Petropoulos, I. N.; Alam, U.; Malik, R. A. Vitamin D for the Treatment of Painful Diabetic Neuropathy. BMJ Open Diabetes Res. Care. 2016, 4, 1–6. DOI: 10.1136/bmjdrc-2015-000148.
- Solanki, N. D.; Patel, R.; Bhavsar, S. K.; Pandya, D. T.; Solanki, N. D.; Bhavsar, S. K.; Pandya, D. T. NAAS Score: 4.11; IC Value: 74.82. UGC-India Approved J. Phytopharm. 2018, 7, 152–161. DOI: 10.31254/phyto.2018.7209.
- Liang, Y. Z.; Xie, P.; Chan, K. Quality Control of Herbal Medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 53–70. DOI: 10.1016/S1570-0232(04)00676-2.
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J. G. D., et al. Current Status of Therapeutic Approaches Against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Bio. Sci. 2020, 16, 116–134. DOI: 10.7150/ijbs.35653.
- Al-Adwani, D. G.; Renno, W. M.; Orabi, K. Y.; D’Mello, S. R. Neurotherapeutic Effects of Ginkgo Biloba Extract and Its Terpene Trilactone, Ginkgolide B, on Sciatic Crush Injury Model: A New Evidence. PLoS One. 2019, 14, e0226626. DOI: 10.1371/journal.pone.0226626.
- Choi, K. M.; Kim, D. R.; Kim, N. H.; Baik, S. H.; Choi, D. S. The Effect of Ginkgo Biloba Extract on Diabetic Peripheral Neuropathy - a 12 Week, Randomized, Placebo-Controlled, Double-Blind Trial -. Korean Diabetes J. 2001, 24, 375–384.
- Numan, A.; Masud, F.; Khawaja, K. I.; Khan, F. F.; Qureshi, A. B.; Burney, S.; Ashraf, K.; Ahmad, N.; Yousaf, M. S.; Rabbani, I., et al. Clinical and Electrophysiological Efficacy of Leaf Extract of Gingko Biloba L (Ginkgoaceae) in Subjects with Diabetic Sensorimotor Polyneuropathy. Trop. J. Pharm. Res. 2016, 15, 2137–2145. DOI: 10.4314/tjpr.v15i10.12.
- da Silva, G. G. P.; Zanoni, J. N.; Buttow, N. C. Neuroprotective Action of Ginkgo Biloba on the Enteric Nervous System of Diabetic Rats. World J. Gastroenterol. 2011, 17, 898–905. DOI: 10.3748/wjg.v17.i7.898.
- Cheung, Z. H.; So, K. F.; Lu, Q.; Yip, H. K.; Wu, W.; Shan, J. J.; Pang, P. K. T.; Chen, C. F. Enhanced Survival and Regeneration of Axotomized Retinal Ganglion Cells by a Mixture of Herbal Extracts. J. Neurotrauma. 2002, 19, 369–378. DOI: 10.1089/089771502753594936.
- Carla, L.; Schneider, L.; Perez, G. G.; Banzi, S. R.; Zanoni, J. N.; Raquel, M.; Natali, M.; Buttow, N. C. Evaluation of the Effect of Ginkgo Biloba Extract (EGb 761) on the Myenteric Plexus of the Small Intestine of Wistar Rats. J. Gastroenterol. 2007, 42, 624–630. DOI: 10.1007/S00535-007-2079-Z.
- Kim, J.; Yokoyama, K.; Araki, S. The Effects of Ginkgo Biloba Extract (GBe) on Axonal Transport Microvasculature and Morphology of Sciatic Nerve in Streptozotocin-Induced Diabetic Rats. Environ. Health Prev. Med. 2000, 5, 53–59. DOI: 10.1007/bf02932004.
- Pottathil, S.; Nain, P.; Morsy, M. A.; Kaur, J.; Al-Dhubiab, B. E.; Jaiswal, S.; Nair, A. B. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica Granatum Leaves in Nicotinamide/streptozotocin-Induced Type 2 Diabetes in Rats. Plants. 2020, 9, 1–15. DOI: 10.3390/plants9111609.
- Guerrero-Solano, J. A.; Jaramillo-Morales, O. A.; Velázquez-González, C.; De la O-Arciniega, M.; Castañeda-Ovando, A.; Betanzos-Cabrera, G.; Bautista, M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants. 2020, 9, 1–18. DOI: 10.3390/plants9040419.
- Li, Y.; Wen, S.; Kota, B. P.; Peng, G.; Li, G. Q.; Yamahara, J.; Roufogalis, B. D. Punica Granatum Flower Extract, a Potent α-Glucosidase Inhibitor, Improves Postprandial Hyperglycemia in Zucker Diabetic Fatty Rats. J. Ethnopharmacol. 2005, 99, 239–244. DOI: 10.1016/j.jep.2005.02.030.
- Raafat, K.; Samy, W. Amelioration of Diabetes and Painful Diabetic Neuropathy by Punica Granatum L. Extract and Its Spray Dried Biopolymeric Dispersions. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–12. DOI: 10.1155/2014/180495.
- Nazma Yesmin, M.; Sarder Nasir Uddin, S. M.; Akond, M. A. Antioxidant and Antibacterial Activities of. Society. 2005, 28, 2225–2230.
- Ahmad, M. B.; Gwarzo, M. Y.; Anwar, S. Antioxidative and Anti-Hyperglycaemic Effect of Calotropis Procera in Alloxan Induced Diabetic Rats. J. Med. Plants Res. 2016, 10, 54–58. DOI: 10.5897/jmpr2014.5704.
- Zafar, S.; Rasul, A.; Iqbal, J.; Anwar, H.; Imran, A.; Jabeen, F.; Shabbir, A.; Akram, R.; Maqbool, J.; Sajid, F., et al. Calotropis Procera (Leaves) Supplementation Exerts Curative Effects on Promoting Functional Recovery in a Mouse Model of Peripheral Nerve Injury. Food Sci. Nutr. 2021, 9(9), 2455.
- Zafar, S.; Anwar, H.; Qasim, M.; Irfan, S.; Maqbool, J.; Sajid, F.; Naqvi, S. A. R.; Hussain, G. Calotropis Procera (Root) Escalates Functions Rehabilitation and Attenuates Oxidative Stress in a Mouse Model of Peripheral Nerve Injury. Pak. J. Pharm. Sci. 2020, 33, 2801–2807.
- Neto, M. C. L.; de Vasconcelos, C. F. B.; Thijan, V. N.; Caldas, G. F. R.; Araújo, A. V.; Costa-Silva, J. H.; Amorim, E. L. C.; Ferreira, F.; de Oliveira, A. F. M.; Wanderley, A. G. Evaluation of Antihyperglycaemic Activity of Calotropis Procera Leaves Extract on Streptozotocin-Induced Diabetes in Wistar Rats. Brazilian J. Pharmacogn. 2013, 23, 913–919. DOI: 10.1590/S0102-695X2013000600008.
- Yadav, S. K.; Nagori, B. P.; Desai, P. K. Pharmacological Characterization of Different Fractions of Calotropis Procera (Asclepiadaceae) in Streptozotocin Induced Experimental Model of Diabetic Neuropathy. J. Ethnopharmacol. 2014, 152, 349–357. DOI: 10.1016/j.jep.2014.01.020.
- Thaifa, M. S.; Roshna, N.; Arya, U. S.; Babu, A. G. A Review on Diabetes Mellitus and Diabetic Neuropathy: A Plant Based Approach. J. Pharmacogn. Phytochem. 2017, 6, 506–510.
- Ghazanfar, K.; Ganai, B. A.; Akbar, S.; Mubashir, K.; Dar, S. A.; Dar, M. Y.; Tantry, M. A. Antidiabetic Activity of Artemisia Amygdalina Decne in Streptozotocin Induced Diabetic Rats. Biomed Res. Int. 2014, 2014, 1–10. DOI: 10.1155/2014/185676.
- Wang, Z. Q.; Ribnicky, D.; Zhang, X. H.; Zuberi, A.; Raskin, I.; Yu, Y.; Cefalu, W. T. An Extract of Artemisia Dracunculus L. Enhances Insulin Receptor Signaling and Modulates Gene Expression in Skeletal Muscle in KK-Ay Mice. J. Nutr Biochem. 2011, 22, 71–78. DOI: 10.1016/j.jnutbio.2009.11.015.
- Watcho, P.; Stavniichuk, R.; Tane, P.; Shevalye, H.; Maksimchyk, Y.; Pacher, P.; Obrosova, I. G. Evaluation of PMI-5011, an Ethanolic Extract of Artemisia Dracunculus L., on Peripheral Neuropathy in Streptozotocin-Diabetic Mice. Int. J. Mol. Med. 2011, 27, 299–307. DOI: 10.3892/ijmm.2011.597.
- Watcho, P.; Stavniichuk, R.; Ribnicky, D. M.; Raskin, I.; Obrosova, I. G. High-Fat Diet-Induced Neuropathy of Prediabetes and Obesity: Effect of PMI-5011, an Ethanolic Extract of Artemisia Dracunculus L. Mediators Inflamm. 2010, 2010, 1–10. DOI: 10.1155/2010/268547.
- Shane-McWhorter, L. Dietary Supplements for Diabetes: An Evaluation of Commonly Used Products. Diabetes Spectr. 2009, 22, 206–213. DOI: 10.2337/diaspect.22.4.206.
- Raju, M. G.; Satyanarayana, S.; Kumar, E. Safety of Gliclazide with the Aqueous Extract of Gymnema Sylvestre on Pharmacodynamic Activity in Normal and Alloxan Induced Diabetic Rats. Am. J. Phytomed. Clin. Ther. 2014, 2, 901–909.
- Fatani, A. J.; Al-Rejaie, S. S.; Abuohashish, H. M.; Al-Assaf, A.; Parmar, M. Y.; Ola, M. S.; Ahmed, M. M. Neuroprotective Effects of Gymnema Sylvestre on Streptozotocin-Induced Diabetic Neuropathy in Rats. Exp. Ther. Med. 2015, 9, 1670–1678. DOI: 10.3892/etm.2015.2305.
- Lingumpelly, R.; Jytothirmaye, P.; Naveen Kumar, G.; Ravi Kumar, M. Anti Diabetic Neuropathy and Pharmacological Evaluation of the Indian Traditional Herb Gymnema Sylvestre. Int. J. Toxicol. Pharmacol. Res. 2015, 7, 60–64.
- Jain, V.; Pareek, A.; Ratan, Y.; Singh, N. Standardized Fruit Extract of Momordica Charantia L Protect Against Vincristine Induced Neuropathic Pain in Rats by Modulating GABAergic Action, Antimitotoxic, NOS Inhibition, Anti-Inflammatory and Antioxidative Activity. South African J. Bot. 2015, 97, 123–132. DOI: 10.1016/j.sajb.2014.12.010.
- Malik, Z. A.; Singh, M.; Sharma, P. L. Neuroprotective Effect of Momordica Charantia in Global Cerebral Ischemia and Reperfusion Induced Neuronal Damage in Diabetic Mice. J. Ethnopharmacol. 2011, 133, 729–734. DOI: 10.1016/j.jep.2010.10.061.
- Malik, Z. A.; Tabassum, N.; Sharma, P. L. Attenuation of Experimentally Induced Diabetic Neuropathy in Association with Reduced Oxidative-Nitrosative Stress by Chronic Administration of Momordica Charantia. Adv. Biosci. Biotechnol. 2013, 04, 356–363. DOI: 10.4236/abb.2013.43047.
- Mahmood, K. T.; Mugal, T.; Haq, I. U. Moringa Oleifera: A Natural Gift-A Review. J. Pharm. Sci. Res. 2010, 2, 775–781.
- Razzaq, A.; Ahmad Malik, S.; Saeed, F.; Imran, A.; Rasul, A.; Qasim, M.; Zafar, S.; Kamran, S. K. S.; Maqbool, J.; Imran, M., et al. Moringa Oleifera Lam.Ameliorates the Muscles Function Recovery Following an Induced Insult to the Sciatic Nerve in a Mouse Model. Food Sci. Nutr. 2020, 8, 1–8. DOI: 10.1002/fsn3.1620.
- Khongrum, J.; Wattanathorn, J.; Muchimapura, S.; Thukhum-Mee, W.; Thipkaew, C.; Wannanon, P.; Tong-Un, T. Moringa Oleifera Leaves Extract Attenuates Neuropathic Pain Induced by Chronic Constriction Injury. Am. J. Appl. Sci. 2012. DOI: 10.3844/ajassp.2012.1182.1187.
- Raafat, K.; Hdaib, F. Neuroprotective Effects of Moringa Oleifera: Bio-Guided GC-MS Identification of Active Compounds in Diabetic Neuropathic Pain Model. Chin. J. Integr. Med. 2017. DOI: 10.1007/s11655-017-2758-4.
- Zahara, K. Clinical and Therapeutic Benefits of Centella Asiatica. Pure Appl. Biol. 2014, 3, 152–159. DOI: 10.19045/bspab.2014.34004.
- Lou, J. -S.; Dimitrova, D. M.; Murchison, C.; Arnold, G. C.; Belding, H.; Seifer, N.; Le, N.; Andrea, S. B.; Gray, N. E.; Wright, K. M., et al. Centella Asiatica Triterpenes for Diabetic Neuropathy: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Study. Esperienze dermatologiche. 2018, 20. DOI: 10.23736/S1128-9155.18.00455-7.
- Khan, M. Y.; Panchal, S.; Vyas, N.; Butani, A.; Kumar, V. Olea Europaea: A Phyto-Pharmacological Review. Pharmacogn. Rev. 2007, 1(1), 114–118.
- Guex, C. G.; Reginato, F. Z.; de Jesus, P. R.; Brondani, J. C.; Lopes, G. H. H.; Bauermann, L. D. F. Antidiabetic Effects of Olea Europaea L. Leaves in Diabetic Rats Induced by High-Fat Diet and Low-Dose Streptozotocin. J. Ethnopharmacol. 2019, 235, 1–7. DOI: 10.1016/j.jep.2019.02.001.
- Kaeidi, A.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M.; Rasoulian, B.; Hajializadeh, Z.; Afrazi, S. Olive (Olea Europaea L.) Leaf Extract Attenuates Early Diabetic Neuropathic Pain Through Prevention of High Glucose-Induced Apoptosis: In vitro and in vivo Studies. J. Ethnopharmacol. 2011, 136, 188–196. DOI: 10.1016/j.jep.2011.04.038.
- Spandana, U.; Ali, S. L.; Nirmala, T.; Santhi, M.; Sipai Babu, S. D. A Review on Tinospora Cordifolia. Int. J. Curr. Pharm. Rev. Res. 2013, 4(2), 61–68.
- Nadig, P.; Aliyar, R.; Dethe, S.; Narayanswamy, S.; Revankar, M. Effect of Tinospora Cordifolia on Experimental Diabetic Neuropathy. Indian J. Pharmacol. 2012, 44, 580. DOI: 10.4103/0253-7613.100380.
- Zangiabadi, N.; Asadi-Shekaari, M.; Sheibani, V.; Jafari, M.; Shabani, M.; Asadi, A. R.; Tajadini, H.; Jarahi, M. Date Fruit Extract is a Neuroprotective Agent in Diabetic Peripheral Neuropathy in Streptozotocin-Induced Diabetic Rats: A Multimodal Analysis. OXID. MED. CELL LONGEV. 2011, 2011, 1–9. DOI: 10.1155/2011/976948.
- Mia, M. A. T.; Mosaib, M. G.; Khalil, M. I.; Islam, M. A.; Gan, S. H. Potentials and Safety of Date Palm Fruit Against Diabetes: A Critical Review. Foods. 2020, 9, 1–21. DOI: 10.3390/foods9111557.
- Heydari, M.; Homayouni, K.; Hashempur, M. H.; Shams, M. Topical Citrullus Colocynthis (Bitter Apple) Extract Oil in Painful Diabetic Neuropathy: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Diabetes. 2016, 8, 246–252. DOI: 10.1111/1753-0407.12287.
- Hussain, A. I.; Rathore, H. A.; Sattar, M. Z. A.; Chatha, S. A. S.; Sarker, S. D.; Gilani, A. H. Citrullus Colocynthis (L.) Schrad (Bitter Apple Fruit): A Review of Its Phytochemistry, Pharmacology, Traditional Uses and Nutritional Potential. J. Ethnopharmacol. 2014, 155, 54–66. DOI: 10.1016/j.jep.2014.06.011.
- Ostovar, M.; Akbari, A.; Anbardar, M. H.; Iraji, A.; Salmanpour, M.; Hafez Ghoran, S.; Heydari, M.; Shams, M. Effects of Citrullus Colocynthis L. in a Rat Model of Diabetic Neuropathy. J. Integr. Med. 2020, 18, 59–67. DOI: 10.1016/j.joim.2019.12.002.
- Cardoso, A. L. B. D.; Frederico, É. H. F. F.; Guimarães, C. A. S.; Moura-Fernandes, M. C.; Guedes-Aguiar, E. O.; da Silva, A. L. P.; Reis-Silva, A.; Francisca-Santos, A.; de Souza, L. F. F.; Mendonça-Guimarães, R., et al. Effects of Coriandrum Sativum L. in Association with Physical Exercise in Alloxan-Induced Type 1 Diabetes Mellitus in Rats. Appl. Sci. 2019, 9, 5409. DOI: 10.3390/app9245409.
- Kajal, A.; Singh, R. Coriandrum Sativum Seeds Extract Mitigate Progression of Diabetic Nephropathy in Experimental Rats via AGEs Inhibition. PLoS One. 2019, 14, e0213147. DOI: 10.1371/journal.pone.0213147.
- Kajal, A.; Singh, R. Coriandrum Sativum Improve Neuronal Function via Inhibition of Oxidative/Nitrosative Stress and TNF-α in Diabetic Neuropathic Rats. J. Ethnopharmacol. 2020, 263, 112959. DOI: 10.1016/j.jep.2020.112959.
- Anjaneyulu, M.; Chopra, K. Quercetin, a Bioflavonoid, Attenuates Thermal Hyperalgesia in a Mouse Model of Diabetic Neuropathic Pain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003, 27, 1001–1005. DOI: 10.1016/S0278-5846(03)00160-X.
- Je, H. D.; Shin, C. Y.; Park, S. Y.; Yim, S. H.; Kum, C.; Huh, I. H.; Kim, J. H.; Sohn, U. D. Combination of Vitamin C and Rutin on Neuropathy and Lung Damage of Diabetes Mellitus Rats. Arch. Pharm. Res. 2002, 25, 184–190. DOI: 10.1007/BF02976561.
- Dodda, D.; Ciddi, V. Plants Used in the Management of Diabetic Complications. Indian J. Pharm. Sci. 2014, 76, 97.
- Maqbool, J.; Anwar, H.; Rasul, A.; Imran, A.; Saadullah, M.; Malik, S. A.; Shabbir, A.; Akram, R.; Sajid, F.; Zafar, S., et al. Comparative Evaluation of Ethyl Acetate and N-Hexane Extracts of Cannabis Sativa L. Leaves for Muscle Function Restoration After Peripheral Nerve Lesion. Food Sci. Nutr. 2023, 00, 1–9. DOI: 10.1002/fsn3.3255.
- Islam, F.; Amer Ali, Y.; Imran, A.; Afzaal, M.; Zahra, S. M.; Fatima, M.; Saeed, F.; Usman, I.; Shehzadi, U.; Mehta, S., et al. Vegetable Proteins as Encapsulating Agents: Recent Updates and Future Perspectives. Food Sci. Nutr. 2023, 00, 1–13. DOI: 10.1002/fsn3.3234.
- Bagdas, D.; Cinkilic, N.; Ozboluk, H. Y.; Ozyigit, M. O.; Gurun, M. S. Antihyperalgesic Activity of Chlorogenic Acid in Experimental Neuropathic Pain. J. Nat. Med. 2013, 67, 698–704. DOI: 10.1007/s11418-012-0726-z.
- Baluchnejadmojarad, T.; Roghani, M. Chronic Epigallocatechin-3-Gallate Ameliorates Learning and Memory Deficits in Diabetic Rats via Modulation of Nitric Oxide and Oxidative Stress. Behav. Brain Res. 2011, 224, 305–310. DOI: 10.1016/j.bbr.2011.06.007.
- Uzar, E.; Alp, H.; Cevik, M. U.; Firat, U.; Evliyaoglu, O.; Tufek, A.; Altun, Y. Ellagic Acid Attenuates Oxidative Stress on Brain and Sciatic Nerve and Improves Histopathology of Brain in Streptozotocin-Induced Diabetic Rats. Neurol. Sci. 2012, 33, 567–574. DOI: 10.1007/s10072-011-0775-1.