ABSTRACT
Wild edible fruits are found globally and play multifarious functions. The present study was undertaken to study the antioxidants and anti-cancerous potential of six wild edible fruits from Mizoram viz. Garcinia lanceifolia, Embelia subcoriacea, Elaeocarpus tectorius, Elaeagnus caudata, Cissus obovata and Averrhoa carambola. The fruits were identified through ITS1 and ITS2 partial genes and the sequences were submitted to GenBank depository and NCBI databases. Physical and physico-chemical characteristics were assessed for their importance. The phytochemical content of the fruits was analyzed qualitatively and shown the presence of saponin, flavonoids, tannin, cardiac glycosides, alkaloids and terpenoids. The extracts were then subjected to quantification of secondary metabolites, such as gallic acid, rutin and quercetin. Further, secondary metabolites, such as gallic acid, rutin, quercetin, hesperidin and naringin were quantified by the HPLC method, which showed significantly high quantity from the extracts. The free radical scavenging activity performed using DPPH and ABTS cations revealed that the extracts possess high potency for scavenging properties, with IC50 ranging from 10.37 to 567.8 µg ml−1. The cytotoxicity of the extracts assessed against A549 cancer cell line showed potency, with IC50 value ranging from 18.2 to 203.2 µg ml−1. Therefore, the present study revealed that wild edible fruits could be a good resource for ethno-medicine as well as income generation for the local people of Mizoram, north-east India.
Introduction
Forests are a direct source of food and income for a large proportion of the world’s poorest people, providing both staple foods and supplemental foods, such as fruits, leaves and nuts. Evidence indicates that above 300 million people throughout the modern world gain part or all of their livelihood and food from forests.[Citation1] From the past, in different parts of world, these wild edible plants play a very important role in the livelihoods of rural communities as being an integral part of the subsistence strategy of people in many developing countries. It constitutes essential components in the diet and food security of many tribal communities, particularly those living in its vicinity. They serve as alternatives to staple foods during periods of food deficit and are the valuable supplements for a nutritional balanced diet as well as one of the primary alternative sources of income for many resource poor communities and the source of species for domestication. They have outstanding adaptation capabilities like drought tolerance, salt tolerance and are also resistant to major pests and diseases.[Citation2] Apart from their traditional use of food, potentially these wild plants have also been used as medicine, fodder, and for rituals and other functions. They also provide minerals like sodium, potassium, magnesium, iron, calcium, and phosphorus. During the recent past, due to the introduction of exotic varieties, the dependency on wild fruits has gradually declined. But many people in tribal areas still use them as a supplement to their food. With ever-increasing population and consequent shortage of food grains, it is considered that special attention should be paid in order to conserve and improve this important source of food supply.
In India, most of the tribal people live close to or within forests and for their food and energy needs they depend on forests as well as wild plants therein. All these tribal people have their own food habits. The wild fruits found in the forests nearby these tribal people have a great role in the socio-cultural and economic lives of these people. A number of studies have been carried out in different locations of India, recorded consumption of wild edibles by tribals and the rural poor in a few locations in India,[Citation3] but information on edible fruits is scattered only in botanical monographs and informal notes. With modernization and settled agriculture, this inherited knowledge is becoming lost, a trend that may lead to decreased diversity of indigenous diets and poorer nutrition.[Citation4] So, it is high time for the scientific community to study and document these non-traditional, noncommercial but high-valued crops in order to validate, quantify and conserve them for future generation.
The north-eastern hill region (NEH) of India, being one of the hot-spots of biodiversity in the Indian gene center, is also known for its richness in ethnic diversity and traditional culture.[Citation5] It falls under the Himalayas and the Indo-Burma biodiversity hotspots, forming a unique biogeographic province harboring major biomes recognized around the world. There are approximately 225 tribes in this region, out of the total 450 tribes that are found in the country.[Citation6] The immense variety of the climatic, edaphic and altitudinal variations in North-eastern India have resulted in a great range of ecological habitats. The lushness of its landscape, favorable climatic conditions, the range of communities and geographical and ecological diversity makes the North-east part of India is quite different from other parts of the subcontinent. The region has been in spotlight for its high biodiversity and traditional knowledge, and this region has been a priority for leading conservation agencies around the world. A large number of ethnic people are also inhabited in these parts of India. These tribes also pose vast traditional knowledge on effective herbal medicines, which was acquired through experience and are usually passed on by oral traditions as a guarded secret of certain families.
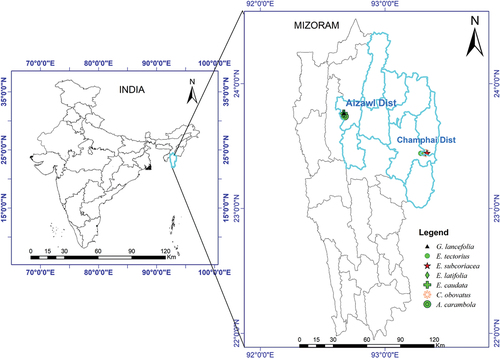
Mizoram, one of the eight states in the NEH region, has the highest proportion of tribal population (94.8%) and is mainly inhabited by Mizos, Maras, Lais, Pangs, Bawms, Hmars, Paites, Brus, Chakmas, and Mogs.[Citation7,Citation8] The state is an expanse of blue-green hills and the 23rd state of the Indian Union in the extremes of the Himalayan ranges and covers an area of 21, 087 sq. km, is situated between Myanmar and Bangladesh (). It is located in between 21º58´ N and 24 º35´N latitudes and 92º16´E and 93 º29´E longitudes and bounded by the Cachar district of Assam in the North, Manipur in the North-east, Myanmar in the East and South and Bangladesh, and the state of Tripura in the West. The state comprises mostly hilly terrains with deep gorges in between the hill ranges running in the North-South direction with an average height of 920 m above msl. The mountain ranges run in north-to-south direction, intercepted by narrow deep valleys and criss-crossed by innumerable small hillocks. The majority of the population is rural and prefers to grow primitive landraces of crop plants by shifting cultivation (“‘Jhum’” farming). In order to fulfill their basic requirements, they depend on the wild economic species and to make these resources easily accessible cultivate some of these plants in their kitchen gardens. The tribal people of the state have been consuming these wild fruits since very ancient times without knowing their nutritive and medicinal values.[Citation8,Citation9] Considerable diversity exists among these indigenous fruit species including variations in plant type, morphological and physiological characteristics, reactions to diseases and pests, adaptability and distribution. However, so far, there has not been any properly documented study on the anti-oxidant and anti-cancerous properties of these valuable wild edible fruits of Mizoram. Hence, the present study was undertaken for scientific validation of wild edible fruits including its anti-oxidant values and anti-cancerous properties in Mizoram, North-East India.
Materials and methods
Sampling
Six different wild edible fruits viz., Averrhoa carambola, Cissus obovata, Elacocarpus tectorius were collected from Champhai District and Elaeagnus caudata, Embelia subcoriacea and Garcinia lanceifolia were collected from Aizawl District, Mizoram () The collected specimens were identified with the help of literature, and by using various floras and monographs including regional flora and the data collected were compared and cross-checked with other recommended literature.[Citation10,Citation11] And after proper identification, the specimens were processed, dried and herbarium specimens were prepared according to conventional herbarium techniques. Voucher specimens of the collected wild edible fruit species were deposited in the department of Horticulture, Aromatic and Medicinal Plants, Mizoram University (MZU-HAMP-GL, MZU-HAMP-ES, MZU-HAMP-ET, MZU-HAMP-EC, MZU-HAMP-CO and MZU-HAMP-AC).
Molecular identification
The molecular identification was performed by DNA Sanger sequencing where ribosomal internal transcribed spacer (ITS1 and ITS2) rRNA gene was used. Genomic DNA was extracted using DNeasy Plant kit Cat No. 69204, Qiagen. The PCR reaction was prepared for 25 µl according to standard protocol (Phire Plant Direct PCR Master Mix Cat. No. F160S) using forward primer 5’- GAA GGA GAA GTC GTA ACA AGG-3’ and reverse primer 5’-TCC TCC GCT TAT TGA TAT GC-3.’ The sequencing was performed using Genetic analyzer, Thermo fisher. The raw sequence was analysed, and Dendrogram was constructed using MEGA7. The sequences were submitted to GenBank depository, NCBI to obtain their accession number. Further, RNA secondary structure was predicted by RNAfold server.[Citation12]
Measurement of fruits attributes
Data on physical parameters like fruit weight was recorded as per standard procedures with the help of an electronic balance. Fruit length and fruit diameter were measured with the help of digital Vernier calipers. Quality parameters like juice, TSS, acidity, ascorbic acid, reducing, non-reducing and total sugars were estimated following standard procedures. The standard method[Citation13] was followed to determine the titratable acidity, reducing, non-reducing and total sugars of fruit. Visual titration method[Citation14] was followed for the estimation of ascorbic acid content of the fruit pulp, and the result was expressed in mg g−100.
Preparation of the Fruit extracts
Wild fruits such as Averrhoa carambola, Cissus obovata, Elacocarpus tectorius, Elaeagnus caudata, Embelia subcoriacea and Garcinia lanceifolia were washed with distilled water, crushed by electric grinder; the juices were collected and filtered. The filtrates were further fractionated with methanol followed by filtration. The filtrates were subjected for rotary evaporation until dryness. The crude extracts were used for experimentation and referred as extract with their respective abbreviated names ACE (Averrhoa carambola), COE (Cissus obovata), ETE (Elacocarpus tectorius), ECE (Elaeagnus caudata), ESE (Embelia subcoriacea) and GLE (Garcinia lanceifolia). The extracts were kept at −20°C until further use.
Preliminary phytochemical screening
The presence of phyto-constituents including saponins, flavonoids, alkaloids, tannins, steroids, terpenoids and cardiac glycosides were screened using standard protocols for fruit extracts, such as ACE, COE, ETE, ECE, ESE and GLE.[Citation15,Citation16]
Total phenols, flavonoid content and free radical activity
The total phenol was determined by Folin-Ciocalteau,[Citation17] and total flavonoids were measured accordingly.[Citation18] The standardization of phenol was prepared using gallic acid, and quercetin or rutin was used for flavonoid calibration. Stable free radical scavenging activity by DPPH assay[Citation19] and ABTS•+ [Citation20] was performed to examine their scavenging efficiency.
High-performance liquid chromatography
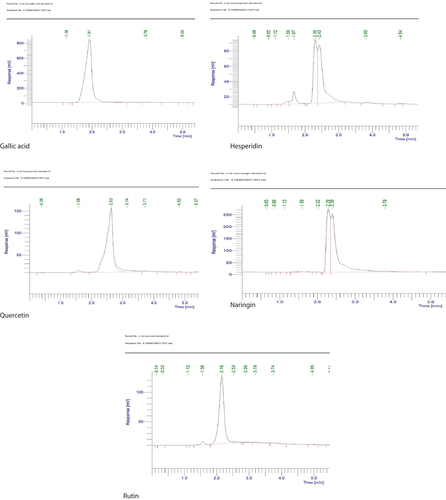
HPLC was carried out to quantify the bioactive compound content of ACE, COE, ETE, ECE, ESE and GLE against standard compounds procured from Himedia (M/s. Warkem Biotech Pvt. Ltd, Mumbai, India), such as rutin (purity ≥ 90%), gallic acid (purity ≥ 98%), naringin (purity ≥ 95%), hesperidin (purity ≥ 98%) and quercetin (purity ≥ 98%) by UltiMate 3000 HPLC v 6.3.1.0504. Their contents were calculated and expressed as the mg g−1 content of the extract.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay
The cytotoxicity of the fruits extract against A549 cells was performed by MTT assay.[Citation21] Approximately, 10000 cells per well were seeded into 96-well plates and incubated for 12–16 h to ensure that the cells were metabolically active. After 16 h, cells were treated with various concentrations (10–100 µg.l−1) and incubated for 48 h followed by the addition of 10 µl MTT (5 mg/ml) solution. The plate was kept in CO2 incubator for 4 h to ensure complete formation of purple crystals by metabolically active cells. The media was removed, and the purple crystal formed was dissolved in 150 µl DMSO. The optical density was measured using a Spectramax-M2e Reader (Molecular Devices Inc.) at 570 nm. The cytotoxicity was determined by untreated cells as 100% viable control and Doxorubicin (DOX) was used as the positive control and the results were shown as Mean ± SEM of triplicates.
Statistical analysis
The data was analyzed using Microsoft Excel 2010 and Graph pad prism (v6). All data were obtained in three different experiments, analyzed and expressed as Mean± SD, N = 3 and the P < .05 was considered significant.
Results
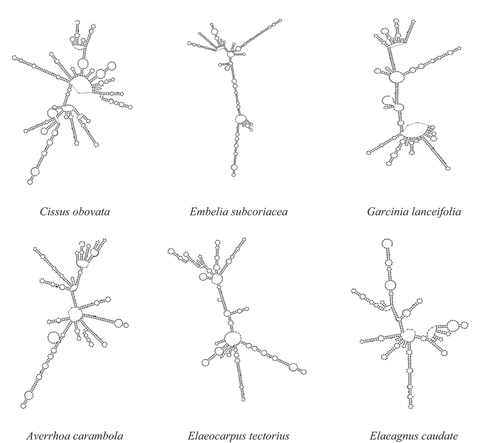
The molecular identification was carried out using ITS1 and ITS2 genes, the sequences had been submitted to GenBank, NCBI database and their accession numbers were MN511173 (Garcinia lanceifolia), MW219729 (Embelia subcoriacea), MW219728 (Elaeocarpus tectorius), MW219727 (Elaeagnus caudata), MN511174 (Cissus obovata) and MN511172 (Averrhoa carambola) (). The genetic distance revealed that wild edible fruits were different from one another with p- distance ranging from 0.00 to 1.14. The phylogenetic tree indicated that the plant species were branched according to their genus ).
Figure 2a. Dendogram of wild edible fruits by Maximum Likelihood method using ITS (ITS1-5.8S-ITS2). Red fonts indicate present study. Number at nodes represent bootstrap support.
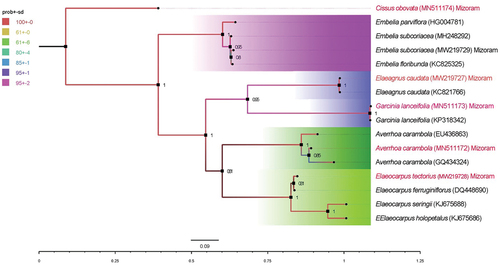
Table 1. The molecular identification of wild edible fruits with uncorrected nucleotide p- distance of ITS gene from Mizoram, north-east India.
The physico-chemical characteristics of wild edible fruits are shown in . Among all the fruits, A. carambola fruit was heaviest, longest and biggest among all fruits study. E. subcoriacea was recorded as the lowest weight and smallest in size of all fruits. The sequence of weight, length and diameter of wild edible fruit recorded as A. carambola (91.97 g, 51.10 mm, 139.7 mm) >G. lanceofolia (70.68 g, 35.78 mm, 51.10 mm) >E. tectorius (38.99 g, 42.78 mm, 42.78 mm) >E. caudata (7.02 g, 20.32 mm, 38.1 mm) >C. obovata (6.32 g, 21.63 mm, 22.70 mm) >E. subcoriacea (1.01 g, 9.52 mm, 11.01 mm). Moreover, their respective moisture content in decreasing order as A. carambola (86.39%) >G. lanceofolia (85.63%) >E. caudata (75.76%) >C. obovata (73.06%) >E. tectorius (63.46%) >E. subcoriacea (54.34%) () The TSS content of the different wild edible fruits was recorded as E. subcoriacea (13.67°B), C. obovata (15.1°B), E. tectorius (8.55°B), A. carambola (9.46°B), G. lanceofolia (12.83°B) and E. caudata (11.13°B). The titratable acidity content of different wild edible fruit recorded is E. subcoriacea (39.36%), C. obovata (19.7%), E. tectorius (0.57%), A. carambola (0.64%), G. lanceofolia (12.16%) and E. caudata (3.904%). The ascorbic acid content of different wild edible fruit are recorded as E. subcoriacea (24.84 mg/100 g), C. obovata (20.01 mg/10 g), E. tectorius (13.8 mg/100 g), A. carambola (15.87 mg/100 g), G. lanceifolia (57.96 mg/100 g) and E. caudata (28.29 mg/100 g). The different wild edible fruit reducing sugar content are recorded as E. subcoriacea (3.33%), C. obovata (3.22%), E. tectorius (3.57%), A. carambola (4.35%), G. lanceifolia (2.46%) and E. caudata (0.8%). The total sugar content was recorded as E. subcoriacea (5.29%), C. obovata (4.28%), E. tectorius (5.7785%), A. carambola (4.65%), G. lanceifolia (3.38%) and E. caudata (4.04%). The non-reducing sugar contents of different wild edible fruit are recorded as E. subcoriacea (1.85%), C. obovata (1.01%), E. tectorius (2.38%), A. carambola (0.52%), G. lanceifolia (1.05%) and E. caudata (3.08%). The sugar: acid ratio of different wild edible fruit is recorded as E. subcoriacea (0.13%), C. obovata (0.22%), E. tectorius (10.04%), A. carambola (7.27%), G. lanceifolia (0.09%) and E. caudata (0.78%). The TSS: acid ratio of different wild edible fruit is recorded as E. subcoriacea (0.35%), C. obovatus (0.77%), E. tectorius (15.1%), A. carambola (14.78%), G. lanceofolia (1.05%) and E. caudata (2.85%).
Table 2. Physico-Chemical Characteristics of wild edible fruits of Mizoram, north-east India.
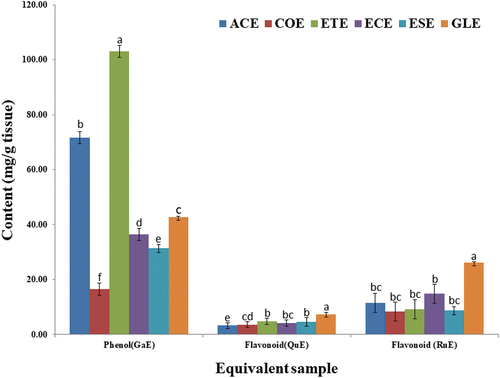
The phytochemical analysis of different wild edible fruit extracts, such as A. carambola (ACE), C. obovate (COE), E. caudate (ECE), E. tectorius (ETE), E. subcoriacea (ESE) and G. lanceifolia (GLE), showed the presence of saponin, flavonoids and terpenoids whereas steroids was absent in all the samples. Tannin was found in ETE, ACE, ECE. Cardiac glycoside was absent only in COE. However, alkaloid was found only in ACE (). In addition, all the wild edible fruits are also a rich source of secondary metabolites as depicted in . The content was shown as gallic acid, rutin and quercetin equivalent content in the parenthesis meta as ACE (71.71, 11.53, and 3.25) COE (23.51, 16.60, and 5.20) ETE (103.51, 18.47, and 5.72) ECE (36.45, 14.8175, and 4.18) ESE (38.21, 17.310, and 5.29) and GLE (42.65, 26.11, and 7.35) ().
Table 3. Preliminary phytochemical analysis of wild edible fruits of Mizoram, north-east India.
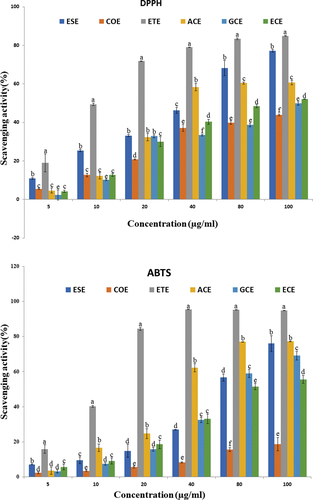
With respect to the antioxidant content of the studied wild edible fruits, all of them are rich sources of antioxidants. The stable DPPH-free radical scavenging activity of wild edible fruits extracts rise with increases in concentration manner up to a certain concentration and declined thereafter. Their 50% inhibitory concentrations (IC50) were ACE (37.35 µg ml−1), COE (125.8 µg ml−1), ECE (88.44 µg ml−1), ESE (30.17 µg ml−1), GLE (91.9 µg ml−1) and ETE (11.93 µg ml−1). The ABTS cations decolorization activity of wild edible fruits extracts with IC50 were ACE (10.37 µg ml−1), COE (567.8 µg ml−1), ECE (61.86 µg ml−1), ESE (62.43 µg ml−1), ETE (10.51 µg ml−1) and GLE (81.7 µg ml−1) ().
HPLC quantification of the secondary metabolites using reference compound such as gallic acid, quercetin, hesperidin, naringin, rutin revealed that the fruits extract contains considerably high quantity (). The gallic acid content was recorded as ESE (0.44 ± 0.08 mg), COE (0.16 ± 0.21 mg), ETE (0.62 ± 0.23 mg), ACE (0.22 ± 0.10 mg), GLE (0.15 ± 0.06 mg) and ECE (0.15 ± 0.05 mg). The quercetin content was recorded as ESE (2.45 ± 0.58 mg), COE (0.90 ± 0.31 mg), ETE (3.43 ± 0.14 mg), ACE (1.21 mg), GLE (0.85 ± 0.54 mg) and ECE (0.83 ± 0.24 mg). The hesperidin content was recorded as ESE (3.49 ± 0.14 mg), COE (1.28 ± 0.87 mg), ETE (4.89 ± 0.15 mg), ACE (1.72 ± 0.95 mg), GLE (1.21 ± 0.12 mg) and ECE (1.19 ± 0.70 mg). The naringin content was recorded as ESE (1.18 ± 0.61 mg), COE (0.43 ± 0.07 mg), ETE (1.66 ± 0.60 mg), ACE (0.58 ± 0.11 mg), GLE (0.41 ± 0.13 mg) and ECE (0.40 ± 0.14 mg). The rutin content was recorded as ESE (4.41 ± 0.50 mg), COE (1.62 ± 0.24 mg), ETE (6.19 ± 0.62 mg), ACE (2.18 ± 0.98 mg), GLE (1.53 ± 0.84 mg) and ECE (1.50 ± 0.18 mg) ().
Table 4. HPLC quantification of bioactive compounds of wild edible fruits extract of Mizoram, north-east India.
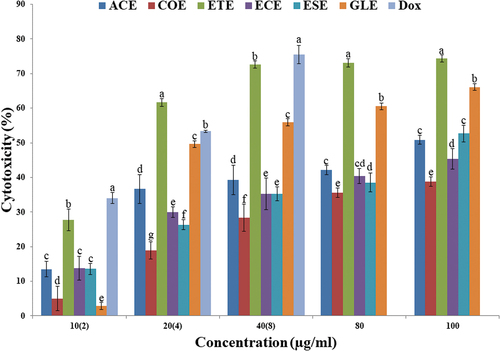
The cytotoxicity of edible fruits extract against A549 cancer cell line showed the increase in cytotoxicity with increase in concentration and with their IC50 values were indicate in the parenthesis such as ACE (101 µg ml−1), COE (203.2 µg ml−1), ECE (142.4 µg ml−1), ESE (89.76 µg ml−1), ETE (18.2 µg ml−1), GLE (21.98 µg ml−1) and DOX (3.25 µg ml−1) ().
Discussion
Wild edible fruits are distributed globally and displayed remarkable multifunctions.[Citation22] It has been studied by many researchers in relation to livelihoods, markets and upliftment of rural areas in many countries. They supply essential nutrients, alkaloids, oils and phenolics derived from secondary metabolism that may be lacking in the normal dietary constituents of rural countryside since the dietary habits of ethnic communities in the different regions are dependent upon obtainability and convenience of seasonal locally grown foods.
The molecular identification was carried out using ITS (ITS1–5.8S-ITS2) partial gene. ITS region sequence analysis is simple, rapid, and significantly reliable that can be used as an effective tool for identification.[Citation23] The genetic distance revealed that edible wild fruits were different from one another with p- distance ranging from 0.00 to 1.14. The phylogenetic trees were reconstructed by the NJ and MP methods and it indicated that the plants species were clustered according to their genus. It could be understood that E. subcoriacea, C. obovata, E. tectorius, A. carambola, G. lanceifolia and E. caudata were nested and shared the highest similarity based on the ITS2 secondary structure.
Physico-chemical characteristics of the fruits consist of various physical and chemical parameters, which determine the quality of fruits and its acceptability by the consumers. Consumers always want to buy the fruits with big size, color and taste. In addition, fruits with high juice content, less acidity high TSS and high ascorbic acid are always preferred by the consumers. Our study recorded that among the six wild fruits reported in the study, A. carambola was heaviest as well as biggest and E. subcoriacea was lightest and smallest. The moisture content in the fruit represents the amount of water present in the fruits and determines the nutritive value of the fruit. It is an important criterion to consider the suitability before consumption since it affects the physico-chemical traits of fruit, which relates with the freshness and stability for the storage of the food for a longer duration as well as the subsequent processing. It also influences the taste, texture, weight, appearance, and shelf life of foodstuffs.[Citation24] It has direct economic importance to the processor and the consumer. In the present study, among the studied fruits, the maximum moisture content was found in A. carambola and minimum in E. subcoriacea. The TSS content of the fruit juice was found to be highly significant ranging from 8.55° to 13.67°Brix. The TSS content was highest in C. obovata and lowest in E. tectorius. The variation in TSS may be due to different genetical constitution of the individual genotypes. Fruits growing in arid regions with limited water tended to accumulate more of dry matter and lower moisture, which may result in higher TSS in fruits.[Citation25] Breeders during selection of superior genotypes should emphasize fruits with higher TSS. It is well-known fact that sugars and organic acids are the most important determinants of fruit quality; organic acids not only influence the fruit flavor but also affect the fruit senescence process and determines storage performance and a high concentration of organic acids or a low pH value, which indicates high storage potential.[Citation26] In the present investigation, among the studied wild fruits, the lowest titratable acidity was reported in E. tectorius, while, it was highest in E. subcoriacea. The reducing sugar was maximum in A. carambola, and the least was in E. caudata. Non-reducing sugar was highest in E. tectorius, and lowest in A. carambola. Total sugar content was highest in E. tectorius and lowest in G. lanceifolia. Ascorbic acid content is also one of the most important criteria in determining the superiority of fruits. In the present investigation, among all the fruits, the maximum ascorbic acid was found in G. lanceifolia, while it was the lowest in E. tectorius. It is a fact that, if TSS increases, the ascorbic acid also increases because the precursor of ascorbic acid is glucose- 6-phosphate,[Citation27] which also confirmed from our study.
Secondary metabolites, such as saponin, phenols and flavonoids, are present in all vascular plants. They stimulate, protect, flavor and contribute pigment to the plants besides playing an important part in plant-micro-organism communication.[Citation28] They also exhibit anti-allergic[Citation29] and anti-osteoporotic[Citation30] activities. In the present study, the phytochemical analysis of the different wild edible fruit extracts has shown the presence of saponin, terpenoids and flavonoids in all the extracts, whereas alkaloids were found only in ACE and none of the fruit extracts has shown the presence of steroids. ACE was found to possess the highest active compounds, and COE was the lowest among fruit extracts.
HPLC quantification of the secondary metabolites revealed that the fruits extracts contain considerably high quantity of the reference compounds, such as gallic acid, quercetin, hesperidin, naringin and rutin. The ETE was found to contain the highest bioactive compounds relating to referenced compounds used among the fruits extracts. Therefore, our study showed that these edible compounds play a vital role in the bio-activities. Quercetin is a known potent antioxidant, which has protective efficiency against various cell injuries caused by the toxic drug[Citation31] and prevents damage to LDL cholesterol, lower cholesterol and inhibit LDL oxidation.[Citation32] It also possesses potential anticancer properties including antiproliferative and growth factor suppression.[Citation33] Our study revealed the presence of quercetin, which might support their biological activity of the extract. Gallic acids are known to induce apoptosis and act as anticancer.[Citation34] Rutin enhances antioxidant activity, acts as a detoxification agent, inhibits proliferation,[Citation35] restores chemo-sensitivity[Citation36] and exhibits anti-neuroblastoma.[Citation37] It acts as an antimicrobial, antiviral, anti-inflammatory, neuroprotective, antiulcerogenic, antiparasitic, antitumoral and antioxidant.[Citation38] Our study showed the presence of gallic acid that juice extracts might also be an essential source of ethnomedicine for tribal people in this region. Ferulic acid is a known potent antioxidant directly involved with the protection of cellular structures and inhibits melanogenesis.[Citation39] Ferulic acid prevents cell proliferation by inducing cell cycle arrest, which results in apoptosis.[Citation40] In the present study, ferulic acid was also found to be significantly high and is believed that it might exhibit similar effects as found in other plants.
Bioactive compounds have significant anticancer effects through various complementary mechanisms of action, including the induction of metabolizing enzymes and the modulation of gene expression; and their impact on cell proliferation, apoptosis and subcellular signaling pathways. Flavonoids have been known to exhibit antioxidant activity. The effectiveness of antioxidant activity of flavonoids depends on the number and position of the free OH groups. The highest flavonoids content indicates a higher antioxidant activity with a greater number of free OH groups. Phenolic compounds have redox properties, which are responsible for antioxidant activity. Studies show that these extracts are a significant source of natural antioxidants, which might be helpful in preventing the progress of various oxidative damages. ETE was found to have the highest DPPH radical scavenging activity as well as cytotoxicity among wild fruits, which corresponds with the highest content of different flavonoids as depicted by HPLC. Hence, it can be revealed that the activities could be due to the presence of flavonoids.
The MTT assay is a simple, widely used and convenient method to study the anticancer activity of a compound. The cytotoxicity against A549 cancer cell line showed the increase in cytotoxicity with increase in concentration, and ETE was the most potent extracts among all other extracts. Based on IC50, the highest cytotoxicity was exhibited by ETE (18.2 µg/ml), whereas the lowest activity was displayed by COE (203.2 µg/ml).
Conclusion
Our study demonstrated that juice extract of the selected six wild edible fruits of north-east India possesses various potent phytochemicals, such as quercetin, ferulic acid, gallic acid, caffeic acid and rutin. Therefore, the extracts might be the favorable ethnomedicinal plant possessing a myriad of active secondary metabolites, which could be suitable for treating various ailments as well as for the preparation of novel natural therapeutical drugs.
Abbreviations
| ITS | = | Internal transcribed spacer |
| NCBI | = | National Center for Biotechnology Information |
| DPPH | = | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ABTH | = | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ACE | = | Averrhoa carambola extract |
| COE | = | Cissus obovata extract |
| ETE | = | Elacocarpus tectorius extract |
| ECE | = | Elaeagnus caudate extract |
| ESE | = | Embelia subcoriacea extract |
| GLE | = | Garcinia lanceifolia extract |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Burlingame, B. Wild Nutrition. J. Food Compos. Anal. 2000, 13, 99–100. DOI: 10.1006/jfca.2000.0897.
- Hazarika, T. K.; Marak, S.; Mandal, D.; Upadhyaya, K.; Nautiyal, B. P.; Shukla, A. C. Undertilized and Unexploited Fruits of Indo-Burma Hot Spot, Meghalaya, North-East India: Ethno-Medicinal Evaluation, Socio-Economic Importance and Conservation Strategies. Genet. Resour. Crop Evol. 2016, 63, 289–304. DOI: 10.1007/s10722-015-0248-0.
- Misra, S.; Maikhuri, R. K.; Kala, C. P.; Rao, K. S.; Saxena, K. G. Wild Leafy Vegetables: A Study of Their Subsistence Dietetic Support to the Inhabitants of Nanda Devi Biosphere Reserve, India. J. Ethnobiol. Ethnomed. 2008, 2008, 15. DOI: 10.1186/1746-4269-4-15.
- Dwebe, T. P.; Mearns, M. A. Conserving Indigenous Knowledge as the Key to the Current and Future Use of Traditional Vegetables. Int. J. Inf. Manag. 2011, 31, 564–571. DOI: 10.1016/J.ijinfomgt.2011.02.009.
- Nayar, M. P. Hot-Spots of Plant Diversity in India-Strate- Gies. In Conservation and Economic Evaluation of Biodiversity;Pushpangadan, P., Ravi, K., and Santosh, V.Eds.;Oxford and IPH Publishing House:New Delhi. 1997, Vol. 1pp. 59–60
- Chatterjee, S.; Saikia, A.; Dutta, P.; Ghosh, D.; Pangging, G.; Goswami, A. K. Biodiversity Significance of North East India; WWF-India: New Delhi, 2006; pp. 1–71.
- Lalramnghinglova, H.; Jha, L. K. New Records of Ethno-Medicinal Plants from Mizoram. Ethnobot. 1999, 11, 57–64.
- Lalramnghinglova, H. Ethno-Medicinal Plants of Mizoram; Bishen Singh Mahendra Pal Singh, Dehra Dun: India, 2003.
- Lalfakzuala Lalramnghinglova, H.; Kayang, H. Ethno-Botanical Usages of Western Mizoram. Indian J. Trad. Knowl. 2007, 6, 486–493.
- Sharma, B. D.; Balakrishnan, N. P.; Rao, R. P.; Hajra, P. K.; Flora of India vol I–III Botanical Survey of India, Bishen Singh mahendrapal Singh, Calcutta, Deep Printers, New Delhi: , 1993.
- Haridasan, K.; Rao, R. R. Forest Flora of Meghalaya- 2 VolumesBishen Singh Mahendrapal Singh; Dehradun, India, 1987. p. 937.
- Lorenz, R.; Bernhart, S. H.; Höner Zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P. F.; Hofacker, I. L. Vienna RNA Package 2.0. Algorithms. Mol. Biol. 2011, 6, 1–26. DOI: 10.1186/1748-7188-6-26.
- A.O.A.C. Official Methods of Analysis, 16th edn ed.; Association of Official Analytical Chemists: Washington, 1995.
- Freed, M. Methods of Vitamin Assay, 3rd edn ed, The Association of Vitamin Chemists, Inc., J. Wiley, Interscience Publishers: New York, 1966; pp. 287–344.
- Harborne, A. J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis, Springer Science & Business Media: Germany, 1998.
- Doughari, J. H. Follicle-Stimulating Hormone Accelerates Mouse Oocyte Development in vivo. Biol. Reprod. 2012, 87, 3, 1–27. DOI: 10.1095/biolreprod.112.099929.
- McDonald, S.; Prenzler, P. D.; Antolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food .Chem. 2001, 73, 73–84. DOI: 10.1016/S0308-8146(00)00288-0.
- Chang, C. C.; Yang, M. H.; Wen, H. M.; Chern, J. C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. DOI: 10.38212/2224-6614.2748.
- Leong, L. P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food. Chem. 2002, 76, 69–75. DOI: 10.1016/S0308-8146(01)00251-5.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free .Radic. Biol. Med. 1999, 26, 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. 1983, 65, 55–63. PMID: 6606682. DOI: 10.1016/0022-1759(83)90303-4.
- Kamatou, G. P.; Vermaak, I.; Viljoen, A. M. An Updated Review of Adansonia Digitata: A Commercially Important African Tree. S. Afr. J. Bot. 2011, 77, 908–919. DOI: 10.1016/j.sajb.2011.08.010.
- Liu, H.; Fang, C.; Zhang, T.; Guo, L.; Ye, Q. Molecular Authentication and Differentiation of Dendrobium Species by rDna ITS Region Sequence Analysis. AMB. Express. 2019, 9, 1–9. DOI: 10.1186/s13568-019-0767-8.
- Isengard, H. D. Water Content, One of the Most Important Properties of Food. Food. Control. 2001, 12, 95–400. DOI: 10.1016/S0956-7135(01)00043-3.
- Meghwal, P. R.; Azam, M. M. Performance of Some Aonla Cultivars in Arid Region of Rajasthan. Indian. J Hortic. 2004, 61, 87–88.
- Sun, Y. J.; Qiao, L. P.; Shen, Y.; Jiang, P.; Chen, J. C.; Ye, X. Q. Phytochemical Profile and Antioxidant Activity of Physiological Drop of Citrus Fruits. J. Food Sci. 2013, 78, C37–42. DOI: 10.1111/j.1750-3841.2012.03002.x.
- Prakash, J.; Maurya, A. N.; Singh, S. P. Studies on Variability in Fruit Characters of Jamun. Indian. J Hort. 2010, 67, 63–66.
- Ghasemzadeh, A.; Ghasemzadeh, N.; Flavonoids and Phenolic Acids: Role and Biochemical Activity in Plants and Human. J. Med. Plants Res. 2011, 5(31), 6697–6703.
- Tanaka, T. Flavonoids as Complementary Medicine for Allergic Diseases: Current Evidence and Future Prospects. OA. Alter Med. 2013, 1(11). DOI: 10.13172/2052-7845-1-2-589.
- Hegarty, V. M.; May, H. M.; Khaw, K. T. Tea Drinking and Bone Mineral Density in Older Women. Am. J. Clin. Nutr. 2000, 71(4), 1003–1007. DOI: 10.1093/ajcn/71.4.1003.
- David, A. V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. DOI: 10.4103/0973-7847.194044.
- Edwards, R. L.; Lyon, T.; Litwin, S. E.; Rabovsky, A.; Symons, J. D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. DOI: 10.1093/jn/137.11.2405.
- Lamson, D. W.; Brignall, M. S. Antioxidants and Cancer, Part 3: Quercetin. Alternative. Medi Review: J Clin Ther. 2000, 5, 196–208.
- Subramanian, A. P.; John, A. A.; Vellayappan, M. V.; Balaji, A.; Jaganathan, S. K.; Supriyanto, E.; Yusof, M. Gallic Acid: Prospects and Molecular Mechanisms of Its Anticancer Activity. R.S.C. Adv. 2015, 5, 35608–35621. DOI: 10.1039/C5RA02727F.
- Sghaier, M. B.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin Inhibits Proliferation, Attenuates Superoxide Production and Decreases Adhesion and Migration of Human Cancerous Cells. Biomed. Pharmacother. 2016, 84, 1972–1978. DOI: 10.1016/j.biopha.2016.11.001.
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E. M.; Kabała‐dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R. D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. DOI: 10.1002/ptr.5878.
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y.;Anti-Tumor Effect of Rutin on Human Neuroblastoma Cell Lines Through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Scient. World J. 2013, 269165. DOI: 10.1155/2013/269165.
- Vyas, D.; Laput, G.; Vyas, A. K. Chemotherapy-Enhanced Inflammation May Lead to the Failure of Therapy and Metastasis. Oncotarget. Ther. 2014, 7, 1015–1023. DOI: 10.2147/OTT.S60114.
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. DOI: 10.1159/000491755.
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Li, Q.; Zhang, S.; Yang, Y.; Wang, Y.; Wang, Y. The Anticancer Effects of Ferulic Acid is Associated with Induction of Cell Cycle Arrest and Autophagy in Cervical Cancer Cells. Cancer. Cell Inter. 2017, 18, 1–9. DOI: 10.1186/s12935-018-0595-y.