ABSTRACT
This study was aiming at the differences in the aroma, flavor compounds, and bioactivities of essential oil of Coreopsis tinctoria flower (CTFEO) and essential oil of Chrysanthemum morifolium flower (CMFEO). Both essential oils were extracted by steam distillation. Their flavor compositions were determined by GC-MS and the antioxidant, antibacterial, and anti-inflammatory activities were also evaluated. It was found that CTFEO possessed a special medicinal herb odor, which was obviously stronger than those of CMFEO. A preferred condition for steam distillation was flower powder–water ratio of 7.5:100 and extraction for more than 3.0 h. Interestingly, the main flavor compounds of CTFEO were determined as D-limonene (34.54%), cis-carveol (5.49%), trans-α-bergamotene (4.44%), and α-pinene (3.90%), which were quite different from those of CMFEO. CTFEO showed better antioxidant activities with IC50 value of 121.4 ± 9.8 μg/mL than those of CMFEO. It also exhibited stronger antibacterial effects than those of CMFEO, with minimal inhibitory concentrations (MICs) ranging from 0.183 to 1.412 mg/mL against six strains of bacteria. Moreover, CTFEO was also found to exhibit strong anti-inflammatory effects that reduced the LPS-induced RAW 264.7 cell mRNA expression of the cytokines IL-1β, IL-6, and TNF-α at a concentration of 10.0 μg/mL. All these results suggest that the aroma, flavor compounds, and bioactivities of the two essential oils were quite different and they could be used as tea, drink, and food additive in the food industry for further development.
Introduction
Coreopsis tinctoria Nutt., belonging to the Asteraceae family, is a kind of small annual herb distributed worldwide.[Citation1] In China, it is known as “Snow chrysanthemum” and mainly grows in the area of Karakorum Mountains (attitude above 3000 m) in Xinjiang province.[Citation2,Citation3] This highland herb is traditionally used as tea, food supplement, and ethnic medicine to treat the diseases like hyperlipidemia, brain aging, hypertension, and bacterial infection.[Citation4–6] A number of bioactive phytochemical compounds have been isolated and identified from C. tinctoria flower, such as polyacetylene glycosides, flavonoids, and phenols.[Citation1,Citation7–9]
As a special kind of tea, food supplement, and ethnic medicine, the essential oil of this plant plays an important role in its flavor and pharmacologic function. It has been known that the essential oils of C. tinctoria flower (CTFEO) and Chrysanthemum morifolium flower (CMFEO) exhibit multiple bioactivities like antimicrobial, antioxidant, N-nitrosamine inhibition, and memory improvement.[Citation2–4] However, the aroma characteristic and hydrodistillation extraction process of CTFEO are not known clearly enough.[Citation2,Citation3] Moreover, there are no reports about the anti-inflammatory effects of CTFEO. In this study, two kinds of chrysanthemum flowers (C. tinctoria and C. morifolium) were chosen and their essential oils were extracted by hydrodistillation. The condition of steam distillation process was optimized and the volatile compositions were determined by GC-MS. The differences in the flavors and the antioxidant, antibacterial, and anti-inflammatory bioactivities of two essential oils were further analyzed.
Materials and methods
General experimental procedures
ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), and analytical grade of solvent (CH2Cl2, ethanol, DMSO, Na2S2O8) were purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Penicillin G and streptomycin were purchased from Solarbio Co., Ltd (Beijing, China). The density of essential oil was determined by SC-3005 U density equipment (Sanchuang Co., Ltd, Xiamen, China). Optical density (OD) values were determined on an F-50 microplate reader (Tecan, Switzerland). GC-MS data were acquired by an Agilent 7890B-5977B GC/MSD (Agilent, Santa Clara, USA).
Plant material
The dry flowers of Coreopsis tinctoria Nutt. and Chrysanthemum morifolium Ramat. were collected from the Karakorum Mountains (Xinjiang province) and Hangzhou (Zhejiang province), respectively. Two voucher specimens were kept in the Key Biotechnology Laboratory of Zhengzhou University of Light Industry. The dry flowers were smashed and sieved using a 40-mesh sieve (pore size 0.42 mm).
Extraction of essential oil
Using Clevenger-type apparatus, the essential oils of two flowers were extracted by hydrodistillation for 1.0–4.0 h.[Citation10] The oils were dried by Na2SO4 and their weights were measured, which were further stored in tightly sealed bottles at 4°C.
Optimization of flower powder–water ratio
The optimization of flower powder–water ratio was carried out using the powder of C. tinctoria flowers. The powders (37.5 g, 75.0 g, 112.5 g, and 150.0 g) were added along with 1500 mL of water in four 2000 mL of round-bottomed flasks, respectively. Then, the hydrodistillation was performed for 4.0 h. The weights of essential oils were recorded and the yields were calculated.
Optimization of the hydrodistillation time
The powders (75.0 g and 112.5 g) were added along with 1500 mL of water in eight 2000 mL of round-bottomed flasks. Afterward, the hydrodistillation was stopped at 1.0 h, 2.0 h, 3.0 h, and 4.0 h, respectively. The weights of essential oils were recorded and the yields were calculated.
GC-MS analysis
The essential oils were diluted with CH2Cl2 to 1.0% (v/v), which were further used for GC-MS analysis. GC-MS measurement was carried on an Agilent 7890B-5977B GC/MSD that equipped with a HP-5 MS column (30 m × 250 μm × 0.25 μm). The gas carrier was helium (flow rate of 1.0 mL/min). The injection volume was 1.0 µL. The column temperature was set as following: 40°C, hold for 4 min; 40°C to 160°C, 10°C/min, hold for 10 min; 160°C to 230°C, 20°C/min, hold for 6 min. The mass spectrometer operating conditions were as follows: ionization voltage of 70 eV, ion source of 230°C, and mass range of 45 amu to 600 amu. The volatile component identification was carried out through comparing their measured mass spectrums with those stored in the mass spectral library 2017 of National Institute of Standards and Technology (NIST), and the components with more than 85% matching scores were chosen.[Citation11] The identification of key flavor compounds was further verified by the retention times with those of the standard compounds available in the authors’ laboratory.[Citation12]
Antioxidant assay
For the ABTS assays, equal volume of Na2S2O8 solution and ABTS solution were mixed and reacted away from light for 16 h. Then, the mixture was diluted around 50 folds to the absorption of 734 nm to 0.70 ± 0.05 with ethanol.[Citation13] The essential oils were dissolved in ethanol and further prepared to the concentrations of 10.0–1000.0 μg/mL. The ABTS solutions (180 μL) and the essential oil solution (20 μL) were added to the 96-well plates, which were further reacted in the darkness for 6 minutes. OD values were further measured at 734 nm. Scavenging rates were determined as: scavenging rate (%) = (1–Atest/Acontrol) × 100.
For the DPPH assays, DPPH powders were dissolved in ethanol to the concentration of 0.25 mM, and essential oils were prepared to the concentrations above. Then, 100 μL of essential oil solutions and 100 μL of DPPH solutions were added to the 96-well plates, which were further reacted in the darkness for 30 minutes. OD values were further measured at 517 nm. Scavenging rates were determined as: scavenging rate (%) = (1–Atest/Acontrol) × 100.
Antibacterial assay
A total of six strains of bacteria were used for the antibacterial assays on the basis of broth microdilution method, namely Pseudomonas aeruginosa ATCC 27853 (gram-negative), Klebsiella Pneumoniae (gram-negative), Escherichia coli ATCC 25922 (gram-negative), Staphylococcus aureus ATCC 25923 (gram-positive), Enterococcus faecalis ATCC 29212 (gram-positive), and Bacillus subtilis ATCC 6633 (gram-positive).[Citation14] In brief, the bacteria were cultivated at 35°C and 120 rpm in MH (Mueller–Hinton liquid broth) for around 5–7 h and diluted to the final concentration of 1.0 × 104–1.0 × 105 CFU/mL. Subsequently, positive controls (penicillin G and streptomycin) were prepared in 0.1–10 μg/mL. The essential oils were dissolved in DMSO and were prepared to 1–200 μg/mL with above-mentioned broth. In a bacteria-free work bench, 100 μL of compound solution and equal volume of bacterial suspensions were added into the 96-well plates, which were further cultivated for 24 h in a 37°C incubator. OD values were recorded at 530 nm and the tests were carried out in triplicate. The compound concentrations that inhibited 50% of the bacteria growth were considered as minimal inhibitory concentration (MIC) values, which were calculated by GraphPad Prism 8.0.
Anti-inflammatory assay
The inhibition on the RAW 264.7 cell mRNA expression of cytokines IL-1β, TNF-α, and IL-6 was determined on the basis of previous method with some modifications.[Citation15,Citation16] In brief, after being treated with essential oil (10.0 μg/mL) and LPS (1.0 μg/mL) for 15.0 h, the total RNA was extracted by Trizol reagent. cDNA was obtained by reverse transcription (KeyGene, Nanjing, China). The mRNA expression was measured by RT-qPCR using the SYBR Green PCR Core Reagent Kit (Vazyme, Nanjing, China), which was further evaluated by the 2−ΔCT method.
Statistical analysis
All the measurements were performed in triplicate, and the results were expressed as mean values ± SD (standard deviations). The differences between individual results were considered as significant when P < .05. The ANOVA (one-way analysis of variance) test was carried out by STATISTICA and GraphPad Prism.
Results and discussion
Characteristic of essential oils
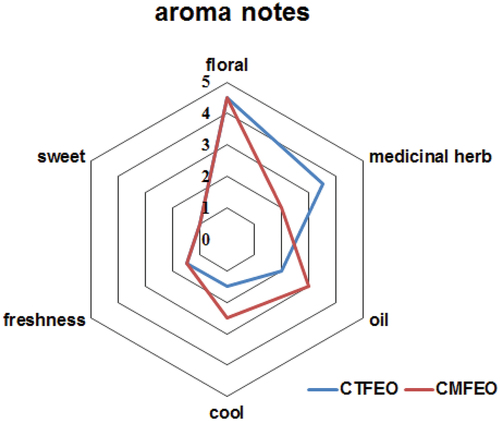
The color of CTFEO was orange, which was obviously darker than that of CMFEO (light yellow-green). The result was in accordance with the color of two flowers. The density of CTFEO and CMFEO was 0.8615 and 0.8542 g/mL, respectively. Through aroma analysis, it indicated that two essential oils mainly possessed floral, medicinal herb, oil, cool, freshness, and sweet smell (). Interestingly, CTFEO exhibited a characteristic medicinal herb odor, which was obviously stronger than that of CMFEO. On the other hand, CMFEO felt cooler and more oiliness. Besides, the floral, freshness, and sweet aromas of the two essential oils were alike.
Optimization of the flower powder–water ratio
The optimization of flower powder–water ratio was carried out using the flower powder of C. tinctoria. As shown in , the flower powder–water ratio of 7.5:100 gave highest yield rate. The yield rate would decrease when the flower powder–water ratio was more than 7.5:100.
Table 1. Optimization of the flower powder–water ratio.
Optimization of the hydrodistillation time
The hydrodistillation time was optimized using the flower powder-water ratios of 5.0:100 and 7.5:100. The hydrodistillation was stopped at 1.0 h, 2.0 h, 3.0 h, and 4.0 h, and the yielded essential oils were weighted. It was found that the yields of the essential oils increased from time beginning to 3.0 h. After 3.0 h, the yields were no longer increased but the color of essential oils could be slightly enhanced (). Thus, a proper hydrodistillation time was suggested as more than 3.0 h.
Table 2. Optimization of the hydrodistillation time.
Chemical composition of essential oils
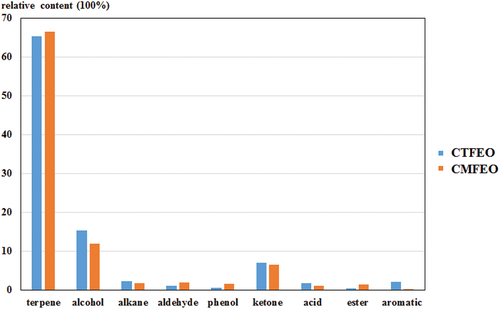
Overall, a total of 161 chemical compositions were identified from two essential oils, and 93 and 92 kinds of flavor compounds were determined from CTFEO and CMFEO, respectively (). Among them, 25 kinds of flavor compounds were both found in CTFEO and CMFEO. Terpenes were revealed as the main compositions in two essential oils (both 45 kinds), which accounted for more than 65% of the whole compounds (). The following were alcohols (CTFEO, 15 kinds; CMFEO, 18 kinds) and ketones (CTFEO, 14 kinds; CMFEO, 11 kinds).
Table 3. Chemical composition of CTFEO and CMFEO identified by GC-MS (100%).
Interestingly, the main flavor compounds of CTFEO were D-limonene (34.54%), cis-carveol (5.49%), trans-α-bergamotene (4.44%), and α-pinene (3.90%), which might be associated with its floral and medicinal herb odor.[Citation3,Citation4] The notably high content of D-limonene was in accordance with those in the literature, which suggested this compound can be one of the most important components in CTFEO.[Citation2,Citation3,Citation4] However, the main flavor components of CMFEO were quite different from CTFEO since the content of D-limonene was determined as only 1.29% in CMFEO. The main flavor compounds of CMFEO were revealed as (–)-β-elemene (8.87%), (–)-β-sesquiphellandrene (5.87%), (+)-ledene (5.53%), and cis-β-farnesene (5.53%). Additionally, the ester compounds were found more in CMFEO (1.45%), which might be the key reason of strong oil aroma of CMFEO ().
Antioxidant activity
In the ABTS assays, CTFEO showed potent antioxidant activity with IC50 value of 121.4 ± 9.8 μg/mL, which was better than those of CMFEO (IC50 = 225.8 ± 13.1 μg/mL; positive control trolox, IC50 = 18.6 ± 2.1 μg/mL). However, no obvious radical scavenging effects were found in the DPPH assays for two essential oils (IC50>1000 μg/mL). The above results were in accordance with those in the literatures.[Citation2,Citation3]
Antibacterial activity
It was known that essential oils possessed antibacterial activities and previous study suggested CTFEO could inhibit the growth of gram-negative Shigella sp. and E. coli.[Citation2,Citation10] In this study, both CTFEO and CMFEO could obviously inhibit the growth of three strains of gram-positive and three strains of gram-negative bacteria (). Moreover, the two essential oils exhibited better antimicrobial activities against gram-positive bacteria than those of gram-negative bacteria. And the best antibacterial activities were found against B. subtilis with MIC values of 0.183 and 0.340 mg/mL, respectively. Overall, CTFEO showed better antibacterial effects than those of CMFEO against all six strains of bacteria. It has been reported that D-limonene possesses antibacterial activities through damaging membrane and inhibiting DNA transcription and translation of bacterial cells.[Citation17,Citation18] Thus, the potent antibacterial effects of CTFEO could be related to its high content of D-limonene.
Table 4. Antibacterial activities of CTFEO and CMFEO (mg/mL).
Anti-inflammatory activity
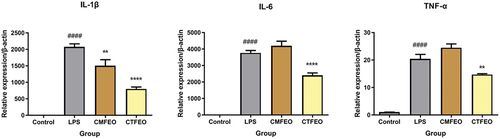
It is worth noting that CTFEO exhibited obvious anti-inflammatory effects that inhibited the LPS-induced mRNA expression of cytokines IL-1β, IL-6, and TNF-α of RAW 264.7 cells at the concentration of 10.0 μg/mL (). Moreover, CTFEO showed the most powerful inhibition on the LPS-induced mRNA expression of IL-1β with the inhibition rate of 61.5%. CMFEO could also inhibit the LPS-induced mRNA expression of IL-1β but have no effects on the other two cytokines. Thus, the anti-inflammatory activities of the two essential oils were disclosed for the first time and CTFEO exhibited better effects than those of CMFEO.
Conclusion
This study suggested that CTFEO possessed a special medicinal herb odor and the other aroma of the two essential oils were alike. A preferred condition for steam distillation was also established. The main flavor compounds especially the content of D-limonene in CTFEO were quite different from those of CMFEO. Moreover, the two essential oils exhibited obvious antioxidant and antibacterial activities, and their anti-inflammatory effects were also determined for the first time. Due to the special flavors and potent biological activities, the essential oils of two chrysanthemum flowers are promising in the application of tea, drink, food additive, and medicine for further development.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang, Y.; Shi, S.; Zhao, M.; Jiang, Y.; Tu, P. A Novel Chalcone from Coreopsis Tinctoria Nutt. Biochem. Syst. Ecol. 2006, 34, 766–769. DOI: 10.1016/j.bse.2006.05.005.
- Yao, X.; Wang, X.; Gu, C.; Zeng, H.; Chen, W.; Tang, H. Chemical Composition, N-Nitrosamine Inhibition and Antioxidant and Antimicrobial Properties of Essential Oil from Coreopsis Tinctoria Flowering Tops. Nat. Prod. Res. 2016, 30, 1170–1173. DOI: 10.1080/14786419.2015.1041943.
- An, L.; Sun, Y.; Huang, J.; Liu, Y.; Yuan, H.; Zhang, R.; Sun, Y. Chemical Compositions and In Vitro Antioxidant Activity of the Essential Oil from Coreopsis Tinctoria Nutt. Flower. J. Essent. Oil-Bear. Plants. 2018, 21, 876–885. DOI: 10.1080/0972060X.2018.1510792.
- Qu, Y.; Guo, Y.; Li, W.; Shen, H.; Cui, J.; Li, J.; Liu, J.; Wu, D. The Improvement of Coreopsis Tinctoria Essential Oil on Learning and Memory Impairment of D-Galactose-Induced Mice Through Nrf2/nf-Κb Pathway. Front Pharmacol. 2022, 13, 994705. DOI: 10.3389/fphar.2022.994705.
- Dias, T.; Bronze, M. R.; Houghton, P. J.; Mota-Filipe, H.; Paulo, A. The Flavonoid-Rich Fraction of Coreopsis Tinctoria Promotes Glucose Tolerance Regain through Pancreatic Function Recovery in Streptozotocin-Induced Glucose-Intolerant Rats. J. Ethnopharmacol. 2010, 132, 483–490. DOI: 10.1016/j.jep.2010.08.048.
- Guo, J.; Wang, A.; Yang, K.; Ding, H.; Hu, Y.; Yang, Y.; Huang, S.; Xu, J.; Liu, T.; Yang, H., et al. Isolation, Characterization and Antimicrobial Activities of Polyacetylene Glycosides from Coreopsis Tinctoria Nutt. Phytochemistry. 2017, 136, 65–69. DOI: 10.1016/j.phytochem.2016.12.023.
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and Qualitative Analysis of Flavonoids and Phenolic Acids in Snow Chrysanthemum (Coreopsis Tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules. 2016, 21, 1307. DOI: 10.3390/molecules21101307.
- Du, D.; Jin, T.; Xing, Z. H.; Hu, L. Q.; Long, D.; Li, S. F.; Gong, M. One New Linear C14 Polyacetylene Glucoside with Antiadipogenic Activities on 3T3-L1 Cells from the Capitula of Coreopsis Tinctoria. J. Asian Nat. Prod. Res. 2016, 18, 784–790. DOI: 10.1080/10286020.2016.1157077.
- Zhang, Y.; Shi, S.; Zhao, M.; Chai, X.; Tu, P. Coreosides A-D, C14-Polyacetylene Glycosides from the Capitula of Coreopsis Tinctoria and Its Anti-Inflammatory Activity Against COX-2. Fitoterapia. 2013, 87, 93–97. DOI: 10.1016/j.fitote.2013.03.024.
- Meriem, A.; Msaada, K.; Sebai, E.; Wannes, W. A.; Abbassi, M. S.; Akkari, H. A. Anthelmintic and Antibacterial Activities of Red Juniper (Juniperus Phoenicea L.) Essential Oil. J. Essent. Oil Res. 2022, 34, 163–172. DOI: 10.1080/10412905.2021.1941338.
- Li, T. X.; Ji, L. B.; Jiang, Z. R.; Geng, Z. Z.; Shentu, H. Q.; Liu, M. C.; Xie, Y. F.; Hu, J.; Liu, Y. F.; Li, D. L. Caramel Products of Glucose with Water During Heating Process and Their Bioactivities. Int. J. Food. Prop. 2020, 23, 971–978. DOI: 10.1080/10942912.2020.1770788.
- Li, T. X.; Xiong, Y. M.; Chen, X.; Yang, Y. N.; Wang, Y.; Jia, X. W.; Yang, X. P.; Tan, L. L.; Xu, C. P. Antifungal Macrocyclic Trichothecenes from the Insect-Associated Fungus Myrothecium Roridum. J. Agric. Food. Chem. 2019, 67, 13033–13039. DOI: 10.1021/acs.jafc.9b04507.
- Liu, Q.; Niu, H.; Zhao, J.; Han, J.; Kong, B. Effect of the Reactant Ratio on the Characteristics and Antioxidant Activities of Maillard Reaction Products in a Porcine Plasma Protein Hydrolysate-Galactose Model System. Int. J. Food. Prop. 2016, 19, 99–110. DOI: 10.1080/10942912.2015.1017048.
- Li, T. X.; Liu, R. H.; Wang, X. B.; Luo, J.; Luo, J. G.; Kong, L. Y.; Yang, M. H. Hypoxia-Protective Azaphilone Adducts from Peyronellaea Glomerata. J. Nat. Prod. 2018, 81, 1148–1153. DOI: 10.1021/acs.jnatprod.7b00663.
- Zhang, H. J.; Zhang, Y. M.; Luo, J. G.; Luo, J.; Kong, L. Y. Anti-Inflammatory Diterpene Dimers from the Root Barks of Aphanamixis Grandifolia. Org. Biomol. Chem. 2015, 13, 7452–7458. DOI: 10.1039/c5ob00674k.
- Wang, Z.; Guan, Y.; Yang, R.; Li, J.; Jia, A. Q. Anti-Inflammatory Activity of 3-Cinnamoyltribuloside and Its Metabolomic Analysis in LPS-Activated RAW 264.7 Cells. BMC Complementary Med. Ther. 2020, 20, 329. DOI: 10.1186/s12906-020-03115-y.
- Su, J.; Guo, Q.; Cai, Y.; Wang, T.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Effect of Ultra-High Temperature Processing on the Physicochemical Properties and Antibacterial Activity of D-Limonene Emulsions Stabilized by β-Lactoglobulin/gum Arabic Bilayer Membranes. Food Chem. 2020, 332, 127391. DOI: 10.1016/j.foodchem.2020.127391.
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic Approach of Multifaceted Antibacterial Mechanism of Limonene Traced in Escherichia Coli. Sci. Rep. 2021, 11, 13816. DOI: 10.1038/s41598-021-92843-3.