ABSTRACT
Kaempferol, found in various plants and foods, has gained attention as a useful flavonoid owing to its potential biological properties, including anticancer characteristics. Recent research studies suggest that kaempferol is effective in the inhibition and treatments of several forms of cancer, e.g. lung, ovarian, breast and lung cancer. One probable mechanism of action of kaempferol is its anti-inflammatory and antioxidant potentials, which may help to prevent DNA damage and inhibit the proliferation of cancerous cells. Besides triggering apoptosis in cancerous cells, Kaempferol may also inhibit the growth and relocation of cancerous cells. Moreover, kaempferol has a very wide range of bioactivities, involving anti-inflammatory and antioxidant effects. This includes understanding the optimal dosing and timing of kaempferol treatment, as well as the potential interactions with other medications and the long-term safety of kaempferol use. Overall, the available evidence and studies suggest that kaempferol may provide a potential natural agent for the prevention and treatments of cancers. Despite the promising findings in preclinical and clinical research, further research is needed to confirm its efficacy and understand the mechanisms of action, and to fully explore the potential of kaempferol in different cancers.
Introduction
Kaempferol is a widespread flavonoid, generally found in various foods of plant origin, including fruits (gooseberries, strawberries and blackberries) and vegetables (cabbages, onions and broccoli) as well as herbal medicines (barbarum and lovage)[Citation1]].[Citation2] It has a very wide range of bioactivities, involving, anti-diabetic, antioxidant anti-inflammatory and anti-cancer effects Breast, prostate, bladder, colon, liver, cervical, ovarian, lungs, and leukemia are just a few of the cancers it has anticancer properties for.[Citation3] Yellow powdered kaempferol is totally soluble in hot ethanol, ether and alkali. It is only weakly soluble in water. 276–278°C is melting point and 6.34 ± 0.40 is acidity coefficient of kaempferol. Kaempferol, also known as 3,5,7-trihydroxy-2(4-hydroxyphenyl)-4 H-chromen-4-one.
Biosynthesis of kaempferol
Kaempferol is comprised of diphenylpropane, which is synthesized by fermentation with the aid of several catalysts. These enzymes are extremely frequent and plentiful in vegetation. Three molecules of malonyl-CoA and one molecule of 4-coumaroyl-CoA are utilized in the condensation procedure. Chalcone synthase is in control of the condensation method that leads to the flavonoid naringenin chalcone synthesis. By closing the C3 ring, the enzyme chalcone isomerase transforms naringenin chalcone to naringenin flavanone using the catalytic performance of the flavanone-3-dioxygenase enzyme, a hydroxyl group is added to the C3 ring of naringenin, resulting in the formation of dihydrokaempferol. In the final stage, flavonol synthase introduces a double bond at C2-C3 of dihydrokaempferol.
Cancer stays among the most imperious human health difficulties, depend on chemopreventive methods as a mode to lessen both occurrence as well as death. The study of kaempferol incline of cancer-preventive characteristics highlights its potentials, along with its biological activities against many diseases kaempferol can selectively hinder carcinomas without distressing vigorous cells.[Citation4] Studies have exhibited a wide range of kaempferol anti-cancer activities, involving metastasis, apoptosis, angiogenesis and inflammation. Consequently, carcinomas that regularly acclimate to inhibition of VEGF, resulting in behavior with kaempferol, might not save various damaging activities brought by the expected flavonoid. However, on the other hand kaempferol is uncertain as a cancer management, it appears to establish a stimulating selection when it comes to well-being. Though, statistics on the long-time influence of kaempferol consumption is rare. However, kaempferol deprived bioavailability symbolizes a noteworthy hindrance, and the usage of nanoparticles based upon kaempferol has carried further expectations on cancer chemopreventive policies. Furthermore, most of the study directed on kaempferol influence against cancer have been done in-vitro, creating it challenging to induce a concluding assumption on its effectiveness. Clinical trials expanding a particular dosage of kaempferol are not found widely. Hence, emphasizing the necessity for further thorough trials fluctuating the amount of kaempferol on its own, such as consuming it by additional flavonoids.
One of the most prevalent phytonutrients in diets high in vegetables and fruits are flavonoids, such as flavones. Flavonoids may be cytotoxic to cancer cells and have anti-inflammatory and antioxidant properties.[Citation5] People may experience a variety of health advantages from flavonoids, such as reduced inflammation, altered disease activity, and reduced chemotherapeutic and antibiotic resistance. It has been demonstrated that they have anti-proliferative, antioxidant, anti-inflammatory, and anti-cancer properties (). They may function as antioxidants, strengthening DNA repair, impeding chemical damage by activating enzymes of phase 2, and altering indicator transducting trails, all while inhibiting DNA destruction by volatile oxygen radicals scavenging.[Citation1,Citation6]
A significant subgroup of the flavonoids family is flavonoids. Two phenyl benzo-pyrone derivatives make up flavonoids. A flavan structure based (C6-C3-C6) 15-carbon skeleton made up of two aromatic rings connected by a heterocyclic pyrone and pyrane ring is a common component in all flavonoids chemical structure.[Citation1] The flavanol category of flavonoids includes kaempferol. It is water insoluble, but it is soluble in alkaline ether and ethanol. Because of its diphenyl-propane structure, kaempferol has hydrophobic characteristics. Kaempferol can be discovered in a variety of components, including flowers, fruits, leaves, seeds and even vegetables (also shown in ). Kaempferol’s anti-inflammatory properties are its most well-known characteristic.[Citation11]
Table 1. Different Sources of Kaempferol.
It can be used as a medicinal agent to treat conditions like cardiovascular diseases, diabetes, obesity, asthma, oxidative stress and microbial infection illnesses, in addition to playing a vital role in lowering cancer. Kaempferol exerts its effects via range of mechanisms, i.e. induction of apoptosis, reduction of cell viability (G2/M phase), downregulation of the protein kinase (PI3K/AKT) and human T-cell lymphoma/leukemia virus-I (HTLV-I) indicating pathways, and suppression of the protein impression of markers related EMT like N- and Snail, E-cadherin, and Slug, metastasis-related markers, for example, matrix metallopeptidase 2.[Citation12]
The main method of preventing cancer is to increase apoptosis, which prevents cancer cells from proliferating.[Citation13] Kaempferol has the ability to slow down or inhibit entirely, the growth of numerous tumor/cancer cells through mechanisms, such as inducing programmed cell death, arresting the cell-cycle at the G2/M phase, downregulating signaling pathways and the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, and affecting markers related to the epithelial-mesenchymal transition (EMT).[Citation14]
Conventional extraction techniques documented include employing the cold maceration process. The recovery rate was 90.1%. The researchers published information on an extraction technique that yields 98.6% more kaempferol from dried Chinese chestnut kernels when methanol and concentrated hydrochloric acid (HCl) are used, followed by 3.5 h in an 85°C water bath. Due to kaempferol’s relative lipophilicity, passive diffusion, active diffusion, and facilitated diffusion are the main methods of absorption in the small intestine.[Citation15] In the xenograft nude mice project of numerous cancer variants, such as breast cancer and gastric cancer, kaempferol’s anti-cancer efficacy in vivo has been proven,[Citation16] lung cancer, osteosarcoma,[Citation17] bladder cancer,[Citation18] esophageal squamous cell malignancy and prostate tumor.[Citation19] Kaempferol inhibits the growth of xenograft tumors primarily through molecular processes that control metastasis, induce apoptosis and regulate associated molecular cell signaling pathways.[Citation20] In this article, data related to anti-cancer properties of kaempferol against cancers of different organs is summarized.
Bioavailability of kaempferol
The fat solubility of absorbed flavonols is a crucial element in bioavailability. When flavonol-rich meals are consumed, the glycoside and aglycones forms breakdown and absorb in distinct ways. Lipophilic aglycons flows unchanged into enterocytes via the intestinal lumen. There these are ingested directly into the hepatic portal vein or alternatively they are metabolized prior to ingestion. Aglycone metabolism in enterocytes includes phase I (oxidation and O-demethylation) and phase II metabolism (glucuronidation, sulfation, and methylation) to create compounds that are transported into the hepatic portal vein via ATP-binding cassette (ABC) transporters.
Antioxidant potential of kaempferol
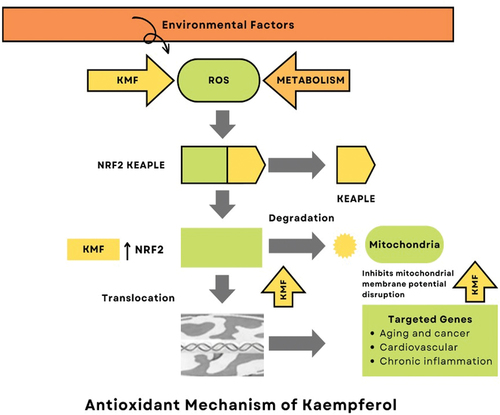
Via scavenging reactive oxygen species, strengthening DNA, reducing chemical damage by inducing phase 2 enzymes, and altering signal transduction pathways, kaempferol functions as an antioxidant and prevents DNA damage.[Citation8] Free radical generation as well as other byproducts like reactive oxygen species can be reduced by kaempferol, its glycosides, and plants that contain reactive oxygen species (ROS). Cancerous cell change might cause by ROS that are the by-products of aerobic metabolism. Then, ROS production suppression can change the phenotypic of aggressive cancer cells. Superoxide dismutase (short form: SOD) converts superoxide anions into hydrogen peroxide (H2O2), which can then react with reduced metal ions, such as cuprous or ferrous ions to produce highly reactive hydroxyl radical groups. Alternatively, SOD can also produce peroxynitrite by reacting with nitric oxide. Both peroxynitrite and the hydroxyl radical are highly reactive species that can cause damage to DNA, proteins, and lipids.[Citation21]
In addition to being a vigorous scavenger of hydroxyl radical, superoxide anion and peroxynitrite at submicromolar concentrations, kaempferol also hinders pro-oxidant-based enzymes like xanthine oxidase, triggers antioxidant enzymes like hemeoxygenase-1, superoxide dismutase and catalase and even stops the hydroxyl radical production by chelating ferrous or cuprous.[Citation4,Citation7] The hydroxyl groups at C3, C5, and C4, as well as the oxo group at C4 and the double bond between C2 and C3, may contribute to the antioxidant properties of kaempferol (). This substance has been shown to inhibit the NF-κB pathway, stimulate the Nrf2 transcriptional pathway, and restore redox homeostasis in cells, potentially helping to prevent cancer through its antioxidant and anti-inflammatory effects.[Citation22]
Anticancer perspectives of kaempferol
The abnormal, uncontrolled division and expansion of cells or tissues that results in cancer is fatal and poses serious health risks. Tumors have a high death rate and are extremely complex errors. Statistics provided by the World Health Organization exhibit about 84 million populations worldwide pass away from cancer each year. Current treatments and medications have a lot of drawbacks, such as adverse effects, lack of selectivity, and ineffectiveness. For the treatment of cancer, more potent medications are required. Cancer treatments have evolved over the past few decades using natural ingredients and their derivatives.[Citation23]
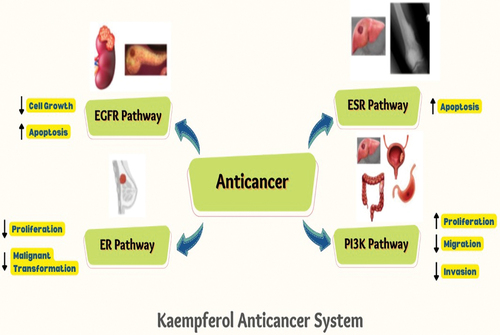
Both in industrialized and developing nations, neoplastic disease morbidity and cancer mortality are rising quickly. According to the latest data obtained from Globocan 2020 on cancers in about 185 countries, there are approx. nineteen million (M) new reported cancer cases. Approx. 9.8 M deaths due to cancers. Cancers continue to be the leading cause of mortality globally despite medical advances in neoplastic disease diagnosis and treatment.[Citation1] Kaempferol anticancer system targeting various oncogenic mechanisms including ERS, ER, PI3K/Akt and EGFR pathways and underlying active mechanisms. The following, different types of cancer and their inhibitory effect by kaempferol are presented .
Figure 3. Kaempferol anticancer system targeting various oncogenic mechanisms including ERS, ER, PI3K/Akt and EGFR pathways and underlying active mechanisms.
Anti-breast cancer activity
Among the most dangerous and fatal type of cancer for women in developed nations is breast-cancer. Since the late 1970s, the prevalence of breast tumour has increased globally. A woman’s lifetime risk of receiving a breast cancer diagnosis is 12.4% higher in the United States. Kaempferol primarily generated anti-breast cancer influences in three ways: by preventing breast cancer cell growth, causing breast cancer cell death, and preventing breast cancer cells’ emigration and seizure.[Citation24]
Kaempferol slows the movement of breast tumor cells by stopping the cell cycle at the G2/M phase in MDA-MB-453 women breast cancer cells. In triad-negative breast malignant cells, a team of researchers discovered similar results. Their research revealed that the number of cells in first gap-G1 phase dramatically reduced following treatment with kaempferol, from 85.48% to 51.35% and number of tumor cells in the G2 phase considerably elevated 9.27% to 37.5%. The results suggest that kaempferol could stop the cell cycle, which can prevent cells from growing.[Citation25]
The apoptosis of breast malignant cells is induced by kaempferol. Caspase, the enzyme at the center of cell apoptosis, uses PARP as a cutting substrate. It is crucial for the repair of DNA impairment and cancer cell death. PARP cleavage is regarded as a key sign of programmed cell death. Bcl2 protein that is anti-apoptotic and protein Bax that was pro-apoptotic were additionally engaged in the procedure. In addition, they discovered that Kaempferol’s ability to stimulate the shear of PARP expression in MCF-7 tumor cells this was combined by a down-regulation of Bcl-2 protein expression as well as a promotion of Bax protein expression.[Citation26]
Breast cancer cells are prevented from migrating and invading by kaempferol. It is believed that the epithelial-mesenchymal-transition (or EMT) is the main factor for the emergence of breast tumour. According to facts, breast cancer cells with EMT will withstand chemotherapy, reducing its effectiveness or perhaps making it ineffective. Exogenous heterologous substances that kaempferol can effectively block the impression of E2, metastatic proteins and triclosan-induced EMT and the metastatic expression of breast cancer produced by endogenic estrogen. All of these studies show that kaempferol may one day be used to treat breast cancers.[Citation25]
Researchers have studied the possible positive potential of kaempferol against growth and proliferation of breast cancer (BC) cells. To assess the inhibitory potential of kaempferol on the growth of cells, the MTS assay was used, and sophisticated flow cytometry was utilized for cell cycle analyses. DNA damage and cellular apoptosis assessed by western blotting. The results showed that kaempferol effectively suppressed the growth of triple-negative breast cancer (TNBC) MDA-MB-231 cells. Observations exhibited kaempferol’s inhibitory effects toward cellular growth and was resilient in MDA MB-231 cell lines compared to estrogen receptor-positive BT-474 cell lines. 48 hours after the kaempferol treatment, the population of cell lines in the G1 stage was reduced considerably, from 85.48% to 51.35%, and population of cell lines in the G2 stage increased considerably, from 9.27% to 37.5%, indicating that kaempferol-induced G2/M phase arrest. Kaempferol-induced DNA damage and apoptosis in MDA MB-231 cell lines, as demonstrated by increase in expression of γH2A×, p-ATM, cleaved caspase-9 and cleaved caspase-3, as compared to control groups. The findings suggest that kaempferol is going to be a potential agent in the treatment of triple-negative breast cancers.[Citation27]
Researchers studied the inhibitory potential of kaempferol on triclosan TCS-induced growth of cells in MCF-7 cancer cells. TCS increased the capability of MCF-7 cells through estrogen receptor α (ERα), similar to the positive control, 17β-estradiol (E2). In contrast, kaempferol considerably inhibited TCS- and E2-induced cellular growth. The molecular process of kaempferol and TCS was also studied, the expression of genes related to, apoptosis, metastasis and cell cycle were analyzed by utilizing western blot assays. TCS treatments upregulated the protein expression of cathepsin D, cyclin-D1 and cyclin E, whereas downregulated bax and p-21 expression. Compound in study “kaempferol” reversed the TCS-induced gene expression. TCS increased phosphorylation of AKT, ERK, IRS-1 and MEK1/2, which indicated that TCS-induced MCF-7 cell growth through the non-genomic estrogen receptor signaling-pathway linked with IGF-1 R. Kaempferol exhibited a negative effect on the signaling process by downregulating protein expressions of, pAkt, pMEK1/2 and pIRS-1 induced by TCS and E2.[Citation25]
An in-vivo xenografted study of a mouse model was devised to study kaempferol, treatments with E2 and TCS-induced growth of tumors, as demonstrated by measurements of, hematoxylin, eosin staining, tumor volume, immunohistochemistry assay and bromodeoxyuridine assay. Similarly, co-treatment with kaempferol had inhibitory effects on TCS- or E2-induced tumor growth in breasts, having almost the same results of in vitro study. The findings suggest, kaempferol exhibit anticancer potential against the pro-tumorigenic activities of TCS or E2, a xeno-estrogen, in breast cancer can be a promising compound for neutralizing the risk of breast cancer.[Citation15]
Anti-bladder cancerous activity
The most common genito-urinary cancers are prostate and bladder cancer, with 1,414,259 and 573,278 cases, respectively, expected to be diagnosed in 2020. Bladder cancer is highly correlated with genomic DNA methylation, both in terms of its development and occurrence. Natural flavonoid kaempferol, which is found in various vegetables and fruits, has powerful anti-cancer effects on bladder cancer.[Citation28]
A typical urological malignant tumor that has a significant negative impact on patients’ quality of life is bladder cancer. Similar to the majority of other malignancies, little is known about the pathophysiology of bladder cancer. Increasing data suggests that bladder cancer and genomic DNA methylation are significantly related.[Citation29] Bladder cancer mortality can be effectively reduced by primary prevention strategies targeting modifiable risk factors before the onset of the disease. The most well-known contributing factors for usual cases of bladder cancer include, age, gender, smoking, occupational and environmental exposures, and infection with Schistosoma haematobium. The most recognized factors of bladder cancers include; lifestyle, ethnicity, age, and family history. Diet also plays a part as a contributing factor of bladder cancer.[Citation30]
A higher intake of processed meat is linked to a higher rate of bladder cancer, whereas a higher uptake of fruit, citrus fruits, vegetables and cruciferous vegetables may reduce that risk. Highly preserved foods are also linked to a higher risk of bladder cancer, while foods rich in lycopene, soy and fish might have a precautionary effect against that probability of causing cancer. Therefore, a preventive approach against bladder cancer may include dietary precautionary strategies, involving the use of nutritious supplements. The anti-neoplastic properties of kaempferol have been demonstrated in numerous pre-clinical projects of bladder and prostate cancer. Kaempferol was administered intraperitoneally at a daily dose of 50–150 mg/kg for a month in a mouse model created by subcutaneously introducing bladder tumor cells into naked mice. Tumor weight was evaluated after the animal was sacrificed, and there was no sign of any harm.[Citation31]
Action mechanism of kaempferol – anti-neoplastic activity concerns about induction of apoptosis, blocking the cell cycle processes, a chloride channel activated by calcium, inhibition of Anoctamin 1 (ANO1), inactivation or inhibition of oncogenic protein kinase FA directed by proline, which is concerned in the stoppage of cyclooxygenase-2, inhibition of glyoxalase 1, neoplastic progression and transformation, blocking of fatty acid synthase activity, DNA methylation modulation, elevated formation of granulocyte-macrophage colony-increasing factor, and onco-suppressor PTEN protein induction.[Citation32]
It was found that kaempferol could inhibit the growth of androgen-sensitive LNCaP cells by thirty-three percent at 5 micro moles, approx sixty percent at 10 micro moles and approx. 100% at 15 micro moles. Kaempferol had limited effect on nonmalignant RWPE-1 cells and PC-3 cell lines. Dihydrotestosterone caused IC-50 for kaempferol, in PC-3 cells, RWPE-1 cells and in LNCaP cells. In a dose-dependent manner, kaempferol-induced apoptosisin LNCaP cells with DHT. Data from the Luciferase assay exhibited significant inhibition of the activation mechanism of androgen receptors by kaempferol, induced by dihydrotestosterone. Downstream targets of androgen receptor, as, TMPRSS2, TMEPA1 and PSA, were reduced due to kaempferol as determined by qPCR assay. Proteins levels of PSA were also found to be decreased. Kaempferol downregulated the vasculogenic mimicry of PC-3 cell lines during the in vitro study. Overall, these results suggest that kaempferol may be a potential therapeutic agent for the reduction of prostate cancer. Chromatin immune-precipitation molecular docking, dual-luciferase reporter assay and cellular immunofluorescence staining, were utilized to study the androgenic potential of kaempferol. The effect on the expression of dihydrotestosterone-induced gene and cell growth was also examined. Rats model of testosterone-induced benign prostatic hyperplasia (BPH) was developed and mechanisms of action of kaempferol on BPH development were evaluated. The data from docking exhibited that kaempferol could bind THR877 and ASN705 residues of an androgen receptor.[Citation33]
Kaempferol directly promoted nuclear translocation process of the androgen receptors in prostate cancer LNCaP cells (androgen-dependent). Additionally, kaempferol stimulation significantly enhanced the in-vivo reaction among androgen receptors and PSA promoter region as well as the transcriptional activities of the androgen receptors. Conversely, pretreatment with kaempferol significantly suppressed the dihydrotestosterone-induced adverse effects, i.e. transcriptional activities of the androgen receptors, cell proliferation of, BPH-1, WPMY-1 and LNCaP cells and the expression of AR and PSA gene. Kaempferol also improved the pathological properties in BPH rats and decreased prostate-index. Kaempferol after administration, significantly reduced upregulated T levels in the serum of BPH rats. These results suggest that kaempferol exhibits androgenic-like activity and acts as a selective androgen receptor modulator, contributing to androgen-related BPH growth.[Citation34]
Anti-head and neck cancer activity
The term “head and neck squamous cell carcinomas” (HNSCC) primarily refers to tumors that start in the gastrointestinal tract and upper respiratory tract. According to statistics from around the world, there are 500,000 incidences of HNSCC every year. Malignant tumors, such as head and neck cancer, have been linked to a significant rise in morbidity and mortality in recent years.[Citation1]
When SCC-1483 cells are exposed to various kaempferol concentrations, apoptosis is induced. Cells were made to undergo apoptosis, and the effect was dose-dependent. Other cell lines, such as SCC-QLL1 and SCC-25, were also looked at conductive to validate an impact of kaempferol on the activation of apoptosis. All malignant cell lines exposed to kaempferol underwent apoptosis. Additionally, a caspase-3 activity test revealed that caspase-3 is required for kaempferol to induce apoptosis. Furthermore, it was shown that kaempferol essentially prevents SCC4 cells from migrating and invading in a dose-depending manner and that it is the cause of the MMP-2 enzyme transduction being inhibited by up to 53% at a maximum dosage of kaempferol (i.e., µ100 M). Kaempferol had a negative impact on the amount of TIMP-2 protein. These findings were supported by the analysis of mRNA expression, which showed that kaempferol significantly suppresses concentration-dependent mRNA MMP-2 expression. Kaempferol had a negative impact on the amount of TIMP-2 protein. These findings were supported by the analysis of mRNA expression, which showed that kaempferol significantly suppresses concentration-dependent mRNA MMP-2 expression.[Citation35]
Additionally, it was shown that kaempferol has a concentration-dependent effect on the activity of the AP-1 bond with the MMP-2 activator and reduces the ability of the AP-1 bond on the MMP-2 gene activator. The kaempferol treatment also reduces the c-Jun protein transformation to the nucleus but has no effect on the c-Fos protein levels in SCC4 cells. Kaempferol decreases MMP-2 production and presses ERK phosphorylation to prevent SCC4 cell migration, but it has little effect on the activation of the p38 and JNK1/1 pathways. Kaempferol primarily suppresses MMP-2 impression by blocking the ERK1/2 signaling pathway.[Citation12]
In an interesting study, the potential of apigenin and kaempferol was checked on viability in various cancer cells. The viability of FaDu, PCI-13 and PCI-15B cells with kaempferol treatment was analyzed in vitro. FaDu explants tumor growth was assessed in athymic mice. The results exhibited decreased the viability of cells in vitro owing to kaempferol, but differences were observed in cell-type-dependent responsiveness. In vivo treatment with apigenin and kaempferol significantly increased the tumor size of FaDu explants, and decreased viability of FaDu cell by kaempferol and apigenin was not exhibited in the results.[Citation36]
Researchers have also evaluated the potential of kaempferol on tumor cells of head and neck and have made efforts to investigate its onset process, including proliferation and apoptosis induction. Additionally, the arrangement of cisplatin and kaempferol on tumor cells of the head and neck were determined. The results showed inhibition of O2 consumption rate and lower intracellular ATP contents in cancer cells. Implying that it may reduce the migratory capacity as well as promote anti-proliferative effect on tumor cells as a result of apoptosis. Data obtained also directed that kaempferol can prepare tumor cells toward the treatments of cisplatin. The findings are evidence that kaempferol is effective against of head and neck cancer.[Citation36]
Brain tumor symptoms can range from mild to severe and both patients and the people who care for them every day must deal with these symptoms. Nevertheless, the usage of specific drugs can frequently be used to manage a variety of symptoms. Drug resistance development is among many reasons contributing to the very bad prognosis of glioblastoma, which is known to be an aggressive and invasive brain tumor. It’s interesting to note that kaempferol has been shown to suppress glioma cell growth and migration when added to kaempferol-loaded nano emulsion and mucoadhesive nano emulsion (KPF-MNE). The superoxide dismutase activity and thioredoxin concentrations are decreased by this flavonoid, which also causes an increase in the levels of monocyte, chemoattractant protein-1, cleaved caspase-3, −8, Bcl-2, anti-apoptotic proteins XIAP and surviving, chemokines, pro-inflammatory cytokines (interleukin-6,8) mitochondrial membrane potential depolarization and immediate decrease in ERK and AKT phosphorylation.[Citation37]
Anti-gastric cancer activity
Including the most frequent health issues with an increased fatality rate worldwide is gastric cancers, which ranks fifth around the globe. Despite the drop in prevalence, clinical, epidemiological, and translational studies continue to center on GC. The rise in GC prevalence will have a significant social and economic impact as well as pose significant difficulties to global healthcare systems. Despite the utilization of cures for GC as in surgeries, radiation, targeted therapy, chemo-therapy, gene therapy, 5-year survival rate was still around 30%. Thus, developing novel medications is crucial for the medication of GC. Kaempferol is found in a variety of plant genera.[Citation12]
Kaempferol demonstrated good oral absorption in both an experiment with healthy humans and the RO5 in PubChem. Particularly, it was claimed that kaempferol had anticancer activities against a number of human malignancies, including GC. Kaempferol consumption seemed to lower the incidence of GC, according a case-control research conducted in Spain. Kaempferol has been shown to dramatically slow the growth of GC tumor xenografts in an in vivo investigation, Kaempferol also has antineoplastic effects by preventing metastasis and proliferation, causing arrest of cell cycle, and encouraging programmed cell death and autophagic cell death. Therefore, the chemical processes governing how kaempferol treats GC are still not completely understood.[Citation38]
To determine the targets for kaempferol, the Swiss Target and PharmMapper Prediction databases were used. To determine the targets for GC, the Online Mendelian Inheritance in Man (OMIM), Therapeutic Target Database (TTD), MalaCards and Coolgen databases were used. To evaluate the interlinkage between the targets and kaempferol, molecular anticipation was carried out. The targets of GC and kaempferol overlap were then found using KEGG and GO enrichment analysis. After that, the hub targets were obtained using a network of interacting protein-protein (PPI), and their expression and overall survival were examined. Finally, the Kaplan-Meier Plotter online tool was used to conduct the overall survival (OS) tests of hub objectives. The 24 possible targets of kaempferol were matched with genes linked to illness in order to produce a total GC connected to 990 genes and 10 overlapping genes. Kaempferol can be fixed to these hub targets, according to the results of molecular docking, with high binding rates. These targets were subsequently portrayed to 11 noteworthy pathways and 140 GO biological process keywords. Three important targets – ESR1, SRC, and EGFR – were discovered using the PPI network analysis. Clearly, greater levels of EGFR and SRC protein and mRNA expression were seen in GC tissues. Poor OS in GC patients was associated with high expression of these targets. The therapeutic techniques of kaempferol on GC were revealed in this study using a unique method, making it easier to use kaempferol clinically in the future to treat GC.[Citation39]
A safe and effective multi-target medication against GC is anticipated to be developed using the promising chemical kaempferol. Through a variety of targets, pathways, and biological processes, kaempferol may exert an anti-GC impact, controlling cell metabolism and apoptosis, according to our network pharmacological study. Furthermore, kaempferol’s suppression of ESR1, SRC and EGFR may be responsible for this outcome. The clinical effectiveness of kaempferol and its mechanisms against GC require additional validation studies.[Citation40]
This study included MKN28, GSE-1, and SGC790 cells. At 37°C and 5% CO2, the cells were grown in RPMI-1640 media (Gibco-BRL) enriched with 10% fetal bovine serum (FBS). Kaempferol was bought. Primary antibodies against Bcl-2, Bax, Bcl-xL, survivin, cleaved-caspase-3, caspase-3, caspase-9, PARP, cleaved-caspase-9, cleaved-PARP, Akt, cyclin B1, p-Akt, Cdk1, GAPDH and Cdc25C and secondary antibodies against rabbit IgG-HRP and (HRP) mouse IgG-horseradish peroxidase (HRP) were acquired. Antibodies were utilized against phospho-ERK1/2, ERK1/2, COX-2, and Ki67.[Citation41]
SGC7901, MKN28, and GSE-1 cells were planted in 96-well plates for 24 h at a density of 3103/well in to study cell viability. Following cell adherence confirmation, the cells were treated with kaempferol at various dosages for 72, 48, or 24 h. Cell viability was determined using a Cell Counting Kit-8. Cell viability was determined as a percent of absorption in treated vs untreated wells. Three separate tests were carried out. Cells were then grown with reagents A-C, and flow cytometry was performed. Double labeling with Annexin V and propidium iodide was used to identify apoptotic cells (PI). Cancer cell lines were incubated with kaempferol (120 or 60 M) for 30 min before being rinsed twice with ice-cold PBS and treated with a 120 l sample buffer on ice Western blotting was finished with a 5-min measurement utilizing an improved chemiluminescence solution. Paraffin-embedded sections fixed with formalin (5 mm) were washed with PBS, blocked for 30 min with 10% bovine serum albumin, and stained overnight with an anti-Ki-67 antibody. A proliferation index (%) was computed by dividing the number of Ki-67-positive cells by the total cell count.[Citation39]
Anti-liver cancer activity
Adults are more likely to develop hepatocellular carcinoma (HCC) than any other primary liver cancer. Human liver tumor cells (Huh7, HepG2, SK-HEP-1) were found to be significantly inhibited in their ability to proliferate by kaempferol in a dose-depending mechanism. In addition, in rats with 2-acetylaminofluorene-induced HCC and diethylnitrosamine, kaempferol and luteolin together suppressed cell development and accelerated cell mortility. Kaempferol does stop the cell cycle and cause cell demise at the G2/M stage, which does stop cell migration and invasion.[Citation42]
Additionally, kaempferol can release cytochrome c by generating reactive oxygen species (ROS), which enlarges the mitochondria and results in membrane potential loss. It also raises the quantity of cleaved caspase-3.[Citation27] In human liver cancer cells, kaempferol reduces the cytokine signaling 3 (SOC33) expression of miR-21, promotor of transcription 3 (STAT3), signal tranolucer, CDK1, P13K/AKT/mTOR, cyclin B, (HIF-1) hypoxia-inducible factor 1 and p-mTOR gesturing trail. Tyrosine kinase 2 (Tyk2), phosphate and tensin homolog (PTEN), Janus kinase 1 (JAK1), endogenous interferon (IFN)-alpha-regulated genes, microtubule connected (LC3–11) protein 1A/1B-light chain 3, beclin1, (Atg) autophagy-related gene 5,7 and 12 and p44/42 MAPK are among the genes whose expression is increased.[Citation43]
An increase in ghrelin, a growth hormone-releasing peptide, promotes a favorable energy balance and is implicated in metabolic regulation. It has been noted that patients with cachexia who have lung cancer have higher plasma ghrelin levels. However, it is yet unknown how ghrelin levels vary in people with liver cancer. Contrarily, B vitamins like (thiamine) vitamin B1, (riboflavin) vitamin B2, (pyridoxine) vitamin B6, folic acid and vitamin B12 are crucial for a number of vital physiological processes like energy metabolism, protein manufacturing, and cell division. Less focus is placed on the variance of these B vitamins in the development of liver cancer cells, in contrast to folic acid. Less knowledge is also available about the connection between ghrelin and B vitamins in people with liver cancer (Sharma et al., 2021).
It appeared during a study that the decreased ghrelin synthesis in these liver cancer patients preferred a negative energy stability, which would eventually cause these individuals to become undernourished. Through mechanisms independent of growth hormone, ghrelin can boost nutrition ingestion and create a favorable balance of energy by reducing the usage of fat. Additionally, it was discovered the significant positive connections between the B6 and B2 vitamins and serum ghrelin contained in RBCs. The metabolism of amino acids is influenced by vitamins B2 and B6, so a lack of either of these nutrients may affect how ghrelin is made. The connection between vitamins B6 and B2 and ghrelin still needs to be clarified by more research. These findings from the current study suggested that measuring ghrelin or RBC (or vitamin B6) vitamin B2 could be utilized as a marker for assessing the nutritious condition of liver cancer patients.[Citation44]
In HCC cells, kaempferol triggered an AMPK-dependent activation of autophagy. Beclin-1/ATG-5 upregulation, Ulk1 phosphorylation, p62 degradation, LC3B-I to LC3B-II conversion and LC3B puncta development were all signs of autophagy activation. HCC cells were shielded against kaempferol by Beclin-1 shRNA or 3-MA -mediated autophagy suppression. Therefore, it appears that kaempferol caused autophagic cell death in HCC cells (but no apoptosis). Surprisingly, AMPK shRNA or mutation almost completely abrogated the autophagy activation caused by kaempferol. In turn, kaempferol also significantly reduced the amount of HCC cell death. Both autophagy and consequent cell expiry in HCC cells are mediated by AMPK stimulation in combination. The study’s second major discovery was that kaempferol administration resulted in complete AMPKα1 overexpression in HCC cells. A broad family of MAGE proteins with a shared homology domain are encoded by the melanoma antigen (MAGE) genes. Male testes are the only organ where MAGEA6 is expressed, and its functions are unknown. However, it is commonly re-expressed in a number of human tumors and is occupied in the development of cancerous cells. MAGEA6 is the AMPKα1 ubiquitin ligase that is particular to cancer. In a study, it was demonstrated that kaempferol-downregulated HCC cell MAGEA6 expression is also found in these cells. The major cause of AMPKα1 activation and subsequent upregulation could be due to MAGEA6 downregulation. The mechanism through which kaempferol inhibits MAGEA6 will undoubtedly be intriguing to further investigate.[Citation15]
Anti-prostate cancer activity
The 2nd most recurrent cancer fatality among males in North America is (PCa) prostate cancer, which is also the most regularly analyzed malignancy. Radiation therapy or surgical prostate removal are the two main treatments for PCa. On the other hand, the cancer often spreads to other parts of the body in men. Androgens have a big impact on how the prostate develops, grows, and stays healthy. When the first therapy fails, the majority of patients with metastatic PCa are given medications that inhibit testosterone synthesis. Almost all men initially respond favorably to androgen restriction therapy, but on average, after two to three years, they all relapse. Prostate cancer (PCa) often becomes fatal after progressing from androgen-dependent to androgen-independent, leaving few treatment options. 1,2,5-Dihydroxy-vitamin D3 (calcitriol), activated metabolite of vit. D, is a therapeutic agent with potential for the treatment of PCa. In addition to regulating bone metabolism as well as calcium homeostasis in the intestines, bones, kidneys, as well as in the parathyroid gland. Proliferation and pro-differentiation inhibition in various tumors and cancerous cells, e.g. PCa. These properties suggest that calcitriol may have potential as an anti-cancer drug for the treatment of PCa.” Further study has been motivated by the need for more effective treatments for prostate malignancy, one of the major risks of mortality among men (Budisan et al., 2019).
Kaempferol-3-O-rhamnoside increases the expression of poly (ADP-ribose) polymerase proteins and caspase-8, −9, −3, which in turn suppresses the stimulation of prostate cancer cells in a dose-depending manner. Granulocyte-macrophage colony-stimulating factor (GM-CSF), known to increase host immunological activity and improve host immunosurveillance via dendritic cells, is a possible treatment for prostate cancer (DC). It has been demonstrated that kaempferol causes PC-3 cells to secrete GM-CSF, which in turn stimulates the phospholipase C (PLC), protein kinase C and MEK1/2 pathways, increasing the chemotaxis of DC (PKC). It is quite likely that kaempferol therapy has a significant effect on the transcriptome of prostate cancer cells given that the androgen receptor gene was down-regulated. Kaempferol was given orally to rats and had no discernible harm. The growth of PCa xenografts decreased and it also markedly enhanced survival in athymic nude mice.
In both androgen’s physiological and pathological roles, the androgen receptor (AR) is a key player via regulation of transcription of targeted genes taking part in a variety of physiological processes as well as pathological diseases, as in benign prostatic hyperplasia (BPH), androgenetic alopecia, acne vulgaris and prostate cancers, the AR regulates androgen activity. Numerous studies have shown that both natural and synthesized kaempferol analogues have anticancer efficacy by having a shared cytotoxic ability against growing cancer/tumor cells. But to inhibit cancerous cell proliferation via specific mechanisms or targeted inside cancer cells is yet to be found. The synthesis, connections between structure and anticancer activity and specific mechanism of novel analogues have recently received substantial study. It has recently been demonstrated that several drugs, including ASC-J9 and its equivalents (3 and 4), limit the growth of prostate cancer by a unique mechanism of increasing AR degradation.[Citation10]
In comparison to the association of androgen receptor with the dihydrotestosterone, kaempferol assumed a comparable posture in the region of the androgen receptor complex, with its binding sides THR877 and ASN705 retained but the direct H-bond ARG752 deleted. The structuralf overlap between kaempferol and dihydrotestosterone to the androgen receptor suggests that kaempferol can interact to the androgen receptor, but with a low affinity. Immunocytofluorescence staining was used to evaluate the impact of kaempferol on androgen receptor nuclear translocation. In the absence of androgen, the androgen receptor was found all through the cell. Nuclear staining of androgen receptor was greatly elevated when stimulated with dihydrotestosterone. Likewise, kaempferol therapy enhanced androgen receptor in the nucleus in a dose-dependent pattern. This research indicated that kaempferol, like dihydrotestosterone, enhanced androgen receptor nuclear translocation, kaempferol boosted the recruitment of androgen receptors to the PSA promoter. Kaempferol also substantially increased androgen receptor transcription of genes, efficiently suppressed dihydrotestosterone-induced ARE luciferase activity, and decreased dihydrotestosterone-induced PSA mRNA production. Kaempferol also effectively prevented the onset of benign prostatic hyperplasia in a testosterone-induced rat benign prostatic hyperplasia model in vivo, indicating its significance as a possible androgen receptor antagonist.[Citation34]
Anti-pancreatic cancer activity
According to estimates, 28900 people in the US died of pancreatic cancer in 2001, making it the fourth most common illness to cause death. This cancer is among the deadliest of all malignancies, having a death-to-incidence ratio of roughly 0.99. Without a continuous improvement in outcome, adjuvant chemotherapy and radiation therapy may only offer a minor survival advantage. Surgery is the only viable treatment, but only 10–15% of individuals have pancreas-specific illness at the time of diagnosis, making resection potentially curative. The majority of these individuals unfortunately pass away from recurrence and metastatic metastasis. Pancreatic cancer has no reliable screening method, and identifying its risk factors is challenging. Additionally, this malignancy is aggressive locally and spreads easily. The prognosis is exceedingly dismal due to all of these problems. It might be possible to find new molecular targets for early detection and treatment with the aid of a better knowledge of the early pathways that cause pancreatic cancer. One of the cytokines in the IL-1 family, interleukin-1-beta (IL-1), promotes inflammation. The pathogenesis of inflammatory disorders has been linked to it. In addition, it has been demonstrated that IL-1 can promote the development and spread of tumors. It has been demonstrated that IL-1 can increase the invasiveness of cancer cells in cases of pancreatic cancer. Constitutive activation of up-regulation of cyclooxygenase-2 (COX-2) and NF-κB by IL-1β contributes to chemo-resistance in pancreatic cancer cells. As demonstrated by a study, IL-1 plays a role in invasion and metastasis by increasing the migratory capability of human pancreatic cancer cell lines.[Citation11]
The development and spread of pancreatic cancer are strongly correlated with inflammation, according to epidemiological and experimental data. Pancreatic cancer risk is increased by persistent pancreatitis. Inflammatory and oncogenic pathways implicated in the onset and spread of pancreatic cancer have been demonstrated to be regulated by comparable mediators, pointing to possible mechanisms that connect persistent pancreatic inflammation to an elevated risk of cancer. In contrast to the fibrotic stroma found in chronic pancreatitis, the stroma of pancreatic cancer is extremely desmoplastic. Most people with pancreatic cancer do not benefit from currently available anti-cancer medications or radiation treatment. As a result, considerable research must be performed to investigate the molecular processes behind these traits and create further techniques to enhance treatment results in pancreatic cancer patients. A phytochemical substance with antioxidant and anti-viability capabilities may operate as a safety anti-migration chemical in patient pancreatic cancer cell lines, providing justification for future research into kaempferol as a possible clinical trial option for benign pancreatic tumors. Both chronic pancreatitis and pancreatic cancer have been linked to elevated levels of pro-invasive substances like (MMP-2) metalloprotease-2 and growth substances like (EGF) epithelial growth factor. Recent research has demonstrated that the EGF receptor is necessary for the development of early Pancreatic Intraepithelial Neoplasia (PanIN) lesions and PDAC in genetically altered mice models. SNU-213, Miapaca-2, and Panic-1, pancreatic cancer cells, are successfully prevented from migrating by kaempferol’s induction of apoptosis and effective inhibition of AKT pathways, the ERK1/2, Src related epidermal growth factor receptor (EGFR). By elevating the FOXP3 expression level, kaempferol recovers the oppressive movement of Tregs, which are T cells that regulate other cells.[Citation26]
SNU-213 and Miapaca-2, Panc-1 pancreatic cancer cell lines of patients were acquired. The cancer cells were developed either in (Miapaca-2 and Panc-1) DMEM or (SNU-213) RPMI-1640 medium complemented with 10% of fetal bovine serum, 100 mg/L Streptomycin and 1 × 105 unit/L Penicillin at temp of 37°C. Pan caspase inhibitor (Gefitinib) GFR inhibitor and usage of antibodies for EGFR, (Tyr416) phospho-Src, (Tyr1068), EGFR, Src, (ERK) 1/2 AKT, phospho-EGFR, (PCNA) proliferating cell nuclear antigen, phospho-extracellular signal-regulatory kinase (Thr202/Tyr204), ERK, (Ser473) phospho-AKT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used to accomplish trials. Kaempferol, kaempferol-4-O-glucoside, and kaempferol-3’-O-glucoside were tried to check results against pancreatic cancer cells. The cell feasibility was evaluated using a WST-1 solution: 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2 H-tetrazolium solution. Invasion assay, migration assay, and western blot tests were performed. Higher quantities of kaempferol had a noteworthy inhibitory result on cell sustainability. As compared with the control cell lines that were handled with PBS, Miapaca-2 cells viability inhibited by approximately 70% when treated with 200 μM kaempferol, 50% in SNU-213 cells, and 45% in Panc-1 cells. At a modest dose, kaempferol selectively reduced serum-induced penetration and migration of patient pancreatic cancer cells, with no cytotoxicity. Kaempferol selectively inhibits serum-induced translocation by blocking the EGFR signaling pathway. It blocks the movement of enzymes, namely; tyrosine kinase, topoisomerase I, DNA topoisomerase II, cAMP-phosphodiesterase and myosin light-chain kinase, connected to development of cell and motioning passageway. Treatment with kaempferol also hinders cell growth, (FAS) fatty acid synthesis and convinces cell death in both breast and prostate cancer cells. This suggests that the suppression of FAS might be related to the activation of apoptosis in certain cancer cell line.[Citation45]
Anti-blood and anti-kidney cancer activity
Deficiency in cell proliferation and apoptotic pathways are the two main characteristics of the deadly blood malignancy known as acute promyelocytic leukemia. Leukemia, myeloma, Hodgkin lymphoma and non-Hodgkin lymphoma are the four major types of blood malignancies. These are currently the 4th most prevalent cancers in both men and women worldwide, making up about 9% of all cancers. When the World Health Organization (WHO) established a recognized classification of hematologic malignancy that distinguished each type based on immunophenotype, genetic abnormalities, and clinical features, it was first noted in 2001 and then again in 2008. However, these four groups contain more than 60 distinct subtypes. For the first time, the morphology, cytochemistry and clinical data were coupled with the genetics of the disease to identify the classification of the illness. It is predicted that in the USA, in 2014, there would have been a total of 157,000 blood cancer diagnoses, and that there would have been approximately 55,000 deaths from hematology malignancies. The high rates of occurrence and mortality underline the requirement for the creation of innovative and efficient therapies. Additionally, a considerably higher rate of market introduction for these medications is required. Despite only 10% of these novel molecular entities (NMEs) having an indication for hematologic malignancies, the FDA authorized 268 NMEs between 2005 and 2014. Overall, blood malignancies were linked to 23 of the 84 FDA-approved cancer drugs in the last five years. Additionally, 11 of the cancer medications that received FDA approval in 2012 cost more than $100,000 per patient per year, making them simply out of reach for the vast majority of patients and practically all medical providers. Repurposing a medicine might only take three to 12 years, whereas it might take up to 17 years to produce a novel drug. Repurposing medications offers a high level of safety because to the wealth of knowledge currently accessible on the pharmacokinetics, toxicities, bioavailability, dosage and procedures of the compounds. Medications have the advantage of a lower failure rate because they have already been examined for safety, which accounts for about 30% of trial drugs failing owing to safety and being repurposed. Phase I clinical studies often permit the establishment, avoidance, or usage of dose limitations with fixed dosage schedules for the trial, making a relaunch less expensive than the launch of a novel chemical.[Citation38]
In HL-60 and NB4 human leukemia cells, kaempferol (12.5–100 M) lowered cell feasibility in a dose-dependent mechanism. Kaempferol also triggers cell cycle halt, DNA disruption, apoptosis at G2-M stage, and it down regulates the impressions of ABCC1, ABCB1, BCL2 and AKT genes, DNA depending on serine/threonine protein kinase (DNA/PK), as well as DN repair system, phosphate-ataxia-telangiectasia, (p-ATM) phosphate-ataxia-telangiectasia mutated and (p-ATR) RAD3-related, p53, (14–3-3gamma) 14-3-3 proteins sigma, MDC1, O(6)-methylguanine-DNA methyl-transferase while up-regulation of cytochrome c expression and caspase-3, −8, p-p53, pH2A×. Kaempferol promoted the formation of secretory granules and mediator accumulation in human leukemic mast cells while decreasing the release of beta-hexosaminidase, in basophilic leukemia (RBL-2H3) cells, that is a hallmark of degranulation in a rat leukemia model.[Citation46]
The most common primary kidney tumor is known as renal cell carcinoma (RCC). In RCC, kaempferol dramatically slows down cell development and induces apoptosis (769-P and 786-O cells). Kaempferol reduces AKT phosphorylation, elevates the activity of focal adhesion kinase (FAK), and stops the MMP-2 protein, all of which contribute to the anticancer effects of the compound. Additionally, it increases cyclin B1 expression, p21 impression, PARP cleavage, and the stimulation of the EGFR indicating pathway.[Citation47] Kaempferol appears to decrease c-Myc mRNA levels while increasing CDKN1A mRNA levels when combined with the chemotherapeutic agent cisplatin. When combined with kaempferol, cisplatin, which by itself is ineffective against cancer cells, efficiently causes apoptosis by inhibiting the expression of c-Myc. It’s interesting to note that kaempferol may not just increase CDKN1A levels via inhibiting c-Myc. Kaempferol appears to increase p53 levels in breast cancer MDA-MB-453 cells. The well-known tumor suppressor protein p53 is sometimes referred to be the genome’s protector. This protein often mediates repairs after cellular DNA is broken and the associated stop of growth. P53 activates p21, also known as CDKN1A, when it detects damaged genomic information.[Citation40]
RCCs, which can spread to the liver, brain, bone, lung, and other organs, make up around 90% of the initial kidney malignancies. The (VHL) von Hippel-Lindau tumor-suppressor gene is largely deactivated in (CCRCC) clear-cell RCC, the most common histologic form of sporadic kidney cancer cells. The accumulation and buildup of hypoxia-inducible factor (HIF) is the result, which impairs normal angiogenesis and cell development. Despite the fact that resistance forms in the majority of patients over time, (TKIs) tyrosine kinase inhibitors that targets HIF-regulated proteins have been created as a first-line treatment for metastatic disease. This is because it is now known how genetic variations in RCC and subsequent trials lead to resistance. RCC is an immunogenic tumor cell, yet there is growing sign that inflammatory pathways and immune cells might promote and immune escape and tumor growth. Current research is starting to clarify the processes of immunological emission in RCC, including the part that cytokines and inflammatory immune cells play in this procedure. An increased interest in using immunotherapy, which includes methods to control inflammatory responses, to treat this illness is the result of recent discoveries.[Citation10]
Anti-oral cancer activity
The sixth utmost important tumor in the world is (OSCC) oral squamous cell carcinoma. The use of tobacco products, including chewing and smoking, have direct relation with an increase in the risk toward onset of mouth cancer and oral pre-cancer. It is believed that tobacco components acting as inflammatory response inducers are to blame for the development of (ROS)/RNS that may cause lipid peroxidation, an increase in NO products, and a compromised antioxidant defense system in tobacco users. The majority of deaths from oral distortions are caused by (OSCC) oral squamous cell carcinoma. Over 350,000 new cases of this tumor, which develops from the oral mucosa, were diagnosed in 2018, and more than 175,000 fatalities were also reported. The formation of tumors, including OSCC, is supported by a number of risk factors and predisposing factors, including fibers, chemicals, pesticides and heavy metals that can result in pro-oncogenic genetic and epigenetic changes.[Citation48]
Although irradiation is one of the clinical methods for treating oral cancer, it has numerous biochemical adverse effects, e.g. immune system changes and damages to cellular DNA and membrane components. The creation of free radicals through their interactions with the components of cells, particularly water, is regarded to be the primary cause of the biological effects they have. The damage caused by radiation is made worse by oxygen, which is typically present in most biological systems. Hydroxyl radicals are produced, and a large portion of the initial damage is caused by them. These radicals can then interact with other cellular components to make organic radicals. A key underlying phenomenon is an oxidative alteration in the lipids of the membrane that can be brought on by free radicals. It is now known that inflammatory diseases, infections, bacterial dysbiosis, oral injuries, and other factors might lead to the development of cancer. In vitro experiments with human tongue squamous cancer (SCC-25, SCC4 and SCC-1483), (Eca-109) human esophageal squamous carcinoma, oral cavity carcinoma (PCI-13), pharynx (FaDu), and kaempferol shown anti-proliferative effects. Kaempferol was exposed to hinder VEGF expression and angiogenesis in human oral carcinomas, and the paths linked with the instruction of HIF-1α. Quercetin and kaempferol-induced apoptosis depend upon caspase-3 in oral cavity cancer cells. The suppression of proliferative ability by kaempferol is caused by a decrease in MMP-2 expression. It also stops cell invasion and migration and significantly elevated apoptosis. The effects of MMP-2, Bcl-2, c-Jun and hexokinase-2 were also diminished by kaempferol, which also caused cell cycle halting at the G0/G1 phase. Kaempferol also increased the expression of PARP, ERK1/2 phosphorylation, EGFR activation, glucose uptake, Bax and cleaved caspase-3 and −9. In a mice xenograft model, kaempferol’s capacity to successfully prevent tumor growth as well as a discernible decline in hexokinase-2 impression and EGFR activity in cancer tissues provided additional proof of the drug’s anticancer efficiency.[Citation12]
Human tongue squamous cell carcinoma cell lines (SCC-4) attained from ATCC were cultivated with an equal capacity of a nutrient combination, 10% fetal bovine serum, F-12 Ham’s medium, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and hydrocortisone in 400 ng/mL. All cell lines were preserved at temp of 37°C in a moistened environment with 5% CO2. SCC4 cancer cells were broadcasted at a density of 5 × 104 cells/well in 24-well plates and handled with kaempferol at an absorption for 24 h. The research used oral squamous cell carcinoma cells (SCC4), and consequences showed that kaempferol suppresses the invasion and migration of SCC4 cells, decreases the enzyme activity and expression of genes of MMP-2, reduces the nuclear translocation of the MMP-2 to AP-1 cofactor, and suppresses the ERK1/2 phosphorylation. Frequent phytochemicals use anti-metastasis capacities by inhibiting the enzyme action or gene expression of MMP-2. Although, MMP-2 was physiologically inhibited by TIMP-2, data indicated that kaempferol decreases protein expression and TIMP-2 and MMP-2 mRNA. TIMP-2 overexpression concentrated angiogenesis and invasion, and sheltered melanoma cells from cell expiry. TIMP-2 enhances an anti-tumoral transcriptomic profile in oral cancer cells by upregulating E-cadherin. Manifestation of TIMP-2 in oral squamous cell carcinoma was interrelated with local recurrence, TNM production, and less advantageous survival proportions. The study exposed elevated average levels of TIMP-2 in cancer tissue than in normal tissue. Kaempferol repressed the connection of the MAPK signal pathway and the phosphorylation of ERK1/2, was well evaluated by using the MEK suppressor in SCC4 cancer cells, therefore, indicating that a treatment using U0126 could lead to SCC4 cell invasion and a suppressive expression of MMP-2 secretion. Kaempferol reduced the MMP-2 activity of SCC4 cancer cells by blocking AP-1 stimulation. Kaempferol represses only the c-Jun appearance in the nucleus without disturbing c-Fos. Kaempferol hinders SCC4 cell invasion and migration by reducing the MMP-2 gene activator-binding activity of AP-1 transcript influences, comprising c-Jun. Kaempferol constrains AP-1 movement, consequently subdues the assault of SCC4 cells and reduces MMP-2 expression. Additionally, Kaempferol obstructs ERK1/2 phosphorylation, efficiently leading to MMP-2 down-regulation. Outcomes of the study proposed that kaempferol may be an influential aspirant in the expansion of mediators used for averting cancer metastasis[Citation10]
Anti-bone cancer activity
Cancer that has spread to the bones can cause excruciating pain. Unfortunately, some current treatments are useless, and even when they are efficient, the patient usually survives longer than the pain is relieved. There is a critical need for new, mechanically based therapeutics. Research on investigational animal models has shed light on the mechanisms underlying the pain of bone cancer and offers a chance to create specialized treatments. Nerve injury induced by tumor, tumor-directed, osteoclast-mediated osteolysis, tumor cells, stimulation of transient receptor potential vanilloid type 1 ion channel, endothelin A and host cell synthesis of nerve growth factor are some of the mechanisms underlying bone cancer pain. External beam radiation, osteoclast-targeted inhibitors, analgesics, transient receptor potential vanilloid type 1 antagonists, anti-inflammatory medicines, and antibody treatments that targets tumor angiogenesis or nerve development factor are some of the current and upcoming treatments. A combination of such remedies will probably be more effective than any one of them by alone. TNF is a powerful osteoclastogenic cytokine that encourages bone absorption and it is primarily produced by bone marrow stromal normal cells, activated macrophages and monocytes. In vivo and in vitro, TNF promotes osteoclast development and multinucleated cell production. By encouraging osteoblasts to release a number of osteoclastogenic cytokines, including monocyte chemoattractant protein-1 and interleukin-6 (IL-6), TNF also increases osteoclastic activity (MCP-1). In order to govern normal bone remodeling and have been linked to the etiology of many bone illnesses, including osteoporosis, these TNF family members and RANKL constitute one of the most powerful osteoclastogenic cytokines.[Citation12]
According to several reports, kaempferol boosts the anticancer affinity when combined with other anticancer medications. For instance, by preventing the efflux of quercetin, the conjunction of quercetin with kaempferol considerably improves the anticancer activity of quercetin. Additionally, it has been shown that kaempferol ingestion considerably increases the cytotoxic activity of cisplatin. Therefore, it may be said that although if kaempferol has a very low bioavailability, it appears that kaempferol boosts the bioavailability of several anticancer medications. Human osteosarcoma cells 143B, HOB and U-2 OS cells that grow and migrate more slowly when exposed to kaempferol, with no damage to human fetal osteoblast progenitor and hFOB cells. urokinase plasminogen activator (uPA), MMP-2, AP-1 DNA binding action and MMP-9, are all downregulated by kaempferol, which then lowers phosphorylated ERK, JNK, and p38. Kaempferol, in BALB/c (nu/nu) mice dramatically decreased the figure of sustainable cells and reduced the cancer cells size that had human osteosarcoma cells injected into them. Additionally, to demonstrate the anti-bone malignancy characters of kaempferol, BALB/c (nu/nu) mice injected with human osteosarcoma cells were used.[Citation8]
Through analyzing the impact of TNFa and RANKL on osteoclast and osteoblast activity, we can better understand the biological pathway through which quercetin and kaempferol prevent bone cancer. It was found that kaempferol, and not quercetin, prevents osteoblasts from producing the osteoclastogenic cytokines MCP-1 and IL-6, and prevents the nuclear translocation of NF-B (p65) that is brought on by TNFa. Additionally, it was demonstrated that quercetin and kaempferol block the RANKL-induced production of c-fos in RAW264.7 macrophage/monocyte cells, which is necessary for osteoclast differentiation. Thus, the discovery offers new physiological explanations for the in vivo observation that quercetin’s antiosteoclastogenic effect prevents ovariectomy-induced bone loss. The findings also suggest that kaempferol’s effect on bone cancer may make it a more effective antiosteoclastic drug in vivo.[Citation49]
Kaempferol might reduce Bcl-2 protein and elevates the appearance of Bax protein, increase caspase-3, −7, and −9 activities and decreased potential of mitochondrial membrane in the U-2 OS cancer cell line. An increase in AIF protein intensities was also detected, representing that cell death was encouraged through a caspase-independent mitochondrial signal pathway. Another mechanism triggered by kaempferol in osteosarcoma cell lines of human is the endoplasmic reticulum stress pathways. The anticancer influences of this bioflavonoid in mouse models were validated in a study. Another study found that kaempferol reduced cell metastasis in U-2 OS cells by blocking several signal transduction mechanisms (e.g., AP-1, ERK, p38, and JNK). Kaempferol showed an anti-osteoclastogenic effect on bone when osteoblasts ceased generating osteoclastogenic cytokines and osteoclast precursor cells stopped developing. It is because it inhibits the function of the TNF receptor family on bone cells.[Citation3]
Anti-cervical cancer activity
For cervical cancer, a variety of treatments are employed, including surgery, chemotherapy, and radiotherapy. Only fertile-age females with cervical cancer who might have lost fertility and patients with early-stage are eligible for surgery. Although radiotherapy and chemotherapy are beneficial, they do not target only cancer cells but also potentially harm all healthy cells. Therefore, efforts to create novel anticancer agents with higher potency and side-effect profiles have continued. Dietary phytochemicals may affect chemotherapy and aid in the treatment of cancer patients, according to researchers. Numerous natural substances have the potential to increase the effectiveness of chemotherapy, reduce drug resistance, and lessen the negative side effects of the treatment. As a result, scientists are attempting to use many herbs and their powerful compounds for cancer therapy both in vivo and in vitro. The majority of herbs have antioxidant properties that can be ingested to fend off cancer or enhance chemotherapy.[Citation16]
Numerous medicated herbal compounds and plants have been shown to have anti-cancerous properties in studies. Additionally, it has been demonstrated that a number of phytochemicals that have been extracted from medicinal plants can rigger apoptosis, slow metastasis, reduce cell proliferation and block angiogenesis. A few of these plant-derived substances are currently employed often in the chemotherapy of cancer patients. For instance, the therapy of such patients has made use of vinca alkaloids (vincristine, vinblastine), taxol analogues and podophyllotoxin analogues. Almost all fruits and vegetables include flavonoids, a class of plant compounds. The yellow flavonoid kaempferol (3, 40, 5, 7 tetrahydroxyflavone) is found worldwide in many different botanical groups. Additionally, kaempferol inhibits tumor growth and their viability in different variations of cancer cell lines of humans, e.g. (FaDu cell-lines) from the pharynx, (PCI-13 cell-lines) oral cavity carcinoma as well as (PCI-15B cell-lines) metastatic lymph nodes and explanted FaDu cells. Kaempferol is cytotoxic to HeLa cells, reducing their survivability in a dose-dependent approach via inhibiting the hTERT and PI3K/AKT pathways, while it lessens sustainability of SiHa cancer cells in both time and dose point manner and prompts apoptosis by distracting mitochondrial membrane prospective. Other research confirmed that kaempferol inhibits the growth of (RCC) renal cell carcinoma lines (769-P and 786-O cells) through a variety of pathways, including cell cycle detention, upregulation of p21 and downregulation of cyclin B1 expressions, inhibition of activation of p38/EGFR signaling mechanisms, apoptotic cell death and initiation of PARP cleavage. Due to their structural variety, flavonoids including kaempferol are more effective than flavanols at preventing cancer. The inhibition and binding in this situation are influenced by the number of hydroxyl ion on the B ring. Flavonols’ ability to bind to DNA has been linked to their structural makeup.[Citation9]
Compared to regular cells and HFF cells, kaempferol was discovered to specifically inhibit the proliferation of human cervical cancer cells for example drug-resistant human cervical cancer, HeLa, SiHa and KB-V1. Apoptosis and cell cycle arrests at G2/M stage were also brought on by kaempferol, and these effects were correlated with decreased activity of the (hTERT) human telomerase reverse transcriptase P13K trials, cyclin B1, Pgp, Rh123 efflux, CDK1, NF-B, Bcl-2 nuclear transposition, and increased activity of the p53 with disruption of mitochondrial cell membrane potential.[Citation16]
Kaempferol can efficiently decrease propagation of (triple-negative BC) TNBC, MB-MDA-231 cells by hindering G2/M alteration and DNA destruction by upgrading ϒH2A× and cleaved caspase—9, 3, compared to the regulator group. Additionally, the extraordinary anti-cancerous characteristics of kaempferol was recognized both in vivo and in vitro situations. Kaempferol prevents the enzymes of COX2 and iNOS, which accordingly diminishes the ROS stage. Additionally, it displays its defensive consequence by upregulating anti-oxidatory enzymes that can absorb the free radicals to shrink infection. The HeLa cells treated with kaempferol 40, 30, and 50 μM confirmed nuclear blebbing, fragmentation, and apoptotic body development, and the influence was established to be more distinct with elevating absorption. Kaempferol effectively reduces DNA integrity and, as a result, induces DNA ladder construction in handled cells in a dose-depending sequence, as demonstrated by agarose gel electrophoresis, while the DNA of control cells stayed integral. Kaempferol-mediated inter-nucleosomal disintegration was confirmed using agarose gel electrophoresis, which revealed that treated cells had DNA ladder design with crisp bands as associated to unprocessed controls. The anti-inflammatory specialty of kaempferol was demonstrated by examining the cell cycle controlling facts through current flow cytometry. Research confirmed that kaempferol prompts its anti-cancerous result by G2/M cell cycle inhibition, as there was a dose-depending proliferation in the proportion of the cancer cells at G2/M, which was supplemented by a minor growth in the sub-G1 population.[Citation50]
Anti-ovarian cancer activity
The procedure of creating new blood nerves, known as angiogenesis, is vital to the development of tumors. Angiogenesis in a healthy adult is essentially dormant, with only 0.01% of endothelial cells going through cell division. However, since active angiogenesis is necessary for tumor growth, anti-angiogenesis becomes a sensible anticancer therapy. Vascular endothelial growth factor (VEGF) is the most crucial positive regulator of angiogenesis, and many human malignancies, including ovarian cancers, express the VEGF gene. Growth factors, oncogenes, hormones and oxygen tension all control the expression of the VEGF gene. (HIF-1) Hypoxia-inducible factor 1, which is made up of the inducible HIF-1α and HIF-1β subunits, stimulates the expression of VEGF in response to hypoxia. The PI3 kinase/AKT pathway is thought to be involved in the control of HIF-mediated VEGF responses in normoxia. The VEGF gene is also stimulated by growth hormones and inflammatory cytokines, such as (TGF) transforming growth factor, epidermal growth factor, interleukin-1 IL-6 and (IL-1).[Citation11]
Through estrogen receptors (ERs) and the estrogen response element, estrogens promote the production of VEGF (ERE). In tumors, the proto-oncogene c-Myc promotes cellular growth and proliferation and works with HIF-1 to increase VEGF production. Subsequently, it was discovered that PI3K/AKT pathways control Myc. The standard method for regulating VEGF is thought to involve PI3Kinase/AKT and HIF, while a recently identified pathway that does not require HIF involves estrogen-related receptor alpha (ESRRA) and peroxisome (PPARGC1A) proliferator-activated receptor gamma coactivator 1 alpha. This pathway involves the orphan nuclear receptor ESRRA, which interacts strongly with ERs and has a significant level of sequence similarity.[Citation19]
A very common natural food supplement called kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4 H–1-benzopyran-4-one) has been shown to lower the incidence of ovarian cancer. Through the activation of MEK-MAPK, it has been discovered that kaempferol hinders the expression of the estrogen receptor alpha in breast tumor cells and cause initiation of apoptosis in glioblastoma and lung cancer cell lines. According to the studies, kaempferol also inhibits the expression of cyclo-oxygenase 2 and interleukin-4 via inhibiting Src kinase and downregulating the NFkB pathway. Additionally, efficient at preventing angiogenesis and causing apoptosis in ovarian cancer cells, the main factor is kaempferol. According to human research, people who consumed the highest quantity of kaempferol had a 40% lower incidence of ovarian cancer than those who consumed the least. Despite encouraging preclinical results, the effectiveness of these substances for chemoprevention in individuals has met with only modest success. This is partly because effective systemic transport and low bioavailability of potential medicines are major obstacles.[Citation51]
Kaempferol was shown to be able to decrease proliferation, tumor angiogenesis and growth by lowering (VEGF) vascular endothelial growth factor impression in studies employing human ovarian cancer cell lines (A2780, A2780, OVCAR-3, and SKOV-3). By upregulating the proteins `Cdc25C, DR4, p38, DR5, p21, JNK, CHOP, caspase-3, Bax, ERK1/2, −7, −8, Bad, and p53 and downregulating a regulator of hypoxia-inducible factor 1 (HIF-1), VEGF impression kaempferol also promotes cell cycle arrest and apoptosis during G2/M phase.[Citation52]
Kaempferol has little cytotoxicity on normal cells SV40, suggesting that it selectively targets cancer cells. To figure out how kaempferol works, we looked into whether it causes apoptotic cell death in OVACAR-3 cells AO/EB stain revealed that kaempferol caused membrane degranulation and programmed cell death. DAPI labeling yielded comparable results, while annexin PI/V staining discovered that the percentage of apoptotic cell populations improved with increasing kaempferol doses. Programmed cell death in OVACAR-3 cells was validated further by looking at the overexpression of apoptosis-relating proteins. Kaempferol reduced the impression of Bcl-2 and elevated the impression of Bax and Caspase 3, 8 and 9. Apoptosis eliminates the flawed or cancer cells and sustains the tissues homeostasis. Moreover, programmed cell death also averts the progress of medication opposition in ovarian cancer cells. Kaempferol convinces apoptotic cell demise in breast cancer, glioblastoma cells, colon cancer and non-small lung cancer cells. Cell cycle inhibition also supports to cessation of the development of cancer cell lines. Kaempferol has also been described to generate cycle arrest in renal and ovarian cancer cell lines. Here, consequences found that kaempferol action hinders the cells at the G0/G1 barrier which was also complemented with dominance of Cyclin Cdc2 and B1 impression. Lastly, the special influences of kaempferol were also examined on the STAT3 and EK/MEK signal transduction paths. These mechanisms have been exposed to be unreasonably initiated in ovarian cancer cell lines and kaempferol might constrain both these mechanisms in the ovarian cancer cells OVACAR-3, indicative of its potential anti-cancer effects.[Citation53]
Anti-colon cancer activity
Colorectal carcinoma is one of the greatest commonly identified cancers in the world with more than 1.8 million new patients, annually. Several human colorectal tumor cell lines, involving SW480 cells, LS174-R, HCT-15, HCT116 and HT-29 have shown cytotoxic effects in response to kaempferol. Although 5-Fluorouracil is susceptible to medicinal failure because of the emergence of resistance, it is still the most frequently advised chemotherapy drug.
In order to continue their rapid growth, tumor cells are believed to primarily depend on the glycolytic pathway; hence, focusing on this pathway as a possible cancer therapy technique. In order to ascertain whether kaempferol has an impact on the glycolysis process in colon cancer cells, it was also determined how much glucose was consumed, how much lactic acid was produced, and how much ATP was produced. Both the HCT116 and DLD1 cells showed reduced glucose intake after kaempferol administration. Additionally, kaempferol therapy greatly reduced the buildup of lactic acid, the byproduct of nonoxidative glucose metabolism. Additionally, ATP levels in cells treated with kaempferol were much lower than in the control group. Together, these findings show that kaempferol prevents cancer cells (colon) from undergoing aerobic glycolysis. 5-fluorouracil and kaempferol were mixed in LS174-R cells, and remarkable anti-proliferative impacts were seen. Additionally, the activity of the death receptor 5 (DR5), and cancer necrosis factor ligand superfamily member is increased by kaempferol which caused colon cancer cells to undergo apoptosis.[Citation54]
Normally, kaempferol is able to activate ATM and induce apoptosis through the p53-caspase-3 mechanism with the association of PUMA and Bax. Kaempferol taken orally in appropriate-dose forms is expected to influence pharmacologically relevant quantities in the colon and display anti-cancer activity effectively. Kaempferol prompts programmed cell death and cell cycle arrest at G2/M stage, and prevents both cell migration and cell invasion. Additionally, kaempferol suppresses the generation of ROS and altered the impression of JAK, MAPK, H2A, ATM, AKT, (H2A×) histone family member, p53, p21, caspase-9, −8, −7, −3, PARP, phospho-p3, 8Bcl-2, p53 increased modulator of programmed cell death, mitochondria release cytochrome c. Additionally, kaempferol markedly decreased the impression of cyclins B1, D1, E, and A, as well as heregulin (HRG)-, CDK2, CDK4, Cdc25C, and CDK2. Additionally, it increased the PARP cleavages and decreased the phosphorylation of the retinoblastoma protein.[Citation9]
Kaempferol prevents the development of the colon cancer cell lines HT-29 and Caco-2, the current reading was achieved to recognize the pathways by which kaempferol suppresses (HT-29) human colon cancer cell growth. The current consequences have exposed that kaempferol hinders DNA development, reduces the impression of cyclin E, D1 and A, reduces the impression of CDK2 and 4, suppresses the action of CDK4 and 2, destroys the phosphorylation of RB, lowers the impression of CDK4 and 2, induces G2/M stage arrest, overturns the impression of Cdc25C, Cdc2, and represses Cdc2 and cyclin B1 activity. Treatment with kaempferol encouraged G1 arrest within 6 h. A broad diversity of cell categories reacts to several growing elements by the initiation of CDK4. Kinase activity of CDK4 involves the binding of the positive monitoring subunit, renowned as cyclin D1. In the current research, kaempferol reduced the points of cyclin D1 and CDK4 proteins within 6 h in a dose-depending sequence. CDK4 activation was extremely reduced within 0.5 h after treatment with kaempferol. As with the decrease in the levels of CDK2 kaempferol additionally inhibits the activity of CDK4 activity, cyclin E and cyclin A proteins, leading toward a reduced CDK2 activity. These effects obviously presented that kaempferol brings a decline in the protein levels of cyclins and CDKs, thus dropping the action of these enzymes in HT-29 cancerous cells.[Citation55]
Programmed cell death can be induced via two different ways: the intrinsic (mitochondrial) mechanisms and the extrinsic (death receptor) pathway. In the death receptor mechanism, TNF or FasL binds to receptors, triggering the cleavage of caspase-8 and leading to Fas-associated death domain. In the mitochondrial mechanism, mitochondria releases cytochrome c and with apoptosis/procaspase-9 protease activating factor-1 forms an apoptosome. This additional persuades cleavage of effector proteins comprising PARP and caspase-3 activation, which lastly leads to cell death. Consequences undoubtedly validate that kaempferol persuades cytochrome c discharge as well as PARP cleavage and caspase-3, proposing that the basic programmed cell death mechanism is accountable for the growth halting properties of kaempferol.[Citation56]
Anti-lung cancer activity
Non-small-cell lung tumor, for instance, has reduced prediction and is presently growing the number of cancer-related expiries worldwide. (NSCLC) Non-small-cell lung cancer impacts about 85% of people with primary lung cancer. The usual course of action for initial-stage NSCLC is surgery. The majority of cancer patients who are detected at a progressive phase, however, are not suitable for extensive mediastinal lymphadenopathy or surgical excision. The current gold standard of care for patients with advanced, irresistible NSCLC is chemotherapy and radiation. However, there are a number of restrictions on chemo- and radiation, such as normal tissue dosage tolerance and tumor radio resistance. Finding efficient medicines that increase tumor susceptibility to radiation and lessen side effects on healthy tissues is therefore essential. Lung cancer evolution was repressed by kaempferol in a concentration-dependent style. A549 cells inhibited colony progress and prompted apoptosis. Even at low doses, oral kaempferol absorbs exceptionally efficiently with little inter-individual variation.[Citation57]
According to reports, kaempferol is helpful against glioma cells, non-small cell lung cancer and pancreatic cancer cells. It has been suggested that it works in concert with quercetin to significantly decrease the propagation of human gastrointestinal and breast cancer cells. Moreover, kaempferol expressively reduced cell relocation, reversed the damage of E-cadherin, and stifled EMT. Sensitive action of AKT/P13K phosphorylation, Bcl-2, MEK1/2, claudin 2, ERK pathways, MMP2, MAPK, Bcl-xL, complicated in apoptotic trail still downregulated by kaempferol. While the normal HFL1 cell line was unaffected, kaempferol had a dose-depending effect on the feasibility of A-549 cells. Such characteristics are important for the creation of an anticancer medicine that, unlike the majority of currently prescribed clinical medications and is not harmful to normal cells. Bax, a member of the Bcl-2 family of apoptosis proteins, may be promoted by inhibition of the PI3K/AKT and ERK mechanism, which will subsequently activate caspase to cause cell death. Kaempferol boosted the expression of Bax, which ultimately triggered caspase-7. It also inhibited the phosphorylation of ERK, PI3K, and AKT. The consequences of the research show that kaempferol suppressed A-549 cells via triggering the mitochondrial route for apoptosis.[Citation58]
Kaempferol was also capable to minimize the amount of hypodermic xenograft and the amount of metastases in a lung metastasis model when linked to the control set. Furthermore, a BALB/c nude mice xenograft model of A-549 cells had an important consequence on the radiation-induced death of cancerous cells. After 6 h of radiation exposure, kaempferol also prevented the phosphorylation of PI3K and ERK sustainability. Using a clonogenic assay, that is also used to evaluate cell reproductive mortality following exposure to ionizing radiation, we then confirmed the radiosensitization impact of kaempferol.[Citation59 Different studies showing the anti-cancer mechanisms of kaempferol were summarized in .
Table 2. Different studies showing the anti-cancer mechanisms of kaempferol summarized.
Another study found that combining radiation with kaempferol (4 h before radiation) led to a substantial reduction in tumor development 25 days after therapy. The sections of the radiation and kaempferol-treated tumors revealed apoptotic signs of nuclear condensation, cell shrinkage, and fragmentation by E&H staining, whereas the control tumors showed a homogeneous distribution of live cells. Kaempferol decreased the proliferation of A-549 cells in a dose-depending mechanism, but had no impact on the normal HFL1 cell line. Such features are important in the creation of an anticancer therapy that is not harmful to normal cells, unlike most therapeutic medicines now in use. Inhibiting the ERK and PI3K/AKT pathways may stimulate the expression of apoptotic proteins from the Bcl-2 family, such as Bax, and subsequently activate caspase to cause cell death. In this study, kaempferol inhibited the kinase activity of PI3K, AKT, and ERK, then triggered caspase-7 and raised the expression of Bax. The findings show that kaempferol suppressed A-549 cells through activating the mitochondrial apoptotic pathway. Prior to determining if kaempferol may enhance radiation’s inhibitory properties on cancer cell development, it was necessary to understand the role of cell cycle control in mediating the risk of local recurrence. Cells’ radiosensitivity varies depending on where they are in the cell cycle. The G2/M phase is the most radiation-sensitive. In the current study, the G2/M cell population increased in a time-dependent manner after being treated with kaempferol. Furthermore, kaempferol therapy before exposure to radiation suppressed the development of A-549 lung cancer cells more efficiently. Kaempferol also decreased the phosphorylation of ERK and PI3K following a 6-h radiation exposure. It then employed a clonogenic assay to confirm the radiosensitization impact of kaempferol, which is also used to evaluate cell reproductive mortality following ionizing radiation therapy. The surviving proportion was found to be considerably lower after kaempferol pretreatment prior to radiation exposure. The results show that kaempferol has the ability to radiosensitize A-549 cells. In vitro and in vivo, kaempferol boosted tumor cell death by radiation by inhibiting the AKT/PI3K and ERK pathways and activating the mitochondrial apoptotic system. The findings demonstrated that kaempferol is a safe and potentially radiosensitizing agent for NSCLC.[Citation60]
Conclusion
In light of the literature presented above, it is evident that kaempferol is an excellent anti-cancer agent. Several studies suggest that kaempferol is effective against numerous types of cancer. As a cancer preventive agent, Kaempferol has proven effective against multiple cancer pathways, in particular prostate and ovarian cancer. Kaempferol should be further studied for the treatment of cancerous cells that are involved in cancer proliferation. Not many artificial drugs possess such a capability. Furthermore, in research studies, kaempferol did not exhibit any adverse side effects. Kaempferol achieves these impacts primarily by eliminating reactive nitrogen and oxygen species, mostly by chelating molecules. Kaempferol has the capacity to inhibit intracellular components such as (NF-B) nuclear factor kappa B, Kaempferol has the capacity to inhibit intracellular components such as (NF-B) nuclear factor kappa B, (MAPKs) mitogen activated protein kinases, (COX-2) cyclooxygenase-2 and (SIRT 1) sirtuin. Since cytokines and chemokines are implicated in both cancer growth and metastasis, another way by which kaempferol function as anti-cancer drugs is through immune cell control. Kaempferol has been shown to actively reduce cancer metastasis elements by modulation of adhesion molecules, such as epithelial-mesenchymal and metalloproteinases transition signals. Angiogenesis is another critical mechanism in cancer development and migration since it is required for the tumor microenvironment to function properly. As a result, one of the ways these chemicals prevent cancer formation is by inhibiting crucial players in the process, such as epithelial growth factor response and vascular endothelial growth factor expression.
Consent for publication
All the authors on this manuscript gave their consent to publish.
Acknowledgments
The authors acknowledge Muhammad Nawaz Shareef University of Agriculture Multan, and Kampala International University in Kampala, Uganda, for providing the facilities used in undertaking this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutr. 2021, 13(3), 845.
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell. Pathol. 2019, 2019, 1–10. DOI: 10.1155/2019/1907698.
- Amjad, E.; Sokouti, B.; Asnaashari, S. A Systematic Review of Anti-Cancer Roles and Mechanisms of Kaempferol as a Natural Compound. Cancer Cell Int. 2022, 22(1), 1–22. DOI: 10.1186/s12935-022-02673-0.
- Egbuna, C.; Awuchi, C. G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K. C.; Singh, O.; Odoh, U. E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S., et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr.Rent Top. Ics in Mmed. Icinal Cchem.Istry. 2021, 21(12), 1067–1095. DOI: 10.2174/1568026621666210509161059.
- Nwozo, O. S.; Effiong, E. M.; Aja, P. M.; Awuchi, C. G. Antioxidant, Phytochemical, and Therapeutic Properties of Medicinal Plants: A Review. Int. J. Food Prop. 2023, 26(1), 359–388. DOI: 10.1080/10942912.2022.2157425.
- Boubekeur, S.; Messaoudi, M.; Awuchi, C. G.; Otekunrin, O.; Sawicka, B.; Idjeri-Mecherara, S.; Bouchareb, S.; Hassani, A.; Sharifi-Rad, M.; Begaa, S., et al. Biological Properties and Polyphenols Content of Algerian Cistus Salviifolius L. Aerial Parts. Eur.Ropean J. Ournal of Biol.Ogical Res.,earch. 2022, 12(2), 163–180.
- Awuchi, C. G.; Twinomuhwezi, H. The Medical, Pharmaceutical, and Nutritional Biochemistry and Uses of Some Common Medicinal Plants. Int. J. Med. Aromat. 2021, 1–32.
- Chen, H. J.; Lin, C. M.; Lee, C. Y.; Shih, N. C.; Peng, S. F.; Tsuzuki, M.; Yang, J. S.; HUANG, W. -W.; YANG, J. -S. Kaempferol Suppresses Cell Metastasis via Inhibition of the ERK-p38-JNK and AP-1 Signaling Pathways in U-2 OS Human Osteosarcoma Cells. Oncol. Rep. 2013, 30(2), 925–932. DOI: 10.3892/or.2013.2490.
- Choi, J. B.; Kim, J. H.; Lee, H.; Pak, J. N.; Shim, B. S.; Kim, S. H. Reactive Oxygen Species and p53 Mediated Activation of p38 and Caspases is Critically Involved in Kaempferol Induced Apoptosis in Colorectal Cancer Cells. J. Agri. Food Chem. 2018, 66(38), 9960–9967. DOI: 10.1021/acs.jafc.8b02656.
- Lin, C. W.; Chen, P. N.; Chen, M. K.; Yang, W. E.; Tang, C. H.; Yang, S. F.; Hsieh, Y. S.; Guan, X. -Y. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS One. 2013, 8(11), e80883. DOI: 10.1371/journal.pone.0080883.
- Ren, J. I. E.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T. A. O.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases. Exp. Ther. Med. 2019, 18(4), 2759–2776. DOI: 10.3892/etm.2019.7886.
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Estevinho, L. M.; Tsouh Fokou, P. V.; Umair Arshad, M.; Khan, H., et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Mol. 2019, 24(12), 2277. DOI: 10.3390/molecules24122277.
- Mishra, A. P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagon. Ther. 2018, 22(3), 281–295. DOI: 10.1007/s40291-018-0329-9.
- Elsharkawy, E. R. Isolation of Phytoconstituents and Evaluation of Anticancer and Antioxidant Potential of Launaea Mucronata (Forssk.) Muschl. Subsp. Pak. J. Pharma. Sci. 2017, 30(2), 411–417.
- Kim, Y. A.; Tarahovsky, Y. S.; Gaidin, S. G.; Yagolnik, E. A.; Muzafarov, E. N. Flavonoids Determine the Rate of Fibrillogenesis and Structure of Collagen Type I Fibrils in vitro. Int. J. Biol. Macromol. 2017, 104, 631–637. DOI: 10.1016/j.ijbiomac.2017.06.070.
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol Increases Apoptosis in Human Cervical Cancer HeLa Cells via PI3K/AKT and Telomerase Pathways. Biomed. Pharmacother. 2017, 89, 573–577. DOI: 10.1016/j.biopha.2017.02.061.
- Chen, Y. C.; Guo, Y. F.; He, H.; Lin, X.; Wang, X. F.; Zhou, R.; Deng, H. W. Integrative Analysis of Genomics and Transcriptome Data to Identify Potential Functional Genes of BMDs in Females. J. Bone Miner. Res. 2016, 31(5), 1041–1049. DOI: 10.1002/jbmr.2781.
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017, 41(4), 1325–1335. DOI: 10.1159/000464435.
- Mamouni, K.; Zhang, S.; Li, X.; Chen, Y.; Yang, Y.; Kim, J.; Bartlett, M. G.; Coleman, I. M.; Nelson, P. S.; Kucuk, O., et al. A Novel Flavonoid Composition Targets Androgen Receptor Signaling and Inhibits Prostate Cancer Growth in Preclinical Models. Neoplasia. 2018, 20(8), 789–799. DOI: 10.1016/j.neo.2018.06.003.
- Yang, L.; Gao, Y.; Bajpai, V. K.; El-Kammar, H. A.; Simal-Gandara, J.; Cao, H.; Xiao, J. Advance Toward Isolation, Extraction, Metabolism and Health Benefits of Kaempferol, a Major Dietary Flavonoid with Future Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–17. DOI:10.1080/10408398.2021.1980762.
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Afshari, A.; Afshari, A. In vitro and in vivo Assessment of Free Radical Scavenging and Antioxidant Activities of Veronica Persica Poir. Cell. Mol. Biol. 2018, 64(8), 57–64. DOI: 10.14715/cmb/2018.64.8.9.
- Verma, A. R.; Vijayakumar, M.; Mathela, C. S.; Rao, C. V. In vitro and in vivo Antioxidant Properties of Different Fractions of Moringa Oleifera Leaves. Food. Chem. Toxicol. 2009, 47(9), 2196–2201. DOI: 10.1016/j.fct.2009.06.005.
- Govindaraju, S.; Roshini, A.; Lee, M. H.; Yun, K. Kaempferol Conjugated Gold Nanoclusters Enabled Efficient for Anticancer Therapeutics to A549 Lung Cancer Cells. Int. J. Nanomed. 2019, 14, 5147. DOI: 10.2147/IJN.S209773.
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A. U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50(1), 1–23. DOI: 10.1186/s40659-017-0140-9.
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The Mechanism of Anticancer Action and Potential Clinical Use of Kaempferol in the Treatment of Breast Cancer. Biomed. Pharmacother. 2019, 117, 109086. DOI: 10.1016/j.biopha.2019.109086.
- Lee, G. A.; Choi, K. C.; Hwang, K. A. Kaempferol, a Phytoestrogen, Suppressed Triclosan-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of MCF-7 Breast Cancer Cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. DOI: 10.1016/j.etap.2016.11.016.
- Zhu, G.; Liu, X.; Li, H.; Yan, Y.; Hong, X.; Lin, Z. Kaempferol Inhibits Proliferation, Migration, and Invasion of Liver Cancer HepG2 Cells by Down-Regulation of MicroRNA-21. Int. J. Immunopathol. Pharmacol. 2018, 32, 20–41. DOI: 10.1177/2058738418814341.
- Peres, R.; Furuya, H.; Pagano, I.; Shimizu, Y.; Hokutan, K.; Rosser, C. J. Angiogenin Contributes to Bladder Cancer Tumorigenesis by DNMT3b-Mediated MMP2 Activation. Oncotarget. 2016, 7(28), 43109. DOI: 10.18632/oncotarget.10097.
- Kitchen, M. O.; Bryan, R. T.; Emes, R. D.; Glossop, J. R.; Luscombe, C.; Cheng, K. K.; Farrell, W. E.; James, N. D.; Devall, A. J.; Mein, C. A., et al. Quantitative Genome-Wide Methylation Analysis of High-Grade Non-Muscle Invasive Bladder Cancer. Epigenetics. 2016, 11(3), 237–246. DOI: 10.1080/15592294.2016.1154246.
- Tarantino, G.; Crocetto, F.; Vito, C. D.; Martino, R.; Pandolfo, S. D.; Creta, M.; Imbimbo, C.; Buonerba, C.; Imbimbo, C. Clinical Factors Affecting Prostate-Specific Antigen Levels in Prostate Cancer Patients Undergoing Radical Prostatectomy: A Retrospective Study. Future Sci. OA. 2020, 7(3). DOI: 10.2144/fsoa-2020-0154.
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D. K.; Jo, S.; Lee, H. K.; Namkung, W.; Mongin, A. A. Inhibition of ANO1 by Luteolin and Its Cytotoxicity in Human Prostate Cancer PC-3 Cells. PLoS One. 2017, 12(3), e0174935. DOI: 10.1371/journal.pone.0174935.
- Crocetto, F.; Di Zazzo, E.; Buonerba, C.; Aveta, A.; Pandolfo, S. D.; Barone, B.; Di Lorenzo, G.; Caputo, V. F.; Scafuri, L.; Ferro, M., et al. Kaempferol, Myricetin and Fisetin in Prostate and Bladder Cancer: A Systematic Review of the Literature. Nutr. 2021, 13(11), 3750. DOI: 10.3390/nu13113750.
- Lenis, A. T.; Lec, P. M.; Chamie, K.; MSHS, M. Bladder Cancer: A Review. Jama. 2020, 324(19), 1980–1991. DOI: 10.1001/jama.2020.17598.
- Wang, X.; Zhu, J.; Yan, H.; Shi, M.; Zheng, Q.; Wang, Y.; Gao, X.; Miao, L.; Gao, X. Kaempferol Inhibits Benign Prostatic Hyperplasia by Resisting the Action of Androgen. Eur. J. Pharmacol. 2021, 907, 174251. DOI: 10.1016/j.ejphar.2021.174251.
- Kang, J. W.; Kim, J. H.; Song, K.; Kim, S. H.; Yoon, J. H.; Kim, K. S. Kaempferol and Quercetin, Components of Ginkgo Biloba Extract (EGb 761), Induce Caspase‐3‐dependent Apoptosis in Oral Cavity Cancer Cells. Phytother. Res. 2010, 24(S1), S77–82. DOI: 10.1002/ptr.2913.
- Jeong, J. C.; Kim, M. S.; Kim, T. H.; Kim, Y. K. Kaempferol Induces Cell Death Through ERK and Akt-Dependent Down-Regulation of XIAP and Survivin in Human Glioma Cells. Neurochem. Res. 2009, 34(5), 991–1001. DOI: 10.1007/s11064-008-9868-5.
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H. F.; Battastini, A. M. O.; Koester, L. S. Kaempferol-Loaded Mucoadhesive Nanoemulsion for Intranasal Administration Reduces Glioma Growth in vitro. Int. J. Pharm. 2018, 543(1–2), 214–223. DOI: 10.1016/j.ijpharm.2018.03.055.
- Pilleron, S.; Sarfati, D.; Janssen‐heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global Cancer Incidence in Older Adults, 2012 and 2035: A Population‐based Study. Int, J, Cancer. 2019, 144(1), 49–58. DOI: 10.1002/ijc.31664.
- Yang, L.; Li, H.; Yang, M.; Zhang, W.; Li, M.; Xu, Y.; Guo, S.; Kang, J.; Zhang, J.; Guo, S. Exploration in the Mechanism of Kaempferol for the Treatment of Gastric Cancer Based on Network Pharmacology. Biomed Res. Int. 2020, 2020. DOI: 10.1155/2020/5891016.
- Zhang, F.; Ma, C. Kaempferol Suppresses Human Gastric Cancer SNU-216 Cell Proliferation, Promotes Cell Autophagy, but Has No Influence on Cell Apoptosis. Braz. J. Med. Biol. Res. 2019, 52(2). DOI: 10.1590/1414-431x20187843.
- Kim, T. W.; Lee, S. Y.; Kim, M.; Cheon, C.; Ko, S. G. Kaempferol Induces Autophagic Cell Death via IRE1-JNK-CHOP Pathway and Inhibition of G9a in Gastric Cancer Cells. Cell Death Dis. 2018, 9(9), 1–14. DOI: 10.1038/s41419-018-0930-1.
- Seydi, E.; Salimi, A.; Rasekh, H. R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer. 2018, 70(4), 594–604. DOI: 10.1080/01635581.2018.1460679.
- Wonganan, O.; He, Y. J.; Shen, X. F.; Wongkrajang, K.; Suksamrarn, A.; Zhang, G. L.; Wang, F. 6-Hydroxy-3-O-Methyl-Kaempferol 6-O-Glucopyranoside Potentiates the Anti-Proliferative Effect of Interferon α/β by Promoting Activation of the JAK/STAT Signaling by Inhibiting SOCS3 in Hepatocellular Carcinoma Cells. Toxicol. Appl. Pharmacol. 2017, 336, 31–39. DOI: 10.1016/j.taap.2017.10.004.
- Huang, W. W.; Tsai, S. C.; Peng, S. F.; Lin, M. W.; Chiang, J. H.; Chiu, Y. J.; Yang, J. S.; TSENG, M. T.; YANG, J. -S. Kaempferol Induces Autophagy Through AMPK and AKT Signaling Molecules and Causes G2/M Arrest via Downregulation of CDK1/Cyclin B in SK-HEP-1 Human Hepatic Cancer Cells. Int. J. Oncol. 2013, 42(6), 2069–2077. DOI: 10.3892/ijo.2013.1909.
- Lee, J.; Kim, J. H.; Scarpa, A. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration Through the Blockade of EGFR-Related Pathway in vitro. PLoS One. 2016, 11(5), e0155264. DOI: 10.1371/journal.pone.0155264.
- Wu, L. Y.; Lu, H. F.; Chou, Y. C.; Shih, Y. L.; Bau, D. T.; Chen, J. C.; Chung, J. G.; Chung, J. -G. Kaempferol Induces DNA Damage and Inhibits DNA Repair Associated Protein Expressions in Human Promyelocytic Leukemia HL-60 Cells. Am. J. Chinese. Med. 2015, 43(02), 365–382. DOI: 10.1142/S0192415X1550024X.
- Hung, T. W.; Chen, P. N.; Wu, H. C.; Wu, S. W.; Tsai, P. Y.; Hsieh, Y. S.; Chang, H. R. Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells Through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 2017, 14(10), 984. DOI: 10.7150/ijms.20336.
- Devi, K. P.; Malar, D. S.; Nabavi, S. F.; Sureda, A.; Xiao, J.; Nabavi, S. M.; Daglia, M. Kaempferol and Inflammation: From Chemistry to Medicine. Pharmacol. Res. 2015, 99, 1–10. DOI: 10.1016/j.phrs.2015.05.002.
- Wong, S. K.; Chin, K. Y.; Ima-Nirwana, S. The Osteoprotective Effects of Kaempferol: The Evidence from in vivo and in vitro Studies. Drug Des. Devel. Ther. 2019, 13, 3497. DOI: 10.2147/DDDT.S227738.
- Afroze, N.; Pramodh, S.; Almutary, A. G.; Rizvi, T. A.; Rais, N.; Raina, R.; Hussain, A.; Alnuqaydan, A. M.; Hussain, A. Kaempferol Regresses Carcinogenesis Through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa. Appl. Sci. 2022, 12(6), 3155. DOI: 10.3390/app12063155.
- Swieca, M.; Herok, A.; Piwowarczyk, K.; Sikora, M.; Ostanek, P.; Gawlik-Dziki, U.; Czyż, J.; Czyż, J. Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells. Mol. 2020, 25(13), 2963. DOI: 10.3390/molecules25132963.
- Gao, Y.; Yin, J.; Rankin, G. O.; Chen, Y. C. Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Mol. 2018, 23(5), 1095. DOI: 10.3390/molecules23051095.
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol Exerts Anti-Proliferative Effects on Human Ovarian Cancer Cells by Inducing Apoptosis, G0/G1 Cell Cycle Arrest and Modulation of MEK/ERK and STAT3 Pathways. J. Buon. 2019, 24(3), 975–981.
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic Compound Kaempferol Overcomes 5-Fluorouracil Resistance in Human Resistant LS174 Colon Cancer Cells. Sci. Rep. 2019, 9(1), 1–20. DOI: 10.1038/s41598-018-36808-z.
- Cho, H. J.; Park, J. H. Y. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013, 18(3), 257. DOI: 10.15430/JCP.2013.18.3.257.
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol Induces Apoptosis in Human HCT116 Colon Cancer Cells via the Ataxia-Telangiectasia Mutated-p53 Pathway with the Involvement of p53 Upregulated Modulator of Apoptosis. Chemi.-Biol. Interact. 2009, 177(2), 121–127. DOI: 10.1016/j.cbi.2008.10.048.
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutr. 2017, 9(6), 597. DOI: 10.3390/nu9060597.
- Boadi, W. Y.; Lo, A. Effects of Quercetin, Kaempferol, and Exogenous Glutathione on Phospho-And Total-AKT in 3T3-L1 Preadipocytes. J. Diet. Suppl. 2018, 15(6), 814–826. DOI: 10.1080/19390211.2017.1401572.
- Han, X.; Liu, C. F.; Gao, N.; Zhao, J.; Xu, J. Kaempferol Suppresses Proliferation but Increases Apoptosis and Autophagy by Up-Regulating MicroRNA-340 in Human Lung Cancer Cells. Biomed. Pharmacother. 2018, 108, 809–816. DOI: 10.1016/j.biopha.2018.09.087.
- Kuo, W. T.; Tsai, Y. C.; Wu, H. C.; Ho, Y. J.; Chen, Y. S.; Yao, C. H.; Yao, C. H. Radiosensitization of Non-Small Cell Lung Cancer by Kaempferol. Oncol. Rep. 2015, 34(5), 2351–2356. DOI: 10.3892/or.2015.4204.
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol Inhibits Gastric Cancer Tumor Growth: An in vitro and in vivo Study. Oncol. Rep. 2015, 33(2), 868–874. DOI: 10.3892/or.2014.3662.
- Oh, S. M.; Kim, Y. P.; Chung, K. H. Biphasic Effects of Kaempferol on the Estrogenicity in Human Breast Cancer Cells. Arch. Pharmacal Res. 2006, 29(5), 354–362. DOI: 10.1007/BF02968584.
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a Flavonoid Compound from Gynura Medica Induced Apoptosis and Growth Inhibition in Mcf-7 Breast Cancer Cell. Afr. J. Traditional, Complementary Altern. Med. 2016, 13(4), 210–215. DOI: 10.21010/ajtcam.v13i4.27.
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Berindan-Neagoe, I.; Raduly, L.; Barbat, A.; Moldovan, A., et al. Inhibitory Effect of CAPE and Kaempferol in Colon Cancer Cell Lines—Possible Implications in New Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20(5), 1199. DOI: 10.3390/ijms20051199.
- Jo, E.; Park, S. J.; Choi, Y. S.; Jeon, W. K.; Kim, B. C. Kaempferol Suppresses Transforming Growth Factor-Β1–induced Epithelial-To-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia. 2015, 17(7), 525–537. DOI: 10.1016/j.neo.2015.06.004.
- Zhang, Y.; Chen, A. Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo Biloba Extract Kaempferol Inhibits Cell Proliferation and Induces Apoptosis in Pancreatic Cancer Cells. J. Surg. Res. 2008, 148(1), 17–23. DOI: 10.1016/j.jss.2008.02.036.
- Luo, H.; Daddysman, M. K.; Rankin, G. O.; Jiang, B. H.; Chen, Y. C. Kaempferol Enhances Cisplatin’s Effect on Ovarian Cancer Cells Through Promoting Apoptosis Caused by Down Regulation of cMyc. Cancer. Cell Inter. 2010, 10(1), 1–9. DOI: 10.1186/1475-2867-10-16.