ABSTRACT
Herbal utilization, as an antidiabetes agent, is an interesting topic to find acceptable herbal drugs to decrease blood glucose levels. The aim of this review is to evaluate the potency of selected herbal medicines to reduce blood glucose levels and to identify the chemical compounds responsible for reducing glucose. The mechanisms of action of different herbal medicines used might be also different. The reduction of blood glucose levels by Aloe vera, Andrographis paniculata, and Trigonella foenum-graecum through minimum 3 mechanisms of action, such as increased GLP-1 secretion and inhibited amylase, glucosidase, and SGLT 2. While Andrographis paniculata has more than 4 mechanisms of action, such as increasing GLP secretion, activating PPAR ɣ-receptor, and also inhibiting amylase, glucosidase, and SGLT 2 but it did not inhibit DPP 4 in diabetic patients.
Introduction
Herbal use is an interesting part of the last decade due to many synthetic drugs and raised side effects for long-time use, especially antidiabetes drugs. Several synthetic drug types such as biguanide, sulfonylurea, thiazolidinediones, a-glucosidase inhibitors, glucagon-like peptide-1 (GLP-1), and dopamine-2 agonists, dipeptidyl peptidase 4 (DPP-4), and sodium-glucose cotransporter-2 (SGLT 2) inhibitors have been existed in the Market [Citation1]. However, they will cause side effects including cancer, hepatitis, allergy, etc. for long consumption [Citation2]. As a consequence, because natural medicine is less toxic than synthetic drugs, many people are turning toward it for disease treatment. We used to say, “Let food be your medicine, and medicine be your food” [Citation3]. We now understand that natural sources of disease treatment will provide numerous benefits because they are safer to consume.
China is one of the countries that has developed natural drug development, namely traditional china medicine (TCM). Many herbals have been approved in China for antidiabetes such as Panax ginseng, Momodica charantia, Lagenaria siceraria, and Psidium guajava [Citation4]. Currently, China has produced more than 30 TCM products known as Yuquan Wan, Xiaokeling Pian, Tangniaoling Pian, etc. [Citation5]. These products contain two or more herbals where they produce synergism activity. Therefore, TCM products significantly affect to reduce diabetes as much as 1.2 folds western drugs [Citation6]. The big problem is no standard for diabetes patients to consume TCM [Citation7]. Based on this issue, a review of selected herbals is critical because it will explain everything before we can combine them to create TCM-like products.
Hopefully, the herbs will support or replace the synthetic diabetes drugs that have been recommended. As a result, this review will investigate several potential herbals for diabetic treatment, including active compound types, potency, mechanism of action, and toxicity. These herbal plants have been widely used for diabetic treatment all over the world. The goal of this review is to assess selected herbal plants as future candidates for antidiabetic drug-like TCM.
Phytochemical of selected herbal candidates as an antidiabetic agent
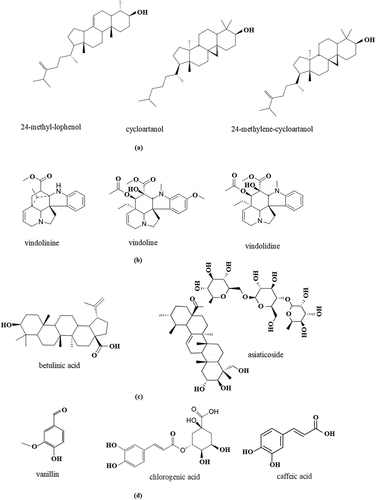
The herbal plants contain several active compounds that are greatly affecting their biological activity including alternative antidiabetic drugs. Secondary metabolites should be reported as a drug discovery from the herb and can be developed in the next research through the synthesis pathway. For example, the synthesis of vitexin derivates becomes new compounds such as vitexin-4’-O-β-glucoside and vitexin-5-O-β-glucoside where both new compounds have better solubility than vitexin [Citation8]. Vitexin is a natural compound that can be isolated from herbal plants including Anredera cordifolia [Citation13]. Moreover, the studies related to isolation, purification, and identification of the putative active compounds are important parts of discovering new drugs or lead compound sources that can be expanded in future research [Citation9]. Besides, the active compounds play a significant role and are able to explain their mechanism compared with crude herbal extract because it contains more complex active compounds. Therefore, the mechanism of action that happened will be explained easier based on the pure compound obtained. depicts several metabolite groups that are reported as anti-diabetes agents.
Figure 1. The putative metabolites have been reported as antidiabetes agents from selected herbal plants: a) phytosterol groups; b) alkaloid groups; c) triterpenoid groups; and d) phenolic groups.
This review showed that the herbal medicines mentioned are potential antidiabetic drugs based on in-vitro and in-vivo evaluations. Several biological pathways of the selected herbal medicine have been mentioned in . Some herbal medicines will increase insulin secretion to control blood glucose levels while other herbal medicines inhibit α-glucosidase, DPP IV, etc. These mechanisms of action were significantly affected by the chemical compounds of the herbals. Chemical compounds such as flavonoids and their derivatives are responsible for diabetes treatment via various mechanisms of action [Citation10]. As a result, the metabolite groups depicted in can be used to treat diabetes via a variety of mechanisms of action.
Table 1. The putative active compound types from herbal plants play a significant role in treating diabetes.
demonstrates that each herbal contains dominant chemical groups as secondary metabolites that are responsible for biological activities such as diabetes. However, antidiabetic agents from selected herbals are not only limited to flavonoids and their derivatives. Even, flavonoid derivatives including rutin are less effective due to only inhibiting 52% of α-glucosidase at 250 µg/ml [Citation27, Citation28]. A lot of active compounds from these herbals affect significantly reducing blood glucose levels that are grouped into triterpenoid groups, phytosterol groups, phenolic groups, and alkaloid groups as shown in . However, each putative active compound will lead to the mechanism of action occurring, especially as inhibitory agents of α-amylase, α-glucosidase, PTP 1B, SGLT 2, and also modulatory agents of GLP 1 and insulin secretion.
Novel triterpenoids have been studied and found to have a variety of mechanisms of action, including plasma glucose, plasma insulin/C-peptide, serum lipid markers, sugar metabolism enzymes, glucose oxidation, and insulin signaling molecules [Citation29]. Despite this, phenolic groups have been linked to diabetes prevention in cell, animal, and clinical studies [Citation30]. Based on this review, we can deduce that differences in the active compounds or metabolite group types of the herbs will result in different mechanisms of action.
Their mechanism of action of selected herbal as antidiabetes
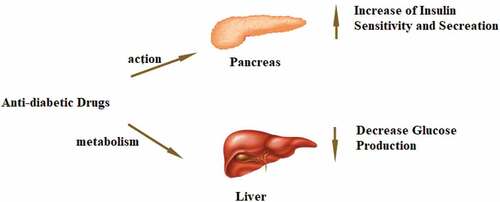
Furthermore, this review explained 11 herbal plants which contain the complex active compounds that lead to their mechanism of action. Several mechanisms of action related to treating diabetes are to improve insulin sensitivity, stimulate insulin secretion, protect pancreatic islets, and even inhibit the intake of intestinal carbohydrates [Citation5]. Generally, anti-diabetic drugs will control blood glucose levels in two ways including action and metabolism. The liver converts glycogen to glucose, so that it is an essential organ for regulating the uptake and release of glucose. While the pancreas increases insulin production from ß-cells while decreasing glucagon production from α-cells for controlling blood glucose levels. The illustration of both pathways is given in .
Therefore, the mechanism of action of herbal medicine may be more than one mechanism of action (). The mechanism of action of the herbal plants sustains the development of herbal use for diabetes medications. However, the combination of herbal use may be improving its potency due to synergistic action may happen [Citation31, Citation32]. Therefore, the information on the mechanism of actions of herbal plants is an important part to support medication fruitfulness. Reference [Citation32] Zang et al. said that the development of traditional Chinese medicine (TCM) is greatly related to biological activities, including the Jun-Chen-Zuo-Shi formula that can be interpreted based on its biological activities. Furthermore, to ensure the effectiveness of the herbal combination, it can be tested by in-vivo tests and clinical trials on humans.
Figure 3. Mechanism of actions from herbal plants in reducing blood glucose levels using in-vitro-based evaluation.
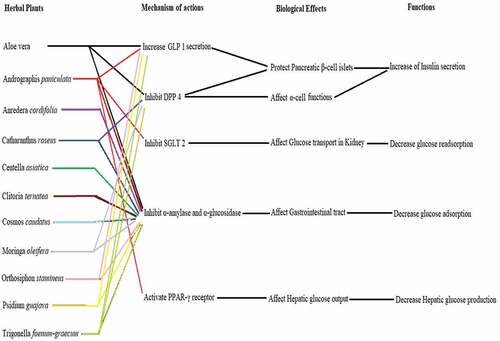
demonstrates how various herb’s mechanisms of action can work together to provide broad effects. These occur as a consequence of the fact that particular various types of potent compounds found in herbs assist in lowering blood sugar levels. Therefore, taking plants instead of synthetic medications may be more advantageous because the chemical compounds in herbs have synergistic effects. The challenging aspect is finding out which substances are responsible as well as where inactive compounds are found. Therefore, herbs can be consumed in larger amounts than synthetic drugs, and implementing their use is difficult to fully understand.
The herbs that prevent the enzymes α-amylase and α-glucosidase from breaking down carbohydrates or oligosaccharides can reduce the quantities of glucose that flow into the bloodstream. Besides, DPP4 inhibitors can avoid GLP-1 from becoming deactivated as well as promote insulin sensitivity and production. A further benefit of SGLT 2 inhibitors is that they prevent glucose readsorption, which could raise blood sugar levels. Even so, increased insulin sensitizers caused by activated PPAR γ-receptors will decrease the amount of glucose produced by the liver. In order to assess these herbs’ effectiveness in regulating blood glucose levels, it is crucial to understand how they work metabolically.
shows that all herbal plants act as α-glucosidase and α-amylase inhibitory agents. Herbal plants also have more than two mechanisms of action in reducing blood glucose levels, such as Aloe vera, Andrographis paniculata, and Trigonella foenum-graecum. Aloe vera extract will increase insulin secretion, which can control blood glucose levels [Citation33]. Reference [Citation11] Tanaka et al. isolated successfully five phytosterols from this herbal that are potentially reducing blood glucose levels. Furthermore, Andrographis paniculata is a herbal candidate for anti-diabetes. This herbal has been reported that it can reduce blood glucose levels through four mechanisms of action (). Tarigan et al., [Citation34] said that Andrographis paniculata has increased GLP-1 levels but does not inhibit DPP 4 in diabetic patients. One of the potent active compounds of A. paniculata as antidiabetes is Andrographolide [Citation12].
In this review, the last herbal, which contains more than 2 mechanisms of action in reducing blood glucose levels, is Trigonella foenum-graecum. One of the isolated active compounds is N-linoleoyl-2- amino – butyrolactone (N55) which can improve GLP-1 excretion [Citation26]. This result was also supported by the clinical studies that Trigonella foenum-graecum seed is able to reduce glucose levels in diabetes patients. Any 10 clinical studies explained the beneficial effect of Trigonella foenum-graecum seed in diabetes patients [Citation35]. Even, the consumption of Trigonella foenum-graecum powder as much of as 25 g can improve lipid metabolism with no adverse effect [Citation36].
Several herbal plants have two mechanisms of action are Catharanthus roseus, Moringa oleifera, Orthosiphon stamineus, and Psidium guajava. Catharanthus roseus contains several alkaloids that play a significant role in reducing blood glucose levels. Reference [Citation15] Tiong et al. have successfully isolated 4 alkaloids from Catharanthus roseus such as vindoline, vindolidine, vindolicine and vindolinine (). These alkaloids act as antidiabetes agents. Furthermore, several active compounds of Moringa oleifera that have a significant effect on diabetes management are coumaroylquinic acid, astragalin, kaempferol-3-O-rutinoside, vitexin, rutin, quercetin-acetyl-glucoside, quercetin-malonyl-glucoside, isoquercetin, kaempferol acetyl glycoside, and quercetin where they have the smaller binding energy of Human pancreatic alpha-amylase than acarbose [Citation21]. This result was supported by the clinical study of the use of Moringa oleifera tea as glycemic control for diabetes patients.
For O. stamineus, it contains active compounds that interact with peptides including GLP-1 and ghrelin levels will stimulate insulin secretion, which contributes to decreasing blood glucose levels [Citation37]. They can even reduce glucose-6-phosphatase activity and increase glucose-6-phosphate dehydrogenase activity and raise glycogen levels [Citation38]. Reference [Citation39] Ngo and Chua reported that rosmarinic acid is one of the active compounds from O. stamineus in controlling diabetes. Another herbal plant is Psidium guajava. It has been evaluated by clinical trials in China. Its potency as an alternative drug’s antidiabetes due to flavonoid derivatives as the putative active compounds reduce blood glucose levels. Besides, this herbal plant also contains lanost-7-en-3β-ol-26-oic acid, anost-7-en-3β, 12β-diol-26-oic acid, lanost-7- en-3β, 12β, 29-triol-26-oic acid, and lanost-7-en-3β-ol-26-oic acid-3β-D-glucopyranoside as lanosterol-type triterpenoids groups [Citation24].
This review also reported several herbal plants which were predicted to only prevent diabetes through the inhibition of α-amylase and α-glucosidase activities. They are A. cardifolia, C. caudatus, C. asiatica, and C. ternatea (). These herbal plants may inhibit blood glucose content through other mechanisms, but in-depth research has not been carried out yet. Therefore, we support that further research can be done in the future so that it can ensure other mechanisms of action in lowering the blood sugar of these herbal plants. However, they have great potential in lowering the blood glucose level of diabetic patients.
Clinical study of the selected herbal plants as an antidiabetic agent
Previous explanations have elaborated that selected herbal plants contributed to reducing blood glucose levels. The selected herbal plants reduced blood glucose levels through one or more mechanisms of action based on in-vitro evaluations. This result depicts that each herbal is responsible to treat diabetes through different mechanisms of action. Previous studies have shown that selected herbals are good candidates as alternative antidiabetes drugs based on in-vitro and in-vivo evaluations.
Furthermore, the clinical study of the selected herbal medicines can be conducted to ensure their safety, efficacy, and efficiency. However, in-vitro and in-vivo testings are the screening evaluation to choose the potent extracts which will be tested further, in particular clinical study. The clinical studies reported that each of herbal medicines are also decreasing blood glucose levels throughout the different mechanisms of action as in vitro evaluation explained before.
According to the literature review, only A. cardifolia and C. roseus have not yet been used in clinical studies. Other medicinal plants have been tested on humans to ensure their efficacy. According to , some herbal remedies have a significant chance of becoming herbal anti-diabetes drugs. The amount of each plant used varies greatly depending on the chemical compounds that are present. This review indicates that between 15 and 100 individuals contributed to these investigations. Unfortunately, this level of evidence remains at 7, with only the use of Trigonella foenum-graecum in capsule and extract forms reaching level 2. As a consequence, more clinical studies to ensure the potency of these herbs should be conducted in the future. Therefore, it is a preliminary investigation before an adequate number of participants is collected. Furthermore, Phase 2, Phase 3, as well as Phase 4 clinical studies can be conducted on these herbs as the forthcoming phase of the study. According to Sharwan et al., [Citation46]reported that In-vitro, preclinical, and clinical research, among other methods, are necessary for officially approved herbs. Thus, the herbs used as antidiabetics in the most recent study were described in this review.
Table 2. The Reported Clinical Study of the Selected Herbal as antidiabetic agents.
Future recommendation
Selected herbal medicines have been speculated as alternative anti-diabetes drugs. The evaluation of nine plants as herbal medicines has been conducted by clinical trial testing (). It is a great opportunity for herbal development in the future, especially nutraceutical products. Nutraceuticals are good role in controlling human body health due to they contain high nutrients and pharmaceutical functions. The herbal medicines that have been evaluated through clinical trials increase their potency including safety, efficacy, and efficiency.
We could deduce through this review that the nine herbal remedies mentioned above have lowered diabetes based on a number of factors, including blood glucose levels, HbA1C, TSS, and so forth. However, further investigation is required before using these herbs as diabetes medications. To confirm the effectiveness of these herbs as well as herbal diabetes drugs, additionally, phase 2, phase 3, and phase 4 clinical trials on their use should be carried out. As a direct outcome of this evaluation, future efforts to produce nutraceutical goods will be supported.
Conclusion
The review revealed that the selected herbal medicines had significant potential as potential anti-diabetes drug alternatives. Furthermore, each of these herbs has an original mechanism of action for lowering blood sugar. This is owing to the fundamental fact that each herb’s power to work is strongly impacted by the presence of different active compounds. Several plants will decrease blood glucose levels following 4, 3, 2 or 1 mechanism of actions. Aloe vera, Andrographis paniculata, and Trigonella foenum-graecum have a minimum of 3 mechanisms of action such as increased GLP 1 secretion and inhibition amylase, glucosidase, and SGLT 2. While Andrographis paniculata has more than 4 mechanisms of action such as increasing GLP secretion, activating PPAR ɣ-receptor, and also inhibit amylase, glucosidase, and SGLT 2 but it did not inhibit DPP 4 in diabetic patients. There is a substantial chance that a lot of mechanisms of action occur, which will boost the impact of herbal medicines in lowering blood sugar.
Author contributions
MA: conceptualization, Data processing, writing-original draft preparation, editing, revising the manuscript and funding acquisition; IJ: supervision; AK: supervision; QUA: supervision; NUR: supervision and revising the manuscript, YDA: supervision, all authors approved the final version of the manuscript.
Acknowledgments
The authors would like to express their gratitude to Universitas Ahmad Dahlan, Kemenristekdikti Republik Indonesia, and The International Islamic University of Malaysia for providing research funding (no: PD-144/SP3/LPPM-UAD/VII/2022; 071E5/PG.02.00.PT/2022; and RMCG20-042-0042) to enable them to carry out this study
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Safavi, M.; Foroumadi, A.; Abdollahi, M. The Importance of Synthetic Drugs for Type 2 Diabetes Drug Discovery. Exp. Opin. Drug Discov. 2013, 8(11), 1339–1363. DOI: 10.1517/17460441.2013.837883.
- Osadebe, P. O.; Odoh, E. U.; Uzor, P. F. Natural Products as Potential Sources of Antidiabetic Drugs. Br. J. Pharm. Res. 2014, 4(17), 2075–2095. DOI: 10.9734/BJPR/2014/8382.
- Rasouli, H.; Farzaei, M. H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food. Prop. 2017, 1–42. DOI: 10.1080/10942912.2017.1354017.
- Covington, M. B. Traditional Chinese Medicine in the Treatment of Diabetes. Diabetes Spectr. 2001, 14(3), 154–159. DOI: 10.2337/diaspect.14.3.154.
- Xie, W.; Zhao, Y.; Zhang, Y. Traditional Chinese Medicines in Treatment of Patients with Type 2 Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2011, 2011, 1–13. DOI: 10.1155/2011/726723.
- Zhao, H. L.; Tong, P. C. Y.; Chan, J. C. N. Traditional Chinese Medicine in the Treatment of Diabetes. Nutr. Manag. Diabetes Mellitus Dysmetabol. Syndr. 2006, 11, 15–29. DOI: 10.1159/000094399.
- Lian, F.; Ni, Q.; Shen, Y.; Yang, S.; Piao, C.; Wang, J.; Wei, J.; Duan, J.; Fang, Z.; Lu, H., Yang, G., Zhao, L., Song, J., Li, Q., Zheng, Y., Lyu, Y., Tong, X., et al. International Traditional Chinese Medicine Guideline for Diagnostic and Treatment Principles of Diabetes. Annal. Palliat. Med. 2021, 9(4), 19–19. DOI: 10.21037/apm-19-271.
- Wu, J. Y.; Wang, T. Y.; Ding, H. Y.; Zhang, Y. R.; Lin, S. Y.; Chang, T. S. Enzymatic Synthesis of Novel Vitexin Glucosides. Molecules. 2021, 26(20), 1–10. DOI: 10.3390/molecules26206274.
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biol. 2020, 9(9), 1–31. DOI: 10.3390/biology9090252.
- Chigurupati, S.; Al-Murikhy, A.; Almahmoud, S. A.; Almoshari, Y.; Saber Ahmed, A.; Vijayabalan, S.; Ghazi Felemban, S.; Raj Palanimuthu, V.; ; , et al. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J Biol Sci. 2022, 29(2), 854–859 DOI:10.1016/j.sjbs.2021.10.021.
- Tanaka, M.; Misawa, E.; Ito, Y.; Habara, N.; Nomaguchi, K.; Yamada, M.; Oida, T., Hayasawa, H., Takase, M., Inagaki, M., HIguchi, R, Identification of Five Phytosterols from Aloe Vera Gel as Anti-Diabetic Compounds. Biol. Pharm. Bull. 2006, 29(7), 1418–1422.
- Nugroho, A. E.; Rais, I. R.; Setiawan, I.; Pratiwi, P. Y.; Hadibarata, T.; Tegar, M., Pramono, S. Pancreatic Effect of Andrograpolide Isolated from Andrographis Paniculata (Bunn F.). Pak. J. Bio. Sci. 2014, 7(1), 22–31.
- Dwitiyanti, D.; Harahap, Y.; Elya, B.; Bahtiar, A. Study of Molecular Docking of Vitexin in Binahong (Anredera cordifolia (Ten.) Steenis) Leaves Extract on Glibenclamide-CYP3A4 Interaction. Pharmacognosy Journal 11 6S . 2019, 1471–1476. DOI: 10.5530/pj.2019.11.227.
- Djamil,; Winarti, W.; Zaidan, S.; Pratiw, S.; Antidiabetic Activity of Flavonoid from Binahong Leaves (Anredera cordifolia) Extract in Alloxan Induced Mice. Journal of Pharmacognosy & Natural Products. 2017, 03 , 1–4. DOI: 10.4172/2472-0992.1000139.
- Tiong, S.; Looi, C.; Hazni, H.; Arya, A., Paydar, M., Wong, W. F., Cheah, S., Mustafa, M. R., Awang, K. Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don. Molecules. 2013, 18(8), 29770–9784. DOI: 10.3390/molecules18089770.
- Macalalad, M. A. B.; Gonzales, P. R.; In-silico screening and identification of phytochemicals from Centella asiatica as potential inhibitors of sodium-glucose co-transporter 2 for treating diabetes. Journal of Biomolecular Structure and Dinamics. 2021, 40 22 , 12221–12238. DOI: 10.1080/07391102.2021.1969282.
- Fitrianda, E.; Sukandar, E. Y.; Elfahmi, E.; Adnyana, I. K.; ; ; Antidiabetic Activity of Extract, Fractions, and Asiaticosida Compound Isolated from Centela Asiatica Linn. Leaves in Alloxan-Induced Diabetic Mice. Asian J. Pharm. Clin. Res. 2017, 10 10 , 268–272. DOI: 10.22159/ajpcr.2017.v10i10.20419.
- Verma, P. R.; Itankar, P. R., Arora, S. K. Evaluation of Antidiabetic Antihyperlipidemic and Pancreatic Regeneration, Potential of Aerial Parts of Clitoria Ternatea. Rev. Bras. Farmacogn 23 . 2013, 819–829. DOI: 10.1590/s0102-695x2013000500015.
- Javadi, N.; Abas, F.; Mediani, A.; Hamid, A. A.; Khatib, A.; Simon, S.; Shaari, K.; Effect of Storage Time on Metabolite Profile and Alpha-Glucosidase Inhibitory Activity of Cosmos Caudatus Leaves-GCMS Based Metabolomics Approach. Saudi J. BJFDA. 2015, 23(), 433–441. DOI: 10.1016/j.jfda.2015.01.005.
- Zainab, B; Ayaz, Z.; Alwahibi, M. S.; Khan, S.; Rizwana, H.; Wafik Soliman, D; Alawaad, A.; Abbasi, A. M> A.; Naz, S.; Waraich, R. S., et al. in-Silico Elucidation of Moringa Oleifera Phytochemicals Against Diabetes Mellitus. Saudi J. Biol. Sci. 2020, 27(9), 2299–2307. DOI: 10.1016/j.sjbs.2020.04.002.
- Hafizur, R. M.; Maryam, K.; Hameed, A., Zaheer, L., Bano, S., Sumbul, S., Sana, A., Saleem, R., Naz, S., Waraich, R. S, Ul-Haq, Z., Faizi, S. nsulin Releasing Effect of Some Pure Compounds from Moringa Oleifera on Mice Islets. Med. Chem. Res. 2018, 27(5), 1408–1418. DOI: 10.1007/s00044-018-2157-1.
- Sunhre, L.; Kar, MA>; Panda, S.; Agnucastoside C, Isolated from Moringa Oleifera Ameliorates Thyrotoxicosis and Liver Abnormalities in Female Mice. lin Phytosci. 2020, 6(1), 1–8. DOI: 10.1186/s40816-020-00165-0.
- Damsud, T.; Grace, M. H.; Adisakwattana, S.; Phuwapraisirisan, P. Orthosiphol a from the Aerial Parts of Orthosiphon Aristatus is Putatively Responsible for Hypoglycemic Effect via α-Glucosidase Inhibition. Nat. prod. communicat. 2014, 9 5 , 1–3 doi:10.1177/1934578x1400900512.
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; IIsolation and Antidiabetic Activity of New Lanostenoids from the Leaves of Psidium Guajava L. Int. J. Pharm. Pharm. Sci. 2016, 8, 14–18.
- Alagesan, K.; Thennarasu, P.; Kumar, P.; Sankarnarayanan, S.; Balsamy, T. Identification of α-Glucosidase Inhibitors from Psidium Guajava Leaves and Syzygium Cumini Linn. Seeds. Internat. J. Pharma Sci. Res (IJPSR). 2012, 3, 316–322. DO I:
- King, K.; Lin, N. P; Cheng, Y. H.; Che, G. H; Chein, R. J Modulator of Glucagon-Like Peptide-1 Signaling fromTrigonella Foenum-graecum(Fenugreek) Seed. J. Biol. Chem. 2015, 290(), 26235–26248. DOI: 10.1074/jbc.m115.672097.
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E. F. T.; Sousa, J. L. C.; Tomé, S. M.; Ramos, M. J.; Silva, A. M. S.; Fernandes, F. A.; Fernandes, E. α-Glucosidase Inhibition by Flavonoids: An in vitro and in silico Structure–Activity Relationship Study. J. Enzyme Inhib. Med. Chem. 2017, 32(1), 1216–1228. DOI: 10.1080/14756366.2017.1368503.
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic Enzyme Inhibitory and Antiglycation Potential of Rutin. Future J. Pharmac. Sci. 2017, S2314724515300480, 1–5. DOI: 10.1016/j.fjps.2017.05.005.
- Indu, S.; Vijayalakshmi, P.; Selvaraj, J.; Rajalakshmi, M. Novel Triterpenoids from Cassia Fistula Stem Bark Depreciates STZ-Induced Detrimental Changes in IRS-1/akt-Mediated Insulin Signaling Mechanisms in Type-1 Diabetic Rats. Molecules. 2021, 26, 6812. DOI: 10.3390/molecules26226812.
- Sun, C.; Zhao, C.; Guven, E. C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K. M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V., et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1–27. DOI: 10.1002/fft2.15.
- Ali, M.; Khan, T.; Fatima, K.; Ali Qu, A.; Ovais, M.; Khalil, A. T.; Ullah, I.; Raza, A.; Shinwari, Z. K.; Idrees, M. Selected Hepatoprotective Herbal Medicines: Evidence from Ethnomedicinal Applications, Animal Models, and Possible Mechanism of Actions. Phytotherapy Res. 2017, 32, 199–215. DOI: 10.1002/ptr.5957.
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front Pharmacol. 2019, 10, 1–22. DOI: 10.3389/fphar.2019.00743.
- Abo-Youssef, A. M. H.; Messiha, B. A. S. Beneficial Effects of Aloe Vera in Treatment of Diabetes: Comparative in vivo and in vitro Studies. Bullet. Faculty Pharmacy, Cairo Univ. 2013, 51, 7–11. DOI: 10.1016/j.bfopcu.2012.03.002.
- Tarigan, T. J. E.; Purwaningsih, E. H.; Yusra Abdullah, M.; Nafrialdi Prihartono, J.; Saraswati, M. R.; Subekti, I. Effect of Sambiloto (Andrographis paniculata) on GLP-1 and DPP-4 Concentration Between Normal and Prediabetic Subject: A Crossover Study. Evid. Based Complement. Altern. Med. 2022, 2022, 1–7. DOI: 10.1155/2022/1535703.
- Neelakantan, N.; Narayanan, M.; de Souza, R. J.; van Dam, R. M. Effect of Fenugreek (Trigonella Foenum-graecumL.) Intake on Glycemia: A Meta-Analysis of Clinical Trials. Nutr. J. 2014, 13, 1–11. DOI: 10.1186/1475-2891-13-7.
- Geberemeskel, G. A.; Debebe, Y. G.; Nguse, N. A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella Foenum-Graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabet. Res. 2019, 1–8.
- Lokman, E. F.; Saparuddin, F.; Muhammad, H.; Omar, M. H.; Zulkapli, A. Orthosiphon Stamineus as a Potential Antidiabetic Drug in Maternal Hyperglycemia in Streptozotocin-Induced Diabetic Rats. Integ. Med. Res. 2019, 8, 173–179. DOI: 10.1016/j.imr.2019.05.006.
- Koteswara Rao, N.; Bethala, K.; Sisinthy, S. P.; Rajeswari, K. S. Antidiabetic Activity of Orthosiphon Staminieus Benth Roots in Streptozotocin Induced Type 2 Diabetic Rats. Asian J. Pharm. Clin. Res. 2014, 7, 149–153.
- Ngo, Y. L.; Chua, L. S. Anti-Diabetic Activity of Rosmarinic Acid Rich Fractions from Orthosiphon Stamineus. Curr. Enzyme Inhib. 2018, 14, 97–103. DOI: 10.2174/1573408014666180101144331.
- Yongchaiyudha S., Rungpitarangsi V., Bunyapraphatsara N., and Chokechaijaroenporn O. 1996. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine, (1996). 3: 241–243. DOI: 10.1016/s0944-7113(96)80060-2
- Agarwal, S.; Sulaiman, S. A.; Muhamed, M. Open Label Clinical Trial to Study Adverse Effects and Tolerance to Dry Powder of the Aerial Part of Andrographis Paniculata in Patients Type 2 with Diabetes Mellitus. Malays J. Med. Sci. 2005, 12, 13–19.
- Lou, J. S.; Dimitrova, D. M.; Murchison, C.; Arnold, G. C.; Belding, H.; Seifer, N.; Le, N.; Andrea, L. B.; Gray, N. E.; Wright, K. M., et al. Centella Asiatica Triterpenes for Diabetic Neuropathy: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Study. Esperienze Dermatol. 2018, 20, 12–22. DOI: 10.23736/S1128-9155.18.00455-7.
- Chusak, C.; Thilavech, T.; Henry, C. J.; Adisakwattana, S. Acute Effect of Clitoria Ternatea Flower Beverage on Glycemic Response and Antioxidant Capacity in Healthy Subjects: A Randomized Crossover Trial. BMC Complementary Altern. Med. 2018, 18, 1–11. DOI: 10.1186/s12906-017-2075-7.
- Cheng, S. H.; Ismail, A.; Anthony, J.; Ng, O. C.; Hamid, A. A.; Barakatun-Nisak, M. Y. Eight Weeks of Cosmos Caudatus (Ulam Raja) Supplementation Improves Glycemic Status in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Evid. Based Complement. Alternat. Med. 2015, 1–7. DOI: 10.1155/2015/405615.
- Gutiérrez, R. M. P.; Mitchell, S.; Solis, R. V. Psidium guajava: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. DOI: 10.1016/j.jep.2008.01.025.
- Sharwan, G.; Jain, P.; Pandey, R.; Shukla, S. S. Toxicity Profile of Traditional Herbal Medicine. Int. J. Ayurvedic Herb. Med. 2015, 1(3), 81–90. AHDA ET AL.