 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the quality characteristics of the following foodstuffs were studied: waxy rice, three varieties of Taiwanese banana, and banana rice cakes prepared using ripe or unripe fruit. The proportions of resistant starch (RS), total starch, and amylose were significantly higher in the ripe bananas than in the unripe bananas (p < .05). Banana rice cakes prepared from the ripe bananas had a higher cooking loss rate and greater a and b values (p < .05); conversely, those prepared from the unripe bananas had greater hardness and L values. The use of unripe bananas led to the rice cakes containing more RS (8.86, 8.53, and 8.44 g/100 g vs. 8.30, 8.15, and 7.36 g/100 g for the ripe bananas). The use of any type of banana led to higher RS content values for the resulting rice cakes than did the use of pure rice (6.67%; p < .05). The rice cakes prepared from the ripe bananas had significantly greater swelling power and solubility, and these characteristics were affected by temperature; higher temperatures were found to result in significantly higher swelling power and solubility levels (p < .05).
Introduction
A staple in the diet of approximately half the global population,[Citation1–4] rice (Oryza sativa L.) provides considerable energy because it contains much starch as well as vitamins, minerals, and proteins.[Citation5] It is hypoallergenic and acts as a carrier of flavor; thus, the cereal has many uses in food processing operations that entail producing foodstuffs such as rice cake, snacks, and wine. Use of rice in value-added products is highly desirable.[Citation6]
Waxy (or sticky) rice is an important economic crop in Taiwan. It is typically used as an ingredient in various types of rice food processing. In grain form, it serves as a staple food. For many dishes, it is used in flour form as an ingredient; for example, it is employed to produce rice cakes. Amylopectin makes up nearly all of the starch in waxy rice, and this compound has a fine structure that is responsible for waxy rice’s texture and properties when cooked.[Citation2] Functional rice food products are widely appreciated in the current market. Because of its sticky texture, waxy rice and its flour form are in high demand by consumers. However, its use in food processing is currently limited because under the extreme conditions that are applied during food processing, waxy rice starch is unstable.[Citation7]
Rice cake is a traditional Chinese rice food in which waxy rice is a primary ingredient. However, other components used in rice cake manufacturing contribute to the food’s qualities and functional properties. Using waxy rice to produce processed rice products does, however, have several limitations; for example, it can lead to poor texture and color, low acceptance by consumers, and a high glycemic index.[Citation8] Extending the multiple applications of waxy rice and finding other ways to use it to add value are thus necessary tasks for the food industry.
Bananas (Musa spp.) constitute a globally important and economical fruit crop.[Citation9] Because they contain relatively little amylase, do not swell considerably, have stability when heated, and are highly resistant to amylase hydrolysis, banana starches are employed as stabilizers, gelling agents, and thickening agents in the food industry. Food processing companies value the aforementioned characteristics and the odorless and soluble capacity of these starches.[Citation10] Raw banana fruit contains considerable starch, termed resistant starch (RS). A study in which cereal and grain starches were compared with banana starch reported several unique properties of banana starch; it was discovered to have a high gelatinization temperature and relative crystallinity and to contain considerable amylose and RS.[Citation11] In tropical countries, bananas are inexpensive and widely grown. RS, employed in rice food processing, has become a health trend. Compared with cereal-derived starch, RS in a hallmark of banana starch and can offer nutritional benefits, making banana starch a valuable addition to manufactured food products. Bananas can be used instead of products with other added value because of their functional properties. As an alternative raw material employed in processing food,[Citation12] bananas can decrease wastage owing to failures that occur when other foods are harvested, stored, and distributed.[Citation13] Starch accounts for the majority of the content of rice[Citation1] and bananas.[Citation8,Citation12,Citation14] Processing bananas into flour is of interest because this banana flour could become an important food resource and be used for various industrial purposes.[Citation15]
Bananas, which are grown widely in Taiwan, are generally consumed fresh[Citation16,Citation17]; sometimes turned into puree, powder, dried fruit, or ingredients for functional foods; or employed to produce numerous types of food items, such as chips, bread, jam, and juice.[Citation16,Citation18] Food processes involving bananas have sometimes failed, however, with such failures due to the bananas being of poor quality or the processed products having too little commercial value. Because of its low commercial acceptance, bananas can be employed as a substitute for, for example, waxy rice within valuable rice-processed foods owing to the beneficial RS that they provide.
The literature contains many articles detailing the components and quality of bananas and rice. However, whether rice can be replaced by ripe or unripe banana flour within food processing to develop new banana flavored rice cakes and to explore functional properties has rarely been investigated. Furthermore, studies on the physicochemical properties of Taiwanese bananas when they are used in waxy-rice-processed products are lacking. Whether waxy-rice-processed products and Taiwanese bananas have sufficient quality and functionality must be investigated. Accordingly, the present study investigated the total starch content as well as proportions of amylose and RS in waxy rice [Taichung Sen glutinous 8 (TCSG 8); grown in Taiwan] and the following varieties of Taiwanese bananas (ripe and unripe): varieties Tai-Chiao No. 5 (TC5), Formosana (FS), and Pei Chiao (PC). Subsequently, rice cakes were produced using these bananas and the waxy rice, and the following characteristics of these rice cakes were determined: hardness, color, solubility, swelling power, and cooking loss.
Materials and methods
Materials
We employed TCSG 8 kernels harvested in 2022. A market in Pingtung (Taiwan) was the source of the samples of the three aforementioned banana varieties. Each of our employed chemicals as well as reagents was established to be of analytical grade.
Methods
Formulation of banana flour: A modification of the method of Bakar et al.[Citation19] was employed to obtain whole flour from ripe and unripe bananas. After cleaning and brushing the bananas, we removed their ends. Slices of thickness 0.5 cm were then cut from the fruit, and immediately afterward, we employed citric acid solution (0.3% w/v) to rinse them. A DKN 612 oven procured from Yamoto Company (Tokyo, Japan) and maintained at 50°C was employed to dry the slices for 12 h. Subsequently, an RT-N08 laboratory grinder procured from Rong Tsong Solutions (Taichung, Taiwan) was employed to grind the slices into a powder; for the derived powder, filtration was executed using a 50-mesh sieve (270 μm) procured from Retsch (Haan, Germany). Until the flour samples were to be analyzed, we maintained them within sealed plastic containers at 7°C.
Amylose content: We used a slight modification to the approach of Li et al.[Citation20] to determine the amylose content of TCSG 8 and the investigated bananas. First, we mixed 90 mg of the sample with 9 mL of 1 N NaOH and 1 mL of 95% ethanol. This was followed by 24 h of incubation of this mixture at 30°C. Volume adjustment to 100 mL was achieved through the addition of distilled water. Subsequently, we collected a 5-mL aliquot of this solution, to which we added iodine solution (2 mL; 0.2% I2 in 2% KI) and 1 N acetic acid (1 mL). Moreover, volume adjustment to 100 mL was achieved again through the addition of distilled water, and the solution was mixed for 20 min. We employed a spectrophotometer (U-1500; Hitachi, Japan) to measure the solution’s absorbance at 620 nm. A standard curve obtained for potato amylose and waxy corn starch (A-0512 and S-9679, respectively; Sigma-Aldrich, St. Louis, MO, USA) was used to calculate amylose content. Each of our study’s analyses was executed in triplicate.
Total starch content: Following a slightly modified version of a reported approach,[Citation20,Citation21] we employed a biochemistry analyzer (model: YSI 7100) that we procured from YSI (Yellow Springs, OH, USA) to determine total starch content. An immobilized enzyme biosensor that measures posthydrolysis glucose levels was used in the approach. Banana powder (weight: 0.5 g) was sampled and subsequently moved to a volumetric flask measuring 100 mL; to this flask, we added 25 mL of distilled water and then 2 N NaOH. After incubating the mixture in a 100°C water bath, we added 2 N HCl. Chemical solubilization was facilitated by cooling the flask to less than 50°C. We then determined the total amount of starch in the sample. The amount of gelatinized starch it contained was discovered by adding 1 N acetate buffer and amyloglucosidase solution to the flask and then incubating it for 70 min at 40°C. Subsequently, we prevented hydrolysis by immediately adding 25% trichloroacetic acid (5 mL). Each of our study’s analyses was executed in triplicate.
RS content: A previously described method[Citation20,Citation22] was employed to measure RS content (RSC). We weighed, on a dry basis, 0.4 g of a sample in a centrifuge tube containing phosphate buffer [20 mL; pH 6.0 (55.6 mM)]; then, we added α-amylase (0.16 g; A-3176, Sigma-Aldrich) to the tube. After 16 h of incubation at 37°C, we used phosphoric acid solution (2 mL/100 mL) to adjust the sample’s pH to 4.5. Next, amyloglucosidase (0.4 mL; A-7095, Sigma-Aldrich) was added, and a 30-min incubation process was performed at 60°C. A CF15R high-speed microcentrifuge procured from Hitachi, Koki, Ltd. (Tokyo, Japan) was employed for 15 min to centrifuge the sample at 4,000 × g. We resuspended the resultant residue in phosphate buffer [20 mL; pH 7.5 (0.08 M)] as well as in protease (0.4 mL; P-2143, Sigma-Aldrich). After incubating the resuspended sample for 4 h at 42°C and then subjecting it to a 15-min centrifugation process executed at 6,000 × g, we employed an oven that was maintained at 60°C (DKN 612, Yamoto Company, Tokyo, Japan) to dry the sample, which was subsequently weighed for RSC determination. Each of our study’s analyses was executed in triplicate. The equation used to determine the RSC was
where all variables were measured in grams.
Banana rice cakes: We kept the employed TCSG 8 at 7°C in a refrigerator before using it. In advance of preparing rice cakes, we removed TCSG 8 from the refrigerator and then deposited it in a stainless-steel container, where it warmed over 2 h to room temperature. Subsequently, we soaked the rice at room temperature for 12 h by weighing out 200 g of the rice in a stainless-steel basin and then adding 200 mL of distilled water. The rice sample was ground for 5 min (Fe-05 grinder, Yung Soon Lih Food Machine, Taichung, Taiwan). A BH-230D thermostatic water bath procured from Yihdder Company (New Taipei City, Taiwan) was employed to heat a stainless-steel basin containing the resultant powder; the bath had a temperature of 85°C, and the heating was conducted for 18 min. After this period of time, we removed the powder and refrigerated it for 2 h at 4°C. We then obtained rice pulp by adding 200 g of water and thoroughly stirring. To this pulp, the powder of the ripe or unripe banana was added, which was followed by thorough mixing (). A rectangular stainless-steel tray of dimensions 25 cm × 8 cm × 15 cm was employed to produce rice cakes. After the rice – banana mixture was poured into the tray, it was steamed for 40 min (in a Ks-610 U steamer, Quickly Food Machinery, Taoyuan, Taiwan). We left the tray to cool to room temperature and then cut banana rice cake strips measuring 4 cm × 4 cm × 1 cm.
Table 1. Ingredients used to produce various steamed banana rice cakes.
Color: We employed the Color Quest XE system procured from Hunter Associates Laboratory (Reston, USA) to evaluate the color of the produced rice cakes. Each of our study’s analyses was executed in triplicate.
Hardness: Following a method described in the literature,[Citation20] with some modifications achieved after the calibration of the method’s accuracy, we employed an EZ Test-500N texture analyzer (TAXTZ-5, Shimadzu Co., Kyoto, Japan) to measure the banana rice cakes’ hardness. Pieces of samples were obtained that measured 4 cm × 4 cm × 4 cm. For a given sample set, we performed two sessions of compression testing. The specifications of the tests were a 30 mm/min compression speed, use of a 500-N load cell and a 10-mm rounded probe, and compression height equal to 50% of the sample’s initial height. Each of our study’s analyses was executed in triplicate.
Cooking loss: A slight modification of the method of Chou[Citation23] was used to measure cooking loss. First, we employed an HP-303D heating plate procured from NewLab Macro Fortunate Company (Taipei, Taiwan) to boil 20 mL of distilled water in a constant-weight (W) crucible. We then added to this water 2 g (denoted X) of a rice cake sample, followed by executing a 5-min heating process. The liquid that was left in the crucible was baked at 105°C in a DKN 612 oven procured from Yamoto Company (Tokyo, Japan) until its weight had ceased to change (weight denoted W1). We calculated the cooking loss as follows:
We made three measurements for each set of samples.
Solubility and swelling power: A slightly modified version of the method of Li et al.[Citation20] was used to evaluate the banana rice cakes’ solubility and swelling power. Using a graduated centrifuge tube of volume 50 mL, we first mixed 0.6 g (dry basis) of banana powder with distilled water such that the final volume was 40 mL and then heated the mixture for 30 min at 50, 60, 70, 80, or 90°C. After this period of heating, we wiped the centrifuge tube dry and reduced its temperature to 30°C. A CF15R high-speed microcentrifuge procured from Hitachi, Koki (Tokyo, Japan) was then used for 20 min of centrifugation at 6,000 × g. We decanted the supernatant into a preweighed crucible. A DKN 612 constant-temperature oven procured from Yamoto Company (Japan) was used to heat it to 105°C. Finally, we weighed the sedimented paste that the process had yielded. The following equations were used to determine swelling power and solubility:
where the sediment, sample, and solution weights were measured in grams.
Statistical analysis
We employed the 2010 version of Microsoft Office Excel (Microsoft Corporation, Redmond, WA, USA) to perform calculations, in addition to employing SAS software (SAS Institute, Cary, NC, USA) to appraise sample characteristics and RSC data under the consideration of variances. The mean values in the various groups were compared using Duncan’s multiple range test. Our definition of significance was p < .05.
Results and discussion
Amylose content, total starch content, and RSC of TCSG 8 and three banana varieties
This study determined that 100 g of TCSG 8 contained 6.07 g of amylose and 90.70 g of total starch (). Starch is the main component of rice,[Citation2,Citation24] and the amount of starch and amylose that rice contains varies considerably between varieties.[Citation24] Our value of 90.7% total starch is in favorable agreement with a figure reported previously (90%–90%).[Citation25] Also comparable to the present results are those of Setyawati et al.,[Citation26] who investigated various types of waxy rice and discovered them to contain 1.43–7.84 g/100 g and 82.1–91.6 g/100 g of amylose and total starch, respectively. We determined the RSC to be 6.67%, a value similar to the 2.90%–9.30% reported by Chung et al.[Citation27] and 9.93%–12.94% discovered by Nakamura et al.[Citation6]
Table 2. Amounts of amylose, total starch, and RS in TCSG 8 and three banana varieties.
displays the amounts of amylose, total starch, and RS in the three investigated banana varieties. The ripe and unripe bananas contained 14.46–32.17 g/100 g of amylose and 23.16–79.05 g/100 g of total starch (The moisture content of ripe and unripe bananas is 18.24–28.19%, data not shown). These values are comparable to those of Mesquita et al.[Citation13] (2016; 25.13–29.01 and 84.94–89.67 g/100 g, respectively) and also those of Ravi and Mustaffa[Citation28](24.41–36.87 and 80.53–86.76 g/100 g, respectively), who investigated the unripe fruit of nine banana varieties.
Scholars previously reported the total starch content to be higher in unripe bananas than in ripe bananas.[Citation29] In this study, we found the ripe bananas to contain significantly less amylose and starch than the unripe bananas did (p < .05; ); this was expected because starch is gradually broken down and converted into soluble sugars during the enzymatic conversion of starch during ripening (;).[Citation6,Citation12,Citation14,Citation29,Citation30] This result indicates a potential use of unripe bananas as a rich source of starch. Because bananas in the ripening stage have higher total starch content than do those that have already ripened, they have greater utility in various types of food processing and could further decrease wastage.[Citation12,Citation13]
As shown in , the RSC was derived to be 15.63–42.33 g/100 g for the various varieties of the ripe and unripe bananas. The RSC was significantly higher in the unripe bananas (). This result accords with that reported previously,[Citation11] and a higher RSC has typically been noted in unripe bananas, particularly under the consideration of RS type II (RS II)[Citation14]; such RSC was reported to be 42.64% in unripe bananas.[Citation8] In other studies, ripe and unripe bananas were discovered to respectively contain 21.9–23.21 g/100 g and 39.76–40.01 g/100 g RS.[Citation30,Citation31] α-Amylase and glucoamylase hydrolysis does not occur easily in RS II.[Citation13,Citation14] In the banana ripening process, the RSC decreases and the sugar content increases because the banana’s starch is converted into soluble solids.[Citation6,Citation12,Citation14,Citation28,Citation30,Citation32] The difference in RSC between ripe and unripe bananas was significant in the present study (p < .05; ). Because it is resistant to enzymatic hydrolysis, unripe banana starch can be incorporated into numerous products to give them high RSC and favorable functional properties.[Citation8]
Banana rice cake color
The appearance of various banana rice cakes produced in this study is shown in . We obtained L values of 39.12–43.12 for the six types of banana rice cake produced in this study (); by contrast, that of the blank group (the group of rice cakes prepared from only rice) was only 51.57. Investigating rice noodles in which unripe banana flour was an ingredient, Tiboonbun et al.[Citation33] reported an L value of 47.82, which is similar to the range that we report herein. Another finding of the present study is that UPC, UFS, and UTC5—the rice cakes prepared from the unripe bananas – had significantly higher L values than did RPC, RFS, and RTC5 (those prepared from ripe bananas; p < .05). Relatively low L values were expected for RPC, RFS, and RTC5 because of the large amount of pigments such as carotenoids in ripe bananas.[Citation16]
Figure 1. Appearance of the various banana rice cakes produced in this study (A sample name beginning with U or R indicates the use of unripe and ripe bananas, respectively. The characters after U and R indicate the banana variety employed).
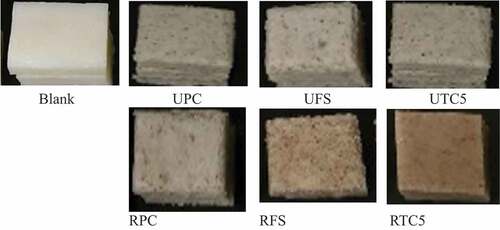
Table 3. Color properties of the various banana rice cakes produced in this study.
shows that we obtained values of 1.01–1.54 and 3.63–4.24 for a and b, respectively. The a values were 1.01, 1.50, and 1.28 and b values were 3.73, 3.63, and 3.79 for UPC, UFS, and UTC5, respectively; the corresponding values for RPC, RFS, and RTC5 were 1.50, 1.54, and 1.44 and 4.24, 4.21, and 4.06, respectively. The differences between all these values and those of the blank (a = −1.54 and b = 2.37) were all significant (p < .05). This was due to the absence of the Maillard reaction, given that waxy rice contains no reducing sugars.[Citation5] In the study of Tiboonbun et al.,[Citation33] increasing the amount of banana flour used to prepare banana rice cakes resulted in significantly higher a and b values for the derived rice cakes because the phenolic compounds in the banana were affected by polyphenol oxidase. Using ripe bananas to prepare rice cakes thus resulted in significantly higher a and b values than when unripe bananas were used (p < .05; ). This difference in a and b values was caused by pigments being produced when polyphenol oxidase reacted with phenolic compounds.[Citation12]
Banana rice cake hardness, adhesiveness and springiness
We obtained hardness values of 0.45–0.70 kgf/mm2, adhesiveness values of 0.21–0.36 kgf.sec and springiness values of 0.36–0.50 in this study (). When the unripe bananas were employed to prepare banana rice cakes, the cakes’ hardness did not significantly differ from that of the blank (RPC, RFS, RTC5, and blank: 0.45, 0.47, 0.49, and 0.46 kgf/mm2, respectively; p > .05). Nevertheless, the use of the unripe bananas resulted in significantly higher hardness than that of the blank (UPC, UFS, and UTC5: 0.67, 0.63, and 0.70 kgf/mm2, respectively; p < .05). Amylose content affects the texture of processed food containing rice,[Citation8] and RPC, RFS, and RTC5 had significantly lower amylose content than did UPC, UFS, and UTC5 (p < .05; ).
Table 4. Hardness, adhesiveness, springiness, cooking loss, and RSC of the various banana rice cakes produced in this study.
Cooked rice has been reported to have hardness of 270–480 g/mm2,[Citation2] which is similar to our derived findings. In the processing of food containing rice, the starch retrogradation degree is generally indicated by the hardness of the food. Chou et al.[Citation21] and Nimsung et al.[Citation6] have noted that a high degree of retrogradation may explain why the use of unripe rather than ripe bananas engenders greater product hardness, possibly because starch with higher amylose content retrogrades more quickly. The banana rice cakes prepared from unripe bananas had significantly greater hardness than did those made from ripe bananas (p < .05).
Cooking loss
The various banana rice cakes produced in this study had cooking loss rates ranging between 1.84% and 2.60% (). RPC (2.21%), RFS (2.32%), and RTC5 (2.60%) had significantly greater cooking loss than did UPC (1.84%), UFS (2.01%), UTC5 (2.24%), and the blank (1.80%; p < .05). Cooking loss was discovered by Fida et al.[Citation12] to be greater when the proportion of banana flour that they used was greater. The cooking loss of extruded restructured rice was found by Patria et al.[Citation5] to be 1.59%–2.55%. Fradinho et al.[Citation24] prepared gluten-free pasta containing Japonica rice flour and discovered cooking loss rates of 1.05%–1.60%. For banana powder – containing noodles, cooking loss rates of 1.5%–2.7% were noted by Wandee et al.[Citation34]; this range is similar to the range of loss values we obtained. Several factors affect cooking loss, including starch degradation during processing and incomplete starch gelatinization.[Citation5] Unripe bananas were proposed by Pragati et al.[Citation35] to be capable of absorbing more water than are ripe bananas; it may thus have been expected that the cooking loss rates of UPC, UFS, and UTC5 were smaller than those of RPC, RFS, and RTC5, as noted in this study (p < .05; ).
RSC
We obtained RSC values in the range 7.36–8.86 g/100 g, as detailed in . The ranges reported by Chung et al.[Citation27] and Nakamura et al.[Citation6]—for cooked and waxy rice, respectively – were 0.05–10.94 and 7.70–8.60 g/100 g, respectively, and these ranges are consistent with our findings. UPC, UFS, and UTC5 had significantly higher RSC values (8.86, 8.53, and 8.44 g/100 g, respectively) than did RPC, RFS, RTC5, and the blank (8.30, 8.15, 7.36, and 6.67 g/100 g, respectively; p < .05). Ripening resulted in greater soluble sugar content due to enzyme enzymolysis,[Citation12,Citation14,Citation29,Citation32] which led to lower RSC values.
These findings can be explained by several unique properties of bananas, including their high amylose content (), the ease with which they retrograde,[Citation29] and decreased enzymatic digestion of starch.[Citation27] Starch gelatinization and retrogradation and the degree to which starch is susceptible to enzymatic hydrolysis are strongly affected by amylose content because amylose is the main component of starch granules’ amorphous fraction.[Citation13,Citation29] Food that contains much amylose is difficult to digest and is beneficial to human health.[Citation13] One study reported high RSC values for rice papads and noodles in which one ingredient was unripe banana flour.[Citation33] We discovered that the unripe bananas led to significantly greater RSC values than did the ripe bananas (p < .05; ), in agreement with the previous findings.
Solubility and swelling power
Our prepared banana rice cakes’ solubility was derived to be 11.67%–38.89% over the temperature range 50–90°C (). A higher temperature was found to result in higher solubility. A similar result was obtained by Krzysztof et al.,[Citation36] who chemically and physically modified banana starch and investigated the same temperature range, and by Thiranusornkij et al.,[Citation37] who used Thai black rice to produce bread and investigated a slightly different temperature range (55–95°C). Increasing the temperature from 55 to 95°C was discovered by Li et al.[Citation30] to significantly increase the solubility of an unripe banana’s flesh and peel, in agreement with our finding. For our banana rice cakes, regardless of whether they were prepared using unripe or ripe fruit, an increase in temperature (within 50–90°C) resulted in a gradual increase in solubility; this effect may have been because at a higher temperature, the starch granules changed shape or size due to stronger fusion, swelling, deformation, and erosional effects.[Citation37] Such changes may have caused the degradation of amylose and amylopectin during gelatinization at high temperatures, leading to smaller molecules, which typically dissolve more easily in water than do large molecules.[Citation5] RPC and RTC5—banana rice cakes produced from ripe fruit – had significantly higher solubility than did UPC and UTC5 (those produced from unripe fruit; p < .05; ).
Table 5. Solubility (%) of the various banana rice cakes produced in this study.
Wani et al.[Citation3] investigated rice and reported its swelling power to be 7.33%–38.8%; they also determined that waxy rice had greater swelling power (equal to 26.9%) than did normal rice (15.5%) and concluded that when the starch granules in waxy rice are swollen and packed too densely, they are softer and disintegrate easily. For our banana rice cakes, we obtained swelling power levels of 10.95–20.52 gg−1 at 50–90°C () and discovered that higher temperatures resulted in greater swelling power. This temperature dependence over the range 50–90°C was also discovered by Krzysztof et al.[Citation36] in their study on banana starch that had been chemically and physically modified. Li et al.[Citation20] reported a similar finding over temperatures of 55–95°C in their investigation of unripe banana’s flesh and peel; the swelling power increased significantly from 2 to 28 gg−1 upon an increase in temperature. In their research on bread prepared using Thai black rice, Thiranusornkij et al.[Citation37] determined the corresponding swelling power to be 4.38–19.00 gg−1. Values of 10.79–14.06 gg−1 were obtained by Setyawati et al.[Citation26] for different varieties of waxy rice; these values are similar those derived for our prepared banana rice cakes, as are those reported by Mesquita et al.[Citation13] for various banana varieties (13.20–15.19 gg−1).
Table 6. Swelling power (gg−1) of the various banana rice cakes produced in this study.
Swelling power is a measure of the ability of starch to bind to water during heating[Citation20,Citation24] and can be used as an index of the forces involved in this binding.[Citation13] At high temperatures, it also reflects how much starch is dispersed.[Citation38] In this study, a higher temperature was discovered to result in starch molecules being more mobile, meaning that the forces underlying water – starch binding were weaker. More soluble components could thus leach from the starch; consequently, the starch was more soluble, and the porosity of its granules was greater. High temperatures thus led to water more effectively diffusing into the granule structure and the retention of more water given the flexibility in the number of starch molecules.[Citation13]
Our banana rice cakes’ swelling power was found to be as low as 10.95 gg−1 at 50°C but as high as 18.92 gg−1 at 80°C (p < .05; ). Higher temperatures meant that amylose chains and the perfect crystalline structure interacted more strongly; thus, the amorphous starch region was not hydrated as easily.[Citation38] The starch granules’ swelling power was even higher at 90°C owing to the gelatinization process[Citation24]; at this temperature, the highest value of the swelling power was 20.52 gg−1 (p < .05; ), and for all banana varieties, banana rice cakes prepared from ripe bananas had significantly greater swelling power than did those obtained using unripe bananas (p > .05; ). For varieties of unripe bananas, a swelling power of < 11.0 gg−1 was determined by Huang et al.[Citation39]; our findings pertaining to the swelling power of the banana rice cakes prepared from the unripe bananas are thus similar to those of Huang et al.
Conclusion
We executed this study to probe the quality characteristics of the following foodstuffs: waxy rice, three varieties of Taiwanese bananas (used either when ripe or unripe), and the banana rice cakes that could be prepared from these ingredients. The unripe bananas were discovered to contain significantly more amylose, total starch, and RS than did their ripe counterparts (p < .05). Regarding rice cake color, we obtained L values and found that the inclusion of any banana type or variety when preparing the rice cake led to a lower L value (p < .05). Additionally, we noted significantly higher cooking loss rates and a and b values for the rice cakes that we obtained using ripe fruit than for those that we obtained using unripe fruit (p < .05). By contrast, use of unripe fruit led to significantly greater hardness. The banana rice cakes prepared using unripe bananas contained significantly more RS than did those prepared using ripe bananas (p < .05), and use of any type of banana led to higher RSC values than those derived when rice cakes were prepared purely from rice (p < .05). UPC and RTC5 contained the most and least RS, respectively. Additionally, RPC, RFS, and RTC5 had greater swelling power and solubility at 50–90°C than did UPC, UFS, and UTC5 (p < .05). Higher temperatures were discovered to correspond to significantly higher swelling power and solubility levels (p < .05). According to our findings, various rice-based food products could be developed using Taiwanese bananas, with the different varieties and choice of unripe versus ripe offering potential for fine-tuning the properties of these food products. Such an approach could lead to rice-based food products with greater quality and functionality and help prevent wastage in relation to bananas and rice.
Acknowledgments
The authors are grateful for support from the Master’s Degree Program in Safety and Health Science, Taiwan, and their permission to use their experimental facilities. This work was supported by Chang Jung Christian University, Taiwan (R.O.C.). The authors have no conflicts of interest to declare.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anugrahati, N. A.; Pranoto, Y.; Marsono, Y.; Marseno, D. W. Physicochemical Properties of Rice (Oryza Sativa L.) Flour and Starch of Two Indonesian Rice Varieties Differing in Amylose Content. Int. Food Res. J. 2017, 24, 108–113.
- Srikaeo, K.; Paphonyanyong, W. Texture, Microstructure and in-Vitro Starch Digestibility of Waxy Rice Cooked with Hydrocolloids. Food Res. 2020, 4, 1089–1097. DOI: 10.26656/fr.2017.4(4).026.
- Wani, A. A.; Singh, P.; Shah, M. A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I. A. Rice Starch Diversity: Effects on Structural, Morphological, Thermal, and Physicochemical Properties—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11(5), 417–436. DOI: 10.1111/j.1541-4337.2012.00193.x.
- Zhang, L.; Zhao, L.; Zhang, X.; Lin, L.; Wei, C. Characterization and Starch Properties of a Waxy Mutant in Japonica Rice Kitaake. J. Agric. Crops. 2018, 4(11), 117–124. DOI: 10.32861/jac.411.117.124.
- Patria, D. G.; Sutrisno, A.; Hsu, J. L.; Lin, J. Physical Properties and Cooking Quality of Extruded Restructured Rice: Impact of Water Temperature and Water Level. Food Res. 2020, 4, 1616–1622. DOI: 10.26656/fr.2017.4(5).141.
- Nakamura, S.; Katsura, J.; Maruyama, Y.; Ohtsubo, K. Evaluation of Hardness and Retrogradation of Cooked Rice Based on Its Pasting Properties Using a Novel RVA Testing. Foods. 2021, 10, 987. DOI: 10.3390/foods10050987.
- Cao, C.; Shen, M.; Hu, J.; Qi, J.; Xie, P.; Zhou, Y. Comparative Study on the Structure-Properties Relationships of Native and Debranched Rice Starch. CyTa – J. Food. 2020, 18(1), 84–93. DOI: 10.1080/19476337.2019.1710261.
- Detchewa, P.; Prasajak, P.; Sriwichai, W.; Moongngarm, A. The Effects of Unripe Banana Flour on Resistant Starch Content and Quality Charateristics of Gluten-Free Rice Cookies. J. Sustain. Sci. Manag. 2021, 16(2), 67–78. DOI: 10.46754/jssm.2021.02.008.
- Parmar, H. C.; Mor, V. B. Quality of Banana Influenced by Different Varieties and Planting Materials. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7(6), 2227–2231. DOI: 10.20546/ijcmas.2018.706.264.
- da Costa, E. L.; Alencar, N. M. M.; Rullo, B. G. D.; Taralo, R. L. Effect of Green Banana Pulp on Physicochemical and Sensory Properties of Probiotic Yoghurt. Food Sci. Technol. 2017, 37(3), 363–368. DOI: 10.1590/1678-457x.01016.
- Jaiturong, P.; Laosirisathian, N.; Sirithunyalug, B.; Eitssayeam, S.; Sirilun, S.; Chaiyana, W.; Sirithunyalug, J. Physicochemical and Prebiotic Properties of Resistant Starch from Musa Sapientum Linn., ABB Group, Cv. Kluai Namwa Luang. Heliyon. 2020, 6, e05789. DOI: 10.1016/j.heliyon.2020.e05789.
- Fida, R.; Pramafisi, G.; Cahyana, Y. Application of Banana Starch and Banana Flour in Various Food Product: A Review. Int. Conf. Food Bio-Industry. 2019, 443(1), 012057. DOI: 10.1088/1755-1315/443/1/012057.
- Mesquita, C. D.; Leonel, M.; Franco, C. M. L.; Leonel, S.; Garcia, E. L.; dos Santos, T. P. R. Characterization of Banana Starches Obtained from Cultivars Grown in Brazil. Int. J. Biol. Macromol. 2016, 89, 632–639. DOI: 10.1016/j.ijbiomac.2016.05.040.
- Chaipai, S.; Kriangsinyot, W.; Srichamnong, W. Effects of Ripening Stage and Cooking Methods on Available Glucose, Resistant Starch and Estimated Glycemic Index of Bananas (Musa sapientum; Nam-Wa Variety). Malays. J. Nutr. 2018, 24, 269–279.
- Waliszewski, K. N.; Aparicio, M. A.; Bello, L. A.; Monroy, J. A. Changes of Banana Starch by Chemical and Physical Modification. Carbohydr. Polym. 2003, 52(3), 237–242. DOI: 10.1016/S0144-8617(02)00270-9.
- Singh, B.; Singh, J. P.; Kaur, A.; Singh, N. Bioactive Compounds in Banana and Their Associate Health Benefits - a Review. Food Chem. 2016, 206, 1–11. DOI: 10.1016/j.foodchem.2016.03.033.
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Kunwisawa, J.; Soponronnarit, S. Determination of Effective Moisture Diffusivity and Assessment of Quality Attributes of Banana Slices During Drying. LWT-Food Sci. Technol. 2011, 44, 1502–1510. DOI: 10.1016/j.lwt.2011.01.003.
- Vu, H. T.; Scarlett, C. J.; Vuong, Q. V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods. 2018, 40, 238–248. DOI: 10.1016/j.jff.2017.11.006.
- Bakar, S. K. S. A.; Ahmad, N. F.; Jailani, F. Chemical and Functional Properties of Local Banana Peel Flour. J. Food Nutr. Res. 2018, 6, 492–496. DOI: 10.12691/jfnr-6-8-1.
- Li, M. C.; Chou, C. F.; Hsu, S. C.; Lin, J. S. Physicochemical Characteristics and Resistant Starch of Different Varieties of Banana from Taiwan. Int. J. Food. Prop. 2020, 23(1), 1168–1175. DOI: 10.1080/10942912.2020.1788077.
- Chou, C. F.; Yen, T. F.; Li, C. T. Effects of Different Cooking Methods and Particle Size on Resistant Starch Content and Degree of Gelatinization of a High Amylose Rice Cultivar in Taiwan. J. Food Agric Environ. 2014, 12, 6–10.
- Chou, C. F.; Li, M. C. A Research of Effect of Three Sweet Potato Varieties and Addition on Resistant Starch Content and Physical Characteristics of Steamed Rice Bowl Cake. J. Food Nutr. Res. 2018, 6(9), 551‒556. DOI: 10.12691/jfnr-6-9-2.
- Chou, C. F. Evaluation of Quality Properties of Emulsified Pork Sausages Containing Sorghum Distillers Grains. J. Food Process Preserv. 2020, 44, e14968. DOI: 10.1111/jfpp.14968.
- Fradinho, P.; Sousa, I.; Raymundo, A. Functional and Thermorheological Properties of Rice Flour Gels for Gluten-Free Pasta Applications. Int. J. Food Sci. Technol. 2018, 54(4), 1109–1120. DOI: 10.1111/ijfs.14001.
- Kusano, M.; Fukushima, A.; Fujita, N.; Okazaki, Y.; Kobayashi, M.; Oitome, N. F.; Ebana, K.; Saito, K. Deciphering Starch Quality of Rice Kernels Using Metabolite Profiling and Pedigree Network Analysis. Mol. Plant. 2012, 5(2), 442–451. DOI: 10.1093/mp/ssr101.
- Setyawati, Y. D.; Ahsan, S. F.; Ong, L. K.; Soetaredjo, F. E.; Ismadji, S.; Ju, Y. H. Production of Glutinous Rice Flour from Broken Rice via Ultrasonic Assisted Extraction of Amylose. Food Chem. 2016, 203, 158–164. DOI: 10.1016/j.foodchem.2016.02.068.
- Chung, H. J.; Lim, H. S.; Lim, S. T. Effect of Partial Gelatinization and Retrogradation on the Enzymatic Digestion of Waxy Rice Starch. J. Cereal Sci. 2006, 43(3), 353–359. DOI: 10.1016/j.jcs.2005.12.001.
- Ravi, I.; Mustaffa, M. Starch and Amylose Variability in Banana Cultivars. Indian J. Plant Physiol. 2013, 18(1), 83–87. DOI: 10.1007/s40502-013-0014-2.
- Nimsung, P.; Thongngam, M.; Naivikul, O. Compositions, Morphological and Thermal Properties of Green Banana Flour and Starch. Kasetsart J. (Nat. Sci.). 2007, 41, 324–330. DOI: 10.1080/23311932.2017.1283079.
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of Physicochemical Properties of Starches from Flesh and Peel of Green Banana Fruit. Molecules. 2018, 23, 2312. DOI: 10.3390/molecules23092312.
- Febriyatna, A.; Damayati, R. P.; Agustin, F. Analyze of Nutrition and Bioactive Compound in Unripe and Ripe Berlin Banana (Musa acuminate) Flour. Proceeding of the 1st International Conference on Food and Agriculture. 616–618. 2018.
- Adiyanti, T.; Subroto, E. Modifications of Banana Starch and Its Characteristics: A Review. Int. J. Sci. Tech. Res. 2020, 9, 3524–3527.
- Tiboonbun, W.; Sungsri-In, M.; Moongngarm, A. Effect of Replacement of Unripe Banana Flour for Rice Flour on Physical Properties and Resistant Starch Content of Rice Noodle. World Academy of Science, Engineering and Technology. Int. J. Nutr. Food Eng. 2011, 5, 9. DOI: 10.5281/zenodo.1083547.
- Wandee, Y.; Uttapap, D.; Puncha-Arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N. Quality Assessment of Noodles Made from Blends of Rice Flour and Canna Starch. Food Chem. 2015, 179, 85–93. DOI: 10.1016/j.foodchem.2015.01.119.
- Pragati, S.; Genitha, I.; Ravish, K. Comparative Study of Ripe and Unripe Banana Flour During Storage. J. Food Process. Technol. 2014, 5(11), 384. DOI: 10.4172/2157-7110.1000384.
- Krzysztof, N. W.; Maria, A. A.; Luı́s, A. B.; Jóse, A. M. Changes of Banana Starch by Chemical and Physical Modification. Carbohydr. Polym. 2003, 52(3), 237–242. DOI: 10.1016/S0144-8617(02)00270-9.
- Thiranusornkij, L.; Thamnarathip, P.; Chandrachai, A.; Kuakpetoon, S.; Adisakwattana, S. Physicochemical Properties of Hom Nil (Oryza Sativa) Rice Flour as Gluten Free Ingredient in Bread. Foods. 2018, 7(10), 159. DOI: 10.3390/foods7100159.
- Wang, L.; Zhang, C.; Chena, Z.; Wanga, X.; Wanga, K.; Lia, Y.; Wanga, X.; Luoa, X.; Lia, Y.; Lia, J. Effect of Annealing on the Physico-Chemical Properties of Rice Starch and the Quality of Rice Noodles. J. Cereal Sci. 2018, 84, 125–131. DOI: 10.1016/j.jcs.2018.10.004.
- Huang, S.; Martinez, M.; Bohrer, B. M. The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana). Foods. 2019, 8, 586. DOI: 10.3390/foods8110586.
