 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to investigate the nutritional and antioxidant composition of dried wild bilberry, blackcurrant, and blackberry pomaces by evaluating the proximate content, titratable acidity, color, total phenolics, total flavonoids, total anthocyanins content, and antioxidant activity. In addition, fatty acid and polyphenolic profiles were assessed using chromatographic methods. Higher fiber, fat, and mineral contents were found in blackberry as compared with bilberry and blackcurrant pomaces. The fatty acid profile revealed high concentrations of monounsaturated (16.7–18.7%) and polyunsaturated fatty acids (72.3–77.4%). The lowest n−6/n−3 ratio was found in bilberry pomace (0.90) followed by blackcurrant (1.28) and blackberry pomaces (4.26). Bilberry pomace showed the highest total phenolic (36.7 mg GAE/g dw) and total anthocyanin content (28.35 mg CGE/g dw). The phenolic profile of the bilberry pomace was dominated by ellagic acid and catechin while in blackberry and blackcurrant pomaces the major phenolic compounds were epigallocatechin and catechin, respectively. High levels of procyanidins B1 have been also quantified in bilberry and blackcurrant pomaces. Due to their healthy lipid profile and richness in antioxidant compounds, berry pomaces are good candidates as food ingredients for enhancing the functionality of food products and for contributing to the sustainability of the food industry.
Introduction
With the increased consumption of resources, humanity has been witnessing a whole series of environmental problems and the exhaustion of natural resources.[Citation1] There is no longer a contradiction between environmental objectives and development but an embedded system, a close relationship between natural resource use, systems of production, consumption, and how they trigger economic prosperity and human development.[Citation2,Citation3] Fruit and vegetable by-products derived from the food processing industry represent nowadays, one of the most pressing environmental concerns.[Citation4,Citation5] With the expansion of fruits production, around 503.3 million metric tons globally, of which 1.4 million metric tons are manufactured into different products for consumption,[Citation6] huge quantities of wastes and by-products are generated such as seed, skin, pod, peel, pomace, husk, stem.[Citation7] In recent years, researchers have established that these by-products are a valuable source of nutrients, phenolic compounds, pigments, dietary fibers, essential oils, enzymes, vitamins, and fatty acids.[Citation8,Citation9] Therefore, valorization and transformation of these by-products into new reusable products can contribute to the mitigation of environmental problems and make a path toward sustainable development.[Citation10] Furthermore, the recovery of these wastes can tackle the circular economy principles by using novel processing methods and technologies[Citation11] with implementation in the food industry[Citation12] as well as in other industries also, like pharmaceutical and cosmetic industries.[Citation4,Citation13]
Currently, berries have gained increased interest as functional food ingredients due to their health benefits and other industrial and nutraceutical applications.[Citation14] Bilberry (Vaccinium myrtillus L.) is a perennial shrub, native to Northern Europe, Asia, and Northern America.[Citation15] Fruits abound in bioactive compounds, like dietary fiber and polyphenols with high antioxidant properties,[Citation16,Citation17] and consequently, several potential health benefits have been associated with their consumption. Alnajjar et al.[Citation18] signaled that the intake of a bilberry extract over some time can ameliorate type 2 diabetes by reducing glycemia while Milenkovic et al.[Citation19] revealed the neuroprotective properties of bilberry extract supplementation applied in mice diet. Bilberries are used as fresh berries or in the manufacture of juices, jams, preserves, purees, and nutraceuticals. After bilberries processing, a fruit pomace in the form of a solid press cake is generated as a by-product, composed overall from seeds and berry peel, that is still abundant in fibers, beneficial phenolic compounds, and other antioxidants.[Citation16] This waste can be recovered and reused as upgraded products in other industries.[Citation20] Svanberg et al.[Citation21] developed a stable food emulsion incorporating bioactive compounds derived from bilberry by-products while Syrpas et al.[Citation22] used enzyme-assisted extraction to achieve a great recovery of saccharide content from bilberry pomace. Zhou et al.[Citation23] used microwave hydrolysis and extraction for the recovery of proteins, saccharides, anthocyanins, and inorganic salts with the goal of converting bilberry pomace into value-added products for the food industry and other applications.
Blackberry (Rubus spp.) is a shrub belonging to the Rosaceae family[Citation24] with a noticeable increase in consumption over the last years due to the beneficial health effects of its fruits, attributed to the high content of nutritional and bioactive compounds.[Citation25] Besides anthocyanins and other phenolic compounds,[Citation26] high amounts of vitamins A, K, and E[Citation27] and plentiful micro and macrominerals[Citation28] are present in these berries. Blackberries can be consumed either fresh or processed into jam, syrups, wine, tea, desserts, jellies, and bakery products.[Citation24] After blackberry juice processing, the resulting pomace can be harnessed as a source of bioactive compounds that can be used as potential dietary additives and natural colorants.[Citation29,Citation30] Recently, Isopencu et al.[Citation31] developed complex edible coatings incorporating blackberry pomace as a valuable source of antioxidants and antimicrobial agents while Tarasevičienė et al.[Citation32] reported that the addition of blackberry pomace improved the quality of beef patties by acting as a thickener, increasing the fiber content, and reducing lipid oxidation.
Blackcurrant (Ribes nigrum L.) is a woody shrub belonging to the Grossulariaceae family, native to central and northern Europe and northern Asia.[Citation33] Although blackcurrant represents a rich source of polyphenols, especially phenolic acids, anthocyanins, flavonols, condensed tannins, and hydrolyzable tannins,[Citation34] this fruit is seldom consumed as fresh fruit because of its strong astringency, acid taste, and high perishability.[Citation35] Therefore blackcurrant is commonly commercialized in the form of processed products like juice, jams, jellies, and alcoholic beverages.[Citation36] After the processing operations of blackcurrant in the berry juice pressing, a high amount of waste in the form of pomace is formed.[Citation37] The pomace is abundant in fiber, fruit acids, and anthocyanins[Citation38] and this has led researchers to conduct studies for the valorization of blackcurrant pomace by using it as a natural ingredient for healthy food products.[Citation39] Previously, Lorenzo et al.[Citation40] proposed the use of blackcurrant pomace as a natural additive for meat processing while Kurek et al.[Citation41] developed food packaging films with blackcurrant processing waste as a source of antioxidant compounds and pigmenting agents. Recently, Alchera et al.[Citation42] optimized the extraction of phenolic compounds from blackcurrant by-products and used the extract to develop an active pad with antimicrobial and antioxidant properties able to extend the storage and shelf-life of fruits.
The present study aimed to explore the nutritional and bioactive content of bilberries, blackberries, and blackcurrants processing by-products in view of their better use in the development of new high-value-added products for the food industry and other applications.
Materials and methods
Chemicals and reagents
Folin-Ciocalteu ’s phenol reagent, 2,2-diphenyl−1-picrylhydrazyl (DPPH), 6-hydroxy−2,5,7,8-tetramethylchroman−2-carboxylic acid (Trolox), 2,2’-azino-bis(3-ethylbenzothiazoline−6-sulfonic acid) (ABTS), gallic acid, and sodium acetate were bought from Sigma-Aldrich (Steinheim, Germany). Aluminum nitrate and sodium carbonate were supplied by Merck (Darmstadt, Germany). The other chemicals were of analytical quality and used without further purification.
Materials
Fresh pomaces, consisting of peels, seeds, and residual pulp, were obtained directly after juice pressing of wild bilberries (Vaccinium myrtillus L.), blackcurrants (Ribes nigrum L.) and blackberries (Rubus fructicosus L.) from Jiancom S.R.L., a commercial juice manufacturer from Vaideeni (Vâlcea county, Romania). The juice processing was performed in duplicate without enzyme treatment and two batches of 5 kg each were collected for each berry. The samples were packed in sealed plastic sacks and quickly moved to the lab, where the pomaces were dried at 57°C in a laboratory dryer with air circulation (Deca +SS Design, Profimatic, Romania). The dried pomaces were ground to break down the seeds using a household electric grinder, sieved using a 0.5 mm screen, packed in polyethylene bags, and preserved at 20°C for further analysis.
Proximate composition
Dry matter, fat, proteins, fiber, and ash contents were analyzed in the pomace powders using standardized methods. Dry matter content was determined using the drying oven method at 103°C (Memmert ULM500, Uden, The Netherlands), Kjeldahl method was performed to determine protein content according to SR EN ISO 5983–2:2009 by using an automated nitrogen analyzer (UDK 149, Velp Scientific, Milan, Italy) and fat was assayed by extraction in organic solvents according to SR EN ISO 6492:2001 by using a Soxhlet automatic extraction system (SER 148/3, Velp Scientific, Usmate, Italy). The crude fiber was assayed by digestion with acid and alkali according to ISO 6865:2002 using an automatic analyzer (Fibertec 2010, Tecator, Sweden) and ash by using a Caloris CL 1206 oven (Romania) according to ISO 2171:2009.
Extraction of phenolic compounds
The extraction of phenolic compounds from dry pomace samples was performed for 60 min at ambient temperature in a Bandelin Sonorex Digital 10P ultrasonic bath (Bandelin Electronic GmbH, Germany) using methanol as the solvent. The extracts were centrifuged at 4032 × g for 5 min. The resulting supernatants were collected, filtered through 0.45 μm polyamide membranes, and assayed for total phenolic content, total flavonoid content, and DPPH radical scavenging activity.
Total phenolic content
The total phenolic content was analyzed in the sample extracts by using the Folin – Ciocalteu spectrophotometric method as previously reported.[Citation43] First, aliquots of extracts (0.1 mL) and 5 mL of ultra-pure water were put together with 0.5 mL of Folin-Ciocalteu reagent in a test tube. After 3 min, 1.5 mL of sodium carbonate solution (20% w/v) and 4.4 mL of ultra-pure water were added. The mixture was shaken, then left for 30 min at 40°C in the dark. After incubation, the absorbance was read at 765 nm using a Varian Cary 50 UV spectrophotometer (Varian Co., USA). A linear calibration curve was established using gallic acid standards and results were expressed as mg gallic acid equivalents (GAE) per 100 g dry pomace sample.
Total anthocyanin content
The total anthocyanin content was estimated spectrophotometrically following the pH differential method previously proposed by Giusti and Wrolstad.[Citation44] Anthocyanins were extracted from the dry pomace powders with 0.1% HCl (v/v) in methanol. The extracts were diluted in 0.025 M potassium chloride at pH 1.0 and 0.04 M sodium acetate at pH 4.5 with a proper dilution insomuch that their absorbances, measured after 15 min in a Varian Cary 50 (Varian Co., USA) spectrophotometer at 510 and 700 nm, were lower than 1000. The results were expressed as milligrams of cyanidin−3-glucoside equivalents (CGE) per gram of dry pomace.
Total flavonoid content
The flavonoid content in the sample extracts was determined using an aluminum nitrate spectrophotometric method previously described.[Citation45] Aliquots of 0.5 mL extract were mixed with 0.1 mL aluminum nitrate aqueous solution (10%, w/v), 0.1 mL sodium acetate (1 M), and 4.3 mL methanol. The absorbance of the mixture was read at 415 nm on a Varian Cary 50 UV-VIS spectrophotometer (Varian Co., USA). The results were expressed as mg of quercetin equivalents (QE) per gram of dry pomace.
DPPH radical-scavenging activity
The investigated dry pomace extracts were tested for their ability to scavenge DPPH using a spectrophotometric method previously described by Oliveira et al..[Citation46] Briefly, aliquots of 50 μL extract were mixed with 3 mL of 0.004% DPPH solution. After shaking and incubation in the dark for 30 min, the absorbance of the reaction mixture was measured at 517 nm using a spectrophotometer (Varian Cary 50 UV, Varian Co., USA). The DPPH radical scavenging activity was calculated as follows:
Trolox was used as the standard for the calibration curve and the results were expressed as micromoles Trolox equivalent (TE) per gram of dry pomace.
ABTS antioxidant activity
The ABTS antioxidant activity was measured using a procedure described by Re et al..[Citation47] The ABTS cation radical solution (ABTS +) was prepared by mixing 5 mL ABTS solution (7.0 mM) and 88 μL potassium persulfate solution (145 mM). After standing for 16 h at room temperature in the dark, the ABTS+ solution was diluted in 80% aqueous ethanol until an absorbance of 0.700 ± 0.005 read at 734 nm. Aliquots of 12 mL ABTS+ were mixed in a Vortex with 120 μl extract, then the absorbance was read at 734 nm. A linear calibration curve was drawn using Trolox solutions in ethanol (100–2000 μM Trolox/L) and the results were expressed in micromoles of Trolox per gram of dry pomace.
Color
The color of dry pomace powders was measured using a PCECSM1 colorimeter (PCE Instruments, Southampton, UK) calibrated against a white standard. The color coordinates L* (brightness), a* (+redness/–greenness) and b* (+yellowness/–blueness) were measured on three replicates from each berry sample at five points on each replicate. Color measurements were made immediately after obtaining the powders (day 0) and after 28 days of storage in the dark (day 28). Color degradation during storage was expressed as the color difference (ΔE), calculated as follows: , where L1, a1, b1 represent color values of the samples on day 28, while L0, a0, b0 are color values of the samples on day 0.
Fatty acids profile
Dry pomace samples were analyzed in duplicate for the fatty acid profile by fatty acid methyl esters (FAME) gas chromatography after conversion of the fatty acids from the lipid extracts to their methyl esters by transesterification in methanol containing 3% sulfuric acid. They were separated on a DB−23 GC capillary column (60 m × 0.25 mm id × 0.25 µm) and detected on a gas chromatograph Perkin-Elmer Clarus 500 (Shelton, MA, USA) equipped with a flame ionization detector. The column temperature was set to increase by 5°C/min from 180°C to 220°C. The carrier gas was hydrogen and the flow rate was 35 cm/s at 180°C. Identification of fatty acid methyl esters was done by comparing their retention times with those of standards from Sigma-Aldrich Chemical Co. (MO, USA). The results were expressed as grams of fatty acid per 100 g of total fatty acids. The total saturated (SFA), total monounsaturated (MUFA), total polyunsaturated (PUFA), total n−3 polyunsaturated (n−3 PUFAs), total n−6 polyunsaturated (n−6 PUFAs) fatty acids, and the n−6/n−3 ratio were calculated in order to characterize the fatty acids profile.
Phenolic compounds
The RP-HPLC protocol involved a binary elution system consisting of 1% (v/v) acetic acid in ultrapure water (A) and acetonitrile (B) following the parameters described elsewhere[Citation48] by using an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA). The analyzed fruit powders were subjected to extraction, as described by Liao et al..[Citation49] Briefly, the pomaces’ powders were mixed with the extraction solvent made of methanol, ultrapure water, and 1N HCl (70:29:1 v/v/v) taking into consideration a 1:10 (w/v) ratio and the mixture was centrifuged at 18,928 × g rpm for 10 minutes (Hettich Universal 32 R, Tuttlingen, Germany). The supernatant was filtered through a 0.22 µm syringe filter and used for chromatographic analysis. For the identified compounds, concentrations were expressed as milligrams per gram dry weight (dw) sample, based on calibration curves for each bioactive compound quantified at 280 nm and 320 nm.
Statistical analysis
Statgraphics Centurion XVI software (StatPoint Technologies, VA, USA) was used to conduct the statistical analysis. All the assays were completed at least in triplicate and the results are presented as mean ± standard deviation. The least significant difference (LSD) test was used to assess multiple comparisons among the means with significant differences tested in ANOVA (p < .05).
Results and discussion
Proximate composition
The proximate composition and titratable acidity of dried berry pomaces are presented in . After drying at 57°C for 6–7 h, all powders resulted in a residual moisture content of around 10%. No significant differences (p < .05) were found between dried berry pomaces in terms of dry matter content.
Table 1. Proximate composition and titratable acidity of dried bilberry, blackberry, and blackcurrant pomace.
As reported in previous studies, the proximate composition of pomaces varied significantly depending on the berry species.[Citation50] In terms of protein, the differences were significant (p < .05), the highest content was found in the dried bilberry pomace (8.13 g/100 g dw), followed by blackberry (7.32 g/100 g dw) and blackcurrant pomace (4.77 g/100 g dw). Similar protein content was reported by Gagneten et al.[Citation35] in dried blackcurrant pomace (4.2%) while a higher protein content (6.9%) was found by Górnás et al.[Citation51] in a similar by-product. Pires et al.[Citation52] found a lower protein content in dried blackcurrant fruits (3%), but it was expected that by-products can be richer in protein as compared to the whole fruit, as they have a higher content of seeds which are richer in protein compared to the pulp and skin.
Significant differences (p < .05) were found also for the fat content, the highest fat content was found in blackberry pomace (9.67 g/100 g dw), which was expected as it was the richest in seeds among the three by-products and it is well-known that the seeds are the main source of lipophilic components in berries. A similar fat content (11.32%) has been reported by Pasquel-Reátegui et al.[Citation53] in the blackberry pomace. The lowest fat content was found in dried blackcurrant pomace (4.25 g/100 g dw). However, other studies reported lower fat content, such as Górnás et al.[Citation51] who found only 0.7% fat, and Gagneten et al.[Citation35] who reported 1.98% fat in dried blackcurrant pomace. Contrariwise, Alba et al.[Citation54] found 5.9% and 10.8% fat in two blackcurrant pomaces of different origins.
Due to the high content of seeds and skins, fiber is an important component of berry by-products.[Citation55] The dried blackberry pomace had the highest fiber content (44.87 g/100 g dw) followed by the dried blackcurrant pomace (15.5 g/100 g dw). However, a higher dietary fiber content was previously reported by Gagneten et al.[Citation35] (32.3%) or by Górnás et al.[Citation51] (38.5%) in dried blackcurrant pomace. Regarding the ash content, the highest was found in dried blackberry pomace (1.59 g/100 g dw), probably also as a consequence of the higher seed content of this by-product. Pasquel-Reátegui et al.[Citation53] reported also 1.62% ash content in blackberry bagasse.
The highest titratable acidity was found in the dried bilberry pomace (5.63 g citric acid/100 g dw), followed by the dried blackcurrant pomace (4.22 g citric acid/100 g dw). A higher titratable acidity was previously reported by Gagneten et al.[Citation35] (6.02 g citric acid/100 g) and by Reißner et al.[Citation56] (9 g citric acid/100 g) in blackcurrant pomace powder. The lowest titratable acidity was found in dried blackberry pomace (2.18 g citric acid/100 g dw), while Zafra-Rojas et al.[Citation57] reported 5.7 g malic acid/100 g dw in Mexican blackberry (Rubus fruticosus) residues.
Fatty acids profile
The berry pomaces have a high content of seeds and, as a result, they are a source of lipophilic compounds. A significant variation of the fatty acid profile has been reported in previous studies even within the same genus of a berry plant.[Citation55] presents the fatty acid profile of the berry pomace powders from our study. The results showed that they are rich in monounsaturated (MUFA) (16.74–18.7%) but especially in polyunsaturated (PUFA) fatty acids (72.25–77.36%). However, a number of significant differences in the fatty acid profile can be noted. The lowest SFA content was found in blackcurrant pomace (5.43%) and the highest in blackberry pomace (9.04%).
Table 2. Fatty acids profile of dried bilberry, blackberry, and blackcurrant pomace (% of total fatty acids).
The dominant fatty acid in the oil of the bilberry pomace powder was linolenic acid (C18:3n−3) while linoleic acid (C18:2n−6) was the dominant fatty acid in blackberry and blackcurrant pomaces. The contents of linoleic and linolenic acids found in blackberry pomace in the present study (58.12% and 12.58%, respectively) were in good compliance with the results previously reported by Van Hoed et al.[Citation58] (61.22% and 17.60%, respectively) or by Piasecka et al.[Citation59] (62.53% and 16.19%, respectively) in the cold pressed blackberry seed oil. Rasheed et al.[Citation27] reported also 57.92% linoleic acid and 15.14% linolenic acid in blackberry seed oil.
In the blackcurrant pomace, linoleic acid was the dominant fatty acid (35.69%), closely followed by linolenic acid (31.52%). Previously, Bada et al.[Citation60] found 41.41% linoleic acid in the blackcurrant seed oil, while Dobson et al.[Citation61] reported 45.6% linoleic acid, but only 12.6% linolenic acid in the blackcurrant pomace. Basegmez et al.[Citation36] found also that linoleic acid was the dominant fatty acid in the blackcurrant pomace oil recovered by different extraction methods (46.89–47.21%), followed by γ-linolenic (14.02–14.08%), linolenic (13.80–13.88%), and oleic (11.84–11.79%) acids.
It should be noted that the blackcurrant pomace was the only one that contained some long-chain polyunsaturated fatty acids, including 7.1% docosadienoic acid (C22:2n−6), 1.78% docosahexaenoic acid (C22:6n−3) and 0.28% docosapentaenoic acid (C22:5n−3). Eicosadienoic acid was previously reported in blackcurrant pomace in the range from 0.06% to 0.3%[Citation60,Citation61] and 0.15% in blackberry pomace,[Citation60] in good agreement with our results (0.17% and 0.18% in blackcurrant and blackberry pomace, respectively). Dried blackcurrant pomace recorded the highest PUFA content (77.36%) and the lowest MUFA content (16.74%). Dobson et al.[Citation61] previously reported 72% PUFA and 11.7% MUFA in blackcurrant pomace, however, it was previously established that the fatty acid composition may depend on the plant cultivar.[Citation36]
The fatty acid profile of blackberry pomace (72.25% PUFA, 18.09% MUFA, and 9.04% SFA) was in good agreement with the findings of Radočaj et al.[Citation62] in blackberry pomace dried at room temperature (74.94% PUFA, 17.87% MUFA, and 7.13% SFA) or by Wajs-Bonikowska et al.[Citation63] (71.4% PUFA, 17.5% MUFA, and 11.1% SFA). In the bilberry pomace, it was found the highest content of linolenic acid of the three by-products (38.11%). Pires et al.[Citation52] previously reported 32.9% linolenic acid in dried bilberry fruits, however, Dulf et al.[Citation64] found only 18.7% linolenic acid in wild blueberry pomace.
Dried bilberry pomace contained 72.93% PUFA, 18.7% MUFA, and 8.08% SFA, close to the results previously reported by Pires et al.[Citation52] in dried bilberry fruits (75.3% PUFA, 16% MUFA, and 8.8% SFA). No significant differences were found between the total PUFAs content in the dried pomaces of the three species. However, the results revealed significant differences regarding the content of n−3 PUFA and n−6 PUFA. The highest content of n−3 PUFA was found in bilberry pomace, followed by the blackcurrant pomace, while the highest n−6 PUFA was found in dried blackberry pomace. As a result, the highest n−6/n−3 ratio was calculated in blackberry pomace (4.26), while very low values of this ratio were found for bilberry (0.90) and blackcurrant (1.28) pomaces. Van Hoed et al.[Citation58] reported also the highest n−6/n−3 ratio (3.58) in the cold-pressed oil from blackberry compared to the seed oils from other berries (e.g. n−6/n−3 ratio = 1.50 in blueberry seed oil). Other studies reported also that the n−6/n−3 ratio was close to 1 in the seed oils of bilberry, while in blackberry and blackcurrant seeds it ranged from 2.7 to 3.6.[Citation65–67]
It is well known that the recent dietary guidelines have promoted the replacement of saturated fatty acid intake with unsaturated fatty acids, especially polyunsaturated fatty acids.[Citation68,Citation69] Besides, numerous biochemical, epidemiological, and clinical studies have proved that the increased dietary consumption of n−6 PUFAs along with lower intake of n−3 PUFAs induces a proinflammatory response, which may cause deleterious effects on health, such as the development of many chronic inflammatory diseases, cardiovascular diseases, autoimmune diseases, and cancers.[Citation70] Therefore, reducing the n−6/n−3 ratio in the human diet by increasing n−3 PUFA and reducing n−6 PUFA intake is suggested as beneficial for human health. This raises a challenge to the food industry for developing new products with an improved fatty acid profile. Besides supplementation or enrichment with n−3 PUFAs, other strategies such as the incorporation of natural ingredients rich in n−3 PUFAs holds great promise to achieve this goal.
Total phenolic, anthocyanin, and flavonoid content and antioxidant activity
The berry skins, along with the seeds, are important components of the berry pomaces. Since anthocyanins and most phenolic compounds are mainly found in the skins,[Citation71] berry pomaces retain various phenolic compounds with high antioxidant potential, especially high levels of anthocyanins, that possess strong antimicrobial, anti-inflammatory, and anti-mutagenic properties.[Citation72] The extraction of these compounds from berry pomaces allows for obtaining natural colorants and bioactive ingredients for the food and pharmaceutical industries. shows the total phenolic content, total flavonoid content, total anthocyanin content, DPPH radical scavenging activity, and ABTS antioxidant activity of dried bilberry, blackberry, and blackcurrant pomaces.
Table 3. Total phenolic content, total flavonoid content, total anthocyanin content, DPPH radical scavenging activity, and ABTS antioxidant activity of dried bilberry, blackberry, and blackcurrant pomace.
The highest total phenolic content was found in the dried bilberry pomace (36.7 mg GAE/g dw) followed by the dried blackcurrant pomace (26.2 mg GAE/g dw). In good agreement with our results, Sójka and Król[Citation73] reported a TPC content in the range of 18.55–22.41 mg EE/g in seedless blackcurrant pomace determined also using the Folin – Ciocalteu method, Basegmez et al.[Citation36] found 24.34 mg GAE/g in blackcurrant pomace while Gagneten et al.[Citation35] reported a total phenolic content of 37.5 mg GAE/g dw in the dehydrated blackcurrant by-product of juice production. Michalska et al.[Citation39] found a total phenolic content of 30.13 mg/g dw in fresh blackcurrant pomace, but only 5.36 mg/g dw in the same product dried at 60°C. Regarding bilberry pomace, Nemetz et al.[Citation50] found around 30 mg/g dw total phenolic content in bilberry pomace powder while Bobinaitė et al.[Citation71] reported between 10 and 18 mg GAE/g in the extracts from blueberry press cake. The lowest TPC content was found in the dried blackberry pomace (14.4 mg GAE/g dw). Jara-Palacios et al.[Citation74] reported a total phenolic content of 16.99 mg GAE/g dw in blackberry pomace while Kalušević et al.[Citation29] found 10.1 mg GAE/g in a similar product. However, Tarasevičienė et al.[Citation32] found only 4.31 mg GAE/g dw total phenolic content in blackberry pomace. Jazić et al.,[Citation75] in a study on polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace, found a total phenolic content of 50.16 mg GAE/g in the pomace of wild blackberry and only 26.30 to 35.40 mg GAE/g dw in the pomace of the cultivated blackberries. They established that wild blackberry varieties had higher total phenolic contents compared to cultivated blackberries.
Many studies previously concluded that the anthocyanin and other secondary metabolite content of the berry pomaces are largely influenced by the berries species, varieties, environmental factors, agricultural practices, as well as by the juice production process, and by the pomace drying conditions.[Citation22,Citation32]
The anthocyanin content determined by the pH differential method was 28.35, 3.68, and 1.86 mg CGE/g dw in dried bilberry, blackcurrant, and blackberry pomace, respectively. Previously, Sójka and Król[Citation73] reported anthocyanins content between 3.44 and 10.46 mg/g in blackcurrant pomace depending on the year of the fruit harvest, Gagneten et al.[Citation35] reported 18.0 mg cyanidin−3-glucoside/g dw in the dehydrated blackcurrant by-product of juice production while Nemetz et al.[Citation50] reported 19.66 mg CGE/g dw in bilberry pomace. However, Varo et al.[Citation76] found an anthocyanin content of only 6.5 mg/g dw in bilberry press residues while Kalušević et al.[Citation29] reported 6 mg/g in blackberry pomace. He et al.[Citation77] found 16.03 mg GAE/g and 4.19 mg CGE/g in blueberry wine pomace for total phenolic content and total anthocyanin content, respectively.
The lowest antioxidant activity was found in dried blackberry pomace (19.2 μmol TE/g dw), which was expected considering the lower content of anthocyanins and other phenolic compounds of blackberry pomace compared to the other two by-products. Machado et al.[Citation78] reported DPPH antioxidant activities between 12.25 and 76.03 μmol TE/g and ABTS antioxidant activities between 21.26 and 68.28 μmol TE/g in the extracts obtained from blackberry pomace through various extraction methods. In the blackcurrant seed press residue, Helbig et al.[Citation65] reported a Trolox equivalent antioxidant capacity of 74.7 and 67.2 μmol/g dry extract in n-hexane and distilled water, respectively. No significant differences (p < .05) were found between the antioxidant activity of dried bilberry and blackcurrant pomaces measured both as DPPH radical scavenging activity and ABTS. The previously reported results for antioxidant activity of the same by-products revealed a large variability that may be attributed to the method used, solvent, pretreatments, and extraction conditions, as well as to the manner of reporting the results.[Citation53,Citation78]
Polyphenolic profile
presents the results of the quantification of phenolic compounds identified in the dried bilberry, blackberry, and blackcurrant pomaces. Chromatograms of the phenolic compounds at 280 nm and 320 nm from bilberry, blackberry, and blackcurrant pomaces are shown in .
Figure 1. Chromatogram of the phenolic compounds at 280 nm from dried bilberry pomace. Peaks identification: 6 - gallic acid, 9 - protocatechuic acid, 10 - procyanidin B1, 14 - catechin, 18 - (–)-epicatechin, 19 - caffeic acid, 20 - syringic acid, 24 - procyanidin A1, 28 - p-coumaric acid, 29 - ellagic acid, 31 - sinapic acid, 32 - quercetin 3-glucoside, 36 - naringin, 1–5, 7, 8, 11–13, 15–17, 21–23, 25–27, 30, 33–35, 37–42 - unidentified compounds.
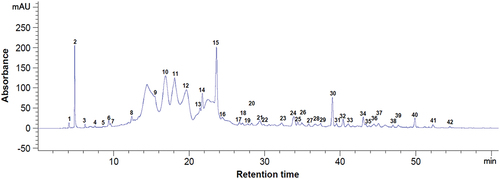
Figure 2. Chromatogram of the phenolic compounds at 320 nm from dried bilberry pomace. Peaks identification: 31 - p-coumaric acid, 35 - quercetin 3-glucoside, 42 - myricetin, 47 - quercetin, 1–30, 32–34, 36–41, 43–46 - unidentified compounds.
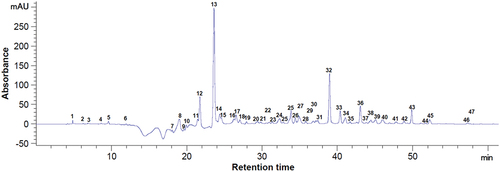
Figure 3. Chromatogram of the phenolic compounds at 280 nm from dried blackcurrant pomace. Peaks identification: 12 - protocatechuic acid, 13 - procyanidin B1, 15 - epigallocatechin, 18 - catechin, 20 - chlorogenic acid, 22 - (–) - epicatechin, 23 - caffeic acid, 24 - syringic acid, 27 - procyanidin A1, 32 - p-coumaric acid, 34 - ellagic acid, 36 - quercetin 3-D-galactoside, 37 - quercetin 3-β-D-glucoside, 42 - hesperidin, 50 - luteolin, 54 - kaempferol, 1–11, 14, 16, 17, 19, 21, 25, 26, 28–31, 33, 35, 38–41, 43–49, 51–53, 55, 56 - unidentified compounds.
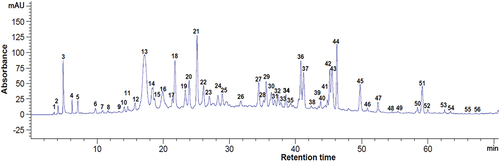
Figure 4. Chromatogram of the phenolic compounds at 320 nm from dried blackcurrant pomace. Peaks identification: 9 - chlorogenic acid, 13 - caffeic acid, 20 - p-coumaric acid, 24 - quercetin 3-glucoside, 28 - hesperidin, 36 - quercetin, 39 - trans-cinnamic acid, 42 - kaempferol, 1–8, 10–12, 14–19, 21–23, 25–27, 29–35, 37, 38, 40, 41, 43 - unidentified compounds.
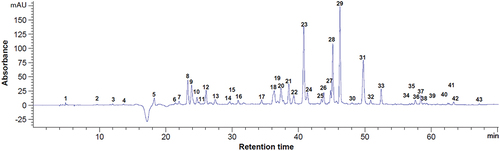
Figure 5. Chromatogram of the phenolic compounds at 280 nm from dried blackberry pomace. Peaks identification: 16 - procyanidin B1, 18 - epigallocatechin, 21 - catechin, 24 - chlorogenic acid, 27 - vanillic acid, 28 - caffeic acid, 29 - syringic acid, 30 - procyanidin C1, 35 - procyanidin A1, 40 - p-coumaric acid, 49 - naringin, 50 - hesperidin, 1–15, 17, 19, 20, 22, 23, 25, 26, 31–34, 36–39, 41–48 - unidentified compounds.
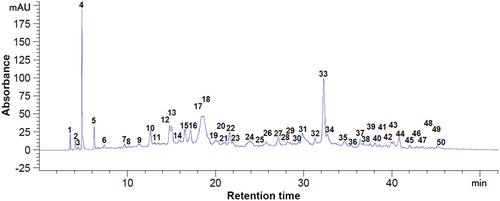
Figure 6. Chromatogram of the phenolic compounds at 320 nm from dried blackberry pomace. Peaks identification: 13 - caffeic acid, 17 - p-coumaric acid, 21 - quercetin 3-glucoside, 23 - hesperidin, 1–12, 14–16, 18–20, 22 - unidentified compounds.
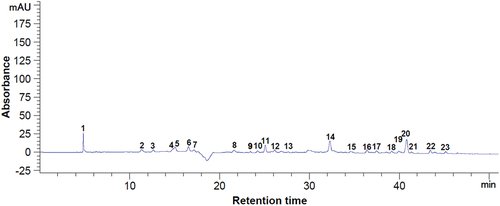
Table 4. Phenolic compounds in the dried bilberry, blackberry, and blackcurrant pomaces.
The phenolic profile of the bilberry pomace was dominated by ellagic acid (13.60 mg/g) followed by catechin (10.86 mg/g) while in blackberry pomace the major phenolic compound was epigallocatechin (22.70 mg/g dw) followed by chlorogenic acid (1.89 mg/g) and catechin (0.97 mg/g). Gođevac et al.[Citation67] identified 47 ellagitannins, 10 ellagic acid derivatives, 4 gallic acid derivatives, and traces of protocatechuic, chlorogenic, and salicylic acids in blackberry seed extracts from three cultivars. Ayoub et al.[Citation79] identified and quantified five phenolic acids in blackberry and blueberry seed meals by HPLC-DAD-ESI-MSn analysis, i.e protocatechuic acid, p-coumaric acid, gallic acid, caffeic acid, and syringic acids. Catechin, epicatechin, quercetin, epigallocatechin, and procyanidin dimer B1 were also identified in berry seed meals. Ayoub et al.[Citation79] found that procyanidins (B1–B4) gave the highest contribution of total flavonoids in blueberries and were the second most abundant compounds in blackberry seed meals.
Procyanidins A1 and B1 have been also quantified in our study in all three berry pomaces, with important levels of procyanidin B1 in bilberry (3.91 mg/g) and blackcurrant (3.56 mg/g) pomaces. Metzner Ungureanu et al.[Citation30] recorded the polyphenolic compounds profile of blackberry by-products and the results of the HPLC analysis showed that the main phenolic acids identified were gallic acid, rosmarinic acid, vanillic acid, syringic acid, caffeic acid, and p-coumaric acid. In addition, catechin and rutin, and in very small amounts quercetin and kaempferol, have also been detected in blackberry by-products. In agreement with our results, catechin was the main flavonol quantified in blackberry by-products.
Color
The color coordinates of the berry pomace powders and their variation during storage are of interest because the berry fruit by-products or their extracts may be used as a source of natural antioxidant pigments in food products.[Citation38,Citation41,Citation50] In the present study, all the values of the color coordinates differed significantly in the three-berry pomace powders ().
Table 5. Color parameters (L*—lightness, a*—redness and b*—yellowness) of dried bilberry, blackberry, and blackcurrant pomace.
Conclusion
Bilberry, blackberry, and blackcurrant pomaces, obtained as by-products of juice processing, are rich and economical sources of nutrients as well as biologically active compounds. Due to their high content of seeds, berry pomaces are a source of oils with a unique fatty acid profile, rich in monounsaturated and polyunsaturated fatty acids. Although the three studied by-products did not differ significantly regarding the total PUFA content, bilberry pomace was the richest in n−3 PUFAs followed by blackcurrant pomace. The red-colored powders obtained after drying and milling of the studied berry pomaces contain considerable amounts of anthocyanins, which make them a potential source of components with colorant properties, able to replace synthetic additives. They are also of interest due to their high content of phenolic compounds, with high antioxidant activity, which has been associated with health benefits. Due to their healthy lipid profile and richness in antioxidant compounds, berry pomaces are good candidates as food ingredients for enhancing the functionality of food products and for contributing to the sustainable development of the food industry. Future studies must focus on the development of new processing technologies allowing the conversion of berry processing by-products into valuable ingredients and additives in order to reduce their environmental impact and to develop new high-value-added foods meeting consumer demands for healthier, all-natural products.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contribution
Nour Violeta – Investigation, Writing original draft, Review and editing, Data curation, Visualization, Resources, and Project administration
Blejan Ana Maria – Formal analysis, Investigation, Writing original draft, Data curation, Validation
Bogdan Păcularu-Burada – Formal analysis, Investigation, Data curation
Popescu Simona Mariana – Writing original draft, Visualization, Supervision
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dawkins, E.; André, K.; Axelsson, K.; Benoist, L.; Swartling, Å. G.; Persson, Å. Advancing Sustainable Consumption at the Local Government Level: A Literature Review. J. Clean. Prod. 2019, 231, 1450–1462. DOI: 10.1016/j.jclepro.2019.05.176.
- Azapagic, A.; Stamford, L.; Youds, L.; Barteczko-Hibbert, C. Towards Sustainable Production and Consumption: A Novel DEcision-Support Framework IntegRating Economic, Environmental and Social Sustainability (DESIRES). Comput. Chem. Eng. 2016, 91, 93–103. DOI: 10.1016/j.compchemeng.2016.03.017.
- George, T. E.; Karatu, K.; Edward, A. An Evaluation of the Environmental Impact Assessment Practice in Uganda: Challenges and Opportunities for Achieving Sustainable Development. Heliyon. 2020, 6(9), e04758. DOI: 10.1016/j.heliyon.2020.e04758.
- Arruda, H. S.; Silva, E. K.; Peixoto Araujo, N. M.; Pereira, G. A.; Pastore, G. M.; Marostica Junior, M. R. Anthocyanins Recovered from Agri-Food By-Products Using Innovative Processes: Trends, Challenges, and Perspectives for Their Application in Food Systems. Molecules. 2021, 26(9), 2632. DOI: 10.3390/molecules26092632.
- Marcillo-Parra, V.; Tupuna–Yerovi, D. S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By–Products for Food Application—A Review. Trends Food Sci. Technol. 2021, 116, 11–23. DOI: 10.1016/j.tifs.2021.07.009.
- Patra, A.; Abdullah, S.; Pradhan, R. C. Review on the Extraction of Bioactive Compounds and Characterization of Fruit Industry By-Products. Bioresour. Bioprocess. 2022, 9(1), 14. DOI: 10.1186/s40643-022-00498-3.
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibers and Its Potential Application as Functional Ingredients. Bioact. Carbohydr. Diet. Fibre. 2022, 27, 100295. DOI: 10.1016/j.bcdf.2021.100295.
- Campos, D. A.; Gómez-García, R.; Vilas-Boas, A. A.; Madureira, A. R.; Pintado, M. M. Management of Fruit Industrial By-Products—a Case Study on Circular Economy Approach. Molecules. 2020, 25(2), 320. DOI: 10.3390/molecules25020320.
- Mohd Basri, M. S.; Abdul Karim Shah, N. N.; Sulaiman, A.; Mohamed Amin Tawakkal, I. S.; Mohd nor, M. Z.; Ariffin, S. H.; Abdul Ghani, N. H.; Mohd Salleh, F. S. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers. 2021, 13(20), 3503. DOI: 10.3390/polym13203503.
- Ben-Othman, S.; Joudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules. 2020, 25(3), 510. DOI: 10.3390/molecules25030510.
- Majerska, J.; Michalska, A.; Figiel, A. A Review of New Directions in Managing Fruit and Vegetable Processing By-Products. Trends Food Sci. Technol. 2019, 88, 207–219. DOI: 10.1016/j.tifs.2019.03.021.
- Fierascu, R. C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products–a Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8. DOI: 10.3389/fbioe.2020.00319.
- Lianza, M.; Marincich, L.; Antognoni, F. The Greening of Anthocyanins: Eco-Friendly Techniques for Their Recovery from Agri-Food By-Products. Antioxidants. 2022, 11(11), 2169. DOI: 10.3390/antiox11112169.
- Adami, R.; Salvo, G.; Meneses, M.; Järvenpää, E.; Huopalahti, R.; Sesti Osséo, L.; Reverchon, E. Innovative Treatment of Bilberry By-Products for a Selective Recovery of Anthocyanin Compounds. In Proceedings of the 10th Conference on Supercritical Fluids and Their Applications, Naples, Italy, 29 April–6 May 2013.
- Aura, A. M.; Holopainen-Mantila, U.; Sibakov, J.; Kössö, T.; Mokkila, M.; Kaisa, P. Bilberry and Bilberry Press Cake as Sources of Dietary Fibre. Food Nutr. Res. 2015, 59(1), 28367. DOI: 10.3402/fnr.v59.28367.
- Dabbou, S.; Ferrocino, I.; Kovitvadhi, A.; Dabbou, S.; Bergagna, S.; Dezzuto, D.; Schiavone, A.; Cocolin, L.; Gai, F.; Santoro, V., et al. Bilberry Pomace in Rabbit Nutrition: Effects on Growth Performance, Apparent Digestibility, Caecal Traits, Bacterial Community and Antioxidant Status. Animal. 2019, 13(1), 53–63.
- Zhang, G.; Dai, X. Antiaging Effect of Anthocyanin Extracts from Bilberry on Natural or UV-Treated Male Drosophila Melanogaster. Curr. Res. Food Sci. 2022, 5, 1640–1648. DOI: 10.1016/j.crfs.2022.09.015.
- Alnajjar, M.; Barik, S. K.; Bestwick, C.; Campbell, F.; Cruickshank, M.; Farquharson, F.; Holtrop, G.; Horgan, G.; Louis, P.; Moar, K.-M.; et al. Anthocyanin-Enriched Bilberry Extract Attenuates Glycaemic Response in Overweight Volunteers without Changes in Insulin. J. Funct. Foods. 2020, 64, 103597. DOI: 10.1016/j.jff.2019.103597.
- Milenkovic, D.; Krga, I.; Dinel, A.; Morand, C.; Laye, S.; Castanon, N. Nutrigenomic Modification Induced by Anthocyanin-Rich Bilberry Extract in the Hippocampus of ApoE-/- Mice. J. Funct. Foods. 2021, 85, 104609. DOI: 10.1016/j.jff.2021.104609.
- Lavecchia, R.; Medici, F.; Piga, L.; Zuorro, A. Factorial Design Analysis of the Recovery of Flavonoids from Bilberry Fruit By-Products. Int. J. Appl. Eng. Res. 2015, 23, 43555–43559.
- Svanberg, L.; Malmberg, K.; Gustinelli, G.; Öhgren, C.; Persson, I.; Brive, L.; Wassén, S. Effect of Anthocyanins on Lipid Oxidation and Microbial Spoilage in Value-Added Emulsions with Bilberry Seed Oil, Anthocyanins and Cold Set Whey Protein Hydrogels. Food Chem. 2019, 272, 273–278. DOI: 10.1016/j.foodchem.2018.06.064.
- Syrpas, M.; Valanciene, E.; Augustiniene, E.; Malys, N. Valorization of Bilberry (Vaccinium Myrtillus L.) Pomace by Enzyme-Assisted Extraction: Process Optimization and Comparison with Conventional Solid-Liquid Extraction. Antioxidants. 2021, 10(5), 773. DOI: 10.3390/antiox10050773.
- Zhou, L.; Lie, Y.; Briers, H.; Fan, J.; Remón, J.; Nyström, J.; Budarin, V.; Macquarrie, D.; McElroy, C. R. Natural Product Recovery from Bilberry (Vaccinium Myrtillus L.) Presscake via Microwave Hydrolysis. ACS Sustain. Chem. Eng. 2018, 6(3), 3676–3685. DOI: 10.1021/acssuschemeng.7b03999.
- Zannou, O.; Koca, I. Greener Extraction of Anthocyanins and Antioxidant Activity from Blackberry (Rubus Spp) Using Natural Deep Eutectic Solvents. LWT. 2022, 158, 113184. DOI: 10.1016/j.lwt.2022.113184.
- Schulz, M.; Seraglio, S. K. T.; Della Betta, F.; Nehring, P.; Valese, A. C.; Daguer, H.; Gonzaga, L. V.; Costa, A. C. O.; Fett, R. Blackberry (Rubus Ulmifolius Schott): Chemical Composition, Phenolic Compounds and Antioxidant Capacity in Two Edible Stages. Food. Res. Int. 2019, 122, 627–634. DOI: 10.1016/j.foodres.2019.01.034.
- Kaume, L.; Howard, L. R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food. Chem. 2012, 60(23), 5716–5727. DOI: 10.1021/jf203318p.
- Rasheed, H. U.; Nawaz, H.; Rehman, R.; Mushtaq, A.; Rashid, U. The Blackberry: A Review on Its Composition and Chemistry, Uses and Bioavailability and Potential Health Benefits. Int. J. Chem. Biochem. Sci. 2017, 11, 120–128. DOI: 10.1021/jf203318p.
- Moraes, D. P.; Chim, J. F.; Barin, J. S.; Vizzotto, M.; Farias, C. A. A.; Ballus, C. A.; Barcia, M. T. Influence of the Cultivar on the Composition of Blackberry (Rubus Spp.) Minerals. J. Food Compos. Anal. 2021, 100, 103913. DOI: 10.1016/j.jfca.2021.103913.
- Kalušević, A.; Salević, A.; Djordjević, R.; Veljović, M.; Nedović, V. Raspberry and Blackberry Pomaces as Potential Sources of Bioactive Compounds. Ukr. Food J. 2016, 5(3), 485–491. DOI: 10.24263/2304-974X-2016-5-3-7.
- Metzner Ungureanu, C.-R.; Lupitu, A. I.; Moisa, C.; Rivis, A.; Copolovici, L. O.; Poiana, M.-A. Investigation on High-Value Bioactive Compounds and Antioxidant Properties of Blackberries and Their Fractions Obtained by Home-Scale Juice Processing. Sustainability. 2020, 12(14), 5681. DOI: 10.3390/su12145681.
- Isopencu, G. O.; Stoica-Guzun, A.; Busuioc, C.; Stroescu, M.; Deleanu, I. M. Development of Antioxidant and Antimicrobial Edible Coatings Incorporating Bacterial Cellulose, Pectin, and Blackberry Pomace. Carbohydr. Polym. Technol Appl. 2021, 2, 100057. DOI: 10.1016/j.carpta.2021.100057.
- Tarasevičienė, Ž.; Čechovičiene, I.; Paulauskienė, A.; Gumbytė, M.; Blinstrubienė, A.; Burbulis, N. The Effect of Berry Pomace on Quality Changes of Beef Patties During Refrigerated Storage. Foods. 2022, 11(15), 2180. DOI: 10.3390/foods11152180.
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic Acylation of Blackcurrant (Ribes nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2019, 281, 189–196. DOI: 10.1016/j.foodchem.2018.12.111.
- Jurčaga, L.; Bobko, M.; Kolesárová, A.; Bobková, A.; Demianová, A.; Haščík, P.; Belej, Ľ.; Mendelová, A.; Bučko, O.; Kročko, M., et al. Blackcurrant (Ribes Nigrum L.) and Kamchatka Honeysuckle (Lonicera Caerulea Var. Kamtschatica) Extract Effects on Technological Properties, Sensory Quality, and Lipid Oxidation of Raw-Cooked Meat Product (Frankfurters). Foods. 2021, 10(12), 2957.
- Gagneten, M.; Archaina, D. A.; Salas, M. P.; Leiva, G. E.; Salvatori, D. M.; Schebor, C. Gluten-Free Cookies Added with Fibre and Bioactive Compounds from Blackcurrant Residue. Int. J. Food Sci. Technol. 2021, 56(4), 1734–1740. DOI: 10.1111/ijfs.14798.
- Basegmez, H. I. O.; Povilaitis, D.; Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Alasalvar, C.; Venskutonis, P. R. Biorefining of Blackcurrant Pomace into High-Value Functional Ingredients Using Supercritical CO2, Pressurized Liquid and Enzyme Assisted Extractions. J. Supercrit Fluids. 2017, 124, 10–19. DOI: 10.1016/j.supflu.2017.01.003.
- Xue, B.; Hui, X.; Chen, X.; Luo, S.; Dilrukshi, H. N. N.; Wu, G.; Chen, C. Application, Emerging Health Benefits, and Dosage Effects of Blackcurrant Food Formats. J. Funct. Foods. 2022, 95, 105147. DOI: 10.1016/j.jff.2022.105147.
- Mäkilä, L.; Laaksonen, O.; Diaz, J. M. R.; Vahvaselkä, M.; Myllymäki, O.; Lehtomäki, I.; Laakso, S.; Jahreis, G.; Jouppila, K.; Larmo, P. Exploiting Blackcurrant Juice Press Residue in Extruded Snacks. LWT-Food Sci. Technol. 2014, 57(2), 618–627. DOI: 10.1016/j.lwt.2014.02.005.
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.; Figiel, A. Effect of Different Drying Techniques on Physical Properties, Total Polyphenols and Antioxidant Capacity of Blackcurrant Pomace Powders. LWT. 2017, 78, 114–121. DOI: 10.1016/j.lwt.2016.12.008.
- Lorenzo, J. M.; Pateiro, M.; Domínguez, R.; Barba, F. J.; Putnik, P.; Bursać Kovačević, D.; Shpigelman, A.; Granato, D.; Franco, D. Berries Extracts as Natural Antioxidants in Meat Products: A Review. Food. Res. Int. 2018, 106, 1095–1104. DOI: 10.1016/j.foodres.2017.12.005.
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F.; Galić, K. Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules. 2021, 26(9), 2569. DOI: 10.3390/molecules26092569.
- Alchera, F.; Ginepro, M.; Giacalone, G. Microwave-Assisted Extraction of Polyphenols from Blackcurrant By-Products and Possible Uses of the Extracts in Active Packaging. Foods. 2022, 11(18), 2727. DOI: 10.3390/foods11182727.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants Using Folin-Ciocalteau Reagent. Methods Enzymol. 1999, 299, 152–178. DOI: 10.1016/S0076-6879(99)99017-1.
- Giusti, M. M.; Wrolstad, R. E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry, Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds. John Wiley & Sons, Inc.: New York, USA, 2001; F1.2.1–F1.2.13.
- Mohammadzadeh, S.; Sharriatpanahi, M.; Hamedi, M.; Amanzadeh, Y.; Sadat Ebrahimi, S. E.; Ostad, S. N. Antioxidant Power of Iranian Propolis Extract. Food Chem. 2007, 103(3), 729–733. DOI: 10.1016/j.foodchem.2006.09.014.
- Oliveira, I.; Sousa, A.; Ferreira, I. C. F. R.; Bento, A.; Estevinho, L.; Pereira, J. A. Total Phenols, Antioxidant Potential and Antimicrobial Activity of Walnut (Juglans Regia L.) Green Husks. Food. Chem. Toxicol. 2008, 46(7), 2326–2331. DOI: 10.1016/j.fct.2008.03.017.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med. 1999, 26(9–10), 1231–1237. DOI: 10.1016//S0891-5849(98)00315-3.
- Vlad, C. C.; Păcularu-Burada, B.; Vasile, A. M.; Milea, Ș. A.; Bahrim, G. E.; Râpeanu, G.; Stănciuc, N. Upgrading the Functional Potential of Apple Pomace in Value-Added Ingredients with Probiotics. Antioxidants 2022, 11(10), 2028. DOI: 10.3390/antiox11102028
- Liao, X.; Greenspan, P.; Pegg, R. B. Examining the Performance of Two Extraction Solvent Systems on Phenolic Constituents from U.S. Southeastern Blackberries. Molecules. 2021, 26(13), 4001. DOI: 10.3390/molecules26134001.
- Nemetz, N. J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules. 2021, 26(9), 2689. DOI: 10.3390/molecules26092689.
- Górnás, P.; Juhņevića-Radenkova, K.; Radenkovs, V.; Mišina, I.; Pugajeva, I.; Soliven, A.; Segliņa, D. The Impact of Different Baking Conditions on the Stability of the Extractable Polyphenols in Muffins Enriched by Strawberry, Sour Cherry, Raspberry or Black Currant Pomace. LWT. Food Sci. Technol. 2016, 65, 946–953. DOI: 10.1016/j.lwt.2015.09.029.
- Pires, T. C. S. P.; Inês Dias, M.; Calhelha, R. C.; José Alves, M.; Santos-Buelga, C.; Ferreira, I. C. F. R.; Barros, L. Development of New Bilberry (Vaccinium Myrtillus L.) Based Snacks: Nutritional, Chemical and Bioactive Features. Food Chem. 2021, 334, 127511. DOI: 10.1016/j.foodchem.2020.127511.
- Pasquel-Reátegui, J. L.; Da Fonseca Machado, A. P.; Barbero, G. F.; Rezende, C. A.; Martinez, J. Extraction of Antioxidant Compounds from Blackberry (Rubus Sp.) Bagasse Using Supercritical CO2 Assisted by Ultrasound. J. Supercrit Fluids. 2014, 94, 223–233. DOI: 10.1016/j.supflu.2014.07.019.
- Alba, K.; Macnaughtan, W.; Laws, A.; Foster, T. J.; Campbell, G.; Kontogiorgos, V. Fractionation and Characterisation of Dietary Fibre from Blackcurrant Pomace. Food Hydrocoll. 2018, 81, 398–408. DOI: 10.1016/j.foodhyd.2018.03.023.
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—a Review. Appl. Sci. 2022, 12(3), 1734. DOI: 10.3390/app12031734.
- Reißner, A.-M.; Alhamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. J. Sci. Food Agric. 2019, 99(3), 1284–1293. DOI: 10.1002/jsfa.9302.
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanis, E.; Añorve-Morga, J.; Quintero-Lira, A.; Castañeda, A.; Ramírez-Moreno, E. Organic Acids, Antioxidants, and Dietary Fiber of Mexican Blackberry (Rubus fruticosus) Residues Cv. Tupy. J. Food Qual. 2018, 1, 5950761. DOI: 10.1155/2018/5950761.
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhe, R. Berry Seeds: A Source of Specialty Oils with High Content of Bioactives and Nutritional Value. J. Food Lipids. 2009, 16(1), 33–49. DOI: 10.1111/j.1745-4522.2009.01130.x.
- Piasecka, I.; Górska, A.; Ostrowska-Ligeza, E.; Kalisz, S. The Study of Thermal Properties of Blackberry, Chokeberry and Raspberry Seeds and Oils. Appl. Sci. 2021, 11(16), 7704. DOI: 10.3390/app11167704.
- Bada, J.; León-Camacho, M.; Copovi, P.; Alonso, L. Characterization of Berry and Currant Seed Oils from Asturias, Spain. Int. J. Food. Prop. 2014, 17(1), 77–85. DOI: 10.1080/10942912.2011.614369.
- Dobson, G.; Shrestha, M.; Hilz, H.; Karjalainen, R.; McDougall, G.; Stewart, D. Lipophilic Components in Black Currant Seed and Pomace Extracts. Eur. J. Lipid Sci. Technol. 2012, 114(5), 575–582. DOI: 10.1002/ejlt.201100313.
- Radočaj, O.; Vujasinović, V.; Dimić, E.; Basić, Z. Blackberry (Rubus Fruticosus L.) and Raspberry (Rubus idaeus L.) Seed Oils Extracted from Dried Press Pomace After Longterm Frozen Storage of Berries Can Be Used as Functional Food Ingredients. Eur. J. Lipid Sci. Technol. 2014, 116(8), 1015–1024. DOI: 10.1002/ejlt.201400014.
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A Comparative Study on Composition and Antioxidant Activities of Supercritical Carbon Dioxide, Hexane and Ethanol Extracts from Blackberry (Rubus fruticosus) Growing in Poland. J. Sci. Food Agric. 2017, 97(11), 3576–3583. DOI: 10.1002/jsfa.8216.
- Dulf, F. V.; Andrei, S.; Bunea, A.; Socaciu, C. Fatty Acid and Phytosterol Contents of Some Romanian Wild and Cultivated Berry Pomaces. Chem. Pap. 2012, 66(10), 925–934. DOI: 10.2478/s11696-012-0156-0.
- Helbig, D.; Böhm, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry Seed Press Residues and Their Valuable Ingredients with Special Regard to Black Currant Seed Press Residues. Food Chem. 2008, 111(4), 1043–1049. DOI: 10.1016/j.foodchem.2008.05.017.
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and Antioxidative Activities of Supercritical CO2-Extracted Oils from Seeds and Soft Parts of Northern Berries. Food. Res. Int. 2011, 44(7), 2009–2017. DOI: 10.1016/j.foodres.2011.02.025.
- Gođevac, D.; Tešević, V.; Vajs, V.; Milosavljević, S.; Stanković, M. Blackberry Seed Extracts and Isolated Polyphenolic Compounds Showing Protective Effect on Human Lymphocytes DNA. J. Food Sci. 2011, 76(7), C1039–C1043. DOI: 10.1111/j.1750-3841.2011.02305.x.
- Zhao, M.; Chiriboga, D.; Olendzki, B.; Xie, B.; Li, Y.; McGonigal, L. J.; Maldonado-Contreras, A.; Ma, Y. Substantial Increase in Compliance with Saturated Fatty Acid Intake Recommendations After One Year Following the American Heart Association Diet. Nutrients 2018, 10, 1486. DOI: 10.3390/nu10101486
- Lenighan, Y. M.; McNulty, B. A.; Roche, H. M. Dietary Fat Composition: Replacement of Saturated Fatty Acids with PUFA as a Public Health Strategy, with an Emphasis on Alpha-Linolenic Acid. Proc. Nutr. Soc. 2019, 78(2), 234–245. DOI: 10.1017/S0029665118002793.
- Mariamenatu, A. H.; Abdu, E. M.; Kostner, G. M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids. 2021, 2021, 1–15. DOI: 10.1155/2021/8848161.
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2015, 52(9), 5898–5905. DOI: 10.1007/s13197-014-1668-0.
- Zorenč, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in Berry Quality of Northern Highbush Blueberry (Vaccinium Corymbosum L.) During the Harvest Season. Turk. J. Agric. For. 2016, 40, 855–864. DOI: 10.3906/tar-1607-57.
- Sójka, M.; Król, B. Composition of Industrial Seedless Black Currant Pomace. Eur. Food Res. Technol. 2009, 228(4), 597–605. DOI: 10.1007/s00217-008-0968-x.
- Jara-Palacios, M. J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F. J.; Escudero-Gilete, M. L. Comparative Study of Red Berry Pomaces (Blueberry, Red Raspberry, Red Currant and Blackberry) as Source of Antioxidants and Pigments. Eur. Food Res. Technol. 2019, 245(1), 1–9. DOI: 10.1007/s00217-018-3135-z.
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic Composition, Antioxidant and Antiproliferative Effects of Wild and Cultivated Blackberries (Rubus Fruticosus L.) Pomace. Int. J. Food Sci. Technol. 2019, 54(1), 194–201. DOI: 10.1111/ijfs.13923.
- Varo, M. A.; Jacotet-Navarro, M.; Serratosa, M. P.; Mérida, J.; Fabiano-Tixier, A. S.; Bily, A.; Chemat, F. Green Ultrasound-Assisted Extraction of Antioxidant Phenolic Compounds Determined by High Performance Liquid Chromatography from Bilberry (Vaccinium Myrtillus L.) Juice By-Products. Waste Biomass Valoriz. 2019, 10(7), 1945–1955. DOI: 10.1007/s12649-018-0207-z.
- He, B.; Zhang, L.-L.; Yue, X.-Y.; Liang, J.; Jiang, J.; Gao, X.-L.; Yue, P.-X. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds and Anthocyanins from Blueberry (Vaccinium ashei) Wine Pomace. Food Chem. 2016, 204, 70–76. DOI: 10.1016/j.foodchem.2016.02.094.
- Machado, A. P. D. F.; Pasquel-Reátegui, J. L.; Barbero, G. F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Blackberry (Rubus Fruticosus L.) Residues: A Comparison with Conventional Methods. Food. Res. Int. 2015, 77, 675–683. DOI: 10.1016/j.foodres.2014.12.042.
- Ayoub, M.; de Camargo, A. C.; Shahidi, F. Antioxidants and Bioactivities of Free, Esterified and Insoluble-Bound Phenolics from Berry Seed Meals. Food Chem. 2016, 197, 221–232. DOI: 10.1016/j.foodchem.2015.10.107.
