?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to characterize the physicochemical, proximal, textural, and visual properties of plant-based meat (PBM) patties blended with natural pigments such as anthocyanin from black beans, Fe-chlorophyll from spinach, and edible red and purple pigments. A comparison was made between these plant-based pigments and the pigments derived from animal-based sources (control). The values of the color coordinates of the developed patties were broadly distributed (L* [lightness], a* [redness], and b* [yellowness] of raw PBMs: 34–53, 2–25, and 4–27; steamed: 29–42, 4–21, and 5–25; and cooked: 28–43, 5–19, and 9–21, respectively). The proximate chemical analysis revealed no (P > .05) significant difference between the PBMs and the control in terms of moisture (63.28–61.25), crude protein (20.98–19.17) crude fat (3.19–2.92) and ash content (3.80–1.71). Nevertheless, significant differences (P < .05) were observed in textural attributes among all samples (S1-S16) in terms of hardness, chewiness, and gumminess. For instance, hardness (6.39–3.72), chewiness (9.27–4.13), and gumminess (3.04–1.60). Conversely, springiness showed considerable variations (P < .05) in a majority of PBM samples except for S1 (dilute red) (3.06) and S4 (paprika) (3.06) than control (3.31). For DPPH radical scavenging activity of patties samples incorporated with, the S10 (Red Cabbage) (19.95), S13 (Grape skin Color) (24.35), S15 (Anthocyanin) (33.12), and S16 (Fe-pheophytin) (18.69) of PBMs had significantly higher activity (P < .05) than the control (5.14). To sum up, the inclusion of natural pigments in PBM resulted in improved overall properties without any deleterious effects.
Introduction
The increasing gap between the supply of and demand for meat products has magnified the need for PBM analogs as alternative and sustainable protein sources.[Citation1,Citation2] PBMs are better sources of nutrition since several evidence-based studies have reported numerous health benefits associated with the substitution of animal-based products, including reduced risk of development or exacerbation of heart disease, stroke, and type 2 diabetes.[Citation3] PBM substitutes have been developed based on textured vegetable protein (TVP), which mimics the fibrillar structure of animal-based meat muscles. TVP is produced by the extrusion and rehydration of proteins from soy, pea, and wheat.[Citation4,Citation5] Despite the optimization and development of technologies for PBM production, the resulting appearance, texture, and flavor of PBM products are still inadequate compared to traditional animal-based meat.[Citation6,Citation7] For instance, most PBM products do not have a color and visible appearance like those of traditional meat-based products, which hinders their economic viability.[Citation7–9]
The goal of PBM is to provide a meat-like experience for those who want to reduce their consumption of animal products for ethical, health, or environmental reasons. Some popular examples of PBM include burgers, sausages, and chicken nuggets produced from ingredients such as soy, pea protein, and mushrooms.[Citation10] As the world population is surging and the gap between the existing supply of meat and its future demand has progressively necessitated a generation of PBM products to overcome shortages of protein in upcoming years.[Citation2,Citation8] Despite optimization and development of technologies for PBM production, the resulting appearance, texture, and flavor of PBM products are still inadequate when compared to traditional animal-based meat.[Citation6,Citation7]
Nevertheless, synthetic food colorants have been widely used for decades. However, these colorants have been associated with numerous side effects and toxicity, allergic reactions, and neurocognitive effects.[Citation11,Citation12] In recent years, plant pigments, as natural colorants, have begun to be recognized as bioactive substances because of their potential health benefits, which has boosted their commercial demand.[Citation13] For instance, anthocyanin, carotenoids, curcuminoids, and betalain extracted from plant resources have been used as pigments in the food industry.[Citation14,Citation15] Similarly, chlorophyll is a pigment abundant in green vegetables and green plants and plays an important role in photosynthesis.[Citation16] Chlorophyll has an Mg2+ ion in the middle of the porphyrin ring structure that plays a role in the coloring and energy-absorption processes.[Citation17] The formation of pheophytin and other derivatives of chlorophyll is irreversible; however, pheophytin is treated with Cu and Zn ions to form a more attractive and stable residue.[Citation15] Moreover, traditional meat consists of myoglobin protein, which is a major pigment in fresh meat. However, during the cooking process, heating caused the denaturation of myoglobin to a dull brown color.[Citation18] In contrast to traditional meat, the color of PBM analogs is tended to be yellow due to the lack of myoglobin in TVP.[Citation19] Therefore this yellowish coloration of TVP and other plant-based ingredients leads to the possible rejection of PBM products among consumers choice of selection.[Citation20]
To overcome this challenge, food colorants and natural pigments can be used to mask undesirable features of PBM products, without affecting their quality and safety. For example, anthocyanin, carotenoids, curcuminoids, and betalain, extracted from plant resources, have been used as pigments in the food industry.[Citation14,Citation15] However, the incorporation of natural colorants into PBM is challenging because these pigments are very sensitive to heat, light, and oxygen. In addition, these pigments can negatively interact with other PBM components, producing undesirable colors and flavor.[Citation16] To date, only beet juice, carrot juice extract, and soy leghemoglobin have been incorporated into PBM.[Citation2,Citation7,Citation21,Citation22] In a series of events, our team developed PBM patties with a fundamental emphasis on taste, texture, and sensory attributes.[Citation22–26] Nonetheless, to validate this experiment, recently we extract and investigated the analytical aspect of natural pigments (leghemoglobin, anthocyanins, and chlorophyll) to be incorporated into our incoming PBM products.[Citation27] In the current study, we developed an ideal and sustainable PBM product patty with optimal physiochemical and textural, characteristics by incorporating pigments from anthocyanin from black beans, chlorophyll from spinach, and edible red and purple pigments. To the best of our knowledge, this is the first report describing the quality characteristics of PBM patties added with these pigments.
Materials and methods
Raw materials and extraction
The PBM generated in this study was composed of TVP isolate, soy protein isolate (ISP), and wheat protein isolate (IWP). The other ingredients are listed in . The natural pigments incorporated into PBMs were edible red and purple pigments (S1 = Dilute Red 1, S2 = Dilute Red 2, S3 = Red color CG2, S4 = Paprika, S5 = Monascus Color No.30, S6 = Red RR, S7 = Purple Grape, S8 = Cherry Red, S9 = Monascus Color 100, S10 = Red Cabbage (liquid), S11 = Red Cabbage 100, S12 = AF Beet Red 30, S13 = Grape skin Color, S14 = Red Color PB, S17 = Myoglobin (control) (ES food ingredient, Gunpo, Korea) and S15=anthocyanin and S16 =Fe-Chlorophyll extracted from black beans and spinach, respectively ().
Figure 1. Pigments extracted from natural sources. 30ml of all samples were prepared in a 50ml conical tube (Φ 3cm) each. The absorbance at 535 nm of all of them is adjusted to 0.700.

Table 1. Composition of PBM.
Chlorophyll extraction and synthesis of Fe-pheophytin
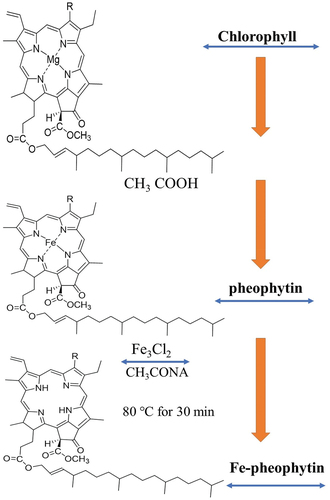
Chlorophyll was extracted from spinach (Spinacia oleracea; Gomgom, Seoul, Korea). Our previous work describes the extraction and synthesis of Fe-pheophytin in detail. ()[Citation27]
Anthocyanin extraction
Black bean was purchased from Morning Crops Co. (Gimpo, Korea) and anthocyanins were extracted from 500 g of black beans. Extraction was performed at RT (25°C) with a mass-solvent ratio of 1:10 w/v in 60% ethanol in distilled water (DW) and dispensed 0.1% acetic acid. The resulting mixture was stirred at 250 rpm for 4 h followed by sedimentation for 1 h. The supernatant was then recovered using a Whatman paper No. 1 filter. The sample was concentrated using a rotary evaporator to eliminate ethanol from the resulting extract. The water bath temperature used was 50°C. Finally, the concentrated extract was lyophilized and the resulting lyophilized powder was stored at − 80°C.[Citation28]
Sample preparation and processing
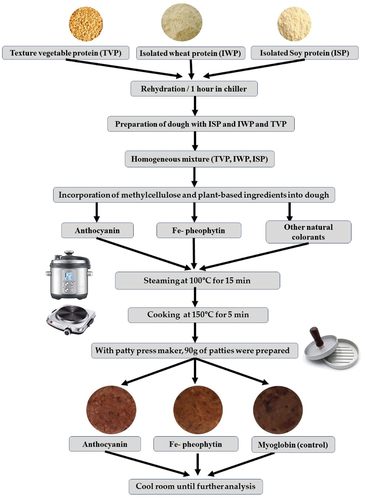
The flow diagram describes the series of events that take place during the formulation and development of PBM patties incorporated with natural pigments (). Edible red and purple pigments, anthocyanin, and Fe-chlorophyll were added to PBM at various mixing ratios and concentrations (). The pigment content was 0.05–1.75 g, and the amount of TVP added was adjusted according to the content of each pigment. A 60 mm × 15 mm cell culture plate (Co. SPL, Gyeonggi-do, Korea) was molded to the standard at 90 g ±0.05 g per sample. A batch of PBM patties was steamed at 100°C for 25 min using a steamer (Jjimgi Kitchenware, Seoul, Korea). Similarly, another batch of patties was cooked at 150°C for 5 min on both sides using a non-sticking pan (Co. Kitchen Art, Incheon, Korea). During the cooking process, the internal temperature of the patties was evaluated using a probe thermometer until it reached 75°C. The cooked PBM patty samples were allowed to cool at room temperature for 30 min. Subsequently, the visible physical, nutritional, physicochemical, and textural features were investigated.
Table 2. Pigment weight and concentration per colorant.
Visible appearance
To evaluate and analyze the visible appearance of the raw, steamed, and cooked patties, the high resolutions photographs of samples were captured by using a digital camera (EOS 700D, Canon, Tokyo, Japan), designating various features in detail.[Citation29]
Color measurements
The chromatic attributes of the uncooked, steamed, and cooked patties were determined using a Konica Minolta Colorimeter (CR−300; Minolta, Minolta, Osaka, Japan). Before measurement, a white plate (L = 95.09, a = 0.31, b = 0.34) was calibrated, and the brightness (L*), redness (a*), and yellowness (b*) of the samples were evaluated using the Hunter scale.[Citation30,Citation31]
where ∆E is total color differences, CIE (Commission International de l’Eclairage) is L*, a*, b*; L*: lightness, a*: redness, b*: yellowness.
Proximate chemical analysis
The proximate composition of the PBM was analyzed at Chungbuk National University (Chungcheongbuk-do, Korea). Approximately 100 g of PBM patty sample was used to determine the moisture content, crude fat, and ash content. The analysis method was based on that of the Association of Official Analytical Chemists. [.Citation32] Moisture content was determined using an air oven (OF−22, JEIO TECH, Daejun, Korea).[Citation33] In a pre-weighed aluminum dish, 10 g of sample were placed in a hot air oven at 105°C for 5 hours. Subsequently after cooling the sample was placed in a desiccator overnight to absorb any extra moisture. After that moisture content was calculated.
Crude protein was quantified using the Kjeldahl method.[Citation33] Accordingly, 1 g of the sample and 1 g of the decomposition accelerator (potassium sulfate: copper (II) sulfate pentahydrate = 9:1) were placed in a Kjeldahl flask, and 20 mL of 95% sulfuric acid was added. The mixture was decomposed for 3–4 h, allowed to cool for 1 h, decomposed in a 250 mL volumetric flask, and the sample was diluted. Subsequently, 10 mL of the diluted sample, 2 drops of the Brunswick indicator, and 10 mL of 30% sodium hydroxide were added during the distillation process, followed by distillation. Next, 100 mL was collected in a flask that contained 10 mL of 0.1N sulfuric acid and 4 drops of the Brunswick indicator and titrated with 0.1N sodium hydroxide. The crude protein was determined.
The crude fat percentage was determined using the Soxhlet extraction method for ether.[Citation34] A round bottom flask was weighed in a dry oven at 105°C, allowed cool in a desiccator, and re-weighed. Based on the Soxhlet procedure, 5-g PBM patty samples were weighed; the samples were kept in a cylinder with filter paper, the top was covered with cotton, and were placed into the Soxhlet extractor. After adding 300 mL of ether into a round-bottom flask, heating was started on a heating mantle connected to the flask, Soxhlet extractor, and a cooler, and heated overnight. After the heating process was completed, the remaining solvent in the flask was heated so that only the crude fat remained. It was then dried completely, the flask was weighed, and crude fat was calculated.
Ash content was determined according to the method reported by.[Citation35] The crucible was heated to 600°C or higher in an electric furnace and was then transferred to a desiccator, cooled, weighed, and heated again at a high temperature for 2 h. Subsequently, it was dried and weighed repeatedly until it reached a constant weight. After placing the sample in the crucible, it was heated in a furnace at 550–600°C for 2–3 h, transferred to a desiccator, cooled, and weighed.
Instrumental texture profile analysis (TPA)
Each PBM sample (30 g) was molded using a 60 mm × 15 mm cell culture plate (Co. SPL, Gyeonggi-do, Korea). The molded samples were steamed for 10 min using a steamer and then baked using an electric grill at 180°C and 3 mL of oil. The baked sample was molded into a block with a size of 10 mm x 10 mm x 10 mm, and the TPA experiment was performed. Experimental conditions were set to trigger load 0.1N, test speed 1 mm/s, and target value 50%, and the experiment was performed using a CT3 Texture Analyzer (Co. Brookfield, St. Ontario, Canada).[Citation36]
Absorbance levels
The absorbance of the anthocyanin, Fe-chlorophyll, and edible red and purple pigments was set to 0.7 based on 535 nm. Each sample was set at an absorbance of 0.7 at 535 nm, and absorbance was measured in the 400–700 nm range using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific, USA).[Citation37]
MW: Molecular weight of pigment, A: absorbance which is measured with a spectrophotometer, L∈: pathlength in cm (standard for 1 cm).
Analysis of 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH radical scavenging activity was determined based on the method established by.[Citation38] Accordingly, 1 mL of extraction solvent was added per 0.1 g of the sample, and extraction was performed at RT for 24 h; methanol was used as the extraction solvent. After extraction, the supernatant was centrifuged at 15,000 rpm for 10 min, transferred to a new tube, and analyzed. The 0.2 mM DPPH solution was prepared by mixing 2.2-diphenyl-picrylhydrazyl (Co. Sigma-Aldrich, St. Missouri, USA) with methanol. A total of 200 μL (40 μL of sample and 160 μL of DPPH solution) were added to a 96-well plate, and the absorbance was measured after the reaction at 37°C for 30 min. Absorbance was measured at 517 nm using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific). The standard material was L-ascorbic acid (Co. Sigma-Aldrich) and the negative control was methanol. DPPH free radical scavenging ability was calculated using the following formula:
Statistical analysis
Values are reported as means ± standard deviation (SD). All data were analyzed using R studio and Duncan’s multiple ranges tests. One-way analysis of variance (ANOVA) was carried out to verify significant differences among the groups at a significance level of p < .05 using R studio (Ver. 4.0.2, NA, USA). All experiments were conducted in triplicate.
Results and discussion
Visible appearance of PBM
The visible features of raw patties with incorporated natural pigments are shown in . Samples S2, S9, and S12 had a stronger red color, while S7, S8, S11, S13, and S14, had a sharper dark purple color compared to the other samples. The control sample (S17) exhibited red color characteristics because of the presence of myoglobin. To determine the visible appearance and color characteristics, the results are associated with the color of the raw materials used and the temperature at which the food was cooked.[Citation39] Meat analogs in which non-animal-based liquid additives have been incorporated exhibited heterogeneity in their external and internal appearance; this result might be attributed to the nature of liquid additives.[Citation29] Furthermore, our previous studies have indicated that cooking methods and different binding agents and natural colorants markedly affect the visible appearance of PBM patties.[Citation23,Citation24]
S1 = Dilute Red 1, S2 = Dilute Red 2, S3 = Red color CG2, S4 = Paprika, S5 = Monascus Color No.30, S6 = Red RR, S7 = Purple Grape, S8 = Cherry Red, S9 = Monascus Color 100, S10 = Red Cabbage (liquid), S11 = Red Cabbage 100, S12 = AF Beet Red 30, S13 = Grape skin Color, S14 = Red Color PB, S15 = Sample Anthocyanin (Sample A), S16 = Sample Fe-pheophytin, S17 = Myoglobin (control)
The visible appearance of steamed PBMs with incorporated natural pigments is shown in . Steamed PBMs S1, S2, S3, S9, and S12 displayed red coloration, which was similar to that of the respective raw PBM. On the other hand, raw S15 (anthocyanin), S16 (Fe-chlorophyll), and S15 (PBM patties) did not show any substantial color characteristics; however, after steaming, purple coloration was observed. In addition, S7, S8, S13, and S14 displayed purple coloration after steaming. shows the visible appearance of cooked PBM patties. Samples S1, S2, S3, and S9 showed a distinct red color compared to the other samples. Meanwhile, S15 (anthocyanin) and S16 (Fe-pheophytin), exhibited a pale purple color. In supporting evidence visible appearance of decolored soy-based protein isolate (SPI and TVP) of PBM patties before and after grilling with hydrogen peroxide as a decolorizer[Citation9] and external and internal appearance of PBM analog patties before and after grilling and reported in detail previously.[Citation2]
Figure 5. Visible appearance of steamed PBM patties incorporated with natural pigments. S1 = Dilute Red 1, S2 = Dilute Red 2, S3 = Red color CG2, S4 = Paprika, S5 = Monascus Color No.30, S6 = Red RR, S7 = Purple Grape, S8 = Cherry Red, S9 = Monascus Color 100, S10 = Red Cabbage (liquid), S11 = Red Cabbage 100, S12 = AF Beet Red 30, S13 = Grape skin Color, S14 = Red Color PB, S15 = Sample Anthocyanin (Sample A), S16 = Sample Fe-pheophytin, S17 = Myoglobin (control).
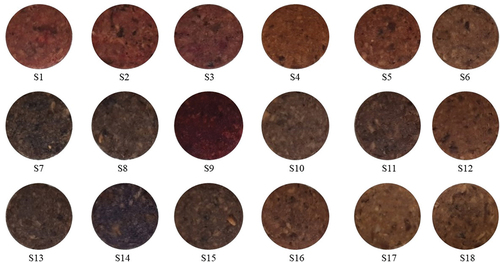
Figure 6. Visible appearance of cooked PBM patties incorporated with natural pigments.

Additionally, during meat preparation, the conversion of nitrosyl myoglobin into nitrosyl hemochrome leads to a color change, usually from red to pink. A similar pattern may have occurred when the PBM patties were cooked.[Citation10] Recently,[Citation40] reported that the color characteristics and visible appearance of TVP-based products are associated with heat treatment. The brown coloration of cooked meat can be attributed to the increased surface temperature and the formation of Maillard molecules and melanin pigment when the threshold of 85°C is exceeded.[Citation41,Citation42]
Color measurements
The chromaticity and color coordinates of the raw PBM patties with incorporated natural pigments are presented in . The results indicated that the lightness (L*) values in S2, S7, S8, S9, S11, S13, S14, and S15 were significantly lower (p < .05) than that in the control. The redness (a*) values of S6, S11, S14, and S16 were also significantly lower (p < .05) than that of the control. On the other hand, the yellowness (b*) values of the PBM patties with incorporated natural pigments were variable. The inconsistent L*, a*, and b* values measured in this study may be because of the following: (1) considerable differences in the color of natural pigments that were used (); (2) different concentration levels of pigments (); and (3) substitution of plant-based proteins, including soy and wheat protein and other plant-based ingredients ().
Table 3. Color coordinates of raw, steamed, and cooked patties incorporated with natural pigments.
The color scores for the steamed PBM patties with incorporated natural pigments are presented in . Regarding the L* scores, S2, S7, S8, S9, S13, S14, and S15 had lower scores than that of the control (p < .05). However, the a* values of S6, S8, S10, S11, S12, S13, S14, S15, and S16 were higher than that of the control. On the other hand, the S3, S4, S7, S8, S9, S11, S12, S13, S14, and S15 samples exhibited significantly different b* values (p < .05). Inconsistent with the current study, the effects of steaming on chicken meat showed similar outcomes, including lightness, redness, and yellowness values.[Citation43] Similarly, higher lightness and lower redness and yellowness values were observed in chicken meat cooked using superheated steam.[Citation44] The color variation observed in the developed PBM products could be due to considerable differences in the color of various pigments and the different cooking methods (steaming, frying).[Citation40,Citation44] Furthermore, the change in coloration of the PBM patties could be due to the combined effects of the oxygen-free environment during steam treatment and the rapid increase in temperature that causes browning reactions in meat and meat products.[Citation45,Citation46] Furthermore, compared to other cooking methods, steaming reduces nutrients but improves the visible appearance of the product by maintaining internal and external moisture levels.[Citation47]
The color values of the cooked PBM patties are listed in . Measurement of chromatic properties revealed that, for cooked samples, the L* value was significantly lower (p < .05) in samples S9, S14, and S16 than in the control. However, the a* values of the cooked PBM patties were variable, with extremely low and high values. Similarly, the b* values of S4, S7, S8, S9, S12, S13, S14, and S16 were significantly different from that of the control. The variations in the color values of the cooked patties might be due to the heating process, which influences the color of meat products due to denaturation and oxidization.[Citation48] According to our previous studies, the color values of PBM patties were markedly affected after cooking with the addition of various levels of methylcellulose incorporated into PBM,[Citation23] comparison of PBM with beef and pork[Citation24] and beef patties replaced by different levels of TVP.[Citation49]
Recently,[Citation2] reported that the incorporation of beet red pigment in meat analogues resulted in decreased a* and increased b* values for (laccase-treated patties containing beet red + sugar beet pectin or beet red + sugar beet pectin + methylcellulose) after grilling. Similarly,[Citation21] reported that natural colorants incorporated in non-meat sausages indicated that treatments containing 0.3 g/kg red yeast rice exhibited lower L* and b* values than the control treatment.
Proximate chemical composition
The proximate chemical composition of the PBM patties colored with natural pigments is presented in . The developed patties showed no significant differences (p > .05) in crude protein and moisture content compared to the control. In contrast, ash content was significantly different (p < .05) between the S15 and S16 samples compared to the control. The current results indicate that natural pigments did not significantly affect the proximate composition of PBMs developed in this study. In other studies, the proximate composition of PBMs was affected by the ratio of ingredients (TVP), rehydration properties, and extrusion process.[Citation4,Citation6] However, in terms of proximate composition, the current results deviated from our previous outcomes, which denoted significant differences in moisture, ash protein, and fat content.[Citation23,Citation24,Citation49] In this study, the moisture, fat, and protein content of the developed PBMs ranged from 62–64%, 2–3%, and 19–21%, respectively.
Table 4. Proximate chemical composition of PBM incorporated with natural pigments.
The present results are similar to the moisture (47.55–49.55%,), fat (6.04–7.22%), and protein (23.70–25.04%) content of seitan with the incorporation of natural pigments.[Citation50] Furthermore, metabolomics and nutritional facts regarding PBM and grass-fed meat have been discussed in detail by.[Citation51] The discrepancy between the results regarding proximate composition obtained in this study and previous reports might be due to the different weights and concentrations of pigments () and the absorbance level of natural pigments incorporated in PBM patties ().
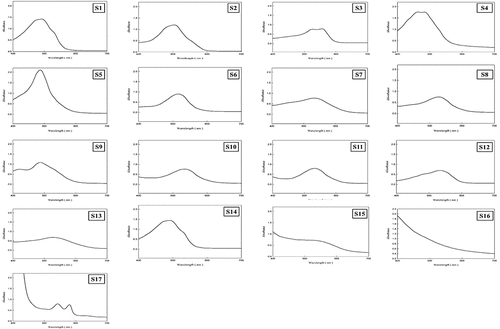
Figure 7. Absorbance measurement results of pigment samples.
Texture profile analysis
Establishing a superior textural profile for meat alternatives is critical as it imitates fibers within PBM that resemble muscle from animal-based meat. The hardness of PBM products is reportedly lower than that of animal-based products.[Citation23,Citation24,Citation52] The lower hardness of PBM products is perhaps due to a higher degree of shrinkage in meat protein. Furthermore, the higher hardness of animal-based meat products has been associated with muscle protein denaturation, which leads to increased hardness compared to PBM products.[Citation2,Citation48,Citation53] presents the chewiness, hardness, gumminess, springiness, cohesiveness, and resilience of the PBM patties in which various natural pigments have been incorporated. The values for the textural attributes (hardness, chewiness, and gumminess) of the developed PBMs were significantly lower (p < .05) than those of the control. Meanwhile, the springiness of the control and all other PBMs were significantly different, except for S1 and S4 (p < .05). However, there was no significant difference (p > .05) between the control and all samples in terms of cohesiveness and resilience.
Table 5. Texture Profile Analysis (TPA) of PBM incorporated with natural pigments.
For instance, techniques such as de-compartmentalization and simple color manipulation, commonly employed in PBM alternatives, can be utilized to achieve a marbling appearance and modify the color of various other plant sources.[Citation54] The authors of the study provided additional clarification by highlighting that novel plant sources have the potential to offer superior taste, texture, and nutritional profiles. By estimating color adjustments, targeted screening of suitable plant pigments for use as natural colorants in diverse products can be facilitated. Notably, the present study outlined precise purification and extraction techniques for a range of color pigments derived from plant sources within the described protocol. Furthermore, in another study[Citation55] the incorporation of microencapsulated carotenoids into muffins was demonstrated, with a focus on their natural color and functional attributes. Consequently, the addition of microencapsulated carotenoids resulted in increased firmness, reduced elasticity, and potential color enhancement in the muffins, leading to higher acceptability during sensory analysis.
Similarly, researchers analyzed the Turkish fermented beef sausages incorporating beetroot extract during an 84-day storage period. The authors provided detailed insights into the textural characteristics, reporting that a concentration of 0.12% resulted in increased hardness (8.78), cohesiveness (0.40), gumminess (3.89), and chewiness (1.84). Moreover, springiness (0.46) exhibited an increase with the addition of 0.35% beetroot powder.[Citation56] Additionally, the inclusion of 0.07% lutein color pigment demonstrated favorable sensory evaluation results (97%), surpassing the scores obtained with 0.05% concentration (86%) and the control (75%). The specific attributes of color, aroma, taste, texture, and overall acceptability received scores of 19, 19, 20, 15, and 13, respectively.[Citation57] Conversely, non-meat sausages utilizing natural colorants[Citation21] and pork sausages incorporating tomato powder as a natural colorant[Citation58] did not exhibit any significant alterations in their textural attributes. To conclude, the findings of this study align with previous reports on the texture attributes of PBM-analog products.[Citation52,Citation59,Citation60]
Absorbance levels
The concentrations of samples S15 (anthocyanin) and S16 (Fe-pheophytin), extracted from vegetable materials, and of edible red and purple dye samples were adjusted so that the absorbance at 535 nm was 0.7, for all pigments. The weights and concentrations of pigments are listed in . The absorbance values at 535 nm of S4 (paprika pigment), S5 (Monascus Color No. 30), and S6 (Red RR) were high; thus, the amount of added dye was less than 0.1 g, which was lower than that of the other samples. After adding 0.54 g of S15, 1.00 g of S16, and 1.76 g of S17, the absorbance value at 535 nm was 0.7. The absorbance measurement spectrum result is shown in . By measuring the absorbance of the samples, we confirmed that the absorbance increased from 400 to 535 nm and then decreased after recording the maximum value at 535 nm. In addition, the ideal extraction conditions were determined for each dye by determining the maximum absorbance level or optical density value at a precise absorbance wavelength using a UV-visible absorbance spectrophotometer.[Citation61] Similarly,[Citation62] confirmed the effective synthesis of Fe-pheophytin derivatives in crude spinach extracts. Moreover, the incorporation of anthocyanins as natural colorants in food additives and their absorbance and toxicity levels have been described in detail by.[Citation63]
The absorbance levels of the pigments used in this study were in the range of previously reported results of optical properties of natural pigments extracted from various flowering plants.[Citation64,Citation65] Similarly, applications of omics in food color science have been described in detail by[Citation66] as has the stabilization of natural pigments in food applications.[Citation67,Citation68]
DPPH radical scavenging activity
The DPPH radical scavenging activity of PBM patties incorporated with natural pigments is shown in . There was no significant difference between the edible and purple pigments and Fe-chlorophyll in both the negative control and the control. However, the anthocyanin showed a significant difference as compared to the control. In general, DPPH radical scavenging activity of PBM analogs is reportedly higher than animal-based meat.[Citation69] This may be due to DPPH activity also depending on the concentration of added substances () and duration of cooking or heat application to meat analogs. The DPPH activity of soybean curd-based meat had higher DPPH radical scavenging activity than animal-based meat such as beef, chicken and pork [.Citation70] Furthermore, assessment of the DPPH radical scavenging activity and peroxide activities of 23 natural plant sources in the meat model system revealed that blueberries, cherries, onions, black tea, and clove buds had higher activity than other plant-based sources (20.6–25.0 mg AA/g > 10.80–16.7 mg AA/) and animal-based meat control.[Citation69]
Figure 8. DPPH radical scavenging activity of PBM incorporated with natural pigments.
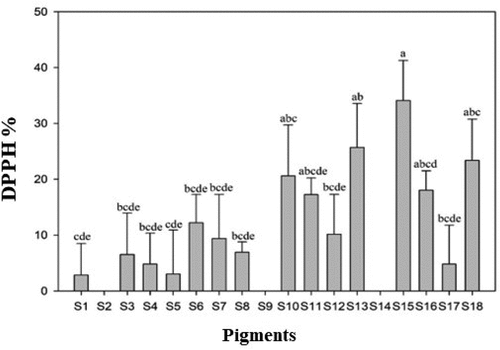
In line with the present study, previous research has also examined thirteen natural plant pigments.[Citation71] Among the pigments analyzed, the red cabbage pigment exhibited the highest DPPH radical scavenging activity, followed by red beet, onion peel, black rice, and mulberry pigments. The elevated DPPH activity of these pigments can be attributed to their capacity to hinder oxidation by donating electrons to free radicals during the scavenging process. Previous research has demonstrated that incorporating 85% wheat flour and 15% beetroot powder into noodles leads to a significant enhancement in color coordinates, along with a DPPH value of 37% and an improved antioxidant potential of 39.22%.[Citation72] Similarly, in the present study, different heating regimes (raw, cooked, and steamed) were employed to evaluate PBM patties.
During the heating process, the application of heat can cause the breakdown of specific compounds and the formation of new compounds in plant-based meat. These reactions can impact the accessibility and reactivity of antioxidant compounds found in the meat substitute. As a result, certain antioxidants may exhibit increased activity or reactivity when subjected to heat, thereby resulting in an elevated DPPH radical scavenging activity.[Citation70] This increase in DPPH radical scavenging activity observed in heated meat substitutes could potentially be attributed to the production of different reductones during the Maillard reaction process. These reductones may contribute to enhanced antioxidant activity. It is important to note that the impact of heating on DPPH radical scavenging activity can vary based on the composition and formulation of the plant-based meat product. Factors such as the ingredients used, processing methods employed, and cooking temperatures applied collectively influence the overall antioxidant activity. Therefore, further research is necessary to gain a deeper understanding of the underlying mechanisms and optimize heating conditions to maximize the DPPH radical scavenging activity of PBM products.
Conclusion
The PBM patties developed in this study incorporated 16 natural pigments, showcasing diverse physiochemical properties, texture, and scavenging activities. Among these patties, those made with Fe-pheophytin and anthocyanins displayed textural and color indices that closely resembled the control. Furthermore, the process of cooking and steaming significantly influenced visible, physiochemical, and texture attributes. When formulating meat alternatives with optimal color stability, it is vital to consider the impact of the chemical composition of natural pigments and their interaction with other ingredients in the PBM matrix. It can be concluded that the utilization of natural red pigments to imitate the color of meat analogs serves a purpose beyond visual appeal, appropriateness, or natural appearance. Thus, further exploration is necessary to comprehend the effects of incorporating natural pigments from various sources in enhancing the physicochemical and visual properties of plant-based meat products. Additionally, investigating the stability of these natural pigments across a wide range of pH and temperature conditions is of utmost importance.
Acknowledgments
The authors are grateful to the Technology Innovation Program (20012411, Alchemist Project) funded by the Ministry of Trade, Industry and Energy (MOTIE). This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01689101)” Rural Development Administration, Republic of Korea.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. Find additional templates here: Writing a data availability statement – Author Services (taylorandfrancis.com). Access to raw data is possible upon justifiable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Cyclodextrins Produced by Cyclodextrin Glucanotransferase Mask Beany Off-Flavors in Plant-Based Meat Analogs. PLoS One. 2022, 17, e0269278. DOI: 10.1371/journal.pone.0269278.
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Synergistic Effects of Laccase and Pectin on the Color Changes and Functional Properties of Meat Analogs Containing Beet Red Pigment. Sci. Rep. 2022, 12, 1–9. DOI: 10.1038/s41598-022-05091-4.
- Godfray, H. C. J.; Aveyard, P.; Garnett, T.; Hall, J. W.; Key, T. J.; Lorimer, J.; Pierrehumbert, R. T.; Scarborough, P.; Springmann, M.; Jebb, S. A. Meat Consumption, Health, and the Environment. Sci. 2018, 361, eaam5324. DOI: 10.1126/science.aam5324.
- Kyriakopoulou, K.; Keppler, J.; van der Goot, A. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods. 2021, 10, 600. DOI: 10.3390/foods10030600.
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A. J. Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Elsevier, 2019; pp 103–126. DOI: 10.1016/B978-0-12-814874-7.00006-7.
- Bohrer, B. M. An Investigation of the Formulation and Nutritional Composition of Modern Meat Analogue Products. Food Sci. Hum. Wellness. 2019, 8, 320–329. DOI: 10.1016/j.fshw.2019.11.006.
- Lee, H. J.; Yong, H. I.; Kim, M.; Choi, Y. S.; Jo, C. Status of Meat Alternatives and Their Potential Role in the Future Meat Market—A Review. Asian-Australas J. Anim. Sci. 2020, 33, 1533–1543. DOI: 10.5713/ajas.20.0419.
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Improved Functional Properties of Meat Analogs by Laccase Catalyzed Protein and Pectin Crosslinks. Sci. Rep. 2021, 11, 1–10. DOI: 10.1038/s41598-021-96058-4.
- Sakai, K.; Okada, M.; Yamaguchi, S. Decolorization and Detoxication of Plant-Based Proteins Using Hydrogen Peroxide and Catalase. Sci. Rep. 2022, 12(1), 1–10. DOI: 10.1038/s41598-022-26883-8.
- Ahmad, M.; Qureshi, S.; Akbar, M. H.; Siddiqui, S. A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S. B. Plant-Based Meat Alternatives: Compositional Analysis, Current Development and Challenges. 2022, 100154. DOI: 10.1016/j.afres.2022.100154.
- Martins, N.; Roriz, C. L.; Morales, P.; Barros, L.; Ferreira, I. C. Food Colorants: Challenges, Opportunities and Current Desires of Agro-Industries to Ensure Consumer Expectations and Regulatory Practices. Trends Food Sci. Technol. 2016, 52, 1–15. DOI: 10.1016/j.tifs.2016.03.009.
- Md Zaki, N. A.; Jai, J. Plant-Based Pigments: Challenges and Future Perspectives for Natural Food Colourants. (MJCET). 2020, 3(1), 44–49. DOI: 10.24191/mjcet.v3i1.10939.
- Leong, H. Y.; Show, P. L.; Lim, M. H.; Ooi, C. W.; Ling, T. C. Natural Red Pigments from Plants and Their Health Benefits: A Review. Food Rev. 2018, 34, 463–482. DOI: 10.1080/87559129.2017.1326935.
- Sigurdson, G. T.; Tang, P.; Giusti, M. M. Natural Colorants: Food Colorants from Natural Sources. Annu. Annu. Rev. Food Sci. Technol. 2017, 8(1), 261–280. DOI: 10.1146/annurev-food-030216-025923.
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A. K.; Yañez, J.; Mojica, L.; Luna-Vital, D. A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods. 2021, 10(3), 634. DOI: 10.3390/foods10030634.
- Wrolstad, R. E.; Culver, C. A. Alternatives to Those Artificial FD&C Food Colorants. Annu. Rev. Food Sci. Technol. 2012, 3(1), 59–77. DOI: 10.1146/annurev-food-022811-101118.
- Hsu, C. Y.; Chao, P. Y.; Hu, S. P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4(8), 1–8. DOI: 10.4236/fns.2013.48A001.
- Mancini, R.; Hunt, M. Current Research in Meat Color. Meat Sci. 2005, 71(1), 100–121. DOI: 10.1016/j.meatsci.2005.03.003.
- Ryu, K. K.; Kang, Y. K.; Jeong, E. W.; Baek, Y.; Lee, K. Y.; Lee, H. G. Applications of Various Natural Pigments to a Plant-Based Meat Analog. LWT - Food Sci. Technol. 2023, 174, 114431. DOI: 10.1016/j.lwt.2023.114431.
- He, J.; Evans, N. M.; Liu, H.; Shao, S. A Review of Research on Plant‐Based Meat Alternatives: Driving Forces, History, Manufacturing, and Consumer Attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19(5), 2639–2656. DOI: 10.1111/1541-4337.12610.
- Akramzadeh, N.; Hosseini, H.; Pilevar, Z.; Karimian Khosroshahi, N.; Khosravi‐Darani, K.; Komeyli, R.; Barba, F. J.; Pugliese, A.; Poojary, M. M.; Khaneghah, A. M. Physicochemical Properties of Novel Non‐Meat Sausages Containing Natural Colorants and Preservatives. J. Food Process Preserv. 2018, 42(9), e13660. DOI: 10.1111/jfpp.13660.
- Bakhsh, A.; Lee, E. Y.; Bakry, A. M.; Rathnayake, D.; Son, Y. M.; Kim, S. W.; Hwang, Y. H.; Joo, S. T. Synergistic Effect of Lactoferrin and Red Yeast Rice on the Quality Characteristics of Novel Plant-Based Meat Analog Patties. LWT -Food Sci. Technol. 2022, 171, 114095. DOI: 10.1016/j.lwt.2022.114095.
- Bakhsh, A.; Lee, S. J.; Lee, E. Y.; Sabikun, N.; Hwang, Y. H.; Joo, S. T. A Novel Approach for Tuning the Physicochemical, Textural, and Sensory Characteristics of Plant-Based Meat Analogs with Different Levels of Methylcellulose Concentration. Foods. 2021, 10, 560. DOI: 10.3390/foods10030560.
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Evaluation of Rheological and Sensory Characteristics of Plant-Based Meat Analog with Comparison to Beef and Pork. Food Sci Anim Resour. 2021, 41, 983–996. DOI: 10.5851/kosfa.2021.e50.
- Bakhsh, A.; Lee, E. Y.; Ncho, C. M.; Kim, C.-J.; Son, Y.-M.; Hwang, Y.-H.; Joo, S.-T. Quality Characteristics of Meat Analogs Through the Incorporation of Textured Vegetable Protein: A Systematic Review. Foods. 2022, 11, 1242. DOI: 10.3390/foods11091242.
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Traditional Plant-Based Meat Alternatives, Current, and Future Perspective: A Review. J. Agirc. Life Sci. 2021, 55(1), 1–11. DOI: 10.14397/jals.2021.55.1.1.
- Bakhsh, A.; Cho, C.; Baritugo, K. A.; Kim, B.; Ullah, Q.; Rahman, A.; Park, S. Production and Analytical Aspects of Natural Pigments to Enhance Alternative Meat Product Color. Foods. 2023, 12(6), 1281. DOI: 10.3390/foods12061281.
- Chávez-Santoscoy, R. A.; Lazo-Vélez, M. A.; Serna-Sáldivar, S. O.; Gutiérrez-Uribe, J. A. Delivery of Flavonoids and Saponins from Black Bean (Phaseolus Vulgaris) Seed Coats Incorporated into Whole Wheat Bread. Int. J. Mol. Sci. 2016, 17, 222. DOI: 10.3390/ijms17020222.
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods. 2020, 9(4), 461. DOI: 10.3390/foods9040461.
- Bakhsh, A.; Ismail, I.; Hwang, Y.-H.; Lee, J.-G.; Joo, S.-T. Comparison of Blood Loss and Meat Quality Characteristics in Korean Black Goat Subjected to Head-Only Electrical Stunning or without Stunning. Korean J. Food Sci. Anim. Resour. 2018, 38, 1286–1293. DOI: 10.5851/kosfa.2018.e64.
- Lee, E. Y.; Rathnayake, D.; Son, Y. M.; Bakhsh, A.; Hwang, Y. H.; Seo, J. K.; Kim, C. B.; Joo, S. Effect of Novel High-Intensity Ultrasound Technique on Physio-Chemical, Sensory Attributes, and Microstructure of Bovine Semitendinosus Muscle. Food Sci Anim Resour. 2023, 43, 85–100. DOI: 10.5851/kosfa.2022.e60.
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, 2002.
- Hwang, Y.-H.; Bakhsh, A.; Ismail, I.; Lee, J.-G.; Joo, S.-T. Effects of Intensive Alfalfa Feeding on Meat Quality and Fatty Acid Profile of Korean Native Black Goats. Korean J. Food Sci. Anim. Resour. 2018, 38(5), 1092–1100. DOI: 10.5851/kosfa.2018.e42.
- Min, D. B.; Steenson, D. F. Crude Fat Analysis. Food Analysis. 1998, 2, 201–216.
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95(5), 1392–1397. DOI: 10.5740/jaoacint.12-129.
- Sabikun, N.; Bakhsh, A.; Rahman, M. S.; Hwang, Y.-H.; Joo, S.-T. Volatile and Nonvolatile Taste Compounds and Their Correlation with Umami and Flavor Characteristics of Chicken Nuggets Added with Milkfat and Potato Mash. Food Chemistry. 2020, 343, 128499. DOI: 10.1016/j.foodchem.2020.128499.
- Fernández-López, J. A.; Angosto, J. M.; Giménez, P. J.; León, G. Thermal Stability of Selected Natural Red Extracts Used as Food Colorants. Plant Foods Hum. Nutr. 2013, 68(1), 11–17. DOI: 10.1007/s11130-013-0337-1.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D. H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. DOI: 10.1016/j.jfca.2006.01.003.
- Zahari, I.; Östbring, K.; Purhagen, J. K.; Rayner, M. Plant-Based Meat Analogues from Alternative Protein: A Systematic Literature Review. Foods. 2022, 11(18), 2870. DOI: 10.3390/foods11182870.
- Choi, H. W.; Lee, Y. Y.; Ryoo, C.; Yoon, H. I.; Hahn, J.; Choi, Y. J. Influence of a Post‐Processing Heat Treatment Method on the Textural Properties of Textured Vegetable Protein. Journal Of Food Sci. 2022, 87(12), 5340–5348. DOI: 10.1111/1750-3841.16367.
- Rabeler, F.; Skytte, J. L.; Feyissa, A. H. Prediction of Thermal Induced Color Changes of Chicken Breast Meat During Convective Roasting: A Combined Mechanistic and Kinetic Modelling Approach. Food Control. 2019, 104, 42–49. DOI: 10.1016/j.foodcont.2019.04.018.
- Kondjoyan, A.; Kohler, A.; Realini, C. E.; Portanguen, S.; Kowalski, R.; Clerjon, S.; Gatellier, P.; Chevolleau, S.; Bonny, J.-M.; Debrauwer, L. Towards Models for the Prediction of Beef Meat Quality During Cooking. Meat Sci. 2014, 97(3), 323–331. DOI: 10.1016/j.meatsci.2013.07.032.
- Singh, S.; Goswami, S.; Pathak, M.; Verma, V.; Rajkumar, A. K. Effect of Steam Cooking on Quality Characteristics of Shelf Stable Chicken Pickle. J. Meat Sci. 2019, 14(1), 12–15. DOI: 10.5958/2581-6616.2019.00003.3.
- Choi, Y.-S.; Hwang, K.-E.; Jeong, T.-J.; Kim, Y.-B.; Jeon, K.-H.; Kim, E.-M.; Sung, J.-M.; Kim, H.-W.; Kim, C.-J. Comparative Study on the Effects of Boiling, Steaming, Grilling, Microwaving and Superheated Steaming on Quality Characteristics of Marinated Chicken Steak. Korean J. Food Sci. Anim. Resour. 2016, 36(1), 1–7. DOI: 10.5851/kosfa.2016.36.1.1.
- Uengkimbuan, N.; Soponronnarit, S.; Prachayawarakorn, S.; Nathkaranakule, A. A Comparative Study of Pork Drying Using Superheated Steam and Hot Air. Dry. Technol. 2006, 24(12), 1665–1672. DOI: 10.1080/07373930601031513.
- Kim, S.-M.; Kim, T.-K.; Kim, H.-W.; Jung, S.; Yong, H. I.; Choi, Y.-S. Quality Characteristics of Semi-Dried Restructured Jerky Processed Using Super-Heated Steam. Foods. 2021, 10(4), 762. DOI: 10.3390/foods10040762.
- Nathakaranakule, A.; Kraiwanichkul, W.; Soponronnarit, S. Comparative Study of Different Combined Superheated-Steam Drying Techniques for Chicken Meat. J. Food Eng. 2007, 80(4), 1023–1030. DOI: 10.1016/j.jfoodeng.2006.04.067.
- Samard, S.; Ryu, G. H. A Comparison of Physicochemical Characteristics, Texture, and Structure of Meat Analogue and Meats. J. Sci. Food Agric. 2019, 99, 2708–2715. DOI: 10.1002/jsfa.9438.
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Characteristics of Beef Patties Substituted by Different Levels of Textured Vegetable Protein and Taste Traits Assessed by Electronic Tongue System. Foods. 2021, 10(11), 2811. DOI: 10.3390/foods10112811.
- Mal’a, P.; Baranová, M.; Marcinčáková, D.; Nagy, J. Organoleptic Evaluation of Poultry Meat Products with Wheat Protein–Seitan, Coloured by Microbial Natural Pigment. Assam Univ. J. 2010, 5, 1–5.
- van Vliet, S.; Bain, J. R.; Muehlbauer, M. J.; Provenza, F. D.; Kronberg, S. L.; Pieper, C. F.; Huffman, K. M. A Metabolomics Comparison of Plant-Based Meat and Grass-Fed Meat Indicates Large Nutritional Differences Despite Comparable Nutrition Facts Panels. Sci. Rep. 2021, 11(1), 1–13. DOI: 10.1038/s41598-021-93100-3.
- Kamani, M. H.; Meera, M. S.; Bhaskar, N.; Modi, V. K. Partial and Total Replacement of Meat by Plant-Based Proteins in Chicken Sausage: Evaluation of Mechanical, Physico-Chemical and Sensory Characteristics. J. Food Sci. Technol. 2019, 56(5), 2660–2669. DOI: 10.1007/s13197-019-03754-1.
- Ismail, I.; Hwang, Y.-H.; Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Kim, C.-J.; Joo, S.-T. Control of Sous-Vide Physicochemical, Sensory, and Microbial Properties Through the Manipulation of Cooking Temperatures and Times. Meat Sci. 2022, 188, 108787. DOI: 10.1016/j.meatsci.2022.108787.
- Ong, S.; Loo, L.; Pang, M.; Tan, R.; Teng, Y.; Lou, X.; Chin, S. K.; Naik, M. Y.; Yu, H. Decompartmentalisation as a Simple Color Manipulation of Plant-Based Marbling Meat Alternatives. Biomater. 2021, 277, 121107. DOI: 10.1016/j.biomaterials.2021.121107.
- Ursache, F. M.; Andronoiu, D. G.; Ghinea, I. O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of Carotenoids from Sea Buckthorn Extract by Microencapsulation and Formulation of Value-Added Food Products. J. Food Eng. 2018, 219, 16–24. DOI: 10.1016/j.jfoodeng.2017.09.015.
- Sucu, C.; Turp, G. Y. The Investigation of the Use of Beetroot Powder in Turkish Fermented Beef Sausage (Sucuk) as Nitrite Alternative. Meat Sci. 2018, 140, 158–166. DOI: 10.1016/j.meatsci.2018.03.012.
- Al-Ali, R. M.; Al-Hilifi, S. A.; Al-Mossawi, A. Extraction of Lutein from Some Plant Source in Different Conditions and Application in Food System. Plant Arch. 2020, 20, 1679–1685.
- Kim, I.-S.; Jin, S.-K.; Mandal, P. K.; Kang, S.-N. Quality of Low-Fat Pork Sausages with Tomato Powder as Colour and Functional Additive During Refrigerated Storage. J. Food Sci. Technol. 2011, 48(5), 591–597. DOI: 10.1007/s13197-010-0182-2.
- Arora, B.; Kamal, S.; Sharma, V. Effect of Binding Agents on Quality Characteristics of Mushroom Based Sausage Analogue. J. Food Process Preserv. 2017, 41(5), e13134. DOI: 10.1111/jfpp.13134.
- De Angelis, D.; Kaleda, A.; Pasqualone, A.; Vaikma, H.; Tamm, M.; Tammik, M.-L.; Squeo, G.; Summo, C. Physicochemical and Sensorial Evaluation of Meat Analogues Produced from Dry-Fractionated Pea and Oat Proteins. Foods. 2020, 9(12), 1754. DOI: 10.3390/foods9121754.
- Prabhu, K.; Bhute, A. S. Plant Based Natural Dyes and Mordants: A Review. J Nat Prod Plant Resour. 2012, 2, 649–664.
- Nelson, R.; Ferruzzi, M. Synthesis and Bioaccessibility of Fe‐Pheophytin Derivatives from Crude Spinach Extract. J. Food Sci. 2008, 73, H86–H91. DOI: 10.1111/j.1750-3841.2008.00783.x.
- He, J.; Giusti, M. M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1(1), 163–187. DOI: 10.1146/annurev.food.080708.100754.
- Amir-Al Zumahi, S.; Arobi, N.; Taha, H.; Hossain, M. K.; Kabir, H.; Matin, R.; Bashar, M.; Ahmed, F.; Hossain, M. A.; Rahman, M. M. Extraction, Optical Properties, and Aging Studies of Natural Pigments of Various Flower Plants. Heliyon. 2020, 6(9), e05104. DOI: 10.1016/j.heliyon.2020.e05104.
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT - Food Sci. Technol. 2022, 153, 112527. DOI: 10.1016/j.lwt.2021.112527.
- Zhou, J.; Wang, M.; Carrillo, C.; Hassoun, A.; Collado, M. C.; Barba, F. J. Application of Omics in Food Color. Curr. Opin. Food Sci. 2022, 46, 100848. DOI: 10.1016/j.cofs.2022.100848.
- Ferreira, A. S.; Pereira, L.; Canfora, F.; Silva, T. H.; Coimbra, M. A.; Nunes, C. Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella Vulgaris. Molecul. 2023, 28(1), 408. DOI: 10.3390/molecules28010408.
- Rodriguez-Amaya, D. B. Natural Food Pigments and Colorants. In Bioactive Molecules in Food; Springer International Publishing, 2019; pp 867–901. DOI: 10.1007/978-3-319-78030-6_12.
- Jin, S.-K.; Kim, G.-D.; Jeong, J.-Y.; Patarata, L. Evaluation of the Effect of Inhibiting Lipid Oxidation of Natural Plant Sources in a Meat Model System. J Food Qual. 2021, 2021, 1–8. DOI: 10.1155/2021/6636335.
- Song, H. S.; Bae, J. K.; Park, I. Effect of Heating on DPPH Radical Scavenging Activity of Meat Substitute. Prev. Nutr. Food Sci. 2013, 18, 80–84. DOI: 10.3746/pnf.2013.18.1.080.
- Boo, H.-O.; Hwang, S.-J.; Bae, C.-S.; Park, S.-H.; Song, W.-S. Antioxidant Activity According to Each Kind of Natural Plant Pigments. Korean J. Plant Res. 2011, 24(1), 105–112. DOI: 10.7732/kjpr.2011.24.1.105.
- Abiodun, O.; Ojo, A.; Abdulganiu, O.; Olosunde, O. Effect of Beetroots Substitution and Storage on the Chemical and Sensory Properties of Wheat Noodles. AGROSH. 2020, 20(1), 1–12. DOI: 10.4314/agrosh.v20i1.1S.