?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The core objective of the current study was to extract heteroxylans from banana peel. Firstly, banana peels were dried to make fine powder, and their nutritional profile including moisture, crude fat, crude ash, crude protein, crude fiber, and nitrogen-free extract (NFE) was noted. Secondly, the heteroxylans were extracted from banana peels, and their bioactive profile was assessed, including total phenols and flavonoids contents, and their activity was assessed through commonly used antioxidant assays including DPPH, FRAP, and ABTS+. Heteroxylans were extracted through alkali extraction method, and their structural characterization was assessed through XRD and FTIR. The results showed that moisture content in banana peels were 60.73 ± 0.85% and 15.77 ± 0.33% in air dried and grounded banana peels, respectively, crude fat 3.94 ± 0.10%, crude protein 6.06 ± 0.15%, and NFE 48.13 ± 0.32%. The total dietary fiber of banana peel powder was 45.3 ± 0.45%, insoluble dietary fiber 34.23 ± 0.30%, and soluble dietary fiber 15.63 ± 0.25%. The bioactive profile of heteroxylans content in banana peels include TPC (19.83 ± 1.10 mg GAE/g), TFC (22.73 ± 0.90 mg QE/g), DPPH (40.73 ± 0.80 mg TE/g), FRAP (39.63 ± 0.73 mg TE/g), and ABTS+ (83.43 ± 0.70 (mg TE/g). The yield of extracted heteroxylans was 9.95 ± 0.17 g/100 g. Further, the structural characterization through XRD showed the amorphous nature of banana peels heteroxylans, and FTIR showed the typical spectra in which different functional groups were observed. Conclusively, the current study showed that banana peels’ heteroxylans has unique nutritional, bioactive, and structural profile and can be utilized for various industrial purposes to enhance technological and nutritional properties using as food additive and way toward the sustainability
Introduction
The world’s population is increasing day-by-day, which results in an increasing demand of food in both perspective, including nutritious and varietal, consequently leadong toward the large scale. industrial processing. In this context, industrial processing produces a huge amount of agro-industrial waste, including peels, seeds, skin, and flesh, of different fruits and vegetables. The leftover of the agricultural residue is being managed through animal feed and remaining is dumped or composted. Moreover, fruits and vegetables are commonly produced seasonally, and overproduction during the season without proper utilization leads to the waste of food, especially in tropical countries.[Citation1]
Banana (Musa sp) is among one of the most widely produced crops globally.[Citation2] Banana peel (banana skin) is the outer covering of banana fruit which contains carotenoids, α-carotene, β-carotene, sterol, and β-sitosterol.[Citation3] The pulp and unripe peel of banana contain α-tocopherol, linolenic acid (ω-3), linoleic acid (ω-6), and β-sitosterol that are used in the treatment of chronic diseases like cardiovascular disease and cancer. The bioactive compounds present in banana, such as rutin, apigenin, quercetin, and kaempferol, also reduces the risk of cancer, including lung, liver, breast, prostate, ovarian, and cervical cancer.[Citation4]
The cell walls of various fruits and vegetables are capable of producing soluble polysaccharides, such as pectin, but they may also supply cellulose, which, when combined with xyloglucans produces insoluble fiber, heteroxylans, and galactoglucomannans in minor amounts.[Citation5] By focusing on the configuration of non-starch polysaccharides from banana (Musa acuminata), it was suggested that the cell wall of banana fruit contains pectin, cellulose, and hemicellulose, together with galacturonans and heteroxylans, likely the cell wall of cereals. The non-starch polysaccharides (NSPs) that are key organizational elements from the cell walls of plants, make up spacious amounts of the dietary fiber consumed by human subjects. Diets enriched with heteroxylans have been shown to improve glycemic management, lipid metabolism, and colon health. According to Gbenga-Fabusiwa et al.,[Citation6] the extract of banana peel flour is considered to be innocuous to healthy cells of human. As a result, peels may be used, without any risk, as food additive, source of antioxidants, and natural food source. Thus, banana peels have been characterized for their nutritional and bioactive profile. The current research aimed to extract and characterize the heteroxylans from the cell wall of banana peels (Agro-industrial waste) and to assess their structure through analytical techniques including fourier transform infra-red (FTIR) and X-ray diffraction (XRD). Further, their phenolic components and scavenging potential were explored through generally used antioxidant assays including DPPH, FRAP, and ABTS+.
Material and methods
Procurement of raw material
Banana peels of Basrai variety were collected from local juice shops in 2022, where banana were utilized and the peels were being thrown away as a waste. The collected peels were sun dried (24–48 hours) at 32–35°C till the chirping of peels became audible. The dried peels were then grinded to make a fine powder through blender, and the powder of the grounded peels was then packed in air-tight zip bags and stored at room temperature. Chemicals used for experimentation including NaOH, ethanol, H2SO4, and distilled water were procured from commercial sources (Sigma–Aldrich) and were of analytical grade.
Proximate analysis of grounded banana peels
Proximate analysis including Moisture, Crude Fat, Crude Fiber, Crude Protein, and Dietary Fibre profile (Soluble and Insoluble) were conducted by standard procedures described in AOAC.[Citation7]
Extraction of heteroxylan from grounded banana peels
Heteroxylans from banana peels were extracted through sequential extraction method by following the method used by Raza et al.,[Citation8] with some modifications. Briefly, grounded banana peels were defatted through overnight soaking in ethanol, and the sample was filtered. The dried sample was then dissolved in 0.5N NaOH solution for 2 hours followed by centrifugation, and supernatant was recuperated with absolute ethanol (65% v/v). The sample was then lyophilized and stored in a zip bag and placed in cool and dry place.
Monosaccharides assessment
Monosaccharides were assessed through the Saeman hydrolysis procedure. Briefly, the sample was hydrolyzed through 75% H2SO4 to convert simple sugars into alditol acetates. Further, GC-MS (Triple Quadruple) equipped with flame ionization detector (Agilent 6890) was used to quantify the monosaccharides in sample as previously used by Deumaga et al..[Citation9]
Bioactive characterization of banana peels heteroxylans
Total Phenolic Content
The total phenolic content (TPC) to determine the antioxidant activity of the extracted sample was evaluated with Folin ciocalteau reagent by subsequent method of Hussain et al.,[Citation10] The absorbance was measured at 760 nm using a spectrophotometer (IRMECO, U2020), and the results were reported as gallic acid equivalent (mg GAE/g).
Total flavonoid content
The total flavonoid content (TFC) was determined by following the method of Turkmen et al.,[Citation11] preparing solutions of 30% ethanol, 5% NaNO2, 10% AlCl3, and 2 g NaOH in 50 ml distilled water. The absorbance was measured at 430 nm using a spectrophotometer (IRMECO, U2020). Flavonoid contents were considered by using a standard calibration curve derived from Quercetin equivalent, and the results were reported as mg QE/g.
Scavenging potential of banana peels heteroxylans
Diphenyl-picrylhydrazyl assay
An antioxidant activity was assessed through diphenyl-picrylhydrazyl assay (DPPH) by using the method of Raza et al..[Citation8] The scavenging potential of the banana peels heteroxylans was quantified by the degree of discolouration. The solution of DPPH (2.8 mg, 10 ml) in methanol was mixed with the extracted sample (1 g), and the mixture was kept in the dark for 30 mins by wrapping the solution tube with aluminum foil. Then the absorbance was assessed at 520 nm until the stability of the absorbance along with control and blank. Trolox was used as an internal standard and inhibition was reported as mg TE/g. The free radical scavenging activity of DPPH was calculated by the following equation:
Ferric reducing antioxidant power
The metal ion chelating capacity is also a variable to evaluate antioxidant activity of heteroxylans. Method of Ali et al.,[Citation12] was used to assess the scavenging potential through FRAP assay Samples were evaluated at absorbance of 700 nm by using a spectrophotometer (IREMCO U2020), and the results were reported as mg TE/g of extract sample.
Aazino-bis, 3-ethylbenzothiazoline-6-sulfonic acid assay
Radical scavenging potential through ABTS+ assay was assessed by deliberating process of Ali et al.[Citation12] The antioxidant activity was described through the standard curve of Trolox, and the results are reported in mg TE/g of extract sample. The wavelength used to assess the absorbance was 734 nm by using a spectrophotometer (IRMECO, U2020).
Structural analysis
Fourier Transform Infra-Red (FTIR)
The functional and structural elucidation of heteroxylans were assessed through FTIR spectroscopy techniques in the range from 650–4000 cm−1 (Agilent Technologies Cary 630 FTIR).
X-ray Diffraction (XRD)
XRD pattern of heteroxylans was accomplished by means of XRD (D8 advance BRUKER) with Nickel-filtered Cu Kα radiation (wavelength = 1.54 Å) ranges from 4–50° at the diffraction angle 2θ.
Statistical analysis
Results obtained from the analyzed samples were statistically assessed through IBM statistics (SPSS version 20.0), and the analysis were performed in triplicates, and the results were written as mean and their standard deviation.
Results and discussion
Nutritional composition of banana peels
Proximate composition is generally used to assess the initial screening of nutritional profiles of different food. In the current study, grounded banana peels were evaluated for their proximate composition, and the results showed that fresh banana peels contained moisture content (60.73 ± 0.85%) and dried/grounded banana peel (15.77 ± 0.33%) as shown in . Further, crude fat content in grounded banana peels assessed in the current study was 3.94 ± 0.10%, which showed that banana peels contain minor concentration of lipophilic components. The mean values regarding the ash content was 9.76 ± 1.02%, which increases after ripening.[Citation13,Citation14] The study of Pyar & Peh[Citation15] showed similar results regarding the ash content i.e. 8.8 ± 0.54%. Further, crude fibers in banana peel was 14.93 ± 0.15%, and the study of Tsado et al.,[Citation16] endorse current results. Further, 6.06 ± 0.15% protein contents were assessed in banana peel powder. Moreover, nitrogen-free extract was 48.13 ± 0.32%, and relatable results were reported by Chaudhry et al..[Citation17] Minor variations in the results were due to varied varieties of banana peel and the stages of maturation. Total dietary fiber depending on the extraction condition and methods generates high quality dietary fibers as shown in .
Table 1. Proximate composition and dietary fiber profile of banana peel powder, n = 3.
Natural sugar assessment
The results regarding the monosaccharide profile of banana peel powder showed that hydrolyzed banana peel powder contains a sugar composition including xylose 10.02 ± 0.35%, arabinose 3.21 ± 0.37%, galactose 1.31 ± 0.07%, and maltose 4.60 ± 0.20%. Similar results were reported by Deumaga et al.,[Citation9]; however, higher glucose content in the results were due to higher concentration of acid during the hydrolysis of sample. Strong acid invert the non-pectic and starchy components into glucose and polysaccharides precipitated with ethanol during recuperation.[Citation18] The results regarding the neutral sugar have been presented in .
Table 2. Yield of Enzymatic and Alkali Extraction Method, n = 3.
Bioactive profile of banana peels heteroxylans
Total Phenolic Content
Phenolic compounds in plants acts as strong antioxidants and have a therapeutic role in normal physiological functioning of body.[Citation19] The total phenolic content of banana peel extract has been given in .
Table 3. Antioxidant Activity of Banana Peel Heteroxylan, (n = 3).
The phenolic contents in banana peel extract were determined as mg GAE/g of dry matter extract, and the mean value of total phenolic contents in banana peel heteroxylans were 19.83 ± 1.10 mg GAE/g of their dry matter. The results of the current study are closely corroborated with the findings of Chaudhry et al.,[Citation17] who reported that banana peels arabinoxylans contain 23.53 mg GAE/g DM and extracted through enzymatic method. Similar study conducted by, Anal et al.,[Citation20] reported that the phenolic content of banana peel extracted with both enzymatic and alkali methods were in the range of 18.21–35.06 mg GAE/g DM.
Total Flavonoid Content
Results regarding the total flavonoid content of banana peel heteroxylans are expressed as mg QE/g dry matter. Due to the hydroxyl group, various flavonoids are discovered to be potent antioxidants that can efficiently scavenge the reactive oxygen species (ROS). The mean value for the total flavonoid contents of the heteroxylan was 22.73 ± 0.90 mg QE/g of their dry matter, and similar results were reported by Aboul-Enein et al.,[Citation21] regarding the total flavonoid content of banana peels heteroxylans.
Antioxidant Activity of Banana peels heteroxylans In vitro efficacy of banana peel heteroxylans was assessed by using diphenyl picryl hydrazyl assay. The result showed that peel extract showed the inhibition value 40.73 ± 0.80 mg TE/g as given in . DPPH activity of the heteroxylan from banana peel was expressed as mg TE/g dry matter. Study conducted by Rebello et al.,[Citation22] reported that the higher antioxidant potential of banana peels extract is due to higher phenolic content. Ferric reducing antioxidant potential was also used to assess the antioxidant potential, and the results showed the FRAP value of 39.63 ± 0.73 mg TE/g DM and given in . Antioxidant potential is measured by their antioxidant’s power to convert ferric to ferrous ion in the FRAP mixture. The study conducted by Rebello et al.,[Citation22] assessed the scavenging potential of banana peels extract and reported the FRAP value as 14.00 ± 1.7 µM TE/g DM.
The results of the antioxidant potential assessed by ABTS assay of heteroxylan, extracted by enzymatic method, was 83.43 ± 0.70 mg TE/g DM and is shown in . Antioxidant activity in plants and fruits may often be determined using the ABTS+ assay.[Citation23] It was dependent on antioxidants’ capacity to scavenge the durable radical cation ABTS.+. The incorporation of banana peel in the food items increases the antioxidant activity and phytochemical diversity. Current study is endorsed by the results of Zaini et al.,[Citation24] who reported the similarity in the ABTS+ scavenging potential in banana peels heteroxylan.
Structural exploration of banana peels heteroxylans
Fourier transform infra-red spectroscopy
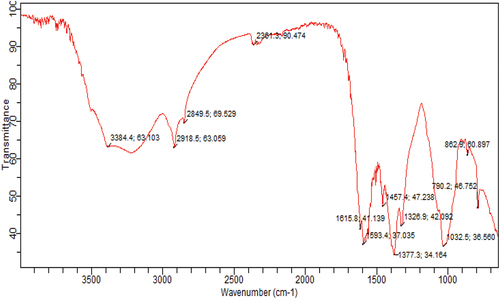
The banana peels heteroxylans were assessed for their structural profile through the application of FTIR spectroscopy, and the spectra is presented in . The spectra showed the diversity of functional groups with different divergence peaks at different wavelengths. The range of 600–1600 cm−1 is populated with various fingerprints of functional groups. The peak at 1032 cm−1 corresponds to the glycosidic linkage that interconnects with the xylan backbone, and the band at this wavenumber indicates that there were more cellulose in banana peel extract. Further, the peak observed at 2355 cm−1 showed the extending vibrations of C=O and stretching of N-H.[Citation25] The stretching at 862 cm−1 was due to C-H vibration of polysaccharides as displayed in . Moreover, the vibration at 3384 endorsed the presence of O-H group of aliphatic carbon.
X-ray diffraction
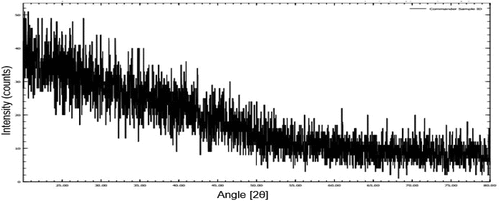
XRD is used to examine the crystalline and amorphous structure of the materials and identify the phase of a crystalline material. The amorphous area of the heteroxylan exhibits faint and broad signals in the XRD mosaic, while heteroxylan crystalline section offers strong signals in XRD peaks.[Citation26] Current XRD graph showed that banana peels heteroxylans were highly amorphous in nature. Rajini et al.,[Citation27] reported that banana peel powder are amorphous in nature and also affect the crystallinity of cellulose-based composite films. XRD diffractogram of extracted heteroxylans has been given in .
Conclusion
Banana peels are generally treated as agro-industrial waste and are being dumped or composted in a large quantity annually. In reality, banana peels are enriched with a variety of polyphenols and have a therapeutic role in human normal physiological functioning. Our current research aimed to extract and characterize the heteroxylans extracted from the cell wall of banana peels. The proximate composition showed that the cell wall of banana peels contain essential nutrients but dietary fibers are present dominantly. Further, the FTIR spectra showed the presence of various functional groups and XRD presented an amorphous nature of heteroxylans. Moreover, polyphenols and their activity showed the scavenging potential conducted through different antioxidant assays, and from the results of current study, banana peels heteroxylans are suitable for the incorporation in food material to attain the desired nutritional as well as technological attributes and sustainable utilization of agro-industrial waste.
Acknowledgement
The authors acknowledge the Advanced Food Analysis Laboratory, Department of Food Science, Government College University, Faisalabad for providing all the chemicals and lab facilities for analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Salim, N. S. M.; Singh, A.; Raghavan, V. Potential Utilization of Fruit and Vegetable Wastes for Food Through Drying or Extraction Techniques. Nov. Tech. Nutr. Food Sci. 2017, 1(2), 1–12. DOI: 10.31031/NTNF.2017.01.000506.
- Food and Agricultural Organization (FAO). Rome, Italy, 2019. http://www.fao.org/faostat/en/#data
- González-Montelongo, R.; Lobo, M. G.; González, M. Antioxidant Activity in Banana Peel Extracts: Testing Extraction Conditions and Related Bioactive Compounds. Food. Chem. 2015, 119(3), 1030–1039. DOI: 10.1016/j.foodchem.2009.08.012.
- Sarma, P. P.; Gurumayum, N.; Verma, A. K.; Devi, R. A Pharmacological Perspective of Banana: Implications on Therapeutical Benefits and Molecular Docking. Food. Funct. 2021, 12(11), 4749–4767. DOI: 10.1039/D1FO00477H.
- Benito-González, I.; Martínez-Sanz, M.; Fabra, M. J.; López-Rubio, A. Health Effect of Dietary Fibers. In Dietary Fiber: Properties, Recovery, and Applications; Academic Press: 2019; pp. 125–163. DOI: 10.1016/B978-0-12-816495-2.00005-8.
- Gbenga-Fabusiwa, F. J.; Jeff-Agboola, Y. A.; Ololade, Z. S.; Akinrinmade, R.; Agbaje, D. O. Waste-To-Wealth; Nutritional Potential of Five Selected Fruit Peels and Their Health Benefits: A Review. Afr. J. Food Sci. 2022, 16(7), 172–183. DOI: 10.5897/AJFS2021.2138.
- AOAC. Official Method of Analysis, Association of Analytical Chemists, 19th ed.; The Association of analytical chemists: Washington, DC, 2012.
- Raza, M. A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Hussain, M.; Al Jbawi, E.; Kashif Mukhtar, M.; Waleed, M.; Al Jbawi, E. Comparative Study of Cross-And Uncross-Linked Arabinoxylans Extracted from Maize Bran with Special Reference to Their Structural and Antioxidant Potential. Int. J. Food Prop. 2022, 25(1), 2495–2504. DOI: 10.1080/10942912.2022.2143524.
- Deumaga, M. F. T.; Emaga, T. H.; Tchokouassom, R.; Vanderghem, C.; Aguedo, M.; Gillet, S.; Richel, A.; Danthine, S.; Magali, D.; Richel, A. Genotype Contribution to the Chemical Composition of Banana Rachis and Implications for Thermo/Biochemical Conversion. Biomass Convers. Biorefin. 2015, 5(4), 409–416. DOI: 10.1007/s13399-015-0158-6.
- Imran, G.; Khan, F.; Jabeen, M.; Ahmed, A.; Hussain, M.; Saeed, M. A.; Shahbaz, F.; Ahmed, A.; Imran, M.; Khan, M. A. Reconnoitring the Impact of Different Extraction Techniques on Ginger Bioactive Moieties Extraction, Antioxidant Characterization and Physicochemical Properties for Their Therapeutic Effect. Pak. J. Pharm. Sci. 2019, 32(5(Supplementary)), 2223–2236.
- Turkmen, F. U.; Takci, H. A. M.; Sekeroglu, N. Total Phenolic and Flavonoid Contents, Antioxidant and Antimicrobial Activities of Traditional Unripe Grape Products. Indian J. Pharm. Educ. Res. 2017, 51(3s2), s489–s493. DOI: 10.5530/ijper.51.3s.72.
- Ali, S. M.; Imran, A.; Arshad, M. U.; Ahmed, R. S.; Imran, M. Physicochemical, Antioxidant and Enzymes Activities of Grape Fruit Peel and Pomace Enriched Functional Drinks. Cell. Mol. Biol. (Noisy-le-Grand). 2021, 67(1), 125–131. DOI: 10.14715/cmb/2021.67.1.19.
- Watharkar, R. B.; Pu, Y.; Ismail, B. B.; Srivastava, B.; Srivastav, P. P.; Liu, D. Change in Physicochemical Characteristics and Volatile Compounds During Different Stage of Banana (Musa Nana Lour Vs. Dwarf Cavendish) Ripening. J. Food Meas. Charact. 2020, 14(4), 2040–2050. DOI: 10.1007/s11694-020-00450-z.
- Amini Khoozani, A.; Birch, J.; Bekhit, A. E. D. A. Production, Application and Health Effects of Banana Pulp and Peel Flour in the Food Industry. J. Food Sci. Technol. 2019, 56(2), 548–559. DOI: 10.1007/s13197-018-03562-z.
- Pyar, H.; Peh, K. K. 2018. Chemical Compositions of Banana Peels (Musa sapientum Fruits Rajendran, K.; Drielak, E.; Sudarshan Varma, V.; Muthusamy, S., & Kumar, G. Updates on the Pretreatment of Lignocellulosic Feedstocks for Bioenergy Production–A Review. Biomass Convers. Biorefin. 8, 2, 471–483.10.1007/s13399-017-0269-3.
- Tsado, A. N.; Okoli, N. R.; Jiya, A. G.; Gana, D.; Saidu, B.; Zubairu, R.; Salihu, I. Z. Proximate, Minerals, and Amino Acid Compositions of Banana and Plantain Peels. J. Biomed. Sci. 2021, 1(1), 032–042.
- Chaudhry, F.; Ahmad, M. L.; Hayat, Z.; Ranjha, M. M. A. N.; Chaudhry, K.; Elboughdiri, N.; Uddin, J.; Uddin, J. Extraction and Evaluation of the Antimicrobial Activity of Polyphenols from Banana Peels Employing Different Extraction Techniques. Separations. 2022, 9(7), 165. DOI: 10.3390/separations9070165.
- Maneerat, N.; Tangsuphoom, N.; Nitithamyong, A. Effect of Extraction Condition on Properties of Pectin from Banana Peels and Its Function as Fat Replacer in Salad Cream. J. Food Sci. Technol. 2017, 54(2), 386–397. DOI: 10.1007/s13197-016-2475-6.
- Azarudeen, A. M.; Nithya, R. Pharmaceutical Aspects of Banana Peel: A Review. J.Pharm.Sci. Res. 2021, 13(2), 112–117.
- Anal, A. K.; Jaisanti, S.; Noomhorm, A. Enhanced Yield of Phenolic Extracts from Banana Peels (Musa Acuminata Colla AAA) and Cinnamon Barks (Cinnamomum Varum) and Their Antioxidative Potentials in Fish Oil. J. Food Sci. Technol. 2014, 51(10), 2632–2639. DOI: 10.1007/s13197-012-0793-x.
- Aboul-Enein, A. M.; Salama, Z. A.; Gaafar, A. A.; Aly, H. F.; Abou-Elella, F.; Ahmed, H. A. Identification of Phenolic Compounds from Banana Peel (Musa Paradaisica L.) as Antioxidant and Antimicrobial Agents. J. Chem. Pharm. Res. 2016, 8(4), 46–55.
- Rebello, L. P. G.; Ramos, A. M.; Pertuzatti, P. B.; Barcia, M. T.; Castillo-Muñoz, N.; Hermosin-Gutierrez, I. Flour of Banana (Musa AAA) Peel as a Source of Antioxidant Phenolic Compounds. Food Res. Int. 2014, 55, 397–403. DOI: 10.1016/j.foodres.2013.11.039.
- Kelebek, H.; Kesen, S.; Selli, S. Comparative Study of Bioactive Constituents in Turkish Olive Oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015, 18(10), 2231–2245. DOI: 10.1080/10942912.2014.968788.
- Zaini, H. M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N. S.; Pindi, W. Banana Peels as a Bioactive Ingredient and Its Potential Application in the Food Industry. J. Funct. Foods. 2022, 92, 105054. DOI: 10.1016/j.jff.2022.105054.
- Nandiyanto, A. B. D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indonesian J. Sci. Technol. 2019, 4(1), 97–118. DOI: 10.17509/ijost.v4i1.15806.
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Dutta, A. Characterization of Ultrasonic-Treated Corn Crop Biomass Using Imaging, Spectral and Thermal Techniques: A Review. Biomass Conv. Bioref. 2020, 12(4), 1393–1408. DOI: 10.1007/s13399-020-00748-4.
- Rajini, N.; Alavudeen, A.; Siengchin, S.; Rajulu, V.; Ayrilmis, N. Development and Analysis of Completely Biodegradable Cellulose/Banana Peel Powder Composite Films. J. Nat. Fibers. 2019, 18(1), 151–160. DOI: 10.1080/15440478.2019.161281.