 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study aimed to evaluate the effect of the incorporation of protein-based nanoencapsulation on the viability and stability of probiotic Lactobacillus rhamnosus in digestion conditions and model food. In the study’s first phase, protein-based nanoparticles were prepared by the pH cycling method, and then the probiotic, Lactobacillus rhamnosus, was nano-encapsulated. Two types of proteins, namely, whey protein and zein protein, were used to encapsulate probiotics individually. The obtained nano-encapsulated probiotics were characterized by performing particle size, SEM, FTIR, and in vitro assay was performed. Then, free and nano-encapsulated probiotics were added to the model food (yogurt) and analyzed for microbiological and sensory evaluation. Nanoencapsulation with both types of proteins significantly (p < .05) improved the stability and viability of Lactobacillus rhamnosus. The particle size for whey and zein nano-encapsulated probiotics ranged between 96 and 100 nm. The encapsulation efficiency for whey and zein nanoparticles was recorded at 96% and 87%, respectively. SEM images for both zein and whey nanoparticles showed their irregular spherical structure. FTIR spectra suggested that the mechanism of entrapment involved in water-insoluble or physical properties was mostly responsible for the generated loaded nanoparticle. In vitro analysis illustrated that whey nanoencapsulation showed the highest viability in SGC and SIC, followed by zein nanoencapsulation and free probiotics showed the minimum viability. Protein nanoencapsulation also significantly affected the sensory properties of food products. Conclusively, the nanoencapsulation of probiotics using protein nanoparticles helps prolong the viability and stability of probiotics under-simulated gastrointestinal digestion conditions and in yogurt.
Introduction
The use of probiotics by incorporating them in functional foods is increasing with time owing to their health benefits to the human body. Probiotics are defined as live microorganisms that provide positive health benefits if are present in appropriate amounts. Besides, they must survive the harsh processing as well as gastrointestinal conditions.[Citation1] For this purpose, researchers have been working to develop new methods to enhance the stability of probiotics. Nanoencapsulation of probiotic bacteria greatly enhances their stability.
Encapsulation involves enclosing a bioactive molecule in its liquid, solid, or gaseous state inside an inert matrix or substance, typically a polymer. The coated product (flavor or food molecules/ingredients) can be preserved by an enclosed wall, enhancing its durability in the appropriate environment. With better control over their release management at the active physiological site, encapsulation can provide increased stability of bioactive components in food.[Citation2,Citation3] Nanotechnology has emerged to enhance the safety and quality of the finished products.[Citation4]
The development and use of mechanisms, materials, or systems at the nm scale are referred to as nanotechnology. In the past 10 years, many researchers have concentrated on using nanotechnology to encapsulate bioactive substances in various industries, including food, cosmetics, and pharmaceuticals.[Citation5,Citation6] The use of films, layers, and coverings to create encapsulation on a nanoscale scale is referred to as nanoencapsulation. The layer or film implemented for encapsulation is nanoscale and serves as a protective covering for components or materials used in food or flavoring.[Citation7] The functional components are transported through nanoencapsulation to the intended site of action. Nanoencapsulation’s homogeneity, which leads to improved encapsulation efficiency and acceptable chemical and physical properties, is one of its key advantages. This method is a desirable substitute for redesigning functional food ingredients that can significantly affect the process-ability, stability bioavailability of goods.[Citation8,Citation9]
Protein nanoparticles are a promising system for encapsulating and delivering drugs and bioactive substances.[Citation10] In recent years, attention has been drawn to the functional qualities of foodstuff proteins, in particular, such as water-binding ability, gelation, foaming, and emulsification, as well as their uses as components in the food industry. Protein-based structures are one of the most practical and often utilized matrices in food uses.[Citation11]
Animal-based proteins (albumin, casein, gelatin, whey, silk proteins) and plant proteins (zein, gliadin, soy proteins, and pea proteins) are commonly utilized for nanoencapsulation. These proteins can be recombinant (like recombinant ferritin silk proteins) or natural sources.[Citation12] Among all these proteins, whey protein is associated with animal proteins and is frequently found in dairy-based food systems and ingested in daily life. They are a blend of globular proteins separated from cheese-making whey and by-products. Immunoglobulin, bovine serum albumin, a-lactalbumin, and b-lactalbumin make up the whey protein.[Citation13] Whey protein hydrolyzates (WPHs), whey protein concentrate (WPC), whey protein isolates (WPI), and native whey proteins are the four main types of isolated whey protein. Whey protein isolate (WPI) is a highly purified type that can provide more than 80% protein, while whey protein concentrate can provide up to 35–80% protein.[Citation14] Zein protein from plant origin, which makes up approximately 50% of the overall protein composition in maize, is the primary protein in the maize. It is made up of lipid-soluble amino acid residues and is a member of the prolamin class.[Citation15]
Zein is a composite protein or polypeptide made up of many proteins, which are often categorized according to molecular weights, solubility, and charges. α-, β-, and γ-zein are the three primary fractions identified based on their solubility and molecular weight. 75–85% of the total zein in maize is made up of the largest fraction, α-zein, whereas the genotype-dependent amounts of β- and γ-zein are only 10–15% and 5–10%, respectively. Mostly used food sources for adding probiotics such as lactobacilli and bifidobacteria, are yogurt and other dairy products.[Citation16] The current study was planned to encapsulate the probiotics “Lactobacillus rhamnoses” in whey protein and zein protein nanoparticles as wall materials and to evaluate the effect of the encapsulation materials under different technological conditions and in simulated gastric and intestinal conditions. At the end of the study, yogurt was prepared and the capsules were added to it to determine the sensory properties of yogurt with the added cells.
Experimental study
The current research was carried out at Food Safety and Biotechnology Lab, Government College University Faisalabad, Pakistan. Raw materials, i.e., whey protein isolate (WPI), were purchased from the local scientific store of Faisalabad. Zein protein was obtained from Rafhan Maize Co. Ltd. Faisalabad. Probiotic (Lactobacillus rhamnosus) culture and Calcium Chloride (CaCl2) were obtained from the National Institute of Food Science & Technology (NIFSAT), University of Agriculture Faisalabad.
Preparation of nanoparticles and probiotic encapsulation
Dispersion of whey and zein protein was prepared individually before nanoparticle preparation. For this purpose, zein protein was dispersed in 100 mL of aqueous-ethanol solution (85% v/v) to a protein concentration of 10% (w/v). It was then stirred using a magnetic stirrer at 500 rpm for 30 min to ensure dissolution by following the method as reported by Hu & McClements.[Citation17] Deionized water was used to disperse whey protein to a 10% (w/v) protein concentration. Then, the pH of both dispersions was adjusted to 7 by adding 1 N NaOH. The dispersions were kept for overnight storage at 4°C. After overnight storage, the dispersions were warmed to room temperature and degassed under a vacuum for 20 min. The whey and zein dispersions were placed in a water bath, and the temperature was increased by 10°C/min until the temperature reached 80°C and kept at that temperature for 15 min. Then, the dispersions were cooled to room temperature in a water bath and diluted immediately to a protein concentration of 2% (v/v).
The nanoparticles were produced by following the pH cycling treatment of Giroux et al., with some modifications.[Citation18] At first, zein and whey protein dispersions were acidified with 0.1 N HCl to the aggregation pH (5.5). CaCl2 was added to a final concentration of 2.5 mM. The pH level was adjusted again to aggregation pH (5.5) after adding CaCl2 if necessary for any dispersion. The protein concentration was 1.9 (w/v) at this point. The preparations were aged for a period of 0, 5, and 24 h at 4°C to allow the formation of disulfide bonds between protein polymers. After the aging period, the dispersions were again neutralized to pH 7 with 0.1 N NaOH solution. The final protein concentration was 1.5% (w/v). This increase in pH level promoted electrostatic repulsions and disrupted non-covalently linked aggregates. The dispersions were immediately homogenized at a speed of 20 MPa to dissociate the large and loosely bound aggregates further. Homogenization reduces the average size of nanoparticles to its minimum. Then, the homogenized whey and zein protein dispersions were centrifuged for 15 min at 4°C. The centrifuged sample was freeze-dried to get the protein nanoparticles in powdered form. Different treatments were named Fp (free probiotics), EWNp (probiotics encapsulated with whey protein nanoparticles), and EZNp (nano encapsulated with zein nanoparticles).
Characterization of probiotic-loaded nanoparticles
Encapsulation efficiency
Encapsulation efficiency expresses the efficiency of nanoparticles to encapsulate probiotics. The encapsulation efficiency of zein nanoparticles and whey protein nanoparticles for probiotics was determined by following the method of Afzaal et al., with slight modifications.[Citation19] The encapsulation efficiency (EE %) of nanoparticles for probiotics encapsulation was calculated as follows:
Particle size
The particle size of the prepared capsules was determined by static light scattering. Each sample of probiotics encapsulated in nanoparticles (2 mL) was added to distilled water (10 ml) and vortexed to prevent multiple scattering effects and ensure homogeneity before analysis. Surface-weighted and volume-weighted mean particle diameters were acquired for all samples.
Scanning electron microscopy
SEM images were obtained to determine the overall appearance of nano-encapsulated probiotics by following the method of Atraki & Azizkhani, with slight modifications.[Citation20] The manufactured nano-encapsulated probiotics samples were entered from the side of the tape for the images, followed by gold spraying was done on it. A scanning electron microscope with high-resolution and low-vacuum pressure was used to examine the materials.
FT-IR (Fourier Transform Infrared Spectroscopy)
FTIR analysis was performed by following the method of Wei et al. (2021) with slight modifications.[Citation21] The FTIR available at NIFSAT, University of Agriculture, Faisalabad, was used to obtain the FTIR spectrum of probiotic-loaded whey nanoparticles and probiotics-loaded zein nanoparticles. The results obtained showed various peaks of the FTIR spectrum for whey nanoparticles as well as zein nanoparticles.
In vitro analysis in simulated gastric and intestinal fluid
In vitro analysis was performed to examine the survival of free and nano-encapsulated probiotic bacteria in simulated gastrointestinal conditions. On purpose, simulated solutions were prepared by using analytical-grade chemicals and aseptic conditions. The viability of free and nano-encapsulated probiotics was determined in simulated gastrointestinal conditions by following the method described by Atraki and Azizkhani, with slight modifications.[Citation20] The pH of the phosphate buffer solution was adjusted to 2 using HCl (1 M). To prepare simulated gastric pepsin, the solution was added to the phosphate buffer solution at 2 g/L. All the solutions and glassware used to prepare artificial gastric fluid were sterilized. To prepare simulated intestinal fluid, 12 g of dipotassium hydrogen phosphate, 2 g of bovine salt, 154 mL of 0.2 M solutions of NaOH, and 20 mL of trypsin were added to deionize distilled water (500 mL). The solution was adjusted to pH 6.8–7.0 using 0.2 M NaOH. Then, the final volume was increased to 1 L using deionized distilled water. The 1 g of probiotics samples (free and nano encapsulated) were added separately to simulated gastric and intestinal fluid (9 mL) and incubated on a shaker at 100 rpm temperature was kept at 37°C. Live cells from each treatment were counted after 0, 5, 30, 60, and 120 min of incubation. The probiotic cells’ ability to survive in a continuous model of gastrointestinal fluid was also assessed. Following a two-hour exposure to simulated gastric fluid (under the identical circumstances as previously described), 1 mL of free and nano-encapsulated probiotics in phosphate buffer solution was introduced into simulated intestinal fluid. Viable cell counting was done for each sample/treatment after 0, 30, 60, 90 and 120 min of incubation at 37°C (Ji et al., 2019). PBS solution was used to make 10-fold serial dilutions from each sample, which were then cultivated on MRS agar and incubated at 37°C for 48 h to count the live cells. Results were recorded in triplicates.
Preparation of model food (Yogurt)
For the preparation of probiotic yogurt, the whole milk was homogenized at 45°C, and to make the yogurt with 18% SNF, skim milk was added with continuous stirring. Then, the milk was heated for 20 min at 80–85°C. The milk was allowed to cool up to 45°C, and a starter culture was added along with free and nano-encapsulated probiotics in different samples. The fermentation process stopped when the milk’s pH reached 4.5, and the yogurt cooled down quickly. Yogurt was allowed to be set at 5°C, and CFU/g of yogurt was determined at a defined interval of time.
Characterization of yogurt
Viability of probiotics
Probiotics viability in prepared yogurt was analyzed by following the method described by Shoji et al,[Citation22,23] with some modifications. Yogurt was kept at 4°C and was examined at intervals of 0, 7, 14, 21, and 28 days. All samples of yogurt were enumerated in MRS agar. The spread plate technique was used for the plating of culture for yogurt. Serial dilutions were prepared with a 20g/L sodium citrate solution, with vigorous agitation to release probiotics in the nanoparticles. The plates were incubated at 37°C for 48 h. The viable cell count was calculated. Media and glassware used for the viability assessment of probiotics were completely sterilized using an autoclave and hot air oven. The plating was performed in duplicate.
Sensory analysis
Sensory analysis of yogurt was done in terms of color, texture, taste, flavor, and overall acceptability on a 9-point hedonic scale with 1=dislike extremely, 5=neither like nor a dislike, 9=like extremely by following the method of Meilgard et al., 2007. A panel of 10 expert judges of Government College University Faisalabad evaluated the yogurt at different intervals (0, 5, 10, and 24 h) for all three treatments.
Statistical analysis
All of the data were put through ANOVA (analysis of variance) to see if there was a significant difference (p < .05) between the different treatments. The results were indicated as the mean values of three replicates.
Results and discussion
Nanoparticles were characterized to determine particle size and encapsulation efficiency and evaluate the stability of prepared nanoparticles during in vitro analysis. SEM, FTIR, and XRD analysis were performed to evaluate nanoparticle efficiency for enhancing encapsulated probiotics’ viability.
Characterization of probiotic-loaded protein nanoparticles
Particle size and zeta potential
The particle size of zein and whey protein nanoparticles were analyzed by static light scattering. There was a significant difference between the average particle size of blank and probiotics-loaded nanoparticles. Blank protein nanoparticles were smaller in size as compared to probiotics-loaded nanoparticles. This increase in size shows the successful entrapment of probiotics in zein and whey nanoparticles. The average size of whey protein nanoparticles was 3.75 µm, and of zein, nanoparticles size was near 3.91 µm, as shown in . These results show that when zein and whey proteins were used alone to prepare nanoparticles, they showed better results than zein nanoparticles, which may be due to the hydrophobic nature of zein or due to change in flow properties of zein dispersions due to its colloidal structure. The results were in line with the results reported by Salem et al.[Citation24] Another study by Hu & McClements (2014) reported similar results. Zhan et al. also reported similar outcomes.[Citation25]
Table 1. Encapsulation efficiency, capsule size, and zeta potential of whey and zein nanoparticles.
Encapsulation efficiency
Encapsulation efficiency is an imperative factor in assessing the efficacy of nanoparticles. The present study used whey and zein as encapsulants to protect probiotics. The encapsulation efficiency of whey and zein nanoparticles is shown in . From the total amount of probiotics added to the nanoparticles, 96% were encapsulated successfully in whey nanoparticles, and 87% were successfully encapsulated in zein protein nanoparticles. The results showed that the whey protein showed slightly better encapsulation efficiency than zein protein nanoparticles, which may be due to the excellent gelling and binding properties of whey protein. Also, the colloidal structure of zein protein can affect the encapsulation efficiency.
All the probiotics encapsulated in whey nanoparticles showed a very low log reduction, providing excellent viability for probiotics. A viable cell count of nearly 107 CFU/g was obtained from whey protein encapsulation, and 106 CFU/g was obtained from zein protein encapsulation. Both values fall under the required range of probiotics. The mean results illustrated that the method applied here was effective in encapsulating/entrapping the probiotics. Similar results for zein encapsulation were reported by Podaralla & Perumal.[Citation24] Picot & Lacroix also conducted a study with similar results.[Citation26]
Scanning electron microscopy
SEM images for probiotics encapsulated in zein protein nanoparticles and whey protein nanoparticles are shown below. Images showed that both the zein and whey nanoparticles attained their irregular spherical structure after encapsulation of probiotics. It is clear from the images that the nanoparticles of zein protein are slightly larger than the whey protein nanoparticles, which may be due to the presence of calcium ions which causes large aggregates, or due to the hydrophobic nature of zein.
Whey nanoparticles showed irregular shapes when SEM images were taken from 1 µm (). Zein nanoparticles showed irregular structure (). This irregularly spherical compact structure of nanoparticles is due to the shrinkage of particles due to loss of moisture during the process of lyophilization or freeze-drying.
Figure 1. SEM images for probiotic-loaded protein nanoparticles; (a) SEM image of probiotic-loaded whey nanoparticles from 1 µm scale; (b) SEM image of probiotic-loaded zein nanoparticles from 1 µm scale.
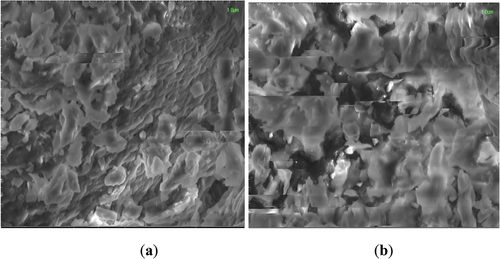
During the comparison of all treatments of nanoparticles, it was observed from a 1 µm scale that zein protein forms slightly larger nanoparticles than whey nanoparticles. It was observed that the probiotics were evenly distributed throughout the nanoparticle’s structure and provided better encapsulation. SEM results are in line with the study results of de Araújo et al..[Citation27] Similar results were described by Parris et al..[Citation28] Salem et al.,[Citation24] also described similar results. A study was conductd by Riaz et al.,[Citation29] in which zein protein was used as a wall material for the encapsulation of probiotics. The results regarding SEM reported by their study suggest that B. bifidum was effectively entrapped in zein-coated microcapsule while in alginate beads the surface was irregular, porous with visible cavities and channels. By increasing the zein concentration, the surface of the cells become smooth, compact, homogeneous with less holes and channels because zein provides maximum protection as compared to SA beads (without coating). The present study also suggests that zein is the better encapsulating material than the whey protein as it provides more fine capsules and better protection to probiotic cells.
FTIR analysis
The FTIR spectra of probiotics-loaded samples of whey protein nanoparticles are shown in . The IR spectrum of probiotics-loaded whey nanoparticles demonstrated a significant signal at 2355 cm−1 corresponding to the amide O=C=O stretch vibration. Another peak was observed at 616.6175 cm−1, corresponding to the C-Br stretching. The examined shifts in certain bands between the probiotics-loaded whey nanoparticles were probably due to hydrophobic interactions in loaded nanoparticles. Wei et al. (2021) reported that whey nanoparticles showed peaks at relatively lower wavenumbers than the present study.
Figure 2. The Fourier Transform Infrared spectroscopy (FTIR) spectra of (a) whey protein Isolate (WPI) and (b) zein capsules loaded with probiotics.
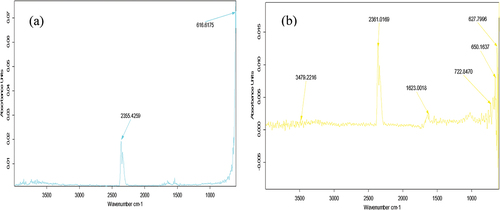
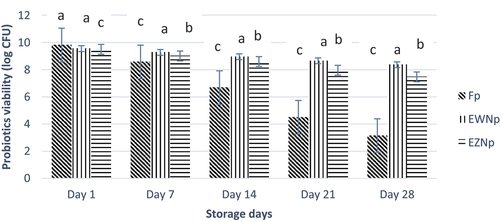
Figure 3. Viability of free and nano encapsulated probiotics (log CFU/g) under simulated gastric conditions (SGC) during storage interval. Each bar represents the mean value for probiotic viability. Fp (Free probiotics), EWNp (encapsulated probiotic with Whey Protein Isolate), and EZNp (encapsulated probiotics with zein protein).
The O=C=O stretching bonds stretch at 2361 cm−1 in the spectrum of probiotics-loaded zein nanoparticles is probably due to the carbon dioxide connected to the cyclohexene end group. The aromatic N-H stretching modes were observed at a lower wavelength than the aliphatic N-H stretching stretches at 3479 cm−1. In comparison, the distinctive signal for the C=C stretches due to the two terminal cyclohexene groups, which appeared at 1623 cm−1. Another characteristic signal of probiotics-loaded zein nanoparticles appeared at 722 cm−1, which is typical for the C-H of conjugated alkene. Wei et al. (2021) reported that FTIR spectra of zein showed characteristic peaks at lower wavenumbers than the present study.[Citation21] Mahalakshmi et al. (2020) also reported peaks at lower wavenumbers.[Citation30,Citation31] A close peak to the present study, 1636 cm−1, was also observed. A study by Reboredo et al.[Citation32] reported similar C-N and N-H stretching vibrations.
Viability of nano-encapsulated probiotic bacteria under simulated gastric conditions
The stability of probiotic bacteria nano-encapsulated in whey and zein nanoparticles was assessed under simulated gastric conditions by artificially making gastric juice and exposing nano-encapsulated probiotics. Probiotics showed a decreasing trend with an increase in exposure time. Unencapsulated probiotics showed a rapid loss of viability compared to nano-encapsulated probiotics. A decrease in viability from 9.63 to 3.37 CFU/mL was observed in the case of unencapsulated probiotics. At the same time, nano-encapsulated probiotics showed a minor loss of viability. Maximum mean viability was observed in treatment EWNp (8.88), followed by treatment EZNp (8.51) and Fp (6.61). The data obtained from statistics illustrated that the results are highly significant for all the treatments and study time and interaction between the study period and treatments. The trend found was EWNp > EZNp > Fp (). The better protection of probiotics in whey nanoparticles may be due to its ability to protect probiotics from the harsh environment of GIT because of the buffering capacity of whey protein. Hence, the results confirmed that nanoencapsulation of probiotics could provide a shielding effect in the gastric environment. Similar results were also reported by Salem et al.[Citation24] The results were also in line with the results described by Mahalakshmi et al.[Citation30]
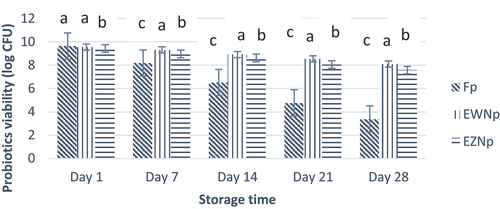
Figure 4. Viability of free and nano-encapsulated probiotics (log CFU/g) under simulated gastric conditions (SIC) during storage interval. Each bar represents the mean value for probiotic viability. Fp (Free probiotics), EWNp (encapsulated probiotic with Whey Protein Isolate), and EZNp (encapsulated probiotics with zein protein).
Viability of nano-encapsulated probiotic bacteria under simulated intestinal conditions
The stability of probiotic bacteria nano-encapsulated in whey and zein nanoparticles was assessed under simulated intestinal conditions by artificially making intestinal juice of pH 7 and exposing nano-encapsulated probiotics. Probiotics showed a decreasing trend with an increase in exposure time. Unencapsulated probiotics showed a rapid loss of viability compared to nano-encapsulated probiotics (). A decrease in viability from 9.83 to 3.15 CFU/mL was observed in the case of unencapsulated probiotics. At the same time, whey nano-encapsulated probiotics showed a minor loss of viability from 9.56 to 8.37 CFU/mL, followed by zein nano-encapsulated probiotics, which showed a decrease from 9.51 to 7.48 CFU/mL. Hence, maximum mean viability was observed in the treatment of EWNp (8.97) followed by the treatment of EWNp (8.52) and Fp (6.55). According to Guerin et al.,[Citation33] milk-based proteins may help bifidobacteria survive under the SGC better than free bacteria when enclosed in pectin, alginate, and whey proteins. Additionally, it has been suggested that milk fat may help protect probiotics from an acidic environment by reducing the diffusion of O2, H+, and organic acids Picot and Lacroix.[Citation16]
Figure 5. Effect of free and nano-encapsulated probiotics on the viability of probiotics in different yogurt treatments. Each bar represents the mean value for probiotic viability. Y1 (control yogurt sample with no or without probiotics), Y2 (yogurt treatment with added probiotic capsules having whey protein isolate wall material), and Y3 (yogurt treatment with added probiotic capsules having Zein protein wall material).
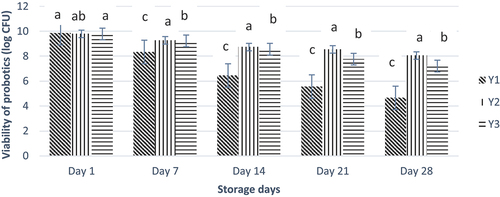
Characterization of probiotic yogurt
Viability of probiotics
Probiotics viability is an important factor when incorporating them into functional food products. The bacteria must survive and pass through the digestive tract of the consumer to provide beneficial effects to the consumer as expected. Encapsulation has provided great protection to the probiotics from the outside environment so that the probiotics can provide the required advantages to consumers.
A decreasing trend was observed for free probiotics’ viability compared to the nano-encapsulated probiotics. Free probiotics showed a decrease in viability from 9.84 to 4.65 CFU/mL. Whey nano-encapsulated probiotics depicted a stable viability decrease from 9.79 to 8.05, whereas zein nanoencapsulation showed a decrease from 9.78 to 7.21 CFU/mL (). Maximum mean viability was observed in treatment Y2 (8.88), followed by treatment Y3 (8.51) and Y1 (6.97). Hence, nanoencapsulation enhances the stability and viability of probiotics. Shoji et al.[Citation23] reported that encapsulation enhances the viability of probiotics in yogurt compared to free probiotics. Menezes et al.[Citation34] explained that similar results and outcomes confirmed the value of encapsulated probiotics with whey protein concentrate, demonstrating better viability for Lactobacillus acidophilus. Mabrouk et al. (2021) also reported that protein encapsulation prevents the probiotic strains from hostile conditions for longer storage than unencapsulated probiotics.[Citation35]
Figure 6. Effect of free and nano-encapsulated probiotics on the viability of probiotics in different yogurt treatments. Each bar represents the mean value for probiotic viability. Y1 (control yogurt sample with no or without pro-biotics), Y2 (yogurt treatment with added probiotic capsules having whey protein isolate wall material), and Y3 (yogurt treatment with added probiotic capsules having Zein protein wall material).
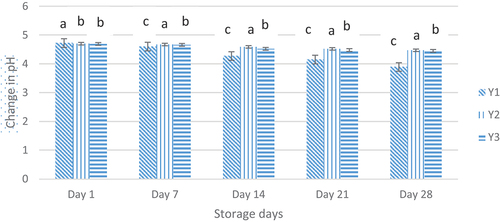
Physicochemical analysis
The bacteria present in yogurt may utilize carbohydrates in yogurt and produce organic acids, which results in the pH level to decrease. The statistical results illustrated that the pH value of yogurt treatment varied significantly (p < .05) with storage days (). The mean result of pH analysis is given in. Mean results illustrated that the highest mean value (4.59) of pH was observed in Y2 (Whey nanoencapsulated probiotic yogurt) followed by Y3 (4.56) while the lowest value (4.4) was observed in Y1 (Free probiotic yogurt). There was a decreasing trend observed for pH in the order Y2 (4.37) > Y3 (4.35) > Y1 (4.08). A lot of research work has confirmed the fact that the pH value of yogurt falls with the storage days such as Kailasapathy et al. (2006). Afzaal et al. (2022) also reported that whey protein encapsulation showed a less decrease in pH level of the yogurt.
Figure 7. Effect of free and nano-encapsulated probiotics on different sensory parameters of yogurt treatments. Y1 (control yogurt sample with no or without probiotics), Y2 (yogurt treatment with added probiotic capsules having whey protein isolate wall material), and Y3 (yogurt treatment with added probiotic capsules having Zein protein wall material).
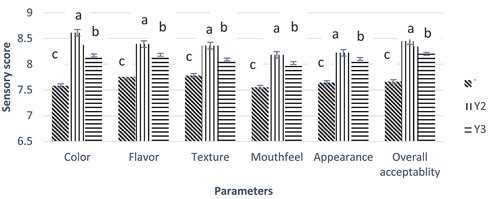
Sensory evaluation
The addition of free or nano-encapsulated probiotics in yogurt affects the sensory parameters of the yogurt samples (). Added probiotics (either free or encapsulated) had no significant effect on the color and flavor parameters of the yogurt. The texture attribute was significantly affected by the addition of probiotics. Compared to free probiotic yogurt, a high sensory score was observed for whey nano-encapsulated probiotic yogurt. The overall sensory properties of yogurt were affected during storage. Yasmin et al. (2019) conducted a study with similar results and described that the whey protein provides a fine texture to nano-encapsulated probiotic yogurt.[Citation36] Kailasapathy reported similar results that describe that the encapsulated probiotics show better sensory scores than unencapsulated probiotics.[Citation37]
Conclusion
Nanoencapsulation enhanced the stability/viability of probiotics as compared to free probiotics. It was illustrated that nano-encapsulated probiotics remain more stable during storage conditions as well as for thermal treatments. Nanoencapsulation influenced the viability of probiotics in simulated gastrointestinal conditions compared to unencapsulated/free probiotics. The addition of nano-encapsulated probiotics greatly enhances the viability of probiotics in the carrier food compared to unencapsulated/free probiotics. Nanoencapsulation improved the microbiological and physicochemical properties of carrier food. The addition of nano-encapsulated probiotics also influenced sensory properties compared to unencapsulated/free probiotics. Hence, the research results concluded that nanoencapsulation of probiotics could be a great opportunity for food industries to develop new functional products.
Author contributions
Conceptualization: F.K; M.A; methodology: F.S; G.A.N; validation: S.A.; formal analysis: M.J.A; S.A.; investigation: H.A; H.Y; data curation: M.J.A; writing – original draft preparation: F.K; H.A; H.Y; A.M.K; M.A.S.; writing – review and editing: M.A; S.A.; F.I.; G.A.N; project administration: M.A; F.S; funding acquisition: S.A.; M.J.A. All authors have read and agreed to the published version of the manuscript.
Institutional review board statementy
This study does not contain any studies with human or animal subjects performed by any authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Afzaal, M.; Saeed, F.; Ateeq, H.; Ahmed, A.; Ahmad, A.; Tufail, T.; Anjum, F. M.; Anjum, F. M. Encapsulation of Bifidobacterium Bifidum by Internal Gelation Method to Access the Viability in Cheddar Cheese and Under Simulated Gastrointestinal Conditions. Food Sci. Nutr. 2020, 8(6), 2739–2747. DOI: 10.1002/fsn3.1562.
- Malekhosseini, P.; Alami, M.; Khomeiri, M.; Esteghlal, S.; Nekoei, A. R.; Hosseini, S. M. H. Development of Casein‐Based Nanoencapsulation Systems for Delivery of Epigallocatechin Gallate and Folic Acid. Food Sci. Nutr. 2019, 7(2), 519–527. DOI: 10.1002/fsn3.827.
- Gómez, B.; Barba, F. J.; Domínguez, R.; Putnik, P.; Kovačević, D. B.; Pateiro, M.; Lorenzo, J. M., Lorenzo, J M. Microencapsulation of Antioxidant Compounds Through Innovative Technologies and Its Specific Application in Meat Processing. Trends In Food Sci. Technol. 2018, 82, 135–147. DOI: 10.1016/j.tifs.2018.10.006.
- Pateiro, M.; Gómez, B.; Munekata, P. E.; Barba, F. J.; Putnik, P.; Kovačević, D. B.; Lorenzo, J. M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules. 2021, 26(6), 1547. DOI: 10.3390/molecules26061547.
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S. M. Lipid Nano Scale Cargos for the Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. DOI: 10.1016/j.tifs.2018.02.001.
- Assadpour, E.; Jafari, S. M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Ann. Rev. Food Sci. Technol. 2019, 10(1), 103–131. DOI: 10.1146/annurev-food-032818-121641.
- Paredes, A. J.; Asensio, C. M.; Llabot, J. M.; Allemandi, D. A.; Palma, S. D. Nanoencapsulation in the Food Industry: Manufacture, Applications and Characterization. Journal of Food Bioengineering and Nanoprocessing, 2016, 1(1), 56–79.
- Human, C.; De Beer, D.; Van Der Rijst, M.; Aucamp, M.; Joubert, E. Electrospraying as a Suitable Method for Nanoencapsulation of the Hydrophilic Bioactive Dihydrochalcone, Aspalathin. Food Chem. 2019, 276, 467–474. DOI: 10.1016/j.foodchem.2018.10.016.
- Mahmoud, K. F.; Ali, H. S.; Amin, A. A. Nanoencapsulation of Bioactive Compounds Extracted from Egyptian Prickly Pears Peel Fruit: Antioxidant and Their Application in Guava Juice. Asian J. Sci. Res. 2018, 11(4), 574–586. DOI: 10.3923/ajsr.2018.574.586.
- Ko, S.; Gunasekaran, S. Preparation of Sub-100-Nm β-Lactoglobulin (BLG) Nanoparticles. J. Microencapsulation. 2006, 23(8), 887–898. DOI: 10.1080/02652040601035143.
- Bourbon, A. I.; Pereira, R. N.; Pastrana, L. M.; Vicente, A. A.; Cerqueira, M. A. Protein-Based Nanostructures for Food Applications. Gels. 2019, 5(1), 9. DOI: 10.3390/gels5010009.
- Fathi, M.; Donsi, F.; McClements, D. J. Protein‐Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17(4), 920–936. DOI: 10.1111/1541-4337.12360.
- Haug, A.; Høstmark, A. T.; Harstad, O. M. Bovine Milk in Human Nutrition–A Review. Lipids Health Dis. 2007, 6(1), 1–16. DOI: 10.1186/1476-511X-6-25.
- Vargas Lopez, L. A. Influence of“added” Whey Protein Isolate on Probiotic Properties of Yogurt Culture Bacteria and Yogurt Characteristics. 2013.
- Pascoli, M.; De Lima, R.; Fraceto, L. F. Zein Nanoparticles and Strategies to Improve Colloidal Stability: A Mini-Review. Front Chem. 2018, 6, 6. DOI: 10.3389/fchem.2018.00006.
- Picot, A.; Lacroix, C. Encapsulation of Bifidobacteria in Whey Protein-Based Microcapsules and Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. Dairy J. 2004, 14(6), 505–515. DOI: 10.1016/j.idairyj.2003.10.008.
- Hu, K.; McClements, D. J. Fabrication of Surfactant-Stabilized Zein Nanoparticles: A pH Modulated Antisolvent Precipitation Method. Food Res. Int. 2014, 64, 329–335. DOI: 10.1016/j.foodres.2014.07.004.
- Giroux, H. J.; Houde, J.; Britten, M. Preparation of Nanoparticles from Denatured Whey Protein by Ph-Cycling Treatment. Food Hydrocoll. 2010, 24(4), 341–346. DOI: 10.1016/j.foodhyd.2009.10.013.
- Afzaal, M.; Khan, A. U.; Saeed, F.; Arshad, M. S.; Khan, M. A.; Saeed, M.; Anjum, F. M.; Khan, M. K.; Ismail, Z.; Ahmed, A. Survival and Stability of Free and Encapsulated Probiotic Bacteria Under Simulated Gastrointestinal Conditions and in Ice Cream. Food Sci. Nutr. 2020, 8(3), 1649–1656. DOI: 10.1002/fsn3.1451.
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. DOI: 10.1016/j.ifset.2021.102750.
- Wei, Y.; Zhan, X.; Dai, L.; Zhang, L.; Mao, L.; Yuan, F.; Liu, J.; Gao, Y. Formation Mechanism and Environmental Stability of Whey Protein Isolate-Zein Core-Shell Complex Nanoparticles Using the Ph-Shifting Method. LWT. 2021, 139, 110605. DOI: 10.1016/j.lwt.2020.110605.
- Yi, J.; Lam, T. I.; Yokoyama, W.; Cheng, L. W.; Zhong, F. Beta-Carotene Encapsulated in Food Protein Nanoparticles Reduces Peroxyl Radical Oxidation in Caco-2 Cells. Food Hydrocoll. 2015, 43, 31–40. DOI: 10.1016/j.foodhyd.2014.04.028.
- Shoji, A. S.; Oliveira, A. C.; Balieiro, J. C. D. C.; Freitas, O. D.; Thomazini, M.; Heinemann, R. J. B.; Fávaro-Trindade, C. S.; Favaro-Trindade, C. S. Viability of L. Acidophilus Microcapsules and Their Application to Buffalo Milk Yoghurt. Food Bioprod. Process. 2013, 91(2), 83–88. DOI: 10.1016/j.fbp.2012.08.009.
- Salem, A.; Ramadan, A. R.; Shoeib, T. Entrapment of β-Carotene and Zinc in Whey Protein Nanoparticles Using the pH Cycle Method: Evidence of Sustained Release Delivery in Intestinal and Gastric Fluids. Food Biosci. 2018, 26, 161–168. DOI: 10.1016/j.fbio.2018.10.002.
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of Curcumin in Whey Protein Isolate and Zein Composite Nanoparticles Using Ph-Driven Method. Food Hydrocoll. 2020, 106, 105839. DOI: 10.1016/j.foodhyd.2020.105839.
- Podaralla, S.; Perumal, O. Influence of Formulation Factors on the Preparation of Zein Nanoparticles. AAPS Pharm. Scitech. 2012, 13(3), 919–927. DOI: 10.1208/s12249-012-9816-1.
- de Araújo Etchepare, M.; Nunes, G. L.; Nicoloso, B. R.; Barin, J. S.; Flores, E. M. M.; de Oliveira Mello, R.; de Menezes, C. R. Improvement of the Viability of Encapsulated Probiotics Using Whey Proteins. LWT. 2020, 117, 108601. DOI: 10.1016/j.lwt.2019.108601.
- Parris, N.; Cooke, P. H.; Hicks, K. B. Encapsulation of Essential Oils in Zein Nanospherical Particles. J. Agric. Food Chem. 2005, 53(12), 4788–4792. DOI: 10.1021/jf040492p.
- Riaz, T.; Iqbal, M. W.; Saeed, M.; Yasmin, I.; Hassanin, H. A.; Mahmood, S.; Rehman, A. In vitro Survival of Bifidobacterium Bifidum Microencapsulated in Zein-Coated Alginate Hydrogel Microbeads. J. Microencapsul. 2019, 36(2), 192–203. DOI: 10.1080/02652048.2019.1618403.
- Mahalakshmi, L.; Leena, M. M.; Moses, J. A.; Anandharamakrishnan, C. Micro-And Nano-Encapsulation of β-Carotene in Zein Protein: Size-Dependent Release and Absorption Behavior. Food Funct. 2020, 11(2), 1647–1660. DOI: 10.1039/C9FO02088H.
- Hu, D.; Lin, C.; Liu, L.; Li, S.; Zhao, Y. Preparation, Characterization, and in vitro Release Investigation of Lutein/Zein Nanoparticles via Solution Enhanced Dispersion by Supercritical Fluids. J. Food Eng. 2012, 109(3), 545–552. DOI: 10.1016/j.jfoodeng.2011.10.025.
- Reboredo, C.; González-Navarro, C. J.; Martínez-Oharriz, C.; Martínez-López, A. L.; Irache, J. M. Preparation and Evaluation of PEG-Coated Zein Nanoparticles for Oral Drug Delivery Purposes. Int. J. Pharmaceutics. 2021, 597, 120287. DOI: 10.1016/j.ijpharm.2021.120287.
- Guérin, D.; Vuillemard, J. C.; Subirade, M. Protection of Bifidobacteria Encapsulated in Polysaccharide-Protein Gel Beads Against Gastric Juice and Bile. J. Food Prot. 2003, 66(11), 2076–2084. DOI: 10.4315/0362-028X-66.11.2076.
- Menezes, M. F. D. S. C. D.; Silva, T. M. D.; Etchepare, M. D. A.; Fonseca, B. D. S.; Sonza, V. P.; Codevilla, C. F.; Menezes, C. R. D.; Silva, C. D. B. D.; Menezes, C. R. D. Improvement of the Viability of Probiotics (Lactobacillus acidophilus) by Multilayer Encapsulation. Ciência Rural. 2019, 49(9). DOI: 10.1590/0103-8478cr20181020.
- Mabrouk, A. M.; Salama, H. H.; El Sayed, H. S.; El Sayed, S. M. Preparation of Symbiotic Whey Protein Gel as a Carrier of Free and Encapsulated Probiotic Bacteria. J. Food Process. Preserv. 2021, 45(7), e15612. DOI: 10.1111/jfpp.15612.
- Yasmin, I.; Saeed, M.; Pasha, I.; Zia, M. A. Development of Whey Protein Concentrate-Pectin-Alginate Based Delivery System to Improve Survival of B. Longum BL-05 in Simulated Gastrointestinal Conditions. Probiotics Antimicrob. Proteins. 2019, 11(2), 413–426. DOI: 10.1007/s12602-018-9407-x.
- Kailasapathy, K. Survival of Free and Encapsulated Probiotic Bacteria and Their Effect on the Sensory Properties of Yoghurt. LWT Food Sci. Technol. 2006, 39(10), 1221–1227. DOI: 10.1016/j.lwt.2005.07.013.
