ABSTRACT
The production of meat using animal stem cell-derived muscle tissue might conceivably do away with the need to sacrifice animals. The creation of “cultured,” “synthetic,” or “in vitro” meat has the potential to produce meat with distinct qualities more quickly and efficiently than normal meat. Although the process of growing muscle tissues in culture from stem cells has been known for a very long time, it has not yet been perfected for the production of cultured meat products for sale. Conditions for applying the technology, which is currently in its infancy, include a phenomenally high level of consumer acceptability and the development of commercially feasible large-scale production techniques. If the meat produced in vitro has physical traits that are identical to those of traditional meat in terms of color, flavor, aroma, consistency, and deliciousness, then it might be realistically viable. Higher the viability of meat production in vitro, the issues including searching for a good stem cell source and talking about the challenges faced throughout the expansion of cultured meat must be resolved. This review highlights the benefits and advancement of cultured meat, highlights its connection problems, and offers prospective solutions for production-related problems.
Introduction
Despite the fact that meat has remained a ubiquitous diet, individuals have voiced increased worry about certain effects of meat consumption. Over the past few years, the news media has featured a number of depressing tales about the manufacturing of beef. Several of the instances are the Bovine Spongiform Encephalopathy (BSE or Mad Cow Disease) outbreaks, the Escherichia coli, and Salmonella outbreaks, the harsh treatment of animals, and the impact of livestock farming on climate change[Citation1]. Those certain types of tales have reinforced buyers’ rising discontent about the meat industry indicating that competitiveness in the food store requires a grasp of customer needs and concerns around livestock farming. Along with market worries about meat consumption, traditional meat producers’ capacity to meet prospective meat requirements is in doubt, raising the meat business’s attention.[Citation2,Citation3]
Cultured meat is meat produced outside of the animal and in vitro. cultivated meat, in particular, is made from animal cells cultivated in a growth medium in a bioreactor rather than being taken directly from slain animals. Cultured meat is thus produced in a significantly different manner than traditional live stock procedures. Scientists, politicians, and artists have long lobbied for the production of meat in vitro. This concept is now becoming a technical reality, as cultured meat is being commercialized. Several firms are now attempting to develop and commercialize their product in the next years.[Citation4]
Cultured meat is a technological revolution, but it also has the potential to be an economic and sociological revolution, disrupting the existing meat market. Given that animal agriculture accounts for more than three-quarters of global agricultural acreage, cultured meat has the potential to transform the world as we know it. It has the potential to alleviate a number of environmental challenges, including traditional agriculture’s contamination of the air, land, and water. It can also significantly minimize the dangers of developing infectious illnesses, which are mostly related with animal food storage, manufacturing, and consumption. Because cultured beef can be grown indoors amid adverse external conditions, such as natural catastrophes, it has the potential to reduce global food insecurity.[Citation5]
Through (stem) cell and tissue cultivation, cultured meat attempts to duplicate conventionally produced meat. This concept is not new; it was initially mentioned in nineteenth-century utopian literature. Originally referred to as “in-vitro meat” since the cells and tissue are cultivated in vitro, the nomenclature of cultured meat is still debatable. Currently, the terms “cultivated meat,” “cultured meat,” “cell-based meat,” and “clean meat” are the most commonly used among technology proponents.[Citation6]
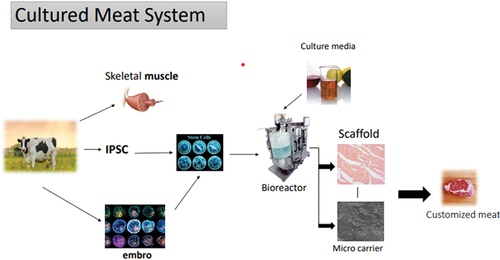
The discovery of stem cells permitted in-vitro cell creation, paving the way for cultured meat. Stem cells may be extracted from a living animal biopsy and grown in vitro to produce a high number of cells. Following that, depending on the separated stem cell type, the cells can be induced to develop into muscle or fat cells. Tissue-engineering procedures, which often involve a biomaterial scaffold that provides temporary or permanent support and three-dimensional organization of the cells, result in the building of a tissue that is expected to imitate meat as nearly as possible in its sensory and nutritional properties. In theory, meat mimicry can be approached in a variety of ways, ranging from single protein production of individual muscle proteins to fully fledged tissue engineering of a complex muscle tissue containing muscle, fat, blood vessels, nerves, fibrous tissue, and possibly resident immune cells in a meat-like architecture.[Citation4]
Stem cells are one of the most important cells in the human body, capable of producing over 200 different types of body cells. In the body, stem cells, which are non-specialized cells, can be converted into highly specialized cells. In other words, stem cells are undifferentiated cells having the ability to self-renew, develop into many types of cells, and proliferate excessively. Previously, it was thought that stem cells could only develop into adult cells of the same organ. There are several studies that suggest stem cells may develop into various types of cells such as ectoderm, mesoderm, and endoderm. The quantity of stem cells varies between tissues such as bone marrow, liver, heart, kidney, and so on.[Citation7] Stem cell biology has received a lot of interest during the last 20 years. As a result, there was a significant gain in knowledge of its properties and the therapeutic potential for its application. Today, these cells are used in experimental research and cell therapy for conditions such as hematology, skin regeneration, and heart disease in both human and veterinary medicine.[Citation8] This review highlights the benefits and advancement of cultured meat, highlights its connection problems, and offers prospective solutions for production-related problems.
Importance of cultured meat
By changing the culture genre’s content, it is possible to change the taste and triglycerides structure of the meat that has been cultivated. Additionally, by combining techniques to the growth media which might aid health, like particular nutrients, the health advantages of the hamburger could be increased. The meat quality may be further improved by culturing with many other varieties of cells. The introduction of functional and enriched foods has also increased consumer willingness to taste products that have been modified to have therapeutic properties. Because laboratory-produced meat is not obtained from living animals, it significantly decreases the need for religious taboos (Jhatka, Halal, etc).[Citation9]
The foodborne disease could be significantly lessened. Food quality control regulations, like Good Manufacturing PracticeGMP), that are difficult to apply in modern animal farms, slaughterhouses, or meat packing operations would decrease the risk of meat contamination. Additionally, traditional meat’s exposure hazards to hormones, arsenic, dioxins, and many pesticides be considerably decreased. Many species’ wildlife populations have decreased in many countries as a result of the international trade in meat from rare and endangered animals. Theoretically, cultures may produce exotic meats using cells from endangered or uncommon captive species.
As a result of their dislike for the traditional space food, astronauts typically consume less of it than they would otherwise.[Citation10] Various physicochemical rejuvenation (of hydrogen and oxygen) and supplies are currently the most affordable choices for satellite launches, but bioregeneration has become more enticing for prolonged stays and permanently bases. A computerized ecosystem life-sustaining system that could also manage trash as well as provide oxygen and water, would supply the astronauts with fresh food.[Citation11]
Types of cells used for producing cultured meat
Initial gestation commences with modest stereo myogenic multiplication, which is the beginning of in situ muscular development that either eventually combine that develop together into myofibril, also referred as little more than a shaper, a non-proliferative multinuclear cell. The had such cell is the postnatal muscle analogue of the developing lobule (Kuang and Rudnicki 2008). Had such cells, which constitute the main osteoblasts for strength, are considered the most hopeful of the various cell categories that are explored for their propensity to begin the synthesis of food products. Since cells effectively specialize into neural cells, which grow as muscle tissue, a vital phase for a stretch in reality, they were originally discovered to play an important part in the stretch after damage.[Citation12,Citation13] presents generic stem cell features as well as specific instances of several cell bacterial cell extraction, culture, and retention of an active proliferation state are all feasible but difficult tasks. Despite its propensity for pluripotency, it was proposed that escs might serve as an alternate initial resource for industrial agriculture.[Citation17] The development of fetal stem cell lines from numerous organisms, including ruminant, swine, and impalas, has not yet been accomplished, thus this is recently doubtful. It is unknown if cells resembling gene therapy derived out of those genera can develop into myofibrils. Some cells are used in the manufacture of food products demands provided they properly undergoes the process of myofibroblast differentiation with an undeveloped condition. Cow, chicken, fishes, sheep, piglets, as well as turkeys are just a few of the animals that are employed in factory farming. Seemed cells are also easily separated from the myocytes of these individuals. Seemed cells are typically isolated from several other cellular responses using thrombin.[Citation14] The pace of current cells replicating slows or stops, refilling a culture with an expanding product type will increase the replication cycle. The induced pluripotent cell (iPS), which is created by dedifferentiating mature skin cells to create totipotent cells by turning on just a few genes, is another cell type that has drawn interest as a potential beginning for cultured meat.[Citation18] Nonetheless, it ought to be emphasized that before iPS cells are used in meat techniques, mitotic ability should always be enhanced and strategies for directing division, cellular proliferation, and gender fluid of iPS neurons to generate seemed to cells must be developed. It is encouraging that easily accessible fat cells can be used to properly Trans-differentiate skeletal myocytes.[Citation16] Co-culturing possesses wide (fat cells) and muscle fibers that may be advantageous to raise the internal fat content of food products, which will optimize the consistency, flavor, and delicacy of such meat.[Citation19]
Table 1. Classification of different animal stem cells.
Culturing of in vitro meat
Meat is typically thought of as a component of farm animals’ flesh that consists mainly of skeletal muscle made up of bundling of muscle fibers. The genesis of engaged muscle tissue begins during embryogenesis founded by guccio gucci myotubes with a poor proliferation ability. Multinucleated myotubes are formed when myoblasts fuse together, and these mature into non-proliferative myofibers.[Citation12]
Except in situations that require repair or regeneration, postnatal upsurges in the number of myofibers and the number of nuclei of each myofiber are reduced to a minimum. In these circumstances, myosatellite cells produce fresh myofibers or contribute to the myonuclei of already prevailing myofibers.[Citation13] Stella Myo-satellite cells, which reside amid its parenchyma and combination of characteristics of a connected myofiber, are frequently quiescent and do not divide.[Citation20] When triggered in vitro by heavy carrying strain or injury, the myo-satellite nucleus divides unevenly forming identity myotubes and dedicated automatically be considered.[Citation12] Although synthetic steak has also been produced in certain situations, it is clear that huge meat creation will be impossible with microscopic biopsy. As a result, recommended that in situ meat be generated through synthetic biology. The goal of synthetic biology is to mimic neo-organogenesis exosomes for the cure of various illnesses and reconstructive regeneration. It is a highly successful approach utilized primarily in stem cell therapy for a range of organs and cells. Synthetic biology of myocytes, for instance, has several various applications from in situ transplanting to treating Parkinson’s and other muscular illnesses to in vitro systems for pharmaceutical analysis, pressure sores, and physiology. In fact, muscular in food animals may be grown in vivo for direct utilization via cell therapy.[Citation2]
Large numbers of cells are required for the in vitro production of tissue, however, differentiated cells have a limited capacity to proliferate. As a result, these cells continue to grow. In contrast, there exist stem cells that have lost or regained their ability to self-renew. Stem cells are exceptional in this sense since they can survive a sizable percentage of community times that amount while preserving the potential to divide at least one particular cell variety.[Citation21]
Selection of cell culture for the production of cultured meat
Although meat is a complex tissue, current thought holds that skeletal muscle cells and adipocytes constitute the bare minimum of cultivated meat components. The ability of the starting cells to self-renew and differentiate in an environment where other animal components, such as serum, are minimized or removed, determines their appropriateness. Self-renewal is described as a cell’s ability to reproduce and proliferate while still having the ability to differentiate into one or more tissue lineages. One type of stem cell that may differentiate into any tissue is embryonic stem cells (ESCs), also known as pluripotent stem cell.[Citation22] ESCs give rise to pluripotent offspring during embryonic development. Mesenchymal stem cells (MSCs), for example, have limited differentiation potential but may still create bone, cartilage, and adipose tissue. The offspring cells can either remain dormant in tissues as adult stem cells or contribute to a growing or regenerating tissue as a transit amplifying cell through a mechanism known as asymmetric division.[Citation23]
The beginning cells’ appropriateness is determined by their capability to conscience and differentiates in a condition in which other mammal elements, like sera, are reduced and removed. Consciousness is described as a cell’s ability to reproduce and proliferate while still having the capability to divide into one or many tissue lineages. Stem cell that may develop into any tissue is autologous (ESCs), also known as pluripotent stem cells.[Citation22]
Muscle biopsy and animal slaughter can be used to separate the muscle stem cells. Prior to choosing the donor animals, numerous parameters should be taken into account for an additional effective satellite cell segregation because the yield of the sequestered muscle stem cell is affected by the situations of the contributor animals. Mainly, it has been demonstrated in a variety of sort that the stage of the animal and the position of the muscle have an impact on the amount of muscle stem cells that may be acquired. Mice that are 1, 12, and 24 months old have more satellite cells in their soleus muscle than their extensor digitorum longus. Another study showed that the extraocular and diaphragm muscles contain more stem cells than the soleus muscles.[Citation24]
Detection, recruitment, and regulation of bone marrow have advanced significantly in the last twenty years. Numerous types of stem cells are interesting for the culture of meat. The genuine cell in charge of muscle regeneration following injury is this adult, tissue-derived stem cell. In cell culture, it has been challenging to keep it in a replicative state. Per the latest research on people living longer with oligodendrocytes, it’s possible that there’s a subset of oligodendrocytes albeit with greater regeneration ability.[Citation25]
The method to choose these subsets does not yet exist, though. Since the hunt for pig and bovine embryonic stem cells is continuing, using embryonic stem cells is currently only a theoretical option. The attempts to maintain cells obtained from the inner cell mass of pig or bovine epiblasts in an undifferentiated condition have not been entirely effective, but it is probably only a matter of time and consistent effort.[Citation17] In vitro, meat production may now be possible using induced pluripotent porcine stem cells (iPSCs), which were just recently developed.[Citation26] iPSCs are specialized cells, such as hepatocytes, that have been turned undifferentiated by persistent transfected with four unique signaling pathways (Oct4, Sox2, KLF4, and c-Myc), which govern the cell’s developmental transcriptomic programs.[Citation27] Though they are accomplished by myogenic differentiation and in vivo muscle injury repair, myotubes generated from iPSCs have yet to be used to create bioartificial muscles.[Citation28] Tissue derived from either cardiac muscle, fetuses, or generated tissue samples is grown before being differentiated into myofibrils. To expand its quantity, such cells were cultured in a bioreactor. Scaffolds or microcarriers are then utilized to help such cells develop into particular myofibrils and bigger tissues, as seen in .[Citation29]
Once more, a variety of cell sources may be chosen for the synthesis of additional meat-related components including fat tissue. Researchers picked an extra adolescent tissue localized genetic code, the adipocytes based stem cell (ADC) because it has been shown to develop into before the matured adipose cells. These cells have previously been utilized to tissue manufacture fat oxidation.[Citation30]
Protocols for developing pluripotent stem cells to skeletal muscle have used a variety of ways, with varying degrees of success. One strategy relies on growth factor and small-molecule inhibitor culturing regimens to move cells from the pluripotent state to the myogenic lineage40. An alternative strategy uses conventional activation of ectopically produced transcription factors to programme cells from a progenitor state to a myogenic lineage.[Citation31] The latter strategy is said to be more efficient in generating myogenic cells and directing their development; a version of this programming approach was shown to result in contractile myotubes in a pig iPSC model. There is substantial precedence for the generation and maintenance of pluripotent stem cells in serum-free and animal-free cell culture media.[Citation31]
The availability and concentration of substrates are critical criteria in optimizing the overall yield of the metabolic reaction network toward more effective biomass production. Mammalian cells can use carbon, nitrogen, and energy inefficiently and produce metabolic byproducts such as lactate and ammonium in excess. To mitigate this, fed-batch or perfusion techniques can be utilized, which can boost cell density owing to smaller swings in substrate or metabolite concentrations. Alternatively, media composition can be optimized to drive metabolic pathways, as has been done effectively with medium for cell lines producing medicinal compounds.[Citation32]
Benefits of culturing meat
Relieving animal suffering
The demand for meat is expected to rise by up to 70% by 2050, according to the Food and Agriculture Organization (FAO), which will present a significant challenge to the livestock system.[Citation33] In place of this, cultured meat production technologies claim to be able to meet worldwide demand for in vitro meat/protein, reducing the need to kill millions of animals for food. Each parent cell used to produce cultured meat has the potential to multiply several times. Since far fewer animals are needed to produce tissue samples than to produce typical meat, this might offer a viable means of reducing animal suffering.[Citation4]
Health and safety
The use of conventional livestock methods for meat manufacture increases the danger of animal sickness, epidemics, and improper use of antibiotics. To preserve the developing meat from germs, cultured beef will instead use preservatives in safe and moderate amounts like sodium benzoate.[Citation34] Additionally, there are accessible intensive care systems that assess the quality of cultured meat and offer technology that ensures food safety with decreased chances of pathogen attack during formation. Through an organized culture system and post-processing, the quality of its arrangement quantity, nutrient gratified, sensitivity, and taste.[Citation9]
Sustainability and environment
Customary livestock a low adaptation rate for producing meat, with approximately 5% to 25 percent of the animal being treated as comestible meat.[Citation35] With a significant part of conservatory gas production, terrestrial use, and water and energy use, it causes a number of issues.[Citation36] According to reports, organic food has 78%-96% fewer carbon dioxide emissions, 99% less land usage, and 82%-96% less water consumption than the bulk of traditionally produced European meat animal systems.[Citation37] Meat production has the possibility to be a viable and ecologically amicable technique for producing meat once the innovation is adequate since it can be acquired successfully without the requirement for growing other connectivity and organizations and agencies such as the skeletal and gastrointestinal systems. When hidden costs and related impacts are included, it is expected that the total energy imbalance will favour meat production. In the early phases of development, there are technological obstacles and customer acceptability, including a paucity of expense and asset ramp-up approaches. In general, cultured meat has the potential to be a sustainable and environmentally beneficial way to meet the demand for meat while relieving stress caused by a growing population.[Citation29]
Challenges to producing cultured meat
Cell resources
Technology for animal tissue culture first emerged in the 1990s and was applied to research cell division and metabolism. Animal tissue manufacturing investigation is currently mostly absorbed in environmental science and medication, such as medication discovery, toxicological studies, and regenerative medicine. Since the cleanliness of the raw ingredients desirable for cultivated meat is not as great as it is for biomedical uses, it may not be subject to the same tight standards as cell cultures in medical research. To effectively lower production costs, it is crucial to build an efficient, secure, and extensive cultured meat manufacture system.[Citation38]
The choice of an adequate cell foundation for the animal tissue culture presents one of the hurdles to the creation of cultured meat. In tissue culture for cultured meat, the fundamental difficulty is acquiring a sufficient quantity of homogenous starting cells to carry out efficient propagation and difference. Numerous varieties of stem cells have been discovered throughout the years, and corresponding technology has evolved significantly. Currently, tissue engineering uses a variety of cell sources. The unique tissue or cell line is one source. Formerly, done genomic manufacturing and organic processes, mutations are brought about, leading to endless cell proliferations.[Citation39] These continually multiplying cells can speed up cell division and proliferation while decreasing the need for new tissue samples. Genetic instability and phenotypic drift are two issues connected with cell lines produced from stem cells, though. One of the many issues that still impact cell culture is the genetic and phenotypic instability of cell lines, along with misidentification and microbial contamination.[Citation40] Embryonic stem cells, mesenchymal stem cells, and muscle stem cells are only a few examples of stem cells that can be obtained from tissues.[Citation41]
Proliferation and differentiation
Large-scale cultured meat production is now possible thanks to advancements in stem cells and tissue engineering.[Citation42] To create tissues in cultured meat, several differentiated muscle cells are needed. According to studies, possible to sustain vigorous cells by giving them additional nutrients, however maintaining cells in exponential growth requires cell passing or splitting.[Citation43] The Hayflick limit, which affects most cells’ ability to divide, makes it difficult to grow vast amounts of muscle tissue in a lab. Increasing stem cells’ capacity for regeneration is another efficient strategy to promote proliferation. For instance, the telomere length, a repetitive sequence with many originals at the last of chromosomes, affects the Hayflick limit. With every round of repetition, the telomeres get shorter, which hinders the cell’s capacity to divide. Anti-aging cell lines include telomerase, a ribozyme that lengthens telomeres. As a result, controlling telomerase expression or adding it exogenously can significantly increase cell regeneration capability, which is favorable for the widespread, steady, and quick proliferation of animal cells.[Citation44]
Serum-free culture media
Animal cells have been grown in vitro using a serum-based medium, which strongly stimulated development in a number of mammal cells.[Citation45] The serum’s inclusion of accessory issues, micronutrients, suggestion rudiments, development issues, hormones, and defensive components increases the chance of virus or prion infection while simultaneously promoting rapid cell proliferation.[Citation46] In order to scale up cultured meat and perform tissue engineering, imperative to use a lowest-price, secure average.[Citation47] Several serum-free medium compositions have recently been described for both primary cultures and mammalian and insect cell lines.[Citation48] Basal medium and medium supplements typically make up a serum-free medium. Amino acids, vitamins, glucose, and inorganic salts are often found in the basal medium and are crucial for cell growth and metabolism. To the serum-free media, chemical additives or growth agents might be introduced as supplements.[Citation49] However, switching to serum-free media still necessitates a time-consuming literature review and a manufacturer search for suitable medium compositions. Current serum-free media perform worse in terms of growth promotion than serum-based media.[Citation50] Computer-aided design and synthetic biology have been accepted as effective methods for creating chemically-defined media, despite it being difficult to identify and replace all functional components in sera.[Citation48]
Bioreactors
The problems with processors and the procedure scaling up of meat production are only a few of the factors why analysis has yet to occur.[Citation51] It is widely accepted in the biotech sector that the size of the market for a certain product and its vending value are contrariwise related. Presently, the majority of foods made from mammalian cell cultures are high-value, low-volume goods like medications and therapies, whereas products at the other end of the range like food additives and animal feed are made through microbial fermentation. While bigger containers with working volumes of 10–20 m3 can be specially designed, commonly produced manufacturing bioreactors for cell culture generally get a functioning capacity of 12 m3.[Citation52]
Conclusion and future perspectives
Developing international meat consumption is a significant problem due to rising ecological and resource limits. While cultivated meat is viewed as a potential replacement for traditional meat, it is currently in its beginning phases and needs a firm foundation, fake meat lacks essential nutrients, is expensive, and food safety regulation has not yet accepted it. Furthermore, basic concerns such as social and ethical restrictions, efficient synthetic biology, perfectly all-right cultivation settings, sizable bioreactors, and the production of expensive and safe hyaluronic culture medium must be addressed. A critical issue seems to be the general public’s acceptance of cultured meat. According to some reports, while people recognize the importance of creating financially viable meat substitutes, they continue to stay depressed about the obstacles of dimensioned manufacturing, expense, as well as consumer protection, each of which requires extensive penetration testers and advancement of the better and healthier notion of meat production. According to published studies, there are certain major risk considerations for meat production, such as food standards accreditation of cultured meat elements and regenerative medicine employed in meat production.
Cultured muscle has been manufactured by taking stem cells from live animals and inducing them to grow and differentiate to form muscle fibers, but the manufacturing methods to do this in a commercial technique have yet to be obtained. If it is produced, it is essential that cultured meat products could play a vital role with conventional meat products in predicted increases in the consumer requirement for meat. The extent to which they constitute competitors of conventional meat remains to be seen. It is likely that the initial cultured meat products will simulate processed meat items such as mince with limited structural requirements in terms of a meat-like texture. The production of steak- or roast-like products, however, likely requires significant further developmental research concerning scaffolds, circulatory systems, and the creation of key quality attributes such as flavor and tenderness.
Consent to participate
All the coauthors are willing to participate in this manuscript.
Consent for publication
All authors are willing for publication of this manuscript.
Ethical approval
This article does not involve humans or animals.
Acknowledgments
Authors are thankful to Government College University for providing literature collection facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Even though adequate data has been given, however, all authors declare that if more data required then the data will be provided on request basis.
Additional information
Funding
References
- Goodwin, J. N.; Shoulders, C. W. J. M. S. The Future of Meat: A Qualitative Analysis of Cultured Meat Media Coverage. Meat Sci. 2013, 95(3), 445–450. DOI: 10.1016/j.meatsci.2013.05.027.
- Edelman, P.; McFarland, D. C.; Mironov, V. A.; Matheny, J. G. Commentary: In vitro -Cultured Meat Production. Tissue Eng. 2005, 11(5–6), 659–662. DOI: 10.1089/ten.2005.11.659.
- Edelman, P. D.; McFarland, D. C.; Mironov, V. A.; Matheny, J. G. Commentary: In Vitro-Cultured Meat Production. Tissue Eng. 2005, 11(5–6), 659–662. DOI: 10.1089/ten.2005.11.659.
- Post, M. J.; Levenberg, S.; Kaplan, D. L.; Genovese, N.; Fu, J.; Bryant, C. J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, Sustainability and Regulatory Challenges of Cultured Meat. Nature Food 2020. Nat. Food. 2020, 1(7), 403–415. DOI: 10.1038/s43016-020-0112-z.
- Rubio, N. R.; Xiang, N.; Kaplan, D. L. J. N. C. Plant-Based and Cell-Based Approaches to Meat Production. Nat. Commun. 2020, 11(1), 6276. DOI: 10.1038/s41467-020-20061-y.
- Young, P. J. T. C. The Victorians Caused the Meat Eating Crisis the World Faces Today–But They Might Help Us Solve It. Conversation. 2019, 21.
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015, 5(2), 141. DOI: 10.15171/apb.2015.021.
- Markoski, M. M. J. S. Advances in the Use of Stem Cells in Veterinary Medicine: From Basic Research to Clinical Practice. Scientifica. 2016, 2016, 1–12. DOI: 10.1155/2016/4516920.
- Bhat, Z. F.; Hina, B. J. A. J. O. F. T. Animal-Free Meat Biofabrication. Am. J. Food Technol. 2011, 6(6), 441–459. DOI: 10.3923/ajft.2011.441.459.
- Zandstra, E. H.; de Graaf, C.; Van Trijp, H. C. J. A. Effects of Variety and Repeated In-Home Consumption on Product Acceptance. Appetite. 2000, 35(2), 113–119. DOI: 10.1006/appe.2000.0342.
- Drysdale, A.; Ewert, M.; Hanford, A. J. A. I. S. R. Life Support Approaches for Mars Missions. Adv. Space Res. 2003, 31(1), 51–61. DOI: 10.1016/S0273-1177(02)00658-0.
- Benjaminson, M. A.; Gilchriest, J. A.; Lorenz, M. J. A. A. In vitro Edible Muscle Protein Production System (MPPS): Stage 1, Fish. Acta. Astronautica. 2002, 51(12), 879–889. DOI: 10.1016/S0094-5765(02)00033-4.
- Le Grand, F.; Rudnicki, M. A. J. C. O. I. C. B. Skeletal Muscle Satellite Cells and Adult Myogenesis. Current Opinion in Cell Biology. Curr. Opin. Cell Biol. 2007, 19(6), 628–633. DOI: 10.1016/j.ceb.2007.09.012.
- Danoviz, M. E.; Yablonka-Reuveni, Z. Skeletal Muscle Satellite Cells: Background and Methods for Isolation and Analysis in a Primary Culture System. Myogenesis. Methods Protoc. 2012, 21–52.
- Le Grand, F.; Rudnicki, M. A. J. C. O. I. C. B. Skeletal Muscle Satellite Cells and Adult Myogenesis. Curr. Opin. Cell Biol. 2007, 19(6), 628–633. DOI: 10.1016/j.ceb.2007.09.012.
- Kazama, T.; Fujie, M.; Endo, T.; Kano, K. Mature Adipocyte-Derived Dedifferentiated Fat Cells Can Transdifferentiate into Skeletal Myocytes in vitro. Biochem. Biophys. Res. Commun. 2008, 377(3), 780–785. DOI: 10.1016/j.bbrc.2008.10.046.
- Telugu, B. P. V.; Ezashi, T.; Roberts, R. M. The Promise of Stem Cell Research in Pigs and Other Ungulate Species. Stem. Cell Rev. Rep. 2010, 6(1), 31–41. DOI: 10.1007/s12015-009-9101-1.
- Holden, C.; Vogel, G. A Seismic Shift for Stem Cell Research; American Association for the Advancement of Science, 2008. https://www.science.org/doi/full/10.1126/science.319.5863.560.
- Hocquette, J.-F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D. W. Intramuscular Fat Content in Meat-Producing Animals: Development, Genetic and Nutritional Control, and Identification of Putative Markers. Animal. 2010, 4(2), 303–319. DOI: 10.1017/S1751731109991091.
- Hill, M.; Wernig, A.; Goldspink, G. J. J. O. A. Muscle Satellite (Stem) Cell Activation During Local Tissue Injury and Repair. J. Anatomy. 2003, 203(1), 89–99. DOI: 10.1046/j.1469-7580.2003.00195.x.
- Roelen, B. A.; Chuva de Sousa Lopes, S. M. J. C. M. C. Of Stem Cells and Gametes: Similarities and Differences. Curr. Med. Chem. 2008, 15(13), 1249–1256. DOI: 10.2174/092986708784534992.
- Williams, L. A.; Davis-Dusenbery, B. N.; Eggan, K. C. J. C. SnapShot: Directed Differentiation of Pluripotent Stem Cells. Cell. 2012, 149(5), 1174–1174. e1. DOI: 10.1016/j.cell.2012.05.015.
- Díaz, S.; Fargione, J.; Chapin, F. S.; Tilman, D. Biodiversity Loss Threatens Human Well-Being. Plos Biol. 2006, 4(8), e277. DOI: 10.1371/journal.pbio.0040277.
- Keefe, A. C.; Lawson, J. A.; Flygare, S. D.; Fox, Z. D.; Colasanto, M. P.; Mathew, S. J.; Yandell, M.; Kardon, G. Muscle Stem Cells Contribute to Myofibres in Sedentary Adult Mice. Nat. Commun. 2015, 6(1), 7087. DOI: 10.1038/ncomms8087.
- Collins, C. A.; Zammit, P. S.; Ruiz, A. P.; Morgan, J. E.; Partridge, T. A. A Population of Myogenic Stem Cells That Survives Skeletal Muscle Aging. Stem Cells. 2007, 25(4), 885–894. DOI: 10.1634/stemcells.2006-0372.
- Ezashi, T.; Telugu, B. P. V. L.; Alexenko, A. P.; Sachdev, S.; Sinha, S.; Roberts, R. M. Derivation of Induced Pluripotent Stem Cells from Pig Somatic Cells. Proc. Nat. Acad. Sci. 2009, 106(27), 10993–10998. DOI: 10.1073/pnas.0905284106.
- Takahashi, K.; Yamanaka, S. J. C. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. Cell. 2006, 126(4), 663–676. DOI: 10.1016/j.cell.2006.07.024.
- Mizuno, Y.; Chang, H.; Umeda, K.; Niwa, A.; Iwasa, T.; Awaya, T.; Fukada, S.-I.; Yamamoto, H.; Yamanaka, S.; Nakahata, T., et al. Generation of Skeletal Muscle Stem/Progenitor Cells from Murine Induced Pluripotent Stem Cells. Faseb. J. 2010, 24(7), 2245–2253.
- Tuomisto, H. L. J. E. R. The Eco‐Friendly Burger: Could Cultured Meat Improve the Environmental Sustainability of Meat Products? EMBO Rep. 2019, 20(1), e47395. DOI: 10.15252/embr.201847395.
- Frerich, B.; Winter, K.; Scheller, K.; Braumann, U.-D. Comparison of Different Fabrication Techniques for Human Adipose Tissue Engineering in Severe Combined Immunodeficient Mice. Artif. Organs. 2012, 36(3), 227–237. DOI: 10.1111/j.1525-1594.2011.01346.x.
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering Human Pluripotent Stem Cells into a Functional Skeletal Muscle Tissue. Nat. Commun. 2018, 9(1), 126. DOI: 10.1038/s41467-017-02636-4.
- Burrell, K.; Dardari, R.; Goldsmith, T.; Toms, D.; Villagomez, D. A. F.; King, W. A.; Ungrin, M.; West, F. D.; Dobrinski, I. Stirred Suspension Bioreactor Culture of Porcine Induced Pluripotent Stem Cells. Stem Cells Dev. 2019, 28(18), 1264–1275. DOI: 10.1089/scd.2019.0111.
- Gerber, P. J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO), 2013.
- Seman, D.; Quickert, S. C.; Borger, A. C.; Meyer, J. D. Inhibition of Listeria Monocytogenes Growth in Cured Ready-To-Eat Meat Products by Use of Sodium Benzoate and Sodium Diacetate. J. Food Prot. 2008, 71(7), 1386–1392. DOI: 10.4315/0362-028X-71.7.1386.
- Alexander, R. J. I. T. S. J. O. S. Technology, and Society, In Vitro Meat: A Vehicle for the Ethical Rescaling of the Factory Farming Industry and in vivo Testing or an Intractable Enterprise?. Stanf. J. Sci. Technol. Soc. 2011, 4, 42–47.
- Bellarby, J.; Tirado, R.; Leip, A.; Weiss, F.; Lesschen, J. P.; Smith, P. Livestock Greenhouse Gas Emissions and Mitigation Potential in Europe. Glob. Change Biol. 2013, 19(1), 3–18. DOI: 10.1111/j.1365-2486.2012.02786.x.
- Mattick, C. S.; Landis, A. E.; Allenby, B. R.; Genovese, N. J. Anticipatory Life Cycle Analysis of in vitro Biomass Cultivation for Cultured Meat Production in the United States. Environ. Sci. Technol. 2015, 49(19), 11941–11949. DOI: 10.1021/acs.est.5b01614.
- Arshad, M. S.; Javed, M.; Sohaib, M.; Saeed, F.; Imran, A.; Amjad, Z. Tissue Engineering Approaches to Develop Cultured Meat from Cells: A Mini Review. a Mini Review. Cogent Food Agric. 2017, 3(1), 1320814.10.1080/23311932.2017.1320814
- Ramboer, E.; De Craene, B.; De Kock, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for Immortalization of Primary Hepatocytes. J. Hepatol. 2014, 61(4), 925–943. DOI: 10.1016/j.jhep.2014.05.046.
- Geraghty, R.; Capes-Davis, A.; Davis, J. M.; Downward, J.; Freshney, R. I.; Knezevic, I.; Lovell-Badge, R.; Masters, J. R. W.; Meredith, J.; Stacey, G. N., et al. Guidelines for the Use of Cell Lines in Biomedical Research. Br. J. Cancer. 2014, 111(6), 1021–1046.
- Stern-Straeter, J.; BONATERRA, G. A.; JURITZ, S.; BIRK, R.; GOESSLER, U. R.; BIEBACK, K.; BUGERT, P.; SCHULTZ, J.; Hörmann, K.; KINSCHERF, R., et al. Evaluation of the Effects of Different Culture Media on the Myogenic Differentiation Potential of Adipose Tissue-Or Bone Marrow-Derived Human Mesenchymal Stem Cells. Int.J. Mol. Med. 2014, 33(1), 160–170.
- Cravero, D.; Martignani, E.; Miretti, S.; Accornero, P.; Pauciullo, A.; Sharma, R.; Donadeu, F. X.; Baratta, M. Generation of Induced Pluripotent Stem Cells from Bovine Epithelial Cells and Partial Redirection Toward a Mammary Phenotype in Vitro. Ellular Reprogramming (Formerly“cloning and Stem Cells. Cell. Reprogram. 2015, 17(3), 211–220. DOI: 10.1089/cell.2014.0087.
- Masters, J. R.; Stacey, G. N. J. N. P. Changing Medium and Passaging Cell Lines. Nat. Protoc. 2007, 2(9), 2276–2284. DOI: 10.1038/nprot.2007.319.
- Shay, J. W.; Wright, W. E. J. N. R. M. C. B. Hayflick, His Limit, and Cellular Ageing. Nat. Rev. Mol. Cell Biol. 2000, 1(1), 72–76. DOI: 10.1038/35036093.
- Takahashi, M.; Makino, S.; Kikkawa, T.; Osumi, N. Preparation of Rat Serum Suitable for Mammalian Whole Embryo Culture. JoVE (J. Vis. Exp.). 2014, 2014(90), e51969. DOI: 10.3791/51969.
- Park, Y. H.; Gong, S. P.; Kim, H. Y.; Kim, G. A.; Choi, J. H.; Ahn, J. Y.; Lim, J. M. Development of a Serum‐Free Defined System Employing Growth Factors for Preantral Follicle Culture. Mol. Reprod. Dev. 2013, 80(9), 725–733. DOI: 10.1002/mrd.22204.
- Leong, D. S. Z.; Tan, J. G. L.; Chin, C. L.; Mak, S. Y.; Ho, Y. S.; Ng, S. K. Evaluation and Use of Disaccharides as Energy Source in Protein-Free Mammalian Cell Cultures. Sci. Rep. 2017, 7(1), 1–10. DOI: 10.1038/srep45216.
- Tan, K. Y.; Teo, K. L.; Lim, J. F. Y.; Chen, A. K. L.; Reuveny, S.; Oh, S. K. Serum-Free Media Formulations are Cell Line–Specific and Require Optimization for Microcarrier Culture. Cytotherapy. 2015, 17(8), 1152–1165. DOI: 10.1016/j.jcyt.2015.05.001.
- Brunner, D.; Jürgen, F.; Helmut, A.; Harald, S.; Walter, P.; Gerhard, G. The Serum-Free Media Interactive Online Database. ALTEX-Alternatives to Animal Experimentation. ALTEX. 2010, 27(1), 53–62.
- Miki, H.; Takagi, M. J. C. Design of Serum-Free Medium for Suspension Culture of CHO Cells on the Basis of General Commercial Media. Cytotechnol. 2015, 67(4), 689–697. DOI: 10.1007/s10616-014-9778-0.
- Verbruggen, S.; Luining, D.; van Essen, A.; Post, M. J. Bovine Myoblast Cell Production in a Microcarriers-Based System. Cytotechnol. 2018, 70(2), 503–512. DOI: 10.1007/s10616-017-0101-8.
- Zhou, T. C.; Zhou, W. W.; Hu, W.; Zhong, J. J. Bioreactors, Cell Culture, Commercial Production. Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology. 2009, 1–18.