ABSTRACT
Oat (Avena sativa) is a cereal crop rich in dietary fibers, which are generally considered beneficial for human health. The objective of this work was to investigate the in vitro starch digestibility-reducing effect of the flour of a high arabinoxylan (AX) content (6.1%) oat line obtained from a random mutagenized oat population, compared to the original variety as a reference. The effect of the oat flours was tested on gelatinized potato starch as a dietary starch model, using a digestion dialysis method to calculate the predicted Glycaemic Index (pGI). The high AX flour reduced the potato starch pGI to larger extent than the flour from the original line. The pGI-lowering effect of the AX in the mutant line was confirmed by its attenuation following pre-treatment of the flour with xylanase. The larger pGI-lowering effect of the high AX oat line suggested this type of mutant as a potential functional food/ingredient for better management of postprandial glycemic responses.
Introduction
Oat (Avena sativa) consumption has increased due to its nutritional value and added (functional) health benefits.[Citation1] Oat is one of the main cereal grains used for human and animal consumption with an annual production of 22 million metric tons.[Citation2] It is a good dietary fiber and nutrient source such as starch, proteins, unsaturated fatty acids as well as micronutrients.[Citation3,Citation4] Whole grain oat contains up to 23% (w/w) of non-starch polysaccharides (NPS) where arabinoxylans (AXs) and β-glucans are the main ones.[Citation5]
Arabinoxylan (AX) is a dietary fiber source in various cereals, including oats.[Citation6] The general structure consists of a linear chain of (1,4)-β-α-xylopyranoside (Xylp) units, with α-L-arabinofuranosyl substitutions through α-(1,2) or/and α-(1,3) glycosidic linkage. Therefore, mono- and/or di-substituted Xylp residues at the O-2 and O-3 positions as well as non-substituted residues can be found.[Citation7] The degree of substitution and the concentration of substitutions can affect the physicochemical properties of AXs.[Citation6]
Consumption of AXs enriched foods has been reported to induce beneficial effects on lipid metabolism by reducing the levels of LDL cholesterol in the blood, improved colonic health by reducing the risk of developing cancer, and improved glycemic control by lowering blood glucose levels.[Citation6,Citation8]
Studies of the effect of AXs on glucose metabolism indicate that AXs slow small intestinal transit, decrease starch availability to digestive enzymes, and reduce the rate of lumen-to-cell glucose diffusion. All of these can contribute to lower the absorption of glucose which attenuates the postprandial glycemic response.[Citation9] The health claim that AX consumption reduces the glucose rise after a meal has been accepted by the European Food Safety Authority.[Citation10]
The production of oat with high content of AX is desired due to all these health benefits. The probability of finding high AX content oat lines has been increased by enlarging the genetic variation in an oat elite cultivar. The chemical ethyl methanesulfonate (EMS) is a mutagen used to induce random point mutations, mostly CG to AT transitions, in plants. It has been widely used in plant mutation breeding.[Citation11] Our group has screened the mutated population, obtained from the reference oat cultivar Belinda used as the target for mutagenesis, looking for lines presenting high levels of AX. The line used in this study has been selected from such a screening.[Citation12]
Carbohydrate-containing foods can produce different effects on postprandial blood glucose excursions depending on their composition. The Glycaemic Index (GI) can be used to quantify the blood glucose response to a test food with respect to a reference one.[Citation13] From a nutritional viewpoint, carbohydrate foods presenting a low GI value are considered beneficial for human health,[Citation14,Citation15] as the intake of this type of foods improves glucose control both in healthy and diabetic people, leading to increased insulin sensitivity and thus reduced glucose intolerance.[Citation16] The rate of starch digestion in vitro is used to predict the rate of blood glucose concentration rise after a meal and is the basis of several methods designed for the prediction of in vivo glycemic responses through the calculation of the so-called predicted glycemic index (pGI).[Citation17,Citation18] The presence of dietary fiber is an important factor influencing starch digestibility in different ways.[Citation16] However, complex food matrices, such as cereal grains, contain different non-starch polysaccharides and it is not always easy to determine the specific contribution of a particular dietary fiber component to the overall starch digestibility modulating effect.
This study aimed to assess the ability of AX in whole oat flour to modulate the in vitro digestibility of gelatinized potato starch, used as a dietary starch model. For this purpose, an oat line with high AX content (6.10%) was investigated in comparison to the reference oat line Belinda, which contains 3.02% AX.
Material and methods
Oat samples
The two lines used in this study originated from a randomly mutagenized population developed by using the mutagen chemic EMS. Belinda line was used as the target for mutagenesis, emerging from the Lantmännen Seed collection (Svalöf, Sweden) and maintained by CropTailor AB (Lund, Sweden). The resulting mutagenized population consisted of ca. 2500 different seed lines.[Citation12] Specifically, Belinda is selected as the reference oat, and CT0320 as the line of study due to its high AX content.
Total starch assay
The dehulled and milled oat seeds and commercial potato starch (Potatismjöl, Coop, Sweden) were assayed for their total starch content using the MegazymeTM Total Starch kit. The assay kit hydrolyses starch in KOH pre-treated samples by incubation with α-amylase and amyloglucosidase (AMG). The final glucose concentrations were measured with the GOPOD reagent.
In vitro starch hydrolysis index
The in vitro digestibility of gelatinized potato starch in the presence of oat flours was assessed with the alpha-amylolysis/dialysis system proposed by Granfeldt et al. (1992) with modifications to better suit the samples and control. This physically restricted method mimics the physiological situation in the small intestine by combining the “luminal” enzymatic digestion of starch with the diffusion of digestion products across a dialysis membrane (“absorption” phase).
The assay was run on samples containing a total of 1000 mg starch (starch in oat flour + potato starch) in a 1:10 ratio. The starch, AX, and β-glucan amounts present in the digestion mixtures are shown in Table S1. Blends of oat flour and potato starch were suspended in 0.022 M amylase buffer and boiled for 10 min with intermittent stirring and then cooled to 37°C in a water bath with constant stirring at 300 RPM. The suspensions were loaded into a dialysis tubing (SpectraPor®2 dialysis membrane 12–14 kD) and added 1 mL of α-amylase enzyme solution (110 U/mL) from porcine pancreas (Sigma Life Science; A6255-25 MG). Each dialysis tubing was then placed in individual beakers containing 800 mL amylase buffer and incubated at 37°C with constant stirring at 150 RPM.
Aliquots from the dialysis buffer were taken every 30 min for 180 min. The DNS reagent was used to stop the reaction and to colorimetrically quantify the reducing sugars present, which were expressed as maltose equivalents.[Citation19] A factor of 0.95 was used to convert maltose to equivalent starch (mg/ml) and further on to the percentage of hydrolyzed starch.
In selected experiments, a pre-treatment of the boiled potato starch:flour suspension with 10 U endo-1,3:1,4-β-D-Glucanase (Bacillus subtilis) or 20 U endo-1,4-β-Xylanase (rumen microorganism) samples was applied, incubating the samples with the enzyme at 50°C for 90 min with constant stirring. The pre-treated samples were finally transferred to the dialysis tubing, added the α-amylase solution, and immersed in 800 mL amylase buffer at 37°C with constant stirring at 150 RPM to monitor the starch hydrolysis reaction. Pilot experiments incubating the potato starch/oat flour suspensions with the ß-glucanase and xylanase enzymes in the absence of pancreatic amylase showed no reducing sugar release (Supplementary material).
The hydrolysis index (HI) was calculated and expressed as the ratio of area under the starch hydrolysis curve (AUC) of the test sample to the corresponding area of pure potato starch, using the trapezoidal rule. The hydrolysis index estimated with this method shows a good correlation with postprandial glycemic responses.[Citation20] The predicted glycemic index (pGI) was calculated from HI values using the empiric formula proposed by Granfeldt (1994): pGI = 0.862 HI + 8.198 (r = 0.026, P < .00001).
Statistics
A Fischer test was performed to determine if the variances between experiments were equal or unequal, followed by a two-sample t-test between replicates to do the mean ± standard deviation. Moreover, to determine a significant difference between samples, ANOVA test, and a two-sample t-test between samples were performed.
Results
The total starch content of the two oat lines flours and the potato starch preparation used as a reference are shown in . Both oat lines, CT0320 and Belinda, showed a similar starch content, which was significantly lower than in the commercial starch sample. These starch contents were used for the calculations needed for preparing the samples for the in vitro hydrolysis experiments.
Table 1. Total starch content of the oat flours and reference potato starch.
Hydrolysis curves for the different samples from the in vitro hydrolysis index experiments are depicted in . Corresponding HI and pGI values are summarized in . The presence of both oat flours decreased the starch digestion rate compared to the potato starch reference (; p ≤ .05). Moreover, the presence of high AX line CT0320 flour resulted in slower hydrolysis compared to the reference Belinda oat line-containing sample (p ≤ .05). Consequently, compared to the potato starch – Belinda sample’s HI and pGI values the high AX line-containing sample were 12.91 and 11.12 units lower, respectively ().
Figure 1. Amylolysis of potato starch blended with oat flours; effect of ß-glucanase and xylanase pre-treatments.
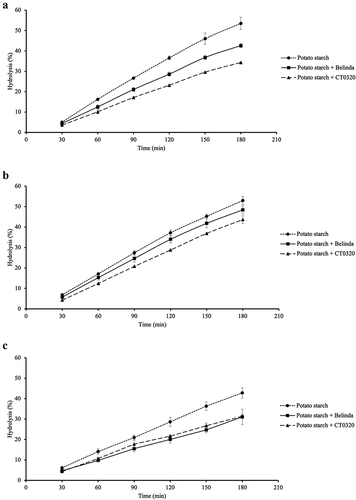
Table 2. Hydrolysis index and predicted Glycaemic Index of Potato starch, Potato starch + Belinda, and Potato starch + CT0320 with different pre-treatments (% + SEM).
In a second set of experiments, a pre-treatment of the blend of potato starch and CT0320 line sample with β-glucanase was performed to hydrolyze the oat β-glucans. As a result, the starch hydrolysis in the presence of the β-glucanase pre-treated CT0320 line was slightly faster compared to that observed in the presence of the untreated sample , with significantly higher HI and pGI values (). A similar experiment was performed where a xylanase pre-treatment of the CT0320 line-containing sample aimed to degrade the AXs present in the oat flour. This treatment erased the slower starch hydrolysis effect observed in the presence of CT0320, presenting significantly higher HI and pGI compared to the untreated sample , and even higher HI and pGI compared to the β-glucanase pre-treated sample ().
Discussion
Although β-glucans play an important role in the modulating effect of oat consumption on the postprandial glycemic response,[Citation21,Citation22] arabinoxylans have also been suggested to contribute to the overall postprandial beneficial action of this and other cereals.[Citation1,Citation23] Aiming to gain knowledge about the contribution of oat AXs to the reduced blood glucose changes observed following the intake of meals containing oats, we investigated the in vitro digestibility of a dietary starch reference (gelatinized potato starch) in the presence of an oat flour with high AX content and the reference Belinda oat flour, the target of the mutagenesis.
In order to assess the starch digestion rate under conditions that simulate the physiological situation, i.e., considering the influence of viscosity-related phenomena, a combined alpha-amylolysis/dialysis method was used.[Citation17] The predicted Glycaemic Indices (pGI) estimated with this procedure show a good correlation with values measured in vivo.[Citation20]
In line with the well-known glycemia-modulating effects of oats,[Citation3] the presence of the investigated oat flours in a mixture with potato starch resulted in a decreased rate of the overall rate of starch digestion (), with consequently lower HI and pGI values (). However, the effect was more marked for the high-AX line (CT0320). Considering that both oat flours provide similar β-glucan concentrations in the assay (Table S1), these observations suggest that AXs in CT0320 make an important contribution to the reduction in the starch digestion rate. The mechanism of action of these polysaccharides involves their viscosity-increasing properties, which may reduce the rate of small intestinal digestion and absorption of the available carbohydrates present in a meal.[Citation1,Citation3] Moreover, recent studies indicate that AX and β-glucans, among other polysaccharides, may interact with the alpha-amylase enzyme in vitro, leading to enzyme inhibition, an effect that might add to the viscosity-related amylolysis retarding effect.[Citation24]
Additional evidence of the role of AX was given by the pre-incubation of the potato starch/CT0320 sample with xylanase which resulted in faster starch digestion (), with higher HI and pGI values compared to those recorded for the untreated potato starch/CT0320 flour blend (), which stresses the significant role that AX in this oat line plays in the starch digestion rate reducing effect. Interestingly, the pre-treatment with ß-glucanase had an intermediate impact on the rate of digestion of the potato starch/CT0320 sample () (), a result that confirms that ß-glucans are also involved in the starch digestion deceleration. Further digestibility characterization through the assessment of nutritionally relevant starch fractions in starch/CT0320 flour blends in vitro, as well as their impact on the glycemic response in vivo, are yet to be investigated.
Conclusion
The present study showed that the flour of the seeds of CT0320, a mutated high arabinoxylan oat line, reduces the predicted Glycaemic Index of a gelatinized potato starch/oat flour blends to a larger extent than the flour of the original low arabinoxylan line and target of the mutagenesis, Belinda. ß-glucans are involved in the pGI-decreasing effect in the CT0320 line, but the greater impact seems to be mainly associated with its higher AX content. These results suggested that the ingestion of high AX oats in a meal may lead to a better modulation of the postprandial glycemic response, adding to the already known beneficial health effects of oats. This new oat line may gain big interest, especially as a potential tool for the dietary prevention and management of Type 2 diabetes. Future clinical studies are granted to confirm these additional health-beneficial properties of arabinoxylan-rich oat lines.
Authors contribution
AB, JZ, and JT planned the research. AB and KV performed experiments and analyzed data. AB and JZ wrote the manuscript and all authors reviewed and approved its final version.
Additional information
This work was made possible through support provided with the funding of this project by the Swedish Strategic Foundation (SSF) and the Scan Oats Industrial Research Center at Lund University.
Supplemental Material
Download ()Acknowledgments
The authors acknowledge support from the Swedish Strategic Foundation (SSF) and ScanOats. We thank the department of Food Technology and the Division of Pure and Applied Biochemistry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data has been provided in the form of tables and figures. More details will be provided on request basis.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2023.2233711
Additional information
Funding
References
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M. C.; Morales, P. Dietary Fiber Sources and Human Benefits: The Case Study of Cereal and Pseudocereals. In Advances in Food and Nutrition Research; Academic Press, Vol. 90 2019, pp. 83–134. 10.1016/bs.afnr.2019.02.002
- Ralla, T.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Oat Bran Extract (Avena Sativa L.) from Food By-Product Streams as New Natural Emulsifier. Food Hydrocoll. 2018, 81, 253–262. DOI: 10.1016/j.foodhyd.2018.02.035.
- Butt, M. S.; Tahir-Nadeem, M.; Khan, M. K. I.; Shabir, R.; Butt, M. S. Oat: Unique Among the Cereals. Eur. J. Nutr. 2008, 47(2), 68–79. DOI: 10.1007/s00394-008-0698-7.
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V. S. Nutritional Advantages of Oats and Opportunities for Its Processing as Value Added Foods-A Review. J. Food Sci. Technol. 2015, 52(2), 662–675. DOI: 10.1007/s13197-013-1072-1.
- Tian, L.; Gruppen, H.; Schols, H. A. Characterization of (Glucurono) Arabinoxylans from Oats Using Enzymatic Fingerprinting. J. Agric. Food Chem. 2015, 63(50), 10822–10830. DOI: 10.1021/acs.jafc.5b04419.
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E.; Makowski, B. The Influence of Arabinoxylans on the Quality of Grain Industry Products. Eur. Food Res. Technol. 2016, 242(3), 295–303. DOI: 10.1007/s00217-015-2549-0.
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L. I.; Zhang, H.; Qi, X.; Rao, Z. Cereal-Derived Arabinoxylans: Structural Features and Structure–Activity Correlations. Trends Food Sci. Technol. 2020, 96, 157–165. DOI: 10.1016/j.tifs.2019.12.016.
- Kellow, N. J.; Walker, K. Z. Authorised EU Health Claim for Arabinoxylan. In Foods, Nutrients and Food Ingredients with Authorised Eu Health Claims; Woodhead Publishing, 2018; pp. 201–218. DOI: 10.1016/B978-0-08-100922-2.00013-9.
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan Structural Characteristics, Interaction with Gut Microbiota and Potential Health Functions. J. Funct. Foods. 2019, 54, 536–551. DOI: 10.1016/j.jff.2019.02.007.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Arabinoxylan Produced from Wheat Endosperm and Reduction of Post-Prandial Glycaemic Responses (ID 830) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Efsa J. 2011, 9(6), 2205. DOI: 10.2903/j.efsa.2011.2205.
- Yan, W.; Deng, X. W.; Yang, C.; Tang, X. The Genome-Wide EMS Mutagenesis Bias Correlates with Sequence Context and Chromatin Structure in Rice. Front. Plant Sci. 2021, 12, 579675. DOI: 10.3389/fpls.2021.579675.
- Zambrano, J. A.; Thyagarajan, A.; Sardari, R. R.; Olsson, O. Characterization of High Arabinoxylan Oat Lines Identified from a Mutagenized Oat Population. Food Chem. 2023, 404, 134687. DOI: 10.1016/j.foodchem.2022.134687.
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro Starch Digestibility and the Glycemic Index of Six Different Indigenous Rice Cultivars from the Philippines. Food Chem. 2003, 83(3), 395–402. DOI: 10.1016/S0308-8146(03)00101-8.
- Chusak, C.; Henry, C. J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria Ternatea Flower Extract on the in vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods. 2018, 7(7), 102. DOI: 10.3390/foods7070102.
- Bergia, R. E.; Giacco, R.; Hjorth, T.; Biskup, I.; Zhu, W.; Costabile, G.; Vitale, M.; Campbell, W. W.; Landberg, R.; Riccardi, G. Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial Nutrients. Nutrients. 2022, 14(3), 706. DOI: 10.3390/nu14030706.
- Kim, H. J.; White, P. J. In vitro Digestion Rate and Estimated Glycemic Index of Oat Flours from Typical and High β-Glucan Oat Lines. J. Agric. Food Chem. 2012, 60(20), 5237–5242. DOI: 10.1021/jf300429u.
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in vitro Procedure Based on Chewing to Predict Metabolic Response to Starch in Cereal and Legume Products. Eur. J. Clin. Nutr. 1992, 46(9), 649–660.
- Li, C.; Hu, Y. In vitro and Animal Models to Predict the Glycemic Index Value of Carbohydrate-Containing Foods. Trends Food Sci. Technol. 2022, 120, 16–24. DOI: 10.1016/j.tifs.2021.12.031.
- Wang, Y.; Yuan, B.; Ji, Y.; Li, H. Hydrolysis of Hemicellulose to Produce Fermentable Monosaccharides by Plasma Acid. Carbohydr. Polym. 2013, 97(2), 518–522. DOI: 10.1016/j.carbpol.2013.05.017.
- Granfeldt, Y. Food Factors Affecting Metabolic Responses to Cereal Products. PhD dissertation, University of Lund, Sweden, 1994.
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients. 2019, 11(8), 1729. DOI: 10.3390/nu11081729.
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch‐Ernst, K. I.; Kearney, J.; Siani, A.; Maciuk, A.; Mangelsdorf, I.; McArdle, H. J.; Naska, A., et al. Beta-Glucans from Oats And/Or Barley in a Ready-To-Eat Cereal Manufactured via Pressure Cooking and Reduction of Blood-Glucose Rise After Consumption: Evaluation of a Health Claim Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFS2. 2021, 19(4), e06493. DOI: 10.2903/j.efsa.2021.6493.
- Kang, J.; Huang-Fu, Z.-Y.; Tian, X.; Cheng, L.; Zhang, J.; Liu, Y.; Liu, Y.; Wang, S.; Hu, X.; Zou, L., et al. Arabinoxylan of Varied Structural Features Distinctively Affects the Functional and in vitro Digestibility of Wheat Starch, Netherlands: Food Hydrocolloids. 2023. DOI: 10.1016/j.foodhyd.2023.108615.
- Li, H.; Dhital, S. α-Amylase Interaction with Soluble Fibre: Insights from Diffusion Experiment Using Fluorescence Recovery After Photobleaching (FRAP) and Permeation Experiment Using Ultrafiltration Membrane. Bioact Carbohydr. Dietary Fibre. 2022, 28, 100319. DOI: 10.1016/j.bcdf.2022.100319.
