ABSTRACT
Significant levels of flavonoids with antioxidant properties can be found in citrus species. Flavonoids are a class of plant chemical known for a wide range of pharmacological activities. Notable among these flavonoids is Hesperidin. It has quite a number of biological properties, like anti-inflammatory and antioxidant properties. Numerous studies have examined the biological impacts of hesperidin and its underlying mechanisms throughout the last few decades. Hesperidin’s antioxidant properties have led to a thorough evaluation of this phytochemical’s cardioprotective and anti-cancer properties. To assess the underlying neuropharmacological processes of hesperidin, numerous cellular and animal models have been created. Additionally, its neuroprotective activity has been validated by clinical data. Hesperidin reduces neuroinflammatory and apoptotic pathways to exert its neuroprotective effects. In preclinical models for disorders of the central nervous system, hesperidin function has been investigated. Hesperidin has been shown to enhance memory and cognition while successfully treating depression. Although the biological activities of hesperidin in neurodegenerative diseases have been evaluated, more investigation into its underlying mechanisms is required to understand its function in a number of central nervous system disorders, including autoimmune demyelinating disease and neurodegenerative diseases. Thus, this study focuses on the potential role of hesperidin in several models of central nervous system neuroinflammation, such as experimental autoimmune encephalomyelitis, and on the potential role of hesperidin in various neurodegenerative diseases, with a focus on its antioxidant, anti-apoptotic, and anti-inflammatory properties. Information about the usage of hesperidin as a nutraceutical to prevent certain CNS illnesses based on recent research is provided.
Introduction
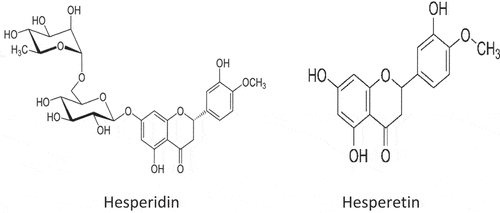
Chemically, hesperidin (C16H14O6) is 3′, 5, 7-trihydroxy-4′ -methoxy flavanone or hesperetin 7-O-rutinoside. It is a naturally occurring flavanone glycoside in citrus[Citation1,Citation2] (). Hesperidin is also present in tea, and olive oil, and it is utilized in traditional Chinese medicine to treat a wide array of ailments.[Citation3] Orally consumed hesperidin is digested right inside the small intestine by the activity of intestinal flora but its absorption takes place in the large intestine, while the aglycone derivative of hesperidin, hesperetin (4′-methoxy-5,7,3′ trihydroxyflavanone), has the small intestine as its site of absorption.[Citation4] According to Camps-Bossacoma et al.,[Citation5] hesperidin regulates the immune system by altering the composition of lymphocyte associated with the intestinal mucosa and tissues connected to the gut.[Citation6] Hesperidin and hesperetin’s chemical structures () have been extensively described in earlier research.[Citation4,Citation7]
Hesperidin possesses a wide range of advantageous biological benefits that have been linked to its anti-inflammatory, anti-apoptotic and antioxidant capabilities.[Citation8,Citation9] Hesperidin produced antidepressive effects in both Parkinson’s disease-affected rats and streptozotocin-induced diabetic rats.[Citation10,Citation11] In addition, studies using animal models of neurodegenerative diseases have shown that hesperidin can lower inflammation and relieve symptoms of disorders like Huntington’s disease,[Citation12] Alzheimer’s disease (AD),[Citation13] neuroimmunological multiple sclerosis (MS),[Citation14,Citation15] depression (El-Marasy et al., Hesperidin also reduced lipopolysaccharide-induced memory impairment in cell-based and cell-free systems, as well as traumatic injury to central nervous system (CNS) tissues.[Citation16] Despite the different and specific etiologies of the aforementioned neuroinflammatory diseases, the damaged brain tissues display oxidative stress and inflammatory responses. However, these aberrations can occasionally be corrected by antioxidant flavonoids.
Hesperidin is one of the citrus flavonoids that has been found to have beneficial health effects. These effects have been discussed in relation to their neuropharmacological characteristics[Citation4] and neuroprotective capacities,[Citation17–19] with a focus on their capacity to combat depression and to prevent learning and memory deficits. Hesperidin’s effects on neurological disorders in animal models are currently the subject of increased research.
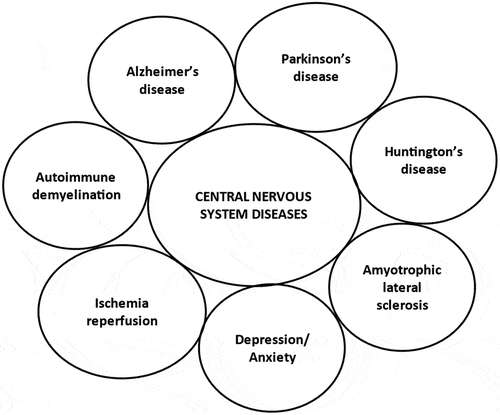
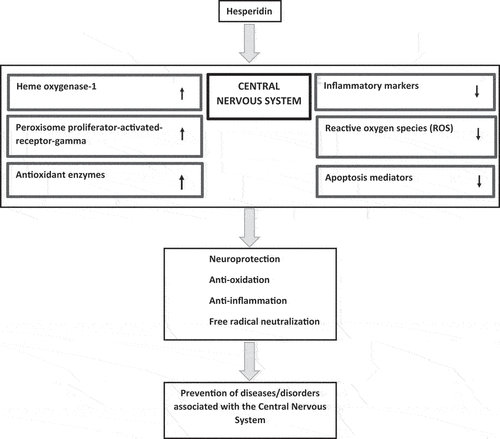
Due to advancements in science and technology, life expectancy is increasing and CNS disorders are receiving attention on a global scale. Hesperidin has been demonstrated to be effective in treating neurological issues despite the fact that there have only been a limited number of studies. Numerous researchers have focused their attention on the neuropharmacological mechanisms underlying its action and the investigation of its molecular targets because of its potentially advantageous neuroprotective characteristics. The disorders of the CNS that have been well studied include but are not limited to neurodegenerative illnesses, psychiatric conditions, demyelinating illnesses, ischemic-reperfusion injuries, and neuroinflammatory conditions (). Many pathways are implicated in the evolution of various neurodegenerative diseases. One of the things that can threaten neuronal populations in the brain is inflammation. The neurons have been shown to be partially or completely annihilated in various neurodegenerative conditions that impair synaptic and cognitive function.[Citation20] Hesperidin’s neuroprotective properties have been shown in numerous research using diverse models of neurodegenerative illness.
We outline the most recent studies on how hesperidin helps alleviate and manage CNS diseases in this review. Due to the dearth of thorough studies, this article attempts to address the gap in the body of knowledge concerning the function of hesperidin in the prevention and treatment of many CNS illnesses. We give a thorough explanation of how hesperidin works and what its molecular targets are in the central nervous system. The current review also offers updates on new studies examining hesperidin’s role in the neuroinflammation model brought on by oxidative stress, such as brain injury from ischemia-reperfusion and chemical-triggered hippocampal anomalies. This review also covers the probable function of hesperidin in autoimmune disorders.
For this review, we used the terms: “hesperidin and Alzheimer’s disease,” “hesperidin and Parkinson’s disease,” “hesperidin and antioxidant and demyelination,” “hesperidin and inflammation,” and “hesperidin and CNS,” to search relevant databases like PubMed, Scopus, Google Scholar, and Science Direct for published studies. All authors of the current review decided which papers were eligible for data extraction.
Citrus fruits contain hesperidin, naringin, naringenin, diosmin, quercetin, and russeting as flavonoids. Flavonoids of citrus origin have anti-inflammatory, anti-apoptotic, and antioxidant abilities. Notable among these flavonoids, hesperidin, has a number of positive health effects.[Citation1] Moreover, the effectiveness of bioactive substances is greatly influenced by absorption, distribution, and metabolism. Hesperidin undergoes a chemical reaction that transforms it into hesperidin 7-O-glycoside after being administered for 20 minutes. Through the action of beta-glucosidase, the small intestine or colon hydrolyzes flavone aglycone.[Citation21]
Antioxidative and anti-inflammatory molecular mechanisms of hesperidin
Over-production of reactive oxygen species (ROS) and/or compromise of the antioxidant defense system often result in an unbalanced redox state. The brain is highly susceptible to oxidative onslaught due to its high demand for oxygen and its high concentration of lipids. Hesperidin’s antioxidant properties have been documented in the past.[Citation1] Hesperidin’s antioxidant properties and enhancement of cellular antioxidant systems are key components of its positive benefits.[Citation8] Hesperidin improves the activity of downregulated antioxidant enzymes associated with the brain of stroke experimental model,[Citation22] irradiation,[Citation23] and lipopolysaccharide-induced endotoxicity.
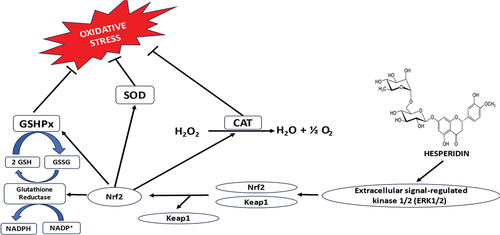
According to a comprehensive study by Evans et al.,[Citation24] oxidative stress has a significant impact on neurodegenerative diseases including AD and PD; agents that reduce excessive ROS are used more frequently in alleviating neurodegeneration. Hesperidin suppresses nuclear factor-kappa B (NF-кB) signaling, the receptor of advanced glycation end products, and stimulates Akt/Nrf2 to annul oxidative onslaught and inflammation.[Citation25] Moreover, the presence of hesperidin prevents differentiation from being inhibited, as seen by an upsurge in differentiation markers and Akt kinase phosphorylation.[Citation26] Moreover, hesperidin may reverse the suppression of oligodendroglial cell development caused by inflammatory cytokines.[Citation27] Heme oxygenase-1 and nuclear factor erythroid 2-related factor-2, which play key roles in the prevention of cells against oxidative onslaught, are selectively upregulated by hesperidin to produce extraordinary antioxidative potentials by neutralizing ROS and improving the defense mechanism of endogenous antioxidants.[Citation28,Citation29] In a study carried out by Li et al.,[Citation30] hesperidin was revealed to increase the level and the activity of antioxidant enzyme via Keap1-Nrf2 (nuclear factor erythroid 2-related factor 2) pathway. This was shown to be the major molecular mechanism behind hesperidin’s antioxidative and anti-inflammatory potentials (). Hesperidin increases the expression of Nrf2, separate the Keap1-Nrf2 complex, and increase the nuclear translocation of Nrf2. Additionally, by binding to the antioxidant response element (ARE) in the gene promoter region, Nrf2 activates gene transcription, increasing the production of anti-oxidant enzymes.
An earlier investigation found that H2O2 bought about oxidative stress in RPE-19 cells treated with hesperidin. Inhibiting apoptosis (by controlling the expression of caspase-3, and B-cell lymphoma 2), preventing the liberation of ROS production, inhibiting the production of malondialdehyde (MDA) from peroxidative damage of cellular membrane, and increasing antioxidant enzymes activities like superoxide dismutase (SOD) and catalase (CAT) and expression of antioxidant molecule like glutathione (GSH), which may stimulate Keap-1 signaling, substantially conferred protection to RPE-19 cells against oxidative onslaught.[Citation31] Zhu et al.[Citation29] reported that rats injected with 250 mg potassium oxonate per kg body weight with or without 5 mg hesperidin per kg body weight and 5 ml orange juice per kg body weight developed hyperuricemia within two weeks of exposure to the test compound. Orange juice and hesperidin increase antioxidant mechanisms and lower lipid peroxidation. Moreover, Hesperidin’s biological effect was experimented on Sprague-Dawley rats at a level of 100 mg/kg/day (4–5 weeks old). Hesperidin was reported to counteract the neurotoxic effect brought on by methotrexate by lowering oxidative stress and boosting hippocampus neurogenesis.[Citation32] One mechanism that may help explain fluoride’s damaging effects is the oxidative stress it causes. Treatment with hesperidin improved neurobehavioral dysfunction and biochemical alterations in the brain, including acetylcholinesterase activity and antioxidant markers. Hesperidin activates peroxisome proliferator-activated receptor-gamma in models of gastric ulcers caused by stress,[Citation33] ischemic heart disease,[Citation34] and cyclophosphamide-induced hepatotoxicity.[Citation34] This is relevant to the signal cascades.[Citation35] Another study suggests that the tendency of hesperidin to mop-up free radicals may be the cause of its neuroprotective effects. Hesperidin markedly reduced MDA levels in mice after inducing mild traumatic brain damage. Hesperidin was chronically given to AD mouse models, which decreased MDA concentration associated with the brain and attenuated peroxidative damage of neuronal lipids.[Citation36] In addition, after N-methyl-D-aspartate assault, hesperidin’s neuroprotective effects were intensified in animals lacking the C/EBP homologue protein.[Citation37] Hesperidin may ultimately lessen high oxidative stress, decreasing the harm brought on by oxidative stress.
Aja et al.[Citation38]reported that hesperidin ameliorated cadmium-induced pancreatitis in rats via regulating insulin secretion, modulation of inducible nitric oxide synthase/nuclear factor-kappa B signaling, and mitigation of redox imbalance. Parhiz et al.[Citation8] reveals that the antioxidant property of Hesperidin correlates with its anti-inflammatory potential. For instance, in a study where aluminum chloride was used to induce neuroinflammation in rat hippocampus and N-methyl-D-aspartate was used to stimulate excitotoxicity in rat retina, hesperidin did well in mitigating the expression of tumor necrosis factor-alpha, a potentiator of pro-inflammation.[Citation39] The extent of phosphorylation of kinase-1 and kinase-2 which is essential for cell signal regulation of the kinases in rat brain, is decreased in normal mice after intraperitoneal injections of hesperidin (Martinez et al.).[Citation40] Consequently, it is possible to hypothesize that hesperidin, like other flavonoids, increases radical-ameliorating activity whilst reducing the inflammatory response, consequently protecting against neuronal death.
Bioactivity of hesperidin in central nervous system diseases
Hesperidin’s neuroprotective actions in neurodegenerative disorders
Hesperidin and Alzheimer’s disease
Alzheimer’s disease (AD) is a neurological condition with complex causative factors that is marked by memory deficit, and cognitive decline. The most typical type of dementia is AD. Hesperidin’s effects on AD were assessed in a lipopolysaccharide-induced neurodegenerative model. It is possible to assess the neuroprotective properties of hesperidin using a variety of animal models (). The advantages of hesperidin have been studied using a rat model of AD. Hesperidin reduced the production of reactive ROS and lipid peroxidation (LPO) caused by lipopolysaccharide. Also, they discovered that hesperidin nanoparticles were more effective than hesperidin powder.[Citation41] Neuroinflammation and neurodegeneration are mediated by antioxidant processes controlled by glial cells via Toll-like receptor 4 (TLR4). Through improving TLR4-mediated Iba-1/glial fibrillary acidic protein expression, hesperidin cotreatment brought about a substantial decrease in inflammatory cytokines’ expression. The Morris Water Maze and Y-maze tests revealed that hesperidin improved cognitive impairment in mice.[Citation42] Lipopolysaccharide is produced by gram-negative bacteria as a component of their outer membranes. Lipopolysaccharides as well as activating transcription factors all target TLR4.[Citation43]
Table 1. Hesperidin’s bioactivity in animal models and the underlying mechanisms.
Hesperidin and Parkinson’s disease (PD)
One of the most prevalent neurodegenerative disorders, along with AD, is Parkinson’s disease. Because the substantia nigra pars compacta is impacted, less dopamine (DA) is accessible for neurotransmission in the striatum.[Citation44] Resting tremors, postural instability, rigidity, bradykinesia, and gait impairment are a few of the motor symptoms linked to PD. The nigrostriatal pathway’s loss of cells of the dopaminergic system causes a malfunction in the basal ganglia.[Citation45] Anxiety and depression are frequently present in PD patients, as non-motor symptoms.[Citation46] In one study, 6-hydroxydopamine, a neurotoxin that causes symptoms like PD, was administered to animals. Administration of 50 mg of Hesperidin per kg body weight, for 28 days proved effective in treating the PD-like symptoms. Hesperidin was found to be efficacious in this investigation against striatal dopaminergic denervation, neurotrophic deficiencies, and neuroinflammatory reactions. Hesperidin dramatically decreased 6-hydroxydopamine-triggered oxidative stress indicators in SH-SY5Y cells.[Citation47] By the activation of these neuropharmacological pathways, 3, 4-dihydroxyphenylacetic acid levels and striatal dopamine were preserved in 6-hydroxydopamine-lesioned mice and emotional-related behaviors were improved.[Citation48] Hesperidin’s usage to confer neuroprotection as in the treatment of PD may have great potential in light of these protective qualities.[Citation10]
Hesperidin and neurodegeneration caused by cadmium
Hesperidin, due to its antioxidant and neuroprotective abilities, can assist in mitigating neurodegeneration brought on by cadmium. Cadmium, an environmental contaminant, builds up in the bodies of both humans and animals. It has been reported to negatively impact the blood-brain barrier; its buildup can lead to oxidative stress and neurodegeneration.[Citation49] Bernhoft[Citation50] reported that hesperidin administration at doses of 10 mg and 50 mg per body weight significantly reduced lipid peroxidation in mice and protected against neurodegeneration through antioxidant enzymes activation. It was also revealed in this same study that the administration of hesperidin at higher doses did not cause neuronal death. Hesperidin’s antioxidant and neuroprotective qualities can therefore be said to be the reason hesperidin can be used to treat neurodegenerative diseases.[Citation51]
Hesperidin and hippocampal dysfunction
The hippocampus has a very essential role in the development and progression of multiple sclerosis (MS), the animal model of experimental autoimmune encephalomyelitis (EAE), cognitive and memory processes. There is currently agreement that MS patients experience cognitive dysfunction[Citation52,Citation53] and that hippocampus shrinkage occurs in EAE mice along with increased oxidative stress and activated microglia.[Citation54,Citation55] Hesperidin produces neuroprotective and other advantageous effects in EAE model organisms, which may be brought about by the inhibition of microglial cells. Hesperidin may confer protection to neurons against excitotoxicity brought on by glutamate in EAE-affected brains, as it is observed in neurodegeneration models.[Citation4,Citation19] The neuroprotective potential of hesperidin against hippocampus excitotoxicity was established through a study that involves neuronal death alongside microglial cells hyperactivation as a result of excitotoxicity caused by aluminum chloride.[Citation56] Hesperidin’s cytoprotective effects are mostly reliant on its anti-inflammatory and antioxidative potentials,[Citation57,Citation58] which suggests that hesperidin therapy can improve cognitive deficits. Donato et al.[Citation59] also found that hesperidin treatment reduced nitrate/nitrite levels, as well as bringing about an upsurge in the amount of neurotrophic factor associated with the hippocampus region of mouse brain.[Citation60] Moreover, via the regulation of acetylcholinesterase activity, hesperidin enhances memory in mouse with cognitive deficits caused by streptozotocin and guards neurons against cell death.[Citation61,Citation62] Hesperidin considerably improves glutathione content after middle cerebral artery blockage and substantially undo deficits in the activity of CAT, SOD, and glutathione peroxidase (GPx). Moreover, hesperidin prevents the expression of inducible nitric oxide synthase and the synthesis of a number of inflammatory mediators, like interleukin-1 and tumor necrosis factor-alpha. This suggests that hesperidin promotes neuronal survival[Citation62] and that neuroprotection brought on by hesperidin depends in part on the inhibition of hippocampal inflammatory processes.[Citation22,Citation63]
Hesperidin’s effects in mental disorders
Hesperidin and depression
Study has shown that hesperidin reduces depression independent of its underpinning factors.[Citation18] Hesperidin decreased the amount of time subjects remained immobile during the forced swimming test and may have even increased hippocampal neuronal activity.[Citation68] Hesperidin’s antidepressant-like effects, according to a study, are also mediated by serotonergic 5-hydroxytryptamine 1A receptors and hippocampal kappa-opioid receptors.[Citation68,Citation69] Hesperidin alters depressive-like behaviors in 6-hydroxydopamine model mice of Parkinson’s disease, mice with lipopolysaccharide-induced endotoxemia, and streptozotocin-triggered diabetic mice, after an olfactory bulbectomy; according to the study, these were attributed to its neuroprotective property and ability to modulate serotonergic neurons.[Citation70] Hesperidin also largely activates the Nrf2/glyoxalase1/antioxidant-responsive element to exert its antidepressant effects.[Citation11] According to a study, hesperidin increases Glo-1 mRNA expression, as well as protein levels in the brain of diabetic rats while decreasing levels of p-Nrf2 in the cytoplasm, as well as Nrf2 within the nucleus. This suggests that these genes are the targets of Nrf2/antioxidant-responsive element communication. Hesperidin treatment allowed SH-SY5Y cells to maintain their neural function in spite of damage brought on by high glucose levels. Hesperidin reduced anxiety and depressive-like symptoms linked to diabetes by stimulating the conventional Nrf2/antioxidant-responsive element pathway.[Citation71] Rats with post-traumatic stress disorder experienced an increase in 5-hydroxytryptamine levels during hesperidin administration, which was partially attributed to a decline in 5-hydroxyindoleacetic acid to 5-hydroxytryptamine ratios. Hesperidin administration also reduced tryptophan hydroxylase-1 expression and blocked the activity of monoamine oxidase-A. After hesperidin therapy, the level of mRNA of tryptophan hydroxylase-1 associated with the hippocampus likewise returned to normal. The committed step in the biosynthetic pathway of serotonin is catalyzed by the enzyme, tryptophan hydroxylase, which is essential for serotonin metabolism.[Citation69] In a different study, high-dose hesperidin administration enhanced the level of neurotrophic factor associated with the hippocampus while suppressing inflammatory and apoptotic signaling. Hesperidin also increased brain-derived neurotrophic factor levels in the hippocampus while decreasing the levels of Interleukin-1 and tumor necrosis factor.[Citation36,Citation72–74] Hesperidin alters cytokines, neurotrophic factors, and dopaminergic innervation in the striatum to ameliorate neurotoxicity brought on by 6-hydroxydopamine.[Citation10] Hesperidin also decreased depression by reducing neuroinflammation linked to kinase B signaling pathways in chronic unusual mild stress (CUMS) mouse models. These findings not only improved our comprehension of the etiology of depression but also speculates that hesperidin could represent a therapeutic candidate for neurodegeneration.[Citation72] Moreover, animals induced with CUMS showed decreased expression of the NLRP3 inflammasome following hesperidin treatment. Additionally, in the CUMS rats, the NLRP3 gene deletion prevents the NF-кB protein complex from becoming activated. Depression-like symptoms were demonstrated in NLRP3 inflammasome-mediated CUMS rats.[Citation74] Hesperidin therapy therefore markedly reduced depressive-related symptoms. Hesperidin with bupropion, agomelatine with bupropion and hesperidin with agomelatine were found to have antidepressant-like effects in an investigation, both in vitro and in vivo.[Citation75] Hesperidin can activate the Nrf2/ARE/Glo-1 pathway to have advantageous effects. In the hippocampus region, hesperidin increases brain-derived neurotrophic factor and norepinephrine (NE) levels while lowering the levels of interleukin-1, and interleukin-6, as well as tumor necrosis factor-alpha. So, it is possible to hypothesize that the antidepressant-like activity of hesperidin is gained in a nonspecific manner through the wide range of neuroprotection outlined above.[Citation4,Citation17,Citation19] The current study investigated the various mechanisms underlying the action of hesperidin because the substance’s antidepressant effects were not well explored. Future research should examine additional factors in preclinical and clinical contexts, including brain-derived neurotrophic factor, melatonin, serotonergic drugs, and glutamate.
Hesperidin and anxiolytic effects
In an investigation by Zhu et al.,[Citation76] hesperidin was presented to reduce anxiety-like behaviors in diabetic rats via its ability to modulate pathways involving protein kinase A, cyclic AMP response element binding protein and brain-derived neurotrophic factor. As a result of the study’s findings, scientists may be able to develop therapies to help address anxiety-like behaviors often time presented in diabetes. In rats with anxiety disorders, hesperidin and nano-hesperidin were found to mitigate anxiety-like behaviors by modulating critical enzymes’ activity and the expression of key molecules associated with this behavior; also, by reducing oxidative onslaught. As confirmed by qRT-PCR, hesperidin, as well as nano-hesperidin impacted CAT, glutathione reductase and SOD genes expression in the cerebral cortex differently than streptozotocin-induced mice.[Citation77] Moreover, 5-hydroxytryptamine concentration was found to be decreased in the hippocampus and in the amygdala, as a result of single persistent stress, which was inhibited by hesperidin therapy. Hesperidin’s beneficial effects on posttraumatic stress can therefore be linked to its anxiolytic characteristics.[Citation69] Hesperidin’s cellular and molecular mechanisms, particularly its impact on healthy brain cells, are still poorly understood.
Hesperidin and memory function
Hesperidin enhances memory in healthy mice and encourages hippocampus synaptogenesis. Hesperidin increases astrocytes’ synaptogenic capacity through modifying signaling associated with transforming growth factor beta-1 in astrocytes. Hesperidin strengthens the link and communication between the hippocampus and the cortical neurons via their respective synapses, directly improving spatial memory ability in healthy adult mice, and indirectly improving it by boosting the secretion of transforming growth factor beta-1, which enhances cortical astrocytes’ synaptogenic activity.[Citation78] Hesperidin reduced motor symptoms like motor incoordination, as well as non-motor symptoms, including anxiety-like behaviors, deficiencies in memory, as well as monoaminergic abnormalities. Hesperidin improves memory and cognition deficits that are caused by neurodegeneration brought on by neuroinflammation.[Citation42] In another investigation, Hesperidin was found to increase α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor trafficking and the balance between antioxidant and oxidant, presumably via reversing age-related deficiencies in emotional memory and synaptic plasticity.[Citation79] The co-administration of Hesperidin and electroconvulsive therapy significantly improved cognition and reduced electroconvulsive therapy-induced memory dysfunction in rats administered reserpine.[Citation80] Hesperidin exhibited a potent anticonvulsant activity in pentylenetetrazole-induced convulsive mice through the modulation of gamma-amino butyric acid/benzodiazepine receptor action.[Citation81] Hesperidin exhibits anticonvulsant activity in a different study using a zebrafish pentylenetetrazole convulsion paradigm. The in silico computational analysis backed by gene expression analyses in this study revealed that the interaction of hesperidin with the central cAMP response element binding protein – brain-derived neurotrophic factor (CREB-BDNF) pathway was responsible for the observed anticonvulsant effect.[Citation82]
Hesperidin and autoimmune demyelinating disease
According to Lassmann,[Citation83] animals exposed to hesperidin treatment displayed less demyelination and glial activation. Diffuse neurodegeneration and chronic inflammatory demyelinating disease does not impact the white matter of the brain but have an impact on the gray matter in multiple sclerosis (MS). In a study carried out by Baradaran et al.,[Citation84] hesperidin therapy was assessed for myelin repair and glial activation in a model of localized demyelination triggered by lysolecithin. Hesperidin was given orally to the study’s animals for 21 days after the lesion was induced at a dose of 20 mg per kg body weight. In a model of focal demyelination brought on by lysolecithin, hesperidin improved visual pathway performance. In this study, visual evoked potential investigation revealed that orally administered hesperidin treatment significantly shortened the N1 wave latency. Results of immunostaining revealed that hesperidin-treated rats had fewer microglia, as well as reduced astrocytes. Experimental animals exposed to hesperidin therapy also exhibited a decrease in the activation of glial cells and a consequent lessened demyelination. Treatment with hesperidin was observed to be successful in myelin sheath repair and preservation, in demyelinating illnesses, particularly MS, indicating its potential as a valuable supplementary therapy. In another study, rats received 20 mg of nano-hesperidin per kg body weight via the oral route for a period of 21 days following lysolecithin injection directly into the optic chiasm. The nano-hesperidin decreased the extent to which demyelination occurs, enhanced oligodendrocytes and myelin basic protein expression, as well as aiding microglia and astrocytes’ activation. Nano-hesperidin increases endogenous remyelination as it activates the glial cells and lessens demyelination levels.[Citation85]
In contrast to neurodegenerative disorders like Alzheimer’s disease, autoimmune demyelinating diseases like multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) are known to cause different types of neuroinflammation.[Citation86] Autoimmune T cells’ development in peripheral lymphoid organs and their subsequent incursion into the parenchyma tissue of the brain, which is believed to be the cause of reactive gliosis and consequent demyelination, are features of EAE.[Citation87] As a result, inhibiting the proliferation of T-cells during the induction phase of experimental autoimmune encephalomyelitis is one of the main objectives for the prevention of this illness. Another crucial step in shielding neurons and the glial cells of the central nervous system (CNS) from onslaught is to stop the migration of inflammatory cells into CNS tissues. Another strategy that may slow the evolution of EAE is the control of oxidative stress and inflammatory mediators.[Citation88] The treatment of EAE would benefit from stopping at least one of these three processes. Recent research has demonstrated that one of the above-mentioned methods is the approach through which the antioxidant and natural flavonoid, apigenin suppresses the course of EAE.[Citation89] Hesperidin reduces EAE-induced paralysis in models of autoimmune CNS illnesses whether given orally at doses of 50, 100, and 200 mg per kg body weight[Citation14] or subcutaneously at a dose of 50 mg daily per kg body weight for a period of 7 straight days.[Citation90] Hesperidin’s potent antioxidant and anti-inflammatory qualities play a key role in the mechanisms behind its effects on EAE.[Citation14,Citation90] Additionally, according to Haghmorad et al.,[Citation14] hesperidin is involved in the reduction of microglial cells in EAE lesions, the stimulation of modulatory T cells, decreased demyelination, and repression of the proliferation of autoimmune T-cells.[Citation88] Hesperidin’s positive effects on EAE, therefore, seem to be mostly based on reducing oxidative stress in tissues that are targeted by EAE and also based on inflammatory response in the immune system, which would prevent demyelination. Nitric oxide can occasionally inhibit T-cell growth and cause cell death, as observed in autoimmune disease models.[Citation91] Nitric oxide levels being lower could therefore encourage the growth of autoimmune T cells and subsequently prevent the apoptotic death of cells associated with the concerned organs. Studies that evaluated the nitric oxide carrier’s treatment in this autoimmune encephalomyelitis provide additional evidence for this conclusion.[Citation92,Citation93]
Hesperidin and ischemia reperfusion injury and brain damage
Through apoptosis and oxidative damage lessening, hesperidin confers protection to ganglion cells against ischemia-reperfusion injury (IRI). IRI happens when ischemic cells’ blood supply is restored and cellular death is hastened. Hesperidin was administered intravenously to mice in the middle cerebral artery to alleviate neurological impairments and control microglia polarization. Hesperidin’s ability to suppress the TLR4-NF-кB pathway in vitro tests demonstrated that it has neuroprotective properties, presenting novel targets and therapeutic options for the treatment of post-stroke neurodegenerative disorders.[Citation94] Blood flow must be reestablished for ischemic tissues to heal. Reperfusion damages the organ even more, compromises its viability, and interferes with its regular operation.[Citation95] Mice may have an IR injury by having their intraocular pressure raised to one hundred and ten millimeter mercury for forty minutes. This may cause damage to ganglion cells, inflammation, oxidative onslaught, and cell death. After giving mice 0.3 mL of either water-soluble hesperidin or normal saline daily, the effects of hesperidin on IRI were evaluated. Through decreasing apoptosis and lipid peroxidation, hesperidin confers protection on ganglion cells from ischemia-reperfusion injury. Hesperidin also decreased inflammation by repressing microglial cells.[Citation96] In a different trial, diets containing 50 mg of carnosine per kg and 150 mg of hesperidin per kg were given to animals seven days before ischemia was induced. There was a substantial reduction in ischemic lesion size by thirty percent, as a result of hesperidin’s neuroprotective capabilities, which is comparable to carnosine. In a study that involves cerebral ischemia rat model, oral administration of hesperidin at doses of 50 mg and 100 mg per kg daily, over a period of 7 days, brought about neuroprotection. Consequently, under identical circumstances, hesperidin conferred neuroprotection in global cerebral ischemia, as well as in focal cerebral ischemia. SOD’s protective action in their investigation of ischemia-affected rats was probably due to the enzyme’s active radical-annulling property. Hesperidin also activated the sirtuin 1 (SIRT1) deacetylase, which increased SOD gene expression in yeast (Devyatov et al.).[Citation97] Hesperidin therefore ameliorated focal cerebral ischemia-reperfusion injury in a preventative manner in rats; conferring a strong neuroprotective effect. Hesperidin decreased inflammatory factor generation and decreased microglial activation. Through its ability to prevent microglial activation via the adenosine monophosphate-activated protein kinase-SIRT1 pathway, hesperidin facilitated neurobehavioral recovery, according to a study that utilizes an in vitro traumatic brain injury (TBI) model. These findings speculate that hesperidin may be a potential therapeutic candidate for reducing or eliminating neuronal inflammation and nerve mutilation after a traumatic brain injury.[Citation98]
Hesperidin and neuroinflammation
Hesperidin plays a well-known role in regulating inflammation, oxidative onslaught, and promoting responses that pertain to acquired and innate immune systems. Hesperidin therapy has been associated with a decrease in inflammatory markers in human studies.[Citation99] Many investigations have been done thus far to show that hesperidin has anti-inflammatory effects. Researchers investigated the underlying mechanism behind hesperidin’s beneficial effects toward neuroinflammation and consequent neurodegeneration brought on by beta-amyloid (Aβ). Hesperidin lessens oxidative damage brought on by beta-amyloid, which in turn lessens activation of astrocytes and microglia. Glial cell suppression was accompanied by a decrease in the phosphorylation of nuclear factor-kappa B and a decrease in the production of inflammatory mediators. Many in vitro experiments have been carried out to demonstrate hesperidin’s rescue effects against Aβ-induced neuroinflammation in order to highlight its anti-inflammatory characteristics. One study looked at how hesperidin prevented beta-amyloid-induced neuroinflammation and neurodegeneration. Hesperidin lowers oxidative damage brought on by beta-amyloid, which in turn lowers activated astrocytes and microglia. Inflammatory mediators were produced and nuclear factor-kappa B phosphorylation was decreased consequent to the inhibition of glial cells. Hesperidin also inhibited toll-like receptor-4 and phosphorylated nuclear factor-kappa B; a process that brought about a reversal in neuroinflammation brought on by beta-amyloid[Citation100]. Hesperidin caused the inflammation to be lessened, which was shown by the decreased production of toll-like receptor-4 and phosphorylated nuclear factor-kappa B, and glial fibrillary acidic protein. Lipopolysaccharide cause neuroinflammation, the emergence of cognitive impairments, and septic shock in animal models.[Citation101] Moreover, tumor necrosis factor-alpha and other oxidative stress and inflammation-related mediators are elevated in mice with lipopolysaccharide-induced neuroinflammation, while hippocampal acetylcholinergic activity is decreased as a result of acetylcholinesterase activation.[Citation101] Hesperidin reduces the effects of lipopolysaccharide exposure by boosting the production of antioxidant enzymes like SOD and CAT, that scavenge free radicals. Hesperidin also lowers the concentration of cytokines of pro-inflammation, such as tumor necrosis factor-α, interleukin-1 and interleukin-6, in the brain of lipopolysaccharide-treated rats.[Citation102] Hesperidin is substantially absorbed in the large intestine following its conversion to hesperetin by intestinal flora,[Citation4] which would then allow it to enter the blood circulation and eventually cross the blood-brain barrier to enter the brain.[Citation103] Hesperidin dramatically reduces the generation of nitric oxide and pro-inflammatory markers like tumor necrosis factor-α and interleukin-1 in the macrophage cell line.[Citation104] Hence, there is general agreement that hesperidin inhibits astrocytes, microglia, or both of their biological activities in a variety of ways. Together, these findings imply that hesperidin prevents inflammation in the brain, as well as in non-neuronal systems. This effect may be accomplished by lowering cytokines of pro-inflammation, boosting the activation of antioxidant enzymes that scavenge free radicals, and reviving neuronal function. All of these cytokines’ expression is controlled by MAPK signaling.[Citation99] By lowering oxidative stress and promoting hippocampus neurogenesis, hesperidin mitigates the negative effects of toxic neurobehavioral substances.[Citation105]
Conclusion
In general, it is still unknown what molecular processes underlie the flavone glycoside hesperidin’s protective actions against CNS illness. Citrus, on the other hand, is well known for its beneficial properties due to the range of nontoxic antioxidants it contains. Hesperidin’s neuroprotective properties have been the focus of numerous investigations over the past ten years. Hesperidin may have positive neuropharmacological effects in addition to its antidepressant, anti-inflammatory, and memory-improving actions. This has been tested in a number of in vivo models of CNS disorders. Research over the past few decades has generated experimental evidence that hesperidin confers significant neuroprotection via antioxidation, anti-apoptotic and anti-inflammation (). Also, the mechanism of action of hesperidin that underlies its beneficial role in immune cells and neurological tissues is directly correlated to the suppression of cell activation signals. Hesperidin’s anti-inflammatory and antioxidant properties will thus be able to support clinical trials for neurodegenerative illnesses; also, more molecular study is required to determine the probable mechanism underlying the anti-depressive and anxiolytic-like effects. Hesperidin may be used as a medicinal agent to treat a number of human ailments as a result of these in-depth studies.
Acknowledgement
The authors are grateful to the management of Kampala International University, Uganda, their colleagues, and peers for their insightful discussions and feedback, which helped to improve the quality of this review article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Garg, A.; Garg, S.; Zaneveld, L. J. D.; Singla, A. K. Chemistry and Pharmacology of the Citrus Bioflavonoid Hesperidin. Phytotherapy Res. 2001, 15(8), 655–669. DOI: 10.1002/ptr.1074.
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules. 2016, 21(6), 708. DOI: 10.3390/molecules21060708.
- Musa, A. E.; Omyan, G.; Esmaely, F.; Shabeeb, D. Radioprotective Effect of Hesperidin: A Systematic Review. Medicina. 2019, 55(7), 370. DOI: 10.3390/medicina55070370.
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological Properties and Pharmacokinetics of the Citrus Flavonoids Hesperidin and Hesperetin—A Mini-Review. Life Sci. 2014, 113(1–2), 1–6. DOI: 10.1016/j.lfs.2014.07.029.
- Camps-Bossacoma, M.; Franch, À.; Pérez-Cano, F. J.; Castell, M. Influence of Hesperidin on the Systemic and Intestinal Rat Immune Response. Nutrients. 2017, 9(6), 580. DOI: 10.3390/nu9060580.
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F. J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin Effects on Gut Microbiota and Gut-Associated Lymphoid Tissue in Healthy Rats. Nutrients. 2019, 11(2), 324. DOI: 10.3390/nu11020324.
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients. 2017, 9(5), 502. DOI: 10.3390/nu9050502.
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti‐Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytotherapy Res. 2015, 29(3), 323–331. DOI: 10.1002/ptr.5256.
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular Mechanisms Behind the Biological Effects of Hesperidin and Hesperetin for the Prevention of Cancer and Cardiovascular Diseases. Life Sci. 2015, 124, 64–74. DOI: 10.1016/j.lfs.2014.12.030.
- Antunes, M. S.; Cattelan Souza, L.; Ladd, F. V. L.; Ladd, A. A. B. L.; Moreira, A. L.; Bortolotto, V. C.; Boeira, S. P.; Araújo, S. M.; Prigol, M.; Nogueira, C. W. Hesperidin Ameliorates Anxiety-Depressive-Like Behavior in 6-OHDA Model of Parkinson’s Disease by Regulating Striatal Cytokine and Neurotrophic Factors Levels and Dopaminergic Innervation Loss in the Striatum of Mice. Mol. Neurobiol. 2020, 57(7), 3027–3041. DOI: 10.1007/s12035-020-01940-3.
- Zhu, X.; Liu, H.; Liu, Y.; Chen, Y.; Liu, Y.; Yin, X. The Antidepressant-Like Effects of Hesperidin in Streptozotocin‐Induced Diabetic Rats by Activating Nrf2/ARE/Glyoxalase 1 Pathway. Front. Pharmacol. 2020, 11, 1325. DOI: 10.3389/fphar.2020.01325.
- Menze, E. T.; Tadros, M. G.; Abdel-Tawab, A. M.; Khalifa, A. E. Potential Neuroprotective Effects of Hesperidin on 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neurotoxicology. 2012, 33(5), 1265–1275. DOI: 10.1016/j.neuro.2012.07.007.
- Lee, D.; Kim, N.; Jeon, S. H.; Gee, M. S.; Ju, Y. J.; Jung, M. J.; Lee, J. K.; Lee, Y.; Lee, S.; Lee, J. K. Hesperidin Improves Memory Function by Enhancing Neurogenesis in a Mouse Model of Alzheimer’s Disease. Nutrients. 2022, 14(15), 3125. DOI: 10.3390/nu14153125.
- Haghmorad, D.; Mahmoudi, M. B.; Salehipour, Z.; Jalayer, Z.; Kokhaei, M.; Rastin, P.; Mahmoudi, M.; Mahmoudi, M. Hesperidin Ameliorates Immunological Outcome and Reduces Neuroinflammation in the Mouse Model of Multiple Sclerosis. J. Neuroimmunol. 2017, 302, 23–33. DOI: 10.1016/j.jneuroim.2016.11.009.
- Yu, S.; Liu, M.; Hu, K. Natural Products: Potential Therapeutic Agents in Multiple Sclerosis. Int. Immunopharmacol. 2019, 67, 87–97. DOI: 10.1016/j.intimp.2018.11.036.
- Balsak, S.; Deveci, E. Effects of Hesperidin on the Changes Made in the Retinal Damage Induced by Traumatic Head Injury. Anal. Quant. Cytopathol. Histopathol. 2021, 43(5), 337–344.
- Cirmi, S.; Ferlazzo, N.; Lombardo, G. E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules. 2016, 21(10), 1312. DOI: 10.3390/molecules21101312.
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S. M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules. 2019, 24(3), 648. DOI: 10.3390/molecules24030648.
- Hwang, S. L.; Shih, P. H.; Yen, G. C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60(4), 877–885. DOI: 10.1021/jf204452y.
- Khan, A.; Ikram, M.; Hahm, J. R.; Kim, M. O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants. 2020, 9(7), 609. DOI: 10.3390/antiox9070609.
- Nectoux, A. M.; Abe, C.; Huang, S. W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) After Single Oral Administration to Sprague-Dawley Rats. J. Agric. Food Chem. 2019, 67(35), 9812–9819. DOI: 10.1021/acs.jafc.9b03594.
- Raza, S. S.; Khan, M. M.; Ahmad, A.; Ashafaq, M.; Khuwaja, G.; Tabassum, R.; Javed, H.; Siddiqui, M. S.; Safhi, M. M.; Islam, F. Hesperidin Ameliorates Functional and Histological Outcome and Reduces Neuroinflammation in Experimental Stroke. Brain Res. 2011, 1420, 93–105. DOI: 10.1016/j.brainres.2011.08.047.
- Said, U. Z.; Saada, H. N.; Abd-Alla, M. S.; Elsayed, M. E.; Amin, A. M. Hesperidin Attenuates Brain Biochemical Changes of Irradiated Rats. Int. J. Radiat. Biol. 2012, 88(8), 613–618. DOI: 10.3109/09553002.2012.694008.
- Evans, J. A.; Mendonca, P.; Soliman, K. F. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients. 2022, 14(11), 2228. DOI: 10.3390/nu14112228.
- Hong, Y.; An, Z. Hesperidin Attenuates Learning and Memory Deficits in APP/PS1 Mice Through Activation of Akt/Nrf2 Signaling and Inhibition of RAGE/NF-Κb Signaling. Arch. Pharm. Res. 2018, 41(6), 655–663. DOI: 10.1007/s12272-015-0662-z.
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57(3), 613–631.
- Nishino, S.; Fujiki, Y.; Sato, T.; Kato, Y.; Shirai, R.; Oizumi, H.; Yamauchi, J.; Ohbuchi, K.; Miyamoto, Y.; Mizoguchi, K. Hesperetin, a Citrus Flavonoid, Ameliorates Inflammatory Cytokine-Mediated Inhibition of Oligodendroglial Cell Morphological Differentiation. Neurol. Int. 2022, 14(2), 471–487. DOI: 10.3390/neurolint14020039.
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The Role of Nrf2 in Oxidative Stress-Induced Endothelial Injuries. J. Endocrinol. 2015, 225(3), R83–99. DOI: 10.1530/JOE-14-0662.
- Zhu, C.; Dong, Y.; Liu, H.; Ren, H.; Cui, Z. Hesperetin Protects Against H2O2-Triggered Oxidative Damage via Upregulation of the Keap1-Nrf2/HO-1 Signal Pathway in ARPE-19 Cells. Biomed. Pharmacother. 2017, 88, 124–133. DOI: 10.1016/j.biopha.2016.11.089.
- Li, X.; Xie, X.; Zhang, L.; Meng, Y.; Li, N.; Wang, M.; Li, P.; Liu, Z.; Di, T.; Zhang, L. Hesperidin Inhibits Keratinocyte Proliferation and Imiquimod-Induced Psoriasis-Like Dermatitis via the IRS-1/ERK1/2 Pathway. Life Sci. 2019, 219, 311–321. DOI: 10.1016/j.lfs.2019.01.019.
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S. I.; Kim, M. O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14(2), 278–294. DOI: 10.1007/s11481-018-9824-3.
- Welbat, J. U.; Naewla, S.; Pannangrong, W.; Sirichoat, A.; Aranarochana, A.; Wigmore, P. Neuroprotective Effects of Hesperidin Against Methotrexate-Induced Changes in Neurogenesis and Oxidative Stress in the Adult Rat. Biochem. Pharmacol. 2020, 178, 114083. DOI: 10.1016/j.bcp.2020.114083.
- Elshazly, S. M.; Abd El Motteleb, D. M.; Ibrahim, I. A. H. Hesperidin Protects Against Stress Induced Gastric Ulcer Through Regulation of Peroxisome Proliferator Activator Receptor Gamma in Diabetic Rats. Chem.-Biol. Interact. 2018, 291, 153–161. DOI: 10.1016/j.cbi.2018.06.027.
- Agrawal, Y. O.; Sharma, P. K.; Shrivastava, B.; Ojha, S.; Upadhya, H. M.; Arya, D. S.; Goyal, S. N.; Das, A. Hesperidin Produces Cardioprotective Activity via PPAR-γ Pathway in Ischemic Heart Disease Model in Diabetic Rats. PLoS. One. 2014, 9(11), e111212. DOI: 10.1371/journal.pone.0111212.
- Mahmoud, A. M. Hesperidin Protects Against Cyclophosphamide-Induced Hepatotoxicity by Upregulation of PPARγ and Abrogation of Oxidative Stress and Inflammation. Can. J. Physiol. Pharmacol. 2014, 92(9), 717–724. DOI: 10.1139/cjpp-2014-0204.
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Abbasi, M. M.; Salari, A. A. Hesperidin Attenuates Depression-Related Symptoms in Mice with Mild Traumatic Brain Injury. Life Sci. 2018, 213, 198–205. DOI: 10.1016/j.lfs.2018.10.040.
- Sato, K.; Sato, T.; Ohno-Oishi, M.; Ozawa, M.; Maekawa, S.; Shiga, Y.; Nakazawa, T.; Yasuda, M.; Himori, N.; Omodaka, K. CHOP Deletion and Anti-Neuroinflammation Treatment with Hesperidin Synergistically Attenuate NMDA Retinal Injury in Mice. Exp. Eye Res. 2021, 213, 108826. DOI: 10.1016/j.exer.2021.108826.
- Aja, P. M.; Izekwe, F. I.; Famurewa, A. C.; Ekpono, E. U.; Nwite, F. E.; Igwenyi, I. O.; Ale, B. A. Hesperidin Protects Against Cadmium-Induced Pancreatitis by Modulating Insulin Secretion, Redox Imbalance and Inos/NF/NF-ĸb Signaling in Rats. Life Sci. 2020, 259, 118268.
- Maekawa, S.; Sato, K.; Fujita, K.; Daigaku, R.; Tawarayama, H.; Murayama, N.; Nakazawa, T.; Yabana, T.; Shiga, Y.; Omodaka, K. The Neuroprotective Effect of Hesperidin in NMDA-Induced Retinal Injury Acts by Suppressing Oxidative Stress and Excessive Calpain Activation. Sci. Rep. 2017, 7(1), 6885. DOI: 10.1038/s41598-017-06969-4.
- Martínez, M. C.; Fernandez, S. P.; Loscalzo, L. M.; Wasowski, C.; Paladini, A. C.; Marder, M.; Viola, H. Hesperidin, a Flavonoid Glycoside with Sedative Effect, Decreases Brain pErk1/2 Levels in Mice. Pharmacol. Biochem. Behav. 2009, 92(2), 291–296.
- Kwon, J. Y.; Jung, U. J.; Kim, D. W.; Kim, S.; Moon, G. J.; Hong, J.; Jeon, M.-T., Shin, M., Chang, J. H., Kim, S. R. Beneficial Effects of Hesperetin in a Mouse Model of Temporal Lobe Epilepsy. J. Med. Food. 2018, 21(12), 1306–1309. DOI: 10.1089/jmf.2018.4183.
- Sun, Z.; Li, X.; Yang, L.; Dong, X.; Han, Y.; Li, Y.; Li, W. SOCE-Mediated NFAT1–NOX2–NLRP1 Inflammasome Involves in Lipopolysaccharide-Induced Neuronal Damage and Aβ Generation. Mol. Neurobiol. 2022, 59(5), 3183–3205. DOI: 10.1007/s12035-021-02717-y.
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J. R.; Kim, M. O. Caffeine May Abrogate LPS-Induced Oxidative Stress and Neuroinflammation by Regulating Nrf2/TLR4 in Adult Mouse Brains. Biomolecules. 2019, 9(11), 719. DOI: 10.3390/biom9110719.
- Bloem, B. R.; Okun, M. S.; Klein, C. Parkinson’s Disease. Lancet. 2021, 397(10291), 2284–2303. DOI: 10.1016/S0140-6736(21)00218-X.
- Brusadin, A. Parkinson’s Disease and Anesthesia. Nurs. Stud. Class Projects. 2021, 474.
- Kwok, J. Y.; Kwan, J. C.; Auyeung, M.; Mok, V. C.; Lau, C. K.; Choi, K. C.; Chan, H. Y. Effects of Mindfulness Yoga Vs Stretching and Resistance Training Exercises on Anxiety and Depression for People with Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76(7), 755–763. DOI: 10.1001/jamaneurol.2019.0534.
- Kesh, S.; Kannan, R. R.; Sivaji, K.; Balakrishnan, A. Hesperidin Downregulates Kinases Lrrk2 and gsk3β in a 6-OHDA Induced Parkinson’s Disease Model. Neurosci. Lett. 2021, 740, 135426. DOI: 10.1016/j.neulet.2020.135426.
- Poetini, M. R.; Araujo, S. M.; de Paula, M. T.; Bortolotto, V. C.; Meichtry, L. B.; de Almeida, F. P.; Prigol, M. Hesperidin Attenuates Iron-Induced Oxidative Damage and Dopamine Depletion in Drosophila Melanogaster Model of Parkinson’s Disease. Chem.-Biol. Interact. 2018, 279, 177–186. DOI: 10.1016/j.cbi.2017.11.018.
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M. O. Caffeine Modulates Cadmium-Induced Oxidative Stress, Neuroinflammation, and Cognitive Impairments by Regulating Nrf-2/HO-1 in vivo and in vitro. J. Clin. Med. 2019, 8(5), 680. DOI: 10.3390/jcm8050680.
- Bernhoft, R. A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 1–7. DOI: 10.1155/2013/394652.
- Khan, M. H. A.; Parvez, S. Hesperidin Ameliorates Heavy Metal Induced Toxicity Mediated by Oxidative Stress in Brain of Wistar Rats. J. Trace Elem. Med. Biol. 2015, 31, 53–60. DOI: 10.1016/j.jtemb.2015.03.002.
- Mandolesi, G.; Grasselli, G.; Musumeci, G.; Centonze, D. Cognitive Deficits in Experimental Autoimmune Encephalomyelitis: Neuroinflammation and Synaptic Degeneration. Neurol. Sci. 2010, 31(Suppl 2), 255–259. DOI: 10.1007/s10072-010-0369-3.
- Planche, V.; Panatier, A.; Hiba, B.; Ducourneau, E. G.; Raffard, G.; Dubourdieu, N.; Tourdias, T.; Lesté-Lasserre, T.; Brochet, B.; Dousset, V. Selective Dentate Gyrus Disruption Causes Memory Impairment at the Early Stage of Experimental Multiple Sclerosis. Brain Behav. Immun. 2017, 60, 240–254. DOI: 10.1016/j.bbi.2016.11.010.
- Kurkowska-Jastrzębska, I.; Świątkiewicz, M.; Zaremba, M.; Cudna, A.; Piechal, A.; Pyrzanowska, J.; Widy-Tyszkiewicz, E.; Członkowska, A. Neurodegeneration and Inflammation in Hippocampus in Experimental Autoimmune Encephalomyelitis Induced in Rats by One–Time Administration of Encephalitogenic T Cells. Neuroscience. 2013, 248, 690–698. DOI: 10.1016/j.neuroscience.2013.06.025.
- Ziehn, M. O.; Avedisian, A. A.; Tiwari-Woodruff, S.; Voskuhl, R. R. Hippocampal CA1 Atrophy and Synaptic Loss During Experimental Autoimmune Encephalomyelitis, EAE. Lab. Invest. 2010, 90(5), 774–786. DOI: 10.1038/labinvest.2010.6.
- Jovanova-Nesic, K.; Shoenfeld, Y.; Herbert Spector, N. Aluminum Excytotoxicity and NeuroAutotoimmunity: The Role of the Brain Expression of CD32+ (FcγRIIa), ICAM-1+ and CD3ع in Aging. CAS. 2012, 5(3), 209–217. DOI: 10.2174/1874609811205030007.
- Justin Thenmozhi, A.; William Raja, T. R.; Manivasagam, T.; Janakiraman, U.; Essa, M. M. Hesperidin Ameliorates Cognitive Dysfunction, Oxidative Stress and Apoptosis Against Aluminium Chloride Induced Rat Model of Alzheimer’s Disease. Nutr. Neurosci. 2017, 20(6), 360–368. DOI: 10.1080/1028415X.2016.1144846.
- Justin-Thenmozhi, A.; Dhivya Bharathi, M.; Kiruthika, R.; Manivasagam, T.; Borah, A.; Essa, M. M. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation Through the AKT/GSK-3β Pathway by Hesperidin in Wistar Rats. Neurotox. Res. 2018, 34(3), 463–476. DOI: 10.1007/s12640-018-9904-4.
- Donato, F.; de Gomes, M. G.; Goes, A. T. R.; Borges Filho, C.; Del Fabbro, L.; Antunes, M. S.; Jesse, C. R.; Boeira, S. P.; Jesse, C. R. Hesperidin Exerts Antidepressant-Like Effects in Acute and Chronic Treatments in Mice: Possible Role of L-Arginine-NO-cGMP Pathway and BDNF Levels. Brain Res. Bull. 2014, 104, 19–26. DOI: 10.1016/j.brainresbull.2014.03.004.
- Chang, C. Y.; Lin, T. Y.; Lu, C. W.; Huang, S. K.; Wang, Y. C.; Chou, S. S. P.; Wang, S. J. Hesperidin Inhibits Glutamate Release and Exerts Neuroprotection Against Excitotoxicity Induced by Kainic Acid in the Hippocampus of Rats. Neurotoxicology. 2015, 50, 157–169. DOI: 10.1016/j.neuro.2015.08.014.
- Javed, H.; Vaibhav, K.; Ahmed, M. E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M. M.; Islam, F. Effect of Hesperidin on Neurobehavioral, Neuroinflammation, Oxidative Stress and Lipid Alteration in Intracerebroventricular Streptozotocin Induced Cognitive Impairment in Mice. J. Neurol. Sci. 2015, 348(1–2), 51–59. DOI: 10.1016/j.jns.2014.10.044.
- Nones, J.; E Spohr, T. C. L. D. S.; Gomes, F. C. A. Hesperidin, a Flavone Glycoside, as Mediator of Neuronal Survival. Neurochem. Res. 2011, 36(10), 1776–1784. DOI: 10.1007/s11064-011-0493-3.
- Gaur, V.; Kumar, A. Hesperidin Pre-Treatment Attenuates NO-Mediated Cerebral Ischemic Reperfusion Injury and Memory Dysfunction. Pharmacol Rep. 2010, 62(4), 635–648. DOI: 10.1016/S1734-1140(10)70321-2.
- Antunes, M. S.; Goes, A. T.; Boeira, S. P.; Prigol, M.; Jesse, C. R. Protective Effect of Hesperidin in a Model of Parkinson’s Disease Induced by 6-Hydroxydopamine in Aged Mice. Nutrition. 2014, 30(11–12), 1415–1422. DOI: 10.1016/j.nut.2014.03.024.
- Verbeek, R.; van Tol, E. A.; van Noort, J. M. Oral Flavonoids Delay Recovery from Experimental Autoimmune Encephalomyelitis in SJL Mice. Biochem. Pharmacol. 2005, 70(2), 220–228. DOI: 10.1016/j.bcp.2005.04.041.
- El-Marasy, S. A.; Abdallah, H. M.; El-Shenawy, S. M.; El-Khatib, A. S.; El-Shabrawy, O. A.; Kenawy, S. A. Anti-Depressant Effect of Hesperidin in Diabetic Rats. Can. J. Physiol. Pharmacol. 2014, 92(11), 945–952. DOI: 10.1139/cjpp-2014-0281.
- Rong, Z.; Pan, R.; Xu, Y.; Zhang, C.; Cao, Y.; Liu, D. Hesperidin Pretreatment Protects Hypoxia–Ischemic Brain Injury in Neonatal Rat. Neuroscience. 2013, 255, 292–299. DOI: 10.1016/j.neuroscience.2013.09.030.
- Carlos Filho, B.; Del Fabbro, L.; de Gomes, M. G.; Goes, A. T.; Souza, L. C.; Boeira, S. P.; Jesse, C. R. Kappa-Opioid Receptors Mediate the Antidepressant-Like Activity of Hesperidin in the Mouse Forced Swimming Test. Eur. J. Pharmacol. 2013, 698(1–3), 286–291. DOI: 10.1016/j.ejphar.2012.11.003.
- Lee, B.; Choi, G. M.; Sur, B. Antidepressant-Like Effects of Hesperidin in Animal Model of Post-Traumatic Stress Disorder. Chin. J. Integr. Med. 2021, 27(1), 39–46. DOI: 10.1007/s11655-020-2724-4.
- Kim, J.; Wie, M. B.; Ahn, M.; Tanaka, A.; Matsuda, H.; Shin, T. Benefits of Hesperidin in Central Nervous System Disorders: A Review. Anat. Cell Biol. 2019, 52(4), 369–377. DOI: 10.5115/acb.19.119.
- Zhu, X.; Zhang, Y. M.; Zhang, M. Y.; Chen, Y. J.; Liu, Y. W. Hesperetin Ameliorates Diabetes-Associated Anxiety and Depression-Like Behaviors in Rats via Activating Nrf2/ARE Pathway. Metab. Brain Dis. 2021, 36(7), 1969–1983. DOI: 10.1007/s11011-021-00785-6.
- Fu, H.; Liu, L.; Tong, Y.; Li, Y.; Zhang, X.; Gao, X.; Wang, H.; Zhao, J.; Xiao, D.; Wen, K. The Antidepressant Effects of Hesperidin on Chronic Unpredictable Mild Stress-Induced Mice. Eur. J. Pharmacol. 2019, 853, 236–246. DOI: 10.1016/j.ejphar.2019.03.035.
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S. R.; Naidu, V. G. M. Hesperidin Alleviates Chronic Restraint Stress and Lipopolysaccharide-Induced Hippocampus and Frontal Cortex Damage in Mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE Signaling. Neurochem. Int. 2020, 140, 104835. DOI: 10.1016/j.neuint.2020.104835.
- Xie, L.; Gu, Z.; Liu, H.; Jia, B.; Wang, Y.; Cao, M.; Song, R.; Zhang, Z.; Bian, Y. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front. Pharmacol. 2020, 11, 1251. DOI: 10.3389/fphar.2020.01251.
- Nadar, J. S.; Kale, P. P.; Kadu, P. K.; Prabhavalkar, K.; Dhangar, R. Potentiation of Antidepressant Effects of Agomelatine and Bupropion by Hesperidin in Mice. Neurol. Res. Int. 2018, 2018, 1–7. DOI: 10.1155/2018/9828639.
- Zhu, X.; Liu, H.; Deng, Z.; Yan, C.; Liu, Y.; Yin, X. Hesperidin Exerts Anxiolytic-Like Effects in Rats with Streptozotocin-Induced Diabetes via PKA/CREB Signaling. Curr. Mol. Pharmacol. 2023, 16(1), 91–100. DOI: 10.2174/1573413718666220314140848.
- Hajizadeh Moghaddam, A.; Ahmadnia, H.; Jelodar, S. K.; Ranjbar, M. Hesperetin Nanoparticles Attenuate Anxiogenic-Like Behavior and Cerebral Oxidative Stress Through the Upregulation of Antioxidant Enzyme Expression in Experimental Dementia of Alzheimer’s Type. Neurol. Res. 2020, 42(6), 477–486. DOI: 10.1080/01616412.2020.1747716.
- Matias, I.; Diniz, L. P.; Buosi, A.; Neves, G.; Stipursky, J.; Gomes, F. C. A. Flavonoid Hesperidin Induces Synapse Formation and Improves Memory Performance Through the Astrocytic TGF-β1. Front. Aging Neurosci. 2017, 9, 184. DOI: 10.3389/fnagi.2017.00184.
- Luo, Y.; Fan, H.; Tan, X.; Li, Z. Hesperetin Rescues Emotional Memory and Synaptic Plasticity Deficit in Aged Rats. Behav. Neurosci. 2021, 135(6), 721. DOI: 10.1037/bne0000475.
- Makvandi, A. A.; Khalili, M.; Roghani, M.; Moghaddam, S. A. Hesperetin Ameliorates Electroconvulsive Therapy-Induced Memory Impairment Through Regulation of Hippocampal BDNF and Oxidative Stress in a Rat Model of Depression. J. Chem. Neuroanat. 2021, 117, 102001. DOI: 10.1016/j.jchemneu.2021.102001.
- Kumar, A.; Lalitha, S.; Mishra, J. Hesperidin Potentiates the Neuroprotective Effects of Diazepam and Gabapentin Against Pentylenetetrazole-Induced Convulsions in Mice: Possible Behavioral, Biochemical and Mitochondrial Alterations. Indian J. Pharmacol. 2014, 46(3), 309. DOI: 10.4103/0253-7613.132180.
- Sharma, P.; Kumari, S.; Sharma, J.; Purohit, R.; Singh, D. Hesperidin Interacts with CREB-BDNF Signaling Pathway to Suppress Pentylenetetrazole-Induced Convulsions in Zebrafish. Front. Pharmacol. 2021, 11, 607797. DOI: 10.3389/fphar.2020.607797.
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8(3), a028936. DOI: 10.1101/cshperspect.a028936.
- Baradaran, S.; Moghaddam, A. H.; Ghasemi-Kasman, M. Hesperetin Reduces Myelin Damage and Ameliorates Glial Activation in Lysolecithin-Induced Focal Demyelination Model of Rat Optic Chiasm. Life Sci. 2018, 207, 471–479. DOI: 10.1016/j.lfs.2018.07.001.
- Baradaran, S.; Ghasemi-Kasman, M.; Moghaddam, A. H. Nano-Hesperetin Enhances the Functional Recovery and Endogenous Remyelination of the Optic Pathway in Focal Demyelination Model. Brain Res. Bull. 2020, 164, 392–399. DOI: 10.1016/j.brainresbull.2020.09.006.
- Schwartz, M.; Deczkowska, A. Neurological Disease as a Failure of Brain–Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016, 37(10), 668–679. DOI: 10.1016/j.it.2016.08.001.
- Shin, T.; Kojima, T.; Tanuma, N.; Ishihara, Y.; Matsumoto, Y. The Subarachnoid Space as a Site for Precursor T Cell Proliferation and Effector T Cell Selection in Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 1995, 56(2), 171–178. DOI: 10.1016/0165-5728(94)00144-D.
- Shin, T.; Ahn, M.; Matsumoto, Y. Mechanism of Experimental Autoimmune Encephalomyelitis in Lewis Rats: Recent Insights from Macrophages. Anat. Cell Biol. 2012, 45(3), 141–148. DOI: 10.5115/acb.2012.45.3.141.
- Ginwala, R.; McTish, E.; Raman, C.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.; Sagar, D., Jain, P., Khan, Z. K. Apigenin, a Natural Flavonoid, Attenuates EAE Severity Through the Modulation of Dendritic Cell and Other Immune Cell Functions. J. Neuroimmune Pharmacol. 2016, 11(1), 36–47. DOI: 10.1007/s11481-015-9617-x.
- Ciftci, O.; Ozcan, C.; Kamisli, O.; Cetin, A.; Basak, N.; Aytac, B. Hesperidin, a Citrus Flavonoid, Has the Ameliorative Effects Against Experimental Autoimmune Encephalomyelitis (EAE) in a C57BL/J6 Mouse Model. Neurochem. Res. 2015, 40(6), 1111–1120. DOI: 10.1007/s11064-015-1571-8.
- van der Veen, R. C. Nitric Oxide and T Helper Cell Immunity. Int. Immunopharmacol. 2001, 1(8), 1491–1500. DOI: 10.1016/S1567-5769(01)00093-5.
- Nath, N.; Morinaga, O.; Singh, I. S-Nitrosoglutathione a Physiologic Nitric Oxide Carrier Attenuates Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2010, 5(2), 240–251. DOI: 10.1007/s11481-009-9187-x.
- Willenborg, D. O.; Staykova, M.; Fordham, S.; O’Brien, N.; Linares, D. The Contribution of Nitric Oxide and Interferon Gamma to the Regulation of the Neuro-Inflammation in Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2007, 191(1–2), 16–25. DOI: 10.1016/j.jneuroim.2007.09.007.
- Zhang, J.; Jiang, H.; Wu, F.; Chi, X.; Pang, Y.; Jin, H.; Zhang, S.; Zhang, S. Neuroprotective Effects of Hesperetin in Regulating Microglia Polarization After Ischemic Stroke by Inhibiting TLR4/NF-Κb Pathway. J. Healthc. Eng. 2021, 2021, 1–10. DOI: 10.1155/2021/9938874.
- Wu, M. Y.; Yiang, G. T.; Liao, W. T.; Tsai, A. P. Y.; Cheng, Y. L.; Cheng, P. W.; Li, C. J.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46(4), 1650–1667. DOI: 10.1159/000489241.
- Kalogeris, T.; Baines, C. P.; Krenz, M.; Korthuis, R. J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317.
- Devyatov, A. A.; Fedorova, T. N.; Berezhnoy, D. S.; Stvolinskii, S. L.; Tutelyan, V. A. Mechanisms of Neuroprotective Action of Hesperetin and Carnosine in Focal Ischemia of the Brain in Rats. Bull. Exp. Biol. Med. 2020, 169, 242–245.
- Song, H.; Ding, Z.; Chen, J.; Chen, T.; Wang, T.; Huang, J. The AMPK-SIRT1-FoxO1-NF-Κb Signaling Pathway Participates in Hesperetin-Mediated Neuroprotective Effects Against Traumatic Brain Injury via the NLRP3 Inflammasome. Immunopharmacol. Immunotoxicol. 2022, 44(6), 970–983. DOI: 10.1080/08923973.2022.2096464.
- Miles, E. A.; Calder, P. C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. DOI: 10.3389/fimmu.2021.712608.
- Ikram, M.; Muhammad, T.; Rehman, S. U.; Khan, A.; Jo, M. G.; Ali, T.; Kim, M. O. Hesperetin Confers Neuroprotection by Regulating Nrf2/tlr4/nf-Κb Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56(9), 6293–6309. DOI: 10.1007/s12035-019-1512-7.
- Rotimi, S. O.; Bankole, G. E.; Adelani, I. B.; Rotimi, O. A. Hesperidin Prevents Lipopolysaccharide-Induced Endotoxicity in Rats. Immunopharmacol. Immunotoxicol. 2016, 38(5), 364–371. DOI: 10.1080/08923973.2016.1214142.
- Li, M.; Shao, H.; Zhang, X.; Qin, B. Hesperidin Alleviates Lipopolysaccharide-Induced Neuroinflammation in Mice by Promoting the MiRNA-132 Pathway. Inflammation. 2016, 39(5), 1681–1689. DOI: 10.1007/s10753-016-0402-7.
- Youdim, K. A.; Dobbie, M. S.; Kuhnle, G.; Proteggente, A. R.; Abbott, N. J.; Rice‐Evans, C. Interaction Between Flavonoids and the Blood–Brain Barrier: In vitro Studies. J. Neurochem. 2003, 85(1), 180–192. DOI: 10.1046/j.1471-4159.2003.01652.x.
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin Ameliorates Behavioral Impairments and Neuropathology of Transgenic APP/PS1 Mice. Behav. Brain Res. 2015, 281, 32–42. DOI: 10.1016/j.bbr.2014.12.012.
- Noshy, P. A.; Azouz, R. A. Neuroprotective Effect of Hesperidin Against Emamectin Benzoate-Induced Neurobehavioral Toxicity in Rats. Neurotoxicol. Teratol. 2021, 86, 106981. DOI: 10.1016/j.ntt.2021.106981.