ABSTRACT
The aim of this review is develop to phytochemicals-based functional products against obesity and cancer management. Natural products have been made from reliable sources in past centuries. Phytochemicals are natural compounds that are available in vegetables, legumes, tea crops, fruits and various spices. Phytochemicals are known as secondary metabolites including carotenoids, phenolics, flavonoids, alkaloids, terpenes, saponins, cyanogenic glycosides, and glucosinolates. These phytochemicals are played direct or indirect role against several chronic diseases. However, these compounds have ability to act against various chronic diseases containing diabetes, hypertension, obesity, and cardiovascular diseases (CVDs). The literature reports the different potential properties of dietary phytochemicals such as anti-oxidants, anti-inflammatory, anti-viral, and anti-bacterial activities.
Introduction
Different chemicals are produced by plants that are emerged to play a novel role in the development and functioning of human body. These are divided into two groups such as primary metabolites and secondary metabolites. Fats, carbohydrates, proteins, etc. are included in the primary metabolites while the secondary metabolites contain carotenoids, phenolics, flavonoids, alkaloids, terpenes, saponins, cyanogenic glycosides, and glucosinolates. In the early researches, a question mark was put on the physiological functioning of these secondary metabolites but the latest literature reports that these metabolites play a very significant role in various functioning of plants along with development[Citation1,Citation2].
Recent years have set trends to use natural products against various types of disorders.[Citation3] Oxidative stress and inflammation are deliberated to be one of the most significant contributors to the happening of various degenerative and chronic diseases. Literature proves that phytochemicals are the potential agents against oxidative stress and inflammation[Citation4]
Intake of dietary phytochemicals (carotenoids, phenolics, flavonoids, alkaloids, terpenes, saponins, cyanogenic glycosides, and glucosinolates) have a promising effect to protect one from chronic diseases (neurodegenerative diseases, CVDs, hypertension, cancer, ulcers, parasitic, viral & microbial infections, diabetes, and obesity). Dietary phytochemicals possessing are potential nutraceutical properties that are abundantly present in various foods. A majority of the foods contain phytonutrients. However, these are plant sources including whole grains, beans, fruit, herbs, and vegetables.[Citation5] This review compiles the sources of dietary phytochemicals and their potential effect and mechanism against cancer and obesity. However, these dietary phytochemicals are extracted from different plant sources.
Methdology
We used Science Direct, Google Scholar, Web of Science, and PubMed to compile the literature on phytochemicals. We received assistance for the paid articles from the Gilgit Baltistan Food and Nutrition Society in Pakistan, and the society gave us free access to the paid articles. We first created an outline to establish the right review structure, and only then did we create the conceptualization. Considering the framework, we created the review’s sections and addressed the phytochemical composition of plants, the bioactive capacity in functional foods, and the ability to fight chronic diseases.
Sources of phytochemicals
Fruits
Among various rich sources of dietary phytochemicals, fruits are considered best sources are fruits. From the major classes of phytochemicals, polyphenols, flavonoids, and anthocyanin are the major phytochemicals present in a significant amount in various fruits. Some of the recent investigations related to phytochemicals have highlighted different fruits as potential sources possessing anti-obese and anti-cancer properties.[Citation6,Citation7]
Berries are very common fruits present in almost all parts of the world. Traditionally, these are considered to possess potential properties for weight management. One type of berries is blueberries which are a very significant source of anthocyanins. Anthocyanins are considered to be potential agents that break down the fats and reduces sugars and weight.[Citation8] Various In vivo studies also highlight the mechanism as reduction in accumulated adipose tissues, which ultimately leads to manage obesity.[Citation9]
Banana is also a very common fruit. It has different varieties with a variable composition. Different parts of banana including peel and pulp are a potential source of various dietary bioactive compounds i.e. syringic acid, dopamine, gallic acid, quercetin, campesterol, and cycloartenol.[Citation10] Mango fruit is also recognized for its potential bioactive compounds and is acknowledged for its anti-cancer properties.[Citation10]
Resveratrol is a very potential bioactive phytochemical which is naturally present in different varieties of grapes, peanuts, and berries.[Citation11] This potential phytochemical decrease adipocyte counts very significantly.[Citation12] It has also been reported that this potential phytochemical when combined with other phytochemicals (quercetin and genistein) decrease the adipogenesis process. Moreover, vitamin D and resveratrol synergistically help in obesity management. Moreover, resveratrol alone or in combination with other bioactive compounds plays a vital role in obesity management.[Citation13]
Pomegranate has been grown in several regions of the world and it is very sweet in taste and rich in flavor. Furthermore, it is a very significant source of several bioactive compounds including anthocyanins, ellagitannins, and various kinds of phenolic acids. These compounds possess significant potential in the management of obesity and cancer. An In vivo and In vitro study has shown that pomegranate, directly or indirectly (juice or in form of extract) exhibits hypoglycemic activity, and helps in the reduction of cholesterol ultimately improving blood lipid profiles (low-density lipoprotein and high-density lipoprotein).[Citation14] It has also be articulated that pomegranate peel is usually wasted. It is a great source of various phytochemicals i.e. polyphenols and flavonoids.[Citation15] Calabash fruit possesses very significant activity in the inhibition of the pancreatic lipase activity, diminishing entry of lipids in the body, and suppressing lipid digestion.[Citation10]
Leaves and oil of olive fruit possess some bioactive phytochemicals that are known as olive bio-phenols collectively. Several health-promoting activities have been associated with these bio-phenols including anti-cancer and anti-obesity properties. Among different bio-phenols, oleuropein is the most significant bio-phenol, followed by luteolin, rutin, verbascoside, hydroxytyrosol and catechin. It has been reported that the addition of oleuropein in a very high-fat diet decreases the chance of weight gaining also improves lipid profiling (low-density lipoprotein and high-density lipoprotein).[Citation16]
Tomatoes are very low caloric fruit that could be consumed in either cooked or in raw form. The most significant phytochemical in tomato is lycopene. This natural phytochemical is reported to be an excellent anti-oxidant, anti-carcinogenic agent, and also a potential agent to manage obesity[Citation9] et al. 2015).
Citrus are very common fruits and readily available in almost all parts of the world. Grapefruit (Citrus paradisi), lemon (Citrus limon), and orange (Citrus sinensis) are the three most commonly available and important economically. Citrus family is a significant source various significant phytochemicals, various phenolic acids, flavonoids, and carotenoids. Lemon peel is usually a waste but is a very significant source of various dietary phytochemicals that possess anti-obesity and anti-cancer properties.[Citation17]
Literature articulates that consumption of grapefruit and other citrus family fruits in the diet prevents several different chronic diseases and disorders including diabetes, obesity, and CVDs. The principal compound present most significantly in grapefruits are “nootkatone.” This is important phytochemical that helped in activate energy metabolism and in the management and prevention of obesity.[Citation18,Citation19]
Vegetables
Vegetables are also a very reliable source of various dietary phytochemicals.[Citation20] Capsaicin is one of the most significant bioactive compounds present in chilies, and this compound boosts the metabolism process and helps the body to burn extra calories.[Citation21] This bioactive compound has been described to be a strong chemo-preventive agent. However, it helps in tumor suppression, ultimately leading to an excellent anti-cancer agent.[Citation22,Citation23]
Red capsicum is a very significant source of different phytochemicals. Comparing to the other colored varieties, red capsicum is the richest source of phenolic compounds including carotenoids, and quercetin.[Citation24] Many bioactive compounds present in ginger exhibit various pharmacological functions including anti-tumorigenic, anti-inflammatory, antioxidant, antihyperglycemic, anti-apoptotic, anti-obesity, anti-lipidemic, and immuno-modulatory effects.[Citation25] Zingiberene, gingerol, bisabolene, hogoals, caffeic acid, curcumin, capsaicin, and salicylate are important bioactive compounds responsible for various properties of ginger.[Citation26]
A group of naturally occurring small bioactive compounds (isothiocyanates) are reported to be excellent anti-cancer. These are significantly available in cruciferous vegetables including cabbage and broccoli.[Citation27] Choi et al.,[Citation28] also reported to possess potential anti-obesity properties.
Consumption of garlic is related with decreasing the body weight level, lowering body cholesterol and glucose levels in the blood. Garlic in synergizes with turmeric emerges to be a potential agent against dyslipidemia, type-2 diabetes, and hyperglycemia.[Citation27] The most significant bioactive compound in garlic is allicin which is known for its various health benefits[Citation29]). Garlic and onion possess different acrid compounds (steroidal, phenolics, volatile sulfur, and thiosulfinates) which are potential agents against various chronic diseases.[Citation27]
Carotenoids are the major class of phytochemicals which possess an excellent activity against cancer and tumors especially breast and prostate. Carrots are very significant source of alpha and beta carotenoids.[Citation30] Cucurbitaceae family includes various plants that have been used in traditional Chinese medicines containing tetracyclic triterpenoids called Cucurbitacins. The result showed that Cucurbitacin B (CuB) is the most important and active component against cancer which is among eight different types of Cucurbitacins.[Citation8]
Legumes
Legumes, clover, peas, chickpeas, lentils, lupines, pigeon pea, peanuts, tamarind mesquite, carob, and soybeans, are regarded as a rich source of several different essential nutrients and phytochemicals.[Citation31] Black soybeans are rich in anthocyanins which are considered as potential medicinal food. Management of obesity and diabetes, excellent anti-inflammatory, and anti-oxidative properties are most substantial health promoting properties that could be associated with black soybeans anthocyanins.[Citation32] Pigeon pea significantly containsphytosterol and linoleic acid which acts synergistically as anti-dyslipidemia and antioxidant effects.[Citation33]
Soybean has been widely grown for its edible pea. It is a species of legume having numerous uses. In disease treatment/prevention, various scientific studies indicate the advantageous of soy such as antidiabetic, anti-obesity, anticancer hypotriglyceridemic and hypocholesterolemic properties. Isoflavones are prominently considered as phytoestrogens. Its represent estrogenic activity having a class of diphenolic molecules (also known as phytoestrogens). Isoflavones (daidzein, genistein, and glycitein) are found abundantly in soybean and their products. Genistein is the highly investigated and abundant isoflavones in soy. When a cross-sectional study was done, it was found that there is a significant opposite relationship between genistein consumption and metabolic syndrome markers including body mass index, weight, total body fat and waist circumference in humans subjects containing Western diets.[Citation34] Due to reported effects, isoflavones are mostly effective in the treatment of conditions depend on hormone such as cardiovascular disease, menopause, cancer and osteoporosis.[Citation35] There are significant reported anti-cancer properties against gastric, prostate, leukemia, breast, non-small lung cell and lymphoma cancer of the isoflavones such as genistein derived from soy.[Citation36] Various studies have indicated the anti-cancer characteristics of genistein in several cancer models like prostate cancer,[Citation37] breast cancer,[Citation38] lung cancer,[Citation37] and neck and head squamous cell carcinoma,[Citation39] cervical cancer,[Citation40] ovarian cancer (Wang et al. 2019), renal cancer, bladder cancer,[Citation41] and liver cancer.[Citation42]
Tea crops
From ancient days, humans have been using tea leaves because of their various medicinal properties. Nowadays tea crops are widely being used in various processed beverages. Green and black tea are two basic types in which tea crops are basically classified. This classification is made as per the way of post-harvest processing. It is reported that green tea is highly effective as compared to black tea.[Citation43] Catechins in green tea have been reported to have a very positive impact on the management of obesity and overweight conditions.[Citation44] Caffeine in synergistic relation to polyphenols leads to enhancement of thermogenesis of brown adipose tissue, by released noradrenaline sympathetically.[Citation45] Green tea aids to enhance the lipolytic pathway, adipose tissue reduction and low-grade inflammation in the animal model of high-fat diet resulting in hypolipidemic and anti-obesity effects.[Citation46] Green tea is also considered to be a potential anti-cancer agent. The most studied flavonoids in green tea is EGCG. Both In vivo and In vitro scientific studies report that EGCG results in the prevention of angiogenesis and tumor cell proliferation inhibition in numerous kinds of tumors (promoted by VEGF), such as prostate, colon, esophagus, and breast.[Citation47,Citation48]
Polyphenols present in coffee have enhanced energy metabolism along with the reduction in abdominal and liver fat accumulation.[Citation49] Caffeine is significant in suppressing fat absorption. Green coffee contains a mixture of feruloyl quinic acid and neochlorogenic acid which suppresses the accumulation of visceral fat and increase in the body weight in mice model.[Citation50] Huang et al.[Citation51] found the effects are significantly stronger with a different compounds combination; orange peel extract (OPE) mixed with black tea extract (BTE) and caffeine have a synergistic effect and was showed to have more prominent anti-obesity actions studied for 10 weeks in mice than individual compounds. The polyphenolic content of the tea is associated with its weight loss properties. Epigallocatechin-3-gallate is one of the highly substantial tea catechins which may result in decreased absorption of energy and increased oxidation of fat.[Citation52]
Spices
Spices are a potential source of various phytochemicals that help the human body in the enhancement of metabolism and management of different chronic diseases like inflammation, diabetes, and obesity. Turmeric is a very common spice used all around the globe, with very significant use in Indian and Chinese foods as a traditional medicine.[Citation25] Curcumin is the most significant and potential phytochemical present in turmeric. It has been reported to be a very potent anti-cancer agent which helps in the management of obesity.[Citation53]
Cuminaldehyde is the most significant phytochemical present in cumin. This natural bioactive compound present in cumin that is reported to have a very significant effect on human health containing anti-allergic, antioxidant, hypoglycemic, and anti-platelet aggregations.[Citation25] Black cumin has been reported to have some potential activities like cholesterol reduction and obesity management in various animal models.[Citation54]
Rosemary is a very common spice consumed all around the globe directly in cooking and indirectly in medicine. Rosmarinic acid and carnosic acid are the most significant phytochemicals in the rosemary along with less significantly present phytochemicals including phenolics, diterpenes, and monoterpenes. Consumption of rosemary is reported to be an excellent approach in the obesity and cancer management.[Citation25,Citation55] shows different phytochemicals, their sources, formulae, and structure.
Table 1. Different phytochemicals, their sources, formulae and structure.
Obesity management
An accusation of excessive body fat is characterized as obesity. Over the last 30 years, the obesity prevention has significantly increased Worldwide. The Western countries have taken lead in this robust rise. Obesity is categorized by body mass index (BMI) which is the ratio of body weight (kg) to body height (meter square). If the value is greater than 25 then an individual is considered as overweight while if the value is over 30 than the individual will be called obese.[Citation71] Obesity is considered to be an alarming onset of the metabolic disorder leading to several different complications (diabetes, hypertension, and CVDs).[Citation71,Citation72]
There are two things that are considered to be the most important in the obesity management including diet (reducing fat) and exercise.[Citation73] While the literature reports that these two simple measures are mostly ignored and the individuals seek alternative simple strategies to combat.[Citation74,Citation75] The major key players in the development as well as management, are adipocytes which are the cells (store fat). An enhancement in the size and/or number of adipocytes leads to obesity. In the adipose tissue, adipocytes show differentiation from pre-adipocytes.[Citation76] The establishment of an adipocyte lifecycle is carried out with the pre-adipocytes metamorphosis to adipocytes. Treatments regulate both the number and size of adipocytes may offer valuable assistance to diminish dietary energy in preventing obesity. The association between inflammation and adiposity is being unraveled gradually with the acknowledgment that the production of inflammatory cytokines by adipocytes instigating an inducement in the inflammatory state which increases obesity progression. The less consumption of fibers, vegetables, whole grains, and fruits results in enhanced weight gain.[Citation77]
The consumption of dietary phytochemicals assists to counteract obesity. The significant interest has been stimulated around the globe in this regard.[Citation78,Citation79] Recently, dietary antioxidants, predominantly phytochemicals have achieved much consideration regarding their impact on adiposity. Phytochemicals are described as non-nutritive bioactive constituents containing polyphenols (flavonoids, phenolic acids, lignans, isoflavones, chalcones, curcuminoids, and stilbenes), phytosterols, terpenoids, and organosulfur.[Citation80] Current results showed that phytochemicals are investigated for antiobesity potential. The pathophysiology and signaling pathways in obesity are epigenetic regulations, regulatory mechanisms, functional ingredients in natural antiobesity products, and therapeutic application of phytochemicals in obesity.[Citation81] The different phytochemicals (quercetin, kaempferol, rosmarinic acid, cyanidin, rutin, catechin, luteolin and ellagic acid) are extracted from different plants sources fruits, honey, vegetables, seeds and nuts. These different compounds are produced as antidiabetic agents.[Citation82] showed the anti-obesity effect of dietary phytochemicals reported by epidemiological studies
Table 2. Anti-obesity effect of dietary phytochemicals reported by epidemiological studies.
Mechanism of action of phytochemicals in the management of obesity
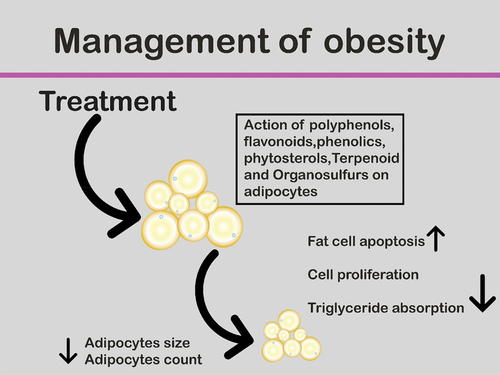
The action of dietary phytochemicals on several stages of the adipocyte’s life cycle has been discussed. Probably, some action mechanisms of these derived products from plants can cause adipose tissue mass reduction by increasing fat cells apoptosis, hindering precursor cell proliferation, and inhibiting triglyceride absorption by decreasing pancreatic lipase formation. They are preadipocyte inhibitors that show differentiation and act as lipolysis stimulators as well.[Citation86] Obesity has been related to the chronic status of inflammation[Citation65,Citation87] and the boosted activity of anti-inflammation is one of the action mechanisms for responding the negative consequence of the obesogenic physiological state.[Citation65]
The relationship between dietary consumption of phytochemicals with adiposity was investigated already in few studies of epidemiology.[Citation56,Citation88,Citation89] illustrates the mechanism of phytochemicals in the management of obesity.
Cancer management
Cancer is characterized as abnormal growth of the cells that enhances to spread irregular/abnormal cells to various other parts of the body by distant metastasis and/or local invasion. Cancer is one of the increasing and foremost problems of public health, presently causing a steady increase in expectancy of life and accounting for over 12% of deaths around the globe. It is reported that the death rate may double in the upcoming 50 years due to cancer and cancer cases may increase up to 15 million by 2020.[Citation90] The reports specify that about a total of 13% of deaths (7.6 million) are persuaded by cancer. The increased burden is globally concerned with both the growth and aging of the world population. Lung, liver, stomach, breast, and colon cancer generate a majority of these particular disorders.[Citation84] Chemoprevention is the practice of biological, synthetic, or natural agents to suppress, prevent or, reverse the preliminary carcinogenesis phase or to inhibit the invading premalignant cells potential.[Citation91] The interest in the chemoprevention area has greatly developed by a rising understanding of cancer biology, molecular targets identification, and achievement in the prostate, colon, and breast cancer inhibition.[Citation92] Chemoprevention of cancer has been differentiated by multiple alteration pathways at the molecular level and develops a critical action in the three elementary carcinogenesis steps including initiation, progression, and promotion.[Citation93] Naturally, phytochemicals from plants serve as vital resources for novel drugs that are in cancer therapy. Some typical examples include taxol analogs, and vinca alkaloids (vincristine, vinblastine, and podophyllotoxin analogs). These phytochemicals often act via regulating molecular pathways implicated in cancer growth and progression. The specific mechanisms include increasing antioxidant status, carcinogen inactivation, inhibiting proliferation, induction of cell cycle arrest and apoptosis.[Citation94] The previous review analyzed the role of phytochemicals-based products from plants in the management of cancer. There are different plant-based dietary factors involved in the management of cancer. Moreover, previous results highlight the process of carcinogenesis and chemoprevention using plants and phytochemicals.[Citation95] showed the anti-cancer effect of dietary phytochemicals reported by epidemiological studies.
Table 3. Anti-cancer effect of dietary phytochemicals reported by epidemiological studies.
Mechanism of action of phytochemicals in the management of cancer
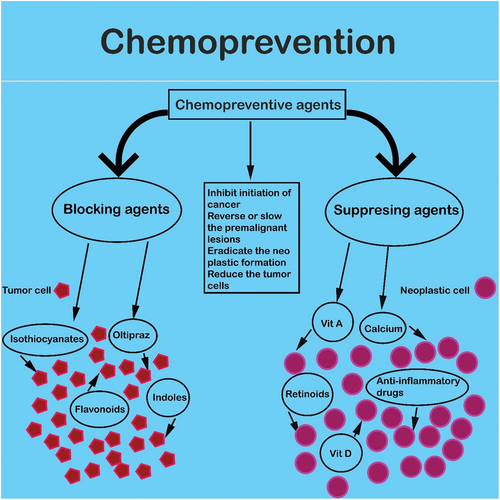
Largely well-defined based on their predominant action mechanisms, chemo-preventive agents can be assembled into two over-all classes: suppressing agents and blocking agents. The blocking agents inhibit carcinogenic constituents from responding or reaching with critical sites of target by stopping the metabolic activation of tumor or carcinogens promoters by increasing systems of detoxification and via trapping responsive carcinogens. Suppressing agents eradicate the neoplastic evolution process in cells otherwise, it would convert into malignant tumors. Usually the suppressing agents act after the attack by carcinogens. Action mechanisms for blocking or suppressing agents are not highly specified. Some cause differentiation, few counteract the genotoxic consequences actions such as activation of the oncogene, some diminish proliferation in the cell, and many have un-understood mechanisms.[Citation101]
An ideal chemo-preventive agent should comprise of 1. No or Little toxic impacts 2. Great effectiveness against multiple spots 3. Oral administration competence 4. A recognized action mechanism 5. Human acknowledgment.[Citation102] A chemo-preventive method accesses and identifies the definite chemical constituents, various occurring naturally in foods, significantly inhibits the initiation of cancer and either reverse or slows the progression of the premalignant lesion to invasive cancer.[Citation103] Various studies of epidemiology propose that diet mainly rich in vegetables and fruits have cancer-inhibiting properties.[Citation104,Citation105] The beneficial and significant diet effects are attributable to bioactive compounds such as polyphenols which have anti-tumor actions both in humans and in animal models.[Citation106,Citation107] illustrates the mechanism of action of phytochemicals in the management of cancer.
Conclusion
It is concluded that various sources of dietary phytochemicals (carotenoids, phenolics, flavonoids, alkaloids, terpenes, saponins, cyanogenic glycosides, and glucosinolates) have potential effect against obesity and cancer. The findings from this review conclude that fruits, vegetables, legumes, tea crops, and various spices are potential sources of different dietary phytochemicals. The epidemiological studies showed that dietary phytochemicals are potential and natural ways in the management of obesity and cancer. The dietary phytochemicals help in management of diabetes by reducing the count and size of adipocytes. While in case of cancer, the dietary phytochemicals inhibit the initiation of cancer and either reverse or slow the progression of the premalignant lesion to invasive cancer. It is recommended that future researches must be directed to investigate the action mechanism of dietary phytochemicals against obesity and various forms of cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Maqbool, Z.; Arshad, M. S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M. F.; Bangar, S. P.; Addi, M.; Hano, C.; Lorenzo, J. M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 1–14. DOI: 10.1155/2022/6480590.
- Kainat, S.; Gilani, S. R.; Asad, F.; Khalid, M. Z.; Khalid, W.; Ranjha, M. M. A. N.; Lorenzo, J. M.; Lorenzo, J. M. Determination and Comparison of Phytochemicals, Phenolics, and Flavonoids in Solanum Lycopersicum Using FTIR Spectroscopy. Food Anal. Methods. 2022, 15(11), 2931–2939. DOI: 10.1007/s12161-022-02344-w.
- Rao, V.; Mans, D.; Rao, L. Phytochemicals in Human Health, Phytochemicals in Human Health. IntechOpen. 2019. DOI: 10.5772/intechopen.77908.
- Souza-Junior, F. J.; Luz-Moraes, D.; Pereira, F. S.; Barros, M. A.; Fernandes, L. M.; Queiroz, L. Y.; Fontes-Junior, E. A. Aniba Canelilla (Kunth) Mez (Lauraceae): A Review of Ethnobotany, Phytochemical, Antioxidant, Anti-Inflammatory, Cardiovascular, and Neurological Properties. Front. Pharmacol. 2020, 11, 699. DOI: 10.3389/fphar.2020.00699.
- Prakash, D.; Gupta, C.; Sharma, G. Importance of Phytochemicals in Nutraceuticals. J. Chin Med. 2012, 1(3), 70–78. https://www.researchgate.net/publication/281369030.
- Ranjha, M. A Critical Review on Pharmaceutical and Medicinal Importance of Ginger. Act. Sci. Nutr. Health 2019, 3(12), 78–82. https://www.researchgate.net/publication/338361864.
- Chaudhry, F.; Ahmad, M. L.; Hayat, Z.; Ranjha, M. M. A. N.; Chaudhry, K.; Elboughdiri, N.; Uddin, J.; Uddin, J. Extraction and Evaluation of the Antimicrobial Activity of Polyphenols from Banana Peels Employing Different Extraction Techniques. Separations. 2022, 9(7), 165. DOI: 10.3390/separations9070165.
- Wu, Q. J.; Yang, Y.; Wang, J.; Han, L. H.; Xiang, Y. B. Cruciferous Vegetable Consumption and Gastric Cancer Risk: A Meta‐Analysis of Epidemiological Studies. Cancer. Sci. 2013, 104(8), 1067–1073. DOI: 10.1111/cas.12195.
- Maruyama, S. A.; Claus, T.; Montanher, P. F.; Bonafé, E. G.; Santos Junior, O. D. O.; SOUZA, N.; Matsushita, M.; Gomes, S. T. M.; Matsushita, M. Evaluation of Lipophilic Antioxidant Capacity and Lycopene Content in Brazilian Tomatoes. Revista. Virtual de Química. 2015, 7(4), 1163–1173. DOI: 10.5935/1984-6835.20150065.
- Afzal, M. F.; Khalid, W.; Akram, S.; Khalid, M. A.; Zubair, M.; Kauser, S.; Abdelsamea Mohamedahmed, K.; Aziz, A.; Anusha Siddiqui, S. Bioactive Profile and Functional Food Applications of Banana in Food Sectors and Health: A Review. Int. J. Food Prop. 2022, 25(1), 2286–2300. DOI: 10.1080/10942912.2022.2130940.
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100(4), 1392–1404. John Wiley and Sons Ltd. DOI: 10.1002/jsfa.10152.
- Murphy, K. P.; Hendley, M. A.; Patterson, A. T.; Hall, H. E.; Carter, G. J.; Isely, C.; Gower, R. M. Modulation of Adipocyte Size and Fat Pad Weight via Resveratrol Releasing Scaffolds Implanted into the Epididymal Adipose Tissue. J. Biomed. Mater. Res. A. 2021, 109(5), 766–778. DOI: 10.1002/jbm.a.37063.
- Rayalam, S.; Della-Fera, M. A.; Baile, C. A. Phytochemicals and Regulation of the Adipocyte Life Cycle. J. Clin. Biochem. Nutr. 2008, 19(11), 717–726. DOI: 10.1016/j.jnutbio.2007.12.007.
- Jungbauer, A.; Medjakovic, S. Anti-Inflammatory Properties of Culinary Herbs and Spices That Ameliorate the Effects of Metabolic Syndrome. Maturitas. 2012, 71(3), 227–239. Elsevier. DOI: 10.1016/j.maturitas.2011.12.009.
- Ranjha, M. M. A. N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M. N.; Jabbar, S.; Murtaza, M. A.; Mahmood, S.; Murtaza, M. A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20(sup3), S1201–S1221. DOI: 10.1080/15538362.2020.1782804.
- Aly, A. H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal Endophytes from Higher Plants: A Prolific Source of Phytochemicals and Other Bioactive Natural Products. Fungal. Diversity. 2010, 41(1), 1–16. DOI: 10.1007/s13225-010-0034-4.
- Maqbool, Z.; Khalid, W.; Taimoor Atiq, H.; Koraqi, H.; Javaid, Z.; Alhag, S. K.; Al-Shuraym, L. A.; Bader, D. M. D.; Almarzuq, M.; Afifi, M., et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules. 2023, 28(4), 1636.
- Fujioka, K.; Greenway, F.; Sheard, J.; Ying, Y. The Effects of Grapefruit on Weight and Insulin Resistance: Relationship to the Metabolic Syndrome. J. Med. Food. 2006, 9(1), 49–54. DOI: 10.1089/jmf.2006.9.49.
- Murase, T.; Misawa, K.; Haramizu, S.; Minegishi, Y.; Hase, T. Nootkatone, a Characteristic Constituent of Grapefruit, Stimulates Energy Metabolism and Prevents Diet-Induced Obesity by Activating AMPK. Am. J. Physiol. Endocrinol. Metab. 2010, 299(2), E266–E275. DOI: 10.1152/ajpendo.00774.2009.
- Kainat, S.; Arshad, M. S.; Khalid, W.; Zubair Khalid, M.; Koraqi, H.; Afzal, M. F.; Noreen, S.; Aziz, Z.; Al-Farga, A. Sustainable Novel Extraction of Bioactive Compounds from Fruits and Vegetables Waste for Functional Foods: A Review. Int. J. Food Prop. 2022, 25(1), 2457–2476. DOI: 10.1080/10942912.2022.2144884.
- Kim, M. J.; Kim, H. K. Effect of Garlic on High Fat Induced Obesity. Acta Biol. Hung. 2011, 62(3), 244–254. DOI: 10.1556/ABiol.62.2011.3.4.
- Oyagbemi, A. A.; Saba, A. B.; Azeez, O. I. Capsaicin: A Novel Chemopreventive Molecule and Its Underlying Molecular Mechanisms of Action. Indian J. Cancer. 2010, 47(1), 53–58. DOI: 10.4103/0019-509X.58860.
- Venier, N. A.; Colquhoun, A. J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N. E.; Klotz, L. H.; Venkateswaran, V. Capsaicin: A Novel Radio‐Sensitizing Agent for Prostate Cancer. Prostate. 2015, 75(2), 113–125. DOI: 10.1002/pros.22896.
- Chapa-Oliver, A. M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules. 2016, 21(8), 931. DOI: 10.3390/molecules21080931.
- Seyedan, A.; Alshawsh, M. A.; Alshagga, M. A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities Against Pancreatic Lipase: A Review. Evid. Based Complementary Altern. Med. 2015, 2015, 1–13. DOI: 10.1155/2015/973143.
- Irfan, F. Dietary Consumption and Cancer Prevention. Adv. Nutri. Food Sci. 2019, 2, 1–15.
- Khalid, W.; Maqbool, Z.; Arshad, M. S.; Kousar, S.; Akram, R.; Siddeeg, A.; Ali, A.; Qin, H.; Aziz, A.; Saeed, A. Plant-Derived Functional Components: Prevent from Various Disorders by Regulating the Endocrine Glands. Int. J. Food Prop. 2022, 25(1), 976–995. DOI: 10.1080/10942912.2022.2070643.
- Choi, Y.; Kim, Y.; Park, S.; Lee, K. W.; Park, T. Indole-3-Carbinol Prevents Diet-Induced Obesity Through Modulation of Multiple Genes Related to Adipogenesis, Thermogenesis or Inflammation in the Visceral Adipose Tissue of Mice. J. Nutr Biochem. 2012, 23(12), 1732–1739. DOI: 10.1016/j.jnutbio.2011.12.005.
- Alam, S. M.; Mahmood, I. A.; Ullah, M. A.; Naseeb, T.; Nawab, N. N.; Haider, S. I.; Aamir, S. S. Growth and Yield of Garlic (Allium Sativum L.) Influenced by Zn and Fe Application. Food. Biol. 1970, 2-19 8, 13–15. DOI: 10.25081/fb.2019.v8.5504.
- Abar, L.; Vieira, A. R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D. A.; Norat, T.; Greenwood, D. C.; Norat, T. Blood Concentrations of Carotenoids and Retinol and Lung Cancer Risk: An Update of the WCRF-AICR Systematic Review of Published Prospective Studies. Cancer. Med. 2016, 5(8), 2069–2083. DOI: 10.1002/cam4.676.
- Zvereva, E. E.; Vandyukova, I. I.; Vandyukov, A. E.; Katsyuba, S. A.; Khamatgalimov, A. R.; Kovalenko, V. I. IR and Raman Spectra, Hydrogen Bonds, and Conformations of N-(2-Hydroxyethyl)-4, 6-Dimethyl-2-Oxo-1, 2-Dihydropyrimidine (Drug Xymedone). Am. J. Clin. Nutr. 2012, 61(6), 1199–1206. DOI: 10.1007/s11172-012-0163-x.
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A Black Soybean Seed Coat Extract Prevents Obesity and Glucose Intolerance by Up-Regulating Uncoupling Proteins and Down-Regulating Inflammatory Cytokines in High-Fat Diet-Fed Mice. J. Agric. Food. Chem. 2011, 59(16), 8985–8993. DOI: 10.1021/jf201471p.
- Dai, F. J.; Hsu, W. H.; Huang, J. J.; Wu, S. C. Effect of Pigeon Pea (Cajanus Cajan L.) on High-Fat Diet-Induced Hypercholesterolemia in Hamsters. Food. Chem. Toxicol. 2013, 53, 384–391. DOI: 10.1016/j.fct.2012.12.029.
- Velasquez, M. T.; Bhathena, S. J. Role of Dietary Soy Protein in Obesity. Int. J. Med. Sci. 2007. 72–82. Ivyspring International Publisher DOI, DOI: 10.7150/ijms.4.72.
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy Isoflavone: The Multipurpose Phytochemical (Review). Biomed. Rep. 2013, 1(5), 697–701. DOI: 10.3892/br.2013.129.
- Sarkar, F. H.; Li, Y. Mechanisms of Cancer Chemoprevention by Soy Isoflavone Genistein. Cancer. Metastasis Rev. 2002, 21(3/4), 265–280. DOI: 10.1023/A:1021210910821.
- Davis, J. N.; Singh, B.; Bhuiyan, M.; Sarkar, F. H. Genistein-Induced Upregulation of p21 WAF1 , Downregulation of Cyclin B, and Induction of Apoptosis in Prostate Cancer Cells. 1998, 32(3), 123–131. DOI: 10.1080/01635589809514730.
- Li, Y.; Upadhyay, S.; Bhuiyan, M.; Sarkar, F. H. Induction of Apoptosis in Breast Cancer Cells MDA-MB-231 by Genistein. Oncogene. 1999, 18(20), 3166–3172. DOI: 10.1038/sj.onc.1202650.
- Alhasan, S. A.; Pietrasczkiwicz, H.; Alonso, M. D.; Ensley, J.; Sarkar, F. H. Genistein-Induced Cell Cycle Arrest and Apoptosis in a Head and Neck Squamous Cell Carcinoma Cell Line. Nutr. Cancer. 1999, 34(1), 12–19. DOI: 10.1207/S15327914NC340102.
- Liu, H.; Lee, G.; Lee, J. I.; Ahn, T. G.; Kim, S. A. Effects of Genistein on Anti-Tumor Activity of Cisplatin in Human Cervical Cancer Cell Lines. Obstetrics. Gynecol. 2019, 62(5), 322–328. DOI: 10.5468/ogs.2019.62.5.322.
- Ali, A.; Manzoor, M. F.; Ahmad, N.; Aadil, R. M.; Qin, H.; Siddique, R.; Riaz, S.; Ahmad, A.; Korma, S. A.; Khalid, W., et al. The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review. Front. Nutr. 2022, 9. DOI: 10.3389/fnut.2022.940514.
- Zhang, Q.; Bao, J.; Yang, J. Genistein-Triggered Anticancer Activity Against Liver Cancer Cell Line HepG2 Involves ROS Generation, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migrationand Inhibition of Cell Migration. Arch. Med. Sci. 2019, 15(4), 1001–1009. DOI: 10.5114/aoms.2018.78742.
- Chacko, S. M.; Thambi, P. T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5(1), 13–19. DOI: 10.1186/1749-8546-5-13.
- Brown, A. L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A. E.; Dadd, T. Health Effects of Green Tea Catechins in Overweight and Obese Men: A Randomised Controlled Cross-Over Trial. Br. J. Nutr. 2011, 106(12), 1880–1889. DOI: 10.1017/S0007114511002376.
- Dulloo, A. G.; Seydoux, J.; Girardier, L.; Chantre, P.; Vandermander, J. Green Tea and Thermogenesis: Interactions Between Catechin-Polyphenols, Caffeine and Sympathetic Activity. Int. J. Obes. 2000, 24(2), 252–258. DOI: 10.1038/sj.ijo.0801101.
- Westerterp-Plantenga, M. S. Green Tea Catechins, Caffeine and Body-Weight Regulation. Physiol. Behav. 2010, 100(1), 42–46. DOI: 10.1016/j.physbeh.2010.02.005.
- Henning, S. M.; Wang, P.; Heber, D. Chemopreventive Effects of Tea in Prostate Cancer: Green Tea versus Black Tea. Mol. Nutr Food Res. 2011, 55(6), 905–920. DOI: 10.1002/mnfr.201000648.
- Yuan, J. M. Cancer Prevention by Green Tea: Evidence from Epidemiologic Studies 1-4. Am. J. Clin. Nutr. 2013, 98(6), 1676S–1681S. DOI: 10.3945/ajcn.113.058271.
- Murase, T.; Yokoi, Y.; Misawa, K.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee Polyphenols Modulate Whole-Body Substrate Oxidation and Suppress Postprandial Hyperglycaemia, Hyperinsulinaemia and Hyperlipidaemia. Br. J. Nutr. 2012, 107(12), 1757–1765. DOI: 10.1017/S0007114511005083.
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory Effect of Green Coffee Bean Extract on Fat Accumulation and Body Weight Gain in Mice. BMC Complement. Altern. Med. 2006, 6(1). DOI: 10.1186/1472-6882-6-9.
- Huang, Y. W.; Liu, Y.; Dushenkov, S.; Ho, C. T.; Huang, M. T. Anti-Obesity Effects of Epigallocatechin-3-Gallate, Orange Peel Extract, Black Tea Extract, Caffeine and Their Combinations in a Mouse Model. J. Funct. Foods. 2009, 1(3), 304–310. DOI: 10.1016/j.jff.2009.06.002.
- Sae-Tan, S.; Grove, K. A.; Kennett, M. J.; Lambert, J. D. Epigallocatechin-3-Gallate Increases the Expression of Genes Related to Fat Oxidation in the Skeletal Muscle of High Fat-Fed Mice. Food. Funct. 2011, 2(2), 111–116. DOI: 10.1039/c0fo00155d.
- Mesa, M. D.; Ramírez Tortosa, M. C.; Aguilera García, C. M.; Ramírez-Boscá, A.; Gil Hernández, Á.; Ríos, A.; Suárez, M. D.; Gil, A. Chronic Diarrhea Impairs Intestinal Antioxidant Defense System in Rats at Weaning. 2000, 45(10), 2044–2050. DOI: 10.1023/a:1005603019800.
- Tauseef Sultan, M.; Butt, M. S.; Anjum, F. M. Safety Assessment of Black Cumin Fixed and Essential Oil in Normal Sprague Dawley Rats: Serological and Hematological Indices. Food. Chem. Toxicol. 2009, 47(11), 2768–2775. DOI: 10.1016/j.fct.2009.08.011.
- Gamboa-Gómez, C. I.; Rocha-Guzmán, N. E.; Gallegos-Infante, J. A.; Moreno-Jiménez, M. R.; Vázquez-Cabral, B. D.; González-Laredo, R. F. Plants with Potential Use on Obesity and Its Complications. EXCLI. 2015, 14, 809.
- Kim, K.; Vance, T. M.; Chun, O. K. Greater Flavonoid Intake is Associated with Improved CVD Risk Factors in US Adults. Br. J. Nutr. 2016, 115(8), 1481–1488. DOI: 10.1017/S0007114516000519.
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal Absorption and Metabolism of Capsaicin and Dihydrocapsaicin in Rats. Toxicol. Appl. Pharmacol. 1984, 72(3), 449–456. DOI: 10.1016/0041-008X(84)90121-2.
- Lee, M. S.; Kim, C. T.; Kim, Y. Green Tea (-)-Epigallocatechin-3-Gallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ann. Nutr. Metab. 2009, 54(2), 151–157. DOI: 10.1159/000214834.
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of Resveratrol Metabolic Profile in Healthy Humans After Moderate Consumption of Red Wine and Grape Extract Tablets. Pharmacol. Res. 2012, 66(5), 375–382. DOI: 10.1016/j.phrs.2012.08.001.
- Mulvihill, E. E.; Allister, E. M.; Sutherland, B. G.; Telford, D. E.; Sawyez, C. G.; Edwards, J. Y.; Markle, J. M.; Hegele, R. A.; Huff, M. W. Naringenin Prevents Dyslipidemia, Apolipoprotein B Overproduction, and Hyperinsulinemia in LDL Receptor–Null Mice with Diet-Induced Insulin Resistance. Diabetes. 2009, 58(10), 2198–2210. DOI: 10.2337/db09-0634.
- Zingg, J. M.; Hasan, S. T.; Meydani, M. Molecular Mechanisms of Hypolipidemic Effects of Curcumin. BioFactors. John Wiley & Sons. 2013, 39(1), 101–121. DOI: 10.1002/biof.1072.
- Kozukue, N.; Han, J. S.; Kozukue, E.; Lee, S. J.; Kim, J. A.; Lee, K. R.; Levin, C. E.; Friedman, M. Analysis of Eight Capsaicinoids in Peppers and Pepper-Containing Foods by High-Performance Liquid Chromatography and Liquid chromatography− Mass Spectrometry. J. Agric. Food. Chem. 2005, 53(23), 9172–9181. DOI: 10.1021/jf050469j.
- Seo, C. R.; Yi, B.; Oh, S.; Kwon, S. M.; Kim, S.; Song, N. J.; Park, K. W.; Park, K.-M.; Ahn, J.-Y.; Hong, J.-W. Aqueous Extracts of Hulled Barley Containing Coumaric Acid and Ferulic Acid Inhibit Adipogenesis in vitro and Obesity in vivo. J. Funct. Foods. 2015, 12, 208–218. DOI: 10.1016/j.jff.2014.11.022.
- Chang, Y. C.; Yang, M. Y.; Chen, S. C.; Wang, C. J. Mulberry Leaf Polyphenol Extract Improves Obesity by Inducing Adipocyte Apoptosis and Inhibiting Preadipocyte Differentiation and Hepatic Lipogenesis. J. Funct. Foods. 2016, 21, 249–262. DOI: 10.1016/j.jff.2015.11.033.
- Goto, T.; Teraminami, A.; Lee, J. Y.; Ohyama, K.; Funakoshi, K.; Kim, Y. I.; Kawada, T.; Uemura, T.; Yu, R.; Takahashi, N. Tiliroside, a Glycosidic Flavonoid, Ameliorates Obesity-Induced Metabolic Disorders via Activation of Adiponectin Signaling Followed by Enhancement of Fatty Acid Oxidation in Liver and Skeletal Muscle in Obese–Diabetic Mice. J. Nutr Biochem. 2012, 23(7), 768–776. DOI: 10.1016/j.jnutbio.2011.04.001.
- Mashmoul, M.; Azlan, A.; Yusof, B. N. M.; Khaza’ai, H.; Mohtarrudin, N.; Boroushaki, M. T. Effects of Saffron Extract and Crocin on Anthropometrical, Nutritional and Lipid Profile Parameters of Rats Fed a High Fat Diet. J. Funct. Foods. 2014, 8(1), 180–187. DOI: 10.1016/j.jff.2014.03.017.
- Shen, Y.; Song, S. J.; Keum, N.; Park, T. Olive Leaf Extract Attenuates Obesity in High-Fat Diet-Fed Mice by Modulating the Expression of Molecules Involved in Adipogenesis and Thermogenesis. Evid. Based Complement. Altern. Med. 2014, 2014, 1–12. DOI: 10.1155/2014/971890.
- Kucuk, O.; Sarkar, F. H.; Djuric, Z.; Sakr, W.; Pollak, M. N.; Khachik, F.; Wood, D. P., Jr; Bertram, J. S.; Wood, D. P. Effects of Lycopene Supplementation in Patients with Localized Prostate Cancer. Exp. Biol. Med. 2002, 227(10), 881–885. DOI: 10.1177/153537020222701007.
- Boyanapalli, S. S.; Li, W.; Fuentes, F.; Guo, Y.; Ramirez, C. N.; Gonzalez, X. P.; Kong, A. N. T.; Kong, A.-N. T. Epigenetic Reactivation of RASSF1A by Phenethyl Isothiocyanate (PEITC) and Promotion of Apoptosis in LNCaP Cells. Pharmacol. Res. 2016, 114, 175–184. DOI: 10.1016/j.phrs.2016.10.021.
- Yar Saglam, A. S.; Alp, E.; Elmazoglu, Z.; Menevse, S. Treatment with Cucurbitacin B Alone and in Combination with Gefitinib Induces Cell Cycle Inhibition and Apoptosis via EGFR and JAK/STAT Pathway in Human Colorectal Cancer Cell Lines. Hum. Exp. Toxicol. 2016, 35(5), 526–543. DOI: 10.1177/0960327115595686.
- Witkamp, R. F. Current and Future Drug Targets in Weight Management. Pharm. Res. 2011, 28(8), 1792–1818. DOI: 10.1007/s11095-010-0341-1.
- Salas-Salvadó, J.; Martinez-Gonzalez, M. A.; Bulló, M.; Ros, E. The Role of Diet in the Prevention of Type 2 Diabetes. Nutrition. 2011, 21, B32–B48. DOI: 10.1016/j.numecd.2011.03.009.
- Astrup, A. Healthy Lifestyles in Europe: Prevention of Obesity and Type II Diabetes by Diet and Physical Activity. Public. Health. Nutr. 2001, 4(2b), 499–515. DOI: 10.1079/PHN2001136.
- Kruger, J.; Galuska, D. A.; Serdula, M. K.; Jones, D. A. Attempting to Lose Weight: Specific Practices Among US Adults. Am. J. Prev Med. 2004, 26(5), 402–406. DOI: 10.1016/j.amepre.2004.02.001.
- Wadden, T. A. Treatment of Obesity by Moderate and Severe Caloric Restriction: Results of Clinical Research Trials. J. Hum. Nutr. Diet. 1993, 119(7_Part_2), 688–693. DOI: 10.7326/0003-4819-119-7_part_2-199310011-00012.
- Furuyashiki, T.; Nagayasu, H.; Aoki, Y.; Bessho, H.; Hashimoto, T.; Kanazawa, K.; Ashida, H. Tea Catechin Suppresses Adipocyte Differentiation Accompanied by Down-Regulation of PPARγ2 and C/EBPα in 3T3-L1 Cells. Biosci. Biotechnol., Biochem. 2004, 68(11), 2353–2359. DOI: 10.1271/bbb.68.2353.
- Vincent, H. K.; Bourguignon, C. M.; Taylor, A. G. Relationship of the Dietary Phytochemical Index to Weight Gain, Oxidative Stress and Inflammation in Overweight Young Adults. J. Hum. Nutr. Diet. 2010, 23(1), 20–29. DOI: 10.1111/j.1365-277X.2009.00987.x.
- Khalid, W.; Arshad, M. S.; Ranjha, M. M. A. N.; Różańska, M. B.; Irfan, S.; Shafique, B.; Kowalczewski, P. Ł.; Khalid, M. Z.; Abdi, G.; Kowalczewski, P. Ł. Functional Constituents of Plant-Based Foods Boost Immunity Against Acute and Chronic Disorders. Open. Life Sci. 2022, 17(1), 1075–1093. DOI: 10.1515/biol-2022-0104.
- Ikram, A.; Khalid, W.; Saeed, F.; Arshad, M. S.; Afzaal, M.; Arshad, M. U. S. Senna: As Immunity Boosting Herb Against COVID-19 and Several Other Diseases. J. Herb. Med. 2023, 37, 100626. DOI: 10.1016/j.hermed.2023.100626.
- Carnauba, R. A.; Chaves, D. F.; Baptistella, A. B.; Paschoal, V.; Naves, A.; Buehler, A. M. Association Between High Consumption of Phytochemical-Rich Foods and Anthropometric Measures: A Systematic Review. Int. J. Food Sci. Nutr. 2017, 68(2), 158–166. DOI: 10.1080/09637486.2016.1229761.
- Kumar, V.; Singh, D. D.; Lakhawat, S. S.; Yasmeen, N.; Pandey, A.; Singla, R. K.; Agbor, G. A. Biogenic Phytochemicals Modulating Obesity: From Molecular Mechanism to Preventive and Therapeutic Approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 1–20. DOI: 10.1155/2022/6852276.
- Egbuna, C.; Awuchi, C. G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K. C.; Singh, O.; Chikwendu, C. J. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Topics. Med. Chem. 2021, 21(12), 1067–1095. DOI: 10.2174/18734294MTE1ENjAgx.
- Ali, F.; Ismail, A.; Esa, N. M.; Pei, C. P.; Kersten, S. Hepatic Genome-Wide Expression of Lipid Metabolism in Diet-Induced Obesity Rats Treated with Cocoa Polyphenols. J. Funct. Foods. 2015, 17, 969–978. DOI: 10.1016/j.jff.2015.06.047.
- Min, S.; Seo, M.; Hajishirzi, H.; Question Answering Through Transfer Learning from Large Fine-Grained Supervision Data. arXiv Preprint arXiv.1702.02171. 2017.
- Lim, H. H.; Lee, S. O.; Kim, S. Y.; Yang, S. J.; Lim, Y. Anti-Inflammatory and Antiobesity Effects of Mulberry Leaf and Fruit Extract on High Fat Diet-Induced Obesity. Exp. Biol. Med. 2013, 238(10), 1160–1169. DOI: 10.1177/1535370213498982.
- Khalid, W.; Arshad, M. S.; Aziz, A.; Rahim, M. A.; Qaisrani, T. B.; Afzal, F.; Anjum, F. M., Ranjha, Muhammad Modassar Ali Nawaz., Khalid, M Z., Anjum, F. Chia Seeds (Salvia Hispanica L.): A Therapeutic Weapon in Metabolic Disorders. Food Sci. Nutrition. 2023, 11(1), 3–16. DOI: 10.1002/fsn3.3035.
- Pan, M. H.; Lai, C. S.; Ho, C. T. Anti-Inflammatory Activity of Natural Dietary Flavonoids. Food. Funct. 2010, 1(1), 15–31. DOI: 10.1039/c0fo00103a.
- Marranzano, M.; Ray, S.; Godos, J.; Galvano, F. Association Between Dietary Flavonoids Intake and Obesity in a Cohort of Adults Living in the Mediterranean Area. Int. J. Food Sci. Nutr. Eng. 2018, 69(8), 1020–1029. DOI: 10.1080/09637486.2018.1452900.
- Vernarelli, J. A.; Lambert, J. D. ‘Flavonoid Intake is Inversely Associated with Obesity and C-Reactive Protein, a Marker for Inflammation, in US Adults. Nutr. Diabet. 2017, 7(5), e276–e276. DOI: 10.1038/nutd.2017.22.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D. M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer. 2015, 136(5), E359–E386. DOI: 10.1002/ijc.29210.
- Sporn, M. B. Approaches to Prevention of Epithelial Cancer During the Preneoplastic Period. 1976.
- Golemis, E. A.; Scheet, P.; Beck, T. N.; Scolnick, E. M.; Hunter, D. J.; Hawk, E.; Hopkins, N. Molecular Mechanisms of the Preventable Causes of Cancer in the United States. Genes. Dev. 2018, 32(13–14), 868–902. DOI: 10.1101/gad.314849.118.
- Pitot, H. C. The Molecular Biology of Carcinogenesis. Cancer. 1993, 72(S3), 962–970.
- Choudhari, A. S.; Mandave, P. C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. DOI: 10.3389/fphar.2019.01614.
- George, B. P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants. 2021, 10(9), 1455. DOI: 10.3390/antiox10091455.
- Sharma, R. A.; McLelland, H. R.; Hill, K. A.; Ireson, C. R.; Euden, S. A.; Manson, M. M.; Steward, W. P.; Marnett, L. J.; Gescher, A. J.; Steward, W. P. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7(7), 1894–1900.
- Gupta, S.; Hastak, K.; Ahmad, N.; Lewin, J. S.; Mukhtar, H. Inhibition of Prostate Carcinogenesis in TRAMP Mice by Oral Infusion of Green Tea Polyphenols. Proc. Natl. Acad. Sci. 2001, 98(18), 10350–10355. DOI: 10.1073/pnas.171326098.
- Tsai, Y. J.; Chen, B. H. Preparation of Catechin Extracts and Nanoemulsions from Green Tea Leaf Waste and Their Inhibition Effect on Prostate Cancer Cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. DOI: 10.2147/IJN.S103759.
- Pramanik, K. C.; Fofaria, N. M.; Gupta, P.; Ranjan, A.; Kim, S. H.; Srivastava, S. K. Inhibition of β-Catenin Signaling Suppresses Pancreatic Tumor Growth by Disrupting Nuclear β-Catenin/TCF-1 Complex: Critical Role of STAT-3. Oncotarget. 2015, 6(13), 11561. DOI: 10.18632/oncotarget.3427.
- Zheng, Q.; LIU, Y.; LIU, W.; MA, F.; ZHOU, Y. I.; CHEN, M.; CHANG, J.; WANG, Y.; YANG, G.; HE, G. Cucurbitacin B Inhibits Growth and Induces Apoptosis Through the JAK2/STAT3 and MAPK Pathways in SH-SY5Y Human Neuroblastoma Cells. Mol. Med. Rep. 2014, 10(1), 89–94. DOI: 10.3892/mmr.2014.2175.
- Wattenberg, L. W. Chemoprevention of Cancer in Preventive Medicine. Academic Press Inc, 1996; pp. 44–45. DOI: 10.1006/pmed.1996.0015.
- Mukhtar, H.; Agarwal, R. Skin Cancer Chemoprevention. In The Journal of Investigative dermatology. Symposium Proceedings. 1996, 209–214.
- Sayed, M.; ElHamid Mahmou, A. A. Cancer Chemoprevention by Dietary Polyphenols, Carcinogenesis; InTech. London, UK 2013. DOI: 10.5772/54945.
- Kontou, N.; Psaltopoulou, T.; Panagiotakos, D.; Dimopoulos, M. A.; Linos, A. The Mediterranean Diet in Cancer Prevention: A Review. J. Med. Food. 2011, 14(10), 1065–1078. DOI: 10.1089/jmf.2010.0244.
- Shu, L.; Cheung, K. L.; Khor, T. O.; Chen, C.; Kong, A. N. Phytochemicals: Cancer Chemoprevention and Suppression of Tumor Onset and Metastasis. Cancer. Metastasis Rev. 2010, 29(3), 483–502. DOI: 10.1007/s10555-010-9239-y.
- Luo, K. W.; Ko, C. H.; Yue, G. G. L.; Lee, J. K. M.; Li, K. K.; Lee, M.; Bik-San Lau, C. Green Tea (Camellia Sinensis) Extract Inhibits Both the Metastasis and Osteolytic Components of Mammary Cancer 4T1 Lesions in Mice. J. Nutr Biochem. 2014, 25(4), 395–403. DOI: 10.1016/j.jnutbio.2013.11.013.
- Norat, T.; Aune, D.; Chan, D.; Romaguera, D. Fruits and Vegetables: Updating the Epidemiologic Evidence for the WCRF/AICR Lifestyle Recommendations for Cancer Prevention. Adv. Nutr. 2014, 35–50.