?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Obesity is one of the major causes of non-communicable diseases (NCDs) associated with a dietary pattern rich in saturated fat. The present study utilized roasted and germinated chickpeas (Cicer arietinum) flour (RCPF and GCPF) as a replacement for fat in biscuits, known to have nutraceutical properties. The fat content was modified using the following ratios: 10%, 20%, and 30% (w/w) of RCPF/GCPF. Based on the physicochemical analysis, increased concentrations of RCPF and GCPF in the flour blends resulted in higher levels of protein, ash, and crude fiber contents. GCPF was found to contain higher levels of protein (20.20%), ash (4.86%), and crude fiber (3.64%) compared to RCPF. Increased RCPF and GCPF levels resulted in reduction of gluten content, which indicated weak gluten network. Scanning electron micrographs (SEM) of biscuit samples further supported these observations. The farinograph properties showed significant increase (P<0.05) in water absorption and dough development time. Furthermore, when RCPF and GCPF were added to flour blends and biscuits samples, significant increase (P<0.05) in antioxidant activity, total phenolic content, and total flavonoid content was observed. These trends were observed to be more prominent with higher quantities of RCPF and GCPF. Notably, the antioxidant properties of chickpeas were found to be significantly improved (P<0.05) by the process of germination compared to roasting. Moreover, improvements in antioxidant activity might be caused by the increased levels of phenolic compounds and ascorbic acid due to the actions of endogenous hydrolytic enzymes during germination. The dimensional, textural, and sensory properties indicated that RCPF 20% and GCPF 10% can effectively serve as an organic fat substitute in bakery products with enhanced concentrations of proteins, fibers, antioxidants, and bioactive compounds with nutraceutical properties.
Introduction
Excessive consumption of food products rich in saturated fats creates many health-issues, including obesity and other non-communicable diseases, i.e., diabetes, cancer, cardiovascular diseases,etc.[Citation1] Biscuits are the highly consumed item among all other bakery products due to their availability in wide varieties, ready-to-eat properties, and cost-effectiveness.[Citation2] However, refined wheat flour, fat, and sugar are the principal ingredients utilized in biscuits manufacturing which contributes to its poor nutritionalprofile.[Citation3] Fat plays multiple desirable roles in baked products, such as providing richness in flavor and enhancing the textural properties and mouthfeel by providing lubrication of ingredients. Moreover, fats maintain structural integrity and extend the shelf life of bakedproducts.[Citation4] Nowadays, consumers are becoming aware of the correlation between diet and the risk of diseases. Therefore, it is the responsibility of food manufacturers to meet consumer demands by reducing fat content in food products without altering their functionalproperties.[Citation5] However, it is difficult to eliminate fat without changing the physicochemical and sensorial properties of the final product, although it is practically possible to partially replace fat with otheringredients.[Citation2,Citation4,Citation6] Fat replacers are classified as the ingredients that can perform some of the functional properties of fats in food product development. They provide zero or few calories than fat. Furthermore, American Dietetic Association states that legumes are organic ingredients that can provide some or all of the functions of fat with lower caloricvalue.[Citation7] Numerous fat replacers, such as inulin, polydextrose, etc., are available in markets, but only very few depict remarkable results without altering the properties of food. Legumes have been reported as an effective fat replacer that not only helps in reducing the fat content of the product but also increases the nutritionalvalue.[Citation8] Legumes are a good source of protein, dietary fibers, vitamins, and minerals compared to other cereals used as staple foods, such as wheat, corn, and rice.
Chickpea (Cicer arietinum L.) is a leguminous crop that grows mainly in cold environments. Worldwide production of chickpeasranks second after commonbeans.[Citation9] Chickpeas are extensively cultivated in temperate and subtropical areas such as Australia, America, West Asia, and Indiansubcontinent.[Citation10] Two main types of chickpeas (Desi and Kabuli) cultivated around the globe are distinguished based on their color, size, and shape. Desi or black chickpeas are produced in Africa and South Asia with higher fiber content, anthocyanins, and bioactive compounds. The global Desi chickpeas production is approximately 85%, whereas 15% is reported for typeKabuli.[Citation10] Chickpeas are rich in polyphenols, i.e., salicylic, gallic, vanillic, anise, isoferulic, chlorogenic, cinnamic, tannic, and ferulicacids.[Citation11] Furthermore, isoflavones, a type of flavonoid in chickpeas, exhibit a wide range of biological activities comprising antioxidant, antifungal, estrogenic, and antibacterialproperties.[Citation12] Chickpeas are a lost-cost source of carbohydrates and proteins. Around 60% of the dry weight of chickpeas consists of carbohydrate content, i.e., monosaccharides, disaccharides, andoligosaccharides.[Citation13] The protein quality of chickpeas is superior to other pulses such as red gram, black gram, and greengram.[Citation14] Chickpeas contain around 66% PUFA, 19% MUFA, and 15% SFA. The Kabuli variety of chickpeas has more oleic acid, while the desi types include more linoleicacid.[Citation15]
Chickpeas contain polar and nonpolar components in their carbohydrates, proteins, and fibers, allowing them to absorb water and oileffectively.[Citation11] Furthermore, higher water absorption of polar compounds of legume flour provides similar functional properties of fat, i.e., lubrication, flow properties, and creaming characteristics. At the same time, the presence of nonpolar compounds in legumes assists in the development of fat-soluble flavor-carrying capacity in partially fat-replaced products. Moreover, chickpeas have been reported to possess higher emulsion capacity, foaming ability, thermal stability, and water absorption properties than other legumes. Due to these functional properties, chickpeas may be utilized as an effective organic fat replacer in baked products. In addition, Grossi et al.[Citation13] depicted the utilization of chickpea aquafaba as a partial replacer of palm oil up to 25%in pound cake. In another research, low-fat beef burger was prepared by the substitution of 10% fat with chickpeaflour.[Citation8] Previously white bean flour had also been reported to possess functional properties for fat replacement in chocolatebiscuits.[Citation8] In another research, muffins were made using peach dietary fibers as a significant fat substitute, owing to fibers functional ability to absorb high amounts ofwater.[Citation6]
Before consumption, many pre-treatments can be applied to legumes, including boiling, roasting, soaking, germination, and fermentation. Germination and fermentation of legumes have been reported to enhance the antioxidant potential and bioavailability of nutrients by the elimination of antinutrients. In addition to textural imperfections, the noticeable beany, earthy, and bitter off-flavor of legume flour may have a negative impact on consumer acceptance of wheat-based products containing legume flour. Roasting tends to be the most promising of the above-mentioned pre-treatments of legumes to eliminate off-flavors. Furthermore, roasting removes undesired volatiles, and other compounds produced during this process (such as pyrazines and alkylated pyrazines) are pleasing. These compounds have the potential to cover up the off-flavors.[Citation16] Additionally, Kotsiou et al.[Citation16] reported that the incorporation of roasted chickpeas flour up to 10% in wheat flour for bread preparation did not have any negative impact on the taste profile. The utmost literature revealed that germinated and roasted chickpea flour (GCPF and RCPF) had not been used before as a fat replacer in baked products. The germination of chickpeas produces enzymes that may improve the technical properties of the flour as fat replacer. Hence, the inclusion of GCPF in biscuits formulation is desired since it is predicted to improve the quality of the final product. Therefore, the aim of this research was to highlight the impact of germination and roasting on techno-functional properties, dough rheology, and quality parameters of fat-replaced biscuits. In addition, the effect of GCPF and RCPF addition on the microstructural properties, antioxidants, bioactive compounds, and nutritional profile of fat-replaced biscuits was also evaluated.
Materials and methods
Materials
In January 2023, Black Desi Chickpeas were purchased from a grocery store located in Metro Cash & Carry in Karachi. The ingredients required for making biscuits, such as wheat flour, fat, sugar, eggs, and salt, were also obtained from the same store. Additionally, glucose, soya lecithin, and baking powder were procured from Sulop Chemicals, a company based in Karachi. All chemicals used for the study were of Analytical Reagent grade, provided by Sigma-Aldrich and Merck.
Preparation of germinated chickpea flour
About 3 kg of chickpeas were sorted manually to remove any undesired particles. Chickpeas were then washed with distilled water, and soaked for 12 h. After soaking, chickpeas were placed between the two sheets of filter paper and kept in dark place at 30°C for 24 h to initiate the germination. The germinated chickpeas were then dried in drying oven (Bionics Scientific, BST/HAO-1124) at 60°C for 8 h. Afterward; it was ground into a fine powder using a laboratory cutter mill (3100 Perten Instruments, USA) and sieved from the 60µ mesh size. It was then collected and stored at 4°C in air-sealed container.
Preparation of roasted chickpea pea flour
Prior to roasting, chickpeas were soaked in tap water in the ratio of 1:2, respectively, for 6 h at room temperature. Then, water was separated, and the chickpeas were left to air dry at room temperature for 15 min. Immediately 200 g of chickpeas were roasted on an exposed pan containing silica (2 mm) for 15 min at 190°C. A digital laser infrared thermometer was used to measure the silica temperature. The heat was applied uniformly throughout the particles of silica by continuous agitation. Roasted chickpeas were separated from silica by sieving from the mesh screen. Roasted chickpeas were then ground and further treated in a similar manner as mentioned for the preparation of germinated flour.
Preparation of flour blends
The incorporation of germinated chickpea flour (GCPF) and roasted chickpea flour (RCPF) in wheat flour (WF) was associated with the level of fat replacement utilized in biscuits preparation. The GCPF and RCPF were individually incorporated in WF with respect to the level of fat replacement in biscuit samples (10%, 20%, and 30% wt/wt). The GCPF-WF blends and RCPF-WF blends were analyzed for techno-functional, proximate composition, rheological, antioxidants, and bioactive compound properties.
Proximate composition of flour blends
A moisture analyzer (Brabender51–55, CW Brabender, Duisbury, NJ, USA) was used to determine moisture content following the method of AACC(2000).[Citation17] Ash and protein contents of flour samples were determined using a Brabender Kernelyzer (Brains Instruments, Germany). Fat content was analyzed by the soxhlet extraction method of AACC(2000).[Citation17] Wet gluten (WG), dry gluten (DG), and gluten index (GI) was determined byGlutomatic-2000.[Citation18]
Techno-functional properties of flour blends
All the functional properties of flour blends were determined in triplicates. Water and Oil Absorption Capacities: Water and oil absorption capacities (WAC and OAC) of flour samples were determined according to the method of Sreerama et al..[Citation19] 1 g of flour sample was added to a tube, and 20 mL of distilled water was added, followed by mixing. Then, tubes were centrifuged at 3000×g for 10 min. The sediments were weighed and analyzed for WAC after the removal of supernatants. For the analysis of OAC of flour samples, soybean oil was used in place of distilled water and was calculated similarly.
Swelling Power: The swelling power (SP) of the sample was determined according to the method described by Olade et al..[Citation20] Briefly, 1 g of the sample was mixed with 10 mL of distilled water in a 20 mL tube. The mixture was then thoroughly mixed and heated in a water bath at 80°C for 30 min. After cooling to room temperature, the tube was centrifuged at 1000×g for 15 min. The resulting paste was analyzed to determine its SP.
Pasting Properties: The effects of varying concentrations of flour blends on pasting characteristics were analyzed using the Micro Visco-Amylo-Graph (Brabender, Duisburg, Germany) in accordance with the AACC Method76–21.02.[Citation17] A predetermined flour sample was mixed with distilled water in a container, heated to 50°C, and stirred at160 rpm for 10 sec. The mixture was left for 1 min at 50°C before being heated for 12.5 min at 95°C. Then, slurry was cooled to 50°C for 7.7 min. The resulting pasting curve was used to determine various pasting parameters, including Gelatinization beginning (G), Peak viscosity (PV), final viscosity (FV), breakdown (BD), and setback (SB) viscosities.
Antioxidant Activity of the Flour Blends: The methanolic extract of samples was prepared by adding 0.5, 1.25, 1.75 and 2.5 g flour in 10 mL methanol and vortexed, followed by sonication for 1 h in ultrasonicator (SONOREX RK 31, Bandelin Electronic KG, Berlin, Germany). The resultant extracts were centrifuged(3500 rpm) for 15 min and used for antioxidantanalysis.[Citation21]
Radical Scavenging Activity by 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH): The protocol developed by Saeed et al.[Citation22] was employed to measure the radical scavenging activity of the samples. Briefly, 1 mL of the sample extract was mixed with 1 mL of a 0.4 mM/L DPPH solution in methanol and incubated for 30 min in the dark. The absorbance at 517 nm was measured using a UV-Vis spectrophotometer (Perkin Elmer, Lambda 25). The DPPH scavenging activity was determined using the equation below:
Ferric/Ferricyanide (Fe3+) Reducing Antioxidant Power (FRAP): Various concentrations of the methanolic extracts of flour blends were analyzed for FRAP by the method of Gawlik et al..[Citation23] The absorbance of the Perl’s Prussian colored complex was determined at 700 nm, and an increase in the absorbance values is dependent on the intensity of color formation, indicating a greater ferric reducing power.
Bioactive compounds
Total Phenolic Content: To determine the total phenol content (TPC), the method described by Sharma et al.[Citation24] was used. A 200 µL aliquot of the methanolic extract was mixed with 1.3 mL of Folin-Ciocalteu reagent and 1.5 mL of saturated sodium carbonate. The mixture was then incubated at 50°C for 30 min, and the absorbance was measured at 725 nm. A calibration curve was prepared using Gallic acid as the standard solution, and TPC was expressed as milligrams of Gallic acid equivalent per 100 g of dry weight.
Total Flavonoid Content: The total flavonoid content (TFC) was calculated following the technique of Sharma et al..[Citation24] The absorbance of samples was computed at 510 nm. The results were represented as Catechin Equivalent (mg CE/100 g dry weight), using Catechin as the reference standard.
Dough rheology
The impact of the incorporation of roasted and germinated chickpea flour in wheat flour on dough mixing properties was evaluated by Farinograph parameters. Rheological properties of flour samples were estimated by using Brabender frainograph (mixer bowl 300 g, Brabender OHG, Duisburg, Germany) according to the method of AACC,54–21.02.[Citation17] Following farinograph parameters were determined, i.e., water absorption (WA), dough development time (DDT), dough stability time (DST), degree of softening (DoS), and Farinograph quality number (FQN).
Preparation offat-replaced biscuits
The biscuit samples were prepared according to the recipe mentioned in . The RCPF and GCPF replaced the fat content in biscuit samples corresponding to the amount of fat in biscuit samples (10%, 20%, and 30%). The traditional biscuit recipe was used, which included flour (RCPF/GCPF and wheat flour were blended in varying proportions of fat replacement), sugar, oil, salt, egg, glucose, baking powder, soy lecithin, and water. Initially, icing sugar and fat were thoroughly creamed for 3 min in a dough mixer (Kenwood KVL4100W, UK). The mixture of fat and sugar was mixed for a further 5 min after the addition of the whole egg and lecithin. Following that, mixture of wheat flour, baking powder, salt, glucose, and water was added. With the help of biscuit cutter, the kneaded dough was sheeted and cut into circular forms. The next step involved baking the biscuits for around 20 min at 180°C in a preheated baking oven (Anex, AG-3079, China). Biscuits were allowed to cool at room temperature and then kept in sealed containers.
Table 1. Composition of biscuits prepared from germinated chickpea flour (GCPF) and roasted chickpea flour (RCPF).
SEM of biscuits
The samples of biscuit samples were evaluated for microstructural properties by using Scanning Electron Microscope (JOEL, Analysis system, Model # JSM-6380, Japan). The sample was placed in an aluminum specimen holder and plated with gold (2 mbar). The samples were then examined under a microscope at 10 kV and 10 µm with 1000X magnification.
End quality of fat-replaced biscuits
Quality attributes of RCPF-WF and GCPF-WF biscuit samples were analyzed as follows. Antioxidants and bioactive compounds analysis of biscuits: Extractpreparation,[Citation21] DPPHinhibition,[Citation22]FRAP,[Citation23]TPC[Citation24] andTFC[Citation24] of biscuit samples were determined according to the similar manner as mentioned for the flour blends.
Physical characteristics of biscuits: The diameter (D) and thickness (T) of biscuits were measured in millimeters (mm) using a Vernier scale. For the thickness calculation, six biscuits were stacked on top of each other, and the total height was measured and divided by six to obtain an average value. The spread ratio of the biscuits was calculated asD/T.[Citation25] All measurements were repeated three times.
Textural analysis: Texture Analyser (UTM, Zwick/Roell, Germany) was used to measure the impact of the substitution of fat with RCPF and GCPF on the breaking strength of biscuit samples. A 5 kg load cell was used, and the force was measured in Newton(N).[Citation25]
Color analysis: The color of each biscuit sample was measured with color meter (NH300, China). L* value is the variable of lightness, b* and a* are the chromaticity values showing (+) yellowness/(-) blueness and (+) redness/(-) greenness, respectively. These values represented an average of measurements when the colorimeter was placed on the surface of biscuits at differentpositions.[Citation25]
Nutritional analysis of biscuits
The primary components of RCPF/GCPF incorporated biscuits, such as carbohydrate, fat, protein, ash, crude fiber, and moisture contents, wereexamined.[Citation17] AACC Method08–01.01 was used to determine the ash content. Protein content was analyzed by using the Kjeldahl apparatus according to the AACC Method46–16.01. Fat content was determined with the help of Soxhlet Apparatus (Thermo Fisher Scientific) by using the AACC Method30–25.01. The crude fiber was determined on a fiber extractor (Marconi, MA-444, Brazil) by using AACC Method32–10.01. Furthermore, AACC Method44–15.02[Citation17] was used in determining the moisture content by the hot air-drying oven method (Lab tech, LDO-060 E). The amount of carbohydrates present in the sample was determined by deducting the combined values of moisture, ash, fat, protein, and crude fiber from 100. We used the Atwater general factor system to determine the kilocalories, with lipid (9 Kcal/g), carbohydrate (4 Kcal/g), and protein (4 Kcal/g) factors.
Sensory analysis of biscuits
The Department of Food Science and Technology at the University of Karachi selected 50 semi-trained panelist, both male and female (ages22–47), to evaluate sensory parameters such as appearance, texture, taste, color, andacceptability.[Citation26] Training of participants takes place in the baking lab, mainly about the sensory method and prototypes ofbiscuits.[Citation27] The samples were graded on the basis of hedonic scale analysis (9* point ranking), 1 indicates the lowest score (dislike extremely), and 9 depicts the highest score (like extremely). The biscuits were analyzed in daylight, and a separate chamber was designated for each participant within the facility. Random 3-digit coding was assigned to each randomly picked biscuit sample and was presented to the panelist with water for rinsing their mouths.
Statistical analysis
All the tests were analyzed statistically through IBM SPSS Statistics Version 17.0. Inc, Chicago, USA software. Duncan’s test was used to observe the 95% level of significance (P ≤ .05) in various parameters. All tests were conducted in triplicate. Data were analyzed statistically by applying one-way analysis of variance (ANOVA).
Results and discussion
Physicochemical properties of flour blends
Chemical compositions of RCPF and GCPF incorporated at different levels in wheat flour (WF) are reported in . The moisture and gluten contents of RCPF-WF blends were decreased and increased, respectively, with the increased concentration of RCPF. Gluten content (dry and wet) and gluten index were reduced as the amount of RCPF and GCPF was increased in wheat flour. Similar observations were reported by Jogihalli et al.[Citation28] when different concentration of sand-roasted chickpea was added to wheat flour. Moreover, the moisture content of wheat flour (14.1%) was observed lower than different ratios of RCPF incorporated in wheat flour, which ranged from 15.34 to 17.76%. The increased values of moisture content of flour blends were due to the higher water absorption capacity of RCPF (). Similarly, increased moisture content was observed for GCPF-WF blends. The increased moisture content depicted the presence of hydrophilic components such as fiber, starch, and protein in RCPF and GCPF, which facilitates more waterabsorption.[Citation25] One of the main advantages of the addition of RCPF and GCPF in flour blends was due to the contents of proteins andfibers,[Citation11] which are well-known sources to enhance the nutritional profile of food products. Furthermore, contents of protein, crude fiber, ash, and fat were increased with the increased incorporation of RCPF and GCPF in the flour blends. Protein content increased from 11.52 to 14.96% and 11.82 to 15.86% for the blends of RCPF-WF and GCPF-WF, respectively. Greater level of fiber content was observed in RCPF (2.56%) and GCPF (3.64%) compared to control (0.21%). Although there are numerous health benefits associated with consuming fiber, such as improved metabolic parameters, microbiota composition, and the synthesis of beneficial metabolites, Western countries continue to have low fiberintake.[Citation29] Consequently, the food industry is focusing on enhancing food products by increasing their fiber content, which presents an opportunity for foodreformulation.[Citation19] The high amount of minerals in RCPF and GCPF increased the ash content in flour blends from 0.42 to 1.16% and 0.42 to 1.84%, respectively. Data reported in indicate that germination resulted in greater increase in protein and ash contents compared to the roasting of chickpeas. Generally, germinations lead to the production of amino acids, which ultimately enhance the crude protein content. While roasting might cause the denaturation of amino acids due to heating, which results in the loss of volatile nitrogenouscompounds.[Citation29] Kumar et al.[Citation29] reported similar observations regarding roasting and germination for black chickpeas.
Table 2. Proximate composition of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) incorporated in wheat flour (WF).
Table 3. Functional characteristics of various ratios of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) mixed with wheat flour.
Water absorption capacity and Oil absorption capacity of flour blends
The WAC of different concentrations of RCPF and GCPF incorporated in wheat flour is reported in . The WAC of RCPF-WF blends and GCPF-WF blends was ranged from 159.92 to 188.56% and 150.12 to 159.18%, respectively. The highest WAC was observed for RCPF 30% (188.56%) and GCPF 30% (159.18%). Furthermore, the lowest WAC was observed for RCPF 10% (159.92%) and GCPF 10% (150.12%). However, the WACs of all the flour blends (RCPF and GCPF) were much higher than the wheat flour (145.60%). The study revealed that roasting significantly (P ≤ .05) enhanced the WAC compared to germination. The less WAC of GCPF blends was due to the enzymatic degradation of hydrophilic components present in proteins andfibers.[Citation29] On the other hand, roasting denatures the proteins and dissociates them into simpler units which ultimately facilitates the binding sites for higher level of waterabsorption.[Citation30] A similar observation has been reported by Kumar et al.(2020)[Citation29] for roasting and germination of black chickpeas. These observations suggested that the addition of RCPF and GCPF to wheat flour influenced the level of water absorption due to the competition of components of chickpea flour with wheat flour for the available water. Moreover, it could be because of the molecular nature and difference in the chemical composition of chickpeas and wheat. The significant WAC of RCPF-WF blends and GCPF-WF blends proposed that these combinations of flour blends could be utilized in bakery products that demand high amount of waterabsorption.[Citation22] Oil absorption is desirable for the improvement of palatability and flavor retention of bakedproducts.[Citation31] The OAC of RCPF-WF blends and GCPF-WF blends ranged from 169.76 to 203.22% and 158.48 to 169.38%, respectively (). The observations reported in show that the increased level of chickpea flour in flour blends resulted in the increased rate of oil absorption. However, germination of chickpeas resulted in decreased level of oil absorption compared to roasting. The possible reason for the increased OAC of roasted samples was the dissociation of protein that ultimately increased the nonpolar sites and facilitated the oil binding to the hydrocarbonchain.[Citation32] On the other hand, germination of chickpeas resulted in the hydrolysis of starch granules which supported both oil and waterabsorption.[Citation28] However, hydrolyzed starch in germinated flour samples has not caused a greater increase in OAC compared to WAC due to the more affinity of starch toward watermolecules.[Citation31] The observations of WAC and OAC of flour blends align with the results of Singh et al.[Citation33] for germinated sorghum flour and Jogihalli et al.[Citation28] for roasted chickpeas.
Swelling power of flour blends
The SP of RCPF-WF blends and GCPF-WF blends was ranged from 5.07 to 5.07 g g−1 and 5.15 to 5.95 g g-1, respectively (). Furthermore, SP of all the flour blends increased with the increased concentration of chickpeas in wheat flour. However, roasted chickpeas caused reduced SP compared to germination. The lower values of SP of RCPF may be due to the formation of insoluble compounds duringroasting.[Citation28] Additionally, SP is an indicator of the degree of starch gelatinization, and during roasting, an increase in SP indicates the release of soluble polysaccharides from the starchgranule.[Citation28] In addition, germination reduced the fat content of chickpeas, which ultimately provided a limited chance for the formation of starch-lipid complexes. Moreover, these complexes are responsible for the reduced SP of foodingredients.[Citation22] Another reason for the greater values of SP for germinated chickpeas might be possible due to the breakdown of polysaccharides into monosaccharides and increased proteinsolubility.[Citation34] Sofi et al.[Citation34] depicted similar findings of increased SP for germinated chickpeas.
Pasting properties
The effects of roasting and germination of chickpeas on the pasting properties of wheat flour are depicted in . The peak viscosity of GCPF-WF blends and RCPF-WF blends, which was related to the SP of starch granules (), increased from 1037.20 to 1272.04 BU and 1037.20 to 1233.12 BU, respectively. Furthermore, the highest peak viscosity was observed for RCPF 30% (1233.2 BU) and GCPF 30% (1272.04 BU). In contrast, the lower peak viscosity was observed for RCPF 10% (1214.43 BU) and GCPF 10% (1263.61 BU). However, compared to roasted and germinated flour blends, the control sample (wheat flour) showed the lowest value of peak viscosity (1037.20 BU). Furthermore, roasting reduced the peak viscosity compared to germination. However, increased concentration of RCPF in the flour blends resulted in increased values of peak viscosities. Moreover, the lower values of peak viscosity observed after roasting might be due to the change in gelatinization and retrogradation temperatures of starch granules, which resulted in rupturing of starch granules, even at low water absorption. Thus, reducing the degree of polymerization duringgelatinization.[Citation35] The pasting temperature of RCPF-WF blends declined from 61.13 to 59.47°C, indicating lower resistance toward swelling. In contrast, the pasting temperature of GCPF-WF blends was increased from 61.38 to 62.35°C. The fragility of starch granules can be assessed through the breakdown viscosity, and the results showed that roasted flour blends had lower values (ranging from 380.52 to 406.01 BU) compared to germinated flour blends (ranging from 457.62 to 486.81 BU). Furthermore, the lower values of breakdown viscosity of RCPF-WF blends indicated that these flour blends were more resistant to shear-thinning duringcooking.[Citation36] The ability of the cooked paste to form a viscous paste during cooling, known as the final viscosity, was observed to range from 1548.03 to 1552.32 BU for roasted flour blends and 1568.23 to 1589.32 BU for germinated flour blends. As the concentration of RCPF and GCPF increased in the flour blends, the setback viscosity, which is associated with the retrogradation of starch granules, significantly decreased. The decreased setback values indicated the higher resistance of roasted and germinated starch of chickpeas for rearrangement andreorientation.[Citation37] Kumar et al.[Citation29] reported similar pasting properties for roasted and germinated black chickpeas. Furthermore, the trend of the pasting characteristics of the germinated flour blends was consistent with the values of germinatedmoong[Citation38] and germinated riceflour.[Citation39] While pasting properties for roasted flour blends were validated by Wani et al.[Citation36] for pan and microwave-roasted chestnuts.
Table 4. Pasting characteristics of different proportions of roasted and germinated chickpea flour (RCPF and GCPF) added to wheat flour.
Dough rheological properties
represents the effect of incorporating various levels RCPF and GCPF in wheat flour on dough mixing properties. There was a continuous increment in WA (from 55.71 to 68.30% and 55.71 to 67.92%) and stability time (from 8.58 to 10.24% and 8.58 to 9.70%) with the increased concentration of RCPF and GCPF in wheat flour, respectively. Moreover, the increased WA may be because of the existence of dietary fibers in flour blends. Saeed et al.(2020)[Citation1] also stated similar observations of increased WA and stability when different ratios of black gram flour were added to wheat flour. The DDT of RCPF-WF and GCPF-WF blends was ranged from 6.23 to 8.44 min and 2.27 to 5.76 min, respectively. The DDT and DoS increased with the inclusion of RCPF and GCPF in wheat flour. The rise in WA of the dough resulted in increased values of DDT. In general, high WA indicates excellent baking performance. Furthermore, higher amount of protein in flour blends provides greater WA in combination with excellent baking performance. reveals the increased level of protein content in flour blends, which was correlated with the increased values of WAC and WA reported in , respectively. However, wheat flour showed lower DDT, a lower DoS, and higher doughstability[Citation2] than the different ratios of RCPF-WF blends and GCPF-WF blends. Furthermore, strong and firm dough formation is proportional to flour strength and dough stability. The higher the stability of dough, the more will be stronger the dough formation. Most importantly, ingredients that lack gluten protein or interfere with gluten formation result in a fragile or less firm dough due to weak gluten matrixdevelopment.[Citation3] The mixing profile of wheat flour can be affected by the addition of RCFP and GCPF because the concentration of fiber, protein, and polysaccharides in the mixture increased as the proportion of RCFP and GCPF increased. Moreover, the inconsistency observed in the mixing profile can be explained by the fact that RCFP and GCPF have different functional properties than wheat flour. Another possibility might be the higher values of farinograph quality number (FQN) for flour blends, which provided the dough strength and hardening effect. Thereby hindered DDT, DS, andDoS.Citation40,Citation41]
Table 5. Farinograph properties of wheat flour and different concentrations of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) incorporated in wheat flour.
Antioxidants activities and bioactive compounds of flour blends and biscuits
The antioxidant activity of roasted and germinated flour blends and their biscuits samples are presented in (a and b), respectively. Germination significantly (P ≤ .05) affected the free radical scavenging activity of germinated samples (flour blends and biscuits). Furthermore, the percentage of radical scavenging activity increased with respect to the incorporation level of GCPF in wheat flour, which ranged from 5.12 to 84.21%. On the other hand, roasted flour blends also showed significant increase (P ≤ .05) in antioxidant activity (5.12 to 72.52%). However, compared to RCPF-WF blends, the radical scavenging activity of GCPF-WF blends demonstrated higher values of DPPH inhibition. Moreover, improvements in antioxidant activity might be caused by the increased levels of phenolic compounds and ascorbic acid due to the actions of endogenous hydrolytic enzymes duringgermination.[Citation34] Similarly, increased antioxidant activity during germination has been reported by Sofi et al.[Citation34] for germinated chickpeas. In contrast to germination, roasted chickpeas depicted lower values of the phenolic compounds as they are heat labile. Generally, phenolic compounds are correlated with the antioxidation of freeradicals.[Citation28] Furthermore, decreased antioxidant activity during roasting has been reported by Joghilial et al.[Citation28] for roasted chickpeas. The results of FRAP of flour blends and biscuit samples are reported in (a and b), respectively. FRAP value of flour blends and biscuit samples increased with the incorporation of RCPF and GCPF. The RCPF 30% and GCPF 30% showed the maximum reducing capacity. Control biscuit samples demonstrated the lowest reducing ability. The FRAP observations showed similar patterns for both RCPF-WF blends and GCPF-WF blends, as evidenced by equivalent DPPH inhibition. Additionally, the germinated flour exhibited higher FRAP values in comparison to the roasted flour. However, higher FRAP values were observed for all samples (flour blends and biscuits) compared to the DPPH inhibition. It could be because of the presence of compounds in the extracts of flour blends and biscuit samples not entirely reactive towards DPPH. For instance, antioxidant compounds like polyphenols of pre-treated chickpeas could have additional potential to act as a reducing agent for ferric iron but certainly may not be able to scavenge DPPH free radicals effectively. Similar observations of FRAP of black gram flour blends used as a fat replacer in biscuits samples were reported by Saeed et al.(2020).[Citation22] The baking process further enhanced the DPPH inhibition and FRAP of biscuit samples formulated from pre-treated flour blends. The antioxidant potential of all the formulated biscuits samples (roasted and germinated) was significantly higher (P ≤ .05) than the control biscuits. The increased antioxidant activity of RCPF and GCPF incorporated biscuits was probably due to the existence of bioactive compounds such as vitamins, flavonoids, and phenols. Another reason was the formation of Maillard reaction products, i.e., melanoidins during baking, which act as antioxidants and scavenge-freeradicals.[Citation42]
Figure 1. (a) Effect of incorporation of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) in wheat flour samples on antioxidants and bioactive compounds (b) Effect of incorporation of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) in biscuits samples on antioxidants and bioactive compounds. The error bars on each bar indicate the standard deviation (n = 3). The mean values on each bar with different letters indicate significant differences (P ≤ .05), as determined by the Duncan method. The abbreviations used in the figure are as follows: DPPH stands for 1,1-Diphenyl-2-picryl-hydrazyl radical scavenging activity, FRAP stands for Ferric/Ferricyanide (Fe3+) reducing antioxidant power, TPC stands for Total phenolic content, and TFC stands for Total flavonoid content.
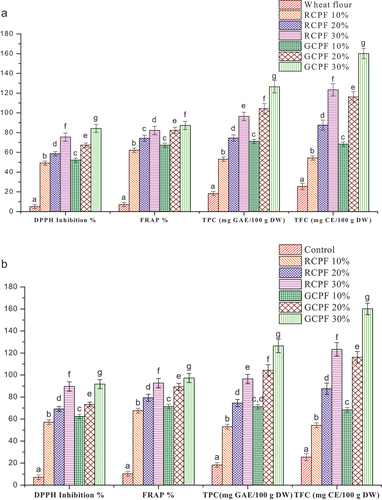
Phenolic compounds are proportional to the nutraceutical potential of any ingredient because they are antioxidants and secondarymetabolites.[Citation22] The TPC of the flour blends significantly increased (P < .05) with the incorporation level of RCPF and GCPF (). Biscuits prepared with RCPF 30% and GCPF 30% showed the highest TPC. Furthermore, the germinated samples (flour blends and biscuits) showed higher values of TPC than the roasted samples. The elevated TPC levels may be caused by endogenous enzymes triggered during germination, making bound phenolic acids readilyaccessible.[Citation43,Citation44] The Maillard end products produced during roasting and baking of RCPF-WF biscuits may be responsible for the antioxidantactivities.[Citation25] However, the phenolic content of biscuit samples decreased during baking compared to their flour blends. Therefore, the reduction in antioxidant capacity compared to germination was attributed to reduced phenolic content during roasting, which cannot be compensated by Maillard reaction endproducts.[Citation28] Moreover, roasting conditions led to the thermal degradation of bioactive compounds and decreasedTPC.[Citation28] The observations of the present study were comparable with the reported values of TPC for roastedchickpeas.[Citation28] Flavonoids are a broad group of phytochemicals with medicinal properties (Saeed et al., 2020). The TFC of RCPF-WF blends and GCPF-WF blends and their biscuit samples are illustrated in , respectively. The TFC in flour blends and biscuit samples increased with the level of addition of RCPF and GCPF. In comparison, control samples (flour blends and biscuits) showed the lowest values of TFC. Moreover, the highest TFC was found in germinated flour blends and their biscuit samples, whereas roasted flour blends and their biscuit samples exhibited lower values. These bioactive compounds have high antioxidant activities, which further increase during germination with the ability to chelate metal ions, thereby lower oxidative stress in the humanbody.[Citation45] Different cooking techniques, such as roasting and baking, have significant (P ≤ .05) impact onTFC.[Citation46] The TFC significantly decreased (P < .05) for the constant roasting temperature of the following incorporation level: 30% > 20% > 10%. According to Mir et al.(2016)[Citation47] and Gujral et al.,[Citation48] TFC content was reduced after the roasting of brown rice and oats, respectively. The lower TFC values of the RCPF-WF blends and their biscuit samples might be attributed to the byproducts of Maillard reaction generated during baking and the loss of heat-sensitive flavonoids duringroasting.[Citation49] However, TFC was increased in biscuit samples prepared from RCPF and GCPF compared to their flour blends.
Microstructure of biscuits
. Illustrates the microscopic surface morphology of control and fat replaced biscuit samples. Fig (S) represents the SEM of biscuit samples at 800X magnification. shows the microscopic image of control biscuits with starch granules and well-developed gluten network. represents the micrographs of RCPF 10%, in which starch granules entrapped in the protein matrix can be observed. Furthermore, images of RCPF 20% () and RCPF 30% () depicted coated starch granules trapped in disrupted protein matrix, and protein bodies of RCPF. The continuity and coherence of the protein matrix appeared prominently disrupted in than in the micrograph of . The decrease in the development of the gluten network or protein matrix was due to the fact that there was insufficient hydration of gluten protein, as RCPF has high water absorptioncapacity.[Citation25] Therefore, endosperm fragments persist unchanged in the resulting biscuits. Moreover, the internal texture of biscuit samples appeared rough as the amount of lubricating fat decreased with the substitution level of fat replacement. However, resembled the micrograph of control biscuits (). Similar observations have been reported by Rajiv et al. (2012) for biscuit samples incorporated with green gram flour. represents the micrographs of biscuit samples made from different levels of GCPF. These micrographs show the morphological characteristics of gelatinized starch contents of wheat flour, GCPF, and the development of continuous sheet-like structures covering these gelatinized starch. Furthermore, the small and large protein molecules can be visualized in . In addition, the appearance of protein bodies and protein-like structures increases in the images () as the level of GCPF increases in biscuit samples. The reason for the higher number of protein bodies in GCPF-WF biscuits was due to the higher protein content of GCPF (20.20%) compared to refined wheat flour (9.80%) (). Furthermore, the baking process gelatinized the starch granules, which ultimately led to the development of a continuous matrix of gelatinized starch that may resemble the proteinmatrix.[Citation42] However, due to the higher protein content of GCPF, all the gelatinized starch contents of wheat flour and GCPF were covered with a continuous sheet-like structure of proteins. Therefore, resembled each other. The images obtained from microscopic analysis of biscuit samples provided additional evidence that the germination process had an impact on the structure, integrity, and interaction of flour components. Specifically, the enzymatic breakdown triggered by α-amylase caused distortion and irregularity on the surface of each starch granule. Furthermore, these disoriented starch granules promoted their association with proteinmatrix.[Citation50] Similar observation has been reported by Singh et al.[Citation50] for composite flour biscuits produced from germinated triticale, kidney bean, and chickpea.
Dimensional and textural analysis
The dimensional characteristics of fat replaced biscuits with different levels of RCPF and GCPF are represented in . The diameter values of RCPF-WF biscuits and GCPF-WF biscuits were ranged from 53.40 to 54.45 mm and 51.74 to 54.38 mm, respectively. The spread ratio of these biscuit samples were varied from 5.10 to 6.23 mm and 4.25 to 6.08 mm, respectively. In comparison, the diameter and spread ratio of control biscuits was 55.33 mm and 6.18 mm, respectively. However, the diameters of RCPF 10%, RCPF 20%, and control biscuits were not differed significantly (P > .05). Similarly, the diameter of GCPF 10% and control biscuits were not significantly different (P > .05) from each other. Moreover, the spread ratio of RCPF 10% and control biscuits were statistically similar (P˃0.05). Furthermore, the values of diameter and spread ratio were decreased with the increased concentration of RCPF and GCPF in biscuit samples. The thickness of biscuit samples increased with the level of fat replacement. The mechanism involved in the reduction of diameter and spread ratio of biscuits might be due to the increased numbers of hydrophilic sites and water-soluble protein in GCPF, which competes for the available limited free water in biscuitsdough.[Citation51] Furthermore, less availability of free water during dough mixing increased dough viscosity, which ultimately leads to decreased values of diameter and spreadratio.[Citation51] Moreover, literature revealed that the diameter and spread ratio is affected by the partial enzymatic degradation of starch content during germination. Therefore, the germination process resulted in increased concentration of dextrins in GCPF, which further enhanced the water absorption ability of dough and reduced biscuits spread ratio anddiameter.[Citation52] Similarly, Patel and Rao et al.(1995)[Citation53] showed the decreased value of diameter and spread ratio of roasted and germination black gram flour-biscuits samples. Another study demonstrated similar findings of reduction in values of diameter and spread ratio of germinated lupine flour-biscuitssample.[Citation54]
Table 6. Effect of roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) as fat replacers on dimensional and textural properties of biscuits.
Texture profiles of roasted and germinated biscuit samples are reported in . The texture of biscuit samples was determined to measure the hardness level, i.e., breakability. The hardness of biscuit samples increased with the level of fat replacement. The hardness value of RCPF 10%, RCPF 20%, and RCPF 30% was observed as 11.46, 15.14, and 23.47, respectively. RCPF 30% showed the highest value of hardness (23.47) among all the biscuit samples. However, the hardness value of RCPF 10% and RCPF 20% was not significantly different (P˃0.05) from the control biscuits. Similar pattern of increase in hardness with the increased concentration of GCPF in biscuit samples was observed. Therefore, GCPF 30% showed the highest value of hardness (20.92). While the texture properties of GCPF 20% and control biscuits were not differed significantly (P˃0.05). The lower level of gluten content due to fat replacement with RCPF and GCPF caused the development of weak gluten network, which affected the hardness ofbiscuits.[Citation55] Furthermore, significant increase in the hardness (P < .05) of biscuits samples was most likely due to the higher fiber contents of RCPF andGCPF.[Citation25] Conversely, roasting increased the degree of hardness of fat replaced biscuits to a greater extent than germination. It may be attributed to the reduced moisture content of RCPF, which increased the concentration of starch, protein, andfiber.[Citation14] These observations revealed that the texture of biscuits is strongly influenced by the presence of fat, with a decrease in the amount of fat leading to harderbiscuits.[Citation42] In addition, solid fat crystals coat the gluten protein during kneading, preventing the development of an extended gluten network, facilitating the lubrication of flour components, and resulting in desired soft texturedbiscuits.[Citation42] Similar observations have been reported by Saeed et al.[Citation22] for the fat replacement in biscuit samples with black gram flour.
Color analysis of biscuits
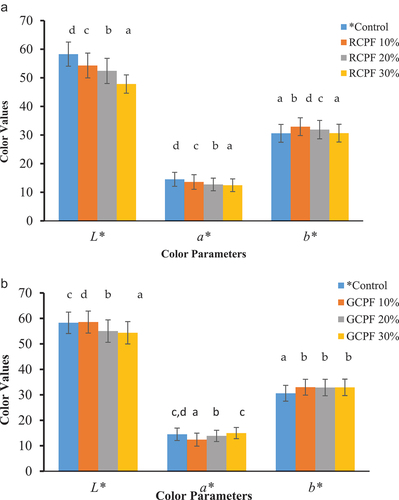
Color is one of the most important parameters which mainly attract the acceptance rate of consumers. Colour parameters significantly (P < .05) varied with the roasting and germination process. (a and b) presented the effect of roasting and germination of chickpeas on biscuit samples, respectively. From , it can be seen that as the concentration of RCPF increases in biscuits samples L* value decreases. The decrease in L* value was due to the development of dark color duringroasting.[Citation29] Similarly, a* and b* values of biscuit samples decreased. It could be due to the formation of Maillard reaction end products during roasting and baking which promoted the deep colour ofbiscuits.[Citation56] In comparison, the germination process showed the higher L* value than the roasting (). However, L* value decreased with the increased level of GCPF in biscuits samples. Furthermore, GCPF 10% and GCPF 30% showed the lowest and highest values of lightness (L*), respectively. Furthermore, GCPF-WF biscuits showed higher a* values than the control biscuits. The characteristics differences observed for the color values of GCPF-WF biscuits were probably the result of increased Maillard browning reactions, which occur due to the higher concentration of free amino acids and sugars released by enzymes during the germinationprocess.[Citation29] Although GCPF-WF biscuits did not achieve the same level of color and intensity as observed for RCPF-WF biscuits. It could be attributed to a slight decrease in the quantity of reducing sugars and free amino acids, which might have been caused by the removal of water during the steam blanching procedure whilebaking.[Citation22] Similar observations have been reported by Kumar et al.[Citation29] for roasted and germinated black chickpeas.
Figure 3. (a) Effect of incorporation of roasted chickpea flour (RCPF) in biscuits samples (b) Effect of incorporation of germinated chickpea flour (GCPF) in biscuits samples. Error bars on each bar represent standard deviation (n=3). The Duncan technique was used to determine the means that have distinct letters in subscripts on each bar which differ significantly (P ≤ 0.05). Where, *Control; biscuits without fat replacement.
Nutritional properties of biscuits
Nutritional analysis of biscuit samples formulated from RCPF and GCPF is reported in . The moisture content of RCPF-WF and GCPF-WF biscuits was ranged from 2.76 to 4.88% and 3.06 to 5.69%, respectively. Furthermore, the increase in moisture content was due to the increased concentrations of dietary fibers and protein contents in biscuit samples which contributes to water absorption. Moreover, the ash content of biscuit samples was also increased with the level of fat replacement. While fat content of biscuit samples decreased when replaced with RCPF and GCPF. Furthermore, the fat content of biscuits formulated from RCPF and GCPF was ranged from 14.02 to 17.21% and 11.32 to 16.01%, respectively. The increase in ash content of biscuit samples was correlated with the increased concentration of minerals (Saeed et al., 2021). The carbohydrate content of RCPF-WF biscuits was varied from 59.34 to 64.55%. The protein content of biscuit samples increased from 11.23 to 24.41% (GCPF) and 11.23 to 21.41% (RCPF). The increase in protein content of biscuit samples might be due to the higher protein content of RCPF and GCPF. Similarly, the increase in fiber content of biscuit samples might be due to the higher crude fiber contents in chickpea flour. Furthermore, GCPF-WF biscuits showed higher protein, dietary fiber, and ash contents than RCPF-WF biscuits. Furthermore, calories of biscuit samples (RCPF and GCPF) were decreased with the level of fat replacement. However, chickpeas contain essential fatty acids, i.e., Linoleic acid, which helps in the synthesis of prostaglandin in human body during its metabolism. Prostaglandin lowers blood pressure and controls the contraction of smoothmuscles.[Citation57] Biscuits formulated from RCPF 30% (449 kcal/100 gm) and GCPF 30% (444 kcal/10 gm) showed lower calories than other biscuit samples. On the other hand, the highest calories were observed for control biscuits (518 kcal/100 gm) without fat replacement. Similar observations of nutritional analysis were reported by Sibian and Riar.(2020)[Citation58] for germinated chickpea biscuits.
Table 7. Effect of fat substitution with roasted chickpea flour (RCPF) and germinated chickpea flour (GCPF) on the nutritional profile of biscuits.
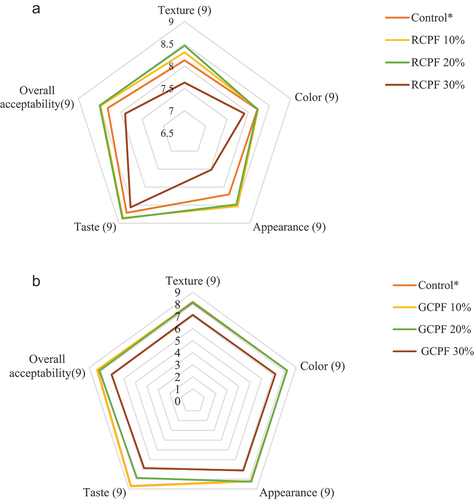
Sensory analysis
(a and b) depicted the effect of incorporation of RCPF and GCPF as a fat substitute on the sensory properties of biscuit samples, respectively. The observations revealed that with the increased level of GCPF in biscuit samples, the sensory scores for taste, texture, color, and appearance of biscuits decreased. However, the biscuits prepared by substituting GCPF 10% were similar to control biscuits with respect to color, taste, appearance, texture, and overall acceptability. Increasing the concentration of GCPF above 10% resulted in significant decrease (P < .05) in the sensory scores. While up to 20% incorporation level of RCPF in biscuit samples demonstrated more excellent sensory scores than control biscuits. The roasting process enhances the sensory profile of biscuits samples. Furthermore, color is an essential parameter in determining baked food products because it reflects the consumer’s acceptance and provides information about the formulation and quality of the end product. The roasting process produced desirable color profile of RCPF-WF biscuits. Higher concentrations of RCPF 30% and GCPF 30% resulted in the darker color of biscuit samples which might be possible due to the increased rate of Millard reaction during the baking process because of the high sugar and protein content. Furthermore, the texture of the biscuits samples was significantly (P ≤ .05) affected by the level of fat substitution. Another reason for the increased hardness values of biscuits samples was due to the increased concentration of starch and non-starch contacts, i.e., fibers and proteins and their resulting interactions with each other, which developed firmetexture.[Citation26,Citation59] Biscuit samples prepared from RCPF 30% and GCPF 30% showed the lowest sensory score of 7.63 and 7.13 for texture, respectively. In comparison, RCPF 20% (8.46), GCPF 10% (8.21), and GCPF 20% (8.20) received greater scores for textural value. Results revealed that RCPF 20% (8.87) had a higher score for taste then than control biscuits (8.71). In contrast, sensory score of the taste of biscuits formulated from GCPF 10% (8.74) was higher than the control. The substitution of fat content beyond 10% of GCPF resulted in an unpleasant mouthfeel and undesirable aroma. The above findings agreed with the observations of Sibian and Riar.(2020)[Citation58,Citation59] for germinated chickpea flour substituted biscuits and Patel and Rao.(1995)[Citation53] for roasted and germinated black gram flour substituted biscuits.
Conclusion
Bakery products are constantly growing, and biscuits are one of them which are recognized and consumed around the world. Therefore, developing baked items with low calories and nutraceutical characteristics may appeal to customers who are highly concerned about their food choices. The present research demonstrated the effect of incorporating RGPF and GCPF on the techno-functional properties of wheat flour and as fat replacer on the quality attributes of biscuit samples. The results showed that adding RCPF and GCPF to wheat flour resulted in desirable biscuits with no adverse effects on the functional properties of wheat flour. Moreover, the results suggested that increased levels of RCPF and GCPF in flour blends improved the functional properties of wheat flour. The proximate analysis interpreted that GCPF blends showed higher concentrations of protein, crude fiber, and ash content than RCPF blends. Biochemical analysis revealed that adding RCPF and GCPF to wheat flour and biscuit samples enhanced antioxidant activities, flavonoid, and phenolic contents. Moreover, biscuits prepared from RCPF 20% and GCPF 10% depicted satisfactory dimensional, textural, and sensory characteristics. The current study revealed that RCPF and GCPF could produce consumer-acceptable fat-replaced biscuits with high protein, fiber, antioxidants, and bioactive compounds with nutraceutical properties. Hence, it is possible to say that many diseases like hypertension, diabetes, cancer, and others can be prevented by including these kinds of fat-replaced functional foods in the diet.
Supplemental Material
Download ()Acknowledgments
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2023.2242602
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lim, J.; Inglett, G. E.; Lee, S. Response to Consumer Demand forReduced-Fat Foods;Multi-Functional FatReplacers. Jpn. J. Food Eng. 2010, 11(4), 147–152. DOI: 10.11301/jsfe.11.147.
- Sudha, M.; Srivastava, A.; Vetrimani, R.; Leelavathi, K. Fat Replacement in Soft Dough Biscuits: Its Implications on Dough Rheology and BiscuitQuality. J. Food Eng. 2007, 80(3), 922–930. DOI: 10.1016/j.jfoodeng.2006.08.006.
- Maache-Rezzoug, Z.; Bouvier, J.-M.; Allaf, K.; Patras, C. Effect of Principal Ingredients on Rheological Behaviour of Biscuit Dough and on Quality ofBiscuits. J. Food Eng. 1998, 35(1), 23–42. DOI: 10.1016/s0260-8774(98)00017-x.
- Ghotra, B. S.; Dyal, S. D.; Narine, S. S. Lipid Shortenings: AReview. Food. Res. Int. 2002, 35(10), 1015–1048. DOI: 10.1016/s0963-9969(02)00163-1.
- Noronha, N.; Duggan, E.; Ziegler, G.; Stapleton, J.; O’riordan, E.; O’sullivan, M. Comparison of Microscopy Techniques for the Examination of the Microstructure ofStarch-Containing ImitationCheeses. Food. Res. Int. 2008, 41(5), 472–479. DOI: 10.1016/j.foodres.2008.02.008.
- Grigelmo-Miguel, N.; Carreras-Boladeras, E.; Martin-Belloso, O. Influence of the Addition of Peach Dietary Fiber in Composition, Physical Properties and Acceptability ofReduced-FatMuffins. Food Sci. Tech. Int. 2001, 7(5), 425–431. DOI: 10.1177/108201301772660484.
- Mattes, R. D. Position of the American Dietetic Association: FatReplacers. J. Am. Diet. Assoc. 1998, 98, 463–469. DOI: 10.1016/s0002-8223(98)00105-9.
- Owon, M.; El-Demery, M. E.; Lotfy, L. M.; Amal, S. E. Quality Attributes of Low–Fat Beef Burgers Formulated with Chickpea Flour. J. Food Dairy Sci. 2014, 5(6), 389–402. DOI: 10.21608/jfds.2014.52992.
- FAOSTAT D. Agricultural Production and Production Indices Data: Crop Primary, 2013.
- FAOSTAT. <http://faostat.fao.org/site/567/desktopdefault.aspx?pageid=567/>.2011.
- Segev, A.; Badan, I. H.; Kapulnik, Y.; Shomer, I.; Oren‐Shamir, M.; Galili, S. Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Colored Chickpea (Cicer ArietinumL.). J. Food Sci. 2010, 75(2), S115–S119. DOI: 10.1111/j.1750-3841.2009.01477.x.
- Zhao, S.; Zhang, L.; Gao, P.; Shao, Z. Isolation and Characterisation of the Isoflavones from Sprouted ChickpeaSeeds. Food. Chem. 2009, 114(3), 869–873. DOI: 10.1016/j.foodchem.2008.10.026.
- Grossi, B. K. G.; Rebellato, A. P.; Joy, S.; Dupas, C.; H, M. ChickpeaAquafaba-Based Emulsions as a Fat Replacer in Pound Cake: Impact on Cake Properties and SensoryAnalysis. Foods. 2022, 16(16), 2484. DOI: 10.3390/foods11162484.
- Kaur, M.; Singh, N.; Sodhi, N. S. Physicochemical, Cooking, Textural and Roasting Characteristics of Chickpea (Cicer Arietinum L.)Cultivars. J. Food Eng. 2005, 69(4), 511–517. DOI: 10.1016/j.jfoodeng.2004.09.002.
- Wang, N.; Daun, J. K. The Chemical Composition and Nutritive Value of CanadianPulses. Canadian Grain. Commission Rep. 2004, 19–29.
- Kotsiou, K.; Sacharidis, D.-D.; Matsakidou, A.; Biliaderis, C. G.; Lazaridou, A. Physicochemical and Functional Aspects of CompositeWheat-Roasted Chickpea Flours in Relation to Dough Rheology, Bread Quality and StalingPhenomena. Food. Hydrocol. 2022, 124, 107322. DOI: 10.1016/j.foodhyd.2021.107322.
- AACC. Approved Methods of the American Association Cereal Chemist.Chemist; USA: St. Pauls, 2000.
- Saeed, S. M. G.; Ali, S. A.; Ali, R.; Sayeed, S. A.; Mobin, L. Exploring the Potential of Bottle Gourd (Lagenaria Siceraria) Flour as a Fat Mimetic in Biscuits with Improved Physicochemical and Nutritional Characteristics andAnti-DiabeticProperties. Ital. J. Food Sci. 2022, 34(2), 50–66. DOI: 10.15586/ijfs.v34i2.1954.
- Sreerama, Y. N.; Sashikala, V. B.; Pratape, V. M.; Singh, V. Nutrients and Antinutrients in Cowpea and Horse Gram Flours in Comparison to Chickpea Flour: Evaluation of Their FlourFunctionality. Food. Chem. 2012, 131(2), 462–468. DOI: 10.1016/j.foodchem.2011.09.008.
- Oladele, A.; Aina, J. Chemical Composition and Functional Properties of Flour Produced from Two Varieties of Tigernut (Cyperusesculentus). African J. Biotech. 2007, 6(21), 2473–2476. DOI: 10.5897/ajb2007.000-2391.
- Sayas-Barberá, E.; Martín-Sánchez, A. M.; Cherif, S.; Ben-Abda, J.; Pérez-Álvarez, J. Á. Effect of Date (Phoenix Dactylifera L.) Pits on the Shelf Life of BeefBurgers. Foods. 2020, 9(1), 102. DOI: 10.3390/foods9010102.
- Saeed, S. M. G.; Ali, S. A.; Ali, R.; Naz, S.; Sayeed, S. A.; Mobin, L.; Ahmed, R. Utilization of Vigna Mungo Flour as Fat Mimetic in Biscuits: Its Impact on Antioxidant Profile, Polyphenolic Content, Storage Stability, and QualityAttributes. Legume. Sci. 2020, 2(4), e58. DOI: 10.1002/leg3.58.
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Kaszuba, K.; Ryszawy, D. C. J.; Czyż, J. Anticancer and Antioxidant Activity of Bread Enriched with BroccoliSprouts. BioMed Resh. Int. 2014, 2014, 1–14. DOI: 10.1155/2014/608053.
- Sharma, P.; Gujral, H. S. Cookie Making Behavior of Wheat–Barley Flour Blends and Effects on Antioxidant Properties. LWT-Food Sci. Technol. 2014, 55(1), 301–307. DOI: 10.1016/j.lwt.2013.08.019.
- Saeed, S. M. G.; Ayesha, R.; Ali, S. A.; Ali, R.; Ahmed, R. Lotus Root (Nelumbo Nucifera Gaertn) Flour a Novel Ingredient for the Formulation of Traditional Unleavened Flatbread: Rheological, Physical and Nutritional Characteristics, and SensoryAttributes. J. Food Process Preser. 2021, 45(12), e16078. DOI: 10.1111/jfpp.16078.
- Saeed, S. M. G.; Ali, S. A.; Faheem, K.; Ali, R.; Giuffrè, A. M. The Impact of Innovative Plant Sources (Cordia Myxa L. Fruit (Assyrian Plum) and Phoenix Dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties ofGluten-FreeBiscuits. Microstruct. Nutr. Sens. Properties Gluten-Free Biscuits. Foods. 2022, 11(15), 2346. DOI: 10.3390/foods11152346.
- Heymann, H.; Lawless, H. T. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: 2013. DOI: 10.1007/978-1-4419-6488-5.
- Jogihalli, P.; Singh, L.; Kumar, K.; Sharanagat, V. S. Physico-Functional and Antioxidant Properties ofSand-Roasted Chickpea (Cicerarietinum). Food. Chem. 2017, 237, 1124–1132. DOI: 10.1016/j.foodchem.2017.06.069.
- Kumar, Y.; Sharanagat, V. S.; Singh, L.; Mani, S. Effect of Germination and Roasting on the Proximate Composition, Total Phenolics, and Functional Properties of Black Chickpea (CicerArietinum). Legume. Sci. 2020, 2(1), e20. DOI: 10.1002/leg3.20.
- Wani, I. A.; Sogi, D. S.; Sharma, P.; Gill, B. S.; Yildiz, F. Physicochemical and Pasting Properties of Unleavened Wheat Flat Bread (Chapatti) as Affected by Addition of PulseFlour. Cogent. Food Agric. 2016, 2(1), 1124486. DOI: 10.1080/23311932.2015.1124486.
- Kaur, M.; Singh, V.; Kaur, R. Effect of Partial Replacement of Wheat Flour with Varying Levels of Flaxseed Flour on Physicochemical, Antioxidant and Sensory Characteristics ofCookies. Bioact Carbohydr. Dietary Fibre. 2017, 9, 14–20. DOI: 10.1016/j.bcdf.2016.12.002.
- Ghavidel, R. A.; Prakash, J. Effect of Germination and Dehulling on Functional Properties of LegumeFlours. J. Sci. Food Food Agri. 2006, 86(8), 1189–1195. DOI: 10.1002/jsfa.2460.
- Singh, A.; Sharma, S.; Singh, B. Effect of Germination Time and Temperature on the Functionality and Protein Solubility of SorghumFlour. J. Cereal Sci. 2017, 76, 131–139. DOI: 10.1016/j.jcs.2017.06.003.
- Sofi, S. A.; Singh, J.; Muzaffar, K.; Majid, D.; Dar, B. Physicochemical Characteristics of Protein Isolates from Native and Germinated Chickpea Cultivars and Their NoodleQuality. Int. J. Gastro. Food Sci. 2020, 22, 100258. DOI: 10.1016/j.ijgfs.2020.100258.
- Sharanagat, V. S.; Jogihalli, P.; Singh, L.; Kumar, K. Effect of Roasting Method onPhysico-Mechanical and Roasting Characteristics of Chickpea (Cicerarietinum). J. Agri. Eng. 2018, 55, 36–46. DOI: 10.1016/j.ijgfs.2020.100258.
- Wani, I. A.; Hamid, H.; Hamdani, A. M.; Gani, A.; Ashwar, B. A. Physico-Chemical, Rheological and Antioxidant Properties of Sweet Chestnut (Castanea Sativa Mill.) as Affected by Pan and MicrowaveRoasting. J. Adv. Res. 2017, 8(4), 399–405. DOI: 10.1016/j.jare.2017.05.005.
- Owuamanam, C.; Ogueke, C.; Iwouno, J.; Edom, T. Use of Seed Sprouting in Modification of Food Nutrients and Pasting Profile of Tropical LegumeFlours. Niger. Food J. 2014, 32(1), 117–125. DOI: 10.1016/s0189-7241(15)30104-1.
- Sharanagat, V. S.; Kumar, P.; Patro, S.; Ghule, P. D.; Naryal, S.; Meena, S.; Singh, L.; Kumar, Y.; Gundev, P.; Nagar, M. Influence of Germination on Physicochemical,Thermo-Pasting, and Antioxidant Properties of Moong Grain (Vignaradiata). J. Food Process. Preser. 2019, 43(5), e13922. DOI: 10.1111/jfpp.13922.
- Cornejo, F.; Rosell, C. M. Influence of Germination Time of Brown Rice in Relation to Flour and Gluten Free BreadQuality. J. Food Sci. Technol. 2015, 52(10), 6591–6598. DOI: 10.1007/s13197-015-1720-8.
- Tavares, B. O.; Silva, E.; Silva, V.; Soares, M. S.; Ida, E. I.; Damiani, C. Stability of Gluten Free Sweet Biscuit Elaborated with Rice Bran, Broken Rice andOkara. Food Sci. Technol. 2016, 36(2), 296–303. DOI: 10.1590/1678-457x.0083.
- Ali, R.; Saeed, S. M. G.; Ali, S. A.; Sayed, S. A.; Ahmed, R.; Mobin, L. Effect of Black Gram Flour as Egg Replacer on Microstructure of Biscuit Dough and Its Impact on EdibleQualities. J. Food Meas. Charact. 2018, 12(3), 1641–1647. DOI: 10.1007/s11694-018-9779-3.
- Saeed, S. M. G.; Urooj, S.; Ali, S. A.; Ali, R.; Mobin, L.; Ahmed, R.; Sayeed, S. A. Impact of the Incorporation of Date Pit Flour an Underutilized Biowaste in Dough and Its Functional Role as a Fat Replacer inBiscuits. J. Food Process. 2021, 45(3), e15218. DOI: 10.1111/jfpp.15218.
- Xu, M.; Rao, J.; Chen, B. Phenolic Compounds in Germinated Cereal and Pulse Seeds: Classification, Transformation, and MetabolicProcess. Crit. Rev. Food Sci. Nutr. 2020, 60(5), 740–759. DOI: 10.1080/10408398.2018.1550051.
- Bubelova, Z.; Sumczynski, D.; Salek, R. N. Effect of Cooking and Germination on Antioxidant Activity, Total Polyphenols and Flavonoids, Fiber Content, and Digestibility of Lentils (Lens CulinarisL.). J. Food Process. Preser. 2018, 42(1), e13388. DOI: 10.1111/jfpp.13388.
- Tiwari, P.; Singh, A.; Singh, U.; Maurya, S.; Singh, M. Chromatographical Analysis of Phenolic Acids in Different Preparations of Pea (Pisum Sativum) and Chickpea (Cicerarietinum). Int. J Altern Med. 2008, 8, 7–13. DOI: 10.5580/622.
- Shem‐Tov, Y.; Badani, H.; Segev, A.; Hedvat, I.; Galili, S.; Hovav, R. Determination of Total Polyphenol, Flavonoid and Anthocyanin Contents and Antioxidant Capacities of Skins from Peanut (Arachis hypogaea) Lines with Different SkinColors. J. Food Biochem. 2012, 36(3), 301–308. DOI: 10.1111/j.1745-4514.2011.00539.x.
- Mir, S. A.; Bosco, S. J. D.; Shah, M. A.; Mir, M. M. Effect of Puffing on Physical and Antioxidant Properties of BrownRice. Food. Chem. 2016, 191, 139–146. DOI: 10.1016/j.foodchem.2014.11.025.
- Gujral, H. S.; Sharma, P.; Rachna, S. Effect of Sand Roasting on Beta Glucan Extractability, Physicochemical and Antioxidant Properties ofOats. LWT-Food Sci. Technol. 2011, 44(10), 2223–2230. DOI: 10.1016/j.lwt.2011.06.001.
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant Properties of Tartary Buckwheat Extracts as Affected by Different Thermal ProcessingMethods. LWT-Food Sci. Technol. 2010, 43(1), 181–185. DOI: 10.1088/1475-7516/2009/06/020.
- Singh, S. M.; Singh, R. C. Optimization and Evaluation of Composite Flour Cookies Prepared from Germinated Triticale, Kidney Bean, andChickpea. J. Food Process Preser. 2021, 45(1), e14996. DOI: 10.1111/jfpp.14996.
- Suriya, M.; Rajput, R.; Reddy, C. K.; Haripriya, S.; Bashir, M. Functional and Physicochemical Characteristics of Cookies Prepared from Amorphophallus PaeoniifoliusFlour. J. Food Sci. Tech. 2017, 54(7), 156–2165. DOI: 10.1007/s13197-017-2656-y.
- Bolarinwa, I.; Lim, P.; Muhammad, K. Quality ofGluten-Free Cookies from Germinated Brown RiceFlour. Food. Resh. 2019, 3(3), 199–207. DOI: 10.26656/fr.2017.3(3).228.
- Patel, M.; Rao, G. V. Effect of Untreated, Roasted and Germinated Black Gram (Phaseolus mungo) Flours on thePhysico-Chemical and Biscuit (Cookie) Making Characteristics of Soft WheatFlour. J. Cereal Sci. 1995, 22(3), 285–291. DOI: 10.1006/jcrs.1995.0065.
- Obeidat, B. A.; Abdul-Hussain, S. S.; Al Omari, D. Z. Effect of Addition of Germinated Lupin Flour on the Physiochemical and Organoleptic Properties ofCookies. J. Food Process. Preser. 2013, 37(5), 637–643. DOI: 10.1111/j.1745-4549.2012.00688.x.
- Saeed, S. M. G.; Tayyaba, S.; Ali, S. A.; Tayyab, S.; Sayeed, S. A.; Ali, R.; Mobin, L.; Naz, S. Evaluation of the Potential of Lotus Root (Nelumbo nucifera) Flour as a Fat Mimetic in Biscuits with Improved Functional and NutritionalProperties. CYTA J. Food. 2020, 18(1), 624–634. DOI: 10.1080/19476337.2020.1812727.
- Žilić, S.; Mogol, B. A.; Akıllıoğlu, G.; Serpen, A.; Delić, N.; Gökmen, V. Effects of Extrusion, Infrared and Microwave Processing on Maillard Reaction Products and Phenolic Compounds inSoybean. J. Sci. Food Agric. 2014, 94(1), 45–51. DOI: 10.1002/jsfa.6210.
- Zia-Ul-Haq, M.; Iqbal, S.; Ahmad, S.; Imran, M.; Niaz, A.; Bhanger, M. Nutritional and Compositional Study of Desi Chickpea (Cicer Arietinum L.) Cultivars Grown in Punjab,Pakistan. Food. Chem. 2007, 105(4), 1357–1363. DOI: 10.1016/j.foodchem.2007.05.004.
- Sibian, M. S.; Riar, C. S. Formulation and Characterization of Cookies Prepared from the Composite Flour of Germinated Kidney Bean, Chickpea, andWheat. Legume. Sci. 2020, 2, e42. DOI: 10.1002/leg3.42.
- Ali, S. A.; Saeed, S. M. G.; Sohail, M.; Elkhadragy, M. F.; Yehia, H. M.; Giuffrè, A. M. (2023). Functionalization of pre-gelatinized Urad bean fermented by Saccharomyces cerevisiae MK-157 as a fat replacer and its impact on physico-chemical, micromorphology, nutritional and sensory characteristics of biscuits. Arab. J. Chem. 16(9), 105029. DOI: 10.1016/j.arabjc.2023.10502.

![Figure 2. SEM of biscuits prepared with roasted chickpeas flour (RCPF) and germenated chickpea flour (GCPF) [Magnification 1000×] (a) 0% RCPF/0% GCPF/ Control (b) 10% RCPF (c) 20% RCPF (d) 30% RCPF (e) 10% GCPF (f)20% GCPF (g) 30% GCPF.](/cms/asset/1ff6c998-9747-461f-a2eb-823632cf1ce9/ljfp_a_2242602_f0002_b.gif)