 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Edible mushrooms are important in the human diet as a source of nutritive compounds with an appreciated taste. The split gill mushroom (Schizophyllum commune) is popularly consumed in Asia owing to its umami taste relating to a meaty flavor. Typically, blanching and drying are performed to preserve food quality and extend shelf-life and may affect the taste characteristics and bioactivity of mushrooms. This work investigated the effect of different methods of blanching – hot-water (HW) or microwave-assisted (MW) – and drying – hot air drying (HAD) and freeze-drying (FD) – on the characteristics, taste profile, and bioactivity of S. commune. The results showed that S. commune contained umami-related compounds, and the umami level was influenced by the processing conditions. Compared to HW blanching, MW blanching was more efficient at prohibiting polyphenol oxidase activity and maintaining the umami taste of the mushroom. Drying, particularly FD, successfully improved the umami level of S. commune. Nevertheless, HAD tended to provide the mushroom with higher bioaccessibility to phenolic compounds and antioxidant activity levels. The results suggested that S. commune could be promising as a functional ingredient with an umami taste. Pre-treatment using MW blanching, followed by FD could preserve appropriately the umami taste and quality of the mushroom.
Introduction
Mushrooms are one of the most consumed fungi owing to their taste, flavor, texture, and the nutritional value of their proteins, polysaccharides, and bioactive compounds.[Citation1,Citation2] Several bioactive characteristics of mushrooms, such as antioxidant, antidiabetic, and immunomodulating activities, of some mushrooms have been reported.[Citation3,Citation4] With the increasing popularity of a vegan diet, consumption of mushrooms is continuing to grow. Mushrooms can be a substitute for meat in several products, such as patties and sausages, owing to their unique taste and flavor, particularly for umami taste. Umami, defined as a savory, brothy, or meaty taste sensation,[Citation5] typically involves the presence of some monosodium glutamate (MSG)-like compounds, including amino acids, such as aspartic acid (Asp) and glutamic acid (Glu), as well as 5”-nucleotides, such as adenosine-5‘-monophosphate (5’−AMP), inosine-5‘-monophosphate (5’−IMP), and guanosine-5”-monophosphate (5’−GMP).[Citation6] These compounds are abundant in several mushrooms, such as Agaricus bisporus,[Citation5] Lentinus edodes,[Citation7] and Flammulina velutipes.[Citation1] Mushrooms with an umami taste are interesting candidates for substitution of MSG for the development of a healthier product. For example, the partial substitution of MSG with F. velutipes extract successfully maintained the consumer’s perception of a vegetable soup.[Citation1] Furthermore, the presence of umami-related compounds makes mushrooms suitable as a flavor enhancer in food products with a lowered salt content.[Citation5] The profiles of the umami-related compounds and bioactive agents of mushrooms depend on several factors, such as species and maturity, as well as the conditions of storage and the processing method.
Mushroom quality rapidly decreases after harvesting due to biochemical processes involving enzymes, especially polyphenoloxidase (PPO, EC 1.14.18.1) resulting in undesirable off-color and off-flavor characteristics.[Citation8] In addition, PPO can induce oxidization of monophenols and diphenols resulting in deterioration of the phenolic compounds present in mushrooms.[Citation9] To preserve the quality and extend the shelf-life of mushrooms, blanching is usually performed as a pre-treatment step to limit biochemical changes, while dehydration helps to retard chemical reactions and microbial growth. As a result of drying, the aromatic intensity of porcini[Citation10] and shitake[Citation11] could be improved, thereby enhancing consumer appreciation of these mushrooms when dried. However, a thermal process may affect umami-related compounds and the bioactive agents of mushrooms. Blanching was reported to result in a loss of umami-related compounds in L. edodes.[Citation7] Furthermore, the processing conditions influenced the microstructure of the mushroom, resulting in the altered bioaccessibility and bioavailability of bioactive compounds.[Citation7]
The split gill mushroom (Schizophyllum commune) is generally found in many areas globally and is widely consumed in Asia because of its taste and medicinal effect.[Citation12] The nutritive value of S. commune can be attributed to its high contents of fiber (ca. 19.9%), protein (ca. 24.5%), and minerals, such as manganese (ca. 64.2 ppm), iron (ca. 2727.3 ppm), and zinc (ca. 188.3 ppm), whereas its fat content is rather low (ca. 1.3%).[Citation13] In addition, S. commune contains bioactive compounds, particularly phenolics that have antioxidant and antidiabetic activities, glucans with antiradical capability, and schizophyllan with antimicrobial, anticancer, antitumor, and immunomodulating activities.[Citation12,Citation13]
The effect of thermal processes on the characteristic of S. commune is still relatively unknown; consequently, its usage has been limited on a commercial scale. The present work aimed to preserve the quality and extend the shelf-life of S. commune by studying the effects of blanching and drying on its umami taste and bioactive compounds. Different methods of blanching – hot water (HW) and microwave-assisted (MW) – and drying – hot air (HAD) and freeze drying (FD) – were conducted to determine the optimal process for the preparation of S. commune with good levels of umami taste and bioactivity. The output of the present work may enhance utilization of S. commune as a nutritive ingredient in food production by lowering the MSG content or the salt content, or both.
Materials and methods
Split gill mushroom samples were collected from a local market (Chumphon Province, Thailand). Folin Ciocalteu phenol reagent was purchased from LOBA Chemie Pvt Ltd (India). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2´-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were procured from Sigma-Aldrich Chemical Co. Ltd. (USA). The digestive components involving α-amylase from porcine pancreas Type VI-B (≥5 U/mg), pepsins from porcine gastric mucosa (≥250 U/mg), lipase from porcine pancreas (100–650 U/mg protein using olive oil as substrate), and porcine bile extract were products of Sigma-Aldrich Chemical Co. Ltd. (USA). NaCl, NaOH, and buffering reagents were purchased from KemAus (Australia). Hexane, ethanol, acetic acid, and nitric acid were products from RCI Labscan (Thailand). All reagents were of analytical grade.
Effect of blanching on characteristics of S. commune
Two blanching methods were studied involving conventional HW blanching and MW-aided blanching. For the HW blanching, the mushroom sample was blanched with water (using a mushroom-to-water ratio of 1:5) at 90°C for 3 or 5 min. In the MW blanching, mushroom and water (ratio also 1:5) were heated in a household microwave (R220; Sharp; Nakhon Pathom, Thailand) at 400 or 800 W for 1, 2, or 3 min. Then, the samples were cooled immediately in an ice bath, freeze-dried, and kept for less than 2 weeks, before analysis.
PPO activity
First, the enzyme was extracted from the mushroom, following the method of Siddiq and Cash.[Citation14] Briefly, the mushroom sample was homogenized with Tris-buffer (100 mM, pH 9.5) and Triton X-100 (0.5%) for 3 min at 4°C. Then, the macerate was filtered and precipitated using acetone. The precipitate was redispersed in phosphate buffer (100 mM, pH 6.5) and purified using CaCl2. The supernatant was taken to determine the PPO activity by reacting with catechol, following the method of Yi et al.[Citation15] with some modifications. Catechol oxidation was monitored by reading the absorbance at 420 nm (UV-1800 Spectrophotometer; Shimadzu; Kyoto; Japan) with a time interval of 4 min. PPO activity was estimated based on regression analysis to obtain a linear absorbance kinetic curve, with the PPO inhibition effect calculated based on the PPO activity of the blanched mushroom compared to fresh mushroom.
Phenolic content and antioxidant activity
First, the extract was prepared by mixing the mushroom sample with ethanol (80%) at a ratio of 1:10, w/v, before homogenizing (10,000 rpm, 3 min). Then, extraction was performed by continuous mixing at room temperature for 4 h. The residue was re-extracted twice; then, all supernatants were combined, centrifuged (4,000 rpm, 10 min, 4°C), and evaporated under vacuum for analysis.
Total phenolic content (TPC) was evaluated based on Folin-Ciocalteu assay according to the method of Javanmardi et al.,[Citation16] quantified using a standard curve of gallic acid, and reported as milligrams of gallic acid equivalent (GAE) per gram of mushroom. Free radical scavenging ability (DPPH- and ABTS-radical scavenging abilities of mushroom) samples were determined based on the methods of Xie et al.[Citation17] and Thaipong et al.,[Citation18] respectively. The DPPH- and ABTS-radical scavenging abilities were quantified as milligrams of Trolox equivalent per gram of mushroom. Reducibility of the mushroom extract was measured using the method described by Xie et al.[Citation17]
Taste-related compounds and umami
The effects of blanching on the profile of the taste-related compounds of S. commune were evaluated based on the following components. Soluble sugars were extracted by mixing the mushroom with ethanol (80%) at the ratio of 1:5, wt/v for 40 min at ambient temperature.[Citation19] Then, the mixture was centrifuged (5,000 rpm, 15 min, 4°C), and the residue was reextracted twice. All supernatants were collected, evaporated under vacuum, redispersed using acetonitrile (75%), and passed through a 0.45 µm micro-filter membrane. The soluble sugars were quantified based on HPLC using a Sugar SZ5532 column (Shodex Asahi-pak; Tokyo; Japan) and a refractive index as a detector. The injection volume of each sample was 20 µL. Acetonitrile (75%) was used as the eluent at a flow rate of 1 mL/min. The contents of the soluble sugars were quantified using external standards of fructose, glucose, trehalose, and mannitol.
Extraction of organic acids was performed using a mixture of KH2PO4 (10 mM, pH 2.86)/methanol (5/95) at the ratio of 1:15, wt/v.[Citation20] After extraction at 45°C for 30 min, the mixture was centrifuged (5,000 rpm, 15 min, 4°C) to collect the supernatant, before passing it through a 0.45 µm micro-filter membrane. The contents of the organic acids in the extracts were determined based on HPLC (Prominence LC-20 series; Shimadzu; Kyoto; Japan) using a Zorbax Eclipse XDB C18 column (250 ☓ 4.6 mm, 5 µm) and a UV detector at absorbance of 210 nm. The sample injection volume was 20 µL and a mixture of KH2PO4 (10 mM, pH 2.86)/methanol (5/95) was used as a mobile phase at 0.5 mL/min. Then, the contents of citric, malic, tartaric, succinic, fumaric, and acetic acids were determined using the external standards.
Extraction of 5”-nucleotides was conducted by mixing the mushroom samples with DI water (1:5, wt/v) at 90°C for 5 min.[Citation21] After cooling, the mixture was centrifuged (5,000 rpm, 15 min, 4°C) and the residue was re-extracted twice. All supernatants were collected and evaporated under vacuum. After adjusting the volume to 10 mL using DI water, the extract was passed through a 0.45 µm micro-filter membrane to determine 5‘-nucleotides using HPLC (Prominence LC-20; Shimadzu; Kyoto; Japan) equipped with a Zorbax Eclipse XDB C18 column (250 ☓ 4.6 mm, 5 µm; Agilent; Germany) and a UV detector at absorbance of 210 nm. The sample injection volume was 20 µL. The mobile phase was DI water/methanol/acetic acid/tetra-butylammonium hydroxide (89.35/10/0.5/0.05%), and the flow rate was 0.6 mL/min. Then, the contents of 5’-GMP, 5‘-AMP, 5’-IMP, and cytidine 5”-monophosphate (5’−CMP) were determined using external standards.
Extraction of free amino acids was performed by mixing the mushroom sample with DI water (1:10, wt/v) at 90°C for 20 min.[Citation22] After cooling, the mixture was centrifuged (5,000 rpm, 15 min, 4°C) and the residue was re-extracted twice. The free amino acid content of the mushroom samples was determined using HPLC (Prominence series; Shimadzu Co.; Japan) equipped with a pre-column of Shim-Pack ISC-30Na (50 ☓ 4 mm; Shimadzu; Kyoto; Japan) and a Shim-Pack Amino-Na column (100 ☓ 6 mm, 5 µm; Shimadzu; Kyoto; Japan). A fluorescence detector was used at excitation and emission wavelengths of 350 and 450 nm, respectively. The injection volume was 10 µL. The mobile phase consisted of methanol/acetonitrile/water (45/45/10) and phosphate buffer (pH 7.5) and the gradient elution program was performed as described by Pei et al.[Citation21]
The equivalent umami concentration (EUC) was calculated based on the MSG content as shown in Equation 1.[Citation6]
where ai is the concentrations (g/100 g) of Asp and Glu; aj is the concentrations (g/100 g) of 5”-GMP, 5‘-AMP, and 5’-IMP; bi is the relative umami concentration (RUC) of each amino acid to MSG (0.077 and 1 for Asp and Glu, respectively); bj is the RUC of each umami 5‘-nucleotide (0.18, 1, and 2.3 for 5’-AMP, 5”-IMP, and 5’-GMP, respectively); and 1218 is a constant based on the percentage calculation.
Effect of drying on characteristic of S. commune
The HAD process involved drying the samples in a tray dryer (BWS-model; Frecon, Bangkok, Thailand) at 60°C and an air velocity of 1.0 m/s. The FD process involved first freezing the mushroom at −20°C for 6 h before dehydrating it in the freeze-drier (Heto LyOLab 3000; Thermo Scientific; Waltham, USA) at −50°C and 0.014 mbar pressure. The drying was performed until the sample contained less than 10% moisture content, which is the moisture level acceptable for a commercial dry product.[Citation23] Then, the characteristics of the dried mushroom were determined.
The moisture content was evaluated using a standard AOAC method.[Citation24] Bulk density was evaluated using a replacement method with sesame and reported as a mass-to-volume ratio.[Citation25] Rehydration ratio (RR) was measured as follows. The dried mushroom sample was submerged in water at 45°C at a ratio of 1:20, wt/v.[Citation23] The sample was weighed every 15 min until it remained constant (that is, the difference in weight from the previous reading was less than 0.05 g). RR was calculated as the ratio of the weight of the fully rehydrated mushroom to the weight of the dried sample.
Color of the mushroom sample was monitored using a Minolta chromameter (CR-200b, Minolta; Tokyo; Japan) and estimated as L*, a*, and b* values. EUC was calculated based on the methods mentioned previously. Values for TPC, DPPH radical scavenging ability, ABTS radical scavenging capability, and reducibility of the mushroom were evaluated using the methods mentioned above.
In vitro bioaccessibility
The effects of blanching and drying on the in vitro bioactivity of S. commune were observed using a simulated human gastrointestinal tract based on the method of Minekus et al.,[Citation26] with some modification. Simulated saliva fluid (SSF) was prepared by dissolving Na2HPO4 (2.38 g/L), KH2PO4 (0.19 g/L), NaCl (8 g/L), and mucin (100 mg/L) in DI water and adjusting to pH 6.75. Simulated gastric fluid (SGF) was prepared by dissolving pepsin in NaCl (30 mM, pH 1.2) to achieve enzyme activity of 300 U/mL. The simulated intestinal fluid (SIF) consisted of pancreatin (300 U/mL) and bile extract in NaCl aqueous solution (0.03 M).
The ground mushroom sample (5 g) was mixed with SSF (15 mL), CaCl2 (0.075 mM), and α-amylase that had been pre-dissolved in SSF to obtain enzyme activity of 200 U/mL, before incubation at 37°C in a shaking water bath (WNB 22, Memmert; Schwabach; Germany) for 10 min. Then, the oral bolus was adjusted to pH 3.0 and added with SGF (15 mL), before incubation at 37°C for 2 h. The gastric chyme was adjusted to pH 7.0, added with SIF (15 mL), NaCl (120 mM), and KCl (5 mM), and incubated at 37°C for 4 h. After the in vitro digestion, the sample was centrifuged to collect a supernatant which was subjected determined for TPC and antioxidant activity as mentioned above. The bioaccessibility index (IAc) was calculated using Equation 2.[Citation27]
where VS and VTotal represent the values of TPC or antioxidant activities of the supernatant after the in vitro digestion and in the mushroom, respectively.
Statistical analysis
The samples were separately prepared in duplicate, and measurements were performed for triplicate. Mean values with standard deviation were shown. The difference between means was statistically evaluated based on one-way analysis of variance using Duncan’s test at a confidence level of 95%.
Results and discussion
Effect of blanching on characteristics of S. commune
First, the effects of the HW and MW blanchings on the relative remaining PPO activity of S. commune were determined, as shown in . As a result of blanching, PPO activity was inhibited, especially with increased blanching time. PPOs are the principal enzymes responsible for browning in fruits, vegetables, and mushrooms.[Citation28] To inactivate PPOs activity, heat treatment is the most generally performed. Effective condition of heating to inactivate PPOs activity is varied depending on plant source.[Citation29] MW blanching, particularly at increased levels of MW power and blanching time, could prohibit PPO activity effectively, with MW blanching at 800 W for 2 or 3 min provided the lowest remaining PPO activity (P ≤.05), which was comparable to HW blanching for 5 min. The shorter operation time of the MW compared to HW blanching suggested better efficiency with MW blanching to inhibit PPO activity. This result was in accord with other reports for A. bisporus[Citation28] and other plants, such as mango, orange, and banana peels.[Citation9] MW blanching involves internal heating because its thermal effect is generated due to microwave energy absorption by the polar molecules resulting in a rotation of the molecular dipoles and agitation of the charged ions that are further converted rapidly to heat.[Citation30] In addition, microwave energy unfolds the secondary and tertiary structures of proteins, thereby inactivating PPO activity effectively.[Citation8] HW blanching for 5 min and MW blanching at 800 W for 2 min were selected for further study, according to the lowest remaining PPO activity. The effect of blanching on the TPC and antioxidant activity of S. commune is shown in . Blanching lowered the levels of TPC and the free radical scavenging abilities of S. commune, which could have been due to heat sensitivity of the phenolic compounds. Destruction of phenolics due to blanching has also been reported.[Citation9] The present results showed there were no significant differences in the levels of TPC and antioxidant activities of the mushrooms blanched using MW or HW.
Figure 1. (a) TPC, (b) DPPH radical scavenging ability, (c) ABTS radical scavenging ability, and (d) reducibility of S. commune as affected by HW and MW blanching methods.
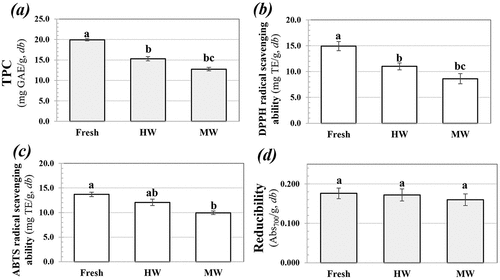
Table 1. Relative remaining PPO activity of S. commune after blanching.
Non-volatile compounds, especially soluble sugars and polyols, organic acids, 5’-nucleotides, and free amino acids, affected the taste characteristics of the mushroom. presents profiles of the taste-related compounds of S. commune as affected by HW and MW blanching. The blanching clearly influenced the profiles of the taste-related compounds, and thereby the umami level of S. commune. The EUC was significantly lower for the S. commune treated by HW blanching, whereas there was no significant difference in the EUC between the fresh mushroom and the sample treated by MW blanching. This suggested that MW blanching was better at maintaining the umami taste of the mushroom compared to HW blanching. The lower EUC of the mushroom treated by HW blanching might have been due to greater leaching of the umami-related compounds by HW blanching. The present trend is in accordant with the other work reporting greater loss of aromatic components in L. edodes due to HW blanching compared to the MW blanching.[Citation31]
Figure 2. Effect of blanching on (a) EUC and profiles of taste-related compounds for (b) 5’-nucleotides, (c) free amino acids with umami taste, (d) free amino acids with sweetness, (e) free amino acids with bitterness, (f) tasteless free amino acids, (g) organic acids, and (h) soluble sugars and polyols of S. commune.
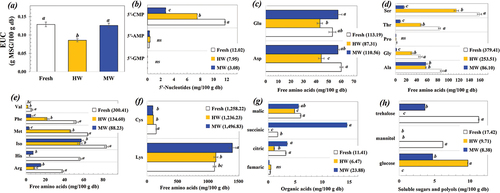
The umami level is highly influenced by the presence of 5”-nucleotides, especially 5‘-GMP, 5’-IMP, and 5‘-XMP that contribute to a meaty flavor.[Citation32] The present results showed that 5’-CMP was predominant in S. commune. Blanching, particularly for MW treatment affected a loss of 5‘-CMP. 5’-CMP was also observed in button mushroom.[Citation21] Although the 5”-CMP has not been attributed to EUC quantification, it has been suggested that 5’-CMP was significantly related to the umami taste of portobello mushroom.[Citation33]
Free amino acids play an important role in determining the taste of mushrooms; they may be classified into four groups, according to their taste characteristics: MSG-like, sweet, bitter, and tasteless.[Citation34] The present results indicated that the total content of the umami-related free amino acids of the fresh S. commune was comparable to that of the mushroom treated by MW blanching, whereas HW blanching resulted in lower total content of the umami-related free amino acids. This trend was in accordance with the EUC as previously mentioned. Blanching reduced the total contents of the bitterness-related free amino acids, particularly with MW blanching. Free amino acids with surface hydrophobicity, such as Arginine, Valine, Isoleusine, and Phenylalanine, as well as their presence in di- or tripeptides, could impart bitterness.[Citation35] The present results suggested an improved taste characteristic of S. commune after blanching by lowering the bitterness. It should be noted that the MW treated mushroom possessed lower contents of the amino acid with bitterness than did the HW treated ones. Blanching could be performed as a pre-treatment to remove undesirable taste and flavor by leaching some water-soluble compounds and volatilizing metabolic gases and volatiles present in the food materials.[Citation30,Citation36]
The most common organic acids in S. commune were succinic, malic, citric, and fumaric acids. Presence of succinic, malic, and citric acids was also reported in L. edodes,[Citation37] whereas citric acid and tartaric acid were the main ones for Agaricus blazei[Citation20] and button mushroom,[Citation21] respectively. Considering the profile of soluble sugars and polyols, S. commune mostly consisted of trehalose, glucose, and mannitol. Glucose was mainly present in portobello mushroom fruit bodies,[Citation33] whereas mannitol and trehalose were significant in button mushroom.[Citation21] Blanching by both HW and MW methods greatly affected to reduce sugars and polyols of S. commune. However, it was previously reported that HW blanching affected to greater loss of soluble sugars of water dropwort.[Citation36] The profiles of the taste-related components of mushrooms depend on several factors involving intrinsic (mushroom strain) and extrinsic (culture substrate, harvesting stage, and processing conditions) factors.[Citation21,Citation37,Citation38] In the present study, MW blanching inhibited PPO activity and preserved the desirable taste characteristics of S. commune more efficiently than HW blanching. Therefore, MW blanching was selected as a pre-treatment step for further study.
Effect of drying on characteristics of S. commune
After blanching, the S. commune was subjected to either HAD or FD and the resultant characteristics of the mushrooms dried by these different methods are summarized in . The drying method had no significant effect on the bulk density of the mushroom. However, there was a significantly higher RR for the mushroom prepared by FD compared to HAD, implying greater porosity, thereby allowing for a higher rehydration capacity of the FD-treated sample.[Citation23] Higher RR was also found for the cabbages treated by FD than did HAD.[Citation39] The sublimation of ice in the FD process resulted in voids without any significant shrinkage in the dried materials, thereby maintaining textural characteristics and rehydration of the sample effectively.[Citation39] A higher RR for the FD-treated S. commune suggested better retention of the mushroom structure which could have been due to lower thermal degree of the FD process.[Citation40] This was confirmed by the appearance of the dried mushrooms, as shown in , where the better shape retention with less shrinkage is clear in the S. commune sample prepared using FD rather than HAD.
Table 2. Effect of HAD and FD on characteristics of S. commune.
Color is an important property determining consumer acceptability in a dried food product. In the present study, the drying method affected the color of S. commune, with greater darkening for the mushroom treated by HAD than by FD, as implied by significant differences, with the values being lower for L* (lamella side), higher for a* (lamella side) and higher for b* (pileus and lamella sides) of the HAD-treated sample. This behavior was also reported following the drying of shiitake.[Citation11,Citation23,Citation40] The higher operational temperature of HAD might have facilitated degradation of the polysaccharides to oligosaccharides and/or sugars that could result in a Maillard reaction and caramelization, which would darken the sample.[Citation2,Citation40] It has been suggested that the degree of Maillard reaction was directly related to the temperature of the drying process.[Citation41] The color change in the S. commune due to drying occurred mostly on the lamella side, which might have been due to the plate-like structure of the lamella providing greater surface areas than the pileus. Therefore, there was a greater degree of chemical change for the lamella, resulting in a more pronounced color change.
Drying method significantly influenced the bioactivity of S. commune. There were significantly lower TPC and antioxidant activity levels in the mushroom dried by HAD compared to FD. This could be expected due to the greater destruction of the bioactive compounds as a result of HAD operating at a higher temperature than FD. This tendency was consistent with other studies of several mushrooms such as Phlebopus colossus,[Citation3] Leccinum scabrum, Hericium erinaceus,[Citation4] and some fruits.[Citation42] The thermal process caused a reduction in the phenolic content and/or antioxidant activity due to degradation and structural change of the compounds.[Citation4,Citation23]
The effects of drying on the EUC and profile of the taste-related compounds of S. commune are shown in . Drying by both HAD and FD significantly enhanced the umami taste of S. commune. Improvement in the umami level after drying has been reported in several mushrooms such as porcini[Citation10] and shitake.[Citation11] Similarly, in the present study, the drying method influenced the umami level of S. commune, with a significantly higher EUC for the FD-treated mushroom compared to processing by HAD. The higher processing temperature in HAD might have affected the flavor profile of the mushroom through several reactions, such as a Maillard reaction, or oxidation, as well as from degradation of the chemical composition of the mushroom, thereby influencing the umami level of S. commune in a different manner compared to the FD process.[Citation2,Citation41] Drying increased the total content of 5”-nucleotides, thereby improving the EUC of the dried mushroom. Drying, especially using FD, significantly increased the content of 5‘-GMP which is a flavor enhancer with a stronger efficiency than MSG.[Citation32] Higher contents of 5’-GMP and 5‘-AMP were also observed for FD treated Suillus granulatus compared to the HAD process.[Citation38] At 5 h, the dried button mushroom prepared by FD had increased contents of 5’-GMP and 5‘-AMP, whereas the contents of 5’-CMP and 5‘-IMP were not affected.[Citation21] Presently, 5’-CMP was predominantly observed in the dried mushroom, and HAD producing higher 5”-CMP content than FD. Considering the profiles of the free amino acids, drying could reduce the total bitterness that is related to free amino acids, suggesting improved taste characteristics of the mushroom after drying. Drying reduced the total contents of the free amino acids related to sweetness. Changes in the profiles of 5’-nucleotides and free amino acids due to drying have varied depending on the mushroom type, drying method, and operating conditions.[Citation21,Citation38]
Figure 3. Effect of drying on (a) EUC and profiles of taste relating compounds for (b) 5’-nucleotides, (c) free amino acids with umami taste, (d) free amino acids with sweetness, (e) free amino acids with bitterness, (f) tasteless free amino acids, (g) organic acids, and (h) soluble sugars and polyols of S. commune.
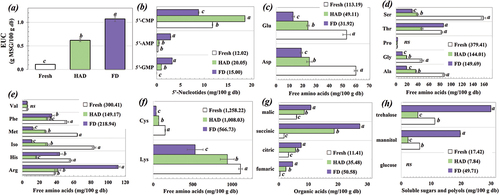
Drying caused a significant increase in the organic acids of S. commune, and succinic acid was a major organic acid present in the mushroom. Improvement on organic acid contents after drying process was also observed for shiitake.[Citation43] Higher total organic acid content was found for the FD – compared to the HAD treated sample. An increase in succinic acid was also observed for the button mushroom after FD, which was ascribed to biochemical changes activated by the increasing temperature of the sample during the sublimation stage of FD.[Citation21] The drying method also affected the profiles of the soluble sugars and polyols of S. commune. After FD, the content of mannitol and trehalose significantly improved, which was in agreement with the drying of button mushroom.[Citation21] The present results showed that the HAD treated S. commune had lower total sugar content than the FD treated mushroom, which might be due to the higher temperature of the HAD, resulting in enhancement of the Maillard reaction, thereby lowering the sugar content.[Citation21] Mannitol could be regarded as a taste-active compound contributing to the sweet perception of mushrooms.[Citation21] Therefore, a higher sugar content of FD S. commune might imply improved taste perception than for the sample prepared using HAD.
From all points of view, FD could provide S. commune with better taste and physical qualities, as well as improved antioxidant activities compared to HAD. The greater efficiency of FD compared to HAD in preserving the quality of shiitake has been confirmed for several aspects, including texture, color, flavor, and the profile of volatile compounds.[Citation11] Better flavor retention similar to the taste of a fresh mushroom based on descriptive analysis was also reported for shiitake dried by FD compared to HAD.[Citation11] The higher process temperature of HAD led to greater destruction of the chemical components, such as sugars, acids, and polysaccharides, leading to altered taste and nutritive profiles of several mushrooms, including L. scabrum, H. erinaceus,[Citation4] and shiitake.[Citation23,Citation40]
In vitro bioaccessibility levels of phenolics and antioxidant activities of S. commune
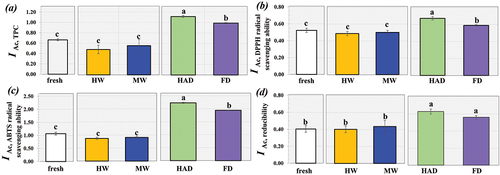
To elucidate the effect of blanching and drying on the bioactivity of S. commune, the in vitro bioaccessibility levels of phenolics and antioxidant activities were determined, as shown in . Compared to the fresh mushroom, blanching had no significant effect on the in vitro bioaccessibility levels of TPC and antioxidant activities, whereas drying significantly affected these levels for S. commune. The reduced less effect from blanching compared to drying on these levels for S. commune might have been due to the shorter operation time of blanching. Although the previous result in suggested lowered TPC, DPPH and ABTS free radical scavenging capabilities of the S. commune dried by HAD compared to FD, there were significantly higher IAC values of TPC and free radical scavenging capabilities for the HAD-treated sample. This might have been due to the relevant compounds breaking out of the mushroom cellular matrix due to the higher operating temperature of HAD, thereby facilitating release of the bioactive compounds with free radical scavenging capability.[Citation44] HAD enhanced the liberation of free and bound phenolics, thereby facilitating more effective bioaccessibility of the compounds.[Citation45] Berries dried using the HAD process had lower levels of TPC and antioxidant activity than their counterparts prepared using microwave-hot air drying, but there was higher bioaccessibility of the phenolics for the HAD-treated berries.[Citation44] Improvement in the bioaccessibility of bioactive compounds due to HAD has been reported for drying persimmon,[Citation42] Rhodomyrtus tomentosa berries,[Citation44] and pumpkin flour.[Citation45] Considering the reducibility of the dried mushroom, there was no significant difference between the IAC values for the samples prepared using HAD and FD. This contradictory behavior might be expected due to the different antioxidant modes of action of the liberated phenolic compounds. For example, after simulated in vitro digestion, the free radical scavenging abilities of the phenolics present in black carrot pomace significantly decreased, whereas reducibility of the sample was not affected.[Citation46]
Figure 4. Effect of blanching (HW and MW blanching methods) and drying (HAD and FD) on in vitro bioaccessibility of (a) TPC, (b) DPPH radical scavenging activity, (c) ABTS radical scavenging activity and (d) reducibility of the S. commune.
Conclusion
The effects of blanching and drying on a profile of taste-related compounds and bioactivity of S. commune were first elucidated in the present work. By using the optimized processing method, S. commune with improved umami taste and prolonged shelf life could be prepared as a nutritive ingredient. MW blanching was more effective at inhibiting PPO activity than the conventional method using HW blanching. The studied blanching method had no effect on the bioaccessibility levels of phenolic compounds or the antioxidant activities of S. commune. Drying, particularly for FD, significantly improved the umami degree of S. commune. Better retention of physical characteristics and higher levels of TPC, and antioxidant activities of S. commune were accomplished using FD compared to HAD. However, HAD provided S. commune with enhanced bioaccessibility levels of phenolics and antioxidant activities. The present work suggested the processing conditions applicable to prepare shelf-stable S. commune as a functional ingredient with improved umami taste, which might be feasibly utilized in the development of food products with lowered MSG or sodium contents, or both. To enlarge the use of S. commune as a functional ingredient, moreover, the effect of processing on other bioactive compounds such as schizophyllan should be further studied.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mau, J. L.; Chen, Y. L.; Chien, R. C.; Lo, Y. C.; Lin, S. D. Taste Quality of the Hot Water Extract from Flammulina Velutipes and Its Application in Umami Seasoning. Food Sci. Technol. Res. 2018, 24(2), 201–208. DOI: 10.3136/fstr.24.201.
- Zhang, L.; Zhang, M.; Mujumdar, A. S. Development of Flavor During Drying and Applications of Edible Mushrooms: A Review. Dry. Technol. 2021, 39(11), 1685–1703. DOI: 10.1080/07373937.2021.1875231.
- Liaotrakoon, W.; Liaotrakoon, V. Influence of Drying Process on Total Phenolics, Antioxidative Activity and Selected Physical Properties of Edible Bolete (Phlebopus Colossus (R. Heim) Singer) and Changes During Storage. Food Sci. Technol. 2018, 38, 231–237. DOI: 10.1590/1678-457x.34116.
- Gasecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szableska, K.; Niedzielski, P.; Mleczek, M. The Effect of Drying Temperature on Bioactive Compounds and Antioxidant Activity of Leccinum Scabrum (Bull.) Gray and Hericium Erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57(2), 513–525. DOI: 10.1007/s13197-019-04081-1.
- Miller, A. M.; Mills, K.; Wong, T.; Drescher, G.; Lee, S. M.; Sirimuangmoon, C.; Schaefer, S.; Langstaff, S.; Minor, B.; Guinard, J. X. Flavor-Enhancing Properties of Mushrooms in Meat-Based Dishes in Which Sodium Has Been Reduced and Meat Has Been Partially Substituted with Mushrooms. J. Food Sci. 2014, 79(9), S1795–S1804. DOI: 10.1111/1750-3841.12549.
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some L-?-Amino Acids and 5’-Nucleotides. J. Food Sci. 1971, 36(6), 846–849. DOI: 10.1111/j.1365-2621.1971.tb15541.x.
- Li, B.; Kimatu, B. M.; Pei, F.; Chen, S.; Feng, X.; Hu, Q.; Zhao, L. Non-Volatile Flavour Components in Lentinus Edodes After Hot Water Blanching and Microwave Blanching. Int. J. Food Prop. 2018, 20(sup3), S2532–S2542. DOI: 10.1080/10942912.2017.1373667.
- Zhang, Z.; Wang, J.; Zhang, X.; Shi, Q.; Xin, L.; Fu, H.; Wang, Y. Effects of Radio Frequency Assisted Blanching on Polyphenol Oxidase, Weight Loss, Texture, Color and Microstructure of Potato. Food Chem. 2018, 248, 173–182. DOI: 10.1016/j.foodchem.2017.12.065.
- Feumba, D. R.; Akdowa, E. P.; Rani, P. A.; Tongwa, Q. M.; Mbofung, C. M. Effect of Microwave Blanching on Antioxidant Activity, Phenolic Compounds and Browning Behaviour of Some Fruit Peelings. Food Chem. 2020, 302, 125308. DOI: 10.1016/j.foodchem.2019.125308.
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and Comparison of Key Aroma Compounds in Raw and Dry Porcini Mushroom (Boletus edulis) by Aroma Extract Dilution Analysis, Quantitation and Aroma Recombination Experiments. Food Chem. 2018, 258, 260–268. DOI: 10.1016/j.foodchem.2018.03.056.
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, A. A. Volatile Composition and Sensory Profile of Shiitake Mushrooms as Affected by Drying Method. J. Sci. Food Agric. 2017, 98(4), 1511–1521. DOI: 10.1002/jsfa.8622.
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Van Greinsven, D. Antioxidative Activities and Chemical Characterization of Polysaccharides Extracted from the Basidiomycete Schizophyllum Commune. LWT-Food Sci. Technol. 2011, 44(10), 2005–2011. DOI: 10.1016/j.lwt.2011.05.010.
- Singh, S.; Raj, C.; Singh, H. K.; Avasthe, R. K.; Said, P.; Balusamy, A.; Sharma, S. K.; Lepcha, S. C.; Kerketta, V. Characterization and Development of Cultivation Technology of Wild Split Gill Schizophyllum Commune Mushroom in India. Sci. Hort. 2021, 289, 110399. DOI: 10.1016/j.scienta.2021.110399.
- Siddiq, M.; Cash, J. N. Physico-Chemical Properties of Polyphenol Oxidase from d’Anjou and Bartlett Pears (Pyrus Communes L.). J. Food Process Preserv. 2000, 24(5), 353–364. DOI: 10.1111/j.1745-4549.2000.tb00424.x.
- Yi, J.; Jiang, B.; Zhang, Z.; Liao, X.; Zhang, Y.; Hu, X. Effect of Ultrahigh Hydrostatic Pressure on the Activity and Structure of Mushroom (Agaricus bisporus) Polyphenoloxidase. J. Agric. Food. Chem. 2011, 60(2), 593–599. DOI: 10.1021/jf203405u.
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J. M. Antioxidant Activity and Total Phenolic Content of Iranian Ocimum Accessions. Food Chem. 2003, 83(4), 547–550. DOI: 10.1016/S0308-8146(03)00151-1.
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant Activity of Peptides Isolated from Alfafa Leaf Protein Hydrolysate. Food Chem. 2008, 111(2), 370–376. DOI: 10.1016/j.foodchem.2008.03.078.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D. H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19(6–7), 669–675. DOI: 10.1016/j.jfca.2006.01.003.
- Tsai, S.; Tsai, H.; Mau, J. Non-Volatile Taste Components of Agaricus Blazei, Agrocybe Cylindracea and Boletus Edulis. Food Chem. 2008, 107(3), 977–983. DOI: 10.1016/j.foodchem.2007.07.080.
- Li, W.; Gu, Z.; Yang, Y.; Zhou, S.; Liu, Y.; Zhang, J. Non-Volatile Taste Components of Several Cultivated Mushrooms. Food Chem. 2014, 143, 427–431. DOI: 10.1016/j.foodchem.2013.08.006.
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A. M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F., et al. Changes in Non-Volatile Taste Components of Button Mushroom (Agaricus bisporus) During Different Stages of Freeze Drying and Freeze Drying Combined with Microwave Vacuum Drying. Food Chem. 2014, 165, 547–554. DOI: 10.1016/j.foodchem.2014.05.130.
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.; Zeng, X. Analysis of Free Amino Acids in Chinese Teas and Flower of Tea Plant by High Performance Liquid Chromatography Combined with Solid-Phase Extraction. Food Chem. 2011, 123(4), 1259–1266. DOI: 10.1016/j.foodchem.2010.05.063.
- Qi, L. L.; Zhang, M.; Mujumdar, A. S.; Meng, X. Y.; Chen, H. Z. Comparison of Drying Characteristics and Quality of Shiitake Mushrooms (Lentinus Edodes) Using Different Drying Methods. Dry. Technol. 2014, 32(15), 1751–1761. DOI: 10.1080/07373937.2014.929588.
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington DC, USA, 1995.
- Gürsiy, S.; Choudhary, R.; Watson, D. G. Microwave Drying Kinetics and Quality Characteristics of Corn. Intl. J. Agric. Biolo. Eng. 2013, 6, 90–99.
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Ellipsis, B. A.; Boutrou, R.; Corredig, M.; Dupont, D. A Standardised Static In Vitro Digestion Method Suitable for Food – an International Consensus. Food Funct. 2014, 5(6), 1113–1124. DOI: 10.1039/C3FO60702J.
- Gawlik-Dziki, U.; Dziki, S.; Świeca, M.; Sęcyzk, Ł.; Różyło, R.; Szymanowska, U. Bread Enriched with Chenopodium Quinoa Leaves Powder – the Procedures for Assessing the Fortification Efficiency. LWT-Food Sci. Technol. 2015, 62(2), 1226–1234. DOI: 10.1016/j.lwt.2015.02.007.
- Bernaś, E.; Jaworska, G. Effect of Microwave Blanching on the Quality of Frozen Agaricus Bisporus. Food Sci. Technol. Int. 2014, 21(4), 245–255. DOI: 10.1177/1082013214529956.
- Nogales-Delgado, S. Polyphenolixidase (PPO): Effect, Current Determination and Inhibition Treatments in Fresh-Cut Produce. Appl. Sci. 2021, 11(17), 7813. DOI: 10.3390/app11177813.
- Ranjan, S.; Dasgupta, N.; Walia, N.; Thara, C. C.; Ramalingam, C. Microwave Blanching: An Emerging Trend in Food Engineering and Its Effects on Capsicum Annuum L. J. Food Process. Eng. 2017, 40(2), 12411–12418. DOI: 10.1111/jfpe.12411.
- Li, B.; Kimatu, B. M.; Li, C.; Pei, F.; Hu, Q.; Zhao, L. Analysis of Volatile Compounds in L. Edodes Blanched by Hot Water and Microwave. Intl. J. Food Sci. Technol. 2017, 52(7), 1680–1689. DOI: 10.1111/ijfs.13442.
- Litchfield, J. H. Morel Mushroom Mycelium as a Food-Flavoring Material. Biotechnol. Bioeng. 1967, 9(3), 289–304. DOI: 10.1002/bit.260090303.
- Wang, J.; Loi, W.; Tang, W.; Wu, X.; Tang, X. Analysis and Evaluation of the Characteristic Taste Components in Portobello Mushroom. J. Food Sci. 2018, 83(6), 1542–1551. DOI: 10.1111/1750-3841.14165.
- Beluhan, S.; Ranogajec, A. Chemical Composition and Non-Volatile Components of Croatian Wild Edible Mushrooms. Food Chem. 2011, 124(3), 1076–1082. DOI: 10.1016/j.foodchem.2010.07.081.
- Zhao, C. J.; Schieber, A.; Gänzle, M. G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentation – a Review. Food Res. Int. 2016, 89, 39–47. DOI: 10.1016/j.foodres.2016.08.042.
- Tang, M.; Sun, H.; Zhang, Z.; Zhao, J.; Cao, J.; Thakur, K.; He, S. Evaluation of Hot Water and Microwave Blanching on Qualities and Sensory Characteristics of Water Dropwort (Oenanthe Javanica DC.). J. Food Process Preserv. 2019, 43(10), e14104. DOI: 10.1111/jfpp.14104.
- Chen, W.; Li, W.; Yang, Y.; Yu, H.; Zhu, S.; Feng, J.; Li, X.; Liu, Y. Analysis and Evaluation of Tasty Components in the Pileus and Stipe of Lentinula Edodes at Different Growth Stages. J. Agric. Food. Chem. 2015, 63(3), 795–801. DOI: 10.1021/jf505410a.
- Zhao, X.; Wei, Y.; Gong, X.; Hu, H.; Xin, G. Evaluation of Umami Taste Components of Mushroom (Suillus granulatus) of Different Grades Prepared by Different Drying Methods. Food Sci. Human Wellness. 2020, 9(2), 192–198. DOI: 10.1016/j.fshw.2020.03.003.
- Rajkumar, G.; Shanmugam, S.; Galvâo, M. S.; Sandes, R. D. D.; Leite Neta, M. T. S.; Narain, N.; Mujumdar, A. S. Comparative Evaluation of Physical Properties and Volatiles Profile of Cabbages Subjected to Hot Air and Freeze Drying. LWT-Food Sci. Technol. 2017, 80, 501–509. DOI: 10.1016/j.lwt.2017.03.020.
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of Different Drying Methods on the Product Quality and Volatile Compounds of Whole Shiitake Mushrooms. Food Chem. 2016, 197, 714–722. DOI: 10.1016/j.foodchem.2015.11.029.
- Cao, C.; Xie, J.; Hou, L.; Zhao, J.; Chen, F.; Xiao, Q.; Zhao, M.; Fan, M. Effect of Glycine on Reaction of Cysteine-Xylose: Insights on Initial Maillard Stage Intermediates to Develop Meat Flavor. Food Res. Int. 2017, 99, 444–453. DOI: 10.1016/j.foodres.2017.06.012.
- Kayacan, S.; Karasu, S.; Akman, P. K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of Different Drying Methods on Total Bioactive Compounds, Phenolic Profile, In Vitro Bioaccessibility of Phenolic and HMF Formation of Persimmon. LWT-Food Sci. Technol. 2020, 118, 108830. DOI: 10.1016/j.lwt.2019.108830.
- Yang, X.; Zhang, Y.; Kong, Y.; Zhao, J.; Sun, Y.; Huang, M. Comparative Analysis of Taste Compounds in Shiitake Mushrooms Processed by Hot-Air Drying and Freeze Drying. Int. J. Food Prop. 2019, 22(1), 1100–1111. DOI: 10.1080/10942912.2019.1628777.
- Zhao, G.; Zhang, R.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, Y.; Ma, Y.; Zhang, M. Different Thermal Drying Methods Affect the Phenolic Profiles, Their Bioaccessibility and Antioxidant Activity in Rhodomyrtus Tomentosa (Ait.) Hassk Berries. LWT-Food Sci. Technol. 2017, 79, 260–266. DOI: 10.1016/j.lwt.2017.01.039.
- Aydin, E.; Gocmen, D. The Influences of Drying Method and Metabisulfite Pre-Treatment on the Color, Functional Properties and Phenolic Acids Contents and Bioaccessibility of Pumpkin Flour. LWT-Food Sci. Technol. 2015, 60(1), 385–392. DOI: 10.1016/j.lwt.2014.08.025.
- Kamiloglu, S.; Demirci, M.; Selen, S.; Toydemir, G.; Boyacioglu, D.; Capanoglu, E. Home Processing of Tomatoes (Solanum lycopersicum): Effects on In Vitro Bioaccessibility of Total Lycopene, Phenolics, Flavonoids, and Antioxidant Capacity. J. Sci. Food Agric. 2014, 94(11), 2225–2233. DOI: 10.1002/jsfa.6546.
