?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to assess the effects of nutritional intervention in breast cancer patients. Anthropometrics serum biomarkers and tumor markers were assessed in breast cancer female patients. Product intervention was done in the breast cancer group (n = 120) which was further segregated into three treatments groups T0 placebo Group (non-supplemented cookies) + chemotherapy, T1 synthetic group (vitamin D and calcium supplement) + chemotherapy, T2 natural group (ESP + CLO supplemented cookies) + chemotherapy. Anthropometrics data showed that body mass index (BMI) and hand grip strength (kg) were varied from 26.48 ± 0.28 to 26.15 ± 0.20 kg/m2 and 22.18 ± 0.36 to 22.21 ± 0.38 kg among T2 group, respectively. After intervention of natural supplement, serum calcium and serum vitamin D levels were significantly improved from 8.45 ± 0.08 to 8.61 ± 0.11 mg/dl and 26.45 ± 0.84 to 30.33 ± 1.37 ng/ml among T2 participants, respectively. Whereas in T1 group the serum calcium slightly improved from 8.46 ± 0.06 to 8.53 ± 0.07 mg/dl and serum vitamin D levels enhanced from 24.18 ± 1.06 to 26.38 ± 1.05 ng/ml among participants. The serum parathyroid hormone level significantly decreased from 5.2 ± 0.07 to 4.9 ± 0.07 pmol/l in T2 group participants. The nutritional intervention of calcium and vitamin D had shown to improved body mass index (BMI), hand grip strength and increased oral intake in patients and reduced inflammatory markers. Chemotherapy-induced dietary issues may be managed by effective oral nutritional intervention. Serum calcium and vitamin D may be modifiable factor for the risk of breast cancer in females.
Introduction
Breast cancer is an uncontrolled proliferation of cells in breast cells that attain malignancy. It is the most prevalent cancer type among female population worldwide. Breast cancer is the fifth most occurring cancer death which contributed to about 28% of new cancer cases every year. Breast cancer is more prevalent in Asian women with age above 40–50 years compared to Western countries where age above 60–70 years is common. Pakistan has a 10-year younger average age for breast cancer diagnoses in females than other developed nations.[Citation1,Citation2] Non-modifiable factors for breast cancer include genes, age, age at menarche and parity, whereas modifiable factors account for ~ 20% such as obesity, physical inactivity and dietary intake contributes to breast cancer onset. The modifiable risk factors offer potential to curb the disease burden by lifestyle modification.[Citation3] A statistical data reported over 268,000 new cases of breast cancer, with nearly 42,000 of women dying from the disease in the same year.[Citation4] The total population accounts for 49.5% of women Worldwide and largest population of women are over age of 60 years. According to a study, 2.1 million women had recently been diagnosed with breast cancer, or one new case every 18 seconds; 626,679 people had also died from breast carcinoma.[Citation5] It has been reported that one in every nine women is likely to be diagnosed with breast cancer. Dietary routine, lifestyle habits and being obese are known as modifiable factors. Evidence research concluded that nutritional intervention plays an important role in determining cancer prognosis, efficacy of anti-tumor therapies and maintain quality of life. Chemotherapy is a common clinical method used to treat breast cancer which may affect quality of patients life.[Citation6,Citation7] Nutrition-based research and nutritional screening of cancer patients have gained much importance. The nutrition-related factors are the key factors for the onset of breast cancer and also it plays role as anti-cancer tool. A cross-sectional, multicenter, epidemiologic study on clinical nutrition reported that cancer patient had an almost three-fold poor nutrition status rate as compared to healthy volunteers.[Citation8] Poor nutrition showed positive correlation with increased, morbidity, mortality, longer hospital stays and higher financial burden in patients. Furthermore, malnutrition is linked with low treatment efficacy and disease prognosis in cancer patients. Nutrition has a vital role in maintaining lifestyle of cancer patient. The physiological and metabolic aspects of patients with cancer can change their nutritional needs (protein, carbohydrate, fats, minerals and vitamins).[Citation9,Citation10] The prevalence of malnutrition in cancer patients accounts for 30–50% in general and 85% longer stay in hospital is due to malnutrition of patients. Nutritional therapy is important to curb the malnutrition rate, better efficacy and prognosis of cancer treatments. Study data observed that there should be a structured coordination between clinical nutritionists and oncologists to perform the practical implementation of nutritional therapy in cancer patients.[Citation11,Citation12] Chemotherapy induced side effects include vomiting, nausea, stomach pain, loss of appetite, sore mouth, diarrhea, loss of taste, constipation and difficulty in swallowing that can affect the dietary intake of patient and hence increases risk of malnutrition. An evidence-based literature showed that a good quality of diet is associated with less chances of developing chronic inflammation consequently.[Citation13,Citation14] It has been proved that dietary or nutritional counseling regarding oral nutritional supplements should be the first line treatment to increase the oral intake and improve clinical treatments outcome. Studies on breast cancer patients undergoing adjuvant therapy observed that dietary counseling improves body weight, increase oral intake, interruptions and treatment related toxicities.[Citation15,Citation16] Effective nutritional screening, oral supplementation are important tools during cancer treatments. Nutritional intervention using oral supplementation might be incorporated in cancer therapeutically treatment to improve both clinical outcomes as well as maintain quality of life. Natural source of vitamin D and calcium supplemented added in food product may be used as vehicle to combat deficiencies in better ways. In clinical oncology practice, vitamin D and calcium supplementation intervention in breast cancer patients can be considered as an integral part of the multimodal therapeutic approach.
Materials and methods
Study design
The present study was conducted at different hospitals and clinics in Rawalpindi/Islamabad-Pakistan. The present study was conducted at hospital which based on case-control trial. Case group participants were patients clinically diagnosed with breast cancer and disease free patients were taken as control group.
Study participants
Study subjects were young, middle-aged and old female patients suffering from breast diseases and healthy volunteers who had no disease or co-morbids and were collected from different hospitals of Rawalpindi, Pakistan.
Study groups
Case study comprised two groups including healthy group and breast cancer group. The case group was further segregated into T0 (placebo group (non-supplemented cookies) + chemotherapy), T1 (synthetic vitamin D and calcium supplement) + chemotherapy) and T2 (natural group (ESP+ CLO supplemented cookies) + chemotherapy). The intervention of different treatments on potential variables was evaluated at 0th day and 90th days.
Study population
Research data was collected through maintained web-based system that includes information of all patients who have had breast cancer. The database provides detailed information on breast tumor type and date of breast cancer occurrence.
Ethical approval and consent to participate
The ethical approval was obtained by Bio-safety and Ethical Review Committee (BERC), University of Sargodha, Sargodha-Pakistan. The written consent was signed from the patients and was informed about the study.
Selection procedure for volunteers
Females were randomly selected for the study with total number of 242 of which 162 volunteers signed the consent; they were further excluded due to not meeting with the selected criteria. Total volunteers selected were n = 150, of eight were dropped off, nine volunteers did not follow up and six volunteers did not meet the criteria. In order to maintain uniformity in data, took 40 volunteers in each group, hence 120 volunteers were included in the study.
Target population and sampling technique
Diagnosed breast cancer female and healthy female were the targeted populace. The selection of the human subjects was completed by using technique of the two-stage sampling which is convenience and purposive sampling technique.[Citation17]
Inclusion exclusion criteria
Clinically diagnosed breast cancer female patients of age between 35–70 years were included in the study. Age group of patient was the inclusion criterion.[Citation18] Volunteers with a history of any other cancer type and age above 70 years were excluded from the study.
Biochemical assays
Serum biochemistry of case study volunteers was assessed to draw their blood samples. The female patients were prescribed to the pathology lab of different hospitals. The blood sample was drawn for different biochemical parameters of volunteers.[Citation19]
Nutritional assessment of study subjects
The complete nutritional assessment of patients was assessed by taking anthropometrical data such as height, weight, mid upper arm circumference, body mass index waist circumference and waist to hip ratio. Bio electric impedance analysis (BIA) was used to assess body composition such as body fat mass, total body water, proteins for building muscles and minerals for strengthening bones. Height and weight measurements of study participants were taken to calculate body mass index (BMI).[Citation20] Weight of breast cancer patients was measured by standard balance (kg). Height was measured using height measuring device attached to the balance.[Citation21] Mid upper arm circumference was measured by standard technique using a non-stretchable tape and estimated to the nearest 0.1 cm. Body mass index was estimated using following formula
The WHO cut off ranges of body mass index are underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, overweight 25–29.9 kg/m2 and obese 30–40 kg/m2.
A standardized nutritional assessment questionnaire was used to obtain data, including age, name, gender and menopausal status, age of menarche, breastfeeding status and parity. Hand grip strength (HGS) in kg was carried out by sitting position with the arms on a table and the average of three measurements was estimated. Hand grip strength was measured by hand held dynamometer. Subjective global assessment (SGA) was used to check the nutritional status of patients and classified as well nourished, severely malnourished and moderately malnourished.[Citation22,Citation23]
Statistical analysis
We examined potential variables categorized in Minitab (version 19) and statistical package for the social sciences (SPSS version16). Interventional parameters including blood biomarkers were expressed in means ± standard error of means (mean ±SEM). Between-groups comparisons were performed using general linear model (GLM) and effect of days on indicative parameters were subjected to two tailed analysis of variance (ANOVA) with post hoc Tukey test. P value < 0.05 was considered statistically significant for all statistical results.[Citation24]
Results
depicted the median, minimum, maximum and mean with SD of serum concentrations of vitamin D among cases and controls. The median concentrations of vitamin D in breast cancer patients (21 ng/ml) were lower than controls (46 ng/ml). Vitamin D deficiency was more prevalent in breast cancer female patients. The cut off value for vitamin D deficiency was <20 ng/ml, insufficient (20–30 ng/ml) and sufficient (>30 ng/ml). The study analyzed median, minimum and maximum with SD of serum calcium levels among cases and controls. The median concentrations of calcium in breast cancer patients were 8.6 mg/dl as compared to the controls with 8.8 mg/dl levels. The standard range of serum calcium levels was 8.5–10.5 mg/dl.
Table 1. Serum concentration of Vitamin D and Calcium among cases and control groups.
presented the characteristics of control group and breast cancer patients including ethnicity, demographics and socioeconomic status compared with chi square tests. Punjabi was 49.7% in control group and 50.3% in case group. The percentages of post-menopausal breast cancer patients were 32.1% as compared to the healthy individuals (15.8%). The participants in breast cancer patients had lower middle income 22.9% as compared to 17.9% in control group. The analysis shows that percentage for age at menarche was 35% in case group who falls in 14–16 years of age. The breast-feeding percentage was only 28.3% among breast cancer patients as compared to 40% frequency of breast feeding in healthy individuals.
Table 2. Characteristics of study participants.
Analysis of variance of weight depicted that effect of treatment group fed with T0, the weight increases from 76.45 ± 1.62 to 76.53 ± 1.70 kg, followed by T1 group weight varied from 75.54 ± 1.14 to 75.46 ± 1.28 kg, T2 treatment fed group, weight showed changed from 74.56 ± 1.49 to 74.23 ± 1.33 kg as presented in .
Table 3. Nutritional parameters among different treatments groups at 0 days and 90 days of interventional period.
It was observed that in T0 fed group, body mass index was found to be increased from 25.26 ± 0.35 to 26.52 ± 0.40 kg/m2. In T2 fed group, body mass index was found to be slightly declined from 26.48 ± 0.28 to 26.15 ± 0.20 kg/m2 as represented in . In group fed with T0 treatment, waist circumference varied from 32.83 ± 0.23 to 32.75 ± 0.34 inches, in group fed with T1 treatment, waist circumference changed from 33.15 ± 0.26 to 33.30 ± 0.27 inches, while in T2 group the waist circumference non-significantly changed from 34.25 ± 0.34 to 34.22 ± 0.31 inches as shown in . Analysis of variance of mid upper arm circumference of treatments fed groups, treatment days and interactive effect of treatment fed groups and treatment days showed non-significant results as depicted in . Group participants fed with T1 treatment showed non-significantly changed from 27.55 ± 0.38 to 27.64 ± 0.36 cm. In T2 fed group treatment, mid upper arm circumference showed non-significant change from 26.76 ± 0.30 to 26.75 ± 0.31 cm. It was observed from analysis of variance that effect of treatment fed groups, treatments days and interactive effect of treatment fed groups and days was found to be non-significant on waist hip ratio. Group fed with T2 treatment showed non-significant changes from 0.81 ± 0.002 to 0.81 ± 0.002 cm as depicted in .
Figure 1. (a, b and c) Effect of treatments on mean values of Anthropometrics (BMI, Waist Circumference and Hand grip strength).
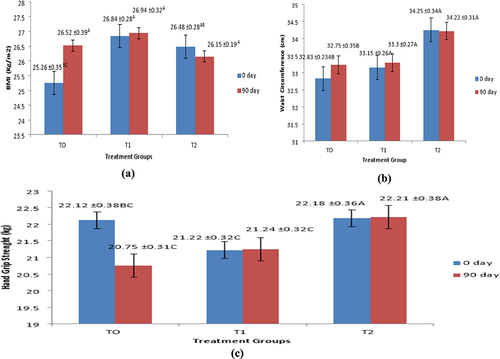
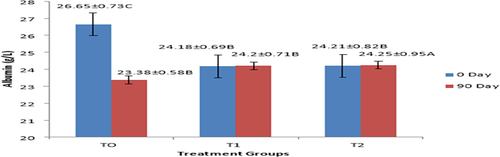
Effect of interactive action between treatment fed groups and treatment days on hand grip strength was found to be significant (p < .05). In group fed with T0 treatment, hand grip strength significantly varied from 22.12 ± 0.38 to 20.75 ± 0.31 kg. In group fed with T1, hand grip strength was slightly varied from 21.22 ± 0.31 to 21.24 ± 0.31, whereas in group which given with T2, hand grip strength slightly improved from 22.18 ± 0.36 to 22.21 ± 0.38 kg as shown in . In group fed with T0 treatment, serum albumin levels were significantly reduced from 26.65 ± 0.73 to 23.38 ± 0.58 g/L. In group fed with T1 (Synthetic vitamin D and calcium supplement + chemotherapy), serum albumin levels were varied non-significantly varied from 24.18 ± 0.69 to 24.20 ± 0.71 g/L. In T2-group serum albumin was found to be slightly increased from 24.21 ± 0.82 to 24.27 ± 0.95 g/L as presented in and .
Impact of intervention intervals and interaction effect of group fed with T0 treatment the serum calcium levels of the breast cancer participants were declined from 8.4 ± 0.08 to 8.3 ± 0.09 mg/dl. In group treated with T1 treatment, the serum calcium was found to be slightly increased from 8.46 ± 0.06 to 8.53 ± 0.07 mg/dl. Treatment group intervenes with T2 treatment showed significant improvement in serum calcium levels after endpoint, the values were noticed to be increased from 8.45 ± 0.08 to 8.61 ± 0.11 mg/dl as presented in .
Figure 3. (a and b) Effect of treatments on mean values of serum calcium and Serum vitamin D concentrations..
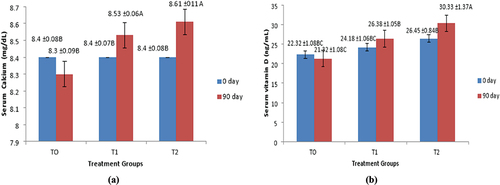
In group fed with T0 treatment, the serum vitamin D levels in patients was significantly reduced from 22.32 ± 1.08 to 21.32 ± 1.08 ng/ml. In group fed with T1 treatment, the serum vitamin D levels in patients were significantly improved from 24.18 ± 1.06 to 26.38 ± 1.05 ng/ml. Serum vitamin D levels were found to be significantly raised from 26.45 ± 0.8 to 30.33 ± 1.37 ng/ml in T2 participants as shown in .
) Effect of treatments on mean values of Anthropometrics (BMI, Waist Circumference and Hand grip strength)
Correlation among anthropometrics parameters with biomarkers at 90th day
After completion of intervention duration, a correlation was analyzed among anthropometrics parameters (body mass index, waist circumference, mid upper circumference and waist hip ratio) with serum calcium, vitamin D and parathyroid hormone among volunteers. It was revealed from Pearson’s correlation that body mass index was negatively correlated with serum calcium levels, serum vitamin D and positive with parathyroid hormone. Mid upper arm circumference was positive correlated with vitamin D, serum calcium and parathyroid hormone. Negative non-significant correlation was observed between waist circumference and serum calcium levels. Negative non-significance of correlation was observed between waist circumference and serum vitamin D levels. Waist hip ratio showed negative non-significant correlation with serum calcium, vitamin D and parathyroid hormone levels at 90 days as depicted in .
Table 4. Pearson’s correlation among anthropometrics with indicative biomarkers 90th day.
Correlation among breast cancer indicative indices and tumor markers at 90th day
After completion of intervention duration, a correlation was found among breast cancer indicative indices and tumor markers in breast cancer volunteer at endline of 90 days. The correlation between serum calcium levels and CA125 and c-reactive proteins was found to be weak and not statistically significant. With CEA and serum calcium status, a favorable non-significant association was discovered. Serum vitamin D levels were found with week positive correlation with tumor marker of CA125. Serum vitamin D represented the negative non-significant correlation with levels tumor marker CEA. Parathyroid hormone was positive correlated with tumor markers. In present findings of correlation of parathyroid hormone with CA125 marker, a week positive but non-significant correlation was present as shown in .
Table 5. Pearson’s correlation among indicative biomarkers with tumor markers 90th day.
Discussion
Above results were in accordance with the study of Casirati et al., [Citation25] which concluded that a nutrition intervention of oral nutritional supplement and eicosatetraenoic acid resulted in weight gain. Further studies concluded that increase in body mass index after nutrition interventions and two finding a reduction in BMI decline after the nutrition intervention.[Citation18,Citation26] Present findings were consistent with the study presented by Arensberg et al.[Citation27] who observed that weight of breast cancer patients was found significantly increased from baseline after chemotherapy from 72.1 ± 0.4 kg to 73.3 ± 0.4 kg. Change in patient’s body weight was observed to be slightly decreased from 73.0 ± 0.4 kg after 6 months of chemotherapy. Recent results are also according to the prospective study conducted in Denmark estimated that females diagnosed with breast cancer with BMI ≥30 kg/m2 had more aggressive disease at diagnosis as with females having with a BMI <25 kg/m2. Furthermore, it was observed that patients with a body mass index of 30 kg/m2 showed significant and more aggressive metastases after 10 years which was increased by 46% and the chance of breast cancer mortality after 30 years increased to 38%. A study observed endocrine therapy and chemotherapy was found to be less effective after 10 or more years for patients with body mass index greater than 30 kg/m2. Therefore, patients should be educated about importance of nutrition intervention and the lifestyle modifications during disease management.[Citation19,Citation28]
Recent findings were in accordance with the study of Lin et al.[Citation8] who observed that breast cancer patients undergoing adjuvant endocrine treatment had reduced hand grip strength. Reduced hand grip might be due to side effects of aromatase inhibitors (AI) and tamoxifen. Another similar finding by Bray et al.[Citation29] who concluded that chemotherapy and radiation such as the taxanes, platinum agents and vinca alkaloids. It may decrease the strength by effecting peripheral and muscles nerve tissue. Above results were in collaborative with Wang et al.[Citation22] who observed association between body mass index and aromatase inhibitors (AI) induced loss of hand grip strength. Present results are inaccordance with the study by Ruan et al.[Citation30] who reported that high body mass index may cause aromatase inhibitors-induced loss of hand grip strength. Another similar trend was observed by Aleixo et al.[Citation31] who concluded that older women with vitamin D deficiency, vitamin D supplementation with dose of 1000 IU per day for a year resulted in a significant improvement in hip muscular strength. A meta-analysis study by Rosendahl-Riise,[Citation32] observed no improvements in muscular strength in community dwelling people above 65 years after vitamin D and calcium supplementation. Serum albumin status is considered as a window into the patient’s nutritional health status but is potential factor for predicting health prognosis of a patient. When compared to patients with low serum vitamin D status, patients with sufficient serum vitamin D levels had considerably higher serum albumin concentrations. As a result, there is a strong positive association between serum albumin levels and serum vitamin D3 status. The current findings were consistent with a study presented by Rosendahl,Riise et al.,[Citation33] who found that hypoalbuminemia could be a risk factor for chemotherapy early termination even if blood albumin levels are a prognostic predictor of prognosis in breast cancer patients. It has stated that serum albumin is known as independent predictor for survival in cancer patients. Inflammation and poor nutrition status may suppress albumin levels. Serum albumin levels serve as a window into patient’s nutritional status but also an important factor for disease prognosis. It has been observed that deficiency of vitamin D is an important contributor factor for poor albumin levels; supplementation with vitamin D and calcium for may improve serum albumin levels with low vitamin D status group.[Citation8]
The current findings are consistent with those reported by Trujillo et al.[Citation34] during neoadjuvant chemotherapy, who noted a significant decrease in serum calcium and vitamin D levels (16 nmol/L). According to a case-control study by Williams et al.[Citation35] volunteers with higher quintile serum vitamin D levels than those with lower quintiles had a less chance of developing breast cancer. A serum vitamin D level of 47 ng/mL was connected to a 50% decreased risk of breast cancer, according to earlier researchers. Similar inverse association was found by Karthikayan et al.[Citation36] in their comprehensive analysis, which revealed that daily 400 IU of vitamin D supplementation, maximum sun exposure and dietary vitamin D intake all reduced the risk of breast cancer and its recurrence in patients. The study’s findings made clear how important it is to take calcium and vitamin D together. According to analysis and findings from the current study, calcium and vitamin D deficiency in breast cancer patients may combat using natural sources of vitamin D and calcium. An evidence based meta-analysis review observed the potential impact of nutritional counseling, oral supplements in cancer patients who underwent adjuvant, neoadjuvant therapy. The authors observed the improved clinical signs of oral intake, appetite, emotional status and overall health of the patients.[Citation37]
A randomized controlled trial observed that intervention of early tailored nutrition counseling during radiotherapy in colorectal cancer improved survival rate and better prognosis in patients after a follow-up period of 6.5 years. Another similar trend was observed in head and neck cancer patients who underwent radiotherapy resulted in better weight maintenance, increased tolerance and protein-calorie intake. A cohort study in colorectal cancer patients performed, and observed long survival rate in 315 patients received dietary counseling and oral nutrition supplements than those 313 patients who had not received nutrition counseling during chemotherapy.[Citation5,Citation38] A clinical data showed that breast cancer female patients who intervened with nutritional guidance had more consumption of vegetables and fruits, low intake of red meat and processed meat but no significant variation anthropometrics parameters were observed. A data observed that breast cancer patients especially older women, there is increased risk of cardiovascular risk who had on adjuvant chemotherapy because of the possible relation between chemotherapy and long-term cardio toxicity. Therefore, a healthy dietary habit and nutritional supplements are important to combat such co-morbidities.[Citation39]
A clinical trial has performed on calcium plus mega dose of vitamin D on cancer onset. In randomized control study, post-menopausal women were given calcium alone (1,400–1,500 mg/day) or in combination with vitamin D3 1,100 IU per day in a 1:2:2 ratio that resulted in fewer total cancer rate in the vitamin D plus calcium supplement as compared with no supplementation group.[Citation40] Studies represented that dose of vitamin D3 (2000 IU/day) was more effective as compared to vitamin D3 intake of 800 IU per day.[Citation41] In randomized controlled trial, the researcher had administered moderate dose of supplementation to achieve desired levels of 25-hydroxyvitamin D levels in both pre and postmenopausal women. The researcher assessed the blood 25-hydroxyvitamin D on biannual basis and also check long-term changes. Researcher concluded that long-term dosage should be adjusted to optimize vitamin D levels.[Citation42]
Research data from case control studies proved that vitamin D supplements have the potential benefit in breast cancer incidence. Consistent results showed that sufficient vitamin D levels are positive linked with better quality of life. In retrospective review of study explored that vitamin D supplements in patients with HER2+ treated with trastuzumab during chemotherapy showed significant disease-free survival rate as compared to no supplementation group. In that particular study mean dose of vitamin D taken by patients was below < 1500/day and 10472IU/week. Results showed better survival rate and better prognosis of the disease.[Citation43]
Efficacy studies observed that tailored intervention of vitamin D supplements regimen compared with a conventional regimen for sufficient vitamin D status in 195 breast cancer patients, who were undergoing neo-adjuvant and adjuvant chemotherapy, the studies concluded that 50, 000 IU/week of vitamin D3 supplementation can reduced the risk musculoskeletal pain.[Citation44] A study suggested that patient receiving the treatment of breast cancer with aromatase inhibitors should consumes daily vitamin D intake between 1000 and 5000 IU to combat the vitamin D deficiency. In a randomized case control study, the efficacy role of high vitamin D supplementation intake remained inconclusive.[Citation12]
In previous randomized control trial and meta-analysis studies have concluded that vitamin D as a potential protective tool. It has been studied that administrating vitamin D supplementation alone for a long period of time had no effect on cancer incidence and mortality rate.[Citation45] Another scientist conducted randomized clinical trial for 5 years, hence proved that administration of vitamin D supplementation for long time have proved to be beneficial for cancer prevention but not for cancer mortality rates. In previous study of cochrane review examined that there could be decreased rate of cancer related mortality in vitamin D given case groups as compared to those who never had it.[Citation46,Citation47]
Interventional study data among old aged individuals have shown that adequate oral intake of vitamin D may help in improving bone mass density, lower risk of osteoporotic fractures and decreased bone loss.[Citation27] Study also observed that calcium supplements along with vitamin D intake are more effective in increasing bone mass density and minimum fracture risk as compared to the participants who were taking vitamin D alone.[Citation7]
Results showed that those who received vitamin D supplements had significantly improved median disease-free survival than those without supplementation after follow-up of 29.5 months. After follow-up of 40.2 months, the intervention of 10,472 IU/week, or < 1500 IU/day resulted in 43.8% survival rate as compared to 32.8% those who had no supplementation.[Citation48] The present results are coherent with study of Arnold,[Citation3] who concluded that a good quality of diet is linked with less chances of chronic inflammation in body and better survival rate. Meta-analysis research on 10 randomized controls by Rinninell et al.[Citation37] studied that vitamin D supplementation helps in lowering inflammation markers in body by 1.08 mg/L.
It has been illustrated from a khan[Citation49] that body mass index was non significantly correlated with serum vitamin D levels and serum calcium levels. Parathyroid hormone has the tumor progression property. It is an important marker to assess in cancer patients. Present results are in accordance with the study of Corre et al.[Citation50] concluded that parathyroid hormone (PTH) is not significantly correlated with anthropometrical data in cancer patients. Anthropometrical data was found to be non-significantly negative correlated with body mass index, waist hip ratio measures in female patients.
Present correlation is in accordance with study by Lin et al.[Citation8] who observed negative relationship between serum vitamin D levels and body weight over a wide range of body mass index. A similar case study reported by Nagarajan,[Citation6] concluded that high body mass index (>30 kg/m2) was positive correlated with high parathyroid hormone levels in elderly adults as reported in present study. A study performed by Cederholm et al.[Citation51] who concluded that vitamin D supplementation was found to be significantly reduction in inflammation marker of c-reactive proteins. Another study conducted by Lotito[Citation19] observed positive association of serum parathyroid hormone with female carcinoma makers. It has been noted that higher the serum parathyroid hormone (PTH) levels in body, the more chances of developing cancer and tumor promoting properties.
Conclusion
It is concluded that low or poor levels of serum vitamin D and calcium were associated with high risk of breast cancer in females. Early intervention of vitamin D and calcium in a food product may help to raise serum calcium and vitamin D levels. Vitamin D and calcium supplementation can be optimized trough natural sources such as fish oils, cod liver oil and egg shell powder. Effective nutritional screening and oral supplementation are important tools during cancer treatments. Nutritional intervention using oral supplementation might be incorporated in cancer therapeutically treatment to improve both clinical outcomes as well as maintain quality of life. Natural source of vitamin D and calcium supplemented added in food product may be used as vehicle to combat deficiencies in better ways. In clinical oncology practice, vitamin D and calcium supplementation intervention in breast cancer patients can be considered as an integral part of the multimodal therapeutic approach. The impact of improved serum vitamin D and calcium levels on patients survival and quality of life needs to be further investigated. Further research of natural supplementation and synthetic interventions in large clinical trials is required for better efficacy studies to improve long-term survival and better quality of life in breast cancer patients. Vitamin D and calcium supplements may help to combat chemotherapy-induced toxicities and improve cancer treatment outcomes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Nguyen, L.-T.; Dang, A.-K.; Duong, P.-T.; Phan, H.-B.-T.; Pham, C.-T.-T.; Le Nguyen, A.-T.; Le, H.-T. Nutrition Intervention is Beneficial to the Quality of Life of Patients with Gastrointestinal Cancer Undergoing Chemotherapy in Vietnam. Can. Med. 2021, 10, 1668–1680. DOI: 10.1002/cam4.3766.
- Jacobson, A. Trastuzumab Deruxtecan Improves Progression Free Survival and Intracranial Response in Patients with HER2-Positive Metastatic Breast Cancer and Brain Metastases. Oncologist. 2022, 27, S3–S4. DOI: 10.1093/oncolo/oyac009.
- Jiang, Y.-Z.; Liu, Y.; Xiao, Y.; Hu, X.; Jiang, L.; Zuo, W. J.; Ma, D.; Ding, J.; Zhu, X.; Zou, J., et al. Molecular Subtyping and Genomic Profiling Expand Precision Medicine in Refractory Metastatic Triple-Negative Breast Cancer: The FUTURE Trial. Cell.Res. 2021, 31(2), 178–186.
- Yan, M.; Ouyan, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z.; Orlandi, A., et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022, 23(3), 353–361.
- Blackwood, H.-A.; Hall, C.-C.; Balstad, T.-R.; Solheim, T. S.; Fallon, M.; Haraldsdottir, E.; Laird, B.-J. A Systematic Review Examining Nutrition Support Interventions in Patients with Incurable Cancer. Support Care Cancer. 2020, 28(4), 1877–1889. DOI: 10.1007/s00520-019-04999-4.
- Nagarajan, D.; McArdle, S. Immune landscape of breast cancers. Biomed. 2018, 6(20), 230–233. DOI: 10.3390/biomedicines6010020.
- Floris, G.; Richard, F.; Hamy, A.-S.; Jongen, L.; Wildiers, H.; Ardui, J.; Punie, K.; Smeets, A.; Berteloot, P.; Vergote, I., et al. Body Mass Index and Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. J. Nat. Can. Inst. 2021, 113(2), 146–153.
- Lin, T.; Yang, J.; Hong, X.; Yang, Z.; Ge, T.; Wang, M. Nutritional Status in Patients with Advanced Lung Cancer Undergoing Chemotherapy: A Prospective Observational Study. Nutr. Cancer. 2020, 72, 1225–1230. DOI: 10.1080/01635581.2019.1675720.
- Luciano-Mateo, F.; Hernandez-Aguilera, A.; Cabre, N.; Camps, J.; Fernandez-Arroyo, S.; Lopez-Miranda, J.; Menendez, J.; Joven, J. Nutrients in energy and one-carbon metabolism: Learning from metformin users. Nutrients. 2019, 9(2), 121. DOI: 10.3390/nu9020121.
- Caccialanza, R.; Goldwasser, F.; Marschal, O.; Ottery, F.; Schiefke, I.; Tilleul, P.; Zalcman, G.; Pedrazzoli, P. Unmet Needs in Clinical Nutrition in Oncology: A Multinational Analysis of Real-World Evidence. Ther. Adv. Med. Oncol. 2020, 12, 775–852. DOI: 10.1177/1758835919899852.
- Murphy, J.-L.; Munir, F.; Davey, F.; Miller, L.; Cutress, R.; White, R.; Lloyd, M.; Roe, J.; Granger, C.; Burden, S., et al. The Provision of Nutritional Advice and Care for Cancer Patients: A UK National Survey of Healthcare Professionals. Support. Care Canc. 2021, 29, 2435–2442. DOI: 10.1007/s00520-020-05736-y.
- François, C.; Anne, C.; Thomas, B.; Jean, Y.; Jean, L.; Florian, C.; Andre, F.; De La Motte Rouge, T.; Pistilli, B.; Dalenc, F., et al. Abstract GS3-05: Fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial. Can. Res. 2022, 82(4), SGS3–05.
- Baumstarck, K.; Boyer, L.; Pauly, V.; Orleans, V.; Marin, A.; Fond, G.; Morin, L.; Auquier, P.; Salas, S. Use of Artificial Nutrition near the End of Life: Results from a French National Population-Based Study of Hospitalized Cancer Patients. Can. Med. 2020, 9(2), 530–540. DOI: 10.1002/cam4.2731.
- Albanesi, B.; Piredda, M.; Marchetti, A.; Mastroianni, C.; Magnani, C.; Artico, M.; D’Angelo, D.; Lusignani, M.; Ianni, A.; De Marinis, M.-G. Oncology and Palliative Care Nurses’ Knowledge and Attitudes Toward Artificial Nutrition and Hydration for Patients at End of Life in Italy: A Cross-Sectional Survey. Can. Nurs. 2021, 44, 99–107. DOI: 10.1097/NCC.0000000000000803.
- Paulsen, M.-M.; Paur, I.; Gjestland, J.; Henriksen, C.; Varsi, C.; Tangvik, R. J.; Andersen, L.-F. Effects of Using the MyFood Decision Support System on Hospitalized Patients’ Nutritional Status and Treatment: A Randomized Controlled Trial. Clin. Nutr. 2020, 39(12), 3607–3617. DOI: 10.1016/j.clnu.2020.03.012.
- Berardi, G.; Antonelli, G.; Colasanti, M.; Meniconi, R.; Guglielmo, N.; Laurenzi, A.; Ferretti, S.; Levi Sandri, G.-B.; Spagnoli, A.; Moschetta, G., et al. Association of Sarcopenia and Body Composition with Short-Term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg. JAMA. Surgery. 2020, 155(11), 203–336.
- Guzman-Prado, Y.; Ben Shimol, J.; Samson, O. Body Mass Index and Immune-Related Adverse Events in Patients on Immune Checkpoint Inhibitor Therapies: A Systematic Review and Meta-Analysis. Can. Immuno. Immunother. 2021, 70, 89–100. DOI: 10.1007/s00262-020-02663-z.
- Meng, L.; Wei, J.; Ji, R.; Wang, B.; Xu, X.; Xin, Y.; Jiang, X. Effect of Early Nutrition Intervention on Advanced Nasopharyngeal Carcinoma Patients Receiving Chemoradiotherapy. J. Can. 2019, 10, 3650–3656. DOI: 10.7150/jca.33475.
- Kim, S.-H.; Lee, S.-M.; Jeung, H.-C.; Lee, I.-J.; Park, J.-S.; Song, M.; Lee, D.-K.; Lee, S.-M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients. 2019, 11, 1145. DOI: 10.3390/nu11051145.
- Renehan, A.-G.; Harvie, M.; Cutress, R.-I.; Leitzmann, M.; Pischon, T.; Howell, S.; Howell, A. How to manage the obese patient with cancer. J. Clin. Onco. 2016, 34(23), 4284–4294. DOI: 10.1200/JCO.2016.69.1899.
- Van den Berg, M.-M.-G.-A.; Kok, D.-E.; Posthuma, L.; Kamps, L.; Kelfkens, C.-S.; Buist, N.; Geenen, M.; Haringhuizen, A.; Heijns, J. B.; van Lieshout, R.-H.-M.-A., et al. Body composition is associated with risk of toxicity-induced modifications of treatment in women with stage IIIIB breast cancer receiving chemotherapy. Br. Can. Res. Treat. 2019, 23(4), 23–26.
- Wang, J.; Wang, X.; Gu, Y.; Liu, M.; Chi, V.-T.-Q.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H., et al. Vitamin D is related to handgrip strength in adult men aged 50 years and over: A population study from the TCLSIH cohort study. Clin. Endocri. 2019, 90, 753–765. DOI: 10.1111/cen.13952.
- Emens, L.-A.; Adams, S.; Barrios, C.-H.; Dieras, V.; Iwata, H.; Loi, S.; Rugo, H. S.; Schneeweiss, A.; Winer, E. P.; Patel, S., et al. First-Line Atezolizumab Plus Nab-Paclitaxel for Unresectable, Locally Advanced, or Metastatic Triple-Negative Breast Cancer: IMpassion130 Final Overall Survival Analysis. Ann. Oncol. 2021, 32(8), 983–993.
- Hipsey, M.-R.; Bruce, L.-C.; Boon, C.; Busch, B.; Carey, C.-C.; Hamilton, D.-P.; Winslow, L. A.; Read, J. S.; de Sousa, E.; Weber, M., et al. A General Lake Model (GLM 3.0) for linking with high-frequency sensor data from the Global Lake Ecological Observatory Network (GLEON). Geosci. Model Dev. 2019, 12(1), 473–523. DOI: 10.5194/gmd-12-473-2019.
- Casirati, A.; Vandoni, G.; Della Valle, S.; Greco, G.; Platania, M.; Colatruglio, S.; Lalli, L.; Gavazzi, C. Nutritional Status and Body Composition Assessment in Patients with a New Diagnosis of Advanced Solid Tumour: Exploratory Comparison of Computed Tomography and Bioelectrical Impedance Analysis. Clin. Nutr. 2021, 40(3), 1268–1273. DOI: 10.1016/j.clnu.2020.08.009.
- Álvaro Sanz, E.; Abilés, J.; Garrido Siles, M.; Pérez Ruíz, E.; Alcaide García, J.; Rueda Domínguez, A. Impact ofWeight Loss on Cancer Patients’ Quality of Life at the Beginning of the Chemotherapy. Supp Care Can. 2021, 29, 627–634. DOI: 10.1007/s00520-020-05496-9.
- Arensberg, M.; Richards, J.; Benjamin, J.; Kerr, K.; Hegazi, R. Opportunities for Quality Improvement Programs (QIPs) in the Nutrition Support of Patients with Cancer. Healthcare. 2020, 8(3), 227–228. DOI: 10.3390/healthcare8030227.
- Socha, M.; Sobiech, K.-A. Socio-demographic and general health factors associated with quality of life in long-term breast cancer survivors from southwestern Poland. Int. J. Envir. Res. Pub. Health. 2021, 18, 9321. DOI: 10.3390/ijerph18179321.
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021, 127, 3029–3030. In press DOI: 10.1002/cncr.33587.
- Ruan, X.; Nakyeyune, R.; Shao, Y.; Shen, Y.; Niu, C.; Zang, Z.; Miles, T.; Liu, F. Nutritional Screening Tools for Adult Cancer Patients: A Hierarchical Bayesian Latent-Class Meta-Analysis. Clin. Nutr. 2020, 261-5614(20)30505-7.
- Aleixo, G.-F.-P.; Williams, G.-R.; Nyrop, K.-A.; Muss, H. B.; Shachar, S. S. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. DOI: 10.1007/s10549-019-05352-3.
- Rosendahl Riise, H.; Spielau, U.; Ranhoff, A.-H.; Gudbrandsen, O.-A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30(33), 3–15. DOI: 10.1111/jhn.12394.
- Ryan, A. M.; Sullivan, E. S. Impact of Musculoskeletal Degradation on Cancer Outcomes and Strategies for Management in Clinical Practice. Proc. Nutr. Soc. 2021, 80, 73–91. DOI: 10.1017/S0029665120007855.
- Trujillo, E.-B.-C.-K.; Dixon, S.-W.; Hill, E.-B.; Lipinski, A.; Braun, E.; Platek, M.-E.; Vergo, M.-T.; Spees, C.; Spees, C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Onco. 2019, 19, 7462–7940. DOI: 10.1155/2019/7462940.
- Williams, D.-G.-A.; Ohnuma, T.; Krishnamoorthy, V.; Raghunathan, K.; Sulo, S.; Cassady, B.-A.; Hegazi, R.; Wischmeyer, P.-E. Postoperative Utilization of Oral Nutritional Supplements in Surgical Patients in US Hospitals. J. Paren and Enter Nutr. 2020, 33, 93–99.
- Karthikayan, A.; Sureshkumar, S.; Kadambari, D.; Vijayakumar, C. Low serum 25-hydroxy vitamin D levels are associated with aggressive breast cancer variants and poor prognostic factors in patients with breast carcinoma. Arch. Endocri. Metabol. 2018, 62(4), 452–459. DOI: 10.20945/2359-3997000000062.
- Rinninella, E.; Fagotti, A.; Cintoni, M.; Raoul, P.; Scaletta, G.; Quagliozzi, L.; Miggiano, G.-A.-D.; Scambia, G.; Gasbarrini, A.; Mele, M.-C. Nutritional Interventions to Improve Clinical Outcomes in Ovarian Cancer: A Systematic Review of Randomized Controlled Trials. Nutrients. 2019, 11, 1404–1407. DOI: 10.3390/nu11061404.
- Jones, R.-H.; Carucci, M.; Casbard, A. C.; Butler, R.; Alchami, F.; Bale, C. J.; Bezecny, P.; Joffe, J.; Moon, S.; Twelves, C., et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER- positive breast cancer (FAKTION): a randomized, double- blind, placebo- controlled, phase II trial. J. Clin. Oncol. 2019, 37(15), 1005–1005.
- Theilla, M.; Cohen, J.; Kagan, I.; Attal-Singer, J.; Lev, S.; Singer, P. Home parenteral nutrition for advanced cancer patients: Contributes to survival?. Nutr. 2018, 54, 197–200. DOI: 10.1016/j.nut.2017.03.005.
- Lope, V.; Castello, A.; Mena-Bravo, A.; Amiano, P.; Aragones, N.; Fernandez-Villa, T.; Guevara, M.; Dierssen-Sotos, T.; Fernandez-Tardón, G.; Castaño-Vinyals, G., et al. Serum 25-hydroxyvitamin D and breast cancer risk by pathological subtype (MCC-Spain). J. Ster. Biochem. Mol. Bio. 2018, 34(2), 23–32.
- Mousavi, S.-E.; Amini, H.; Heydarpour, P.; Amini Chermahini, F.; Godderis, L. Air pollution, environmenta chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Inter. 2019, 122(44), 67–90. DOI: 10.1016/j.envint.2018.11.052.
- Del Pup, L.; Codacci-Pisanelli, G.; Peccatori, F. Breast cancer risk of hormonal contraception: counseling considering new evidence. Crit Review. Onco. Hematol. 2019, 137, 123–130. DOI: 10.1016/j.critrevonc.2019.03.001.
- Alkan, A.; Koksoy, E.-B. Vitamin D deficiency in cancer patients and predictors for screening (D-ONC study). Curr Prob Can. 2019, 43(5), 421–428. DOI: 10.1016/j.currproblcancer.2018.12.008.
- Ahmed-Mohamad, E.; Falasiri, S.; Hajiran, A.; Chahoud, J.; Spiess, P.-E. The Immune Microenvironment in Penile Cancer and Rationale for Immunotherapy. J. Clin. Med. 2020, 9(10), 3334–3340. DOI: 10.3390/jcm9103334.
- Goulão, B.; Stewart, F.; Ford, J.-A.; MacLennan, G.; Avenell, A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 25(4), 652–663. DOI: 10.1093/ajcn/nqx047.
- Sofi, N.-Y.; Jain, M.; Kapil, U.; Seenu, V.-R.-L.; Yadav, C.-P.; Yadav, C. P.; Pandey, R. M.; Sareen, N. Reproductive factors, nutritional status and serum 25(OH)D levels in women with breast cancer: A case control study. J. Ster. Biochem. Molicu. Bio. 2018, 175(23), 200–204. DOI: 10.1016/j.jsbmb.2017.11.003.
- Keum, N.; Lee, D.-H.; Greenwood, D.-C.; Manson, J. E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Annals Oncol. 2019, 30(5), 733–743.
- Sadeghian, M.; Rahmani, S.; Khalesi, S.; Hejazi, E. A Review of Fasting Effects on the Response of Cancer to Chemotherapy. Clin. Nutr. 2020, 261-5614(20), 30580.
- Hurvitz, S.-A.; Saura, C.; Oliveira, M.; Trudeau, M.-E.; Moy, B.; Delaloge, S.; Gradishar, W.; Kim, S.-B.; Haley, B.; Ryvo, L., et al. Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial. Oncol. 2021, 26(8), e1327–e1338.
- Corre, R.; Greillier, L.; Le, C. H.; Audigier-Valette, C.; Baize, N.; Bérard, H.; Falchero, L.; Monnet, I.; Dansin, E.; Vergnenègre, A., et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non–Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J. Clin. Onco. study. 2019, 34(13), 1476–1483.
- Cederholm, T.; Jensen, G.-L.; Correia, M.-I.-T.-D.; Gonzalez, M.-C.; Fukushima, R.; Higashiguchi, H.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A., et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. ClinNutr. 2019, 38, 1–9. DOI: 10.1016/j.clnu.2019.02.033.