?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The intake of nitrite in sausages is a concern for health-conscious consumers. This study investigated the effects of heating method (smokehouse, water bath, or ohmic heating), temperature (40, 60, or 75°C), sodium nitrite level (80 or 250 ppm), and frozen storage period (≤3 days or 4 weeks) on the residual nitrite, pigments, and curing efficiency of chicken sausages. The results indicated that the electrical conductivities of samples were sufficiently high for ohmic heating. The extension of frozen storage duration to 4 weeks could substantially lessen the residual nitrites in all sausage samples compared to less than 3 days of storage, particularly for the raw sausage. The total pigment, nitrosylhemochrome, and curing efficiency values of the normal-sodium nitrite sausages were considerably higher than their counterparts, especially for the samples heated at high temperatures. Almost all attributes of the sausages were rather similar among the three heating techniques.
Introduction
Sausages are one of the most popular ready-to-eat meat products for consumers with a modern lifestyle. However, the production of sausage commonly uses sodium nitrite/nitrate as an inhibitor of heat-resistant spores, especially for the neurotoxin-producing Clostridium botulinum. Applications of sodium nitrite/nitrate together with sodium chloride and the heating process result in the inhibition of both spoilage and the growth of food pathogens,[Citation1] as well as enhancing the color, flavor, and aroma of sausages, while obstructing lipid oxidation.[Citation2] Although the addition of sodium nitrite/nitrate in the sausage formulation provides essential benefits, these are deemed undesirable preservatives that raise concerns by health-conscious consumers due to the formation of nitrosamines that are recognized as carcinogens.[Citation3,Citation4] Eichholzer and Gutzwiller[Citation5] claimed that the intake of sodium nitrite/nitrate in cured meat products was associated with the occurrence of carcinogenic and mutagenic nitroso compounds, particularly N-nitrosamines that are correlated to the development of some tumors, red blood cell obstruction, and the increased risk of leukemia in children.
Currently, there is no single perfect substitute for sodium nitrite/nitrate to be applied in sausages when considering cost, functionality, stability, and efficiency. A number of studies proposed alternative methods to replace or reduce the nitrite/nitrate quantity used in sausage production. Alirezalu et al.[Citation6] applied natural antimicrobial compounds, comprising nisin, ε-polylysine, and chitosan together with a mixed plant extract, to replace nitrite in a frankfurter-type sausage. They reported that the application of 1% chitosan and 0.2% ε-polylysine combined with the mixed plant extract was the most promising natural alternative to nitrite in frankfurter-type sausage production. Nonetheless, there is strong consumer demand for improved color stability of sausages during processing and refrigerated storage, especially for redness. Some studies proposed the replacement of chemical nitrites in the formulation of meat products with natural sources of nitrate, such as spinach, celery, parsley, leek, and beetroot.[Citation7–13] However, the application of chemical nitrites is apparently more effective and convenient than using these natural ingredients because of their concentration, stability, and effect on sensorial quality. Due to the fact that nitrite is still considered as the most efficient preservative for commercial sausage production, it is added in most sausage products sold in the marketplace, although a number of approaches for manufacturing nitrite-free sausage have been proposed. Therefore, a reduction of the applied nitrite amount to a low level, together with the application of some processing technique that can provide sausage products with satisfactory levels of food safety and comparable quality to the sausage using normal nitrite level, would be of great interest to lessen the health risk to sausage consumers.
Thermal treatments using smokehouse, steaming or boiling are generally applied to pasteurize sausages and extend the shelf life. The principle of these thermal treatments relies on conventional mechanisms, composed of heat conduction, convection, and radiation. Ohmic heating, an innovative thermal treatment, is not dependent on these heating mechanisms. In ohmic heating, the heat is directly generated inside the food material by passing an electrical current through the food. The amount of heat occurring inside the food depends on the values of electrical conductivity of the food and the electrical field strength.[Citation14] Ohmic heating is considered as a potential method to improve the quality of low-nitrite sausage to be similar to normal-nitrite-level sausage because during ohmic heating, ions such as nitrite usually move faster within the food material than in conventional heating methods as a result of the applied electrical field. The rapid movement of nitrite is expected to raise the reaction and effect of nitrite in a low-nitrite sausage and subsequently improve sausage quality. In addition, the formation of pores in cell membranes as a result of electroporation occurring during ohmic heating might help in facilitating the reaction between the nitrite and the constituents in meat batter. Cho et al.[Citation15] investigated samples treated using the ohmic method and concluded that the naturally porous cell walls permitted the cell membrane to accumulate charges, which initiated disruptive pores resulting in electroporation. In addition, the greater uniformity of heat distribution inside the sausage during ohmic heating compared to a conventional heating method might help to produce a high quality sausage. Engchuan et al.[Citation14] reported that the uniform temperature inside meat ball samples during ohmic heating was the main cause of the variations between the qualities of meat balls cooked by conventional and ohmic heating methods. Inmanee et al.[Citation16] applied ohmic heating in the post pasteurization of sausages after cooking and vacuum packaging and claimed that the consumers could not detect any sensorial quality differences between the sausages pasteurized using ohmic or conventional methods. They also pointed out that ohmic heating has potential to be used for the sausage pasteurization, especially from the aspects of food safety and process efficiency.
To date, there has been negligible study on the possibility of producing low-nitrite sausage with comparable product qualities to normal-nitrite level sausage by lessening the applied nitrite amount together with the application of an ohmic heating technique. Therefore, the current work added sodium nitrite to the sausage formulation at two concentration levels (80 or 250 ppm). The electrical conductivities of the meat batters, sodium chloride solution, and collagen casing were measured to determine the possibility of applying ohmic heating in sausage cooking. Different heating methods, heating temperatures, and storage times were investigated. Three different heating methods comprising smokehouse, water bath, and ohmic were applied because smokehouse is generally used in the commercial production while the ohmic method is an innovative technique that was interested in this study. The sausage heating by water bath was more similar to the ohmic heating than that of the smokehouse since the sausages were surrounded by the liquid instead of hot air and steam in case of smokehouse. Thus, the comparison between the ohmic and water bath heating techniques would clarify the effects of different heating mechanisms under the similar surrounding of sausage samples. The main objective was to investigate the effects of heating method, temperature, added sodium nitrite level, and storage time on the residual nitrite, total pigment, nitrosylhemochrome, and curing efficiency of chicken sausages.
Materials and methods
Sausage preparation
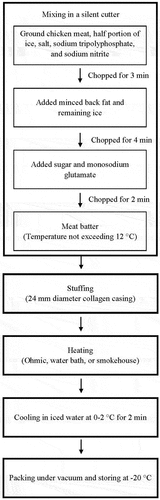
The chicken and pork back fat were purchased from a local supermarket (produced by the CP group, Bangkok, Thailand). The chicken and back fat were cut and minced through a grinder (Savioli 32 Classic; Mantova, Italy) using a 3 mm plate. The food grade chemical ingredients (sodium tripolyphosphate and sodium nitrite) were supplied by Vicchi Enterprise Co., Ltd. (Bangkok, Thailand), while the sugar and monosodium glutamate were bought from a local supermarket in Bangkok, Thailand. The sausages were prepared using two different sodium nitrite concentration levels (80 or 250 ppm). These concentrations were considered as the minimum and maximum levels that could be applied in sausages. According to the studies of Gunvig et al.[Citation17] and Lee et al.[Citation18] the added nitrite level required for controlling microbial activity especially Clostridium botulinum in meat products should not be lesser than 80 ppm. The maximum amount of residual nitrite containing in meat products specified by Thai FDA[Citation19] is 80 ppm. Therefore, the nitrite level at 250 ppm was applied as the maximum level since the preliminary test result indicated that after processing the nitrite content in sausage decreased from 250 ppm to around 80 ppm. The formulations for preparing the sausage emulsions are presented in . The ground chicken meat, half a portion of ice, salt, sodium tripolyphosphate, and sodium nitrite were mixed in a silent cutter (CM-14; Mainca; Barcelona, Spain) for 3 min. After that, the minced back fat and remaining ice were added to the mixture and mixed for 4 min prior to adding the sugar and monosodium glutamate and chopping for 2 min. The temperature of the meat batter was maintained below 12°C before stuffing into 24 mm diameter collagen casing (Fcase; Myślenice, Poland) using a hydraulic stuffer (FC-12; Mainca; Barcelona, Spain). The weight of each sausage piece was 45 ± 2 g. Then, the sausages were heated using the different methods before cooling in iced water at 0–2°C for 2 min, packing under vacuum in polyethylene bags (LLDPE/Nylon, size 127 × 229 mm) and storing in a chest freezer (Mirage FZ-380; Puramun Co., Ltd.; Bangkok, Thailand) at −20°C for ≤3 days or 4 weeks before quality determination. The sausage preparation procedure is summarized in .
Table 1. Formulation of sausages with 80 or 250 ppm of sodium nitrite.
Electrical conductivity measurement
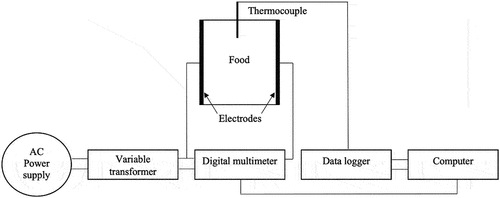
The electrical conductivities of the sausage emulsions, sodium chloride solution (concentration 0.75% w/w), and collagen casing were measured to evaluate the possibility of heating the sausages using an ohmic method. The schematic diagram of the ohmic heating system for measuring the electrical conductivity is shown in . The small ohmic cell used with the sausage emulsions and collagen casing was made of 15 mm inner diameter acrylic pipe, whereas the inner diameter of the glass tube was 45 mm for the larger ohmic cell used for the sodium chloride solution. The electrodes were made from stainless-steel grade 316 L and were installed on both sides of the ohmic cell. The distances between electrodes for the small and the larger ohmic cells were 18.5 mm and 47 mm, respectively. The samples were placed in the ohmic cell and manually pressed to avoid air space inside the cell. The collagen casing sample was soaked in the sodium chloride solution for 2 min before moving it into the ohmic cell for electrical conductivity measurement to simulate the actual condition of its moisture content during ohmic heating. The electric field strength at around 13.5 V/cm was supplied in the measurement using a variable transformer (model TSGC2-5K; Stable Transformer Co., Ltd.; Pathum Thani, Thailand) with input/output voltages of 220 V AC/0–250 V AC. The sample temperature was recorded every 1 min using a type-K thermocouple and a data logger (MidiLogger GL240; Graphtec Corporation; Yokohama, Japan). The electrical current and voltage values were monitored using a digital multimeter (model 8808A; Fluke Corporation; Everett, WA, USA). The electrical conductivity was calculated using Equationequation (1)(1)
(1) :
where σ is the electrical conductivity (S/m), I is the electrical current (A), L is the distance between the two electrodes (m), A is the cross-sectional area of the electrode (m2), and V is the supplied voltage (V).
Smokehouse heating
The smokehouse (CS 700; Kerres; Backnang, Germany) was used to heat the sausage samples by setting the chamber temperature at 85°C whereas the actual chamber temperatures were between 85 ± 5°C. In each batch of heating experiments, approximately 180–185 g of sausage samples (4 pieces) were placed in the smokehouse chamber before starting cooking mode. Then, the sausage temperature increased during the cooking time until the core temperature reached the required temperatures of 40, 60, or 75°C after 405, 803, and 1,501 seconds, respectively. Notably, a core temperature of approximately 75°C is generally used as the criterion for heating raw poultry products to be safe from foodborne illness.[Citation20] The core temperature of each sausage was measured using a type-K thermocouple and a datalogger (MidiLogger GL240; Graphtec Corporation; Yokohama, Japan). After heating, the sausages were immediately exposed to the cooling step, as previously mentioned. Notably, every time a sample reached the required temperature, the operation of the smokehouse was stopped and the chamber was opened to cool to ambient temperature before loading another sample batch into the chamber.
Water bath heating
The water bath (Memmert, model W200; Schwabach, Germany) was used to heat sausages by setting the water temperature at 75°C. After the water temperature reached the set point, the sausages were placed in the hot water until their core temperatures reached the specified temperatures of 40, 60, or 75°C after 170, 360, and 700 seconds, correspondingly. Then, the sausages were cooled in iced water, as formerly described. The amounts of sausage and water used in each heating batch were approximately 180–185 g of sausage samples and 1 L of water, respectively.
Ohmic heating
The schematic diagram of the ohmic heating apparatus was similar to that used for the electrical conductivity measurement, except that the shapes and sizes of the ohmic cells were different. The ohmic cell used for heating the sausage samples was made from 10 mm thickness acrylic plate. The inner dimension of the ohmic heating cell was 130 × 180 × 140 mm (width × length × height, respectively). The electrodes were made from Ti-Gr-2 titanium plate of thickness 2 mm and were installed on both sides of the ohmic cell. The distance between electrodes was 130 mm. Around 1 L of sodium chloride solution at a concentration of 0.75% w/w was poured into the ohmic cell at room temperature before placing around 180–185 g of the sausages and turning on the ohmic heating system. The sodium chloride solution was used instead of water to raise the electrical conductivity of the mixture. The core temperature of each sausage and the surrounding solution temperature were monitored during the heating process until the specified temperatures of 40, 60, or 75°C of sausages were obtained after 227, 371, and 574 seconds, respectively. The electrical voltage between the two electrodes of the ohmic heater was manually controlled using a variable transformer during the heating period to obtain core temperature profiles for the samples during heating that were analogous to those from the water bath heating. After heating, the sausages were cooled, as previously stated.
Residual nitrite
A sample (5 g) of each sausage that had been minced using a Chopper (Tefal; model DPA1; Lourdes, France) was mixed with 25 mL of double-deionized water at 60°C in a 125 mL Erlenmeyer flask before manual stirring using a glass rod until uniform. After that, 25 mL of acetonitrile was added to the mixture. The mixture was left at room temperature to cool before passing through Whatman no.1 filter paper. The filtrate volume was adjusted to 100 mL using double-deionized water. A sample (1 mL) was passed through a 0.2 µm syringe filter (VertiClean; Vertical Chromatography Co. Ltd.; Nonthaburi, Thailand) into a vial to determine the residual nitrite content using high-performance liquid chromatography in a Shimadzu LC-20, including an SPD-M20A diode array detector, an SIL-20AC HT autosampler, a CTO-10AS VP column oven, a DGU-20A 5 R degasser, and an LC-20AD pump (Shimadzu; Kyoto, Japan). The buffer solution was prepared using 34 g of boric acid, 19.6 mL of gluconic acid, 11 g of lithium hydroxide anhydrous, and 125 mL of glycerol and was made up to 1,000 mL with double-deionized water. Then, 34 mL of buffer solution was mixed with 250 mL of acetonitrile and made up to 2,000 mL with double-deionized water to be utilized as the mobile phase. Its pH was adjusted to 6.5 ± 1 with 1.8 and 0.1 M hydrochloric acid solutions. The mobile phase was passed through a 0.2 µm nylon membrane filter (Vertical Chromatography Co. Ltd.; Nonthaburi, Thailand) and degassed for 15 min before use. A filtrate sample (40 µL) was injected into an IC-Pak Anion HC Column, 4.6 mm x 150 mm (Water Corp.; Milford, Massachusetts, USA) at a flow rate of 2 mL/min at 30°C. The diode array detector (SPD-M20A; Shimadzu; Kyoto, Japan) was used at a wavelength of 205 nm to detect the nitrite peak. The residual nitrite was calculated using the nitrite peak area.[Citation21]
Total pigment, nitrosylhemochrome, and curing efficiency
The methods of AMSA[Citation22] were applied for determination of total pigment, nitrosylhemochrome, and curing efficiency. A sample (10 g) of minced sausage was mixed with the acidified acetone solution and then kept in the dark for 1 h with intermittent stirring prior to passing through Whatman no.1 filter paper. The acidified acetone solution was prepared using 40 mL of acetone, 2 mL of deionized water, and 1 mL of hydrochloric acid solution at a concentration of 37%. The absorbance value was measured of the filtrate at a wavelength of 640 nm (A640) using a T60U UV-Vis spectrophotometer (PG instruments Limited; Lutterworth, UK). The total pigment content (total heme concentration; ppm acid hematin) was calculated using Equationequation (2)(2)
(2) :
For the nitrosylhemochrome content determination, a 10 g sample of minced sausage was mixed with 40 mL of acetone and 3 mL of deionized water before leaving the mixture in the dark for at least 5 min with intermittent stirring and then passing it through Whatman no.1 filter paper. The absorbance of the filtrate was examined at a wavelength of 540 nm (A540) using the spectrophotometer. The nitrosylhemochrome content (NO-heme concentration; ppm acid hematin) was calculated using applying Equationequation (3)(3)
(3) :
The curing efficiency, an indicator presenting the proportion of total pigment converted to the nitroso pigment and also the degree of cured color fading, was calculated using equation (4)[Citation22]:
Curing efficiency (%) = (nitrosylhemochrome content/total pigment content) × 100 (4)
Moisture content
The moisture content was examined using 3 g of minced sausage sample and drying temperature at 105°C for 18 h in a hot air oven. Afterwards, the sample was cooled down in a desiccator and weighed. The weight loss after drying was used for calculating the moisture content value and expressed on a wet basis (w.b.).
Statistical analysis
Electrical conductivity measurements and the heating experiments were conducted in three replicates. The sausage sample obtained from each heating experiment was determined for its quality attributes using three replications. Each result was presented as the mean ± standard deviation of all replications. The statistical software package SPSS/PC version 12.0 (SPSS Inc.; Chicago, IL, USA) was used for the one-way analysis of variance. Mean separations were carried out using Duncan’s multiple-range test and differences at P < .05 were deemed significant.
Results and discussion
Electrical conductivity
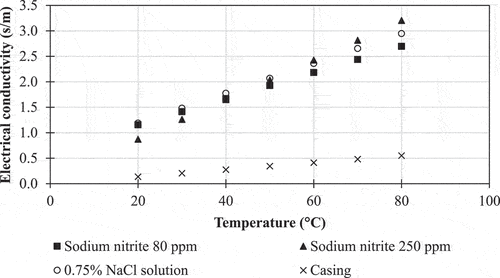
The electrical conductivities of the sausage emulsions, sodium chloride solution, and collagen casing at temperatures between 20 and 80°C are shown in and were in the ranges 1.16–2.69, 0.87–3.20, 1.19–2.95, and 0.14–0.55 S.m−1 for sausage emulsions with 80 and 250 ppm of sodium nitrite, sodium chloride solution, and collagen casing, respectively. All the samples were considered suitable for ohmic heating. According to Piette et al.,[Citation23] food materials have potential to be heated using the ohmic method if their electrical conductivities are in the range 0.01–10 S.m−1. Furthermore, the correlations between electrical conductivities of samples and temperatures followed a linear pattern that was consistent with many published research articles, such as Icier and Ilicali,[Citation24] Khuenpet et al.,[Citation25] and Aamir and Jittanit.[Citation26] Usually, the electrical conductivity of a food material is strongly influenced by its ionic content, moisture mobility, and physical structure.[Citation27] Therefore, it was unsurprising that the electrical conductivities of all samples escalated with increasing temperature, as a result of the more rapid movement of ions at the higher temperature. The increase in the sodium nitrite concentration in sausage emulsions from 80 to 250 ppm did not result in greater electrical conductivities at sample temperatures of 20–40°C because sodium nitrite made up only a small part of the recipe compared to the other ingredients. However, at a temperature greater than 40°C, the escalation of sodium nitrite concentration from 80 to 250 ppm visibly affected the increase in the electrical conductivity values, presumably due to the more rapid movement of the ions decomposing from the sodium nitrite. As expected, the electrical conductivity of the collagen casing was the lowest among all samples. Although the casing was composed of bovine gelatin, glycerin, cellulose, and oil (which have low electrical conductivity), its electrical conductivity was in the range 0.14–0.55 S.m−1 which was high enough for ohmic heating as a result of the exposure to the sodium chloride solution for 2 min before measurement.
Residual nitrite
presents the residual nitrite contents of the sausage samples heated using three different methods following frozen storage for ≤3 days or 4 weeks after applying sodium nitrite concentration levels of 80 or 250 ppm. The use of a 250 ppm sodium nitrite level is considered normal in the commercial sausage production, whereas 80 ppm is considered low. The storage of sausage products at a temperature below freezing is commonly used for either product exportation or expansion of the product’s shelf life.
Table 2. Residual nitrite, total pigment, nitrosylhemochrome, and curing efficiency of samples from different conditions after frozen storage for less than 3 days and 4 weeks.
For the sausage formulation with low sodium nitrite (80 ppm), the residual nitrite content of the raw meat batter (unheated) after frozen storage for less than 3 days was 22.95 ppm and this decreased to 12.47 ppm after storage for 4 weeks. After ≤3 days of storage, heating the sample to 40 or 60°C did not cause any significant change in the residual nitrite content; however, the residual nitrite content significantly decreased compared to the raw meat batter when applying the water bath or ohmic heating techniques at 75°C. This reduction might have occurred due to nitrite loss from the sample to the surrounding solution during the heating process to 75°C. On the other hand, heating in the smokehouse did not cause this significant reduction. The explanation was that during smokehouse heating the samples were heated by hot air and steam instead of the surrounding liquid; therefore, the loss of substances to the surrounding solution did not occur. This result implied that if the consumers heated their sausages by boiling them in a water bath or ohmic heater before consumption, the nitrite risk would be less compared to heating techniques without a liquid medium such as a smokehouse, air fryer, hot-air oven, or microwave.
Notably, after frozen storage for 4 weeks, the residual nitrites declined in all samples with the 80 ppm nitrite formulation. The residual nitrite of the raw meat batter reduced by 45.7% compared to that after ≤3 days storage, while the reduction of residual nitrites in the sausage samples that were heated to core temperatures of 40, 60, and 75°C were in the ranges 38.4–43.8, 25.5–31.0, and 14.7–19.6%, respectively with minor variations between the heating techniques. The greater heating temperatures led to significantly lower decreases in residual nitrites during frozen storage. The reduction in nitrites during storage could have been caused by various reasons, such as the enzymatic reaction and chemical reactions, especially oxidation. Nitrite can disintegrate to nitric oxide, nitrous acid and nitrate. Jo et al.[Citation28] reported that nitrite is typically decomposed to nitric oxide (NO) when added to meat products. In addition, nitrite can accept a hydrogen ion to generate nitrous acid (HNO2) which is volatile and easily decomposed. Azeem et al.[Citation29] claimed that the decomposition of nitrite can occur via oxidization to nitrate and nitrites can interact with myoglobin to produce nitrosylhemochrome as a pink color in cooked sausage and also interact with protein or other substances in meat products.[Citation2,Citation30] Besides, Higuero et al.[Citation31] reported a gradual reduction in the residual nitrite contents in dry cured loins from day 0 to day 40 during cold storage at 4°C as a consequence of its chemical conversion to nitric oxide and reactions with myoglobin and other constitutes in the meat or due to oxidation to nitrate. A higher temperature causes more protein denaturation and stability of the sausage sample microstructure, leading to lower rates of either enzymatic or chemical reactions of nitrites during frozen storage. Carballo et al.[Citation32] reported that around 5.5–12% of the added nitrites reacted with protein in pink bologna and became protein-bound nitrite. Ishiwatari et al.[Citation33] reported that meat protein was denatured at different temperatures; myosin started to denature at 35°C compared to 65°C for actin. Myoglobin is one of the sarcoplasmic proteins in meat. Okayama et al.[Citation34] demonstrated that the denaturation of sarcoplasmic protein occurred at 50–60°C during cooking.
The trend in the residual nitrite contents for the samples prepared using the normal-sodium nitrite level (250 ppm) in the formulation were similar to those for the low-sodium nitrite (80 ppm) samples; however, the values of residual nitrites were greater. For example, the residual nitrite content of raw batter after frozen storage for ≤3 days was 81.41 ppm and declined to 37.75 ppm at the fourth week. Sallan et al.[Citation35] demonstrated that the residual nitrite amount in sausages depended on the applied amount of nitrite. Overall, heating temperatures between 40 and 75°C did not cause any significant change in the residual nitrite content of samples after ≤3 days storage compared to the raw meat batter for all three different heating methods applied. After frozen storage for 4 weeks, the residual nitrite of the raw meat batter reduced by 53.6% from that after ≤3 days. In addition, the decreases in the residual nitrites of the samples that were heated to 40, 60, or 75°C were in the ranges 45.2–51.7, 37.9–44.9, and 24.0–32.0%, respectively. These results confirmed that the higher heating temperature did not lead to any significant changes in the residual nitrites in sausages within 3 days after heating. However, the higher heating temperature caused a slight reduction in the residual nitrites during frozen storage. This finding was notable because if the residual nitrites of both low-sodium nitrite and normal-sodium nitrite sausages noticeably declined during frozen storage, especially for the raw sausage, this could be useful for informing health-conscious consumers about a potential way to reduce nitrite risk in their sausage consumption. It must be noted that although the commercial sausages are typically stored under refrigerated condition, the sausage products produced for exportation are transported and preserved under freezing temperature. In addition, in some cases of household consumption the sausage might be kept in the freezer for long term storage. Therefore, the finding of this study provided the benefits for the practical application. Importantly, according to US FDA,[Citation36] the amount of sodium nitrite in a finished meat product is limited to not more than 200 ppm for safe use, whereas a maximum amount of 80 ppm is specified by Thai FDA.[Citation19] Therefore, all the samples in the current study were acceptable under the US FDA regulation, whereas the raw batter and sausages prepared using the normal-sodium nitrite level and then heated in the smokehouse at 60 or 75°C before frozen storage for less than 3 days had slightly greater residual nitrites than the maximum allowable by Thai FDA.[Citation19] The sausage manufacturer and consumer should be aware that the sodium nitrite level must be in the suitable range. Excessive amount could lead to the health risk of consumer due to the carcinogenic compounds; on the other hand, too low level would result in the insufficient effect on the microbial growth inhibition especially for the Clostridium botulinum.
Total pigment, nitrosylhemochrome, curing efficiency, and moisture content
The results for the total pigment, nitrosylhemochrome, curing efficiency, and moisture content determinations are shown in . For the sausage formulation with low-sodium nitrite (80 ppm), the total pigment content of raw meat batter after frozen storage for ≤3 days was 10.20 ppm, whereas this value significantly increased when applying heating between 40 and 60°C. On the other hand, heating to the 75°C produced a decreasing trend in the total pigment contents in samples. The results were comparable among the three different heating techniques. After storing for 4 weeks, the total pigment contents were not significantly different from those for less than 3 days. The increase in total pigments was related to the values of nitrosylhemochrome that significantly rose after heating to 40, 60 and 75°C. Nitrosylhemochrome is a cured meat pigment (pink color) that can occur from the interaction between the added nitrites and the myoglobin in meat products, with heat being the critical factor that expedites the reaction. Zając et al.[Citation37] claimed that in their study only 6–15% of the total amount of sodium nitrite that was added to a meat product could bind with the heme pigment in the meat to produce the pink color. The results of nitrosylhemochrome in demonstrated that increasing the heating temperatures resulted in greater amounts of nitrosylhemochrome in the samples. However, the total pigment contents in the samples prepared using low-sodium nitrite (80 ppm) and heating at 75°C were less than those at 40 and 60°C, probably because at 75°C, some pigments were lost, especially those that are heat sensitive. Overall, although the nitrosylhemochrome contents of the heated samples prepared using low-sodium nitrite increased after frozen storage for 4 weeks, the total pigment contents were not significantly different between ≤3 days and week 4 because the increased amounts of nitrosylhemochrome contents during the storage period might be comparable to the lost amounts of some pigments occurring due to chemical reactions, such as oxidation. The curing efficiency values rose with increasing temperature and storage period because they were directly correlated to the ratio of nitrosylhemochrome content-to-total pigment content.
The total pigment, nitrosylhemochrome, and curing efficiency values of the samples prepared using the normal-sodium nitrite level (250 ppm) in the formulation were much higher than those for the low-sodium nitrite (80 ppm) samples, presumably due to the increased amount of sodium nitrite. Honikel[Citation2] reported that the applications of sodium nitrite enhanced sausage color and product stability while impeding lipid oxidation during storage. For the sausage formulation with normal-sodium nitrite level, the total pigment content of raw meat batter after frozen storage for ≤3 days was 10.68 ppm, which was similar to that for the low-sodium nitrite level; however, after heating to 40, 60, and 75°C, the total pigment contents clearly increased. The results were rather similar among the three heating techniques. The increase in total pigments was associated with the values of nitrosylhemochrome that significantly rose with the heating temperature. The amounts of nitrosylhemochrome were much greater for sodium nitrite at 250 ppm because the nitrite acted as a substrate for the occurrence of nitrosylhemochrome. After storing for 4 weeks, the total pigment and nitrosylhemochrome contents of some heated samples significantly declined, possibly because the concentrations of nitrosylhemochrome in the samples were rather high, leading to greater rates of chemical reactions that altered nitrosylhemochrome to other substances. Marches et al.[Citation38] reported that the reduction of total pigment and nitrosylhemochrome in meat product could be caused by light, oxygen, and temperature during storage, affecting protein oxidation, including the heme pigment group. In the current study, although the sausages were vacuum-packed during frozen storage, there was some residual oxygen inside the package. Sindelar et al.[Citation39] stated that for vacuum-packed conditions, the total pigment values of sausages decreased during storage at temperatures of 0–2°C. The curing efficiency values of samples using sodium nitrite at 250 ppm were considerably higher than those for 80 ppm, owing to their much higher nitrosylhemochrome contents, with only slightly greater values for the total pigment content. These results demonstrated that the reduction of sodium nitrite in the sausage formulation from 250 to 80 ppm led to apparent differences in the nitrosylhemochrome (cured meat pigment) content in the products; however, these sausage products could be stored under frozen conditions for a long duration without any obvious effect on their pigment values. In addition, if cured meat pigments in the products were required, the sausages should be heated in the manufacturing process. It could be expected that the application of the ohmic heating technique might help to increase the cured meat pigment content in the low-nitrite sausage due to the faster movement of nitrites as a result of the applied electrical field and the pore disruption in the sausage microstructure due to electroporation. However, the results showed that the three different heating techniques produced similar effects because the faster movement of nitrites when ohmic heating was applied did not significantly affect the curing efficiency as most of the nitrosylhemochrome had been initiated in the sample preparation process before heating. Ozaki et al.[Citation40] reported that the color of meat batter rapidly changed during the sample preparation process because of pigment formation occurring from the presence of nitrite and metmyoglobin. Furthermore, the heating methods had less influence on the nitrosylhemochrome content than the heating temperature. In addition, the samples were made from meat emulsions that had already been minced in the grinder; therefore, the pore disruption generated by electroporation during ohmic heating did not significantly affect the curing efficiency of the samples.
Conclusion
The electrical conductivities of sausage emulsions and their soaked collagen casing were high enough to apply ohmic heating. The nitrite content of sausages could be reduced if the sausages were heated by boiling in a water bath or ohmic heater before consumption. The residual nitrite levels in sausages notably declined during frozen storage, especially for the raw sausage. Therefore, consumers could reduce the risk of excessive nitrite in their sausage consumption by storing their raw or partially cooked sausage products under frozen conditions for some period. The total pigment, nitrosylhemochrome, and curing efficiency values of the samples prepared using the normal-sodium nitrite level in the formulation were much higher than those for the low-sodium nitrite samples. The increase in total pigments correlated to the nitrosylhemochrome contents that significantly increased with heating temperature. In summary, it was possible to produce sausages using a low amount of added sodium nitrite amount together with the application of ohmic heating; however, the sausage characteristics were dissimilar to normal-sodium nitrite sausages. The total pigment, nitrosylhemochrome, and curing efficiency values were rather comparable between three heating techniques. The possible advantages of ohmic heating technique compared to its counterparts are its energy efficiency, rapid and uniform heating rate within the sausage products. The results in the lab-scale experiments in this study can be applied for the upscaling if in the large-scale process the key parameters consisting of the electrical conductivity of sample, electrical field strength, electrical field distribution, and heating period are controlled to be analogous to those of the lab-scale study.
Author contributions
Suveena Jantapirak: Methodology, Investigation, Formal analysis, Data curation, Writing- Original Draft, Visualization, Funding acquisition. Kanithaporn Vangnai: Conceptualization, Methodology, Resources, Data curation, Supervision, Project administration. Titaporn Tumpanuvatr: Methodology, Investigation, Resources. Weerachet Jittanit: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Writing- Reviewing and Editing, Visualization, Supervision, Project administration.
Acknowledgments
The research presented in this paper was funded by the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand (FF(KU) 6.65). The first authors are grateful to the Royal Thai Government Scholarship (Ministry of Higher Education, Science, Research and Innovation), Thailand for funding the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Skibsted, L. H. Nitric Oxide and Quality and Safety of Muscle Based Foods. Nitric Oxide - Biol. Chem. 2011, 24(4), 176–183. DOI: 10.1016/j.niox.2011.03.307.
- Honikel, K.-O. The Use and Control of Nitrate and Nitrite for the Processing of Meat Products. Meat. Sci. 2008, 78(1), 68–76. DOI: 10.1016/j.meatsci.2007.05.030.
- Zhu, Y.; Wang, P.; Guo, L.; Wang, J.; Han, R.; Sun, J.; Yang, Q. Effects of Partial Replacement of Sodium Nitrite with Lactobacillus Pentosus Inoculation on Quality of Fermented Sausages. J. Food Process Preserv. 2019, 43(5). DOI: 10.1111/jfpp.13932.
- Zhu, Y.; Guo, L.; Yang, Q. Partial Replacement of Nitrite with a Novel Probiotic Lactobacillus Plantarum on Nitrate, Color, Biogenic Amines and Gel Properties of Chinese Fermented Sausages. Food. Res. Int. 2020, 137. DOI: 10.1016/j.foodres.2020.109351.
- Eichholzer, M.; Gutzwiller, F. Dietary Nitrates, Nitrites and N-Nitroso Compounds and Cancer Risk with Special Emphasis on the Epidemiological Evidence. In Food Safety: Contaminants and Toxins, D’Mello, J. P. F. Ed.; CABI Publishing Edinburgh: Scottish Agricultural College, 2003; pp 217–234. DOI: 10.1079/9780851996073.0217
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P. E. S.; Barba, F. J.; Lorenzo, J. M. Combined Effect of Natural Antioxidants and Antimicrobial Compounds During Refrigerated Storage of Nitrite-Free Frankfurter-Type Sausage. Food Res. Int. 2019, 120, 839–850. DOI: 10.1016/j.foodres.2018.11.048.
- Ahmad, S.; Jafarzadeh, S.; Ariffin, F.; Zainul Abidin, S. Evaluation of Physicochemical, Antioxidant and Antimicrobial Properties of Chicken Sausage Incorporated with Different Vegetables. Ital. J. Food Sci. 2020, 32(1), 75–90. DOI: 10.14674/IJFS-1574.
- Ayaseh, A.; Alirezalu, K.; Yaghoubi, M.; Razmjouei, Z.; Jafarzadeh, S.; Marszałek, K.; Mousavi Khaneghah, A. Production of Nitrite-Free Frankfurter-Type Sausages by Combining ε-Polylysine with Beetroot Extracts: An Assessment of Microbial, Physicochemical, and Sensory Properties. Food Biosci. 2022, 49, 101936. DOI: 10.1016/j.fbio.2022.101936.
- Horsch, A. M.; Sebranek, J. G.; Dickson, J. S.; Niebuhr, S. E.; Larson, E. M.; Lavieri, N. A.; Ruther, B. L.; Wilson, L. A. The Effect of pH and Nitrite Concentration on the Antimicrobial Impact of Celery Juice Concentrate Compared with Conventional Sodium Nitrite on Listeria Monocytogenes. Meat. Sci. 2014, 96(1), 400–407. DOI: 10.1016/j.meatsci.2013.07.036.
- Jin, S.-K.; Choi, J. S.; Yang, H.-S.; Park, T.-S.; Yim, D.-G. Natural Curing Agents as Nitrite Alternatives and Their Effects on the Physicochemical, Microbiological Properties and Sensory Evaluation of Sausages During Storage. Meat. Sci. 2018, 146, 34–40. DOI: 10.1016/j.meatsci.2018.07.032.
- Kim, T. K.; Kim, Y. B.; Jeon, K. H.; Park, J. D.; Sung, J. M.; Choi, H. W.; Hwang, K. E.; Choi, Y. S. Effect of Fermented Spinach as Sources of Pre-Converted Nitrite on Color Development of Cured Pork Loin. Korean J. Food Sci. Anim. Resour. 2017, 37(1), 105–113. DOI: 10.5851/kosfa.2017.37.1.105.
- Palamutoğlu, R.; Fidan, A.; Kasnak, C. Spinach Powder Addition to Sucuk for Alternative to Nitrite Addition. Bull Transilva. Univ Brasov, Series II: Forestry, Wood Ind, Agri Food Engine. 2018, 11(12–60), 155–162.
- Riel, G.; Boulaaba, A.; Popp, J.; Klein, G. Effects of Parsley Extract Powder as an Alternative for the Direct Addition of Sodium Nitrite in the Production of Mortadella-Type Sausages – Impact on Microbiological, Physicochemical and Sensory Aspects. Meat. Sci. 2017, 131, 166–175. DOI: 10.1016/j.meatsci.2017.05.007.
- Engchuan, W.; Jittanit, W.; Garnjanagoonchorn, W. The Ohmic Heating of Meat Ball: Modeling and Quality Determination. Innov. Food Sci. Emerg. Technol. 2014, 23, 121–130. DOI: 10.1016/j.ifset.2014.02.014.
- Cho, H. Y.; Yousef, A. E.; Sastry, S. K. Growth Kinetics of Lactobacillus acidophilus Under Ohmic Heating. Biotechnol. Bioeng. 1996, 49(3), 334–340. DOI: 10.1002/(SICI)1097-0290(19960205)49:3<334:AID-BIT12>3.0.CO;2-E.
- Inmanee, P.; Kamonpatana, P.; Pirak, T. Ohmic Heating Effects on Listeria Monocytogenes Inactivation, and Chemical, Physical, and Sensory Characteristic Alterations for Vacuum Packaged Sausage During Post Pasteurization. LWT-Food Sci. Technol. 2019, 108, 183–189. DOI: 10.1016/j.lwt.2019.03.027.
- Gunvig, A.; Hansen, F.; Borggaard, C. A Mathematical Model for Predicting Growth/no-Growth of Psychrotrophic C. Botulinum in Meat Products with Five Variables. Food Control. 2013, 29(2), 309–317. DOI: 10.1016/j.foodcont.2012.06.046.
- Lee, S.; Lee, H.; Kim, S.; Lee, J.; Ha, J.; Choi, Y.; Oh, H.; Choi, K.-H.; Yoon, Y. Microbiological Safety of Processed Meat Products Formulated with Low Nitrite Concentration—A Review. Asian-Australas J. Anim. Sci. 2018, 31(8), 1073. DOI: 10.5713/ajas.17.0675.
- Thai FDA (Thailand Food and Drug Administration). Announcement of the Ministry of Public Health No. 418 (B.E.2563): Prescribing the principle, conditions, methods and proportion of food additives (No.2). 2020, 356.
- USDA (U.S. Department of Agriculture). Safe Minimum Internal Temperature Chart. Food Safety Basics. 2020. https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/safe-temperature-chart
- Wood, R.; Foster, L.; Damant, A.; Key, P. 9 - E249–50: Nitrites. In Analytical Methods for Food Additives, Wood, R., Foster, L., Damant, A. Key, P., Eds.; Woodhead Publishing, 2004; pp 98–127.
- AMSA (American Meat Science Association). Section XI: Detail of Analytical Analyses Related to Meat Color. In Meat Color Measurement Guidelines, Hunt, M. C., and King, A. Eds.; Champaign, IL, USA: American Meat Science Association, 2012; pp 80–81.
- Piette, G.; Dostie, M.; Ramaswamy, H. Ohmic Cooking of Processed Meats - State of the Art and Prospects. 47th International Congress of Meat Science and Technology; Krakow, Poland, 2001, 62–67.
- Icier, F.; Ilicali, C. Temperature Dependent Electrical Conductivities of Fruit Purees During Ohmic Heating. Food. Res. Int. 2005, 38(10), 1135–1142. DOI: 10.1016/j.foodres.2005.04.003.
- Khuenpet, K.; Fukuoka, M.; Jittanit, W.; Sirisansaneeyakul, S. Spray Drying of Inulin Component Extracted from Jerusalem Artichoke Tuber Powder Using Conventional and Ohmic-Ultrasonic Heating for Extraction Process. J. Food Eng. 2017, 194, 67–78. DOI: 10.1016/j.jfoodeng.2016.09.009.
- Aamir, M.; Jittanit, W. Ohmic Heating Treatment for Gac Aril Oil Extraction: Effects on Extraction Efficiency, Physical Properties and Some Bioactive Compounds. Innov. Food Sci. Emerg. Technol. 2017, 41, 224–234. DOI: 10.1016/j.ifset.2017.03.013.
- Srivastav, S.; Roy, S. Changes in Electrical Conductivity of Liquid Foods During Ohmic Heating. Int. J. Agric. Biol. Eng. 2014, 7(5), 133–138. DOI: 10.3965/j.ijabe.20140705.015.
- Jo, K.; Lee, S.; Yong, H. I.; Choi, Y.-S.; Jung, S. Nitrite Sources for Cured Meat Products. LWT-Food Sci. Technol. 2020, 129. DOI: 10.1016/j.lwt.2020.109583.
- Azeem, S. M. A.; Madbouly, M. D.; El-Shahat, M. F. Determination of Nitrite in Processed Meat Using Digital Image Method and Powdered Reagent. J. Food Compos. Anal. 2019, 81, 28–36. DOI: 10.1016/j.jfca.2019.05.003.
- Karwowska, M.; Kononiuk, A.; Wojciak, K. M. Impact of Sodium Nitrite Reduction on Lipid Oxidation and Antioxidant Properties of Cooked Meat Products. Antioxidants. 2019, 9(1). DOI: 10.3390/antiox9010009.
- Higuero, N.; Moreno, I.; Lavado, G.; Vidal-Aragón, M. C.; Cava, R. Reduction of Nitrate and Nitrite in Iberian Dry Cured Loins and Its Effects During Drying Process. Meat. Sci. 2020, 163, 108062. DOI: 10.1016/j.meatsci.2020.108062.
- Carballo, J.; Cavestany, M.; Jiménez-Colmenero, F. Effect of Light on Colour and Reaction of Nitrite in Sliced Pork Bologna Under Different Chilled Storage Temperatures. Meat. Sci. 1991, 30(3), 235–244. DOI: 10.1016/0309-1740(91)90069-3.
- Ishiwatari, N.; Fukuoka, M.; Sakai, N. Effect of Protein Denaturation Degree on Texture and Water State of Cooked Meat. J. Food Eng. 2013, 117(3), 361–369. DOI: 10.1016/j.jfoodeng.2013.03.013.
- Okayama, T.; Fujii, M.; Yamanoue, M. Effect of Cooking Temperature on the Percentage Colour Formation, Nitrite Decomposition and Sarcoplasmic Protein Denaturation in Processed Meat Products. Meat. Sci. 1991, 30(1), 49–57. DOI: 10.1016/0309-1740(91)90034-N.
- Sallan, S.; Kaban, G.; Çelik, M.; Kaya, M.; Kaya, M. Nitrosamine Formation in a Semi-Dry Fermented Sausage: Effects of Nitrite, Ascorbate and Starter Culture and Role of Cooking. Meat. Sci. 2020, 159, 159. DOI: 10.1016/j.meatsci.2019.107917.
- US FDA (U.S. Food and Drug Administration). Code of Federal Regulations. Title 21, Chapter I, Subchapter B, Part 172: Food additives permitted for direct addition to food for human consumption, Subpart B, Sec. 172.175 Sodium nitrite. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.175 (accessed Nov 29, 2022).
- Zając, M.; Zając, K.; Dybaś, J. The Effect of Nitric Oxide Synthase and Arginine on the Color of Cooked Meat. Food Chem. 2022, 373, 131503. DOI: 10.1016/j.foodchem.2021.131503.
- Marches, C. M.; Cichoski, A. J.; Zanoelo, E. F.; Dariva, C. Influência das condições de armazenamento sobre os pigmentos cárneos e a cor do salame italiano fatiado. Ciênc. Tecnol. Aliment. 2006, 26, 697–704. DOI: 10.1590/S0101-20612006000300033.
- Sindelar, J.; Cordray, J.; Sebranek, J.; Love, J.; Ahn, D. Effects of Vegetable Juice Powder Concentration and Storage Time on Some Chemical and Sensory Quality Attributes of Uncured, Emulsified Cooked Sausages. J. Food Sci. 2007, 72(5), S324–S332. DOI: 10.1111/j.1750-3841.2007.00369.x.
- Ozaki, M. M.; Munekata, P. E.; Jacinto-Valderrama, R. A.; Efraim, P.; Pateiro, M.; Lorenzo, J. M.; Pollonio, M. A. R. Beetroot and Radish Powders as Natural Nitrite Source for Fermented Dry Sausages. Meat. Sci. 2021, 171, 108275. DOI: 10.1016/j.meatsci.2020.108275.