ABSTRACT
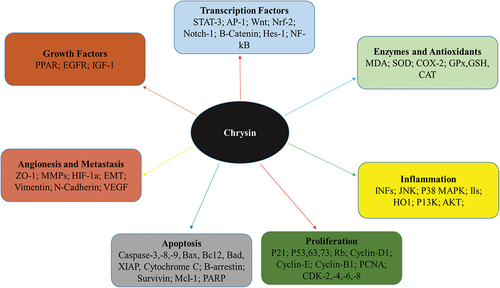
Honey, propolis, and passionflower contain a flavone known as chrysin. Researchers are studying chrysin to prove its potential to stop cancer cells growth. Chrysin cancer therapeutic potential is of great interest and numerous studies have been done to illustrate this potential. It is associated protein pathways effectively suppress tumor growth within the body. It is shown to have the ability to kill breast, prostate, lung, liver, and stomach cancerous cells. It can block Phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and Mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling in different animals against various cancers. Chrysin has the potential to kill breast cancer cells in a laboratory setting by inhibiting their cell division. Chrysin strongly suppresses Matrix metalloproteinase-9 (MMP-9), Urokinase plasminogen activator (uPA) and Vascular endothelial growth factor (VEGF), i.e. factors that can cause cancer. Chrysin has the ability to suppress the androgen receptor (AR), a protein necessary for prostate cancer development and metastasis. It starts the caspase cascade and blocks protein synthesis to kill lung cancer cells. Unnecessary apoptosis can be prevented by stopping certain protein pathways. Chrysin significantly decreased lung cancer metastasis in various animal-modeled studies. Chrysin induces apoptosis and stops colon cancer cells in the G2/M cell cycle phase. Chrysin suppresses colon cancer-promoting cyclin B1 and cyclin-dependent kinase 2. Chrysin suppressed cyclin B1 and CDK2 production in order to stop cancerous growth. Chrysin prevents tumor growth and cancer spread by blocking blood vessel expansion. Chrysin’s solubility, accessibility and bioavailability may limit its medical use. Chrysin targets numerous cancer-related communication pathways present in cells. Chrysin may reduce the chances of the onset of cancer, it can also serve as an alternative treatment as a whole to prevent and treat various cancers, but more clinical trials and research studies are needed to fully unlock its potential.
Introduction
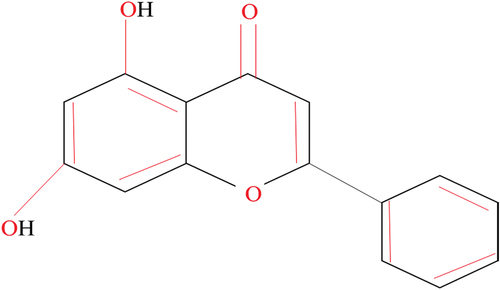
This flavone can also be found in high quality in passion fruit and chrysin (5, 7-dihydroxyflavone). Both propolis and honey are excellent food sources of this compound. Studies find that Radix scutellariae and Pleurotus ostreatus may be utilized for chrysin extraction.[Citation1] This compound is composed of two benzene rings (A and B) joined to an oxygen-containing ring and a heterocyclic ring. It has double bonds and OH groups, making it a ring structure. In contrast to the B rings of other flavonoids, chrysin’s B ring does not have any oxygen.[Citation2] Chemical structure of chrysin was shown in .
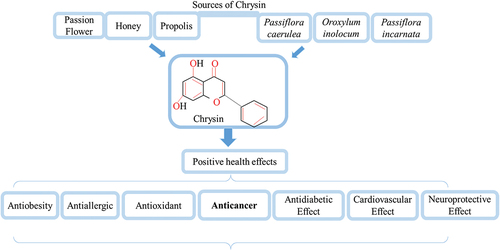
Natural occurrences of this compound have been documented in a variety of plant sources, including propolis, honey, passion fruit, and even mushrooms, while its synthetic analogues are used in the pharmaceutical industry.[Citation3] Chrysin (5,7-dihydroxyflavone) is one of several naturally occurring polyphenols found in honey, propolis, the bitter melon (Momordica charantia), the wild Himalayan pear (Pyrus pashia), and many other therapeutic plants and fruits.[Citation4] Recent studies have shown that chrysin may be located in several parts of the passion fruit Passiflora edulis Sims, Diaphragma juglandis fruit, walnut pellicle, and common walnut flowers.[Citation5] Banxia Xiexin is a medicinal plant used in traditional Chinese medicine to treat gastrointestinal issues. Chrysin, either as 6-C-arabinoside-8-C-glucoside or as a glucuronic acid ester, i.e., chrysin-7-O-glucuronide, was identified in this plant. Both of these crystalline and amorphous forms of chrysin were found in the plant.[Citation6] The aerial parts of the Scutellaria schachristanica plant also contained chrysin glucuronides. An Indian green marine alga provides chrysin, and as an endophytic fungus called Chaetomium globosum.[Citation2] When it comes to global health issues, cancer is at the top of the list. One in twelve cases is caused by lung cancer, one in eleven by breast cancer, and one in ten by colon cancer. Cancers of the liver account for 9.1% of all cancer-related deaths, followed by cancers of the stomach at 8.2%. Surgery, radiation therapy, and chemotherapy are the gold standard treatments for cancer, but they seldom succeed in curing the disease and always come with a long list of unpleasant side effects. Chemotherapy drugs are chemical treatments with poor therapeutic effectiveness and significant negative effects. This is because they are poorly soluble, cannot target particular parts of the body, and are eliminated rapidly. As a result, it’s clear that more study into complementary cancer therapies is required.[Citation7] The search for effective and safe natural and synthetic substances for use in cancer therapy and prevention has received a great deal of attention in recent years. These chemicals may be found in nature or manufactured in a lab.[Citation8] Research has shown that this chemical is effective against free radicals, inflammation, and allergens. As chrysin has been shown to limit cell growth and promote cell death via the apoptotic pathway in a number of different cancer cell lines, it represents a promising therapeutic strategy. This provides hope that it may be utilized to combat the disease. Although chrysin has been shown to have anticancer characteristics, it faces significant barriers to being used in cancer therapy due to its insolubility in water, low absorption rate, and rapid elimination from the body.[Citation9] Chrysin sources and health benefits were shown in .
The anti-cancer effects of chrysin are magnified by a factor of ten when combined with a metallic complex. The metal complex makes it easier for chrysin to insert into DNA at hydrophobic sites, which is why this happens. Using the DNA intercalator structure as a blueprint, five chrysin derivatives were created to enhance chrysin’s anti-cancer properties. The MTT assay was used to determine if chrysin and its derivatives slowed the growth of cancer cells (Hela, BGC823, MCF-7, and HepG2) and normal cells (HEK-293). All cancer cells were killed by the new chrysin derivative 5-(2′-amino) phenyl-7-cyclohexanemethylchrysin (Ch-1) at a concentration of 62.5 mol/L, but over 60% of normal HEK-293 cells were unharmed. To get this result, the chemical concentration was raised to 62.5 mM. Sixty percent or more of those cells were still alive after being exposed to chrysin concentrations between 250 and 500 mM. Ch-1 was able to intercalate DNA, but chrysin was unable, as evidenced by their respective circular dichroism spectra. Chrysin simply couldn’t pull it off. Interestingly, the proportion of surviving HeLa cells dropped from 95% to 10% when exposed to Ch-1 at concentrations of 20 M and 30 M, respectively. Both the intrinsic and extrinsic apoptotic pathways were shown to be involved in Ch-1-induced cell death in HeLa cells. After being exposed to 25 M Ch-1, Hela cells significantly up-regulated p53, a key regulator of the apoptotic pathway. The apoptosis-related protein responses and the suppressive effects of Ch-1 were both eliminated in Hela cells upon treatment with the p53 inhibitor pifithrin (Pft). In conclusion, p53-independent apoptosis is the major regulator of Ch-1’s suppressive effects in HeLa cells. At concentrations (2.5–10 mol/L) that were well tolerated by HeLa, BGC823, and MCF-7 cells, Ch-1 greatly increased the anti-cancer activity of 10-hydroxycamptothecin (HCPT).[Citation10] Anticancer properties of chrysin was shown in .
Antioxidant status of chrysin
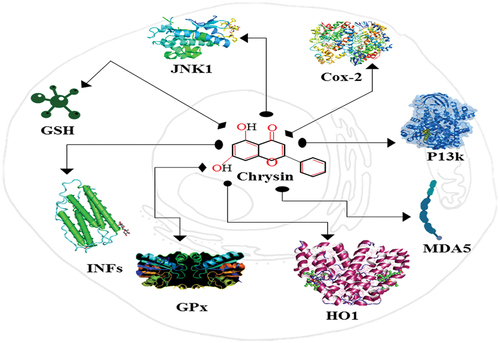
Chrysin, a natural flavone, may be found in honey, propolis, and even some fruits and vegetables. Antioxidants prevent cell damage, cellular aging, and disease caused by free radicals, which are unstable molecules. Antioxidants get rid of the free radicals that cause oxidative stress. Recent research suggests it may have antioxidant properties and help the body get rid of damaging free radicals.[Citation11] Which has piqued the curiosity of the scientific community. As chrysin reduced oxidative stress and lipid peroxidation in rat liver cells exposed to a toxic chemical agent. Protecting the brain against oxidative stress (GPx) may be aided by increasing levels of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). The antioxidant properties of chrysin may explain its anti-inflammatory effects. Research has connected inflammation-induced free radical and reactive oxygen species (ROS) generation to oxidative stress. A decrease in oxidative stress and an increase in antioxidant capacity may result from chrysin’s anti-inflammatory properties.[Citation12] Chrysin’s antioxidant strength has been evaluated using a number of in vitro assays. Chrysin’s capacity to inactivate DPPH, ABTS, and hydroxyl radicals proved its antioxidant status.[Citation13] The goal of this investigation was to see if chrysin may help lower oxidative stress in diabetic rats. Liver and kidney tissues from treated rats were shown to have dramatically increased activity of antioxidant enzymes, including SOD and catalase, and significantly decreased activity of oxidative stress markers.[Citation14] In a clinical trial with healthy human volunteers, the impact of chrysin supplementation on oxidative stress and inflammation was examined. The participants consumed 500 milligrams of chrysin each day for a period of four weeks. This suggests that chrysin supplementation, by reducing oxidative stress markers and pro-inflammatory cytokines, may have beneficial effects on health and well-being. A look into how to make the chemotherapy medication cisplatin more efficient in killing human cervical cancer cells. Treatment with chrysin rendered cancer cells more susceptible to cisplatin, which led to a greater reduction in cell viability and an increase in apoptosis.[Citation15] The study aimed to examine the antioxidant status of chemically-induced colon cancer animals. Supplementation with chrysin increased the activity of antioxidant enzymes like SOD and catalase and reduced the levels of oxidative stress markers like malondialdehyde (MDA) in the colon tissue of the rats. The cell signaling pathways involved in the development and progression of cancer have been linked to chrysin’s ability to suppress cancer cell growth, induce apoptosis, and regulate cell proliferation. It has been highlighted that chrysin’s potential to improve the efficacy of other cancer medicines. The study examines the antioxidant effects of chrysin in relation to the prevention of breast, prostate, and lung cancer.[Citation16] Chrysin has been shown to inhibit cancer cell growth and induce apoptosis via many mechanisms, including modulation of oxidative stress and antioxidant pathways.[Citation17] Antioxidant levels in chrysin-treated mice with chemically induced liver cancer. Antioxidant enzyme activity (SOD, CAT) and oxidative stress marker (MDA) levels were both enhanced by chrysin supplementation in mouse liver tissue. The proliferation of liver cancer cells was also inhibited by chrysin.[Citation18] How chrysin could enhance paclitaxel’s capacity to kill human ovarian cancer cells during treatment by increasing their sensitivity to paclitaxel, cancer cells treated with chrysin saw a greater reduction in cell viability and a greater rise in apoptosis. The reduction of reactive oxygen species (ROS) and oxidative stress markers in the cancer cells further indicated the antioxidant activity of chrysin.[Citation19] Anti-oxidant and anti-inflammatory factors regulated by Chrysin were presented in .
Pharmacokinetics study of chrysin
Pharmacokinetics is the study of how drugs are absorbed, transported, metabolized, and excreted by the body. Both human and animal subjects have been studied to determine the pharmacokinetics of chrysin. Oral administration of chrysin has subpar results. Studies in rats show that less than 5% of the dose gets absorbed into the circulation. This is because it has a low solubility in water and is metabolized rapidly in the digestive tract. Once ingested, chrysin rapidly distributes itself throughout the body. After crossing the blood-brain barrier, it has been shown to accumulate there.[Citation20] The chrysin metabolism is highly dependent on the liver. It is initially methylated into 7-O-methylchrysin (COMT) by Catechol-O-methyltransferase. The additional metabolic processes of glucuronidation and sulfation make this molecule more water-soluble and excreteable. Chrysin and its metabolites are mostly excreted in the urine and feces. Rats can excrete as much as 70% of an oral dose in their feces, but humans only excrete 10% in their urine.[Citation21] Pharmacokinetic studies have been conducted with human participants. After administering 400 mg of chrysin to healthy individuals, chrysin-sulfate was shown to be the most abundant form in plasma, with an AUC of 1490 485 ng/mLhr. Systemic exposure to chrysin was around 20 times higher than its AUC of 64.33 ng/mL/h. Despite its strong in vitro activity, in vivo experiments have shown conflicting findings for chrysin. The author projected that less than 1% of chrysin would be absorbed orally. It was discovered that aglycone, rather than chrysin or chrysin-glucuronide, accounts for the majority of chrysin excreted in the feces, with the remaining 10% eliminated in the urine. Possible causes of increased feces output include enterohepatic recycling and low water solubility. The majority of chrysin may be secreted in conjugate forms (such as glucuronide or sulfate) through the bile, which may be hydrolyzed back to chrysin by gut bacteria in the terminal ileum or the colon. Enterohepatic recycling, as it is known, will be discussed in further depth down below. Methods that target first-pass metabolism and enterohepatic recycling (such as metabolic enzyme inhibitors) should be developed if these mechanisms are responsible for the limited oral bioavailability of chrysin. However, due to its low solubility in water, chrysin is not well absorbed. When chrysin is taken orally, a lot of it is lost because of the high apparent fecal clearance. If inadequate water solubility is the major cause of chrysin’s poor absorption when taken orally, then new strategies are required to increase its water solubility (such as a nanoparticle formulation) to improve its oral bioavailability.[Citation22] The administration routes for chrysin have included orally, intravenously, and intraperitoneally. Most chrysin dosages are given orally. The bioavailability of chrysin after oral administration is low. Studies in rats have shown that the bioavailability of chrysin when taken orally is less than 5%. This is due to the fact that it is poorly soluble in water and undergoes fast metabolism in the digestive tract. The half-life of chrysin in rats is predicted to be close to 2 hours. The human chrysin half-life has not been studied extensively. Taking chrysin with food may increase the effectiveness of the supplement. One study found that the bioavailability of chrysin was increased by a factor of 1.8 when taken with a high-fat meal as opposed to when administered on an empty stomach. Compounds including 7-O-methylchrysin, apigenin, luteolin, and their conjugates are derived from chrysin. The pharmacokinetics of chrysin may differ depending on sex, according to rat studies. Female rats were shown to eliminate chrysin at a faster rate than male rats.[Citation19] First-pass metabolism of chrysin upon oral administration is carried out by the liver enzymes CYP1A1 and CYP1B1. This results in the formation of metabolites including 7-hydroxyflavone, 7, 8-dihydroxyflavone, and 4’-O-methylchrysin.[Citation23] Anticancer mechanisms of Chrysin was presented in .
Chrysin’s potential against gastric cancer
The third leading cause of cancer-related mortality worldwide is gastric cancer, also known as GC. Despite a general decline in the prevalence of GC, the number of newly diagnosed cases continues to increase. This trend demonstrates that the rising incidence of GC in younger age groups is consistent with a bimodal onset distribution. In 2019, it is anticipated that there will be 27,510 newly diagnosed cases of GC in the United States, with an associated death toll of 11,140. This represents a 21% increase from the prior year (2014) and a 1.4% increase from the prior year (2010). Those with advanced GC had the lowest 5-year survival rates (5.3%), while those with localized GC had the highest (58%). East Asia has a higher incidence rate than any other region on the globe.[Citation24] In China, gastric cancer is a significant public health concern. Despite the fact that the risk of developing stomach cancer is comparatively minimal in comparison to other cancers, it is the second leading cause of cancer-related mortality in China. Several factors, including smoking, consuming an inordinate amount of sodium, hereditary gastric cancer syndrome, and infection with Helicobacter pylori, are associated with an increased risk of stomach cancer. Over the past decade, both in China and in other countries, the risk factors for gastric cancer have decreased consistently.[Citation25] The food supply may contain a number of cancer-fighting compounds that occur naturally. Honey contains the flavone chrysin, which has been demonstrated to inhibit the growth of malignancies. Hepatocellular carcinoma (HCC) is treated with chrysin because it inhibits tumor glycolysis and induces apoptosis in malignant cells. In addition, chrysin has been demonstrated to inhibit the metastasis and invasion of melanoma cells.[Citation26]
Human gastric malignancy AGS and AGS/FR cells were grown in 96-well plates at a cell density ranging from 0.5 to 1.0104 cells per well for 24 hours. After either 24 or 48 hours of incubation, the cells were treated with a solution of chymotrypsin and 5-fluorouracil (FU) in 0.5% dimethyl sulfoxide (DMSO). The MTT test showed whether or not these cells have DNA that is still alive. The media that had been treated with MTT for a total of three hours was thrown away and replaced with new media. The cells were exposed to DMSO for 30 minutes. Setting the reading frequency of a microplate reader to 560 nm made it possible to measure absorption. During the experiment, the Chou-Talalay method was used to see how well chrysin and 5-FU worked together. By first washing the cells in ice-cold phosphate-buffered saline (PBS), it was possible to figure out how much apoptosis had happened. According to the study, the Western blotting method was used to measure the relative amounts of proteins that are known to play a role in apoptosis. The researchers looked at how the health of AGS cells changed when chrysin, 5-FU, or both were given in increasing amounts and how the changes changed the ability of AGS cells to live. For Conditions 1, 2, and 3, Chrysin was given at concentrations of 40, 50, 60, and 80 M, while 5-FU was given at concentrations of 20, 25, 30, and 40 M for the same three conditions. When chrysin was added to 5-FU, cell survival was much lower than when 5-FU was used alone. Both chrysin by itself and chrysin in combination with 5-fluorouracil (5-FU) were tested on AGS/FR cells that had stomach cancer. Because AGS/FR cells are so sensitive, the damaging effects of chymotrypsin hit them very hard. Results showed that when chrysin and 5-FU were given to cells at the same time for 48 hours, there was a combined effect on the loss of cell survival.[Citation27] Human gastric epithelial GES-1 and gastrointestinal stromal (GC) MKN-45 cell types have been the subject of a lot of study. These two different cell types were the focus of the study. After treating GC cells in the lab with chrysin, quantitative real-time PCR and Western blotting were used to find out how much TET1 is expressed in those cells. This lets figure out how much the genes are being expressed. Immunofluorescence labeling was used to figure out how much 5mC and 5hmC were being expressed in comparison to each other. The effects of chrysin, si-TET1, and TET1-KO on GC cell growth, cell cycle development, apoptosis, migration, and invasion were tested with wound repair and Matrigel invasion. These factors were also looked at with flow cytometry. Researchers made a naked mouse xenograft model to analyze more about how TET1 expression affects how tumors grow. The production of TET1 was much higher in GC cells that had been treated with chrysin, according to the results of quantitative real-time polymerase chain reaction and Western blot. Immunofluorescence research showed that the amounts of TET1 and 5hmC in MKN45 cells went up a lot after they were treated with chrysin. There is a link between chrysin, cell death (also called apoptosis), and a drop in the number of cells that move and invade. The researchers examined how TET1 expression affects cell death, cell migration, and cell invasion in MKN45 cells by either stopping it or making it too strong. When TET1 was overexpressed, the number of cells that died went up a lot, while the number of cells that moved or entered the surrounding tissue went down. Also, the CRISPR/Cas9 technology was used to make a creature without the TET1 gene. Researchers found a link between how much TET1 is expressed and how fast GC tumors grow in real life. The results also showed that the anti-tumor effects of GC were caused by chrysin, which changed the expression of TET1.[Citation28] This makes it more likely that TET1 could be a potential therapeutic target for treating GC. Scanning electron microscopy, nuclear magnetic resonance, and Fourier transform infrared spectroscopy were all used to look at chrysin-coated PLGA-PEG nanoparticles. Cytotoxicity was done to a cell line from a human gut, and the MTT test was used to see how this affected the rate at which the cell line grew. Researchers used real-time PCR to look at the amounts of expression of three miRNAs – miR-22, miR-34a, and miR-126—in RNA from cells that had been treated with a set amount of pure and contained chrysin. Both kinds of enzyme had been used on the cells. The treatment was previously administered to the cells. It was demonstrated that the IC50 value for chrysin loaded in PLGA-PEG nanoparticles was substantially lower than that for free chrysin as a function of the quantity of chrysin loaded. It was demonstrated that this is the case. Gene expression of miR-22, miR-34a, and miR-126 is higher in response to Nanoencapsulated chrysin than it is in response to unbound chrysin, confirming the study’s findings. This conclusion was reached during the course of the investigation. The results revealed that PLGA-PEG-chrysin inhibits the proliferation of human gastric cell lines more effectively than unbound chrysin. RNA sequencing was conducted to investigate the differential mRNA expression that occurred in gastric cancer cells following treatment with chrysin. COPB2, H19, and let-7a were also investigated using both knockdown and overexpression techniques.[Citation27] In addition to these characteristics, researchers also investigated cell proliferation, apoptosis, migration, and invasion. To investigate tumor formation in vivo, the COPB2 gene was targeted with the CRISPR/Cas9 system. On the basis of the findings, it was determined that chrysin affected the expression of the COPB2 mRNA. According to the findings, chrysin is not only capable of causing cell demise but also of preventing cell migration and invasion. Researchers are analyzing the expression of the lncRNA H19 and the microRNA let-7a to obtain a deeper understanding of the mechanism that regulates COPB2 production. Following treatment with chrysin, the expression of H19 and COPB2 was inhibited, whereas the expression of let-7a was greatly enhanced. Additionally, it has been shown that reduced levels of COPB2 expression directly increase the rate of cellular apoptosis. In vivo experiments have demonstrated that COPB2 expression is associated with the development of malignancies. Chrysin’s anti-tumor properties appeared to be mediated by the H19/let-7a/COPB2 axis.[Citation26]
Chrysin’s impact on colon cancer
Colon cancer was found to be the third most common type of cancer in 2022. It starts in the big intestine and then moves on to other parts of the digestive system. Research done in 2022 showed that colon cancer is one of the three types of cancer that kill the most people and is the most common in the United States. China has more people than any other country in the world, so colon cancer is a big problem there. Colon cancer makes up about 10% of all cancers that are being found and handled right now. Cancers that start in the colorectal tract are called “colorectal cancers,” and this term includes both cancers of the colon and cancers of the rectum. The vast majority of cases of each of these cancers are of the adenocarcinoma form. Metastasis, which is when the main disease spreads to other parts of the body, is the main reason why people who have this type of cancer die. According to the results of a study, colon cancers most often spread to the liver and peritoneum. Most of the time, this means that the person’s illness has become so bad that it can’t be fixed and will eventually kill them. This is what leads to death in the end.[Citation29] Colorectal cancer, also called CRC, is the third most common type of cancer in men and the second most common type of cancer in women, according to the American Cancer Society. It’s the cause of 10% of all tumors that later turn out to be cancerous. The rate of occurrence changes a lot from one country to the next, but men are usually 25% more likely to be affected than women. Risk factors include being overweight, not getting enough exercise, eating a lot of red meat, smoking cigarettes, and drinking alcohol. The process that leads to colon cancer, called carcinogenesis, is thought to involve things that change the gut bacteria. People think that several things may have something to do with it. In 23 countries, the death rate in a five-year period ranges from 28.5% for men to 57.0% for women and from 30.9% for women to 60% for men. The average death rate for men is 46.8% and the average for women is 48.4%. A study found that a person’s lifestyle or behavior, along with their genes, can either increase or lower their chance of getting colon cancer.[Citation30] CRC gets worse because of three major factors: chromosomal instability, the CpG island methylator trait, and microsatellite instability. More and more people are looking at bioactive chemicals from plants as a possible treatment for colon cancer because they are safe for the body and stop tumors from growing just as well as chemotherapy. They can slow the spread of colon cancer in a few ways: by stopping the cell cycle at the G1 phase, G1/S phase, S-phase, and G2/M phase to increase apoptosis; by lowering the levels of anti-apoptotic proteins like BCL2 and BCL-XL; and by increasing the activity of superoxide dismutase. Expression of PI3K, AKT, and MMP goes down, while expression of cell cycle inhibitors (p53, p21, and p27) and apoptotic markers (BCL2-associated causes of cell death) goes up. Flavonoids, phenolics, terpenoids, saponins, and quinones are all examples of secondary plant chemicals that protect against CRC cells. This defense is also provided by alkaloids. The study says that this can be done by causing apoptosis and stopping the cell cycle, changing tumor-suppressing microRNAs, blocking oncogenes, and lowering the amounts of anti-apoptotic proteins. Instead of 5-fluorouracil, chrysin has been suggested as a possible way to treat colon cancer (CRC).[Citation31] It was found that when CRC cells were treated with chrysin (5–50 M), the number of cells that survived was cut down by a lot. The results are the same for cancer cell lines (HCT116, DLDD, and SW837) made from tissue from either the colon or the rectal. After 24 hours of growing in DMEM-supplemented media, cells were taken out of 96-well plates, where there were about 104 cells in each well. After 24 hours, the cells had grown in the liquid. After 24 hours, the XTT proliferation test was done to count the number of living cells both before (10 mol/L, 50 mol/L, or 100 mol/L) and after (10 mol/L, 50 mol/L, or 100 mol/L) treatment with chrysin (10 mol/L, 50 mol/L, or 100 mol/L concentrations of DMSO). A spectrophotometer was used to measure the absorbance at 460 nm, while 750 nm was used to measure the reference, and a device called a CellTiter-Fluor cell viability test to check whether or not the cells were still alive. A SpectraMax Plus 384 microplate reader was used to measure how bright the light was. The process started at a wavelength of 390 nm and finished at a wavelength of 460 nm. The ApoTox-Glo Triplex Assay was used by experts to figure out how chrysin causes cells to die. Caspase-Glo 3/7 Reagent was used to figure out if apoptosis was happening or not. The Dead-end Fluorometric TUNEL System was used to find out how far along the process of apoptosis the cells were. Total RNA was taken out of the cells with the help of the RNeasy solution that Qiagen provided. With the help of a High Capacity cDNA Reverse Transcription Kit and the RNAs that were found, cDNA was made. With the help of TaqMan Universal PCR Master Mix, the amount of mRNA that was made was found.[Citation32] Researchers used the RT Profiler PCR array to look into how genes are expressed during the apoptosis process. Researchers looked at how well HCT116, DLD-1, and SW837 colon cancer cell lines could keep living so they could see how chrysin treatment affected the cells. They did this to find out what role the protein plays in each of these different types of cancer. After 24 hours of being exposed to 10–100 M chrysin, the viability of all types of cells dropped in a way that depended on the quantity. The doses could be anywhere from 1 to 100. When chrysin was present in 50 and 100 micromolar amounts, the overall number of living cells in all three cell lines went down by a large amount. When exposed to chrysin, HCT116 cells had the highest rate of death (61.4% (50 M) and 37.9% (100 M) of the control) of the four cell lines that were tested. According to the results, the factor that causes colon and rectal cancer cells to die by apoptosis is chrysin. Colorectal cancer (CRC) cell lines of both the HT-29 and HCT-116 types were grown in McCoy’s 5A medium. On the other hand, the SW48, SW480, and SW620 cells were kept alive by keeping them in L15 medium. 10% fetal bovine serum was added to the medium for growth that already had CRC in it. The enzymes that were used in the study were chymotrypsin, 3-methyladenine (3-MA), and anti-tubulin antibodies. In dimethylsulfoxide (DMSO), chrysin, 5-fluorouracil (5-FU), and oxaliplatin were dissolved to make a stock solution with a concentration of 50 mM. Before the working solutions were used, new amounts were made, and 95% of 3-MA was dissolved in hot ethanol. This was done before the methods that were found to work were used. To find out if the cells were still alive or not, colorimetric tests were done with 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). Using an antibody labeling method and a fluorescence lens, it is possible to see the GFP-LC3 molecules. Ten millimeters of cell granules were colored with thirty minutes of CM-H2DCFDA in the dark at 37 degrees Celsius. Ten millimeters of CM-H2DCFDA were used. It was found that flow cytometry is the most accurate way to measure the amount of reactive oxygen species that are found inside cells.[Citation33] Researchers tested how well chrysin, 5-FU, and oxaliplatin worked as treatments for CRC cells by putting them on a small group of CRC cells and watching how they responded. It was agreed that the amount of chrysin should be the same as the amount of 5-FU and oxaliplatin added together. This was done to make sure that all the numbers were fair and correct. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) test was used to measure how many CRC cells were still alive after being treated with the right amount of each drug for three days. This treatment was done to find out if the CRC cells were still alive or not. Researchers showed that the mixture of 5-fluorouracil and oxaliplatin is better than chrysin at killing CRC cell lines. However, they also showed that chrysin is just as effective. Chrysin is better than the mixture of 5-fluorouracil (5-FU) and oxaliplatin (Oxaliplatin) at killing many types of cells. Cleaved poly-ADP-ribose polymerase (cleaved-PARP) and active caspase-3, which is also known as cleaved-PARP, were looked at to see if they were signs of apoptosis or autophagy. Based on these results, it is very likely that autophagy is the way that chrysin affects the health of CRC cells. In contrast to 5-FU/oxaliplatin, chrysin increases the production of reactive oxygen species (ROS), which in turn causes autophagy by stopping Akt and mTOR from doing their jobs.[Citation33] In order to help the SW620 human colon cancer cell line grow, 10% fetal bovine serum (FBS), 100 units/ml of penicillin, and 100 mg/ml of streptomycin were added to Leibovitz’s L-15 medium. The cells were kept in an environment with a temperature of 37°C, a CO2 level of 5%, and a humidity level of 37%. Using the sulforhodamine-B (SRB) method, the material was put through a number of tests to find out if it was safe to eat or not. After keeping the SW620 cells at a warm temperature for 24 hours, they were buried in the ground. After being treated with chrysin and daidzein for three days, the cells were fixed with 150 liters of trichloroacetic acid (TCA) and put in the refrigerator at 4 degrees Celsius for one hour. After adding 70 liters of an SRB solution with a weight-to-volume ratio of 0.4%, the cells were washed three times with clean water and then left to rest for ten minutes at room temperature and without light. Five times, the cells were washed with a fluid that had 1% acetic acid in it. After mixing the SRB dye with 150 l of 10 mM TRIS, researchers used a BMG to measure how much light the solution received at 540 nm. The IC50 numbers for chrysin and daidzein show that they can both stop SW620 colon cancer cells from growing. The value of chrysin is 70.18 mM, while the value of daidzein is 23.50 mM. Based on these numbers, both medicines worked. The Western blotting method was used to find out which proteins are in charge of sending messages after the epidermal growth factor receptor (EGFR). Some of these enzymes are proteins like extracellular signal-regulated kinase (ERK) and protein kinase B (AKT). When compared to SW620 cells that had not been treated, SW620 cells that had been treated with the IC50 values of chrysin and daidzein had much lower amounts of phosphorylated ERK and AKT proteins. The results of the investigation showed this to be true. Because both ERK and AKT can be stopped from working by chrysin and daidzein, it was found that these two flavonoids should play a big role in the treatment of colon cancer.[Citation34] Another thing that was looked into was the role that Chrysin (Chr) played in the damage that Dic caused in human colon cancer HT-29 cells. Chrysin is an example of a certain kind of enzyme. The mRNA amounts of apoptotic and anti-apoptotic genes were studied using the quantitative real-time PCR method. By using the WST-1, LDH leaks, and TUNEL tests together, researchers were able to find out if cells were dividing and if they were damaged. The goal of all of these tests was to find out how much caspase-3 protein was being made in total. Chr stopped Dic from killing cells and turned around the effects that Dic had on ROS production, amounts of malondialdehyde, the release of lactate dehydrogenase, total antioxidant activity, and catalase activity. Chr also stopped Dic from stopping catalase from working. Chr was also able to stop Dic from making reactive oxygen species (ROS), which are the main thing that causes cells to die. The markers p53, cas-3, cas-8, Bax, and cytochrome c were more present in the group that got Dic, while they were less present in the group that got Chr. The results suggest that antioxidant vitamins, especially Chr, may make Dic less successful at killing colon cancer cells.[Citation35]
Targeting eye cancer with chrysin
There are many types of eye cancer, but the most common ones are uveal melanoma, retinoblastoma, orbital rhabdomyosarcoma, medulloepithelioma, and ocular non-Hodgkin’s lymphoma. Most people with uveal melanoma are older than 15 years old. Most of the time, retinoblastoma is found in very young children. The average age at detection is 5. Uveal melanoma does less damage to the eye and the body of the ciliary than it does to the choroidal area. Research shows that retinoblastoma attacks cone photoreceptors in particular. This kind of cancer is not hard to figure out.[Citation36] Even though the rate of uveal melanoma (UM) is low, with only about 6 new cases per million people each year.[Citation37] It is still the most common type of primary eye cancer seen in people. Retinoblastoma is the name of the disease that happens when cancerous tumors grow in the cells of the eye. Studies show that people with a type of retinoblastoma that can be passed down have a higher chance of getting more cancers in the future.[Citation38] This number is equivalent to about 40% of all cases of retinoblastoma. Fluorescein angiography (FA), ultrasound (US), and fundoscopy have all been used to diagnose UM in the past.[Citation37] In the past few years, a lot of epidemiological studies and clinical tests have been done to find out if certain foods can help improve or stop the loss of eyesight that comes with getting older in older people.[Citation39] Chrysin, which is also called 5, 7-di-OH-flavone, is a flavonoid that has been getting a lot of attention lately. Research studies have found that the main ways chrysin works are by stopping cell growth, speeding up cell death through apoptosis, and reducing inflammation.[Citation40]
Researchers have studied the effects of chrysin on eye cancer in mice. Mice were given 25 milligrams per kilogram of body weight of chrysin dissolved in 10 liters of dimethyl sulfoxide (DMSO) and 140 liters of phosphate-buffered saline (PBS) by oral gavage starting three days before vaccination (day 3) and continuing until they were killed 21 days after vaccination.[Citation41] An EAU model made by IRBP and CFA was used to look into the possibility of chrysin as a treatment for uveitis. Mice were given injections of chrysin every day, starting on day 3 and going through day 21. Inspections of the fundus began on day 12 and went on until day 21. This made it possible to keep track of each mouse’s medical history and how sick it was. Papilledema, vasculitis, retinal degeneration, and retinal separation were some of the signs of EAU. A study found that mice who were given chrysin had much less inflammation and tissue damage than mice who were not given any treatment. The focus of the study was also on raw human RPE cells. Before chrysin was added to a culture of living cells, it was dissolved in dimethyl sulfoxide (DMSO). The total amount of DMSO in the culture was less than 0.5%. The quantity of chymotrypsin that is used ranges from 1 M to 20 M. In a Western blot experiment, 3.5 105 RPE cells were planted in each well, and those cells were used to make whole cell lysates, which were then used to study. The results showed that RPE cells started to grow after three days in an incubator where they were exposed to 33 mM of glucose. But the growth of these cells was stopped when harmless chrysin was present in amounts ranging from 1 to 20. A study found that the release of cytokines, growth factors, and parts of the extracellular matrix by cells of the retinal pigment epithelium (RPE) contributes to the growth of retinal and choroidal neovascularization.[Citation42] Researchers used an extra H22 xenograft mouse model to study how chrysin affects the growth of tumors and how PD-L1 is expressed in tumors. The amount of cytotoxicity caused by chrysin in HepG2 cells that had been treated with interferon gamma (IFN) was measured with the MTT test. Researchers used flow cytometry, ELISA, and RT-PCR to measure how much PD-L1 was expressed, and Western blotting was used to look at how much STAT3 and NF-B pathway proteins were expressed. Chrysin was shown to stop tumors from growing in an H22 xenograft mouse model, and it also improved the mice’s ability to fight tumors by making more CD4+ and CD8+ T cells show up in tumor tissues. The picture below shows how both of these things turned out. Not only did chrysin block the STAT3 and NF-B pathways, but it also greatly reduced PD-L1 production both in vivo and in vitro. Chrysin did this by going after the group of proteins that make up PD-L1. In a setting where T cells and IL-2 are grown together, it has been shown that chrysin increases both the rate of T cell growth and the amount of IL-2.[Citation43] A diode laser was used to break Bruch’s membrane in male brown Norway rats that had been put to sleep. Fluorescein angiography was used to look for CNVs in the rats’ eyes two weeks after they got an intravitreal dose of five liters of 15 mg/ml of chrysin. Researchers used fluorescein angiography and histology to figure out what effect chrysin had on CNV. After two weeks of laser treatment, there was a statistically significant change (p = .044) in the amount of fluorescein that leaked out of the lesions in the photocoagulated lesion group compared to the control group. After dividing the tumors into those with low leakage and those with high leakage, there was a statistically significant link between chrysin treatment and leakage grade (p = .028). Compared to the group that was given chrysin, the chance of high-leakage tumors was 3.18 times higher in the group that wasn’t given chrysin.[Citation44]
Effect of chrysin in oral cancer
The term “oral cancer,” which is often shortened to “OC,” refers to cancerous tumors that grow in the mouth and throat. However, when policy choices are made, the effects of this type of cancer on society and businesses are rarely taken into account. In 2018, OC was the cause of death for 177,384 people, and 354,864 new cases of OC were reported around the world. According to a study, smoking and using smokeless tobacco (SLT), drinking alcohol, and being infected with the human papillomavirus (HPV) are some of the main causes of mouth cancer.[Citation45] The study shows that this is true for all types of oral and oropharyngeal cancer. Cancers of the oropharynx, which are more commonly called cancers of the mouth and throat, are the sixth most common type of cancer in people. Even though the number of people under 60 who have been told they have mouth cancer has gone up, most people who get it are over 60. Overall, the five-year death rate for mouth cancer is as low as 40%, but it can go up to over 80% if it is found early (in stages I and II). If mouth cancer is not found early, 40% of people will die from it and up to 50% of cases of mouth cancer are found in stages III and IV, which are much later. Oral squamous cell carcinomas, or OSCCs for short, are the most common type of mouth cancer. They make up more than 90% of all cases of mouth cancer. Melanomas, lymphomas, and smaller tumors that start in the salivary glands are other types of cancer that can show up in the mouth and throat. Lymph nodes, which are a common place where OSCC can spread, can go through a lot of different kinds of changes at a lot of different levels.[Citation46] In order to achieve R0 resection, reduce surgery margins, and make the cancer easier to remove. This would improve both DFS (survival without sickness) and OS (total survival). Even after trying all of these different things, some food ingredients, like antioxidants, can still help. Antioxidants are made by combining enzymes and other chemical molecules. They can stop the effects of things in the body that might cause cancer, like reactive oxygen species. Antioxidants are a group of natural substances that have been shown to lower the risk of getting cancer. According to the study, vitamin E and flavonoids are both good examples of safe nutrients. Flavonoids can be found in both the food people eat and the supplement industry, which is growing quickly. Flavonoids are polyphenolic plant parts that appear spontaneously.[Citation47] Researchers say that chrysin (5,7-dihydroxyflavone), a natural bioactive flavone that is found in large amounts in bee wax, has anticancer, anti-inflammatory, antioxidant, hepatoprotective, antibacterial, and anti-diabetic effects. Also, it has been shown that chrysin helps protect the liver. People with diabetes may also benefit from chrysin.[Citation48]
After putting the cells in 96-well plates, the researchers treated the SCC-25 (Squamous Cell Carcinoma) and CAL-27 (Centre Antoine Lacassagne) cells for 12 hours with chrysin (50, 100, and 200 M) and cisplatin (2.5, 5, 7.5, and 10 M). After 4 hours at 37 degrees Celsius, 5 mg/mL of methyl thiazolyl tetrazolium (MTT) was added to a 20-liter solution of methyl thiazolyl tetrazolium. After dissolving the intracellular formazan product that came from making the formazan crystals in 200 mL of dimethyl sulfoxide (DMSO), the absorbance at 490 nm was measured by putting the product in a microplate reader. The chemical 8-hydroxy-2′-deoxyguanosine, also known as 8-oxo-dG, is being looked at as part of research on reactive DNA damage. This chemical is, without a doubt, very important. An H2AX test was done on the samples to see if the DNA double-strand breaks that were found in the SCC-25 and CAL-27 cell nuclei had been fixed. After giving the fluorescent marker carboxy-2“,7”-dichlorodihydrofluoropsin diacetate (DCFHDA) to SCC-25 and CAL-27 cells in a 96-well plate for forty minutes, the cells were looked at. After taking off the supernatant and rinsing the cells in PBS, the light strength was recorded with a plate reader. After the liquid was taken out, this was done. When all of the fluid had been drained, this job was done.[Citation48] Scientists used a colorimetric caspase 8 test kit to measure the amount of caspase 8, an enzyme that plays a key role in the process of innate apoptosis. The MTT test showed that a dose of 6.4 M of cisplatin was enough to kill 50% of SCC-25 and CAL-27 cells. On the other hand, at a concentration of 5.77 M, 50% of CAL-27 cells were killed. Also, the 50% inhibitory concentration (IC50) of chrysin was found to be 56 millimoles for SCC-25 cells and 46 millimoles for CAL-27 cells, respectively.[Citation49] Chrysin and cisplatin were used to treat the cells, and the fact that chrysin was present made cisplatin work better. Checking the amount of 8-oxo-dG in SCC-25 and CAL-27 cells showed that cisplatin causes more 8-oxo-dG to form in both of these cell lines. After being treated with chrysin and then given cisplatin, both cell types made a lot more 8-oxo-dG than they did before. As with the last finding, these ones were made because of research into the link between cisplatin and 8-oxo-dG. The results showed that chrysin, cisplatin, and the mixture of chrysin and cisplatin caused the SCC-25 and CAL-27 cell lines to make more oxygen free radicals. After treatment with chrysin, cisplatin, or both, the amount of reactive oxygen species (ROS) was found to have gone up. When chrysin was given to SCC-25 and CAL-27 cells at the same time, Cas-8 activity was much higher than in the control group. This was the case at the level of importance called P 0.05. This result is in line with what was found in previous studies, which showed that cisplatin therapy alone may increase Cas-8 activity in SCC-25 cells by a lot but that chrysin therapy mixed with cisplatin therapy has a much stronger effect. In CAL-27 cells, cisplatin caused Cas-8 activity to go up a little bit. But chrysin can change the way the MMP9 gene is expressed and, as a result, change how cancer cells spread.[Citation49] This needs a full source. This is what happens in stomach and skin cancer cells. The effects of chrysin at 1, 2, 4, 8, 16, and 32 mol/L were also looked at in KB cells, which are a model for mouth cancer. The JC-1 test was used to figure out the membrane potential of the mitochondria in KB cells.[Citation50] The MMT test was used to measure cell growth, flow cytometry was used to measure cell death, and a chemiluminescent experiment was used to measure the activity of caspase-3/7. Through Western blotting, the authors were able to figure out how active AKT and PI3K were. Researchers have found that more chrysin can stop AKT and PI3K from getting phosphorylated, which makes it more likely for KB cells to die. It has also been shown that chrysin can lower the potential of the mitochondrial membrane and turn on caspase-3/7.
A recent study shows that mitochondrial malfunction and PI3K/AKT pathway dysfunction may be to blame for how chrysin causes KB cells to die. The human TSCC cell lines SCC9 and CAL27 were also studied. The extracellular vesicles (EVs) from the SCC9 cell cultures that were treated with chrysin (EVs-chrysin) and the SCC9 cell cultures that were treated with PBS (EVs-Con) were examined. Feeding was stopped for the SCC9 cells after they had been in contact with each other for 70% of the time. During the cell growth process, the supernatant fluid was filtered after being spun at 300 g for 30 minutes, 2000 g for 30 minutes, and 12,000 g for 45 minutes. EVs that had chrysin in them were treated with HAuCl4 (50 mM, Sigma), and the treatment lasted all night at 37 degrees Celsius. Nano-flow cytometry’s dynamic light scattering (DLS) method was used to measure the size and number of EVs-Con and EVs-chrysin particles. The shape of the EVs-Con, EVs-chrysin, and Au-EVs was looked at with transmission electron microscopy (TEM). Both the EVs-Con and the EVs-chrysin samples were treated so that their total RNA could be recovered. In order to find out how quickly SCC9 cells moved through a cut, an experiment was done to measure how quickly wounds heal. Five hundred and 105 SCC9 cells were looked at after they had been treated for 48 hours with either let-7a-3p models or inhibitors. The cells were then subcultured in FBS medium after going through many more rounds of the passage process. The authors looked at the scratched area 12, 24, and 48 hours after it was made. The study into cellular invasion used 3,444 SCC9 cells. The authors used a chemical called annexin V-FITC/PI to study apoptosis.[Citation50] According to what the study found, flow cytometry helped figure out which cells had died. It was found that EVs-Con and EVs-chrysin are round molecules in their normal states. In a DLS study, the size distribution of EVs-Con and EVs-chrysin was found to be between 50 and 150 nm. NanoFCM’s study showed that EVs-chrysin had all three proteins at the same time: CD9, CD63, and CD81. Also, both the standard EVs and the chrysin EVs were used to treat the SCC9 cells at the same time. EVs-Con and EVs-chrysin were found to be able to get into the cytoplasm of SCC9 cells. Based on these results, SCC9 cells released extracellular vesicles (EVs) that contained chrysin, which were then eaten by other SCC9 cells. The protein’s ability to fight cancer was improved by both growing EVs-chrysin in HAuCl4 and making Au-EVs. To figure out how much EVs-chrysin should be used to make Au-EVs, the results from using 10 grams and 30 grams of the solution were compared. The results showed that when AuNPs were grown on top of 10 and 30 g of EVs-chrysin, a new nanomaterial called Au-EV was made. This tiny piece of matter is called Au-EV. The way that Au-EVs and EVs-chrysin were taken in by SCC9 cells was very similar. The cells were happy to get both kinds of external vesicles. Based on these facts, Researchers say that SCC9 cells are constantly taking in Au-EVs. According to the results, chrysin works on the transcription factor let-7a-3p to speed up the death of SCC9 cells and stop their growth at the same time. The squamous cell line CAL-27 was used to find out what role chrysin plays in the tissue of the tongue. A test called methyl thiazolyl tetrazolium (MTT) was used to see if the flavonoids under study could kill CAL-27 cells. MTT is basically Carmichael’s test that has been improved. According to the Mitochondrial Tetrazolium Bromide (MTT) test, the mitochondrial enzyme succinate dehydrogenase can turn a yellow tetrazolium bromide MTT solution into purple formazan derivatives. This is the most important idea behind the test. The authors were able to hit a confluence level of 70% by growing the cells at a rate of 105 cells per milliliter in 96 well plates. After that, the polyphenols that were the focus of the study were given to the cells for a full 24 hours.[Citation51] Researchers used Western blotting as well as tests for cell growth and survival in their study. Chrysin, caffeic acid, p-coumaric acid, and ferulic acid were put through the MTT test to see if they affected the survival of the human tongue squamous cell cancer cell line (CAL-27). None of the polyphenolic chemicals that were tried failed when it came to stopping CAL-27 cells from growing. The study showed that chrysin made it less likely for cells to live by 82%, 62%, 32%, and 29%, respectively.[Citation52]
Emerging role of chrysin in brain cancer
The number of people who die from brain cancer is much higher in Asia than it should be. When cancer spreads to the brain or spinal cord, it shows up in these areas. Primary brain tumors start in the brain or spinal cord and don’t spread to other parts of the body. Malignant brain tumors are rare but likely.[Citation53] Brain cancer, both cancerous and nonmalignant, is diagnosed in about 28.57 out of every 100,000 people each year, on average. But while survival rates are going up for most types of cancer, they are going down for tumors in the central nervous system. Only 33.3% of people with these tumors are still alive after 5 years. Most kinds of cancer are getting easier to live with. It was found that the average amount of time a patient lived after their diagnosis was only 15 months. Benign brain tumors, like meningiomas, pituitary tumors, and astrocytomas, are made up of cells that rarely attack the healthy cells in the area around them, and the tumors grow very slowly. Oligodendrogliomas, high-grade astrocytomas, and other malignant brain tumors tend to grow quickly, have unclear edges, and affect nearby brain or spinal cord cells. Some of the signs of brain cancer are problems with balance, regular headaches, changes in mood, speech changes, trouble focusing, seizures, and memory loss. Primary and secondary brain tumors can be told apart by where they started. Main brain growth starts in a brain cell that was there before and grows from there.[Citation54] A metastasis is when cancer starts in another part of the body and then spreads to the brain. It could also be called a second brain tumor. Brain tumors are also ranked from low to high in terms of how bad they are based on how quickly the cancerous cells spread.[Citation53] Antioxidants are a very important part of fighting brain cancer, and they are used in all of the above treatment methods. Antioxidants, either natural or made in a lab, can be added to food to keep it from going bad during the “farm to plate” process. This is done by keeping the food from getting damaged by oxidation while it is being processed and stored.[Citation55] This is true, according to research. Most of the polyphenolic chemicals found in plants belong to a group called flavonoids. Flavonoids, which have qualities that make them protective, anti-inflammatory, anti-viral, and anti-carcinogenic, are involved in a number of different cellular signaling pathways and play an important role in keeping the body healthy as a whole. Chrysin is a type of flavone that is also known as chrysinic acid. Honey, propolis, and different fruits and vegetables are some of the most important sources.[Citation56] According to a study, it can work as an anti-inflammatory, anti-tumor, anti-asthmatic, anti-hyperlipidemic, cardioprotective, neuroprotective, and renoprotective agent.[Citation57]
A Kit-8 test was used to count the number of cells, and a plate colony formation experiment was used to figure out how fast the cells were growing. A wound-healing experiment was used to measure the movement of cells. An experiment was used to see how well cells could move and spread. The Authors were able to stop Nrf2 from being made by transfecting it with shRNA. Western blotting and fluorescent tagging were used to look at how proteins were being made.[Citation58] It was found that tumor xenografts in naked mice were used to test how well the drug worked against cancer in real life. Chrysin was found to stop glioblastoma cells from growing, moving, and spreading in a way that depends on the amount and time. Chrysin didn’t have a big effect on how much p-JNK and p-P38 proteins were expressed, but it did hurt how much p-extracellular signal-regulated kinases 1 and 2 (ERK1/2) proteins were expressed. A study found that chrysin finally slowed down the growth of tumors in U87 xenografts. Researchers also found that glioma cells treated with chrysin stopped dividing in the G1 phase of the cell cycle. This is shown by a rise in the protein P21 (waf1/cip1) and the activation of P38-MAPK. Chrysin and pine-needle extracts may be able to stop the inhibition of O-6-methylguanine-DNA Methyltransferase (MGMT) and the activation of AKT, which are both needed for the growth of gliomas. Chrysin worked better against glioblastoma in GBM8901 cells than any of the other medications being tried. When GBM8901 cells were exposed to amounts between 25 mM and 100 mM, their growth was halted in a way that depended on the concentration. This relationship changed over time.[Citation59] According to the study, the fact that chrysin did not hurt other glial cell lines as much as other chemicals did shows that it may have specific anti-glioblastoma effects without hurting normal cells. At high amounts, chrysin cuts caspase-3 and poly (ADP-Ribose) polymerase (PARP), which stops cells from growing and makes them die.[Citation57] A172 and LN18 human glioblastoma multiforme (GBM) cell lines have also been looked into. In both types of GBM models, Chrysin and Tmz use the intrinsic apoptosis mechanism. This is done by turning off Akt and ERK1/2 and turning on the p38 MAPK protein at the same time. The MTT method was used to look at the cells after they had been exposed for 48 hours to different mixtures of chrysin (M) and Tmz (IC50 concentration range and below). In order to find out the IC50 values for chrysin and Tmz in the A172 and LN18 cell lines, GBM cells were treated with 20 L of each drug at concentrations ranging from 0 to 60 M for 48 hours. From 0 to 60 million, the amounts varied. MTT was used to find out if the cells were still alive or not. The MuseTM Cell Viability Kit was used as the main tool in a cytometric cell viability experiment, which was done to find out how harmful individual and mixed GBM cell treatments were. The MuseTM Annexin V and Dead Cell Assay was used to find out that apoptosis was happening in A172 and LN18 cells. The IC50 values for chrysin were found by exposing Tmz-sensitive A172 and Tmz-resistant LN18 cell lines to increasing amounts of chrysin (3.125 to 100 M) or Tmz for 48 hours. This was done to find out which cell line could handle Tmz better. The IC50 numbers for chrysin were also much lower, which showed that it killed GBM cells much more effectively. To find the perfect mix that kills the most cells, For future study, a cytometric cell viability experiment was done because, compared to the MTT test, it gives a more accurate numeric measurement of cell viability. The number of A172 cells that died after being treated with either 20 M chrysin on its own or 20 M chrysin plus 50 M Tmz was much higher. The mixture of chrysin and Tmz can cause apoptosis more effectively than either medicine alone, even though the quantity of Tmz is lower in the combination. When chrysin was added to Tmz, it made the medicine much more effective at causing apoptosis.[Citation60] This was done by lowering the amount of proteins that stop cells from dying and increasing the amount of proteins that make cells die. Most of the work has been done on the human glioma cell line U251 and the two main glioblastoma cell lines, GBM28 and GBM37. To show that the death of cells in all three GBMs was caused by chrysin and DCA, which are two inhibitors of HIF1 and PDK. So that the cells would stick together, 3103 GBM cells were planted in each well of a 96-well plate. The cells were then grown for 24 hours at 37°C in an environment with 5% CO2 and 90% relative humidity. After that, the cells were put in a container for another 72 hours, where they were exposed to 1% oxygen and hypoxia. In order to make the control groups, the normoxic chamber, which had 20% oxygen, had to be used. Over the course of three days, the Cell Counting Kit-8 was used every hour for 24 hours to find out how hypoxic and normoxic conditions affected the growth of individual cells. After the first two hours of incubation, ten liters of the CCK solution were poured into each well. Using the directions from the maker of the Multiscan MS spectrophotometer, the absorbance of the reaction solution was measured at 450 nm. This was done to measure how many cells were being made. Each experiment was done at least three times, and all three times the same findings were found. With the help of the RNeasy Mini Kit, GBM cells were broken up and their total RNA was taken out.[Citation61] Chrysin is a flavonoid that is found in nature and can stop HIF1 from working. When chrysin was added to GBMs, it caused a lot of cell death in both normoxia and hypoxia. Cells that grew in hypoxia were much healthier than those that grew in normoxia, which suggests that hypoxia-dependent tolerance is still being maintained. Even though the effects of chrysin depended on the amount in all cell types, this was still the case. After the chrysin was injected, the genes PDK1, PDK3, and GLUT1 that are involved in glycolysis had less expression. Chrysin also stopped the HIF1 gene from being copied. Even though hypoxia made some GBM cells less likely to live, the hypoxia-dependent rises in these genes were still found in some cells of all types. Chrysin seems to have increased cell death in all of the GBMs that were studied by lowering the expression of genes linked to hypoxia and glycolysis. In a recent study, the researchers found that when DCA and chrysin were added to the treatment of GBMs, they made the cells die faster.[Citation61]
Chrysin’s therapeutic impact on breast cancer
Breast cancer, which mostly affects women, is the most dangerous type of the disease. Because of this, most women who die from cancer do so in countries that are still getting started. Cancer is the top cause of death for people under the age of 65 in Canada.[Citation62] To put it another way, about 244,000 younger women in the United States are diagnosed with breast cancer (BC) every year. By the year 2050, it is expected that 3.2 million women will be diagnosed with breast cancer for the first time. The most common type of breast cancer, which makes up between 60 and 70% of all cases, is called a Luminal Breast Tumor. There is a link between estrogen and progesterone receptors and these cancers. This is a very dangerous type of breast cancer that spreads quickly. Triple-negative breast cancer (TNBC), which is also called “basal breast cancer,” is a type of breast carcinoma that doesn’t have estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) expression.[Citation63] Another study says that triple-negative breast cancer, also called TNBC, is different because it doesn’t have any of these three hormone receptors. The estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) are all examples of these receptors. Researchers found that antioxidants are an important part of treating cancer. Many people think that flavonoids are a good place to start looking for new cancer treatments that might be helpful. The flavonoid called chrysin comes from plants. It has a backbone made up of 15 carbon atoms. In the scientific world, it is known as 5,7-dihydroxyflavone.[Citation64] A study says that chrysin’s most reliable pharmacological properties are that it is anticancer, neuroprotective, antiviral, antibacterial, anti-asthmatic, anti-inflammatory, hepatoprotective, nephroprotective, cardioprotective, antidiabetic, antidepressant, anxiety-relieving, and antiarthritic. Chrysin is also good at killing bacteria, viruses, and fungi. When double-strand breaks (DSBs) are present, chrysin stops 53BP1 from being recruited and slows down the recruitment of RAD51.[Citation65]
In clonogenic studies, it was found that some mixtures of chrysin and etoposide were effective at killing breast cancer cells. Chrysin hurts the ability of MCF-7 cells to live and makes them more vulnerable to the effects of chemotherapy. Also, it hurts the DNA of MCF-7 and BT474 cells. Chrysin stops DSB repair, which means that DNA damage builds up. Double-strand breaks can be fixed with DSB repair. Also, it stops the recruitment of 53BP1, which is an important part of NHEJ, and lowers the recruitment of RAD51, which is an HR factor.[Citation66]
The T47D breast cancer cell line was used to show what happened when PCL-PEG-PCL was tested with chrysin. Moreover, 1 H NMR, FT-IR, and SEM help in understanding the structure of chrysin and how it stores medicines. The MTT method was used to test the cytotoxicity of both pure chrysin and nano-chrysin in a test tube. Using real-time PCR, part of the genes FTO, hTERT, and BRCA1 were identified in the body. Based on the results of the MTT test, the effect of chrysin on the T47D cell line changes over time and, in the end, causes the cells to die. A study found that the effect of encapsulated chrysin on the expression of FTO, BRCA1, and hTERT genes was much more anti-cancer than that of free chrysin. Over the course of three days, different amounts of the enzyme chrysin were added to human breast cancer cells (MCF-7) in a Petri dish. A test called the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was done to find out if the cells were still alive.[Citation9] The researcher used flow cytometry with Annexin V-fluorescein isothiocyanate to figure out how many cells had died. The MTT test showed that chrysin stopped the growth of MCF-7 cells in different ways depending on how much and how long the cells were exposed to the chemical. After 48 and 72 hours of treatment at 19.5 M and 9.2 M concentrations, chrysin was able to stop the growth of MCF-7 cells by a factor of 50%. Flow cytometry showed that chrysin caused MCF-7 cells to want to kill themselves. Chrysin may help fight cancer in a number of ways, including by making cancer cells go through a process called apoptosis, which stops the cells from multiplying. The study also shows that chrysin can kill cancer cells and has the potential to be turned into a chemotherapeutic treatment that could be used to treat breast cancer cells in the future.[Citation67]
The researchers looked at a few different samples of Chinese propolis. Propolis is a natural material made by honey bees that is very good at fighting cancer. Cancer cells can’t grow as fast when propolis is around. The amount of chrysin in these samples was pretty high, ranging from 2.52 to 6.38%. An enzymatic study has shown that chrysin has a big effect on how the enzyme HDAC8 works (EC50 = 40.2 M). When breast cancer cells (MDA-MB-231) were treated with chrysin, their growth slowed down a lot and they changed into many different types of cells. For 42 days, an HDAC8 inhibitor (90 mg/kg per day) was given by mouth to an MDA-MB-231 transgenic mouse. During this time, the growth grew much more slowly than it would have otherwise.[Citation67]
A study shows that adding Chrysin to cells raises both the mRNA and protein amounts of p21, which is an inhibitor of cyclin-dependent kinase. Using a test tube, the effect of Chrysin on hypoxia-treated mouse breast cancer cells (4T1) has been studied. This medicine caused the cells to die because it stopped STAT3 from getting modified when there wasn’t enough air. Chrysin was also able to stop the rise in VEGF gene expression that had been caused by hypoxia. He also found that giving Chrysin to Balb/c mice every day through their mouths stopped the growth of 4T1 cells that had been transferred into the animals. Researchers looked into how chrysin fights hepatocellular cancer (HCC) and how HK-2 helps chrysin do its job.[Citation68]
The researchers used western blotting and antibody tagging to find out if HCC cell lines and tumor tissue had HK-2 or not. The researchers looked into the link between chrysin and the growth of HCC cells and how sugar is used by tumors. Flow cytometry was used to find out what effect chrysin has on apoptosis. How chrysin affected the production of HK-2 and how it caused human colon cancer cells to die by apoptosis were studied. Researchers looked at the effects of chrysin on cell death and the stopping of glycolysis in HK-2 cells that made too much of a certain protein. Researchers looked to see what happened to HK-2 in the tumor cells after the chrysin treatment. They also used an HCC cell transplant model to show that chrysin worked against cancer in real animals. Chrysin has been shown to stop the growth of cancer cells in animals that are still alive. Compared to normal cell lines and tissues, the expression of HK-2 was found to be much higher in most of the HCC cell lines and tumor tissue that were looked at. When chrysin was added to HCC cells, they took in a lot less glucose and made a lot less lactate than usual because HK-2 expression went down a lot. When chrysin is used to treat a cell, Bax moves from the cytoplasm to the mitochondria, where it works with VDAC-1 to start the cell death process. This is because chrysin causes HK-2, which works with VDAC-1 on mitochondria, to drop by a lot. When chymotrypsin was used on cells with too much HK-2 to stop apoptosis and metabolism, it caused a number of serious problems. In HCC xenograft models, chrysin treatment stopped tumors from growing by a lot, and the amount of HK-2 mRNA in tumor tissue that had been treated with chrysin therapy went down by a lot. According to a study, people with HCC who have a high amount of HK-2 expression may benefit the most from treatment with chrysin or a drug that is similar to it. This is because chrysin stops glycolysis, which is what HCC cells need to do to live.[Citation69]
Chrysin in lung cancer treatment
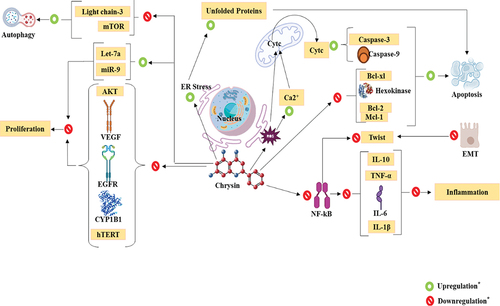
Lung cancer is the most common type of cancer and the main reason why people die from it all over the world. This makes it a major public health problem. According to the study, it is responsible for 20% of all cancer-related deaths, or 1.8 million deaths among men and women. Lung cancer is the most common way for both men and women who have the disease to die. It has a higher death rate than colon, breast, and pancreatic cancers when all of them are added together. Lung cancer patients have a recovery rate of about 17.8% after five years, but within one year of being diagnosed, the death rate is over 50%. Small-cell lung carcinoma, which makes up 15% of cases, and non-small-cell lung carcinoma, which makes up 85% of cases, are the two most common types of lung cancer. Non-small-cell lung cancer includes squamous-cell carcinoma, adenocarcinoma, and large-cell carcinoma. Between 25% and 30% of lung cancers are caused by a type of cancer called squamous cell carcinoma. It starts in the middle of the lung, in the bronchial tubes, where the squamous cells are. Non-small cell lung cancer is much more likely to happen to people who smoke cigarettes. Adenocarcinomas are the most common type of lung cancer, and about 40% of people who are diagnosed with lung cancer have them. This chemical is made by type II alveolar cells in the lungs. These cells line the very small tubes in the lungs and make this chemical. Large cell carcinoma, also called “undifferentiated carcinoma,” makes up 5–10% of all lung cancers. Because the cells in this cancer don’t change into squamous or glandular cells, it is usually only found after the patient has died and all other possible reasons have been ruled out.[Citation70] Reactive oxygen species, also called ROS, are needed for a wide range of biological processes when they are present in normal amounts. This is because they turn on signaling pathways that are needed for cell growth and development. But too much of what are called reactive oxygen species, or ROS, can be bad for DNA, proteins, and lipids. Through the modification of cell signaling pathways, chrysin causes its anticancer effects. Nuclear factor-kappa B (NF-kB), a transcription factor that is essential for the development of cancer and inflammation, has been shown to be inhibited by chrysin. Chrysin can slow the growth of cancer cells by inhibiting NF-kB and reducing the expression of genes that promote inflammation and cancer. Additionally, it has been discovered that chrysin controls the expression of several genes linked to apoptosis and the course of the cell cycle. For instance, it has been demonstrated that in cancer cells, chrysin upregulates the expression of pro-apoptotic genes like Bax and caspase-3 and decreases the expression of anti-apoptotic genes like Bcl-2. Additionally, chrysin has been demonstrated to have anti-angiogenic properties, which means that it can stop the growth of new blood vessels needed for the expansion and progression of cancer cells. Additionally, it has been discovered to increase the cytotoxicity of chemotherapy medications, raising the possibility that it could be a helpful addition to traditional cancer treatments.[Citation71]
Chrysin prevents Swiss albino rats exposed to benzo (a) pyrene (B (a) P), from developing lung cancer. For four weeks, 50 mg/kg of B(a)P was given orally twice a week to mice in order to make them get lung cancer. Estimates were made for a number of biomarkers and parameters, such as body mass index, lung capacity, tumor incidence, lipid peroxidation, carcinoembryonic antigen, enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase), and non-enzymatic antioxidants (reduced glutathione, vitamins E and C). Lung tissue was also looked at using histopathology and western blotting. In these tests, PCNA, COX-2, and NF-B were looked for. After the B(a)P treatment, the levels of enzyme antioxidants went down while the levels of other antioxidants went up. This caused the amount of carcinoembryonic antigen and lipid peroxides to go up. When people with lung cancer were treated with chrysin (250 milligrams per kilogram of body weight), it helped a lot.[Citation72]
Lung histology and immunoblotting studies of PCNA, COX-2, and NF-B showed that adding chrysin stopped the production of these proteins and maintained the balance of cells. This was shown by the fact that chrysin kept the balance of cells. These results show that chrysin could be used to fight cancer. Researchers have also shown that chrysin slows down the growth of a lung cancer cell line called L132. L-132 was grown at 37 degrees Celsius in culture plates or flasks with Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% Fetal Bovine Serum and 1% drug solution with penicillin, streptomycin, and amphotericin. The air was moist and had 5% carbon dioxide in it. Every time the stock culture went through a multilayer culture with 0.02% EDTA, its size grew by an order of magnitude. When the cells were kept alive in complete medium, they had to be reseeded often. The MTT and NRU tests were used to find out how harmful the sample was to cells. DAPI was used so that the number of dead cells could be counted. The Western blot and RT-PCR methods were used to find out if proteins were pro- or anti-apoptotic. Researchers looked into the protein chrysin and used computers to figure out how to stop it from doing the job it was made to do. After 48 hours, chrysin made TRAIL (tumor necrosis factor related apoptosis-inducing ligand) a lot more effective than TNF (tumor necrosis factor) at killing L132 lung cancer cells. This was shown by the way the shape of the nucleus changed when DAPI (4,6-diamidino-2-phenylindole) was used to color the cells. Treatment. When cells are treated with chrysin, they make more proteins linked to apoptosis, such as caspase-3, 8, 9, and Bax, but less Bcl-2. They did a study in which they mixed chymotrypsin with caspase-3, caspase-8, caspase-9, bax, and bcl-2 to try to find out what factors stop it from working. The IC50 concentration was found by using the MTT test and lung cancer cell lines from normal, PANC-1, and A549 cases. Chrysin nanoparticles, which are sometimes called CCNPs, were also looked into. Chrysin and CCNPs were put to the test to see if they could stop cytochrome oxidase (CI), superoxide dismutase (SOD), and mitochondrial swelling from doing their jobs.[Citation73] Researchers used RT-qPCR to look at how much genes like SDH C and D subunits, sirtuin-3 (SIRT-3), and hypoxia-inducible factor (HIF-1) were being expressed. The value of the IC50 found when CII subunits C and D bound to chrysin was then used to see how ubiquinone oxidoreductase affected the activity of SDH. The drop in expression of SDH C and D, SIRT-3, and HIF-1 mRNA (CCNPs chrysin 5-FLU) backs up the fact that enzyme activity went down (chrysin CCNPs 5-FLU and CCNPs chrysin 5-FLU, respectively). Compared to non-cancerous cells, PANC-1 and A549 cells had much higher levels of apoptotic effects (CCNPs > chrysin > 5-FLU) and mitochondrial expansion (CCNPs > chrysin > 5-FLU and CCNPs > chrysin > 5-FLU, respectively). By increasing the activity and expression of succinate-ubiquinone oxidoreductase, CCNP treatment may be more effective than chemotherapy at stopping spread and the growth of new blood vessels in PDAC and lung cancer. This is what a study that came out in 2023 showed.[Citation74]
In addition, the experts looked at SCC tissue from people. Human recombinant Akt, chymosin, luteolin, Lipofectamine 2000, and LY-294002 proteins, as well as recombinant LY-294002 proteins, in each cell of a 96-well plate with a flat bottom, there were 733 cells. Anticancer drugs like chrysin and luteolin were given to the cells for twenty-four hours, along with a solution that didn’t have FCS. The researchers used a Premix WST-1 Cell Proliferation Assay Kit to figure out how healthy the cells were in general. With the help of ImageJ, researchers measure the relative brightness of DXR and LOX-1, which is a test for low oxygen. The TRI solution was used to get all of the RNA out of the cells. Five grams per milliliter of human recombinant Akt were pumped into the flow cell at a rate of 50 liters per minute. Using a QCM sensor device in the experiments helped to figure out how chrysin works with Akt. Changes in the CH2 and CH1 frequencies were used to figure out how the sensor would respond. It was found that flavonoids called chrysin in goods made from propolis change the way CLDN is expressed. Both luteolin between 5 and 50 M and chrysin above 50 M were able to kill more cells at higher concentrations than at lower concentrations. Both 10 M chrysin and 10 M luteolin were shown to lower the levels of CLDN11 protein and mRNA. However, chrysin only changed the amounts of CLDN1. CLDN1 protein levels could not be lowered by quercetin or kaempferol. Based on these results, they said that chrysin may be able to stop the phosphorylation of Akt as well as the link between PDK1 and Akt by attaching directly to Akt.[Citation75]
Exploring chrysin’s role in ovarian cancer
Ovarian cancer is one of the most dangerous diseases a woman can have. It is right up there with cervical cancer and uterine cancer in terms of how often it kills. A lot of people have died, and it doesn’t look like things will get better in the near future. Even though ovarian cancer is less common than breast cancer now, it is expected that a lot of people will die from it by the year 2040. A study found that non-Hispanic white women were most likely to get ovarian cancer, with a rate of 12.0 per 100,000. After that, the rate was 10.3 per 100,000 for women of Hispanic background, 9.4 per 100,000 for women of African background, and 9.2 per 100,000 for women of Asian or Pacific Islander background. About 152,000 women die every year from ovarian cancer (OC), a type of cancer that affects the ovaries. Every year, about 239,000 women are told they have ovarian cancer. A woman has a 1 in 75 chance of getting OC in her lifetime, and if she does, she has a 1 in 100 chance of dying from it. Because the signs don’t show up until later, only 29% of people who are identified will still be alive in their fifth year. There are three main types of ovarian cells that can turn into ovarian cancer. These are epithelial cells, stromal cells, and germ cells. The group of diseases called “epithelial OC” includes a lot of different types of tissues.[Citation76] One study found that OC may have come from one of the other reproductive systems to the ovary. If a woman has had ovarian cancer before or comes from a family with a history of the disease, she is more likely to get it again. Even if no one in their family has had breast or ovarian cancer, people with ovarian cancer are more likely to have the BRCA genes, which are responsible for most inherited cancers. When a person hits the age of 70, those with the BRCA1 mutation have a 40–50% chance of getting ovarian cancer. Those with the BRCA2 mutation, on the other hand, have a 10–20% chance.[Citation77] Antioxidants could be used as a possible form of treatment. Chrysin is a member of the flavone family. It is a hydroxylated flavonoid. You can find this vitamin in honey and propolis. When reactive oxygen species (ROS) and calcium levels in the cytoplasm rise because of chrysin, OC cells die. A Scientist’s study says that chrysin stops OC tumors from growing by turning on PI3K/Akt and mitogen-activated protein kinase (MAPK). Human ovarian cancer cell lines OVCAR3, OVCAR8, and ES2 have been used in research on serous carcinoma (OSC) and clear cell carcinoma (OCCC). The chrysin that was used in the study had selenium (Se) in it. Researchers used flow cytometry to count how many cells died after being exposed to encapsulate SeChry (SeChry@PUREG4-FA) alone or in combination with cysteine and/or carboplatin. Even though SeChry@PUREG4-FA cut down on the total number of dead cells, the ovarian cancer cells (ES2, OVCAR3, and OVCAR8) were still killed by the treatment. SeChry@PUREG4-FA nanoparticles caused OVCAR8 cells to die, and carboplatin did not work to protect them. When tested on HaCaT and HK2 cells, it was found that SeChry@PUREG4-FA nanoparticles were much less dangerous than SeChry on its own. Another study found that SeChry@PUREG4-FA nanoparticles were more likely to kill ovarian cancer cells than noncancerous cells, which were mostly keratinocytes. The role chrysin plays in human ovarian cancer stem cells is another thing that has been looked into. After the OVCAR-3 cells were treated with TNF and TGF, the growth medium was given 5, 10, and 20 umol/L of chrysin (ChR).[Citation78] Several studies have found that On top of that, a Western blot and mending of wounds were done. Tumor necrosis factor alpha and transforming growth factor beta were used to treat OVCAR-3 cells so that the role of ChR in EMT and CSLC traits could be studied. ChR might be able to stop the bad things that EMT does to the body. Sphere-making and wound-healing tests showed that ChR stopped OVCAR-3 cells from growing and moving when they were treated with TNF and TGF. It was found that increasing the amount of ChR upregulated the protein expression of E-cadherin while decreasing the expression of N-cadherin, CD133, and CD44. When TNF and TGF were given together, the levels of the proteins NF-Bp65 and Twist went up. However, ChR was able to stop this more than it would have been able to without treatment. They found that when both TNF and TGF were present in the surroundings, ChR stopped both EMT and CSLC in OVCAR-3 cells. In this study, ES2 and OV90 cells were used to find out how chrysin affects the cells’ ability to live, divide, and die. Chrysin and dimethyl sulfoxide were used to treat not only the ES2 ovarian clear cell cancer cell line, but also the OV90 cystic serous adenocarcinoma cell line. 5 105 cells were put in a CO2 oven set to 37 degrees Celsius with chrysin at one of the following amounts for 48 hours: 0, 5, 10, 20, or 50 millimoles. Before being looked at under a microscope, the cells were first spun down, then washed twice in cold 0.1% BSA in PBS, and then stored in 70% ethanol at 4 degrees Celsius for 24 hours. After the ES2 and OV90 cells were separated by centrifuging them, the leftover material was thrown away. After being dyed for 30 minutes at room temperature with PI in 100 ng/ml RNase A, the pellets were washed twice with 0.1% BSA in PBS. Both washes had 0.1% BSA in them. With the help of a flow cytometer, the amount of light was recorded. The authors did a study with the help of a fluorescein isothiocyanate (FITC) Annexin V apoptosis detection kit I to find out what effect naringenin has on apoptosis in endometriosis cells. Researchers used immunofluorescence imaging to find out how much PCNA (proliferating cell nuclear antigen) there was. The BrdU enzyme-linked immunosorbent test (ELISA) kit was used to find out how many cells were being made. The reactive oxygen species test (ROS) and the mitochondrial membrane potential test (MMP) were also done.[Citation79]
Researchers used the Bradford test to figure out how much protein was in the whole-cell samples. To find out how the protein affects the growth of cancer cells, chrysin was added to ovarian cancer cell lines ES2 and OV90 in the following amounts: 0, 5, 10, 20, 50, and 100 M. When ES2 cells were exposed to chrysin at doses of 5, 10, 20, 50, and 100 M, the growth of the cells was slowed by 43%, 55% (both p 0.01), 63%, 64%, and 68% (all p 0.001). Also, chrysin at doses of 10, 20, 50, and 100 M slowed the growth of OV90 cells a lot compared to untreated control cells by 28% (p 0.05), 41% (p 0.01), 75% (p 0.001), and 71% (p 0.001). Then, a fluorescent test was done to find out where in the cells the protein PCNA was. This protein is a sign that DNA replication is happening in cells that are growing. When ES2 and OV90 cells were left alone, there was a lot of protein cyclin A (PCNA) in the nucleus. On the other hand, ES2 and OV90 cells stopped making too much PCNA protein when chrysin was present. Based on these tests, chrysin was able to stop ovarian cancer cells from growing in people. The study shows that chrysin-induced apoptosis changed how ovarian cancer cells moved through the cell cycle. They found that one of the main ways chymase stopped the growth of ovarian cancer cells was by sending messages through PI3K and MAPK. Chymase also prevented the growth of other types of cancer cells.[Citation80]
Chrysin’s anticancer potential in kidney cancer
Kidney cancer starts in the tissues of the kidney. Clear cell renal carcinomas make up more than 70% of all cases of kidney cancer found in people. Kidney cancer is the thirteenth most common type of cancer in the world. Every year, about 330,000 new cases are found. According to a study, this type of cancer is the sixth deadliest in Europe, North America, Australia/New Zealand, and Japan. Most people who have renal cell cancer, also called RCC, die from it. It is expected that there will be 62,700 new cases of kidney cancer in the United States and 66,800 in China in 2016. There will be 14,200 deaths in the US and 23,400 deaths in China. People think that between 3% and 5% of kidney cancers are passed down from parents to children.[Citation81] Renal cell cancer (RCC) makes up about 5% of all cancers found in men and about 3% of all cancers found in women. Hypertension is more common in men than in women. When compared to women, men with hypertension were 1.32 times more likely to get RCC than women with the same disease. But it seems that hypertension seems to affect the formation of RCC in women more than in men. The second and third most common types of RCC are papillary carcinoma (pRCC; types I and II) and chromophobe carcinoma (chRCC; 5% of cases).[Citation82] Flavonoids are used as an active ingredient in all of the above cancer medicines. Polyphenolic substances like flavonoids are often found in high amounts in plant foods. Chrysin can come from a number of natural sources, such as flowers (especially the blue passion and the Indian trumpet), mushrooms, honey, and propolis. In addition to lowering anxiety and stress, it helps fight cancer, diabetes, inflammation, and apoptosis. It also has effects that help fight anxiety and stress.[Citation83]
For eight weeks, 40 male albino rats were kept at room temperature and given food and water. Rats averaged 160 grams. After two weeks in their new home, forty rats were put into four groups of ten. The chrysin group (CNT) got dissolved chrysin at 50 mg/kg/BW twice a day for four weeks, while the saline group (CNT) got a sugar pill. Both groups got the same amount of saline. Chrysin and GA3 were given to the protective group in similar amounts. The people in the group that got both treatments got chrysin an hour before GA3. On day 28, people were given isoflurane to make them sleepy and had their tail veins bled. The blood was kept at 20°C after being spun at 3,000 g for 10 minutes. To check the chemicals and metabolites in the blood. After taking the rats’ blood, took their kidneys and heads off. They were cleaned and cut after being washed in cold salt water. Samples for histology and immunohistochemistry were kept in 10% formalin in a neutral buffer. Qiazol kept tissue samples for RT-PCR and RNA extraction. Researchers used a colorimetric spectrophotometer and kits from Bio Diagnostics to check the amounts of catalase, reduced glutathione (GSH), nitrous oxide (NO), and malondialdehyde (MDA) in the blood. The tissue RNA extraction and screening were measured at 260/280 nm. There are gene primers for antioxidation, anti-inflammatory, and death genes. Quantitative RT-PCR was used to figure out how much each gene was being expressed.[Citation84] The amounts of creatinine, urea, and uric acid were all much higher than the levels in the control group. All three of these things are signs that your kidneys aren’t working well. Compared to the animals that had only been given GA3, the rats that had also been given chrysin had much less albumin and total protein in their bodies. Based on these results, chrysin seemed to lessen the effects that GA3 had on the biomarkers in the kidneys. The amounts of IL-1, IL-6, TNF, and NO in the chrysin + GA3 group were significantly lower than those in the GA3 group. According to what was found, chrysin worked as an antioxidant. These studies show that chrysin may have properties that make it good at lowering inflammation.[Citation84] In addition, experts watched how 35 male Sprague-Dawley rats behaved. The mice used in the study were between ten and twelve weeks old and weighed between two hundred fifty and two hundred seventy grams. Chrysin (5,7-dihydroxyflavone, which was 97% pure) and lead acetate (lead (II) acetate trihydrate) were the two things used in the experiment. The right amounts of PbAc and CR have been found. Twenty-one male rats were used in the experiment. Each of the rats was put into one of five groups of seven rats. The rats in Group 1, which was the control group, were given saline by mouth once a week. The rats in group 2 (the CR-50 group) received the CR by mouth once daily for seven days at a dose of 50 milligrams per kilogram of body weight. The third group, which was called the PbAc group, was given 30 milligrams per kilogram of body weight by mouth every day for seven days. For 7 days, rats in Group 4 (PbAc + CR-25) received 25 mg/kg/BW CR by mouth. Then, 30 minutes later, they were given 30 mg/kg/BW PbAc. The rats in group 5 (PbAc + CR-50) got CR at a dose of 50 mg/kg body weight 30 minutes before they started getting PbAc at a dose of 30 mg/kg body weight each day for 7 days. Blood was taken from the patient by sticking a needle into their Vena jugularis. After the blood samples were taken, they were spun at 1200 g for 15 minutes and then looked at. After getting serum from the blood, the sample was used to test how well the kidneys were working. After being cleaned in ice-cold physiologic saline (0.85% NaCl), one set of rat kidneys was flash-frozen at 80°C so that it could be used for biochemical testing, and the other set of rat kidneys was put in a 10% buffered formalin solution so that it could be looked at histologically. Then, each set of rat kidneys was put in its own jar. To find out how much creatinine and urea were in the blood, a commercial kit was used. At a frequency of 532 nanometers, the amount of malondialdehyde (MDA) was tested to find out the level of lipid peroxidation. To find out how much P53 there was, a rat ELISA kit was used. Tissue samples were taken from the kidney and kept in a solution with 10% formalin for 48 hours. This was followed by a histological analysis. The results showed that the damage to the kidneys caused by PbAc went down with higher doses (25 and 50 mg/kg/BW) and that the amounts of urea and creatinine in the blood went down as well. The results also showed that the kidney damage had stopped. Statistically, there wasn’t a big difference between the control group and the CR group. Also, it was found that after CR treatment, the rats who had been given PbAc went back to their normal weight, making them the same size as the rats who had been used as a control group. When compared to the PbAc group, SOD, CAT, and GPx functions, as well as GSH levels, were found to be much higher in the CR group. AQP-1 levels were much higher in the CR group than in the PbAc group, which shows that the CR treatment worked to reduce kidney damage. A study showed that CR stops cells from going through apoptosis by stopping p53 from being expressed. Male Wistar rats that were between 6 and 8 weeks old and weighed between 150 and 200 g were also used in the study. Based on the results of a small, dose-dependent pilot study that was done in the lab, a treatment plan for CH (Chrysin) was made, and its promise as a chemopreventive drug against kidney cancer was looked into. The title of the study asks, “Can CH Help Counteract the Biochemical and Serological Changes Seen in Rats after Being Poisoned with Fe-NTA?”. So researchers chose to do an experiment to find out by placing the 24 male Wistar rats into four groups of about the same size. After Fe-NTA was injected into each animal, they were all killed exactly 12 hours later. Before a biochemical test can be done, the kidney’s cells must be handled. Donor serum was made ready so that it could be used in immunology studies. After the kidneys were surgically removed, they were put through histopathological, immunohistochemical, and genetic tests.[Citation85] Another experiment also measured creatinine, blood urea nitrogen (BUN), protein, lipid peroxidation, Catalase activity (CAT), reduced glutathione (GSH), glutathione reductase (GR), and glutathione peroxidase (GPx) activities. According to the results, CH was able to stop Fe-NTA from lowering antioxidant defenses and causing kidney poisoning markers to rise. The number of people who got kidney tumors (RTs) in each treatment group. There were no signs that the people in Group I had kidney cancer. In group III, where CH (50 mg/kg bwt) was given along with DEN start and Fe-NTA promotion, there were 50% tumors. In group IV, where CH (100 mg/kg bwt) was given, there were only 28.57% tumors. It seems that CH stopped the growth of kidney cells because there were a lot fewer PCNA-positive cells in the kidneys of rats in Group IV that got 100 mg/kg bwt of CH than there were in the kidneys of rats in Group II. Because of this, it is very important to learn how CH can stop the spread of disease. They found that treatment with CH scavenged ROS, reduced proliferation and inflammation, dropped the number of tumors, and caused apoptosis.[Citation86]
Anticancer potential of chrysin against liver cancer
One of the top five cancers that kill the most people and on the rise every year at a statistically significant rate is liver cancer. There are many things that can increase the chance of getting liver cancer. These include the viruses hepatitis B and C, nonalcoholic fatty liver disease, cirrhosis caused by drinking too much alcohol, smoking, being overweight, having diabetes, having too much iron in your body, and a number of food risks. The future is not good for people with liver cancer. Surgical excision is only a good option for 5–15% of people because the process is least risky in the early stages of the disease. PLC, or primary liver cancer, is the seventh most common type of liver cancer. The most common types of primary liver cancer (PLC) are hepatocellular carcinoma, intrahepatic cholangiocarcinoma, hepatoblastoma, and combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (cHCC-ICC). The fourth main type of PLC is hepatoblastoma.[Citation87] The presence of viral hepatitis, alcoholism, nonalcoholic steatohepatitis, carbon tetrachloride (CCl4), 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), and a necroptosis-associated hepatic cytokine milieu due to hepatocyte necrosis are all important factors in the development of PLC.[Citation11] Getting an early evaluation can be done in a few different ways. Antioxidants like vitamins, flavonoids, and carotenoids also help fight cancer, which is a big factor in liver cancer. Flavonoids are a group of polyphenolic chemicals that are found naturally in plants. These substances have a number of good therapeutic effects, such as anti-inflammatory, anti-apoptotic, antioxidant, and anticancer properties. Cancer cells can’t grow as fast when flavonoids are around. Another study says that the flavonoid chrysin may be found in both Passiflora leaves and bee propolis. It has medicinal properties that make it neuroprotective, anti-diabetic, anti-cancer, nephroprotective, cardioprotective, anti-arthritic, and anti-asthmatic, just to name a few.[Citation88] Researchers found that chrysin can stop the effects of a number of hepatotoxins, such as ethanol, carbon tetrachloride, ammonia, and cisplatin. The anticancer potential of chrysin against HCC is shown by the way it turns on the p53/Bcl-2/caspase-9 pathway and goes after hexokinase-2. Chrysin also stops hexokinase-2 from working. It shows that changing the extracellular matrix is a very important part of controlling how HCC changes, grows, and spreads.[Citation88]
Also, both HepG2 and QGY7701 human HCC cells were used to study how chrysin causes apoptosis (cell death). The cells were grown in a humid setting with 5% carbon dioxide in RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 g/ml streptomycin. The growing medium was kept at a temperature of 37 degrees Celsius. Cell growth media was used as the medium of choice to dilute chymotrypsin to different amounts. After the cells had grown to a size of 60% confluence, they were given chrysin amounts ranging from 0 to 50 g/ml. Before a 96-well plate was put in an incubator, 5,000 to 10,000 cell seeds were put in each well. Over the course of twenty-four hours, different amounts of chrysin (zero, ten, fifteen, twenty-five, thirty, forty, and fifty g/ml; five samples for each amount) were added to cell cultures. After 4 hours of running the experiment at 37 degrees Celsius and 5% carbon dioxide, the supernatants were thrown away. The chemical Annexin V-FITC/PI was used to find cells that had died. Flow cytometry was used to find out which cells died from apoptosis. Apoptosis is the process by which a drug-treated cell dies. This happens when the mitochondria don’t work right and apoptotic signaling pathways are turned on.[Citation89]
Researchers say that apoptosis can damage mitochondria, and both caspase-9 and caspase-3 have been linked to this. The effects of chrysin on QGY7701 and HepG2 cells were a slower rate of growth, bending, and rounding, as well as separation from the bottom of the plate. As the dose of the medicine went up, it was also seen that more cells were breaking off. After chrysin was added to QGY7701 and HepG2 cells, the MTT test was done to see how healthy the cells were. After 24 hours of treatment with chrysin, the number of living HCC cells was significantly lower, and this effect was based on the amount. The half-maximal effective dose, also called the IC50, is a way to measure how well a medicine works. The IC50 values for chrysin in QGY7701 and HepG2 cells were found to be 18 and 25 g/ml, respectively, using the GraFit-Erithacus IC50 formula. It was decided that these numbers were scientifically important. When Annexin V and PI were used to mark QGY7701 and HepG2 cells and flow cytometry was used to look at them, it was clear that both types of cells had gone through apoptosis. When chrysin was present, HCC cells went through a process called apoptosis. Chrysin was found to increase apoptosis in HepG2 and QGY7701 cells in a way that depended on how much of it was in the cells. Higher amounts of apoptosis show that QGY7701 cells are more susceptible to the effects of chrysin treatment than HepG2 cells. Based on what was found, apoptosis was induced, which included caspase-9 and caspase-3. The protein amounts of p53, Bcl-2, Bax, Bad, and Bak were also checked to see if the p53/Bcl-2 signaling system had been turned on. The study showed that treating both HCC cell lines with chrysin set off their natural way of killing themselves (apoptosis). Western blotting and antibody tagging were used on tumor tissue and HCC cell lines, respectively, to look at how much HK-2 they had. Researchers looked at how Chrysin affected the growth of HCC cells and the way tumors broke down sugar. Flow cytometry was used to find out more about how chrysin causes apoptosis. The Research looked into how chrysin caused death in HCC cells and how it affected HK-2 expression. Researchers looked for changes in the way chrysin caused cell death and stopped glycolysis in HK-2 cells with exogenous increases.[Citation90] Researchers used an HCC cell xenograft model to test how well chrysin fights cancer in living things. Researchers looked at how chrysin affected HK-2 in tumor tissue that had been treated with chrysin. Most of the HCC cell lines and tumor tissue that were looked at had very high levels of HK-2 expression. This was compared to how normal cell lines and tissues expressed themselves. When chrysin was added to HCC cells, the expression of HK-2 went down. This made it much harder for the cells to take in glucose and make lactate. After being treated with chrysin, Bax goes from the cytoplasm to the mitochondria. This triggers cell death because HK-2, which works with VDAC-1 on mitochondria, drops a lot. This happened because HK-2 was in the mitochondria. Exogenous abundance made it hard for chymotrypsin to control planned cell death (apoptosis) and slow down glycolysis in HK-2 cells. In HCC xenograft models, chrysin treatment greatly slowed the growth of tumors, and HK-2 mRNA was greatly reduced in tumor tissue that had been treated with chrysin. Tumor growth was also slowed down a lot by chrysin treatment. They said that patients with a high amount of HK-2 expression may get the most out of treatment with chrysin or a version of the drug. This is because chrysin slows glycolysis and makes HCC cells more likely to die by apoptosis. The H22 xenograft mouse model was used to study how chrysin affects the growth of tumors and how PD-L1 is expressed inside tumors.[Citation91] The authors used the MTT test on HepG2 cells that had been treated with interferon gamma (IFN) to measure how dangerous chrysin is to cells. Flow cytometry, ELISA, and RT-PCR were used to measure how much PD-L1 was being made. On the other hand, Western blotting was used to measure the expression of proteins in the STAT3 and NF-B pathways. In this work, the CCK-8 test was used to measure the growth of co-cultured HepG2 and Jurkat T cells, and an ELISA reagent was used to measure the amount of IL-2.[Citation43]
Researchers found that using chrysin greatly slowed down the growth of tumors and made the mice’s immune systems stronger against cancer. This was done by making the mice’s tumor tissues have more CD4+ T cells than CD8+ T cells. For the study, the xenograft mouse model of H22 cancer was used. In addition to blocking the STAT3 and NF-B pathways, chrysin greatly reduced the expression of PD-L1 both in vivo and in vitro. In co-culture setups, there is also some proof that chrysin can increase the number of T cells and the amount of IL-2. Based on the information provided here, chrysin could be used as a preventative measure or as an extra treatment for HCC.[Citation43] Researchers wanted to find out how chrysin stops HCC cell lines from making spheres, so they looked at how the protein affects the control of SHP-1 and STAT3, which is a signal molecule that is downstream of SHP-1. MHCC97H and SMMC-7721 cells that had been grown in standard culture were taken out and put on six-well plates with ultra-low attachment. In line with what a Western blot showed. It was found that both SMMC-7721 cell spheres and MHCC97H cell spheres had much lower levels of SHP-1 protein production. Chrysin had a strong effect on both SMMC-7721 and MHCC97H cells to stop them from making spheres. At the same time, it increased the production of SHP-1 and decreased the levels of p-STAT3 and Twist1. Also, blocking SHP-1 in SMMC-7721 cells stopped chrysin from stopping the formation of spheres and led to more p-STAT3 and Twist1 proteins being made. The results showed that chrysin could be used to treat HCC because it changes the way SHP-1 and STAT3 talk to each other.[Citation92]
Chrysin and pancreatic cancer: a new approach to cancer suppression
In terms of the number of deaths caused by cancer around the world, pancreatic cancer (PC) ranks sixth. Pancreatic cancer, also called PADC, starts in the exocrine head of the pancreas. PADC is a type of cancerous epithelial neoplasia that causes the growth of ducts. Some things that put you at risk for pancreatic cancer are smoking cigarettes, having a family history of the disease, having diabetes mellitus, being overweight, eating different things, drinking alcohol, and not being active enough. It’s not clear where pancreatic cancer risk factors come from. GLOBOCAN 2018 says that pancreatic cancer will be the 11th most common disease in the world in 2018. It is expected to cause 458,918 new cases and 432,242 deaths, which is 4.5% of all cancer deaths. Pancreatic cancer was the 11th most common disease in the world in 2018. People think that pancreatic ductal adenocarcinoma, or PDAC, is one of the worst kinds of cancer. Since the disease makes less than 20% of people resectable, the benefits of treatment are not that great. According to a study, doctors are using combo therapy to reduce the side effects of individual drugs, improve the success of treatment, and deal with the problems caused by different types of tumors.[Citation93] Most of the cells in the pancreas are acinar cells, but because they are flexible, they can change into ductal-like cells through a process called metaplasia. Acinar-to-ductal metaplasia (ADM) is a metaplastic process that can happen in both acute and chronic pancreatitis. It may be the first step in the development of pancreatic intraepithelial neoplasia (PanIN).[Citation94] ADM is a change that happens in both short-term and long-term pancreatitis. Less than 8% of people who are diagnosed with pancreatic ductal adenocarcinoma (PDAC) live for 5 years after being told they have it. A recent study by Trovato and his colleagues (2019) has shown that when PDAC cells make pro-inflammatory chemicals, this helps myeloid-derived suppressor cells, or MDSCs, form and grow. The bioflavonoid chrysin can be found in large amounts in honey, propolis, and blue passion flowers (Passiflora caerulea). Passiflora is another name for a blue passion flower. Flavonoids are the most common type of secondary substance found in plants that is good for you. You can find the flavonoid chrysin in both Passiflora leaves and the honey that bees make. It works as an antioxidant, a pain reliever, an anti-inflammatory, an anti-diabetic, a neuroprotective, a liver protector, a heart protector, and a lipid-lowering agent. These are just a few of the many health benefits that it has. They said that chrysin could be used as a substitute medicine because it has good therapeutic benefits.[Citation32]
To learn more about how well therapy works, pancreatic tumor cell chrysin (CHY) was tested on solid lipid nanoparticles (SLNs), which were then decorated with folate-bound chitosan. In order to make CHY-SCF-NPs and study them, homogenizing and sonication methods were used. FA The therapeutic effectiveness of CHY-SCF-NPs was measured by looking at the following parameters: binding and encapsulation efficiency (HPLC), antioxidant capacity (ABTS and DPPH), cell survival (MTT), analysis of programmed cell death (fluorescent staining, flow cytometry, and quantitative real-time PCR), and angiogenesis (CAM and molecular). It was found that coating CHY-SLNs with folate-bound chitosan made them bigger (PS: 125 nm, ZP: +34.9 mV) and changed their surface charge (PS: 84.3 nm, ZP: 18 mV). It was found that 53, 55, 249, and >250 g/mL were the limiting concentrations (IC50) of CHY-SCF-NPs against PANC, MCF-7, A2780, and HepG2 cancer cells, as well as normal HFF cells. In the CAM and qPCR tests, CHY-SCF-NPs were able to get rid of free radicals and stop the formation of new blood vessels. ABTS had an IC50 of 123.73 g/mL, while DPPH had an IC50 of 108.7 g/mL. Fluorescence marking and information about the cell cycle, as well as the increase of the genes for Bax and caspase 9, were used to prove that CHY-SCF-NPs were pro-apoptotic. CHY-SCF-NPs have shown a lot of promise as a possible treatment for cancer in both animal and clinical studies of pancreatic cancer.[Citation95] MIA PaCa-2 cells and a xenograft model were used to figure out how chrysin-induced GPER activation stops PC from growing. Experiments were done with clonogenicity, cytotoxicity, Western blotting, and flow cytometry. In addition, the MIA PaCa-2 xenograft model was used to study how the treatment affected the growth of the tumor. Western blotting, immunohistochemistry, and protein analysis were used to look at samples of tumor tissue. Chrysin was able to stop cells from dividing and stop the cell cycle. When 17-estradiol was added to the treatment process, it made the effect of chrysin on cell growth even worse. In a xenograft model, the growth of the tumor was greatly slowed by both chrysin and G1, which is a GPER agonist. Along with this, the amounts of Ki-67, which is a sign of growth, and c-Myc in the tumor tissues went down. After treatment with chrysin, tumor tissues were put through a proteomic analysis, which showed that the protein levels of rho-associated coiled-coil containing protein kinase 1 (ROCK1), transgelin 2 (TAGLN2), and FCH and Mu domain containing endocytic adaptor 2 (FCHO2) were much lower. The Kaplan-Meier survival study showed that having high expressions of ROCK1, TAGLN2, and FCHO2 was linked to having a low PC survival rate. Researchers say that these results support the idea that chrysin prevents PC development by increasing the expression of GPER and decreasing the amounts of ROCK1, TAGLN2, and FCHO2. Researchers looked at how adding 5-fluorouracil (5-FLU) to chrysin and chrysin nanoparticles (CCNPs) affected the activity and production of mitochondrial complex II (CII) in pancreatic (PANC-1) cells in order to cause death. After making, describing, and testing chrysin nanoparticles (CCNPs), the IC50 was found in normal PANC-1 cell lines using the MTT assay.[Citation96] After the chrysin nanoparticles (CCNPs) were made, this was done. Chrysin and CCNPs were tested to see if they could stop cytochrome oxidase (CI), superoxide dismutase (SOD), and mitochondrial swelling from doing their jobs. Flow cytometry was used to measure apoptosis, and RT-qPCR was used to look at the expression of several genes, such as SDH C and D subunits, SIRT-3, and HIF-1.[Citation74] The value of the IC50 found when CII subunits C and D bound to chrysin was then used to see how ubiquinone oxidoreductase affected the activity of SDH. The decreased expression of SDH C and D, SIRT-3, and HIF-1 mRNA (chrysin CCNPs 5-FLU) confirmed that the enzyme activity (chrysin CCNPs 5-FLU and CCNPs chrysin 5-FLU, respectively) went down. Compared to non-cancerous cells, PANC-1 cells had much higher levels of apoptosis (CCNPs > chrysin > 5-FLU) and mitochondrial enlargement (CCNPs > chrysin > 5-FLU and CCNPs > chrysin > 5-FLU, respectively). In pancreatic ductal adenocarcinoma (PDAC), treatment with CCNPs increased the effect of chrysin on the activity and expression of succinate-ubiquinone oxidoreductase.[Citation74] This suggests that this may be a better way than chemotherapy to stop HIF-1-driven metastasis and angiogenesis.
Chrysin and skin cancer
In many parts of the world, there are a lot of people who get skin cancer. There may be more than one reason why skin cancer has become more common over the past few decades. Changes in the climate, especially in the ozone layer, as well as individual and group habits could be among these causes. Melanoma and other kinds of skin cancer are the ones that doctors see most often. Basal cell carcinoma (also called BCC) and squamous cell carcinoma (also called SCC) are two types of non-melanoma cancer that can be further broken down. People with this kind of cancer usually have a very bad outlook on their health.[Citation97] Basal cell carcinoma is the main cause of 80% of all skin cancers that aren’t melanomas. The most important thing that can cause basal cell cancer (BCC) is being exposed to UV rays. Even though aggressive melanomas only make up 1% of all skin cancers, they cause 60% of skin cancer deaths. Melanoma is more likely to be found in guys than in women. Researchers found that this disease affects white people in the United States fifteen times more often than it affects black people. Some of the things that should be looked at and found more quickly are: more time in the sun, bad environmental conditions, a family history of the condition, and the need for more tracking. Changes in a person’s genes can make a difference in how likely they are to get skin cancer. UV rays from the sun cause DNA damage, gene changes, immune inhibition, oxidative stress, and inflammatory reactions.[Citation98] All of these things contribute to the development of skin cancer. UV rays are also a major cause of skin aging, which is another important factor in the growth of skin cancer. Melanoma patients who are in stages I to III usually have surgery as their first treatment. Polyphenols help fight cancer in a lot of different ways. Polyphenols are a large group of phytochemicals that include flavonoids, stilbenes, lignans, and phenol, among other things. In this group of phytochemicals, flavonoids are also included. Flavonoids are a type of phytochemical that is known to be good for human health. Chrysin, which is a type of flavonoid, has the ability to be used as a medicine. Chrysin, 5-hydroxy-7-flavone. Chrysin is an antioxidant that can be found in nature and has been linked to a number of health benefits.[Citation99] They also said that this product may help people with cancer, heart disease, inflammation, liver disease, kidney disease, diabetes, allergies, and nervousness.[Citation100]
Researchers tried Chrysin on the human cancer cell line A375 and looked at the effects. Cell type S2 metastasizes in vitro. The A375 will be sent out the next day with a MEM cover. The next day, one day after 1 105 S2 cells were put into each well of a 24-well plate, the cells were treated with chrysin in triplicate at final concentrations of 0, 5, 10, and 15 mM. The cells were then taken out, washed twice with PBS, labeled with PI (5 g/mL), looked at with a contrast-phase microscope, photographed at a magnification of 200, and the results were written down. Flow cytometry was then used to figure out what percentage of cells were still alive. Since the tests were done three times, there was no transfer of data. Image J was used to do the math that helped figure out the relative size of the cut. More study is needed on cell migration and invasion than just the Transwell invasion experiment mentioned above. They used Gelatin zymography, Western blotting analysis, and confocal laser scanning microscopy. When A375.S2 cells were exposed to 10–15 M chrysin for 24 and 48 hours in vitro, the shape of the cells changed and the number of living cells went down. This shows that the lethal action caused these changes by looking at the effects of 0, 5, 10, and 15 M of chrysin with a scratch wound healing test, a cell migration/invasion experiment, western blotting, and confocal laser imaging. When A375.S2 cells were exposed to 10-15 mM of chrysin for 24 and 48 hours, they killed other cells in the lab. This was shown by changes in the shape of the cells and a drop in the number of healthy cells. At doses between 5 and 15 mM, chytrid was able to stop A375.S2 cells from moving around a lot. After 48 hours of being introduced to 15 mM chrysin, A375.S2 cells were much less likely to invade. Chrysin reduced the activity of MMP-2 by a lot when given for 24 and 48 hours. However, this effect was only noticeable at 10–15 M for 48 hours. After 48 hours of treatment with chrysin at amounts between 5 and 15 millimoles, p-FAK and RhoA were greatly lowered, with the biggest change happening at 15 millimoles. Their study found that the production of GRB2, SOS-1, PKC, and p-AKT is strongly stopped by chrysin at amounts between 5 and 15 mM. In a different set of tests, the effects of chrysin pretreatment on A375 cells were also seen. The cells were washed twice with ice-cold PBS after a total of 48 hours. To break up the cytoplasmic proteins, RIPA that had been added with protease and phosphatase inhibitors was used. Cell scrapers were used to get the cell lysate, and the sample was then whirled around for ten minutes while it was chilled to get rid of any leftover cell waste. After the protein was taken out of the cells, it was mixed with the sample loading water and heated for ten minutes at 95 degrees Celsius. To find out how much protein there was all together, a BCA protein test kit was used. In this study, the amounts of FOXM1 and catenin expression were looked at with the help of RNA extract, reverse transcription PCR, and quantitative real-time PCR.[Citation101] After the A375 cells were marked with IF, they were looked at with confocal imaging. Researchers first looked at how the protein chrysin affected wound healing and cell movement in the human melanoma cell line A375. This helped them figure out what role chrysin plays in the spread of melanoma. At a distance of 10 meters, cells moved much more slowly when chymotrypsin was present. After 48 hours, chrysin was able to stop the p-EGFR protein from being made. The results of a Western blot study of the expression of key EMT markers showed that chrysin treatment can also stop EMT. Also, treatment with chrysin was able to stop the production of MMP-2 and MMP-9, which shows that migrating melanoma cells were less able to break down matrix. The anoikis rate of the A375 cell line was used to find out what happened to melanoma cells when FOXM1 expression was raised. In a lab setting, A375’s health did not get better when FOXM1 was overexpressed. When FOXM1 was overexpressed, the rate of anoikis caused by chrysin went from 31.06% to 16.42%, which is a big change. They found that the pro-apoptotic effect of chrysin did not have anything to do with the fact that melanoma cell spread was stopped both in vivo and in vitro.[Citation102] The results of the studies stated above showed this to be true. Researchers looked into what B16F10 cells that had been treated with Chrysin could do in terms of metabolism and apoptosis. After B16F10 melanoma cells were inserted under the skin of BALB/c mice, they were treated with chrysin (50 mg/kg b.w.) for two weeks to fight cancer.[Citation103] For two weeks, chrysin was given to these animals. The study found that chrysin stopped the growth of cancer cells in a way that depended on the amount. It did this by causing apoptosis and stopping the cell cycle in the G2/M phase. Also, after 21 days of treatment with chrysin, the average number of new melanoma tumors was lowered by 71% compared to the group that already had tumors (after 14 days of treatment, 60% of new tumors were stopped). The chrysin treatment also made natural killer (NK) cells, cytotoxic T lymphocytes (CTL), and macrophages kill more cancer cells. They used a mouse model of melanoma to study the effects of chrysin on tumors. They found that chrysin had a strong anticancer effect, which suggests that it could be used to make new anti-melanoma drugs in the near future.[Citation103] Researchers made a melanoma vaccine that used an antigen made from a soluble protein extract from B16F10 melanoma cells to test how well Chrysin works as a vaccine adjuvant. It was found that the B16F10 soluble protein antigen combined with other antigens in a combination vaccine (CHR) led to a higher mortality rate than the B16F10 antigen vaccine alone. A split vaccination method was used to make the cancer model. In vivo and in vitro experiments were done to find out if CHR could stimulate antigen-presenting cells (APCs) and if it could work as a possible adjuvant. Research done both in vivo and in vitro showed that cytotoxic T lymphocytes (CTLs) were better at fighting cancer when APC stimulation was caused by CHR. After transferring CD8+ T cells from vaccinated mice into B16F10 tumor-bearing mice, researchers saw a big jump in the number of tumor-bearing animals that lived.[Citation104] This finding was reported in his journal. The study proves beyond a doubt that CD8+ T cells are an important part of stopping the growth of melanoma. According to a study, the results suggest that CHR could be used as an adjuvant to make antigens more immunogenic, slow the growth of B16F10 tumors in mice, and stimulate the immune system to fight cancer.[Citation104]
Chrysin’s anticancer impact on prostate cancer
Cancer of the prostate is the second most common cause of death in men who have it. Men who have prostate cancer can be cured about 80% of the time with the help of radiation treatment and surgery to remove the growth. On the other hand, about 20% of men with prostate cancer have a form of the disease that is very invasive.[Citation105] A study shows that between 50 and 60% of solid tumors already have hypoxia zones when they are found. Men between the ages of 75 and 79 have the highest risk of prostate cancer, and it continues to grow quickly after that. It was thought that for every 100,000 guys in that age group, there were 155 new cases. By the time it hit the age group of 65–69, the rate had risen to 510 per 100,000 people. The rate was 751/100,000 in men aged 75–79, which was five times higher than in younger men. Even though a biopsy is the best way to diagnose prostate cancer, there are other ways, such as the digital rectal examination (DRE), transrectal ultrasonography (TRUS), serum PSA (Prostate-Specific Antigen), total PSA, and the ratio of free PSA to total PSA.[Citation106] Prostate cancer can be both cancerous and non-cancerous at the same time. Even though it affects men in their 30s and 40s most often, prostatitis can happen to men of any age. But most people do it in those decades. Prostatitis can be caused by bacterial and mycoplasmal illnesses, urine reflux, autoimmune diseases and muscle conditions.[Citation5] Chrysin is a naturally occurring flavone that can help fight cancer and can be found in honey, propolis, and many plant products. The amount of expression of the proliferating cell nuclear antigen was lower in prostate cancer cell lines that had been treated with chrysin than in prostate cancer cell lines that had not been treated with chrysin. They also showed that the amount of chrysin increases reactive oxygen species (ROS) and lipid breakdown while lowering the mitochondrial membrane potential (MMP).[Citation107]
In the study, both DU145 and PC-3 prostate cancer cell lines were used to look at the direct and secondary effects of chrysin on cancer. After putting twice as many DU145 cells and PC-3 cells in each well of a six-well plate, the cultures were left to grow for 24 hours in serum-free media until they hit confluence (70–80% cell coverage). After that, the cells were treated with chrysin in a way that depended on the amount for 48 hours in a carbon dioxide-filled chamber at 37 degrees Celsius. After trypsin-EDTA was used to separate attached cells from each other, gathering tubes were used to gather the supernatants. After being spun twice in ice-cold PBS, the cells were fixed in 70% ethanol at 4°C for one night. Before the cells were looked at with flow cytometry, they were washed twice with PBS. Then, for 30 minutes at room temperature, they were treated with RNase A (Sigma-Aldrich) and propidium iodide (PI) in PBS. The experiment was done when there was no light. Immunofluorescence imaging was used to find out how chrysin affects how the proliferating cell nuclear antigen (PCNA) is expressed. With the help of 2“,7”-dichlorofluorescin diacetate, researchers were able to find out how many reactive oxygen species (ROS) cancer cells produced. They also did Western blotting and stained the cells with annexin V and propidium iodide (PI). Also, the tunnel test and the lipid peroxidation test were done. At amounts between 0 and 100 M, chrysin was found to cause DU145 and PC3 cancer cells to die by a process called apoptosis. The authors were able to find apoptotic cells with flow cytometry after marking them with FITC-coupled Annexin V and PI and comparing the number of these cells to the number of control cells. When 50 or 100 times the average amount of chrysin was added to DU145 cells, the rate of apoptosis went up by 199% (p = .05) and 269% (p = .01), respectively. Compared to the control PC-3 cells, chrysin increased the rate of death in PC-3 cells by 211% (p 0.01) at 50 M and by 227% (p 0.01) at 100 M. TUNEL stands for “tetramethyl-rhodamine-dUTP-labeled DNA fragments,” and it is a test intended to find apoptosis. After being treated with chrysin, prostate cancer cells show more red light, which is a sign of apoptosis. Chrysin treatment stopped prostate cancer cells from multiplying and increased the number of cells in the sub-G1 part of the cell cycle. These results show that chrysin is the cause of death in both types of prostate cancer cells. It does this by depolarizing mitochondrial membrane potential (MMP), making reactive oxygen species (ROS), and starting lipid peroxidation. The study showed that when chrysin is present in DU145 and PC-3 cells, the expression of a group of proteins that control ER stress goes up. Hypoxic PC-3 cancer cells and a PC-3 xenograft animal model were used to study how chrysin fights cancer. Chrysin was found to help with both kinds of cancer. PC-3 cells are a type of human cancer cell line that is resistant to castration. They reach their full potential when grown in RPMI 1640 media with 10% fetal bovine serum (FBS) and 1% drugs.[Citation107] A chamber with 37°C and 5% CO2 was used to keep the cells alive and healthy. Cells were raised to 37 degrees Celsius and exposed to 1% oxygen, 5% carbon monoxide, and 94% nitrogen to make them act like they were in a low-oxygen environment. The cobalt chloride was then given to the cells in amounts of 150 millimolars. After 48 hours of being treated with 10 mM chrysin, 1 104 PC-3 cells were planted in each well of a 96-well plate that had been covered with 10 mg/mL of Matrigel. After the spheroids were made, photos were taken with a zoom of less than 20 × . A CELLOMAXTM Viability Kit was used to find out how healthy the cells were. Western blotting was done to figure out what was going on with the samples. CellEvent can be used to find apoptotic cell death.[Citation105] The 3D growth model has proven to be a good way to study how cancer cells respond to treatment. This is because the model is based on cancers that have been found in real animals. Chrysin was put into wells that had been covered with Matrigel and had cells in them for 48 hours. This was done to see how it affected the growth of tumors. Chrysin was able to stop PC-3 cells from making tumor spheroids that were up to 100 micrometers in diameter. Using a different method that involved making 3D spheroids, the effect of chrysin on stopping tumors from growing was studied. After the tumor spheroids had grown for four days, chrysin was added to the culture for a total of 48 hours. Four days after being inserted, the PC-3 tumor spheroids had grown to be between 280 and 350 mm in size. The spheroids made from cells treated with chrysin were smaller than those made from normal PC-3 cells. The typical end tumor spheroid diameter in the control group was 342 30.7 m on day 6 after seeding, but it was only 217.3 39 m in the chrysin group. Chrysin cut the size of the tumors down by a lot. In hypoxic PC-3 cells that had been treated with CoCl2, it was shown that chrysin stopped AKT and GSK-3 from getting phosphorylated and shut down SPHK-1 and HIF-1 in a big way. These results suggest that chrysin might block ROS-related pathways, making it less likely that hypoxia-induced HIF-1 and SPHK-1 will be made.[Citation105] The results suggested that HIF-1/SPHK-1 reduction is necessary for chrysin-induced death in PC-3 cells when there isn’t enough oxygen. This is because the apoptotic markers were affected more by the combination treatment with chrysin and SPHK-1 or HIF-1 siRNA than by the single treatments. Research and testing have also been done to see if chrysin might help avoid BPH in an animal model where testosterone causes BPH. In line with what the OECD suggested, female Sprague-Dawley rats were used to test the acute oral toxicity of chrysin. After the animals had gone without food the night before, they were given a dose of chrysin through an IV that was 2000 milligrams per kilogram and 10 milliliters per kilogram. The rats were split into five groups of eight, and each group got one of the following treatments for two weeks, starting on the first day of each week: Rats in group 1 were given a mix of pure DMSO and corn oil an hour before they were injected with olive oil. In group 2, rats were given pure DMSO and corn oil. An hour later, they were injected with 3 mg/kg of testosterone in olive oil through the skin. Each group of rats got either 25 mg/kg of chrysin dissolved in pure DMSO and corn oil (group 4) or 50 mg/kg of chrysin dissolved in pure DMSO and corn oil (group 5). After their last shot of testosterone, the rats were given another 72 hours to live before they were put to sleep and their genital cells were removed. The rats’ prostate glands were taken out and weighed. Researchers could figure out the prostate index for each rat by taking the rat’s total weight and dividing it by the weight of the prostate. Using rats as test subjects, an ELISA kit was used to measure the amount of PSA in the blood. Real-time polymerase chain reaction, immunohistochemistry to find PCNA, NF-kappa B (p65) binding activity evaluation, and tissue study were all done. When the animals were looked at 24 hours after being given an amount of chrysin equal to 2,000 mg/kg by mouth, there were no clear signs of death. When the same amount was given to three other animals, they all had the same symptoms. At doses of 50 and 100 mg/kg, chrysin reduced growth, hyperplasia, and intraluminal projections in the BPH group by a lot, while the histological structure stayed the same. Expression of mRNA for P53, P21, Bax, and Bcl-2 was the same in rats that were given chrysin and rats that were used as controls. Chrysin stopped this rise in testosterone and helped a lot to bring back both of these factors. The levels of the antioxidant enzymes glutathione (GSH), catalase, and superoxide dismutase (SOD) dropped a lot when testosterone was present.[Citation108]
Combating thyroid cancer with chrysin
More than 90% of all other cancers in the endocrine system are caused by cancers of the thyroid. About 56,870 new cases of cancer were found in 2017, which was 3.4% of all cancer cases in the country. After five years, people with pathologically differentiated thyroid cancer have a better than 98% chance of being alive. Papillary thyroid cancer, which is also called differentiated thyroid cancer (DTC), is the most common type of thyroid cancer. It makes up more than 80% of all cases of thyroid cancer. Follicular and medullary thyroid cancers are the other two types of differentiated thyroid cancer. Based on what Saini and his colleagues found in 2018, these cancers start in the follicular and Para follicular cells. They said that badly differentiated, Hurthle cell, and follicular thyroid cancers are high-risk tumors because they tend to spread through the blood to places like the lungs and bones.[Citation109] Researchers also said that the undifferentiated type of thyroid cancer called anaplastic thyroid carcinoma (ATC) has a typical survival rate of about six months, which makes it nearly impossible to treat.[Citation110] Even though it only makes up about 2% of all thyroid cancer cases, it is responsible for between 14% and 50% of all deaths from the disease.[Citation45] Standard treatments for thyroid cancer include removing the growth surgically, using radioactive iodine (RAI), and blocking the thyroid stimulating hormone (TSH).[Citation27] According to the study, one of the many benefits of cancer screenings is that they can help treat and avoid cancer. Flavonoids, which are a big group of naturally occurring aromatic phytochemical chemicals,[Citation111] have been shown to have an effect on how well the thyroid does its job. The flavonoid called chrysin stands out.[Citation112] Chrysin is a phytochemical that is found in a lot of plants. Honey, passion fruit, and mushrooms are three more healthy foods. Chrysin has been found to stop the growth of cancer cells in a number of ways, such as by causing apoptosis, stopping the cell cycle, reducing angiogenesis, and stopping invasion and spread. All of these things happen without hurting good cells.[Citation113] Researchers said that chrysin does this by controlling the different cell signaling pathways that are involved in inflammation, cell growth, angiogenesis, cancer cell entry and spread, and cancer cell survival. Even though chrysin has a molecule that is similar to estrogen, it doesn’t do much of what estrogen does. These phytoestrogens might affect both how thyroid hormone is made and how it is used in the body. So, whether flavonoids are good or bad for your health depends on both the setting and the amount you take. But taking too much phytoestrogen, especially soy isoflavones, which can stop the iodination of human thyroid hormones, can cancel out the benefits of flavonoids.[Citation114]
In vitro, the methyl thiazolyl tetrazolium (MTT) test was used to see if iodo-chrysin stopped the growth of SW-579 and TT thyroid cancer cell lines. Biological tests showed that these chemicals were better at fighting thyroid cancer than 5-FU. Compound 10 was found to kill TT cell lines better than 5-FU (IC50 = 6.2 M), while compound 21 was found to kill SW-579 cell lines better than 5-FU (IC50 = 3.4 M). Also, chrysin, an antioxidant, was looked into to see if it could be used to help ATC cells. The cells were grown by following the instructions that were given by DSMZ. In short, 10% of SW1736 and 8505C cells were grown in RPMI 1640 media with fetal bovine serum (FBS), 100 uM/l of penicillin, non-essential amino acids, and sodium pyruvate. Monolayers of cells grew on plastic plates in an incubator with 95% air, 5% carbon dioxide and a humidifier. The incubator was set to a temperature of 37 degrees Celsius. Human anaplastic thyroid cancer SW1736 and 8505C cells were treated with 50 M Chrysin for 72 hours after being transfected with plasmid SLUG cDNA or small interfering RNA (siRNA) targeting Notch-1 or PUMA for 24 hours. During the treatment, the amount of Chrysin stayed the same. Flow cytometry was used to study the processes of apoptosis and cell survival. The dye 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2 H-tetrazolium bromide (MTT) and the protein Annexin V-FITC were used.[Citation115]
In order to determine the effect of chrysin on the proteins involved in signal transmission pathways, western blot analysis was used. The proteins like Notch-1, SLUG, and PUMA, as well as pro and anti-apoptotic proteins as in Bad, Bid, Bim, Noxa, and Bcl-2. After being treated with 50 nM Chrysin, the findings showed that ATC cell growth was greatly slowed down, and the grown cells began to die. Notch-1 and PUMA became active after being treated with chrysin, while Slug became inactive. Chrysin-induced PUMA activation caused a drop in cell survival and death, but this could be fixed by overexpressing Slug with Slug cDNA transfection or by using siRNA to shut down Notch-1 or PUMA. Both of these ways needed siRNA. Chrysin has shown promise as a possible medicinal medicine because it can stop ATC cells from dividing and speed up their death through apoptosis. When Notch-1 is turned on or when SLUG is turned off, PUMA is released from Slug, where it had been kept. A study found that chrysin works well against ATC cells (SW1736 and 8505C). In the lab, it has been found that chrysin can slow or stop the growth of thyroid cancer cells. The anticancer effects of chrysin on ATC cells (HTH7 and KAT18) were a decrease in cell growth, changes in protein levels, and a rise in the ratio of Bax expression to Bcl-2 expression. These were just a few of the ways that anticancer drugs worked.[Citation90] The expression of cyclin D1, Mcl-1, and XIAP was seen to go down, while the expression of cleaved caspase-3 and cleaved PARP was seen to go up. Chrysin was also found to be able to trigger Notch in ATC cells (HTh7 and KAT18), which is interesting. Chrysin increased the production of Notch 1 and hairy/enhancer of split 1 at the protein and mRNA levels, which stopped cells from dividing. On both levels, Chrysin worked well. The fact that chrysin turned on Notch1-induced apoptosis explained why it could slow the growth of ATC cells. This, in turn, led to a rise in the amount of cleaved PARP protein in living cells, which helped explain how chrysin can stop cells from dividing. A study showed that chrysin not only slowed the growth of ATC xenografts but also made it take on average longer for tumors to form in vivo.[Citation113]
Chrysin’s impact on bone cancer
Cancer is the top cause of death in the world right now, causing one out of every four deaths each year. Cancer of the bones is a very dangerous type of the disease. Researchers say that people with bone cancer have a high death rate, which can be caused by things like a late diagnosis, not enough research and tracking, and not enough testing to see how well medicines are working. Because primary bone tumors are so rare, there isn’t much known about how they happen or what makes them more likely to happen. Even though every year there are 0.9 new cases of cancer in the bones and joints for every 100,000 people, only 0.2% of all cancers that can happen in the body are bone sarcomas. The American Cancer Society predicts that 3,300 new cases of cancer will be found in people in 2016 and that 1,490 people will die from the disease. Metastasis and non-neoplastic diseases like inflammation, bone lumps, and fibrous dysplasia can sometimes look like tumors in the bones. This makes it hard to tell if someone has a real tumor or something else. This is because bone cancers can look like other health problems. Osteosarcomas, are a type of primary bone cancerous tumor that usually affects young people and affects the leg bones. “Metastasis” is the process by which cancer moves to other parts of the body, especially the bones.[Citation116][Citation117]Another study said that more than 80% of people with advanced breast or prostate cancer will have bone metastases by the end of their cancer treatment. Some of the steps in metastatic spread are the breakdown of intercellular adhesion, cell migration, angiogenesis, systemic circulation, circulation survival, avoiding local immune reactions, and growth in faraway organs. Bone metastases are one of the main causes of morbidity because they can cause a wide range of symptoms, such as excruciating pain, inability to move easily, pathologic fractures, spinal cord compression, aplasia of the bone marrow and hypercalcemia.[Citation17] In osteosarcoma cells, chrysin was discovered to dose-dependently cause cell death. Additionally, the researchers discovered that chrysin reduced the expression of matrix metalloproteinases (MMPs), which are vital for the spread of cancer cells to other body regions. By increasing the expression of the pro-apoptotic protein Bax and decreasing the expression of the pro-survival protein Bcl-2, chrysin reduced the proliferation and invasion of osteosarcoma cells.[Citation93] Chrysin reduced the expression of the cancer-causing protein c-Myc and increased the expression of the tumor-suppressor protein p53, which in turn prevented osteosarcoma cells from proliferating and migrating. A natural therapy option for this form of bone cancer, chrysin has powerful anticancer effects against osteosarcoma. To completely comprehend its modes of action and establish its efficacy and safety in humans, additional research is necessary.[Citation118]
According to the study, a person’s future ability to feel pain may depend on how much radiation reduces bone-resorbing osteoclasts in the injured area. In contrast to these other medicines, antioxidants stand out as being especially helpful in the fight against cancer. Antioxidant chemicals like vitamins, flavonoids, and carotenoids have all been looked into to see if they might help keep people from getting sick.[Citation119]
According to another study, chemicals called flavonoids that are made from polyphenols have molecular functions. Chrysin is a very popular flavonoid for many reasons, such as its anti-inflammatory, anti-aging, anti-viral, antioxidant, anti-diabetic, aromatase, and anticancer benefits.[Citation112] Chrysin also stops aromatase from working. Further data has also shown that chrysin helps bone cells grow and change by turning on the ERK/MAPK pathway.[Citation120]
At 37 degrees Celsius and 5% carbon dioxide, a human osteosarcoma (MG-63) cell line was grown in DMEM with 10% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin. It was studied while it was in this environment. About 1.30 hours were spent in a 100% ethanol solution that was bubbling and had chrysin (0.5 mmol) and vanadyl acetylacetonate (0.25 mmol) in it. The end pH was 5. The solid was taken out of the hot green solution with a filter. It was then washed three times in clean ethanol and dried with air. The complex was first made as a fresh stock solution (20 mM) in DMSO. It was then diluted to the right concentrations to get the final concentrations. So that the final amounts could be found, this had to be done. Using a change to the hanging drop method, scientists were able to make multiple spheroids. In 96-well plates, multicellular spheroids were grown for 72 hours in either 1% DMSO in DMEM plus 10% FBS (control) or 100 M of VOchrys in DMEM plus 10% FBS (treatment). After 72 hours, the spheroids were taken out. The loss of spheroid cell survival was then measured using image analysis and the acid phosphatase test. In any future study that looks into how Vochrys might help fight cancer in osteosarcoma, female mice should be used instead of male mice. This is because tumors grow faster and are worse in female mice than in male mice.[Citation121]
In another study, it was found that the complex Vochrys reduced the size of tumors in rats without hurting their health. This suggests that it may help fight cancer. At 37 degrees Celsius and 5% carbon dioxide, the human osteosarcoma cell lines Saos-2 and MG-63 were grown in RPMI-1640 media with 10% fetal bovine serum and 100 units/ml of penicillin/streptomycin. After that, the cells were looked at by the experts. After chrysin (Cayman Chemical) and TRAIL (Sigma Aldrich) were given to cells either separately or together, the MTT test was used to find out how healthy the cells were. After the MTT solution was taken away, the violet crystals that had formed were broken up by adding DMSO. The optical density was found to be 570 nm with the help of a microplate reader. The researchers used a Cell Death Detection ELISA kit to see how the presence of chrysin changed how likely Saos-2 and MG-63 cells were to die when TRAIL was present. The effects of chrysin and TRAIL on the activity of caspase-8, an enzyme that is very important in the early stages of apoptosis, were tested using a colorimetric method. To get total RNA, samples of MG-63 and Saos-2 cells were treated with an Accuzol specific RNA extraction kit (Bioneer).[Citation121] Researchers used Western blotting to figure out how much target gene protein was there. Based on the results of the MTT test, both types of cells grew much slower when they were exposed to chrysin. For chrysin, the IC50 for Saos-2 cells was 45.1%, but it was only 38.5% for MG-63 cells. Both cell types were killed by TRAIL’s cytotoxicity in a similar way. In Saos-2 cells, the IC50 number for TRAIL was 1782 nM, but in MG-63 cells, it was 1750 nM. When 45 M chrysin was used with large amounts of TRAIL to stop Saos-2 cell lines from growing, the IC50 value for TRAIL dropped to 1358 nM. This was also true for MG-63 cell types, which showed that 38 M and TRAIL, when used together, were very good at stopping this cell line from growing. The IC50 value for TRAIL dropped from 2448 nM to 1276 nM when chrysin was also used to treat the cells. This was a pretty big drop. When the CDI was less than 1, all of the doses of TRAIL that were tried together with chrysin made both cell lines grow faster. When the harmful effects of TRAIL on osteosarcoma were studied, it was found that chrysin could lower the IC50 number.[Citation122]
Chrysin’s anticancer impact on blood cancer
Leukemia, lymphoma, myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma are all forms of blood cancer; however, leukemia and lymphoma are the most fatal forms of blood cancer. Leukemia, a form of malignancy, affects white blood cells. Plasma and the three major categories of cells that can be found in blood (white blood cells, red blood cells, and platelets) collaborate to ensure that each component of the blood is able to carry out the functions that are uniquely associated with it. White blood cells are responsible for antibody production. Red blood cells are responsible for transporting oxygen from the lungs to the remainder of the body, including all the diverse tissues. Platelets are blood cells that have the ability to cluster together to stop further hemorrhaging. Leukemia could potentially affect additional categories of blood cells. These aberrant cells, which are typically white blood cells, do not operate properly and appear distinct from healthy blood cells in their mode of operation and appearance. The immune system of the body detects abnormal cells the overwhelming majority of the time. This condition is also harmful to other blood cell types, such as red blood cells and platelets, which may be present in the blood. There are two distinct types of abnormal white blood cells that can contribute to the progression of leukemia. These cells are known respectively, as lymphoid cells and myeloid cells. Myelogenous leukemia is a type of leukemia in which myeloid cells are affected. It is occasionally called myeloid leukemia. Lymphocytic leukemia, on the other hand, is a form of leukemia that originates in lymphoid cells. The rate at which cancerous cells divide is one of the factors that determines whether a case of leukemia is “acute” or “chronic.” Lymphoma is a type of blood cancer that can develop when the uncontrolled development of lymphocytes, a specific type of white blood cell, is permitted to continue for an extended period of time. Lymphocytes are one of the white blood cell categories. The lymphocyte is a type of white blood cell that searches the body’s circulatory system and lymphatic system for diseases that are normally found outside the body. Lymphoma can be caused by any combination of the numerous distinct cell types enumerated above or by any one of these numerous distinct cell types alone. An enlarged lymph node is caused by abnormal lymphocytes outnumbering healthy lymphocytes, which occurs when abnormal lymphocytes develop in an inaccessible region. This results in aberrant lymphocytes being more numerous than healthy lymphocytes. As a direct result of this factor, the size of the lymph node increases.[Citation123] Myelodysplastic syndrome (MDS) can be distinguished from other myeloid disorders by peripheral blood cytopenias, dysplastic hematopoietic differentiation, and the absence of acute leukemia-specific characteristics. This spectrum includes clonal hematopoiesis of uncertain potential (CHIP) and secondary acute myeloid leukemia (sAML).[Citation124] According to the study, the origin of multiple myeloma has been linked to the frequent alteration of more than fifty genes. These modifications have been associated with the diagnosed condition. These genes are responsible for encoding the proteins that play essential functions in a wide variety of cellular processes. Some of these processes include transcription, epigenetic regulation, and pre-mRNA splicing.[Citation125] Included in this category are the diseases juvenile myelomonocytic leukemia, atypical chronic myeloid leukemia, and myelodysplastic syndrome/myelodysplastic neoplasm with ring sideroblasts.[Citation126] According to a study, Multiple myeloma is a type of B-cell malignancy characterized by the unrestrained production of plasma cells. The researchers provided this definition. An abnormally high rate of immunoglobulin synthesis, which consists of heavy- and light-chain monoclonal proteins, is caused by plasma cells. Plasma cells produce immunoglobulins. These plasma cells are the source of this abnormally elevated production rate. Due to this, an abnormally large amount of the Para protein is produced in the bone marrow. Multiple myeloma is estimated to have been diagnosed in 5,500 new patients in the United Kingdom in 2013, making it the seventeenth most prevalent form of cancer in the country. As a direct consequence of their advanced ages (the median age at diagnosis is approximately 70 years), comorbidities influence both the health status of patients and the efficacy of their treatments. This is due to the fact that comorbidities are a direct consequence of patients receiving treatment at such advanced ages.[Citation127] Chrysin is an antimicrobial agent effective against a broad spectrum of pathogens, including bacteria, viruses, and even parasites. DNA topoisomerases and histone deacetylase were inhibited, along with the synthesis of the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-alpha) and interleukin 1 beta (IL-1 beta), while the activity of protective signaling pathways was increased.[Citation65]
Also investigated were the effects of chrysin on mitochondria isolated from normal B-lymphocytes and B-lymphocytes with chronic lymphocytic leukemia (CLL). Chrysin is used to treat chronic lymphocytic leukemia. Cytotoxicity, intracellular reactive oxygen species, the collapse of mitochondrial membrane potential, the ADP/ATP ratio, caspase 3 activation, and death were all found to be substantially and selectively increased in chrysin-treated CLL B-lymphocytes. Chrysin was also responsible for the demise of B-lymphocytes in CLL patients. Chrysin was utilized as a lymphocyte treatment. It was an important discovery that chrysin inhibits complex II and ATPases in the mitochondria of cancer cells. It was determined that chrysin is responsible for this inhibition. In the study, the researchers determined that chrysin induces programmed cell death (apoptosis) in CLL B-lymphocytes by targeting mitochondria preferentially. The researchers are examining human lymphocytes for signs of cell mortality (also known as cytotoxicity), oxidative damage (also known as genotoxicity), and the protective effects of chrysin (CH). It has been demonstrated that chrysin induces apoptosis in CLL B-lymphocytes. Chrysin can also protect human lymphocytes from oxidative stress-induced damage. After isolating blood lymphocytes and treating them with AA (50 M), AA (50 M) + CH (10, 25, 50 M), or CH (50 M), cell viability, mitochondrial and lysosomal damage, and oxidative DNA damage were evaluated.[Citation128] In contrast to AA, CH protected human cells from oxidative DNA damage, the collapse of mitochondrial membrane potential, and the formation of reactive oxygen species, as determined by the study. This was demonstrated by contrasting the two compounds. When CH is used as a treatment for human cells that have been exposed to AA, there is also an increase in glutathione (GSH) levels. This occurrence occurs whenever CH is used to treat human lymphocytes. At doses of 10, 25, and 50 millimolar, CH demonstrated a protective effect against AA-induced oxidative stress, mitochondrial damage, and DNA oxidative damage.[Citation129] Chrysin is being investigated to determine how it protects B-lymphocytes from the onset of lymphocytic leukemia (CLL), the subject of the current study. This investigation is being carried out in the United States. In addition to an increase in intracellular reactive oxygen species (ROS), these pathways include cytotoxicity, a collapse of mitochondrial membrane potential (MMP), activation of caspase-3, an ADP/ATP ratio, and ultimately mortality. At concentrations of 25, 50, and 75 g/ml, chrysin promoted cytotoxicity and cell death in murine lymphoma cells but not in normal fibroblast or lymphocyte cells. According to the research, chrysin was also responsible for a significant decrease in complex II and ATPase activity in the mitochondria of cancer cells. At these concentrations of chrysin, there was a dose-dependent increase in the accumulation of S-phase cells, which led to an arrest in the G1/S-phase phase of the cell cycle. Utilizing flow cytometry to study the cell cycle led to the discovery of this phenomenon. Flow cytometry revealed that a dose-dependent increase in the number of cells that were positive for annexin V and active caspase-3 occurred as the concentration of the test material increased.[Citation3] Researchers discovered that administering chrysin at a dose of 1.3 milligrams per kilogram of body weight to mice with Dalton’s lymphoma ascites significantly decreased the quantity of tumor and increased the mice’s overall probability of survival by 52.6%. The findings revealed that the therapy had chemo protective properties when administered in conjunction with cyclophosphamide, which contributed to a decrease in drug-induced toxicity. This was demonstrated by the fact that there was a decrease in the medication’s toxicity. In addition to hematological indications, evaluations of the function of the liver and kidneys obtained from animal research supported these findings. In addition to hematological indicators, these findings were supported by hematological indicators. Another study found that when using combination therapy, it may be possible to use lesser doses of cyclophosphamide to achieve the same results as when the two medications were administered separately. This is the case because chrysin and cyclophosphamide have a synergistic effect when combined. Chrysin and Hesperetin are two examples of flavonoids that have been researched for their potential immunomodulatory effects. Chrysin and Hesperetin are two examples of flavonoids that have been investigated for their potential immunomodulatory effects. The effect of flavonoids on the proliferation of B and T cells in splenocytes was examined, regardless of whether the cells had been activated by mitogens. Using splenocytes that had been previously co-incubated with target cells, the effect of this interaction on the activity of cytotoxic T lymphocytes (CTL) and natural killer cells (NK) was examined. The purpose of this evaluation was to ascertain the significance of this interaction. It was discovered that a concentration of 3.12 M of any of the investigated flavonoids was sufficient to stimulate lymphocyte proliferation.[Citation130] Chrysin was discovered to have a significant inhibitory effect on lipopolysaccharide (LPS), and lectin was found to stimulate splenocyte proliferation. These two discoveries were made simultaneously and independently by two distinct research organizations. Both of these bits of information were independently discovered by separate researchers at separate times. After treatment with hesperetin, the proliferation capacity of splenocytes was enhanced in response to LPS and lectin. In addition, the presence of the two flavonoids results in a substantial enhancement of the functions of both NK cells and CTLs. According to the study’s findings, flavonoid molecules are responsible for regulating macrophage activity. They accomplish this by affecting lysosomal activity and the production of nitric oxide (NO), which, depending on the concentration, may have anti-inflammatory effects.[Citation131] Mechanisms of action of chrysin against cancers was shown in .
Effect of chrysin combined with chemotherapy drugs
Chrysin has been studied in combination with different drugs used in chemotherapy. The clinical studies specifically based on the use of chrysin for the treatment of cancers or chrysin combined with chemotherapy drugs, are not widely available. Most of the studies have been done in-vitro using chemotherapy drugs.
Researchers used chrysin and silibinin in combination to assess their combined efficiency against breast cancer cells. Results exhibited down-regulation of cyclin-D1 and hTERT-genes, leading to the anti-cancer effects due to synergistic activity of chrysin combined with silibinin. Similarly, in another study, chrysin was complexed with poly lactic-co-glycolic acid (PLGA) and PEG i.e. poly (ethylene glycol) to obtain chrysin-PLGA-PEG complex. Authors reported that PLGA-PEG-chrysin complex exhibited superior anticancer effects by inhibited the proliferation of against gastric cell lines.[Citation132]
Cisplatin, a chemotherapy drug, is widely used against cancers, however, drug resistance is a challenge in the application of cisplatin. Chrysin and cisplatin were used in combination against liver cancer cells (Hep G2). This combination enhanced the activation of ERK1/2 in Hep G2 cancer cells. As a result of which expression of anti-apoptotic protein Bcl-2 was suppressed and the pro-apoptotic proteins DR5 and Bax overexpressed. This phenomenon lead to increased apoptosis in liver cancer cells. The results of the study exhibited the synergistic effect of chrysin with cisplatin, where chrysin reduced the degree of resistance to cisplatin leading to enhanced drug efficiency.[Citation133]
In a recently conducted study, chrysin was used in combination with 5‑FU; 5‑fluorouracil (5‑FU) against gastric cancer AGS cells. Data indicated enhanced 5 FU chemotherapeutic effects on gastric cancerous AGS cells as well as AGS/FR-cells through cell-cycle arrest. The obtained results suggested possible synergy of chrysin with 5-FU against cancer cells.[Citation27] In another study, chrysin was complexed with selenium polyurea dendrimer (a novel chemotherapy drug carrier). The anticancer effects in ovarian cancer cells were studied. The cytotoxic effects of the selenium-chrysin polyuria dendrimer were enhanced toward cancerous cells as well increased GSH depletion was observed. Authors suggested that chrysin could be used in combination with other chemotherapy drug carriers based on the obtained results.[Citation78] summarized the results of chrysin against different cancers
Table 1. Chrysin against different cancers (Summarized).
Conclusion
Chrysin is a type of polyphenol that is found in nature and has many properties that stop cancer cells from growing. In this study, data has been summarized to show that there is proof that chrysin may help treat cancers of the breast, digestive system, liver, prostate, and other organs. Chrysin may also help treat cancers in several other parts of the body as well. Several studies have shown that chrysin has positive potential, which stop cancer cells from growing and dividing through several mechanisms. It can cause death in almost all types of cancer cells. Not only did chrysin stop Nrf2 and the genes it controls from working, but it also caused MCF-7 breast cancer cells to die via apoptosis. Based on how chrysin is thought to work, chrysin probably works by making caspases and stopping the Akt signaling pathway from causing cancers. According to the results of studies done by experts, chrysin treatment lowers the development of the Toll-4 receptor gene (TLR-4), as well as the production of the caspase-3 protein, liver necrosis, and astrocyte edema. Apoptosis is set off in ATC cells by chrysin, which also stops these cells from growing. Its effect depends on both Notch-1 being turned on and SLUG being turned off at the same time. By stopping the ROS-driven Akt/mTOR signaling pathway, Chrysin has shown us its ability to boost autophagy in EC cells, which led to more autophagic breakdown. In other studies, it was found that when chrysin activates AMPK, it shuts down Akt, stops human lung cancer cells from growing, and makes them commit suicide (i.e. apoptosis). Some of the processes are the activation of GPER and the decrease of ROCK1, TAGLN2, and FCHO2 expressions, that to prevent onset of cancers. Mitogen-activated protein kinases (MAPK), ERK1/2, and P38 proteins were turned on in prostate cancer cells when Chrysin was used. On the other hand, it can stop phosphoinositide 3-kinase (PI3K) from working and lower the levels of the proteins AKT, P70S6K, S6, and P90RSK. Phosphoinositide 3-kinase (PI3K) levels were found to go down when chrysin was present. Chrysin has the potential to be used as a medicine because it stops cancer cells from dividing, which means it could be used to treat cancers. Based on the fact that chrysin stops tumors from growing and is safe and doesn’t hurt or mutate normal cells, this substance could be used with chemotherapeutic drugs to treat cancer. Both the process of apoptosis of cancer cells caused by chrysin and the aftermath of the process need more research studies.
Acknowledgments
Authors are thankful to the Muhammad Nawaz Shareef University of Agriculture Multan, Pakistan, Government College University Faisalabad Pakistan and Agricultural Extension Directorate, MAAR, Damascus, Syria for providing library facilities for data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry. 2018, 145, 187–196. DOI: 10.1016/j.phytochem.2017.09.016.
- Kamat, S.; Kumari, M.; Sajna, K. V.; Jayabaskaran, C. Endophytic Fungus, Chaetomium Globosum, Associated with Marine Green Alga, a New Source of Chrysin. Sci. Rep. 2020, 10(1), 18726. DOI: 10.1038/s41598-020-72497-3.
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I. E.; Shariati, M. A.; Shahbaz, M.; Qaisrani, T. B.; Shah, Z. A.; Plygun, S.; Heydari, M. Chrysin: Pharmacological and Therapeutic Properties. Life Sci. 2019, 235, 116797. DOI: 10.1016/j.lfs.2019.116797.
- Maasomi, Z. J.; Soltanahmadi, Y. P.; Dadashpour, M.; Alipour, S.; Abolhasani, S.; Zarghami, N. Synergistic Anticancer Effects of Silibinin and Chrysin in T47D Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18(5), 1283.
- Zhang, L.; Wang, Y.; Qin, Z.; Gao, X.; Xing, Q.; Li, R.; Wang, W.; Song, N.; Zhang, W. Correlation Between Prostatitis, Benign Prostatic Hyperplasia and Prostate Cancer: A Systematic Review and Meta-Analysis. J. Cancer. 2020, 11(1), 177. DOI: 10.7150/jca.37235.
- Coleman, R. E.; Brown, J.; Holen, I. Bone metastases. Abeloff’s Clin. Oncol. 2020, 809–830. DOI: 10.1016/B978-0-323-47674-4.00056-6.
- Sulaiman, G. M.; Jabir, M. S.; Hameed, A. H. Nanoscale Modification of Chrysin for Improved of Therapeutic Efficiency and Cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2018, 46(sup1), 708–720. DOI: 10.1080/21691401.2018.1434661.
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11(1). DOI: 10.4081/oncol.2017.321.
- Eatemadi, A.; Daraee, H.; Aiyelabegan, H. T.; Negahdari, B.; Rajeian, B.; Zarghami, N. Synthesis and Characterization of Chrysin-Loaded PCL-PEG-PCL Nanoparticle and Its Effect on Breast Cancer Cell Line. Biomed. Pharmacother. 2016, 84, 1915–1922. DOI: 10.1016/j.biopha.2016.10.095.
- Tang, Q.; Ji, F.; Guo, J.; Wang, J.; Li, Y.; Bao, Y. Directional Modification of Chrysin for Exerting Apoptosis and Enhancing Significantly Anti-Cancer Effects of 10-Hydroxy Camptothecin. Biomed. Pharmacother. 2016, 82, 693–703. DOI: 10.1016/j.biopha.2016.06.008.
- Wang Qin, Z.; Gao, X.; Xing, Q. Chrysin Enhances Cisplatin Sensitivity of Human Cervical Cancer Cells by Modulating the MAPK Pathway. Molecules. 2020, 25(13), 2941.
- Atessahin, A.; Al-Matubsi, H.; Alghamdi, A. Chrysin Prevents 1,2-Dimethylhydrazine-Induced Oxidative Damage and Inflammation in Rat Colon. Anticancer. Res. 2019, 39(3), 1193–1200.
- Rana, S. V.; Pal, R.; Vaiphei, K.; Sharma, S. K.; Ola, R. P. Protective Effect of Chrysin on Oxidative Stress and Inflammatory Markers in Liver and Kidney of Galactosamine-Induced Hepatitis in Rats. J. Environ. Pathol. Toxicol. Oncol. 2013, 32(2), 145158.
- Tang, H.; Singh, R. P.; Canè, S. Anticancer Effects of Chrysin on Hepatocellular Carcinoma Progression by Targeting IL-6/STAT3 Signaling Pathway. Nutrients. 2019, 11(2), 389.
- Chen, Y.; Jiang, Z.; Li, C.; Hu, H.; Wang, T. Chrysin Induces Cell Apoptosis and Inhibits COX-2 Expression in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19(2), 597.
- Biswas, S. K.; Chowdhury, A.; Das, J.; Roy, A.; Ashfaq, M. K. Effect of Raspberry Ketone on Normal, Obese and Health-Compromised Obese Mice: A Preliminary Study. J. Diet. Suppl. 2021, 18(1), 1–13. DOI: 10.1080/19390211.2019.1674996.
- Sun, L. R.; Zhou, W.; Zhang, H. M.; Guo, Q. S.; Yang, W.; Li, B. J.; Sun, Z. H.; Gao, S. H. Modulation of multiple signaling pathways of the plant-derived natural products in cancer. Frontiers in oncology. 2019, 9, 1153.
- Zheng Wang, J. N.; Xiong, X. K.; Yang, X. F. Chrysin Suppresses Tumor Progression and Metastasis in Nasopharyngeal Carcinoma by Downregulating CC Chemokine Ligand. J. Cell. Biochem. 2018, 119(7), 5845–5854.
- Choi, E. J.; Lee, S.; Lee, J.; Kim, Y. J.; Kim, K. T.; Kim, H. Y.; Kim, Y. K. Pharmacokinetics and Anti-Inflammatory Effects of Chrysin-Loaded Solid Lipid Nanoparticles in Mice. Int. J. Nanomed. 2019, 14, 4519.
- Kim, K. M.; Lim, H. K.; Shim, S. H.; Jung, J. Improved Chemotherapeutic Efficacy of Injectable Chrysin Encapsulated by Copolymer Nanoparticles. Int. J. Nanomed. 2015, 12, 1917. DOI: 10.2147/IJN.S132043.
- Al-Rimawi, F.; Al-Matubsi, H.; Alghamdi, A. Pharmacokinetics and Oral Bioavailability of Chrysin-Loaded Solid Lipid Nanoparticles in Rats. Pharmaceutics. 2019, 11(10), 503.
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing Nutritional Component Chrysin as a Therapeutic Agent: Bioavailability and Pharmacokinetics Consideration, and ADME Mechanisms. Biomed. Pharmacother. 2021, 142, 112080. DOI: 10.1016/j.biopha.2021.112080.
- Al-Rimawi, F.; Al-Matubsi, H.; Alghamdi, A. Enhanced Oral Bioavailability and Anti-Inflammatory Activity of Chrysin Using Chitosan Nanoparticles. AAPS Pharm Scitech. 2018, 19(2), 919–926.
- Chandra, R.; Balachandar, N.; Wang, S.; Reznik, S.; Zeh, H.; Porembka, M. The Changing Face of Gastric Cancer: Epidemiologic Trends and Advances in Novel Therapies. Cancer Gene Ther. 2021, 28(5), 390–399. DOI: 10.1038/s41417-020-00234-z.
- Wang, F. H.; Zhang, X. T.; Li, Y. F.; Tang, L.; Qu, X. J.; Ying, J. E.; Zhang, J.; Sun, L. Y.; Lin, R. B.; Qiu, H., et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer, 2021. Cancer Commun. 2022, 41(8), 747–795. DOI: 10.1002/cac2.12193.
- Chen, L.; Li, Q.; Jiang, Z.; Li, C.; Hu, H.; Wang, T.; Gao, Y.; Wang, D. Chrysin Induced Cell Apoptosis Through H19/let-7a/COPB2 Axis in Gastric Cancer Cells and Inhibited Tumor Growth. Front. Oncol. 2021, 11, 651644. DOI: 10.3389/fonc.2021.651644.
- Lee, S.; Lee, S. K.; Jung, J. Potentiating Activities of Chrysin in the Therapeutic Efficacy of 5fluorouracil in Gastric Cancer Cells. Oncol. Lett. 2021, 21(1), 1–1. DOI: 10.3892/ol.2020.12285.
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells. OncoTargets Ther. 2020, 13, 3277. DOI: 10.2147/OTT.S246031.
- Jia, S. N.; Han, Y. B.; Yang, R.; Yang, Z. C. Chemokines in Colon Cancer Progression. In Seminars in Cancer Biology, Academic Press, 2022; Vol. 86. p 400–407. DOI: 10.1016/j.semcancer.2022.02.007.
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J., et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31(10), 1291–1305. DOI: 10.1016/j.annonc.2020.06.022.
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Maqsummiya, Z. U.; Banerjee, A.; Pathak, S.; Duttaroy, A. K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. DOI: 10.1016/j.biopha.2022.113384.
- Moghadam, E. R.; Ang, H. L.; Asnaf, S. E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A. P. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules. 2020, 10(10), 1374. DOI: 10.3390/biom10101374.
- Lin, Y. M.; Chen, C. I.; Hsiang, Y. P.; Hsu, Y. C.; Cheng, K. C.; Chien, P. H.; Pan, H. L.; Lu, C. C.; Chen, Y. J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells Through Autophagy Induction Unlike 5-Fluorouracil/oxaliplatin. Int. J. Mol. Sci. 2018, 19(6), 1763. DOI: 10.3390/ijms19061763.
- Salama, A. A.; Allam, R. M. Promising Targets of Chrysin and Daidzein in Colorectal Cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021, 892, 173763. DOI: 10.1016/j.ejphar.2020.173763.
- Ozbolat, S. N.; Ayna, A. Chrysin Suppresses HT-29 Cell Death Induced by Diclofenac Through Apoptosis and Oxidative Damage. Nutr. Cancer. 2021, 73(8), 1419–1428. DOI: 10.1080/01635581.2020.1801775.
- Goswami, M.; Ferreira, A. C. Deep Learning Models for Benign and Malign Ocular Tumor Growth Estimation. Comput. Med. Imaging Graph. 2021, 93, 101986. DOI: 10.1016/j.compmedimag.2021.101986.
- Ferreira, T. A.; Grech Fonk, L.; Jaarsma-Coes, M. G.; van Haren, G. G.; Marinkovic, M.; Beenakker, J. W. M. MRI of Uveal Melanoma. Cancers. 2020, 11(3), 377. DOI: 10.3390/cancers11030377.
- Beddard, M.; Baharara, J.; Amini, E. Anticancer Properties of Chrysin on Colon Cancer Cells, in vitro and in vivo with Modulation of Caspase-3,-9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14(3), 177. DOI: 10.15171/ijb.1374.
- Khoo, H. E.; Ng, H. S.; Yap, W. S.; Goh, H. J. H.; Yim, H. S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants. 2019, 8(4), 85. DOI: 10.3390/antiox8040085.
- Webb, P. M.; Jordan, S. J. Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. DOI: 10.1016/j.bpobgyn.2016.08.006.
- Meng, X.; Fang, S.; Zhang, Z.; Wang, Y.; You, C.; Zhang, J.; Yan, H. Preventive Effect of Chrysin on Experimental Autoimmune Uveitis Triggered by Injection of Human IRBP Peptide 1–20 in Mice. Cell. Mol. Immunol. 2017, 14(8), 702–711. DOI: 10.1038/cmi.2015.107.
- Kang, M. K.; Lee, E. J.; Kim, Y. H.; Kim, D. Y.; Oh, H.; Kim, S. I.; Kang, Y. H. Chrysin Ameliorates Malfunction of Retinoid Visual Cycle Through Blocking Activation of AGE-RAGE-ER Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes. Nutrients. 2018, 10(8), 1046. DOI: 10.3390/nu10081046.
- Rong, W.; Wan, N.; Zheng, X.; Shi, G.; Jiang, C.; Pan, K.; Gao, M.; Yin, Z.; Gao, Z. J.; Zhang, J. Chrysin Inhibits Hepatocellular Carcinoma Progression Through Suppressing Programmed Death Ligand 1 Expression. Phytomedicine. 2022, 95, 153867. DOI: 10.1016/j.phymed.2021.153867.
- Song, J. H.; Moon, K. Y.; Lee, S. C.; Kim, S. S. Inhibition of Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor by Chrysin in a Rat Model of Choroidal Neovascularization. Int. J. Mol. Sci. 2020, 21(8), 2842. DOI: 10.3390/ijms21082842.
- Zhang, S. Z.; Xie, L.; Shang, Z. J. Burden of Oral Cancer on the 10 Most Populous Countries from 1990 to 2019: Estimates from the Global Burden of Disease Study 2019. Int. J. Environ. Res. Public Health. 2022, 19(2), 875. DOI: 10.3390/ijerph19020875.
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; Dipalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I. A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health. 2020, 17(9), 3168. DOI: 10.3390/ijerph17093168.
- Vyas, T.; Sood, P.; Kaur, M. Antioxidants in Oral Diseases and Future Prospects and Their Application in Dentistry. J. Adv. Med. Dent. Sci. 2018, 6(5), 53–62.
- Mehnath, S.; Arjama, M.; Rajan, M.; Annamalai, G.; Jeyaraj, M. Co-Encapsulation of Dual Drug Loaded in MLNPs: Implication on Sustained Drug Release and Effectively Inducing Apoptosis in Oral Carcinoma Cells. Biomed. Pharmacother. 2018, 104, 661–671. DOI: 10.1016/j.biopha.2018.05.096.
- Chen, J.; Li, Q.; Jiang, Y. Chrysin Promotes Cisplatin-Induced Apoptosis via Oxidative DNA Damage in Oral Squamous Cell Carcinoma. Biochem. Syst. Ecol. 2023, 108, 104623. DOI: 10.1016/j.bse.2023.104623.
- Xie, Y.; Peng, X. Effects of Chrysin on the Apoptosis in Oral Squamous Carcinoma KB Cell Line and the Underlying Mechanisms. Zhong Nan da Xue Xue Bao. J. Cent. South University Med. Sci. 2019, 44(5), 522–527.
- Yang, Z.; Liu, D.; Zhou, H.; Tao, B.; Chang, L.; Liu, H.; Luo, H.; Wang, D.; Liu, W. A New Nanomaterial Based on Extracellular Vesicles Containing Chrysin-Induced Cell Apoptosis Through Let-7a in Tongue Squamous Cell Carcinoma. Front. Bioeng. Biotechnol. 2021, 9, 766380. DOI: 10.3389/fbioe.2021.766380.
- Celińska-Janowicz, K.; Zaręba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, P-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336. DOI: 10.3389/fphar.2018.00336.
- Tandel, G. S.; Biswas, M.; Kakde, O. G.; Tiwari, A.; Suri, H. S.; Turk, M.; Laird, J. R.; Asare, C. K.; Ankrah, A. A.; Khanna, N. N., et al. A Review on a Deep Learning Perspective in Brain Cancer Classification. Cancers. 2019, 11(1), 111. DOI: 10.3390/cancers11010111.
- Viswanadh, M. K.; Singh, R. P.; Mehata, P.; Agrawal, A. K.; Pawde, D. M.; Sonkar, R.; Muthu, M. S.; Sonkar, R.; Muthu, M. S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics. 2018, 2(1), 70. DOI: 10.7150/ntno.21638.
- Tu, L.; Luo, Z.; Wu, Y. L.; Huo, S.; Liang, X. J. Gold-Based Nanomaterials for the Treatment of Brain Cancer. Cancer Biol. Med. 2021, 18(2), 372. DOI: 10.20892/j.issn.2095-3941.2020.0524.
- Wilson, D. W.; Nash, P.; Buttar, H. S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants. 2017, 6(4), 81. DOI: 10.3390/antiox6040081.
- Mishra, A.; Mishra, P. S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y. N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules. 2021, 26(21), 6456. DOI: 10.3390/molecules26216456.
- Marques, J.; Silva, A. M.; Marques, M. P. M.; Braga, S. S. Ruthenium (II) Trithiacyclononane Complexes of 7, 3′, 4′-Trihydroxyflavone, Chrysin and Tectochrysin: Synthesis, Characterisation, and Cytotoxic Evaluation. Inorganica. Chimica. Acta. 2019, 488, 71–79. DOI: 10.1016/j.ica.2019.01.003.
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin Suppresses Proliferation, Migration, and Invasion in Glioblastoma Cell Lines via Mediating the ERK/Nrf2 Signaling Pathway. Drug Des. Devel. Ther. 2018, 12, 721–733. DOI: 10.2147/DDDT.S160020.
- KAMARUDIN, M. N.; Parhar, I. Chrysin Promotes Temozolomide-Induced Apoptosis by Activating p38 MAPK and Suppressing Akt and ERK1/2 in Human Glioblastoma. 2021. DOI: 10.21203/rs.3.rs-196841/v1.
- Han, J. E.; Lim, P. W.; Na, C. M.; Choi, Y. S.; Lee, J. Y.; Kim, Y.; Park, H. W.; Moon, H. E.; Heo, M. S.; Park, H. R., et al. Inhibition of HIF1α and PDK Induces Cell Death of Glioblastoma Multiforme. Exp. Neurobiol. 2017, 26(5), 295. DOI: 10.5607/en.2017.26.5.295.
- Daly, A. A.; Rolph, R.; Cutress, R. I.; Copson, E. R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer: Targets And Therapy. 2021, Volume 13, 241–257. DOI: 10.2147/BCTT.S268401.
- Seely, J. M.; Alhassan, T. Screening for Breast Cancer in 2018—What Should We Be Doing Today? Current Oncol. 2018, 25(s1), 115–124. DOI: 10.3747/co.25.3770.
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O. B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8(5), 1483–1507. DOI: 10.1007/s13346-018-0551-3.
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D. M.; Bernatoniene, J.; Samarghandian, S. Emerging Cellular and Molecular Mechanisms Underlying Anticancer Indications of Chrysin. Cancer. Cell Inter. 2021, 21(1), 1–20. DOI: 10.1186/s12935-021-01906-y.
- Geng, A.; Xu, S.; Yao, Y.; Qian, Z.; Wang, X.; Sun, J.; Zhang, J.; Shi, F.; Chen, Z.; Zhang, W., et al. Chrysin Impairs Genomic Stability by Suppressing DNA Double-Strand Break Repair in Breast Cancer Cells. Cell Cycle. 2022, 21(4), 379–391. DOI: 10.1080/15384101.2021.2020434.
- Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Hasanzadeh, M.; Jabbari, F.; Farkhondeh, T.; Samini, M. Inhibitory and Cytotoxic Activities of Chrysin on Human Breast Adenocarcinoma Cells by Induction of Apoptosis. Pharmacogn. Mag. 2016, 12(Suppl 4), S436. DOI: 10.4103/0973-1296.191453.
- Ganai, S. A.; Sheikh, F. A.; Baba, Z. A. Plant Flavone Chrysin as an Emerging Histone Deacetylase Inhibitor for Prosperous Epigenetic‐Based Anticancer Therapy. Phytotherapy Res. 2021, 35(2), 823–834. DOI: 10.1002/ptr.6869.
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin Inhibited Tumor Glycolysis and Induced Apoptosis in Hepatocellular Carcinoma by Targeting Hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36(1), 1–11. DOI: 10.1186/s13046-017-0514-4.
- Ramalingam, S. S.; Yang, J. C.; Lee, C. K.; Kurata, T.; Kim, D. W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y., et al. Osimertinib as First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36(9), 841–849. DOI: 10.1200/JCO.2017.74.7576.
- Wang, G.; Wang, Q.; Liang, N.; Xue, H.; Yang, T.; Chen, X.; Qiu, Z.; Zeng, C.; Sun, T.; Yuan, W., et al. Oncogenic Driver Genes and Tumor Microenvironment Determine the Type of Liver Cancer. Cell Death Dis. 2019, 11(5), 313. DOI: 10.1038/s41419-020-2509-x.
- Kasala, E. R.; Bodduluru, L. N.; Barua, C. C.; Madhana, R. M.; Dahiya, V.; Budhani, M. K.; Mallugari, R. R.; Maramreddy, S. R.; Gogoi, R. Chemopreventive Effect of Chrysin, a Dietary Flavone Against Benzo (A) Pyrene Induced Lung Carcinogenesis in Swiss Albino Mice. Pharmacol. Rep. 2016, 68(2), 310–318. DOI: 10.1016/j.pharep.2015.08.014.
- Mehdi, S. H.; Zafaryab, M.; Nafees, S.; Khan, A.; Ahmad, I.; Hafeez, Z. B.; Rizvi, M. A. Chrysin Sensitizes Human Lung Cancer Cells to Tumour Necrosis Factor Related Apoptosis-Inducing Ligand (TRAIL) Mediated Apoptosis. Asian Pac J Cancer Biol. 2019, 4(2), 27–33. DOI: 10.31557/apjcb.2019.4.2.27-33.
- Ragab, E. M.; El Gamal, D. M.; Mohamed, T. M.; Khamis, A. A. Impairment of Electron Transport Chain and Induction of Apoptosis by Chrysin Nanoparticles Targeting Succinate-Ubiquinone Oxidoreductase in Pancreatic and Lung Cancer Cells. Genes Nutr. 2023, 18(1), 1–15. DOI: 10.1186/s12263-023-00723-4.
- Maruhashi, R.; Eguchi, H.; Akizuki, R.; Hamada, S.; Furuta, T.; Matsunaga, T.; Endo, S.; Ichihara, K.; Ikari, A. Chrysin Enhances Anticancer Drug-Induced Toxicity Mediated by the Reduction of Claudin-1 and 11 Expression in a Spheroid Culture Model of Lung Squamous Cell Carcinoma Cells. Sci. Rep. 2019, 9(1), 13753. DOI: 10.1038/s41598-019-50276-z.
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Women’s Health. 2019, Volume 11, 287–299. DOI: 10.2147/IJWH.S197604.
- Reid, B. M.; Permuth, J. B.; Sellers, T. A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14(1), 9. DOI: 10.20892/j.issn.2095-3941.2016.0084.
- Santos, I.; Ramos, C.; Mendes, C.; Sequeira, C. O.; Tomé, C. S.; Fernandes, D. G.; Mota, P.; Pires, R. F.; Urso, D.; Hipólito, A., et al. Targeting Glutathione and Cystathionine β-Synthase in Ovarian Cancer Treatment by Selenium–Chrysin Polyurea Dendrimer Nanoformulation. Nutrients. 2019, 11(10), 2523. DOI: 10.3390/nu11102523.
- Li, G.; Li, Y.; Wang, J.; Gao, X.; Zhong, Q.; He, L.; Li, C.; Liu, M.; Liu, Y.; Ma, M., et al. Guidelines for Radiotherapy of Prostate Cancer (2020). Precis. Radiat. Oncol. 2021, 5(3), 160–182. DOI: 10.1002/pro6.1129.
- Lim, W.; Ryu, S.; Bazer, F. W.; Kim, S. M.; Song, G. Chrysin Attenuates Progression of Ovarian Cancer Cells by Regulating Signaling Cascades and Mitochondrial Dysfunction. J. Cell. Physiol. 2018, 233(4), 3129–3140. DOI: 10.1002/jcp.26150.
- Scelo, G.; Larose, T. L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36(36), 3574. DOI: 10.1200/JCO.2018.79.1905.
- Wu, J.; Wang, H.; Ricketts, C. J.; Yang, Y.; Merino, M. J.; Zhang, H.; Shi, G.; Gan, H.; Linehan, W. M.; Zhu, Y., et al. Germline Mutations of Renal Cancer Predisposition Genes and Clinical Relevance in Chinese Patients with Sporadic, Early‐Onset Disease. Cancer. 2019, 125(7), 1060–1069. DOI: 10.1002/cncr.31908.
- Posadas, E. M.; Limvorasak, S.; Figlin, R. A. Targeted Therapies for Renal Cell Carcinoma. Nat. Rev. Nephrol. 2017, 13(8), 496–511. DOI: 10.1038/nrneph.2017.82.
- Soliman, M. M.; Aldhahrani, A.; Gaber, A.; Alsanie, W. F.; Mohamed, W. A.; Metwally, M. M.; Elbadawy, M.; Shukry, M. Ameliorative Impacts of Chrysin Against Gibberellic Acid-Induced Liver and Kidney Damage Through the Regulation of Antioxidants, Oxidative Stress, Inflammatory Cytokines, and Apoptosis Biomarkers. Toxicol. Res. 2022, 11(1), 235–244. DOI: 10.1093/toxres/tfac003.
- Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F. M.; Bengu, A. S.; Ayna, A.; Caglayan, C.; Dortbudak, M. B. Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: A Biochemical and Histopathological Approach. Biol. Trace Elem. Res. 2021, 199(4), 1501–1514. DOI: 10.1007/s12011-020-02268-8.
- Rashid, S.; Nafees, S.; Vafa, A.; Afzal, S. M.; Ali, N.; Rehman, M. U.; Hasan, S. K.; Siddiqi, A.; Barnwal, P.; Majed, F., et al. Inhibition of Precancerous Lesions Development in Kidneys by Chrysin via Regulating Hyperproliferation, Inflammation and Apoptosis at Pre-Clinical Stage. Archiv. Biochem. Biophys. 2016, 606, 1–9. DOI: 10.1016/j.abb.2016.07.004.
- Anwanwan, D.; Singh, S. K.; Singh, S.; Saikam, V.; Singh, R. Challenges in Liver Cancer and Possible Treatment Approaches. Biochim. Biophys. Acta - Rev. Cancer. 2020 1, 1873(1), 188314. DOI: 10.1016/j.bbcan.2019.188314.
- Sherif, I. O.; Al-Mutabagani, L. A.; Sabry, D.; Elsherbiny, N. M. Antineoplastic Activity of Chrysin Against Human Hepatocellular Carcinoma: New Insight on GPC3/SULF2 Axis and LncRNA-AF085935 Expression. Int. J. Mol. Sci. 2020, 21(20), 7642. DOI: 10.3390/ijms21207642.
- Pai, S. A.; Munshi, R. P.; Panchal, F. H.; Gaur, I. S.; Juvekar, A. R. Chrysin Ameliorates Nonalcoholic Fatty Liver Disease in Rats. Naunyn-Schmiedeberg’s Archives Pharmacol. 2019, 392(12), 1617–1628. DOI: 10.1007/s00210-019-01705-3.
- Zhang, Q.; Ma, S.; Liu, B.; Liu, J.; Zhu, R.; Li, M. Chrysin Induces Cell Apoptosis via Activation of the p53/Bcl2/Caspase9 Pathway in Hepatocellular Carcinoma Cells. Exp. Ther. Med. 2016, 12(1), 469–474. DOI: 10.3892/etm.2016.3282.
- Xu, S.; Liu, C.; Zong, Y.; Chen, S.; Lu, Y.; Yang, L.; Ng, E. Y.; Wang, Y.; Wang, Y.; Liu, Y., et al. An Early Diagnosis of Oral Cancer Based on Three-Dimensional Convolutional Neural Networks. IEEE Access. 2019, 7, 158603–158611. DOI: 10.1109/ACCESS.2019.2950286.
- Zhang, Y.; Chen, F.; Xiao, X.; Pan, W.; Yuan, Q.; Cao, J. Chrysin Inhibits Sphere Formation in SMMC-7721 Cells via Modulation of SHP-1/STAT3 Signaling Pathway. Cancer Manag. And Res. 2019, 11, 2977. DOI: 10.2147/CMAR.S193647.
- Huang, C.; Lan, W.; Fraunhoffer, N.; Meilerman, A.; Iovanna, J.; Santofimia-Castaño, P. Dissecting the Anticancer Mechanism of Trifluoperazine on Pancreatic Ductal Adenocarcinoma. Cancers. 2019, 11(12), 1869. DOI: 10.3390/cancers11121869.
- Liot, S.; Balas, J.; Aubert, A.; Prigent, L.; Mercier-Gouy, P.; Verrier, B.; Bertolino, P.; Hennino, A.; Valcourt, U.; Lambert, E. Stroma Involvement in Pancreatic Ductal Adenocarcinoma: An Overview Focusing on Extracellular Matrix Proteins. Front. Immunol. 2021, 12, 612271. DOI: 10.3389/fimmu.2021.612271.
- Farhadi, A.; Homayouni Tabrizi, M.; Sadeghi, S.; Vala, D.; Khosravi, T. Targeted Delivery and Anticancer Effects of Chrysin-Loaded Chitosan-Folic Acid Coated Solid Lipid Nanoparticles in Pancreatic Malignant Cells. J. Biomat. Sci. Polym. Ed. 2022, 34(3), 1–19. DOI: 10.1080/09205063.2022.2121589.
- Lim, H. K.; Kwon, H. J.; Lee, G. S.; Moon, J. H.; Jung, J. Chrysin-Induced G Protein-Coupled Estrogen Receptor Activation Suppresses Pancreatic Cancer. Int. J. Mol. Sci. 2021, 23(17), 9673. DOI: 10.3390/ijms23179673.
- Khazaei, Z.; Ghorat, F.; Jarrahi, A. M.; Adineh, H. A.; Sohrabivafa, M.; Goodarzi, E. Global Incidence and Mortality of Skin Cancer by Histological Subtype and Its Relationship with the Human Development Index (HDI); an Ecology Study in 2018. World Cancer Res. J. 2018, 6(2), e13.
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. North Am. 2020, 100(1), 1–12. DOI: 10.1016/j.suc.2019.09.005.
- Domingues, B.; Lopes, J. M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, Volume 7, 35–49. DOI: 10.2147/ITT.S134842.
- Islam, M. M.; Nagaraja, S.; Hafsa, N. E.; Meravanige, G.; Asdaq, S. M. B.; Anwer, M. K. Polyphenol Chrysin for Management of Skin Disorders: Current Status and Future Opportunities. J King Saud Univ Sci. 2022, 34(5), 102026. DOI: 10.1016/j.jksus.2022.102026.
- Chen, H. Y.; Jiang, Y. W.; Kuo, C. L.; Way, T. D.; Chou, Y. C.; Chang, Y. S.; Chung, J. G. Chrysin Inhibit Human Melanoma A375. S2 Cell Migration and Invasion via Affecting MAPK Signaling and NF‐Κb Signaling Pathway in vitro. Environ. Toxicol. 2019, 34(4), 434–442. DOI: 10.1002/tox.22697.
- Yufei, Z.; Yuqi, W.; Binyue, H.; Lingchen, T.; Xi, C.; Hoffelt, D.; Fuliang, H. Chrysin Inhibits Melanoma Tumor Metastasis via Interfering with the FOXM1/β-Catenin Signaling. J. Agric. Food Chem. 2020, 68(35), 9358–9367. DOI: 10.1021/acs.jafc.0c03123.
- Sassi, A.; Maatouk, M.; Bzéouich, I. M.; Hatira, S. A. B.; Ghedira, S.; Jemni-Yacoub, K.; Chekir-Ghedira, L.; Chekir-Ghedira, L. Chrysin, a Natural and Biologically Active Flavonoid Suppresses Tumor Growth of Mouse B16F10 Melanoma Cells: In vitro and in vivo Study. Chem.-Biol. Interact. 2018, 283, 10–19. DOI: 10.1016/j.cbi.2017.11.022.
- Lu, R.; Wang, S.; Jiang, S.; Li, C.; Wang, Y.; Li, L.; Wang, Y.; Ma, G.; Qiao, H.; Leng, Z., et al. Chrysin Enhances Antitumour Immunity Response Through the IL‐12‐STAT4 Signal Pathway in the B16F10 Melanoma Mouse Model. Scand. J. Immunol. 2022, 96(2), e13177. DOI: 10.1111/sji.13177.
- Han, H.; Lee, S. O.; Xu, Y.; Kim, J. E.; Lee, H. J. SPHK/HIF-1α Signaling Pathway Has a Critical Role in Chrysin-Induced Anticancer Activity in Hypoxia-Induced PC-3 Cel. Cells. 2022, 11(18), 2787. DOI: 10.3390/cells11182787.
- Grozescu, T.; Popa, F. Prostate Cancer Between Prognosis and Adequate/Proper Therapy. J. Med. Life. 2017, 10(1), 5.
- Ryu, S.; Lim, W.; Bazer, F. W.; Song, G. Chrysin Induces Death of Prostate Cancer Cells by Inducing ROS and ER Stress. J. Cell. Physiol. 2017, 232(12), 3786–3797. DOI: 10.1002/jcp.25861.
- Shoieb, S. M.; Esmat, A.; Khalifa, A. E.; Abdel-Naim, A. B. Chrysin Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats. Food Chem. Toxicol. 2018, 111, 650–659. ls, 11(18), p.2787 DOI: 10.1016/j.fct.2017.12.017.
- Cabanillas, M. E.; McFadden, D. G.; Durante, C. Thyroid Cancer. Lancet. 2016, 388(10061), 2783–2795. DOI: 10.1016/S0140-6736(16)30172-6.
- Saini, S.; Tulla, K.; Maker, A. V.; Burman, K. D.; Prabhakar, B. S. Therapeutic Advances in Anaplastic Thyroid Cancer: A Current Perspective. Mol. Cancer. 2018, 17(1), 1–14. DOI: 10.1186/s12943-018-0903-0.
- Gonçalves, C. F.; De Freitas, M. L.; Ferreira, A. C. Flavonoids, Thyroid Iodide Uptake and Thyroid Cancer—A Review. Int. J. Mol. Sci. 2017, 18(6), 1247. DOI: 10.3390/ijms18061247.
- Dastmalchi, N.; Baradaran, B.; Latifi-Navid, S.; Safaralizadeh, R.; Khojasteh, S. M. B.; Amini, M.; Roshani, E.; Lotfinejad, P. Antioxidants with Two Faces Toward Cancer. Life Sci. 2020, 258, 118186. DOI: 10.1016/j.lfs.2020.118186.
- Li, Y.; Zhang, J.; Zhou, H.; Du, Z. Anticancer Effects of Natural Phytochemicals in Anaplastic Thyroid Cancer (Review). Oncol. Rep. 2022, 48(3), 1–13. DOI: 10.3892/or.2022.8368.
- Yang, Y.; Chen, Q.; Yu, W. Y.; Zhang, H. H.; Zhong, Y. S.; Zhang, S. Z.; Wang, J. F.; Yu, C. H. Herbal Active Ingredients: An Emerging Potential for the Prevention and Treatment of Papillary Thyroid Carcinoma. Biomed Res. Int. 2020, 2020, 1–10. DOI: 10.1155/2020/1340153.
- Wei, Y.; Zheng, Q.; Tang, G.; Song, C.; Wang, G.; Zhang, Y.; Xiao, Y.; Zeng, X.; Wang, Z.; Xiao, J., et al. Synthesis and Anti-Thyroid Cancer Effect of Iodo-Chrysin Derivatives. Med. Chem. 2016, 12(5), 441–447. DOI: 10.2174/1573406411666150921111220.
- Hutanu, D.; Popescu, R.; Stefanescu, H.; Pirtea, L.; Candea, A.; Sarau, C.; Boruga, O.; Mehdi, L.; Ciuca, I.; Tanasescu, S. The Molecular Genetic Expression as a Novel Biomarker in the Evaluation and Monitoring of Patients with Osteosarcoma-Subtype Bone Cancer Disease. Biochem. Genet. 2017, 55(4), 291–299. DOI: 10.1007/s10528-017-9801-1.
- Fernandes, R. S.; dos Santos Ferreira, D.; de Aguiar Ferreira, C.; Giammarile, F.; Rubello, D.; de Barros, A. L. B. Development of Imaging Probes for Bone Cancer in Animal Models. A Systematic Review. Biomed. Pharmacother. 2016, 83, 1253–1264. DOI: 10.1016/j.biopha.2016.08.039.
- Zhang, C.; Yu, M.; Hao, F.; Dong, A.; Chen, D.; Zhang, K. Chrysin Inhibits Growth and Induces Apoptosis of Anaplastic Thyroid Cancer Cells via Notch-1/slug/puma Signals. Int. J. Clin. Exp. Pathol. 2017, 9(9), 9038–9047.
- Tseng, Y. D.; Jordan. Radiation Therapy for Painful Bone Metastases: Fractionation, Recalcification, and Symptom Control. Seminars In Radiation Oncol. April 2022, 33(2), 139–147. DOI: 10.1016/j.semradonc.2022.11.004.
- Kim, D. H.; Kim, S. Y.; Park, J. H. Pharmacokinetics of Chrysin and Its Metabolite, Apigenin, After Oral Administration of a Standardized Extract of Propolis in Humans. J. Agric. Food Chem. 2017, 65(12), 2565–2571.
- León, I. E.; Cadavid-Vargas, J. F.; Resasco, A.; Maschi, F.; Ayala, M. A.; Carbone, C.; Etcheverry, S. B. In vitro and in vivo Antitumor Effects of the VO-Chrysin Complex on a New Three-Dimensional Osteosarcoma Spheroids Model and a Xenograft Tumor in Mice. J. Biol. Inorg. Chem. 2016, 21(8), 1009–1020. DOI: 10.1007/s00775-016-1397-0.
- Xie, B.; Yang, J.; Zhang, J. Chrysin Sensitizes Osteosarcoma Cells Against TRAIL‐Induced Apoptosis. Cell Biol. Int. 2022, 46(11), 1825–1833. DOI: 10.1002/cbin.11879.
- Saritha, M.; Prakash, B. B.; Sukesh, K.; Shrinivas, B. Detection of Blood Cancer in Microscopic Images of Human Blood Samples: A Review. In 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), Chennai, India; IEEE, March 2016; p. 596–600.
- Crees, Z. D.; Ghobadi, A. Cellular Therapy Updates in B-Cell Lymphoma: The State of the CAR-T. Cancers. 2021, 13(20), 5181. DOI: 10.3390/cancers13205181.
- Sperling, A. S.; Gibson, C. J.; Ebert, B. L. The Genetics of Myelodysplastic Syndrome: From Clonal Haematopoiesis to Secondary Leukaemia. Nat. Rev. Cancer. 2017, 17(1), 5–19. DOI: 10.1038/nrc.2016.112.
- Bewersdorf, J. P.; Zeidan, A. M. Risk-Adapted, Individualized Treatment Strategies of Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML). Cancers. 2021, 13(7), 1610. DOI: 10.3390/cancers13071610.
- Zappa, C.; Mousa, S. A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung. Cancer Res. 2016, 5(3), 288. DOI: 10.21037/tlcr.2016.06.07.
- Salimi, A.; Hashemidanesh, N.; Seydi, E.; Baghal, E.; Khodaparast, F.; Ghobadi, H. Restoration and Stabilization of Acrylamide-Induced DNA, Mitochondrial Damages and Oxidative Stress by Chrysin in Human Lymphocyte. Expert Opin Drug Metab. Toxicol. 2021, 17(7), 857–865. DOI: 10.1080/17425255.2021.1940951.
- Salimi, A.; Roudkenar, M. H.; Seydi, E.; Sadeghi, L.; Mohseni, A.; Pirahmadi, N.; Pourahmad, J. Chrysin as an Anti-Cancer Agent Exerts Selective Toxicity by Directly Inhibiting Mitochondrial Complex II and V in CLL B-Lymphocytes. Cancer Invest. 2017, 35(3), 174–186. DOI: 10.1080/07357907.2016.1276187.
- Lakshmi, S.; Suresh, S.; Rahul, B. S.; Saikant, R.; Maya, V.; Gopi, M.; Padmaja, G.; Remani, P. In vitro and in vivo Studies of 5, 7-Dihydroxy Flavones Isolated from Alpinia Galanga (L.) Against Human Lung Cancer and Ascetic Lymphoma. Med. Chem. Res. 2019, 28(1), 39–51. DOI: 10.1007/s00044-018-2260-3.
- Sassi, A.; Bzéouich, I. M.; Mustapha, N.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory Potential of Hesperetin and Chrysin Through the Cellular and Humoral Response. Eur. J. Pharmacol. 2017, 812, 91–96. DOI: 10.1016/j.ejphar.2017.07.017.
- Mohammadian, F.; Abhari, A.; Dariushnejad, H.; Nikanfar, A.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Effects of Chrysin-PLGA-PEG Nanoparticles on Proliferation and Gene Expression of miRnas in Gastric Cancer Cell Line. Iran J. Cancer Prev. 2016, 9(4). DOI: 10.17795/ijcp-4190.
- Li, X.; Huang, J. M.; Wang, J. N.; Xiong, X. K.; Yang, X. F.; Zou, F. Combination of Chrysin and Cisplatin Promotes the Apoptosis of Hep G2 Cells by Up-Regulating p53. Chem.-Biol. Interact. 2015, 232, 12–20. DOI: 10.1016/j.cbi.2015.03.003.